1. Introduction
Duchenne muscular dystrophy (DMD) is an X-linked muscle wasting disorder that affects approximately 1 in 5000 live male births [
1]. Patients suffer from progressive muscle weakness and become wheelchair bound in their early teens. DMD is caused by a mutation in the dystrophin gene, resulting in loss of functional dystrophin protein. Dystrophin is a key component of the dystrophin-associated protein complex that plays an important role in the stabilization of the sarcolemma by connecting the intracellular cytoskeleton to the extracellular matrix. Dystrophin deficiency compromises the sarcolemmal integrity of myofibers and fibers are more susceptible to contraction-induced damage, leading to repetitive cycles of muscle degeneration and regeneration, inflammation, and fibrosis [
2,
3,
4].
In muscle samples from DMD patients, edema and excessive sodium levels are present [
5], suggesting osmotic perturbations play a role in DMD pathology. When cells are subjected to osmotic stress, they import or synthetize osmolytes to counteract the osmotic imbalance. This build-up of organic osmolytes is orchestrated by nuclear factor of activated T cells 5 (NFAT-5)-induced activity of genes coding for members of osmolyte pathways. The latter include aldose reductase (AR), an enzyme that catalyzes the reduction of glucose to sorbitol, and osmolyte transporters such as taurine transporter (TauT), betaine GABA transporter 1 (BGT), and the sodium/myo-inositol transporter 1 (SMIT), responsible for cellular import of osmolytes, respectively taurine, betaine, and myo-inositol. Osmolytes can also contribute to membrane stabilization and serve as anti-oxidants [
3]. In muscle samples from DMD patients, increased protein levels of AR, TauT, and SMIT have been observed [
6], which might support the regenerative processes taking place in dystrophin-deficient tissue.
The most widely used animal model for DMD is the
mdx (X-chromosome-linked muscular dystrophy) mouse. Similar to DMD pathology in humans, the lack of dystrophin in the
mdx mouse model manifests as loss of muscle force, inflammation, and myofiber necrosis. While the diaphragm of
mdx mice shows progressive and severe deterioration over the whole life span, hindlimb muscle damage appears early in life and stabilizes afterwards. Approximately 3 to 4 weeks after birth, necrosis is reported in 30–60% of the myofibers in the tibialis anterior [
7,
8]. This period of extensive muscle damage is followed by repetitive cycles of degeneration and regeneration, after which necrosis persists further at lower levels. Within 7 to 10 days after necrosis, myoblasts become activated, proliferation and differentiation take place, and myoblasts fuse into myotubes, which mature over time [
8,
9,
10]. Regenerated myofibers are identified by the presence of central nuclei, which appear around 1 to 4 days after replication of myoblasts and migrate from the center to the periphery in approximately 50–100 days [
7,
10,
11]. Myofibers with one or more central nuclei thus represent the regenerative response to myofiber necrosis. Hence, the severity and progressive nature of the human disease is not fully reflected by the condition of skeletal muscles of the
mdx mouse. Nonetheless, the unique features of this model allow the detailed assessment of changes in expression of osmolyte pathway members in relation to the age-dependent degenerative and regenerative processes occurring in the skeletal muscle of the
mdx mouse.
Expression of TauT has been studied in the
mdx mouse model, showing its significant downregulation in the quadriceps of
mdx mice [
12]. Our current study is, however, the first to investigate multiple osmoregulatory components in maturing
mdx mice. The nature of osmo-deregulation in DMD remains poorly understood, therefore we aimed to further study the relation between osmoregulation and dystrophinopathology. We examined the expression of osmolyte pathway members using qPCR, immunofluorescence, and Western blot in the skeletal muscles and diaphragm of
mdx mice at different ages, revealing differential expression patterns.
3. Discussion
Scientific evidence has accumulated for the involvement of osmotic stress in muscular dystrophy, and we previously observed upregulation of osmolyte pathway members TauT, SMIT, and AR in muscle tissue from DMD patients [
6]. The aim of the current study was to investigate possible disease stage-related effects on osmolyte pathway activation in the
mdx mouse model and, somewhat unexpectedly, our results revealed a differential regulation of individual osmolyte transporters, with upregulation of SMIT and downregulation of TauT protein levels in the
mdx mouse model. These findings suggest a more complex regulation of osmolyte pathway members than general stress damage control in the
mdx mouse model. Our results indicate variations of osmolyte pathway activation that seem to follow the trajectory of disease progression, with lower TauT protein levels in the most active phase of muscle degeneration/regeneration and normal levels in hind limb muscle of 26 week-old
mdx mice, at which time muscle damage stabilizes. These results reveal complex and underappreciated involvements of osmolyte pathway members in DMD pathology, affirming the topic as a research interest and future therapeutic target in dystrophinopathy. This descriptive study in the
mdx model contributes to the understanding of differences in pathological features between the mouse model and DMD patients.
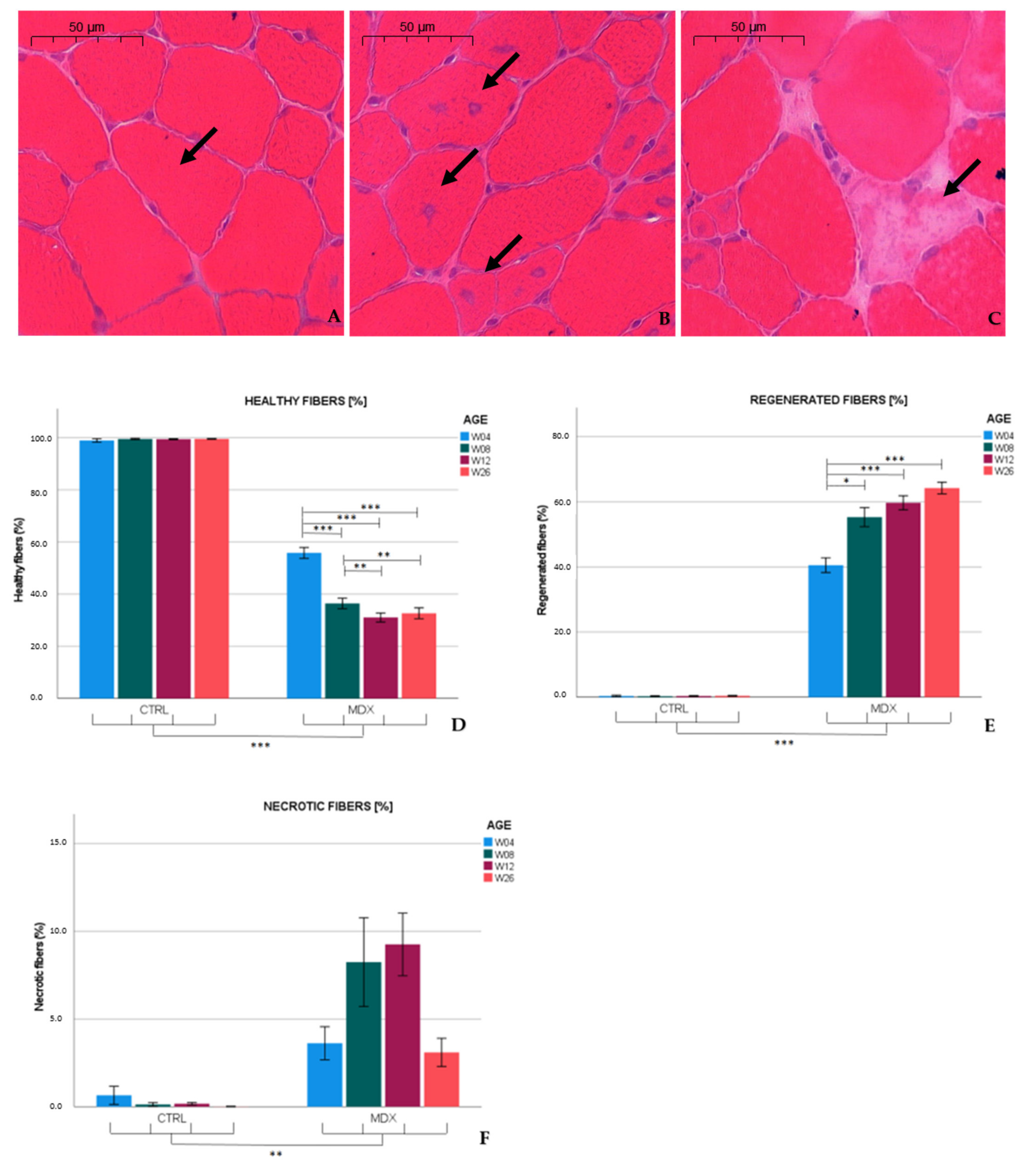
Similar to published observations [
13,
14], we found necrotic hallmarks (3.6%) and myofibers with central nuclei (40.7%), indicative of regenerated fibers, in the tibialis anterior of 4 week-old
mdx mice. At age 12 weeks, necrotic and regenerated fibers were observed in 9.2 and 59.9% of the myofibers, respectively. Our results are in line with previous studies that reported necrosis varying from 1 to 7% in 12 week-old
mdx mice [
15,
16,
17], whereas 70% of the myofibers exhibited a central nucleus at age 13 weeks [
15]. In the adult
mdx mouse, low graded necrosis has been described [
7,
18], which is consistent with our observations (≈3.1% of the myofibers affected) in 26 week-old
mdx mice. Furthermore, the fraction of regenerated fibers increased to 64.2%. Higher levels of regenerative fibers might have been hypothesized; however, these fibers might be repeatedly subjected to necrosis [
11], which could explain the non-linearity between the total amount of fibers subjected to necrosis and the percentage of regenerated fibers observed. These results indicate that necrosis was most prominent in skeletal muscle of 12 week-old
mdx mice, and persists at slower pace in older mice.
In muscle biopsies of young DMD patients, the amount of necrotic fibers remains stable at 1.5–1.8% in patients older than 3 years, and the percentage of fibers with centralized nuclei significantly increase from age 1 (1.31%) to 10 years (8.9%). Connective tissue (30%) is already highly abundant in young (7–10 years) DMD patients [
19], a feature that becomes conspicuous in older
mdx mice [
7].
We were the first to recently show the involvement of NFAT-5-mediated pathways and general activation of its downstream osmolyte pathway members in the pathogenesis of DMD [
6,
20]. In the
mdx mouse model, we observed that protein levels of TauT and SMIT were altered in
mdx mice in comparison to age-matched controls, though without significant changes at the mRNA level. Presumably, these factors could be regulated via post-translation modifications [
21,
22].
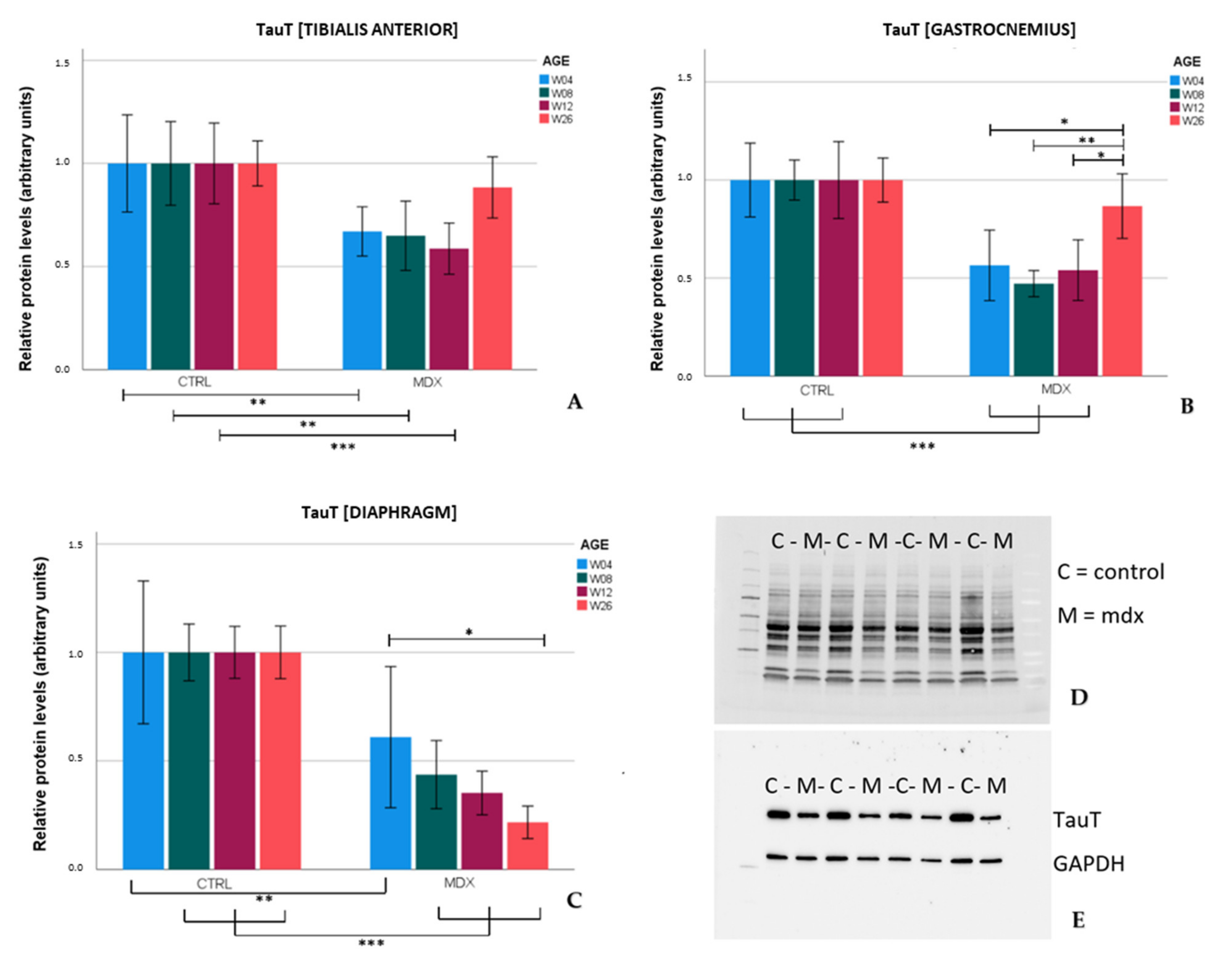
TauT protein levels were significantly lower in the tibialis anterior and gastrocnemius of
mdx mice aged 4, 8, and 12 weeks in comparison to age-matched control mice. These results were in line with the low levels of TauT reported in quadriceps of 18 and 42 day-old
mdx mice [
12]. In the skeletal muscles of older
mdx mice (aged 26 weeks), TauT protein levels practically normalized to control levels, which coincided with lower graded necrosis. Protein levels of the transporter were significantly downregulated in the diaphragm of
mdx mice as well, most explicitly at age 26 weeks. Unlike stabilization of dystrophinopathy in the skeletal muscle by age 26 weeks, damage to the diaphragm continuously increases with age [
7], which could fit the tissue’s progressive decrease in TauT levels. Diminished levels of TauT, and subsequently a low intramuscular taurine concentration, are presumably associated with muscle wasting. In evidence, inhibition of TauT by guanodinoethane sulfonate supplementation in mice reduced taurine muscle content and resulted in a small but significant decline in force output when compared to controls [
23]. Furthermore,
TauT knockout mice suffer from muscle fatigability and increased serum creatine kinase levels [
24], both hallmarks of disease in DMD. Our results indicate that protein levels of TauT are reduced, at which time degeneration/regeneration is most active and TauT could act as a biomarker. Given the detrimental effect of low TauT levels, diminished levels of taurine and/or its transporter might actively contribute to disease progression in the
mdx mouse model; however, further investigation is required to support this theory.
Taurine supplementation appears to be effective in the
mdx mouse model.
mdx mice that received taurine treatment showed significantly higher numbers of intact myofibers with peripheral nuclei, together with reduced markers of inflammation and oxidative stress [
25,
26]. However, high doses of taurine have been shown to alter taurine synthesis in the liver, with unwanted growth restraint as a consequence [
26]. Another possible limitation of taurine supplementation relates to the discrepancy in taurine regulatory pathways between the
mdx mouse model and DMD patients. In contrast to our observations in the muscles of the
mdx mouse, TauT has been described to be upregulated in muscle of 8 month-old
golden retriever muscular dystrophy dogs [
27] and DMD patients aged 4 to 9 years [
6]. Presumably, the difference in TauT regulation is more likely species-specific than age-related, since the age of 3 to 4 week-old
mdx mice corresponds to the age of 10 years in DMD patients [
7]. Furthermore, patients with a homozygous mutation in the
SLC6A6 gene and subsequent low taurine levels suffer from retinal degradation, without any description of muscle impairment mentioned [
28]. Additionally, chronic taurine supplementation in humans failed to increase muscle taurine content [
29], compromising the possible benefit of long-term taurine treatment. These findings point towards strict regulation of endogenous osmolyte levels in the human body. Taurine function, metabolism, and compensatory mechanisms might vary between species. Up until now, taurine supplementation as a therapeutic strategy has not yet been tested in DMD patients. Both our former studies and others’ firmly link taurine metabolism to dystrophin deficiency. We suggest that the taurine pathway and its regulation in both
mdx mice and DMD patients needs to be characterized further to successfully explore the therapeutic potential of taurine.
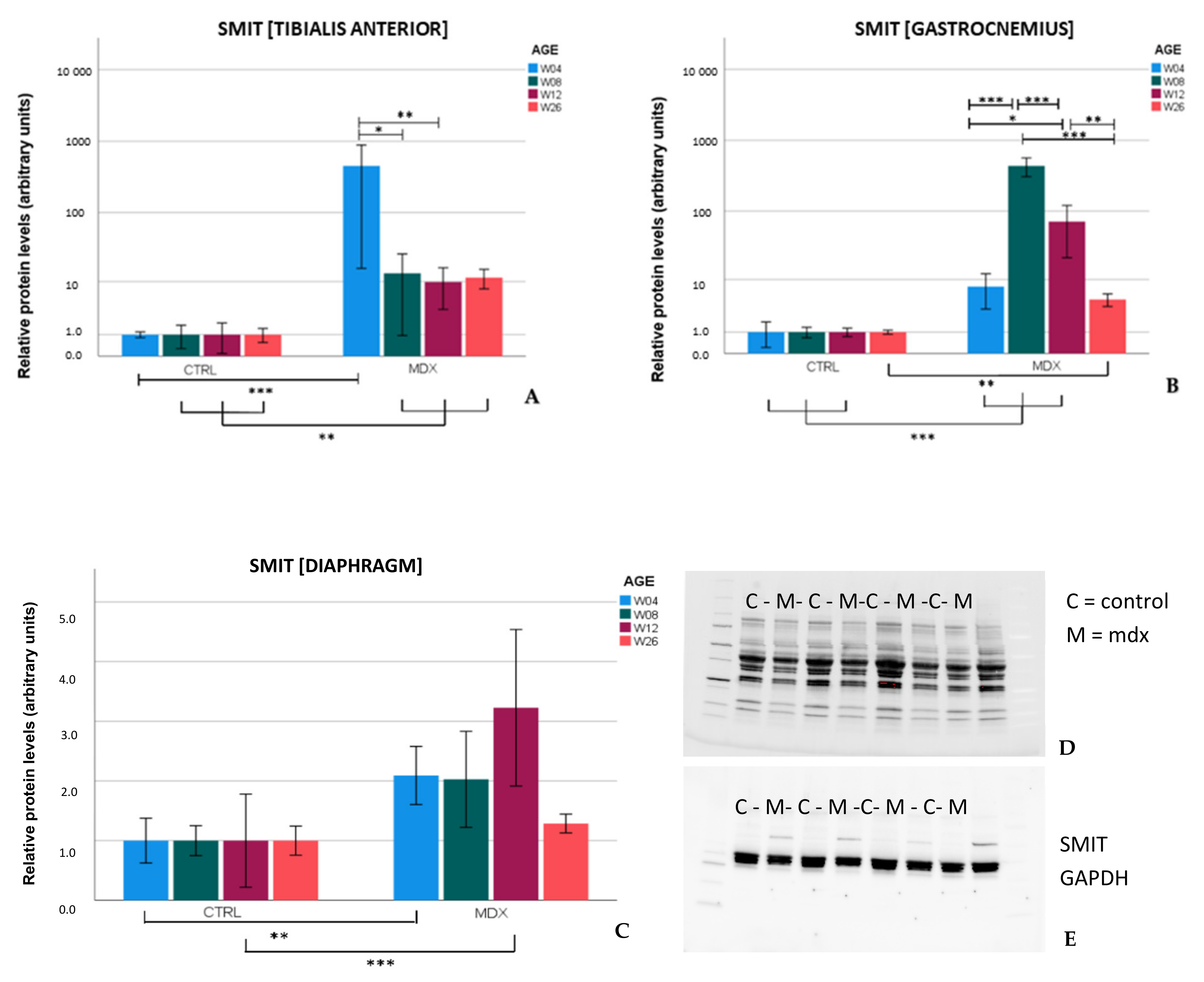
Upregulation of SMIT protein levels in the
mdx mouse model is described here for the first time. In the skeletal muscles of
mdx mice, SMIT protein levels were readily detected, whilst in control mice, the protein was almost undetectable, resulting in high fold changes. In the diaphragm, clear bands were present in both
mdx and control mice, pointing to muscle subgroup-specific protein levels of SMIT. We noticed that the observed molecular weight (≈±53 kDa) was lower than the expected molecular weight of the full-length SMIT protein (≈79 kDa). This phenomenon might be explained by alternative splicing [
30], post-translational modifications, or it can refer to the uncharacterized SMIT protein (≈58 kDa) known as (UniRef100_Q3UMR9) [
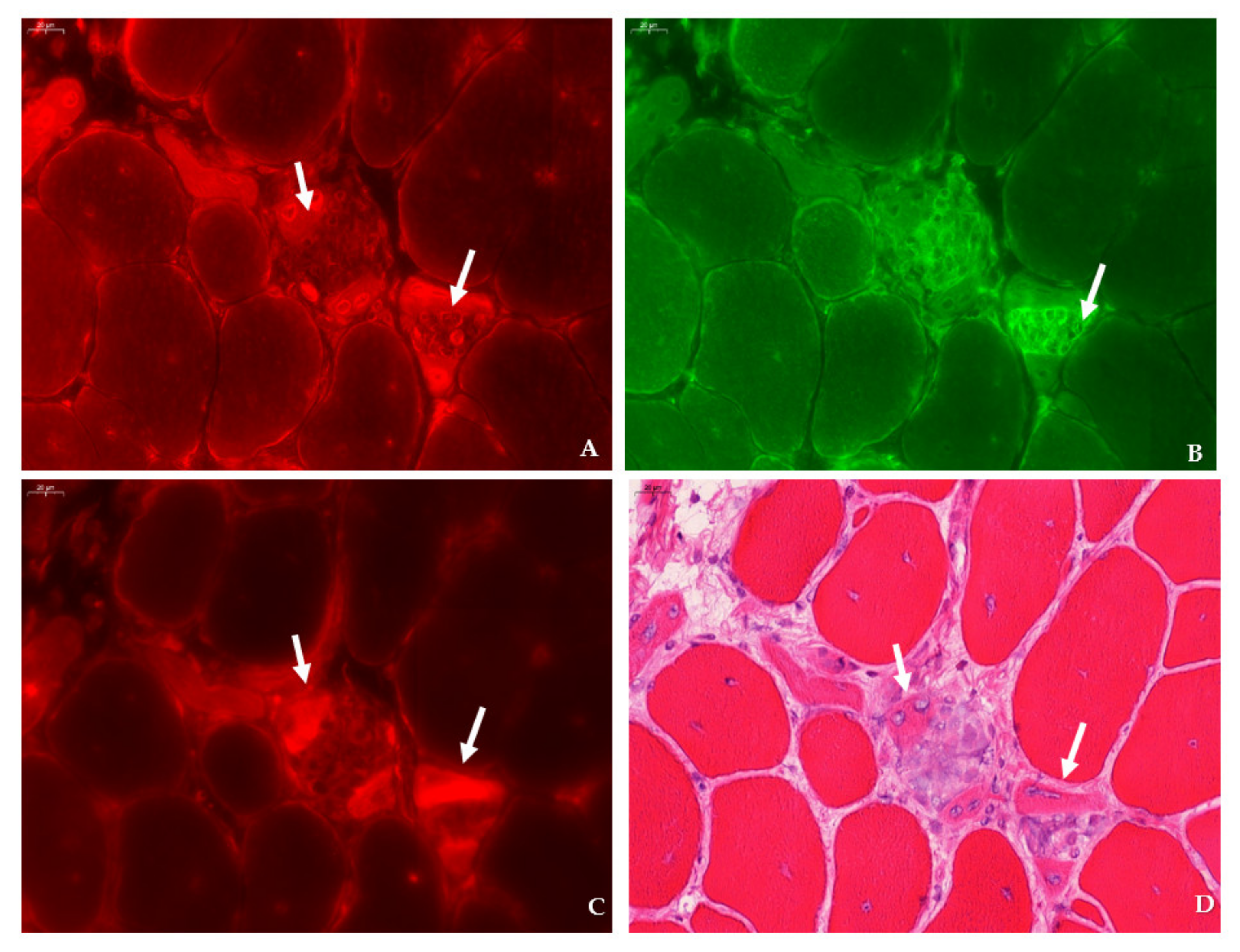
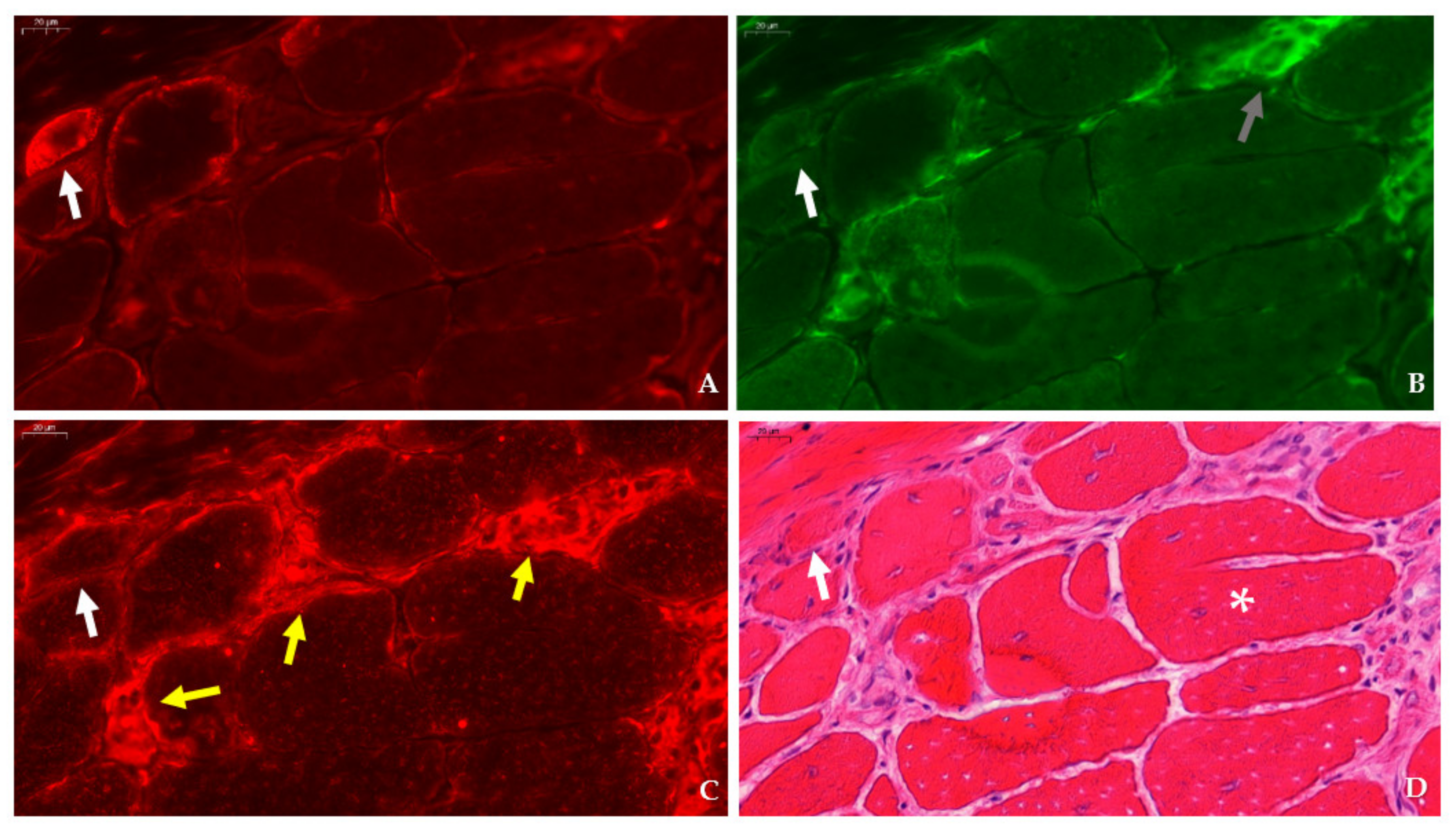
31]. Immunofluorescent detection in the tibialis anterior of
mdx mice revealed SMIT expression was most conspicuous in necrotic myofibers with fragmented sarcoplasm, whereas sporadic expression was observed in CD206+ and F4F80+ macrophages. Presumably, myofibers are the main source of SMIT expression. In DMD patients, upregulation of SMIT was observed at the protein level and colocalization studies showed that expression coincided with CD56+ fibers, a subset of CD206+ macrophages and T-cells, pointing towards involvement in the inflammatory and the successive regenerative response [
6]. In vitro experiments have shown a significant upregulation of SLC5A3 mRNA in muscle cells exposed to pro-inflammatory cytokines [
32]. Taken together, our results show increased protein levels of SMIT in
mdx mice, especially in damaged muscle fibers. Supposedly, SMIT is upregulated in order to counteract osmotic imbalance, the latter being a consequence of leaky membranes that are conspicuous in dystrophin-deficient muscle [
6]. Previously, upregulation of myo-inositol levels in
mdx brain has been reported [
33].
AR levels have been described to be upregulated in the skeletal muscle of DMD patients [
6]. However, we could not detect significant differences in AR protein levels in skeletal muscle nor in the diaphragm of
mdx mice, yet marked expression of AR was present in the necrotic myofibers and small, regenerating myofibers of
mdx muscle tissues. Using liquid chromatography-mass spectrometry, a fold change of 1.38 in AR protein was reported in the heart of aging
mdx mice when compared to 7 week-old
mdx mice [
34].
In conclusion, we revealed a substantial difference in regulation between TauT and SMIT, two members of the osmolyte pathway downstream of the NFAT-5 cascade, in the mdx mouse model. These results point towards a specific regulation of osmolyte pathways in the mdx mouse model, which are more complex than a general response to stress. In the mdx mouse model, active degeneration/regeneration occurs early in the species’ lifetime. Our results indicate that TauT protein levels during this period are diminished in the mdx mouse, whereas these levels normalize to control levels in older mice, at which time histopathological characteristics are less apparent. Whether TauT levels are affected by muscle damage or actively contribute to dystrophinopathology remains unclear. The results discussed in this paper highlight the potential of osmolyte pathway members as a research interest and future therapeutic target in dystrophinopathy.
4. Materials and Methods
4.1. Ethical Statement
Mice were bred at the central specific pathogen free animal facility of Ghent University (ECD 17/130). All experimental procedures were approved by the Animal Ethics Committee of Ghent University, faculty of Medicine and Health Sciences (ECD 19/77 and 19/110) and performed in accordance with European guidelines (Directive 2010/63/EU) and ARRIVE guidelines.
4.2. Animals
C57BL/10ScSn-Dmdmdx/J (mdx) mice and C57BL/10SnJ control mice were purchased from Jackson Laboratory (Bar Harbor, ME, US). All animals had access to food and water ad libitum. The male mice were selected, weighed, and euthanized by cervical dislocation at different ages: day 28(±1), 56(±1), 84 (±1), and 182 (±7) after birth. A total of 8 animals per age group (week 4, 8, 12, 26) of each strain were used for molecular experiments and 5 animals per age group of each strain for immunohistological analysis. Following sacrifice, specific skeletal muscle groups were dissected, those being the tibialis anterior, gastrocnemius, and diaphragm, and were then either isopentane (for histology) or snap (for other techniques) frozen, and stored at −80 °C until further processing. The number of siblings within age groups was kept as low as possible to avoid litter-dependent bias.
4.3. Western Blot
Tissue was ground in 2 units of extraction buffer (50 mM TrisHCL, 2mM EDTA pH 7.4) supplemented with protease and phosphatase inhibitor tablets (TM mini protease inhibitor cocktail and PhosStop; Roche, Indianapolis, IN, US). Muscle extracts were centrifuged twice at 15,000 RPM for 20 min, supernatant was collected, and protein concentration was measured using the Biodrop µLite device (Isogen, PW Utrecht, The Netherlands). Loading buffer, containing 2-mercapto-ethanol and 4x Laemmli sample buffer (Bio-Rad Laboratories, Hercules, CA, USA), was added to muscle extracts in a 1 to 3 ratio. Samples were boiled for 2 min and 15–20 µL of total lysate was loaded (
Supplementary Table S2) onto 4–20% Mini-Protean TGX Stain-Free gel (Bio-Rad Laboratories, Hercules, CA, US) for electrophoresis. Next, proteins were transferred to ethanol-treated low fluorescent polyvinylidene membrane (Bio-Rad Laboratories, Hercules, CA, US). Prior to overnight antibody incubation, non-specific binding was avoided by 1 h treatment in a tris-buffered saline with 0.1% Tween20 (TBST) blocking solution containing 0.2% non-fat dry milk. The following antibodies were used: rabbit anti-GADPH antibody-loading control (0.2 µg/mL, ab 9485, Abcam, Cambridge, UK); rabbit anti-beta tubulin polyclonal antibody (0.5 µg/mL, PA5-16863, Invitrogen, Waltham, MA, US); rabbit anti-BGT-1 antibody (2 µg/mL, ab 200676, Abcam, Cambridge, UK); rabbit anti-SLC6A12 antibody (10 µg/mL, NBP1-88641, Novus Biologicals, Centennial, CO, US); rabbit anti-SLC6A12/BGT-1 antibody (10 µg/mL, LS-C411851, LifeSpan Biosciences, Seattle, WA, US); rabbit anti-AKR1B1 polyclonal antibody (1 µg/mL, PA5-12316, Invitrogen, Waltham, MA, US); rabbit anti- slc6a6/TauT antibody (0.125 µg/mL, ab 196821, Abcam, Cambridge, UK); rabbit anti-SLC5A3 antibody (2 µg/mL, ABS 518, Sigma-Aldrich, St. Louis, MO, US). Membranes were washed with TBST and exposed to the corresponding horseradish peroxidase-conjugated secondary antibodies. Protein bands were visualized with chemiluminescent Clarity Western ECL substrate (Bio-Rad Laboratories, Hercules, CA, US) or with the chromogenic Western Breeze kit (Invitrogen, Waltham, MA, US) using the Chemidoc Imaging System (Bio-Rad Laboratories, Hercules, CA, US), except for BGT, since no protein bands could be obtained by Western blotting using three different commercial antibodies. Densities were quantified with Image-Lab 6.0 software, normalized to total protein using Stain-Free technology (Bio-Rad Laboratories, Hercules, CA, US), or normalized against a housekeeping protein (GAPDH/beta tubulin) to correct for loading errors. Protein data per age category was analyzed relative to control animals (=1). Data of SMIT levels in the tibialis anterior, gastrocnemius, and diaphragm were log-transformed to adhere to normality criteria and univariate ANOVA analysis was performed. Other datasets were evaluated by mixed model analysis with the following specifications: the variables ‘group’, ‘age’, and ‘age by group’ were defined as fixed effects and were nested in this study. Bonferroni correction was used to compare relative differences in protein levels of osmolyte pathway in
mdx and control mice of different ages.
4.4. qPCR
RNA was isolated from the tibialis anterior using the RNeasy Mini kit, following the manufacturer’s instructions (Qiagen, Hilden, Germany). In short, skeletal muscle tissue and Qiazol lysis reagent were added to tubes containing ceramic beads for homogenization with the Precellys system (Peqlab, Erlangen, Germany). Chloroform was added to muscle extracts prior to centrifugation. Next, supernatant was mixed with 200 µL of 70% EtOH solution and transferred to an RNEasy spin column. Washing steps were carried out according to the manufacturer’s protocol and each step was followed by centrifugation. RNAse free water was added to the spin column membrane, and, after centrifugation, purified RNA was obtained. RNA concentration was measured with a Nanodrop 1000 (ThermoFisher Scientific, Waltham, MA, US). Next, cDNA was prepared by adding 500 µg/mL oligo (dT)s, 5x First Strand Buffer, 0.1M DTT, 10mM dNTPs, and SuperScript II Reverse transcriptase (Invitrogen, Waltham, MA, US) according to the manufacturer’s protocol. PCR reactions were run in triplicates on a 7500 Real Time PCR system by supplementing 1 µL cDNA with 10 µL Taqman Gene Expression Mastermix (Applied Biosystems, Foster City, CA, USA), 8 µL RNAse free water, and 1 µL specific primers for: SLC5A3 (Mm00444330_s1), SLC6A6 (Mm00436909_m1), SLC6A12 (Mm00446665_m1), AKR1B3 (Mm01135578_g1), CCL2 (Mm00441242_m1), SPP1 (Mm00436767_m1), (Applied Biosystems, Waltham, MA, US). Analysis was carried out using Quantstudio and presented as 2-DCT relative to GADPH mRNA expression. Results that displayed statistically significant changes in the first test were confirmed by a second round of qPCR reactions. Data were analyzed using the mixed model approach.
4.5. Histology
Left and right tibiales anteriores were carefully dissected and embedded in Tissue Tek O.C.T. (Sakura, Alphen aan den Rijn, The Netherlands) prior to snap freezing in isopentane-cooled nitrogen. Sections (8 µm) were cut transversally with a microtome and stained with H&E according to the protocol TREAT-NMD SOP: DMD_M.1.2.007. Whole muscle cross-sections were scanned and digitalized by the Pannoramic 250 Flash III (3DHistech, Budapest, Hungary) at a 20× enlargement. Sections were manually analyzed for the following parameters: number of healthy muscle fibers, number of regenerated muscle fibers with centralized nuclei, and number of necrotic myofibers (fibers invaded by macrophages, associated with loss of membrane integrity, and/or pale cytoplasm). In addition, non-muscle area (including necrotic surface, inflammatory regions, and interstitial area) was quantified both manually and automatically using color deconvolution with QuPath 0.2.3 software [
35]. In total, 5 sections per animal (
n = 5 animals per age group) with an interval of approximately 150 µm between consecutive sections were cut, stained, and analyzed. Per section, an average of 2338 fibers, representing a surface of 4.67 × 10
6 µm
2, were examined. The number of healthy, regenerating, and necrotic fibers to total fibers was compared between
mdx and control groups of a specified age using the negative binomial regression model with log link distribution and compound symmetry as covariance structure. The variable ‘section’ was defined in repeated measures, since 5 sections were analyzed per animal. Log total fibers was used as offset to correct for the total amount of fibers counted per section. Fixed effects were determined by ‘group’, ‘age’, and ‘age by group’ and Bonferroni correction was used to compare results between different ages.
4.6. Immunofluorescence
Sections were permeabilized for 2 min in acetone and left to dry. Next, sections were incubated with PBS blocking solution that contained 2% bovine serum, 5% donkey serum, and 10% heat-inactivated human serum. The following primary antibodies were diluted in PBS blocking serum and incubated overnight at 4 °C: rabbit anti-SLC5A3 antibody (20 µg/mL, ABS 518, Sigma-Aldrich, St. Louis, MO, US), rat anti-F4/F80 antibody (5 µg/mL, ab6640, Abcam, Cambridge, UK), rabbit anti-CD206 antibody (400 µg/mL, PA5-101657, Invitrogen, Waltham, MA, US), rabbit anti-SLC6A12 antibody (400 µg/mL, NBP 1-88641, R&D systems, Minneapolis, MN, US) rabbit anti- SLC6A6/TauT (20 µg/mL, LS-C386313, LifeSpan Biosciences, Seattle, WA, US) and rabbit anti-AKR1B1 polyclonal antibody (400 µg/mL, PA5-12316, Invitrogen, Waltham, MA, US), goat anti-NCAM/CD56+ antibody (5 µg/mL, AF 2408, R&D systems, Minneapolis, MN, US), or rabbit anti-MYH8 (20 µg/mL, PA5-72846, ThermoFisher Scientific, Waltham, MA, US). Sections were consecutively stained with rat anti-F4/F80 in combination with rabbit antibody, directed to an osmolyte pathway member, and rabbit anti- CD206 antibody in combination with goat anti-NCAM/CD56 antibody. Sections were rinsed three times with PBS and incubated for 1 h with secondary antibodies labelled with CY3 (Jackson ImmunoResearch Laboratories, Westgrove, PA, US) and AlexaFluor 488 (ThermoFisher Scientific, Waltham, MA, US). Sections were rinsed three times with PBS and mounted with Fluoromount G (Southern Biotech, Birmingham, AL, USA). After digitalization, sections double stained for osmofactors and F4/F80 were subsequently stained with hematoxylin–eosin.
4.7. Statistical Analysis
Statistical analysis was performed in IBM SPSS Statistics version 27.0.0. Normality assessment was based on raw data and residual data. Statistical significance was set at a p-value of ≤ 0.05.