Cuticle Protein LmACP19 Is Required for the Stability of Epidermal Cells in Wing Development and Morphogenesis of Locusta migratoria
Abstract
:1. Introduction
2. Results
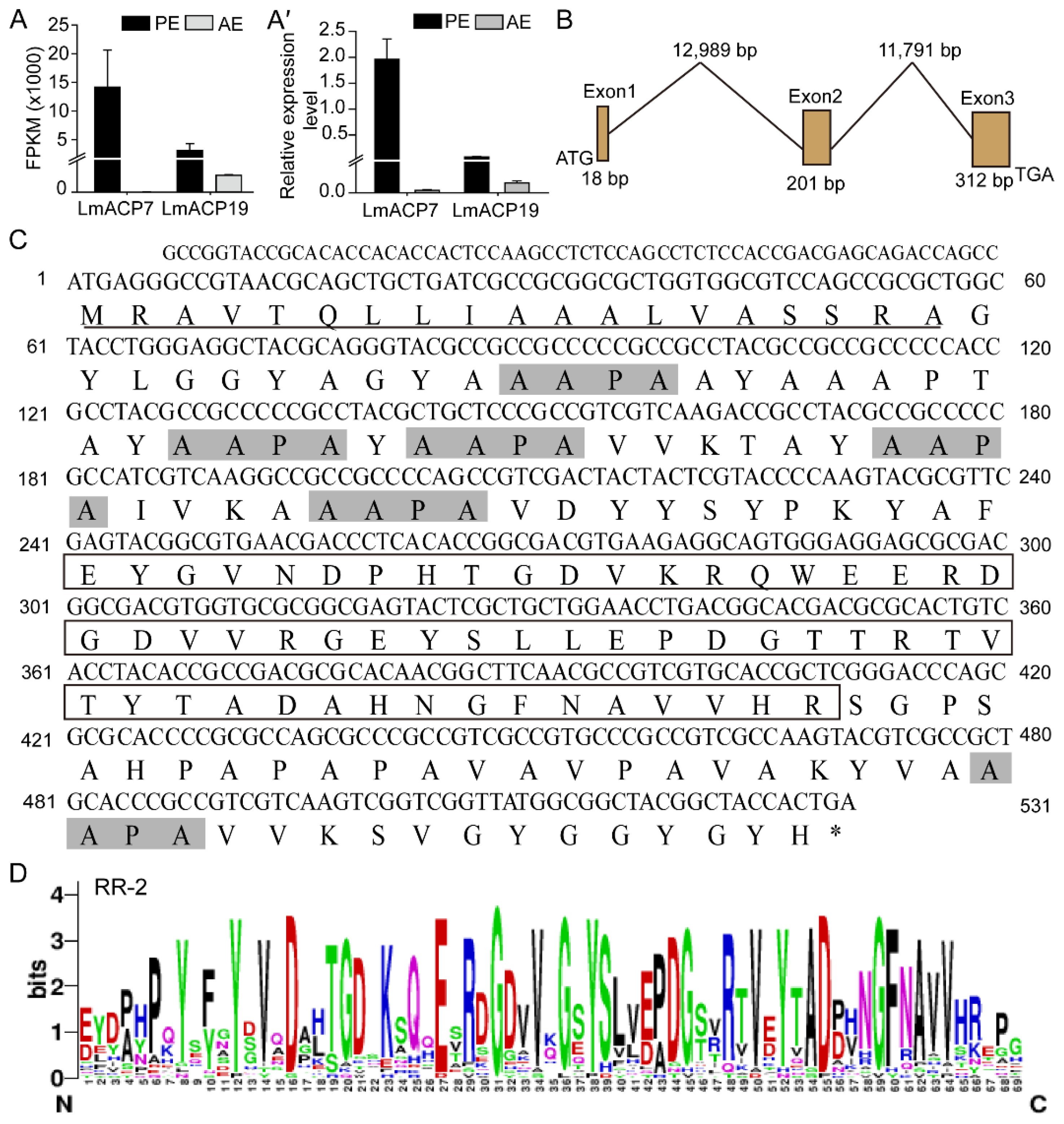
2.1. Identification and Characterization of LmACP19
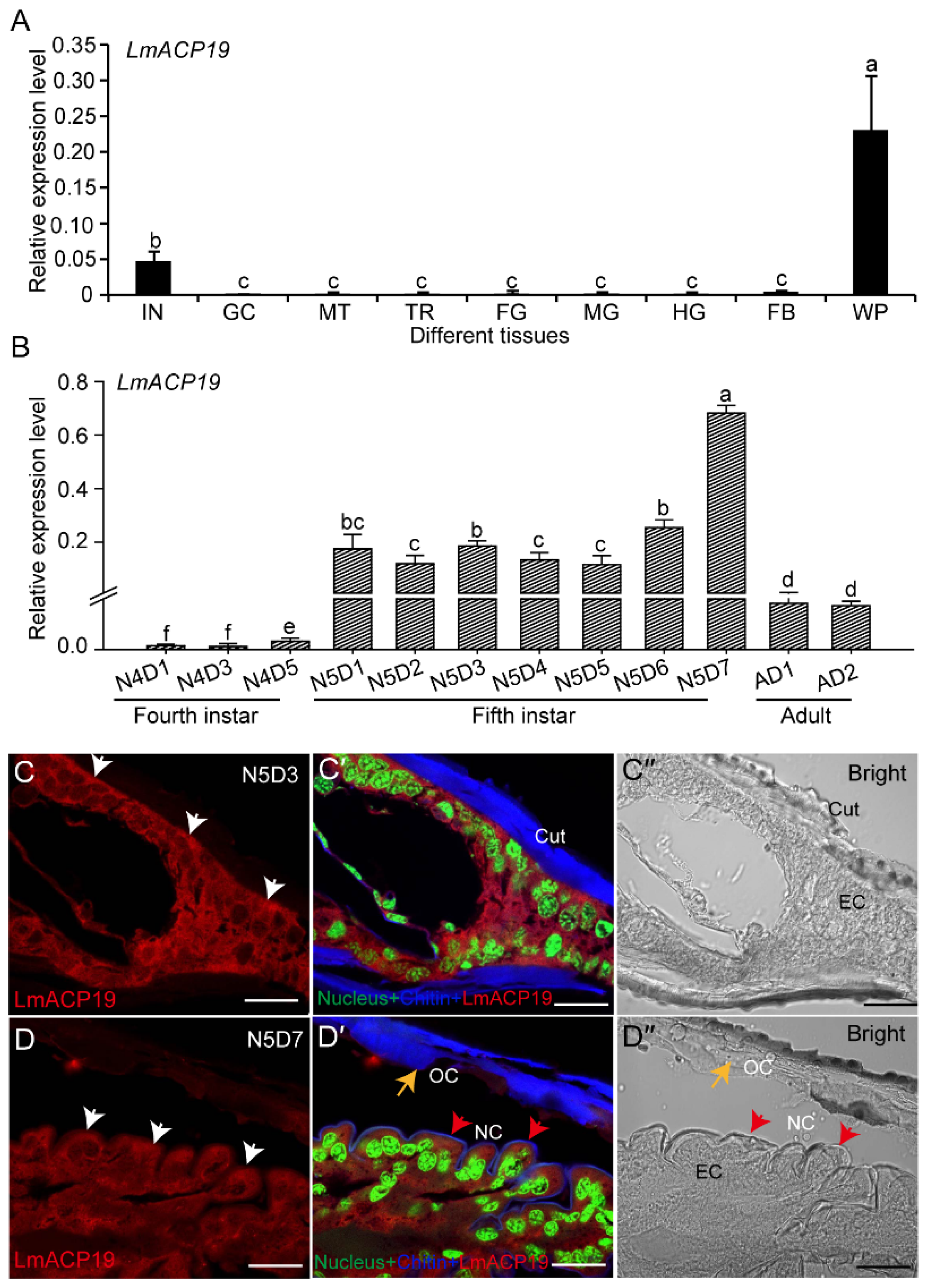
2.2. LmACP19 Is Mainly Expressed in Wing Pad Cells
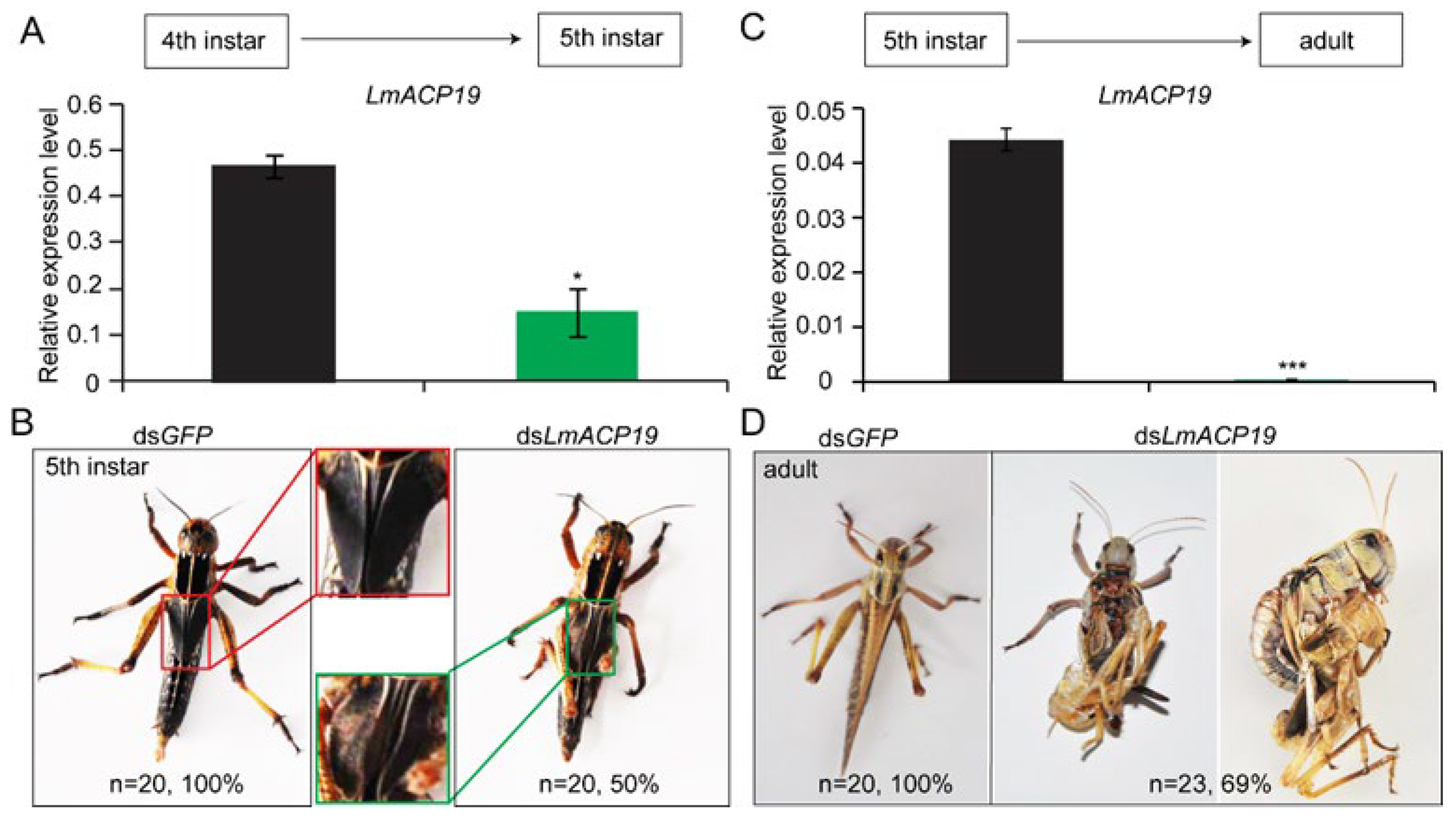
2.3. LmACP19 Is Required for the Wing Morphogenesis
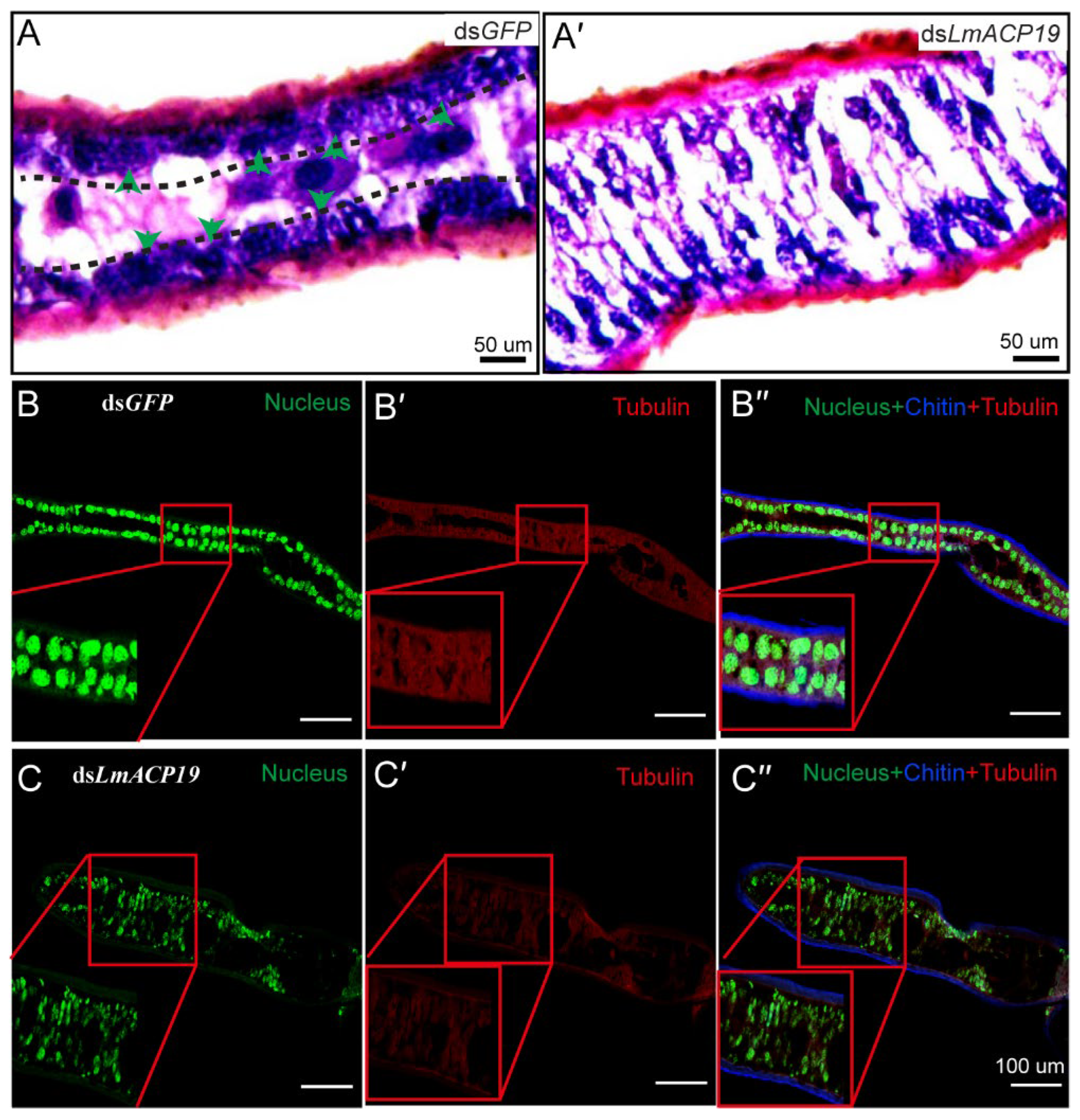
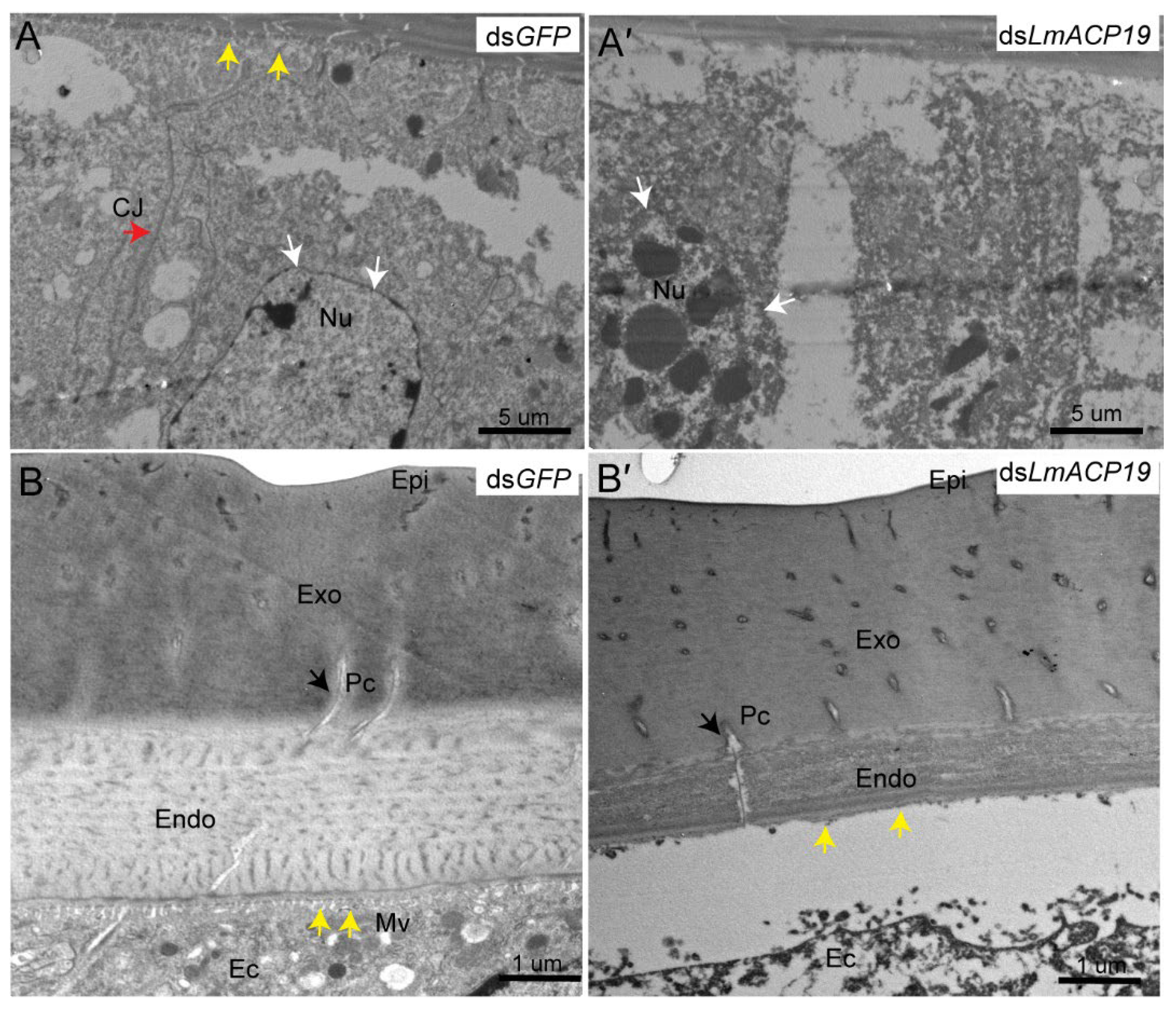
2.4. Deficiency of LmACP19 Affected the Arrangement of Epidermal Cells
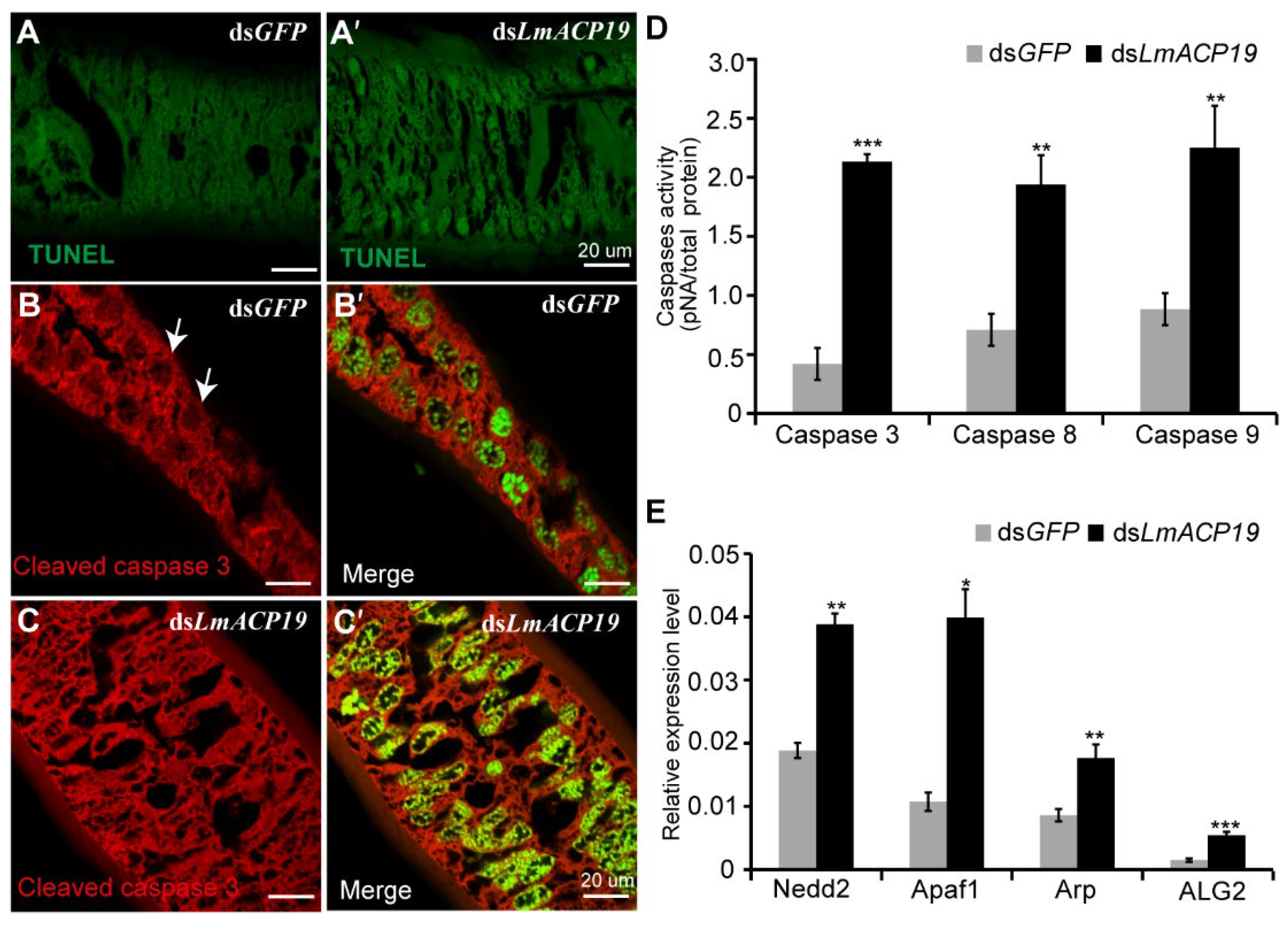
2.5. Deficiency of LmACP19 Results in Apoptosis of Epidermal Cells
3. Discussion
4. Materials and Methods
4.1. Insects
4.2. Bioinformatics Analysis
4.3. Tissue-Specific and Developmental Expression Analysis
4.4. Antibody Preparation and Immunohistochemistry
4.5. RNA Interference (RNAi)
4.6. Hematoxylin–Eosin Staining and Transmission Electron Microscope
4.7. TdT-Mediated dUTP Nick-End Labeling (TUNEL) and Caspase Activity
4.8. Staining of Cytoskeleton and Caspase-3 by Immunohistochemistry
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moussian, B.; Seifarth, C.; Muller, U.; Berger, J.; Schwarz, H. Cuticle differentiation during Drosophila embryogenesis. Arthropod Struct. Dev. 2006, 35, 137–152. [Google Scholar] [CrossRef]
- Noh, M.Y.; Muthukrishnan, S.; Kramer, K.J.; Arakane, Y. Development and ultrastructure of the rigid dorsal and flexible ventral cuticles of the elytron of the red flour beetle, Tribolium castaneum. Insect Biochem. Mol. Biol. 2017, 91, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Klowden, M.J. Physiological Systems in Insects, 2nd ed.; Academic Press: New York, NY, USA, 2007; pp. 1–703. [Google Scholar]
- Chen, J.; Dai, G.; Xu, Y. Optimal composite structures in the forewings of beetles. Compos. Struct. 2007, 81, 432–437. [Google Scholar] [CrossRef]
- Grodnitsky, D.L. Form and function of insect wing the evolution of biological structures. Ann. Entomol. Soc. Am. 2000, 93, 1195–1196. [Google Scholar] [CrossRef]
- Bloor, J.W.; Kiehart, D.P. RhoA regulates the cytoskeleton and cell-cell adhesion in the developing epidermis. Development 2002, 129, 3173–3183. [Google Scholar] [CrossRef] [PubMed]
- Charles, J.P. The regulation of expression of insect cuticle protein genes. Insect Biochem. Mol. Biol. 2010, 40, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Karouzou, M.V.; Spyropoulos, Y.; Iconomidou, V.A.; Cornman, R.S.; Hamodrakas, S.J.; Willis, J.H. Drosophila cuticular proteins with the R&R Consensus: Annotation and classification with a new tool for discriminating RR-1 and RR-2 sequences. Insect Biochem. Mol. Biol. 2007, 37, 754–760. [Google Scholar] [PubMed]
- Futahashi, R.; Okamoto, S.; Kawasaki, H.; Zhong, Y.-S.; Iwanaga, M.; Mita, K.; Fujiwara, H. Genome-wide identification of cuticular protein genes in the silkworm, Bombyx mori. Insect Biochem. Mol. Biol. 2008, 38, 1138–1146. [Google Scholar] [CrossRef]
- Cornman, R.S.; Togawa, T.; Dunn, W.A.; He, N.; Emmons, A.C.; Willis, J.H. Annotation and analysis of a large cuticular protein family with the R&R Consensus in Anopheles gambiae. BMC Genom. 2008, 9, 22. [Google Scholar]
- Dittmer, N.T.; Tetreau, G.; Cao, X.; Jiang, H.; Wang, P.; Kanost, M.R. Annotation and expression analysis of cuticular proteins from the tobacco hornworm, Manduca sexta. Insect Biochem. Mol. Biol. 2015, 62, 100–113. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Badgett, M.; Bowen, J.; Vannini, L.; Orlando, R.; Willis, J. Distribution of cuticular proteins in different structures of adult Anopheles gambiae. Insect Biochem. Mol. Biol. 2016, 75, 45–57. [Google Scholar] [CrossRef] [Green Version]
- Rebers, J.E.; Riddiford, L.M. Structure and expression of a Manduca sexta larval cuticle gene homologous to Drosophila cuticle genes. J. Mol. Biol. 1988, 203, 411–423. [Google Scholar] [CrossRef]
- Lomakin, J.; Huber, P.A.; Eichler, C.; Arakane, Y.; Kramer, K.J.; Beeman, R.W.; Kanost, M.R.; Gehrke, S.H. Mechanical properties of the beetle elytron, a biological composite material. Biomacromolecules 2011, 12, 321–335. [Google Scholar] [CrossRef]
- Qiao, L.; Xiong, G.; Wang, R.X.; He, S.Z.; Chen, J.; Tong, X.L.; Hu, H.; Li, C.L.; Gai, T.T.; Xin, Y.Q.; et al. Mutation of a cuticular protein, BmorCPR2, alters larval body shape and adaptability in silkworm, Bombyx mori. Genetics 2014, 196, 1103–1115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arakane, Y.; Lomakin, J.; Gehrke, S.H.; Hiromasa, Y.; Tomich, J.M.; Muthukrishnan, S.; Beeman, R.W.; Kramer, K.J.; Kanost, M.R. Formation of rigid, non-flight forewings (elytra) of a beetle requires two major cuticular proteins. PLoS Genet. 2012, 8, e1002682. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Gou, X.; Qin, Z.; Li, D.; Wang, Y.; Ma, E.; Li, S.; Zhang, J. Identification and expression of cuticular protein genes based on Locusta migratoria transcriptome. Sci. Rep. 2017, 7, 45462. [Google Scholar] [CrossRef]
- Willis, J.H. Structural cuticular proteins from arthropods: Annotation, nomenclature, and sequence characteristics in the genomics era. Insect Biochem. Mol. Biol. 2010, 40, 189–204. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Gou, X.; Liu, W.; Ma, E.; Moussian, B.; Li, S.; Zhu, K.; Zhang, J. The wing-specific cuticular protein LmACP7 is essential for normal wing morphogenesis in the migratory locust. Insect Biochem. Mol. Biol. 2019, 112, 103206. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yang, J.; Gou, X.; Liu, W.; Zhang, J. Cuticular protein gene LmACP8 is involved in wing morphogenesis in the migratory locust, Locusta migratoria. J. Integr. Agr. 2021, 20, 1596–1606. [Google Scholar] [CrossRef]
- Dittmer, N.T.; Hiromasa, Y.; Tomich, J.M.; Lu, N.; Beeman, R.W.; Kramer, K.J.; Kanost, M.R. Proteomic and transcriptomic analyses of rigid and membranous cuticles and epidermis from the elytra and hindwings of the red flour beetle, Tribolium castaneum. J. Proteome Res. 2012, 11, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Talbo, G. Determination of the covalent structure of an N- and C-terminally blocked glycoprotein from endocuticle of Locusta migratoria. Eur. J. Biochem. 1991, 195, 495–504. [Google Scholar] [CrossRef]
- Jespersen, S.; Hojrup, P.; Andersent, S.O.; Roepstorff, P. The primary structure of an endocuticular protein from two locust species, Locusta migratoria and Schistocerca gregaria, determined by a combination of mass spectrometry and automatic Edman degradation. Comp. Biochem. Physiol. 1994, 109, 125–138. [Google Scholar] [CrossRef]
- Nohr, C.; Hojrup, P.; Andersen, S.O. Primary structure of two low molecular weight proteins isolated from cuticle of fifth instar nymphs of the migratory locust, Locusta migratoria. Insect Biochem. Mol. Biol. 1992, 22, 19–24. [Google Scholar] [CrossRef]
- Andersen, S.O. Insect Cuticular Proteins. Insect Biochem. Mol. Biol. 1995, 25, 153–176. [Google Scholar] [CrossRef]
- Jensen, C.; Andersen, S.O.; Roepstor, P. Primary structure of two major cuticular proteins from the migratory locust, Locusta migratoria, and their identification in polyacrylamide gels by mass spectrometry. Biochim Biophys Acta 1998, 1429, 151–162. [Google Scholar] [CrossRef]
- Shahin, R.; Iwanaga, M.; Kawasaki, H. Cuticular protein and transcription factor genes expressed during prepupal-pupal transition and by ecdysone pulse treatment in wing discs of Bombyx mori. Insect Mol. Biol. 2016, 25, 138–152. [Google Scholar] [CrossRef] [PubMed]
- Vannini, L.; Willis, J.H. Localization of RR-1 and RR-2 cuticular proteins within the cuticle of Anopheles gambiae. Arthropod Struct. Dev. 2017, 46, 13–29. [Google Scholar] [CrossRef] [Green Version]
- Xiong, G.; Tong, X.; Gai, T.; Li, C.; Qiao, L.; Monteiro, A.; Hu, H.; Han, M.; Ding, X.; Wu, S.; et al. Body shape and coloration of silkworm larvae are influenced by a novel cuticular protein. Genetics 2017, 207, 1053–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Wei, W.; Chu, Y.; Zhang, L.; Shen, J.; An, C. De novo transcriptome analysis of wing development-related signaling pathways in Locusta migratoria Manilensis and Ostrinia furnacalis (Guenée). PLoS ONE 2014, 9, e106770. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Qin, Z.; Liu, W.; Liu, X.; Moussian, B.; Ma, E.; Li, S.; Zhang, J. Nuclear receptor HR3 controls locust molt by regulating chitin synthesis and degradation genes of Locusta migratoria. Insect Biochem. Mol. Biol. 2018, 92, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Li, Z.; Cao, J.; Zhang, S.; Zhang, H.; Wu, X.; Zhang, Q.; Liu, X. Selection and assessment of reference genes for quantitative PCR normalization in migratory locust Locusta migratoria (Orthoptera: Acrididae). PLoS ONE 2014, 9, e98164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.M.; Liu, C.; Li, Q.Y.; Hu, W.B.; Zhou, M.T.; Nie, H.Y.; Zhang, Y.X.; Peng, Z.C.; Zhao, P.; Xia, Q.Y. Basic helix-loop-helix transcription factor Bmsage is involved in regulation of fibroin H-chain gene via interaction with SGF1 in Bombyx mori. PLoS ONE 2014, 9, e94091. [Google Scholar] [CrossRef] [PubMed]
- Toprak, U.; Baldwin, D.; Erlandson, M.; Gillott, C.; Hegedus, D.D. Insect intestinal mucins and serine proteases associated with the peritrophic matrix from feeding, starved and moulting Mamestra configurata larvae. Insect Mol. Biol. 2010, 19, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Song, T.Q.; Yang, M.L.; Wang, Y.L.; Liu, Q.; Wang, H.M.; Zhang, J.; Li, T. Cuticular protein LmTwdl1 is involved in molt development of the migratory locust. Insect Sci. 2016, 23, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Xie, Y.; Xue, J.; Gao, Y.; Zhang, Y.; Zhang, X.; Tan, J. Histopathological changes of Ceroplastes japonicus infected by Lecanicillium lecanii. J. Invertebr. Pathol. 2009, 101, 96–105. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Shao, T.; Su, Y.; Zhang, J.; Gou, X.; Liu, W.; Zhang, J. Cuticle Protein LmACP19 Is Required for the Stability of Epidermal Cells in Wing Development and Morphogenesis of Locusta migratoria. Int. J. Mol. Sci. 2022, 23, 3106. https://doi.org/10.3390/ijms23063106
Zhao X, Shao T, Su Y, Zhang J, Gou X, Liu W, Zhang J. Cuticle Protein LmACP19 Is Required for the Stability of Epidermal Cells in Wing Development and Morphogenesis of Locusta migratoria. International Journal of Molecular Sciences. 2022; 23(6):3106. https://doi.org/10.3390/ijms23063106
Chicago/Turabian StyleZhao, Xiaoming, Ti Shao, Yazhi Su, Jing Zhang, Xin Gou, Weimin Liu, and Jianzhen Zhang. 2022. "Cuticle Protein LmACP19 Is Required for the Stability of Epidermal Cells in Wing Development and Morphogenesis of Locusta migratoria" International Journal of Molecular Sciences 23, no. 6: 3106. https://doi.org/10.3390/ijms23063106
APA StyleZhao, X., Shao, T., Su, Y., Zhang, J., Gou, X., Liu, W., & Zhang, J. (2022). Cuticle Protein LmACP19 Is Required for the Stability of Epidermal Cells in Wing Development and Morphogenesis of Locusta migratoria. International Journal of Molecular Sciences, 23(6), 3106. https://doi.org/10.3390/ijms23063106





