The Role of Insulin-like Peptide in Maintaining Hemolymph Glucose Homeostasis in the Pacific White Shrimp Litopenaeus vannamei
Abstract
:1. Introduction
2. Results
2.1. Glucose Is the Main Component of Shrimp Hemolymph Sugar
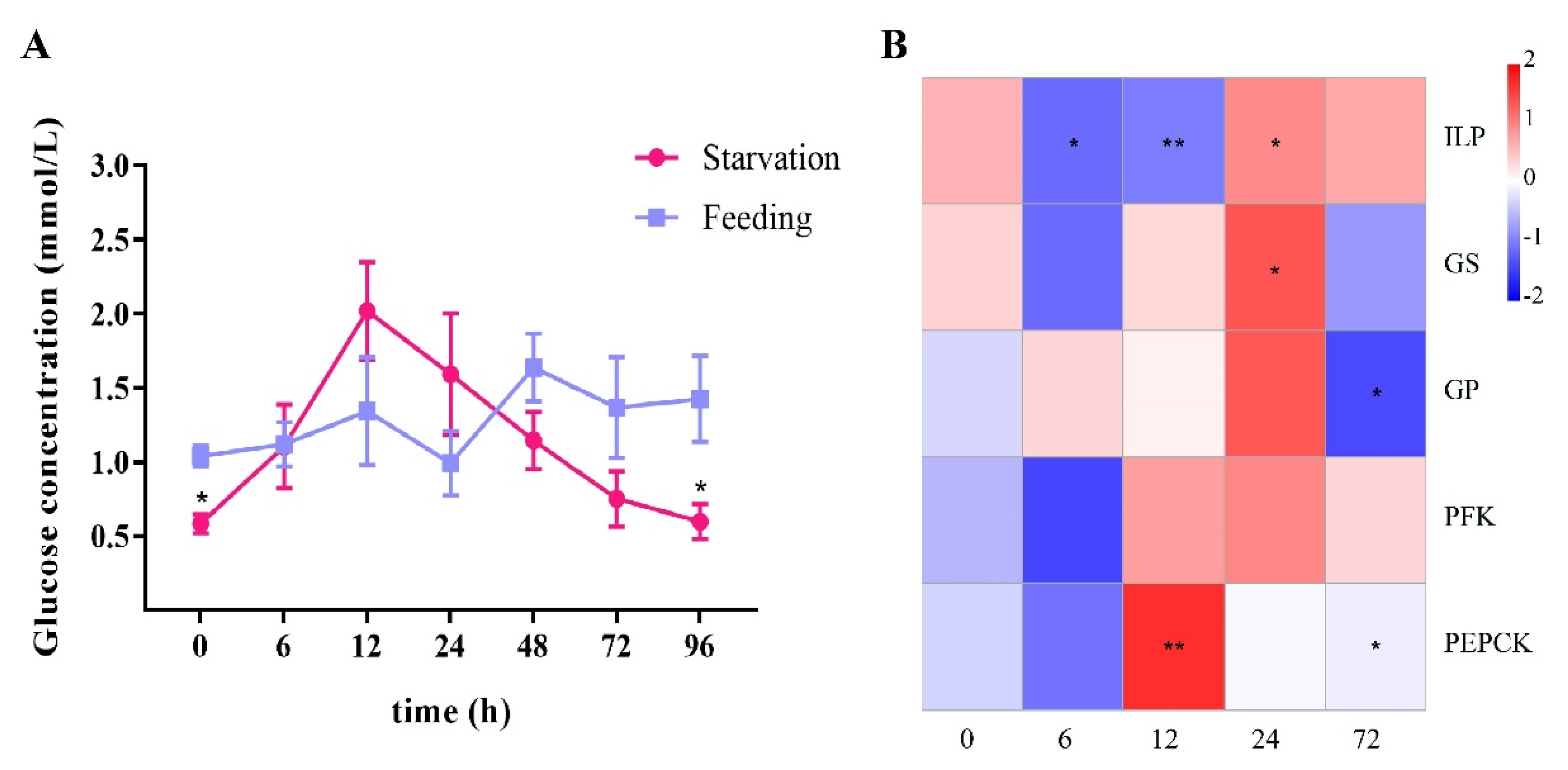
2.2. Starvation Stress Affected Hemolymph Glucose and the Expression of LvILP and Glucose Metabolism Genes
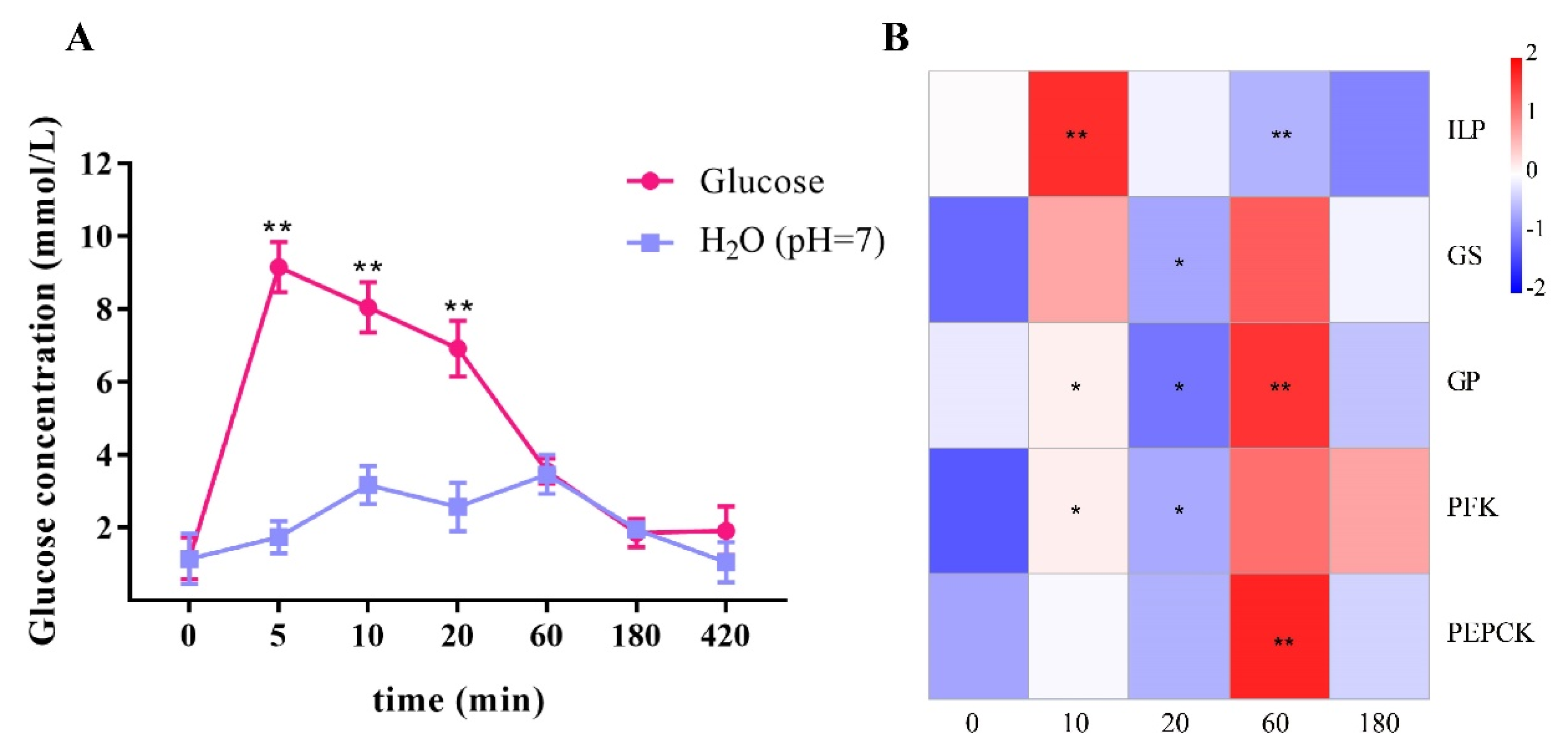
2.3. Exogenous Glucose Was Rapidly Cleaned Up by Activating LvILP and Glucose Metabolism Genes
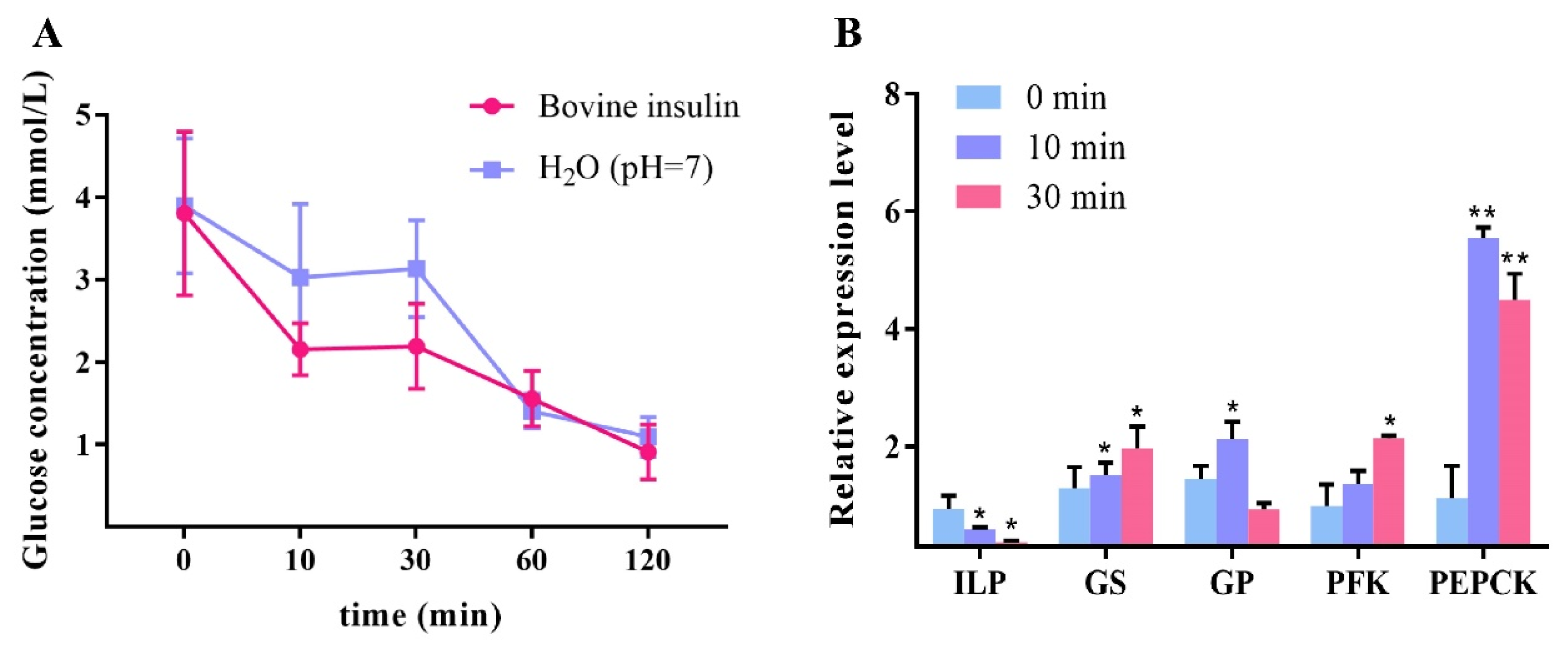
2.4. Bovine Insulin Reduced Hemolymph Glucose and Affected Glucose Metabolism Genes in L. vannamei
2.5. Recombinant LvILP Protein Accelerated the Decline of Hemolymph Glucose in L. vannamei
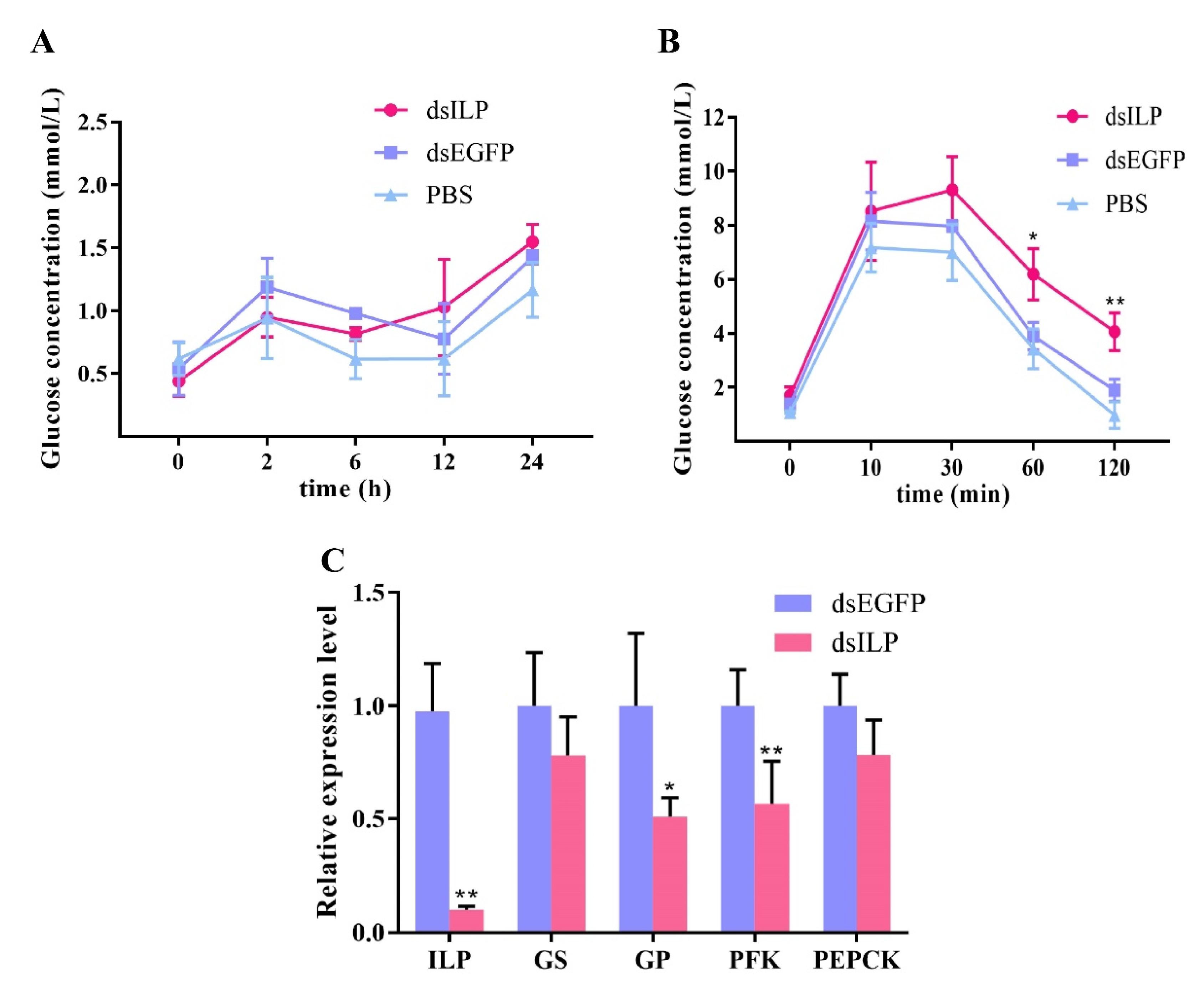
2.6. RNA Interference of LvILP Inhibited the Clearance of Exogenous Glucose and Downregulated the Glucose Metabolism Genes
3. Discussion
3.1. The Main Component of Hemolymph Sugar in Shrimp
3.2. Shrimp Have a Mechanism to Maintain Hemolymph Glucose Homeostasis
3.3. LvILP May Flexibly Regulate Hemolymph Glucose in Multiple Ways
3.4. LvILP Regulates Glucose Metabolism
4. Materials and Methods
4.1. Experimental Animals
4.2. Identification and Cloning of LvILP Gene
4.3. Detection of Hemolymph Sugar Components in L. vannamei
4.4. Starvation Experiment
4.5. Glucose Injection
4.6. Bovine Insulin Injection
4.7. Injection of Recombinant LvILP Protein
4.8. RNA Interference of LvILP Gene
4.9. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saltiel, A.R. Insulin signaling in the control of glucose and lipid homeostasis. Handb. Exp. Pharmacol. 2016, 233, 51–71. [Google Scholar] [CrossRef] [PubMed]
- Norris, D.; Yang, P.; Shin, S.Y.; Kearney, A.L.; Kim, H.J.; Geddes, T.; Senior, A.M.; Fazakerley, D.J.; Nguyen, L.K.; James, D.E.; et al. Signaling heterogeneity is defined by pathway architecture and intercellular variability in protein expression. iScience 2021, 24, 102–118. [Google Scholar] [CrossRef] [PubMed]
- Titchenell, P.M.; Lazar, M.A.; Birnbaum, M.J. Unraveling the regulation of hepatic metabolism by Insulin. Trends Endocrinol. Metab. 2017, 28, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Santoleri, D.; Titchenell, P.M. Resolving the paradox of hepatic insulin resistance. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 447–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wyatt, G.R.; Kale, G.F. The chemistry of insect hemolymph. II. Trehalose and other carbohydrates. J. Gen. Physiol. 1957, 40, 833–847. [Google Scholar] [CrossRef] [PubMed]
- Candy, D.J.; Kilby, B.A. The biosynthesis of trehalose in the locust fat body. Biochem. J. 1961, 78, 531–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, T.A.; Wyatt, G.R. The enzymes of glycogen and trehalose synthesis in silk moth fat body. J. Biol. Chem. 1965, 240, 1500–1508. [Google Scholar] [CrossRef]
- Becker, A.; Schlöder, P.; Steele, J.E.; Wegener, G. The regulation of trehalose metabolism in insects. Experientia 1996, 52, 433–439. [Google Scholar] [CrossRef]
- Shukla, E.; Thorat, L.J.; Nath, B.B.; Gaikwad, S.M. Insect trehalase: Physiological significance and potential applications. Glycobiology 2015, 25, 357–367. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Hong, Y. Regulation of hemolymph trehalose level by an insulin-like peptide through diel feeding rhythm of the beet armyworm, Spodoptera exigua. Peptides 2015, 68, 91–98. [Google Scholar] [CrossRef]
- Wu, Q.; Brown, M.R. Signaling and function of insulin-like peptides in insects. Annu. Rev. Entomol. 2006, 51, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Broughton, S.; Alic, N.; Slack, C.; Bass, T.; Ikeya, T.; Vinti, G.; Tommasi, A.M.; Driege, Y.; Hafen, E.; Partridge, L. Reduction of DILP2 in Drosophila triages a metabolic phenotype from lifespan revealing redundancy and compensation among DILPs. PLoS ONE 2008, 3, e3721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belgacem, Y.H.; Martin, J.R. Disruption of insulin pathways alters trehalose level and abolishes sexual dimorphism in locomotor activity in Drosophila. J. Neurobiol. 2006, 66, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Brent, C.S.; Fennern, E.; Amdam, G.V. Gustatory perception and fat body energy metabolism are jointly affected by vitellogenin and juvenile hormone in honey bees. PLoS Genet. 2012, 8, e1002779. [Google Scholar] [CrossRef] [Green Version]
- Al Baki, M.A.; Lee, D.W.; Jung, J.K.; Kim, Y. Insulin-like peptides of the legume pod borer, Maruca vitrata, and their mediation effects on hemolymph trehalose level, larval development, and adult reproduction. Arch. Insect Biochem. Physiol. 2019, 100, e21524. [Google Scholar] [CrossRef]
- Li, Y.N.; Ren, X.B.; Liu, Z.C.; Ye, B.; Zhao, Z.J.; Fan, Q.; Liu, Y.B.; Zhang, J.N.; Li, W.L. Insulin-like peptide and FoxO mediate the trehalose catabolism enhancement during the diapause termination period in the Chinese oak silkworm (Antheraea pernyi). Insects 2021, 12, 784. [Google Scholar] [CrossRef]
- Telford, M. The effects of stress on blood sugar composition of the lobster, Homarus americanus. Can. J. Zool. 1968, 46, 819–826. [Google Scholar] [CrossRef]
- Meenakshi, V.R.; Scheer, B.T. Metabolism of glucose in the crabs Cancer magister and Hemigrapsus nudus. Comp. Biochem. Physiol. 1961, 3, 30–41. [Google Scholar] [CrossRef]
- Parvathy, K. Blood sugar metabolism during moulting in the isopod crustacean Ligia exotica. Mar. Biol. 1971, 9, 323–327. [Google Scholar] [CrossRef]
- Gallardo, N.; Carrillo, O.; Moltó, E.; Deás, M.; González-Suárez, R.; Carrascosa, J.M.; Ros, M.; Andrés, A. Isolation and biological characterization of a 6-kDa protein from hepatopancreas of lobster Panulirus argus with insulin-like effects. Gen. Comp. Endocrinol. 2003, 131, 284–290. [Google Scholar] [CrossRef]
- Chandler, J.C.; Aizen, J.; Elizur, A.; Hollander-Cohen, L.; Battaglene, S.C.; Ventura, T. Discovery of a novel insulin-like peptide and insulin binding proteins in the Eastern rock lobster Sagmariasus verreauxi. Gen. Comp. Endocrinol. 2015, 215, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Jiang, Z.; Gu, S.; Qian, L.; Li, X.; Gao, X.; Zhang, X. Insights into carbohydrate metabolism from an insulin-like peptide in Macrobrachium rosenbergii. Gen. Comp. Endocrinol. 2020, 293, 113478. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, H.; Wang, L.; Song, L. An insulin-like peptide serves as a regulator of glucose metabolism in the immune response of Chinese mitten crab Eriocheir sinensis. Dev. Comp. Immunol. 2020, 108, 103686. [Google Scholar] [CrossRef]
- Kleinholz, L.H.; Havel, V.J.; Reichart, R. Studies in the regulation of blood-sugar concentration in crustaceans. II. Experimental hyperglycemia and the regulatory mechanisms. Biol. Bull. 1950, 99, 454–468. [Google Scholar] [CrossRef] [PubMed]
- Sanders, B. Insulin-like peptides in the lobster Homarus americanus. III. No glucostatic role. Gen. Comp. Endocrinol. 1983, 50, 378–382. [Google Scholar] [CrossRef]
- Chung, J.S. An insulin-like growth factor found in hepatopancreas implicates carbohydrate metabolism of the blue crab Callinectes sapidus. Gen. Comp. Endocrinol. 2014, 199, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, A.; Nieto, J.; Pozo, F.; Stern, S.; Schoofs, L. Effect of insulin/IGF-I like peptides on glucose metabolism in the white shrimp Penaeus vannamei. Gen. Comp. Endocrinol. 2007, 153, 170–175. [Google Scholar] [CrossRef]
- Wang, S.S.; Li, G.Y.; Liu, Y.K.; Luo, Y.J.; Xu, C.D.; Li, C.; Tang, B. Regulation of carbohydrate metabolism by trehalose-6-Phosphate Synthase 3 in the brown planthopper, Nilaparvata lugens. Front. Physiol. 2020, 11, 575485. [Google Scholar] [CrossRef]
- Elbein, A.D.; Pan, Y.T.; Pastuszak, I.; Carroll, D. New insights on trehalose: A multifunctional molecule. Glycobiology 2003, 13, 17R–27R. [Google Scholar] [CrossRef]
- Frison, M.; Parrou, J.L.; Guillaumot, D.; Masquelier, D.; François, J.; Chaumont, F.; Batoko, H. The Arabidopsis thaliana trehalase is a plasma membrane-bound enzyme with extracellular activity. FEBS Lett. 2007, 581, 4010–4016. [Google Scholar] [CrossRef] [Green Version]
- Tang, B.; Chen, X.; Liu, Y.; Tian, H.; Liu, J.; Hu, J.; Xu, W.; Zhang, W. Characterization and expression patterns of a membrane-bound trehalase from Spodoptera exigua. BMC Mol. Biol. 2008, 9, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grunewald, R.W.; Weber, I.I.; Kinne, R.K. Renal inner medullary sorbitol metabolism. Am. J. Physiol. 1995, 269, F696–F701. [Google Scholar] [CrossRef]
- Amo, K.; Arai, H.; Uebanso, T.; Fukaya, M.; Koganei, M.; Sasaki, H.; Yamamoto, H.; Taketani, Y.; Takeda, E. Effects of xylitol on metabolic parameters and visceral fat accumulation. J. Clin. Biochem. Nutr. 2011, 49, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, W.H.; Liu, Y.L.; Jiang, Y.Q.; He, S.F.; Wang, Q.Q.; Yang, Z.N.; Xu, H.J. Molecular characterization of insulin-like peptides in the brown planthopper, Nilaparvata lugens (Hemiptera: Delphacidae). Insect Mol. Biol. 2020, 29, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Sheng, Z.; Palli, S.R. Juvenile hormone and insulin regulate trehalose homeostasis in the red flour beetle, Tribolium castaneum. PLoS Genet. 2013, 9, e1003535. [Google Scholar] [CrossRef] [Green Version]
- Baker, P.F.; Carruthers, A. Insulin regulation of sugar transport in giant muscle fibres of the barnacle. J. Physiol. 1983, 336, 397–431. [Google Scholar] [CrossRef] [Green Version]
- Trapp, M.; Valle, S.C.; Pöppl, A.G.; Chittó, A.L.F.; Kucharski, L.C.; Da Silva, R.S.M. Insulin-like receptors and carbohydrate metabolism in gills of the euryhaline crab Neohelice granulata: Effects of osmotic stress. Gen. Comp. Endocrinol. 2018, 262, 81–89. [Google Scholar] [CrossRef]
- Shi, Z.K.; Wang, S.; Wang, S.G.; Zhang, L.; Xu, Y.X.; Guo, X.J.; Zhang, F.; Tang, B. Effects of starvation on the carbohydrate metabolism in Harmonia axyridis (Pallas). Biol. Open 2017, 6, 1096–1103. [Google Scholar] [CrossRef] [Green Version]
- Tang, B.; Xu, Q.; Zou, Q.; Fang, Q.; Wang, S.; Ye, G. Sequencing and characterization of glycogen synthase and glycogen phosphorylase genes from Spodoptera exigua and analysis of their function in starvation and excessive sugar intake. Arch. Insect Biochem. Physiol. 2012, 80, 42–62. [Google Scholar] [CrossRef]
- Feng, J.Y.; Chen, J.X.; Luo, L.; Lin, S.M.; Chen, Y.J.; Wang, D.S. Molecular and metabolic adaption of glucose metabolism in the red and white muscle of the omnivorous GIFT tilapia Oreochromis niloticus to a glucose load. Gen. Comp. Endocrinol. 2019, 277, 82–89. [Google Scholar] [CrossRef]
- Jiang, D.; Wu, Y.; Huang, D.; Ren, X.; Wang, Y. Effect of blood glucose level on acute stress response of grass carp Ctenopharyngodon idella. Fish Physiol. Biochem. 2017, 43, 1433–1442. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Yang, Y.; Zhu, X.; Han, D.; Liu, H.; Xie, S. Effects of glucose administration on glucose and lipid metabolism in two strains of gibel carp (Carassius gibelio). Gen. Comp. Endocrinol. 2018, 267, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Lee, V.M. Insulin and insulin-like growth factor-1 regulate tau phosphorylation in cultured human neurons. J. Biol. Chem. 1997, 272, 19547–19553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacAulay, K.; Hajduch, E.; Blair, A.S.; Coghlan, M.P.; Smith, S.A.; Hundal, H.S. Use of lithium and SB-415286 to explore the role of glycogen synthase kinase-3 in the regulation of glucose transport and glycogen synthase. Eur. J. Biochem. 2003, 270, 3829–3838. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]

| Carbohydrate | Substance Class | Concentration (mmol/L) |
|---|---|---|
| Xylitol | Monosaccharide | 0.109 |
| Inositol | Monosaccharide | 0.053 |
| Glucose | Monosaccharide | 1.726 |
| D-Sorbitol | Monosaccharide | 0.049 |
| L-Rhamnose | Monosaccharide | - |
| L-Fucose | Monosaccharide | - |
| D-Galactose | Monosaccharide | - |
| D-Fructose | Monosaccharide | - |
| D-Arabinose | Monosaccharide | - |
| Trehalose | Disaccharide | 0.073 |
| Maltose | Disaccharide | 0.151 |
| Sucrose | Disaccharide | - |
| Lactose | Disaccharide | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, M.; Zhang, X.; Yuan, J.; Zhang, X.; Li, F. The Role of Insulin-like Peptide in Maintaining Hemolymph Glucose Homeostasis in the Pacific White Shrimp Litopenaeus vannamei. Int. J. Mol. Sci. 2022, 23, 3268. https://doi.org/10.3390/ijms23063268
Su M, Zhang X, Yuan J, Zhang X, Li F. The Role of Insulin-like Peptide in Maintaining Hemolymph Glucose Homeostasis in the Pacific White Shrimp Litopenaeus vannamei. International Journal of Molecular Sciences. 2022; 23(6):3268. https://doi.org/10.3390/ijms23063268
Chicago/Turabian StyleSu, Manwen, Xiaojun Zhang, Jianbo Yuan, Xiaoxi Zhang, and Fuhua Li. 2022. "The Role of Insulin-like Peptide in Maintaining Hemolymph Glucose Homeostasis in the Pacific White Shrimp Litopenaeus vannamei" International Journal of Molecular Sciences 23, no. 6: 3268. https://doi.org/10.3390/ijms23063268
APA StyleSu, M., Zhang, X., Yuan, J., Zhang, X., & Li, F. (2022). The Role of Insulin-like Peptide in Maintaining Hemolymph Glucose Homeostasis in the Pacific White Shrimp Litopenaeus vannamei. International Journal of Molecular Sciences, 23(6), 3268. https://doi.org/10.3390/ijms23063268





