Periodic Exposure of Plasma-Activated Medium Alters Fibroblast Cellular Homoeostasis
Abstract
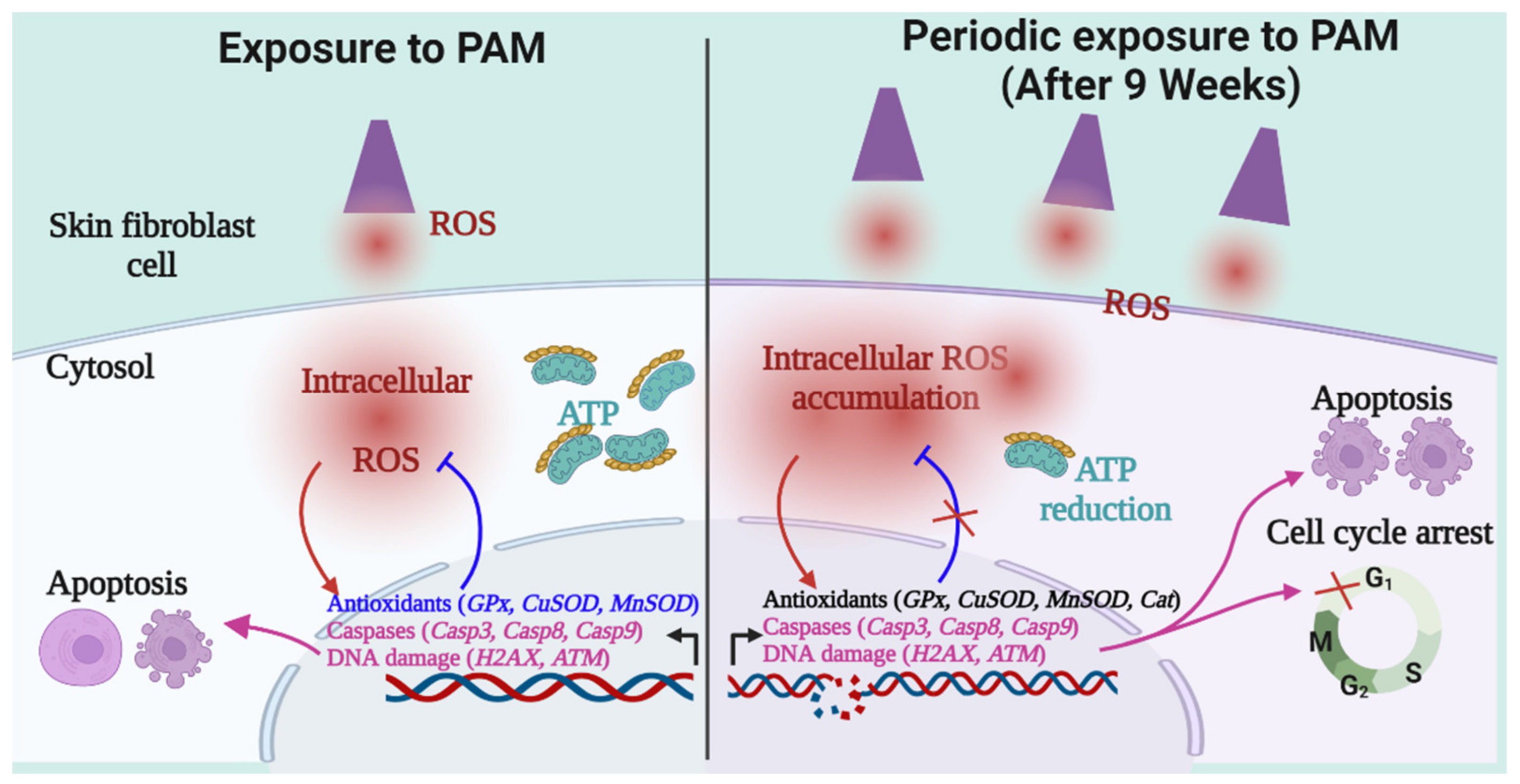
:1. Introduction
2. Results
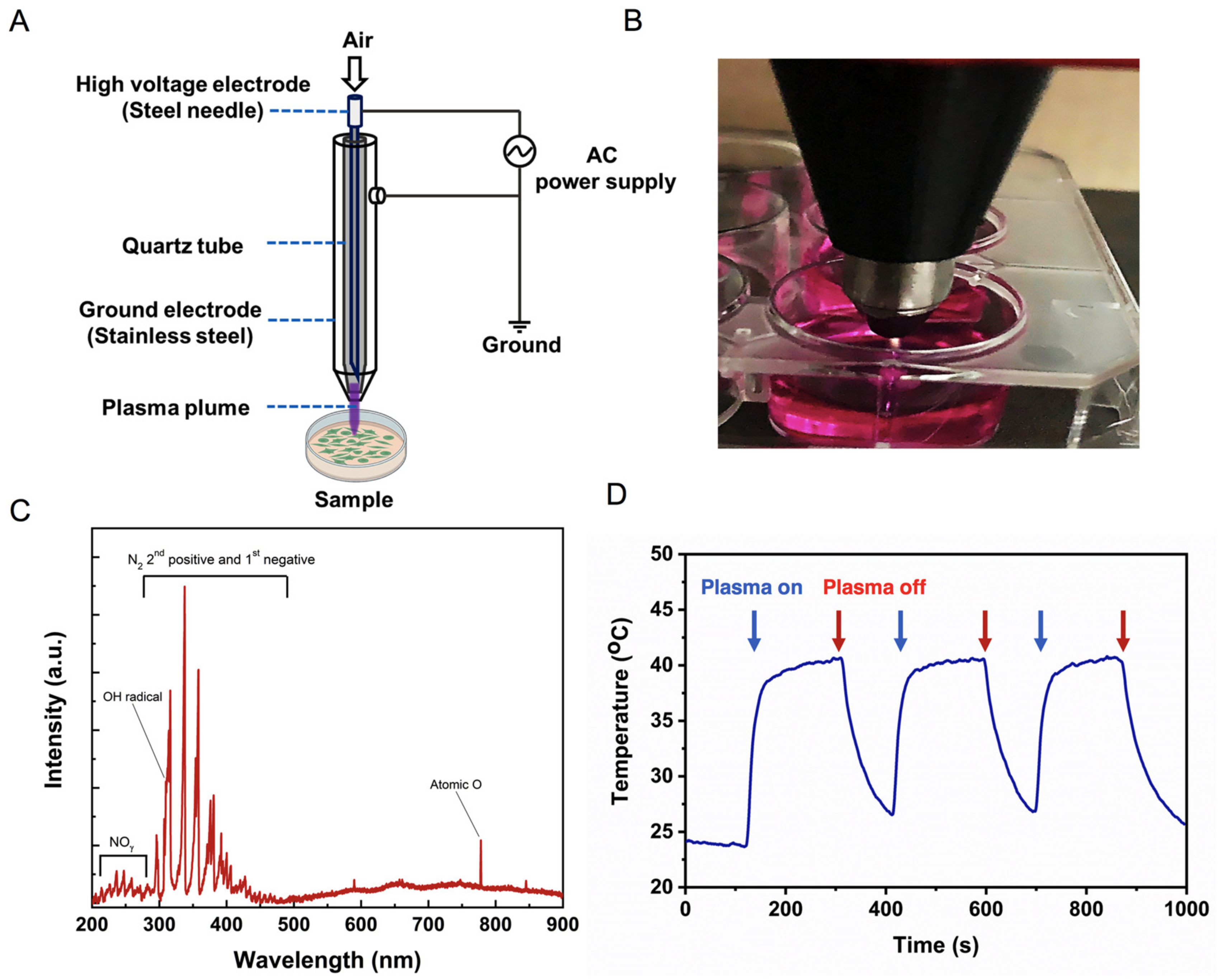
2.1. Physical Characteristics of Soft Jet Plasma
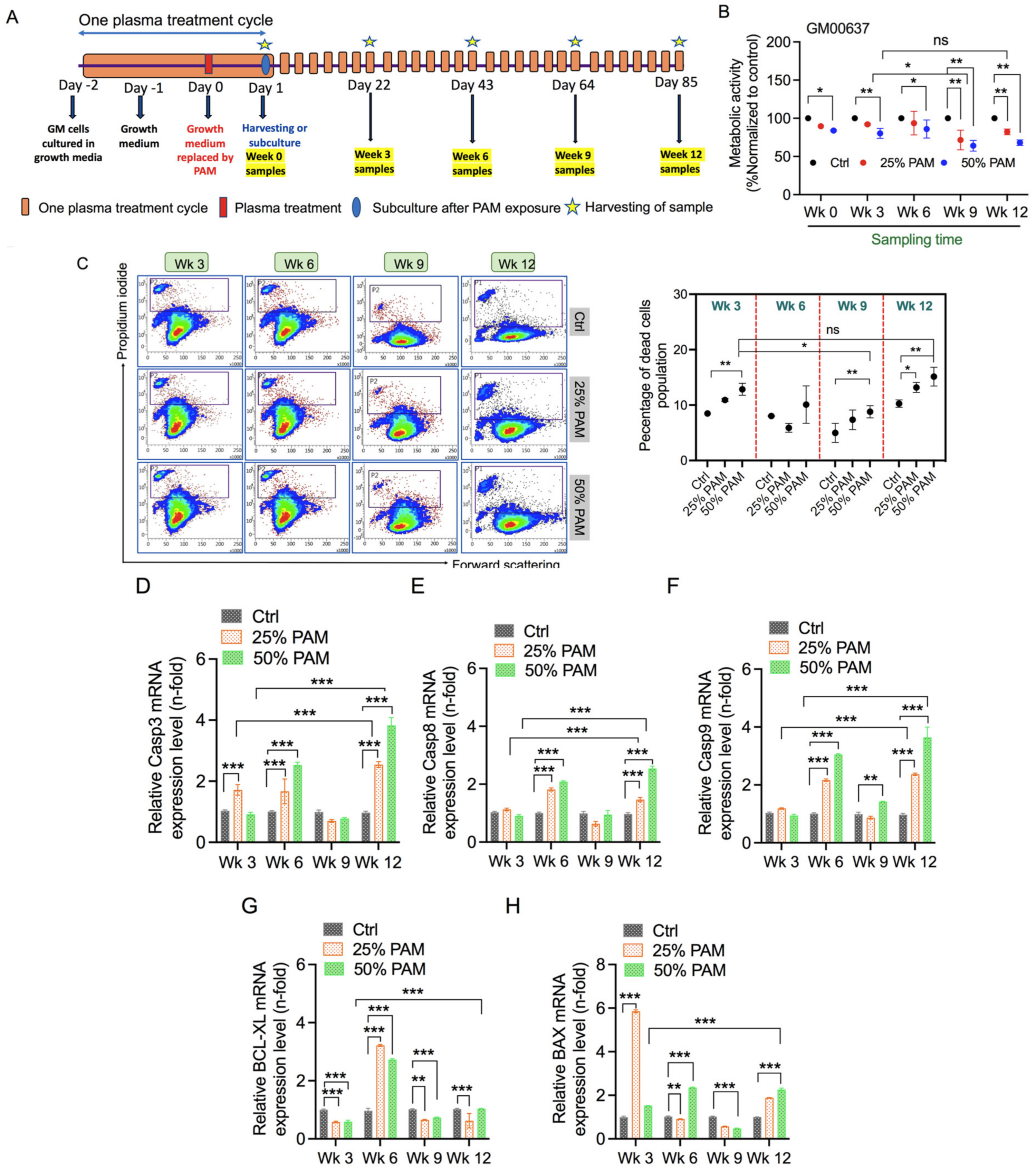
2.2. Single and Periodic Exposure to PAM Leads to Reduced Viability of Fibroblasts
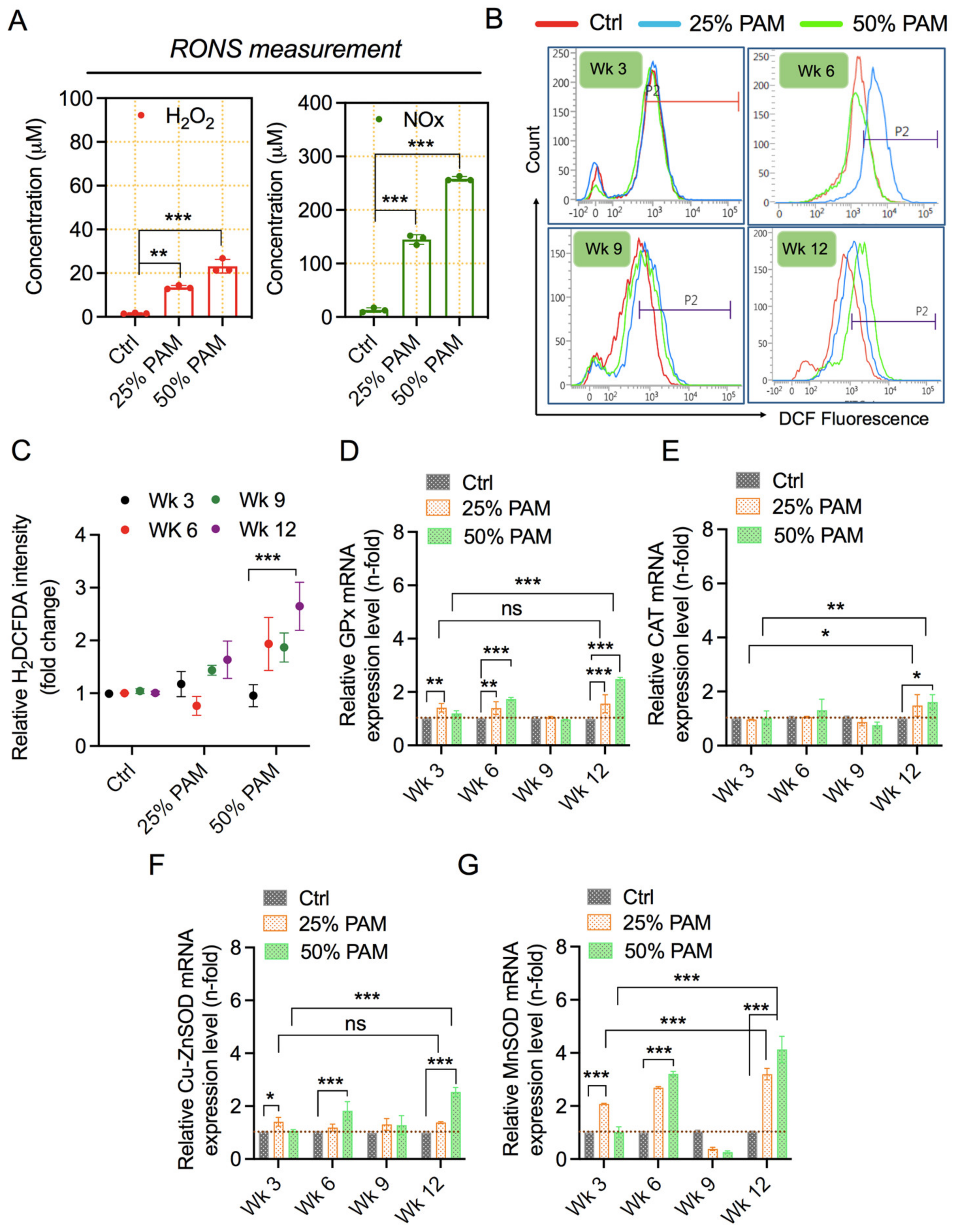
2.3. PAM Exposure Increases Reactive Species Accumulation and Triggers Cellular Antioxidant Proteins
2.4. Periodic PAM Exposure Effect on Fibroblast Cell Energetics
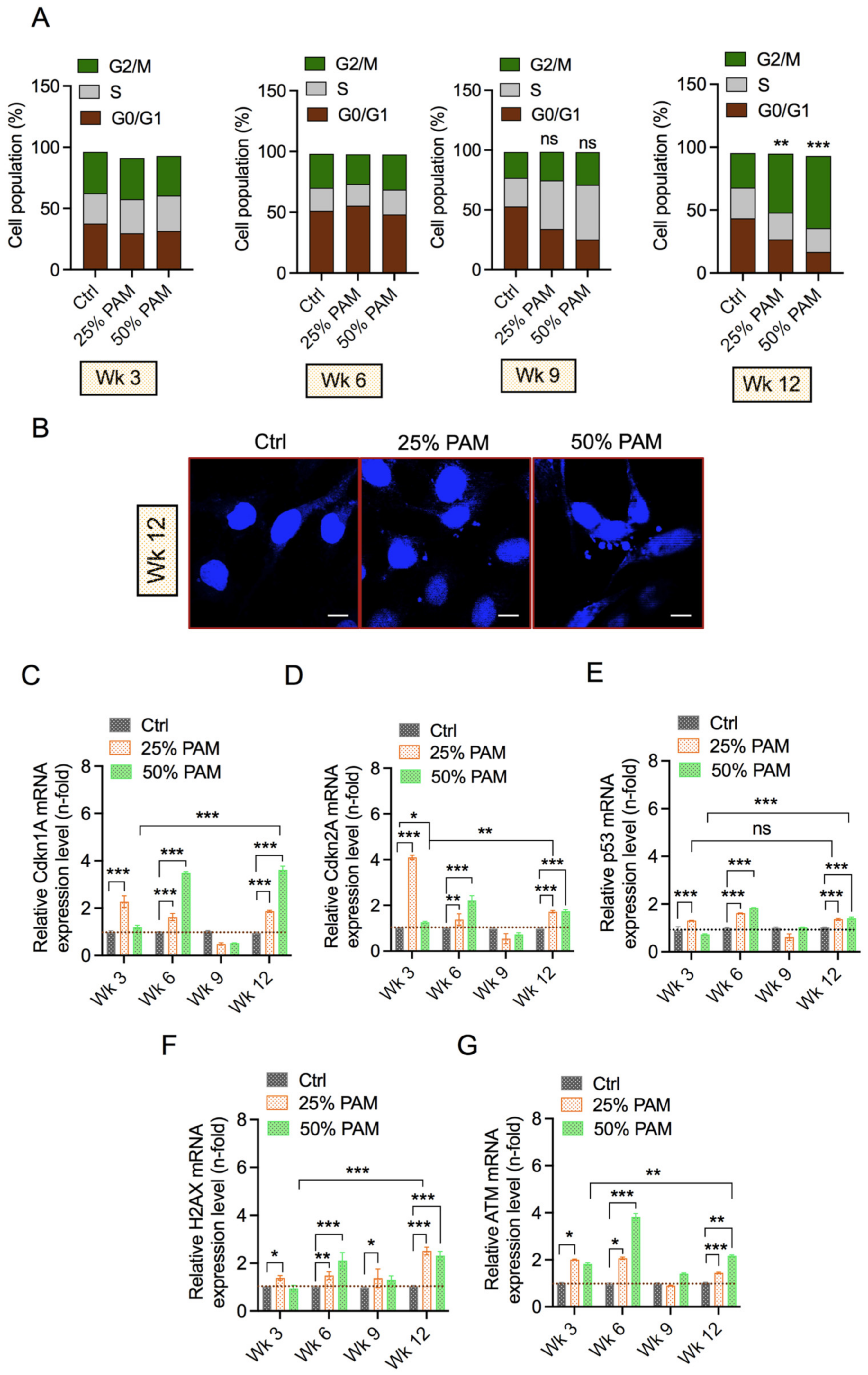
2.5. Periodic PAM Exposure Induces the DNA Damage Response and Cell Cycle Arrest in Fibroblast Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. PAM Generation and Treatment
4.3. Cell Growth Assay
4.4. Terminal Cell Death
4.5. Quantitative Real-Time PCR Analysis
4.6. Reactive Species (RONS) Detection
4.7. Cell Cycle Analysis
4.8. Cell Energetics Assay (ATP Measurement)
4.9. Flow Cytometry for Mitochondria Membrane Potential
4.10. Confocal Microscopy
4.11. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Thiele, J.J.; Podda, M.; Packer, L. Tropospheric ozone: An emerging environmental stress to skin. Biol. Chem. 1997, 378, 1299–1306. [Google Scholar] [PubMed]
- Vierkötter, A.; Krutmann, J. Environmental influences on skin aging and ethnic-specific manifestations. Dermatoendocrinology 2012, 4, 227–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tobin, D.J. Introduction to skin aging. J. Tissue Viability 2017, 26, 37–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatem, S.; Nasr, M.; Elkheshen, S.A.; Geneidi, A.S. Recent advances in antioxidant cosmeceutical topical delivery. Curr. Drug Deliv. 2018, 15, 953–964. [Google Scholar] [CrossRef]
- Shain, A.H.; Bastian, B.C. From melanocytes to melanomas. Nat. Rev. Cancer 2016, 16, 345–358. [Google Scholar] [CrossRef]
- Doebel, T.; Voisin, B.; Nagao, K. Langerhans cells—The macrophage in dendritic cell clothing. Trends Immunol. 2017, 38, 817–828. [Google Scholar] [CrossRef]
- Simpson, C.L.; Patel, D.M.; Green, K.J. Deconstructing the skin: Cytoarchitectural determinants of epidermal morphogenesis. Nat. Rev. Mol. Cell Biol. 2011, 12, 565–580. [Google Scholar] [CrossRef] [Green Version]
- Woo, S.-H.; Lumpkin, E.A.; Patapoutian, A. Merkel cells and neurons keep in touch. Trends Cell Biol. 2015, 25, 74–81. [Google Scholar] [CrossRef] [Green Version]
- Rognoni, E.; Watt, F.M. Skin cell heterogeneity in development, wound healing, and cancer. Trends Cell Biol. 2018, 28, 709–722. [Google Scholar] [CrossRef] [Green Version]
- Sriram, G.; Bigliardi, P.L.; Bigliardi-Qi, M. Fibroblast heterogeneity and its implications for engineering organotypic skin models in vitro. Eur. J. Cell Biol. 2015, 94, 483–512. [Google Scholar] [CrossRef] [Green Version]
- Ross, M.H.; Pawlina, W. Histology: A Text and Atlas with Correlated Cell and Molecular Biology; Lippincott Williams & Wilkins: Philadephia, PA, USA, 2018. [Google Scholar]
- Janson, D.G.; Saintigny, G.; van Adrichem, A.; Mahé, C.; El Ghalbzouri, A. Different gene expression patterns in human papillary and reticular fibroblasts. J. Investig. Dermatol. 2012, 132, 2565–2572. [Google Scholar] [CrossRef] [Green Version]
- Mine, S.; Fortunel, N.O.; Pageon, H.; Asselineau, D. Aging alters functionally human dermal papillary fibroblasts but not reticular fibroblasts: A new view of skin morphogenesis and aging. PLoS ONE 2008, 3, e4066. [Google Scholar] [CrossRef] [Green Version]
- Bernhardt, T.; Semmler, M.L.; Schäfer, M.; Bekeschus, S.; Emmert, S.; Boeckmann, L. Plasma medicine: Applications of cold atmospheric pressure plasma in dermatology. Oxid. Med. Cell. Longev. 2019, 2019, 3873928. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Ha, C.S.; Hwang, S.W.; Lee, H.J.; Kim, G.C.; Lee, K.-W.; Song, K. Non-thermal atmospheric pressure plasma preferentially induces apoptosis in p53-mutated cancer cells by activating ROS stress-response pathways. PLoS ONE 2014, 9, 91947. [Google Scholar] [CrossRef]
- Lin, A.; Truong, B.; Patel, S.; Kaushik, N.; Choi, E.H.; Fridman, G.; Fridman, A.; Miller, V. Nanosecond-pulsed DBD plasma-generated reactive oxygen species trigger immunogenic cell death in A549 lung carcinoma cells through intracellular oxidative stress. Int. J. Mol. Sci. 2017, 18, 966. [Google Scholar] [CrossRef] [Green Version]
- Kaushik, N.K.; Kaushik, N.; Wahab, R.; Bhartiya, P.; Linh, N.N.; Khan, F.; Al-Khedhairy, A.A.; Choi, E.H. Cold atmospheric plasma and gold quantum dots exert dual cytotoxicity mediated by the cell receptor-activated apoptotic pathway in glioblastoma cells. Cancers 2020, 12, 457. [Google Scholar] [CrossRef] [Green Version]
- Graves, D.B. The emerging role of reactive oxygen and nitrogen species in redox biology and some implications for plasma applications to medicine and biology. J. Phys. D Appl. Phys. 2012, 45, 263001. [Google Scholar] [CrossRef]
- Von Woedtke, T.; Schmidt, A.; Bekeschus, S.; Wende, K.; Weltmann, K.-D. Plasma medicine: A field of applied redox biology. In Vivo 2019, 33, 1011–1026. [Google Scholar] [CrossRef] [Green Version]
- Privat-Maldonado, A.; Schmidt, A.; Lin, A.; Weltmann, K.-D.; Wende, K.; Bogaerts, A.; Bekeschus, S. ROS from physical plasmas: Redox chemistry for biomedical therapy. Oxid. Med. Cell. Longev. 2019, 2019, 9062098. [Google Scholar] [CrossRef] [Green Version]
- Reczek, C.R.; Chandel, N.S. The two faces of reactive oxygen species in cancer. Annu. Rev. Cancer Biol. 2017, 1, 79–98. [Google Scholar] [CrossRef]
- Srinivas, U.S.; Tan, B.W.Q.; Vellayappan, B.A.; Jeyasekharan, A.D. ROS and the DNA damage response in cancer. Redox Biol. 2019, 25, 101084. [Google Scholar] [CrossRef]
- Colin, D.J.; Limagne, E.; Ragot, K.; Lizard, G.; Ghiringhelli, F.; Solary, E.; Chauffert, B.; Latruffe, N.; Delmas, D. The role of reactive oxygen species and subsequent DNA-damage response in the emergence of resistance towards resveratrol in colon cancer models. Cell Death Dis. 2014, 5, e1533. [Google Scholar] [CrossRef] [Green Version]
- Salehi, F.; Behboudi, H.; Kavoosi, G.; Ardestani, S.K. Oxidative DNA damage induced by ROS-modulating agents with the ability to target DNA: A comparison of the biological characteristics of citrus pectin and apple pectin. Sci. Rep. 2018, 8, 13902. [Google Scholar] [CrossRef]
- Perillo, B.; di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative stress in aging human skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef] [Green Version]
- Liebel, F.; Kaur, S.; Ruvolo, E.; Kollias, N.; Southall, M.D. Irradiation of skin with visible light induces reactive oxygen species and matrix-degrading enzymes. J. Investig. Dermatol. 2012, 132, 1901–1907. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, A.; von Woedtke, T.; Bekeschus, S. Periodic exposure of keratinocytes to cold physical plasma: An in vitro model for redox-related diseases of the skin. Oxid. Med. Cell. Longev. 2016, 2016, 9816072. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, A.; Liebelt, G.; Nießner, F.; von Woedtke, T.; Bekeschus, S. Gas plasma-spurred wound healing is accompanied by regulation of focal adhesion, matrix remodeling, and tissue oxygenation. Redox Biol. 2021, 38, 101809. [Google Scholar] [CrossRef]
- Schmidt, A.; Liebelt, G.; Striesow, J.; Freund, E.; von Woedtke, T.; Wende, K.; Bekeschus, S. The molecular and physiological consequences of cold plasma treatment in murine skin and its barrier function. Free Radic. Biol. Med. 2020, 161, 32–49. [Google Scholar] [CrossRef]
- Schmidt, A.; Nießner, F.; von Woedtke, T.; Bekeschus, S. Hyperspectral imaging of wounds reveals augmented tissue oxygenation following cold physical plasma treatment in vivo. IEEE Trans. Radiat. Plasma Med. Sci. 2020, 5, 412–419. [Google Scholar] [CrossRef]
- Norberg, S.A.; Johnsen, E.; Kushner, M.J. Formation of reactive oxygen and nitrogen species by repetitive negatively pulsed helium atmospheric pressure plasma jets propagating into humid air. Plasma Sources Sci. Technol. 2015, 24, 35026. [Google Scholar] [CrossRef] [Green Version]
- Sakiyama, Y.; Graves, D.B.; Chang, H.-W.; Shimizu, T.; Morfill, G.E. Plasma chemistry model of surface microdischarge in humid air and dynamics of reactive neutral species. J. Phys. D Appl. Phys. 2012, 45, 425201. [Google Scholar] [CrossRef]
- Kossyi, I.A.; Kostinsky, A.Y.; Matveyev, A.A.; Silakov, V.P. Kinetic scheme of the non-equilibrium discharge in nitrogen-oxygen mixtures. Plasma Sources Sci. Technol. 1992, 1, 207. [Google Scholar] [CrossRef]
- Zhou, R.; Zhang, T.; Zhou, R.; Wang, S.; Mei, D.; Mai-Prochnow, A.; Weerasinghe, J.; Fang, Z.; Ostrikov, K.K.; Cullen, P.J. Sustainable plasma-catalytic bubbles for hydrogen peroxide synthesis. Green Chem. 2021, 23, 2977–2985. [Google Scholar] [CrossRef]
- Takeuchi, N.; Ishibashi, N. Generation mechanism of hydrogen peroxide in dc plasma with a liquid electrode. Plasma Sources Sci. Technol. 2018, 27, 45010. [Google Scholar] [CrossRef]
- Corina, B.; Kinga, K.; Magureanu, M.; Puac, N.S.Ž. Reactive nitrogen species in plasma-activated water: Generation. J. Phys. D Appl. Phys. 2020, 53, 223001. [Google Scholar]
- Schneider, C.; Arndt, S.; Zimmermann, J.L.; Li, Y.; Karrer, S.; Bosserhoff, A.K. Cold atmospheric plasma treatment inhibits growth in colorectal cancer cells. Biol. Chem. 2019, 400, 111–122. [Google Scholar] [CrossRef]
- Lin, L.; Wang, L.; Liu, Y.; Xu, C.; Tu, Y.; Zhou, J. Non-thermal plasma inhibits tumor growth and proliferation and enhances the sensitivity to radiation in vitro and in vivo. Oncol. Rep. 2018, 40, 3405–3415. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Ghimire, B.; Li, Y.; Adhikari, M.; Veerana, M.; Kaushik, N.; Jha, N.; Adhikari, B.; Lee, S.-J.; Masur, K.; et al. Biological and medical applications of plasma-activated media, water and solutions. Biol. Chem. 2019, 400, 39–62. [Google Scholar] [CrossRef]
- Antico Arciuch, V.G.; Elguero, M.E.; Poderoso, J.J.; Carreras, M.C. Mitochondrial regulation of cell cycle and proliferation. Antioxid. Redox Signal. 2012, 16, 1150–1180. [Google Scholar] [CrossRef] [Green Version]
- Wende, K.; Barton, A.; Bekeschus, S.; Bundscherer, L.; Schmidt, A.; Weltmann, K.-D.; Masur, K. Proteomic tools to characterize non-thermal plasma effects in eukaryotic cells. Plasma Med. 2013, 3, 81–95. [Google Scholar] [CrossRef] [Green Version]
- Kaushik, N.; Lee, S.J.; Choi, T.G.; Baik, K.Y.; Uhm, H.S.; Kim, C.H.; Kaushik, N.K.; Choi, E.H. Non-thermal plasma with 2-deoxy-D-glucose synergistically induces cell death by targeting glycolysis in blood cancer cells. Sci. Rep. 2015, 5, 8726. [Google Scholar] [CrossRef] [Green Version]
- Choi, E.H.; Uhm, H.S.; Kaushik, N.K. Plasma bioscience and its application to medicine. AAPPS Bull. 2021, 31, 10. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Kaushik, N.; Bhartiya, P.; Nguyen, L.N.; Choi, E.H. Glycolytic inhibitor induces metabolic crisis in solid cancer cells to enhance cold plasma-induced cell death. Plasma Process. Polym. 2021, 18, 2000187. [Google Scholar] [CrossRef]
- Quijano, C.; Trujillo, M.; Castro, L.; Trostchansky, A. Interplay between oxidant species and energy metabolism. Redox Biol. 2016, 8, 28–42. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.-R.; Xu, G.-M.; Shi, X.-M.; Zhang, G.-J. Low temperature plasma promoting fibroblast proliferation by activating the NFκB pathway and increasing cyclinD1 expression. Sci. Rep. 2017, 7, 11698. [Google Scholar] [CrossRef] [Green Version]
- Ermakov, A.M.; Ermakova, O.N.; Afanasyeva, V.A.; Popov, A.L. Dose-dependent effects of cold atmospheric argon plasma on the mesenchymal stem and osteosarcoma cells in vitro. Int. J. Mol. Sci. 2021, 22, 6797. [Google Scholar] [CrossRef]
- Kaushik, N.; Uddin, N.; Sim, G.B.; Hong, Y.J.; Baik, K.Y.; Kim, C.H.; Lee, S.J.; Kaushik, N.K.; Choi, E.H. Responses of solid tumor cells in DMEM to reactive oxygen species generated by non-thermal plasma and chemically induced ROS systems. Sci. Rep. 2015, 5, 8587. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, L.N.; Kaushik, N.; Bhartiya, P.; Gurmessa, S.K.; Kim, H.-J.; Nguye, L.Q.; Kaushik, N.K.; Choi, E.H. Plasma-synthesized mussel-inspired gold nanoparticles promote autophagy-dependent damage-associated molecular pattern release to potentiate immunogenic cancer cell death. J. Ind. Eng. Chem. 2021, 100, 99–111. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Kaushik, N.; Adhikari, M.; Ghimire, B.; Linh, N.N.; Mishra, Y.K.; Lee, S.-J.; Choi, E.H. Preventing the solid cancer progression via release of anticancer-cytokines in co-culture with cold plasma-stimulated macrophages. Cancers 2019, 11, 842. [Google Scholar] [CrossRef] [Green Version]
- Verbon, E.H.; Post, J.A.; Boonstra, J. The influence of reactive oxygen species on cell cycle progression in mammalian cells. Gene 2012, 511, 1–6. [Google Scholar] [CrossRef]
- Lim, S.; Kaldis, P. Cdks, cyclins and CKIs: Roles beyond cell cycle regulation. Development 2013, 140, 3079–3093. [Google Scholar] [CrossRef] [Green Version]
- Satyanarayana, A.; Kaldis, P. Mammalian cell-cycle regulation: Several Cdks, numerous cyclins and diverse compensatory mechanisms. Oncogene 2009, 28, 2925–2939. [Google Scholar] [CrossRef] [Green Version]
- Chen, J. The cell-cycle arrest and apoptotic functions of p53 in tumor initiation and progression. Cold Spring Harb. Perspect. Med. 2016, 6, a026104. [Google Scholar] [CrossRef]
- Souliotis, V.L.; Vlachogiannis, N.I.; Pappa, M.; Argyriou, A.; Ntouros, P.A.; Sfikakis, P.P. DNA damage response and oxidative stress in systemic autoimmunity. Int. J. Mol. Sci. 2020, 21, 55. [Google Scholar] [CrossRef] [Green Version]
- Martins, S.G.; Zilhão, R.; Thorsteinsdóttir, S.; Carlos, A.R. Linking oxidative stress and DNA damage to changes in the expression of ECM components. Front. Genet. 2021, 12, 1279. [Google Scholar] [CrossRef]
- Bhartiya, P.; Mumtaz, S.; Lim, J.S.; Kaushik, N.; Lamichhane, P.; Nguyen, L.N.; Jang, J.H.; Yoon, S.H.; Choi, J.J.; Kaushik, N.K.; et al. Pulsed 3.5 GHz high power microwaves irradiation on physiological solution and their biological evaluation on human cell lines. Sci. Rep. 2021, 11, 8475. [Google Scholar] [CrossRef]

| Gene Name | Sequence (5′-3′) |
|---|---|
| ACTIN-forward | GGC ATC CTC ACC CTG AAG TA |
| ACTIN-reverse | AGG TGT GGT GCC AGA TTT TC |
| Casp3-forward | ATG TCG ATG CAG CAA ACC TC |
| Casp3-reverse | TCC TTC TTC ACC ATG GCT CA |
| Casp8-forward | CCC AAA TCA ACA AGA GCC TGC |
| Casp8-reverse | TCA GAC AGT ATC CCC GAG GTT |
| BCL-XL-forward | TCA GTG AGT GAG CAG GTG TT |
| BCL-XL-reverse | GGC CTC AGT CCT GTT CTC TT |
| BAX-forward | AAG AAG CTG AGC GAG TGT CTC |
| BAX-reverse | GCT GGC AAA GTA GAA AAG GGC |
| GPx-forward | TTG ACA TCG AGC CTG ACA TC |
| GPx-reverse | CAA GGT GTT CTT CCC TCG TA |
| CAT-forward | TCT GGA GAA GTG CGG AGA TT |
| CAT-reverse | AGT CAG GGT GGA CCT CAG TG |
| CuZnSOD-forward | GAA GGT GTG GGG AAG CAT TA |
| CuZnSOD-reverse | ACA TTG CCC AAG TCT CCA AC |
| MnSOD-forward | TGT ACC GGT TCC GAG TTT TC |
| MnSOD-reverse | TTC AGG CCC TAC AAT TCA CC |
| ATP5A-forward | TTT TGC CCA GTT CGG TTC TG |
| ATP5A-reverse | GAT ATC CCC TTA CAC CCG CA |
| p53-forward | GCC CCT CCT CAG CAT CTT ATC |
| p53-reverse | AAA GCT GTT CCG TCC CAG TAG |
| H2AX-forward | CAA CAA GAA GAC GCG AAT CA |
| H2AX-reverse | CGG GCC CTC TTA GTA CTC CT |
| ATM-forward | TCC GTC AGC AAA GAA GTA GAA |
| ATM-reverse | TGG GAT AGA GCG AAT ACA CAG |
| Cdkn1A-forward | TTG GCT CCC CTG TAC CTT TT |
| Cdkn1A-reverse | CCT TCC CCT TCC AGT CCA TT |
| Cdkn2A -forward | CCC AAC GCA CCG AAT AGT TA |
| Cdkn2A -reverse | ACC CCT TCT GAA AAC TCC CC |
| Casp9-forward | CGA CAT CTT TGA GCA GTG GG |
| Casp9-reverse | GAA AGC TTT GGG GTG CAA GA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhartiya, P.; Kaushik, N.; Nguyen, L.N.; Bekeschus, S.; Masur, K.; Weltmann, K.-D.; Kaushik, N.K.; Choi, E.H. Periodic Exposure of Plasma-Activated Medium Alters Fibroblast Cellular Homoeostasis. Int. J. Mol. Sci. 2022, 23, 3120. https://doi.org/10.3390/ijms23063120
Bhartiya P, Kaushik N, Nguyen LN, Bekeschus S, Masur K, Weltmann K-D, Kaushik NK, Choi EH. Periodic Exposure of Plasma-Activated Medium Alters Fibroblast Cellular Homoeostasis. International Journal of Molecular Sciences. 2022; 23(6):3120. https://doi.org/10.3390/ijms23063120
Chicago/Turabian StyleBhartiya, Pradeep, Neha Kaushik, Linh N. Nguyen, Sander Bekeschus, Kai Masur, Klaus-Dieter Weltmann, Nagendra Kumar Kaushik, and Eun Ha Choi. 2022. "Periodic Exposure of Plasma-Activated Medium Alters Fibroblast Cellular Homoeostasis" International Journal of Molecular Sciences 23, no. 6: 3120. https://doi.org/10.3390/ijms23063120
APA StyleBhartiya, P., Kaushik, N., Nguyen, L. N., Bekeschus, S., Masur, K., Weltmann, K.-D., Kaushik, N. K., & Choi, E. H. (2022). Periodic Exposure of Plasma-Activated Medium Alters Fibroblast Cellular Homoeostasis. International Journal of Molecular Sciences, 23(6), 3120. https://doi.org/10.3390/ijms23063120










