The Endocannabinoid System and Physical Activity—A Robust Duo in the Novel Therapeutic Approach against Metabolic Disorders
Abstract
:1. Introduction
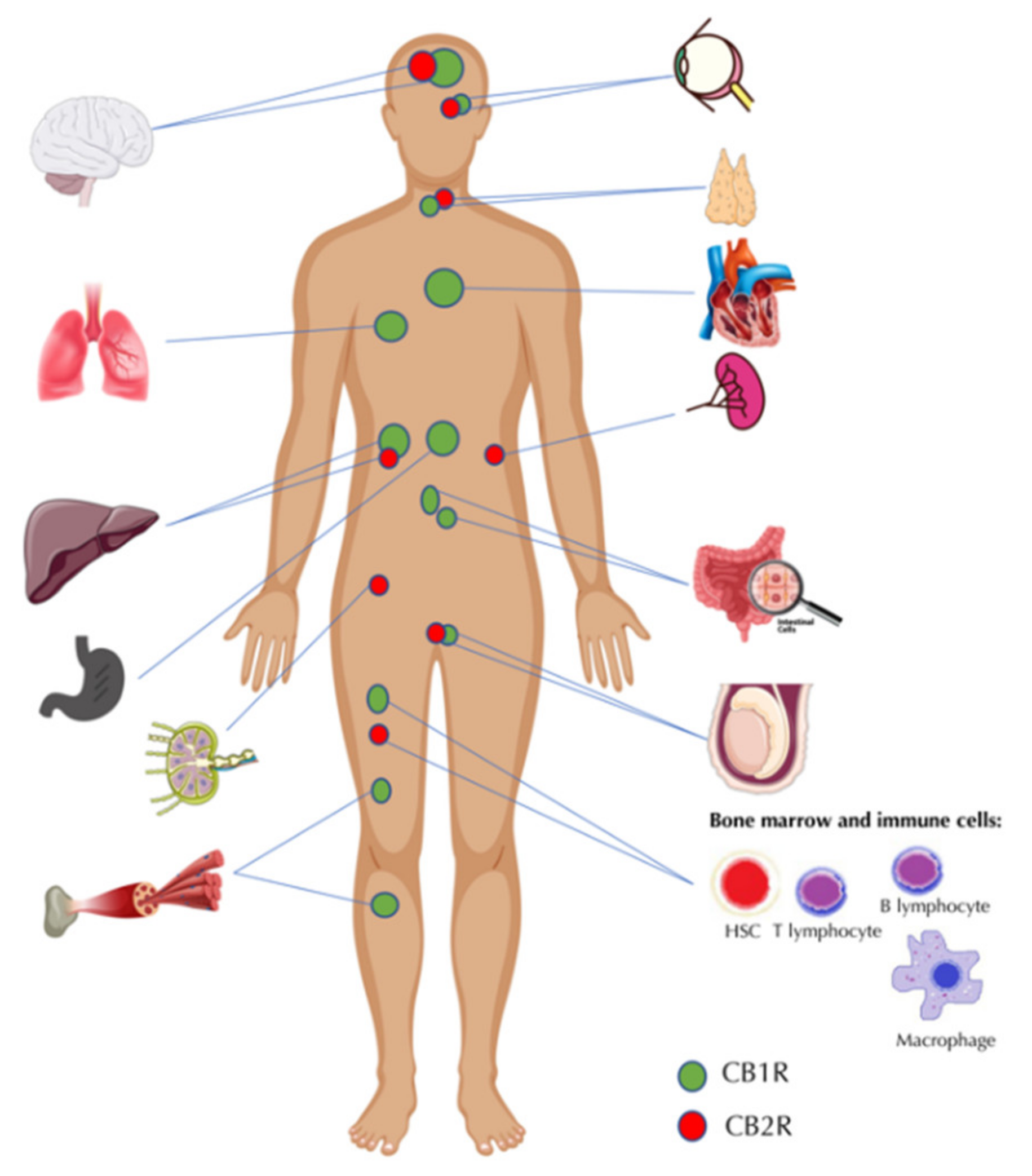
2. The Endocannabinoid System—A Brief Overview of the Key Functions and Elements
3. The Endocannabinoid System and Metabolic Pathologies
3.1. Obesity and Non-Alcoholic Fatty Liver Disease (NAFLD)
3.2. Insulin Resistance and Type 2 Diabetes
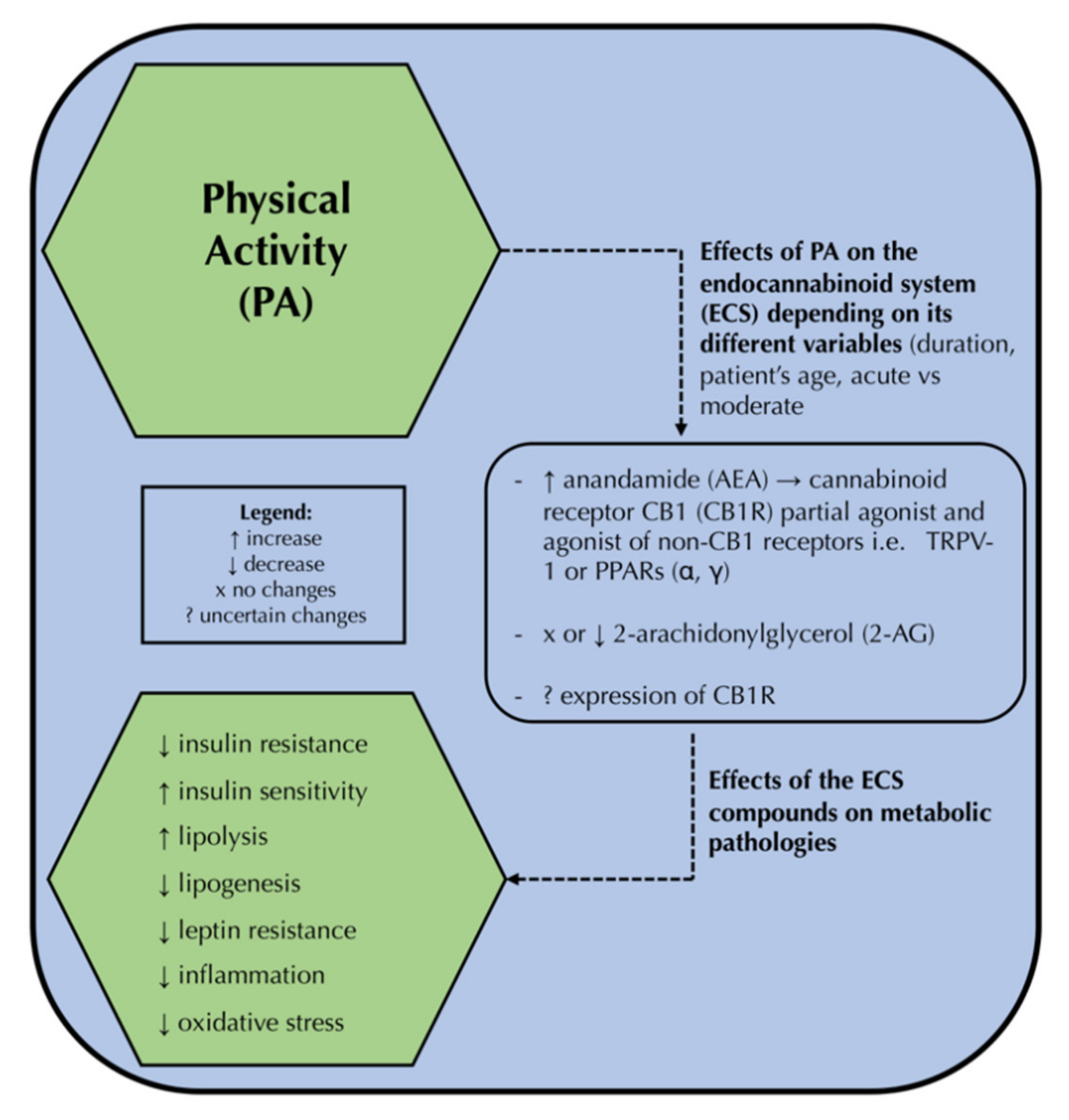
4. The Endocannabinoid System and Physical Activity
5. The Triad—Physical Activity, the Endocannabinoid System, and the Novel Therapeutic Approach to Metabolic Disorders—How All These Components May Be Linked?
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Caballero, B. Humans against Obesity: Who Will Win? Adv. Nutr. 2019, 10, S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Mayoral, L.P.-C.; Andrade, G.B.; Mayoral, E.P.-C.; Teresa, H.H.; Socorro, P.C.; Francisco, J.R.C.; Héctor, A.C.-F.; Cruz, M.M.; Santiago, A.D.P.; Alpuche, J.J.; et al. Obesity subtypes, related biomarkers & heterogeneity. Indian J. Med. Res. 2020, 151, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.B.; Smith, M.S. Obesity Statistics. Prim. Care Clin. Off. Pract. 2016, 43, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Yaribeygi, H.; Butler, A.E. Insulin resistance: Review of the underlying molecular mechanisms. J. Cell. Physiol. 2019, 234, 8152–8161. [Google Scholar] [CrossRef]
- Antonopoulos, A.S.; Tousoulis, D. Themolecularmechanisms of obesity paradox. Cardiovasc. Res. 2017, 113, 1074–1086. [Google Scholar] [CrossRef]
- Mazier, W.; Saucisse, N.; Gatta-Cherifi, B.; Cota, D. The Endocannabinoid System: Pivotal Orchestrator of Obesity and Metabolic Disease. Trends Endocrinol. Metab. 2015, 26, 524–537. [Google Scholar] [CrossRef]
- Tantimonaco, M.; Ceci, R.; Sabatini, S.; Catani, M.V.; Rossi, A.; Gasperi, V.; Maccarrone, M. Physical activity and the endocannabinoid system: An overview. Cell. Mol. Life Sci. 2014, 71, 2681–2698. [Google Scholar] [CrossRef]
- Watkins, B.A. Diet, endocannabinoids, and health. Nutr. Res. 2019, 70, 32–39. [Google Scholar] [CrossRef]
- Heyman, E.; Gamelin, F.X.; Aucouturier, J.; Di Marzo, V. The role of the endocannabinoid system in skeletal muscle and metabolic adaptations to exercise: Potential implications for the treatment of obesity. Obes. Rev. 2012, 13, 1110–1124. [Google Scholar] [CrossRef]
- Gamelin, F.X.; Aucouturier, J.; Iannotti, F.A.; Piscitelli, F.; Mazzarella, E.; Aveta, T.; Leriche, M.; Dupont, E.; Cieniewski-Bernard, C.; Montel, V.; et al. Effects of chronic exercise on the endocannabinoid system in Wistar rats with high-fat diet-induced obesity. J. Physiol. Biochem. 2016, 72, 183–199. [Google Scholar] [CrossRef]
- Meccariello, R. Endocannabinoid system in health and disease: Current situation and future perspectives. Int. J. Mol. Sci. 2020, 21, 3549. [Google Scholar] [CrossRef] [PubMed]
- Nesto, R.W.; Mackie, K. Endocannabinoid system and its implications for obesity and cardiometabolic risk. Eur. Hearth J. Suppl. 2008, 10, 34–41. [Google Scholar] [CrossRef] [Green Version]
- Mechoulam, R.; Parker, L.A. The Endocannabinoid System and the Brain. Annu. Rev. Psychol. 2013, 64, 21–47. [Google Scholar] [CrossRef] [Green Version]
- Bovolin, P.; Cottone, E.; Pomatto, V.; Fasano, S.; Pierantoni, R.; Cobellis, G.; Meccariello, R. Endocannabinoids are involved in male vertebrate reproduction: Regulatory mechanisms at central and gonadal level. Front. Endocrinol. 2014, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Iannotti, F.A.; Di Marzo, V. The gut microbiome, endocannabinoids and metabolic disorders. J. Endocrinol. 2021, 248, R83–R97. [Google Scholar] [CrossRef] [PubMed]
- Freitas, H.R.; Isaac, A.R.; Malcher-Lopes, R.; Diaz, B.L.; Trevenzoli, I.H.; De Melo Reis, R.A. Polyunsaturated fatty acids and endocannabinoids in health and disease. Nutr. Neurosci. 2018, 21, 695–714. [Google Scholar] [CrossRef] [PubMed]
- Cristino, L.; Bisogno, T.; Di Marzo, V. Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat. Rev. Neurol. 2020, 16, 9–29. [Google Scholar] [CrossRef]
- Berk, K.; Bzdega, W.; Konstantynowicz-nowicka, K.; Charytoniuk, T.; Zywno, H.; Chabowski, A. Phytocannabinoids—A green approach toward non-alcoholic fatty liver disease treatment. J. Clin. Med. 2021, 10, 393. [Google Scholar] [CrossRef]
- Bozkurt, T.E. Endocannabinoid system in the airways. Molecules 2019, 24, 4626. [Google Scholar] [CrossRef] [Green Version]
- Joshi, N.; Onaivi, E.S. Endocannabinoid System Components: Overview and Tissue Distribution. Adv. Exp. Med. Biol. 2019, 1162, 1–12. [Google Scholar] [CrossRef]
- Mackie, K. Cannabinoid receptors: Where they are and what they do. J. Neuroendocrinol. 2008, 20, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Zagozdzon, R.; Jorda, M.A.; Parmar, K.; Fu, Y.; Williams, J.S.; Wood, J.A.T.; Makriyannis, A.; Banu, N.; Avraham, S.; et al. Endocannabinoids are expressed in bone marrow stromal niches and play a role in interactions of hematopoietic stem and progenitor cells with the bone marrow microenvironment. J. Biol. Chem. 2010, 285, 35471–35478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, S.; Kumar, U. Cannabinoid receptors and the endocannabinoid system: Signaling and function in the central nervous system. Int. J. Mol. Sci. 2018, 19, 833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Marzo, V. New approaches and challenges to targeting the endocannabinoid system. Nat. Rev. Drug Discov. 2018, 17, 623–639. [Google Scholar] [CrossRef] [PubMed]
- Karwad, M.A.; Macpherson, T.; Wang, B.; Theophilidou, E.; Sarmad, S.; Barrett, D.A.; Larvin, M.; Wright, K.L.; Lund, J.N.; O’Sullivan, S.E. Oleoylethanolamine and palmitoylethanolamine modulate intestinal permeability in vitro via TRPV1 and PPARα. FASEB J. 2017, 31, 469–481. [Google Scholar] [CrossRef] [Green Version]
- Karwad, M.A.; Couch, D.G.; Wright, K.L.; Tufarelli, C.; Larvin, M.; Lund, J.; O’Sullivan, S.E. Endocannabinoids and endocannabinoid-like compounds modulate hypoxia-induced permeability in CaCo-2 cells via CB1, TRPV1, and PPARα. Biochem. Pharmacol. 2019, 168, 465–472. [Google Scholar] [CrossRef]
- Gatta-Cherifi, B.; Cota, D. New insights on the role of the endocannabinoid system in the regulation of energy balance. Int. J. Obes. 2016, 40, 210–219. [Google Scholar] [CrossRef]
- Bermudez-Silva, F.J.; Cardinal, P.; Cota, D. The role of the endocannabinoid system in the neuroendocrine regulation of energy balance. J. Psychopharmacol. 2012, 26, 114–124. [Google Scholar] [CrossRef]
- Balsevich, G.; Sticht, M.; Bowles, N.P.; Singh, A.; Lee, T.T.Y.; Li, Z.; Chelikani, P.K.; Lee, F.S.; Borgland, S.L.; Hillard, C.J.; et al. Role for fatty acid amide hydrolase (faah) in the leptin-mediated effects on feeding and energy balance. Proc. Natl. Acad. Sci. USA 2018, 115, 7605–7610. [Google Scholar] [CrossRef] [Green Version]
- Buettner, C.; Muse, E.D.; Cheng, A.; Chen, L.; Scherer, T.; Pocai, A.; Su, K.; Cheng, B.; Li, X.; Harvey-White, J.; et al. Leptin controls adipose tissue lipogenesis via central, STAT3-independent mechanisms. Nat. Med. 2008, 14, 667–675. [Google Scholar] [CrossRef]
- Rossi, F.; Punzo, F.; Umano, G.R.; Argenziano, M.; Miraglia Del Giudice, E. Role of cannabinoids in obesity. Int. J. Mol. Sci. 2018, 19, 2690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gruzdeva, O.; Borodkina, D.; Uchasova, E.; Dyleva, Y.; Barbarash, O. Leptin resistance: Underlying mechanisms and diagnosis. Diabetes, Metab. Syndr. Obes. Targets Ther. 2019, 12, 191–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tam, J.; Szanda, G.; Drori, A.; Liu, Z.; Cinar, R.; Kashiwaya, Y.; Reitman, M.L.; Kunos, G. Peripheral cannabinoid-1 receptor blockade restores hypothalamic leptin signaling. Mol. Metab. 2017, 6, 1113–1125. [Google Scholar] [CrossRef]
- DiPatrizio, N.V.; Piomelli, D. The thrifty lipids: Endocannabinoids and the neural control of energy conservation. Trends Neurosci. 2012, 35, 403–411. [Google Scholar] [CrossRef] [Green Version]
- Bazwinsky-Wutschke, I.; Zipprich, A.; Dehghani, F. Endocannabinoid system in hepatic glucose metabolism, fatty liver disease, and cirrhosis. Int. J. Mol. Sci. 2019, 20, 2516. [Google Scholar] [CrossRef] [Green Version]
- Karaliota, S.; Siafaka-Kapadai, A.; Gontinou, C.; Psarra, K.; Mavri-Vavayanni, M. Anandamide increases the differentiation of rat adipocytes and causes PPARλ and cB1 receptor upregulation. Obesity 2009, 17, 1830–1838. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.; Lynch, D.L.; Hurst, D.P.; Reggio, P.H. A Closer Look at Anandamide Interaction With TRPV1. Front. Mol. Biosci. 2020, 7, 1–8. [Google Scholar] [CrossRef]
- Lee, E.; Jung, D.Y.; Kim, J.H.; Patel, P.R.; Hu, X.; Lee, Y.; Azuma, Y.; Wang, H.F.; Tsitsilianos, N.; Shafiq, U.; et al. Transient receptor potential vanilloid type-1 channel regulates diet-induced obesity, insulin resistance, and leptin resistance. FASEB J. 2015, 29, 3182–3192. [Google Scholar] [CrossRef] [Green Version]
- Lotteau, S.; Ducreux, S.; Romestaing, C.; Legrand, C.; Van Coppenolle, F. Characterization of Functional TRPV1 Channels in the Sarcoplasmic Reticulum of Mouse Skeletal Muscle. PLoS ONE 2013, 8, e58673. [Google Scholar] [CrossRef] [Green Version]
- Christie, S.; Wittert, G.A.; Li, H.; Page, A.J. Involvement of TRPV1 Channels in Energy Homeostasis. Front. Endocrinol. 2018, 9, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Zhang, X.; Lee, N.R.; Jin, H.S. TRPV1 Gene Polymorphisms Are Associated with Type 2 Diabetes by Their Interaction with Fat Consumption in the Korean Genome Epidemiology Study. J. Nutrigenet. Nutrigenomics 2016, 9, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Muller, T.; Demizieux, L.; Troy-Fioramonti, S.; Gresti, J.; de Barros, J.P.P.; Berger, H.; Vergès, B.; Degrace, P. Overactivation of the endocannabinoid system alters the antilipolytic action of insulin in mouse adipose tissue. Am. J. Physiol. Endocrinol. Metab. 2017, 313, E26–E36. [Google Scholar] [CrossRef] [Green Version]
- Blüher, M.; Engeli, S.; Klöting, N.; Berndt, J.; Fasshauer, M.; Bátkai, S.; Pacher, P.; Schön, M.R.; Jordan, J.; Stumvoll, M. Dysregulation of the peripheral and adipose tissue endocannabinoid system in human abdominal obesity. Diabetes 2006, 55, 3053–3060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Côté, M.; Matias, I.; Lemieux, I.; Petrosino, S.; Alméras, N.; Després, J.P.; Di Marzo, V. Circulating endocannabinoid levels, abdominal adiposity and related cardiometabolic risk factors in obese men. Int. J. Obes. 2007, 31, 692–699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vähätalo, L.H.; Ruohonen, S.T.; Mäkelä, S.; Ailanen, L.; Penttinen, A.M.; Stormi, T.; Kauko, T.; Piscitelli, F.; Silvestri, C.; Savontaus, E.; et al. Role of the endocannabinoid system in obesity induced by neuropeptide y overexpression in noradrenergic neurons. Nutr. Diabetes 2015, 5, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cota, D.; Marsicano, G.; Tschöp, M.; Grübler, Y.; Flachskamm, C.; Schubert, M.; Auer, D.; Yassouridis, A.; Thöne-Reineke, C.; Ortmann, S.; et al. The endogenous cennabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J. Clin. Invest. 2003, 112, 423–431. [Google Scholar] [CrossRef]
- Müller, T.D.; Clemmensen, C.; Finan, B.; Dimarchi, R.D.; Tschöp, M.H. Anti-obesity therapy: From rainbow pills to polyagonists. Pharmacol. Rev. 2018, 70, 712–746. [Google Scholar] [CrossRef] [Green Version]
- Bennetzen, M.F.; Wellner, N.; Ahmed, S.S.; Ahmed, S.M.; Diep, T.A.; Hansen, H.S.; Richelsen, B.; Pedersen, S.B. Investigations of the human endocannabinoid system in two subcutaneous adipose tissue depots in lean subjects and in obese subjects before and after weight loss. Int. J. Obes. 2011, 35, 1377–1384. [Google Scholar] [CrossRef] [Green Version]
- Izzo, A.A.; Piscitelli, F.; Capasso, R.; Aviello, G.; Romano, B.; Borrelli, F.; Petrosino, S.; Di Marzo, V. Peripheral endocannabinoid dysregulation in obesity: Relation to intestinal motility and energy processing induced by food deprivation and re-feeding. Br. J. Pharmacol. 2009, 158, 451–461. [Google Scholar] [CrossRef] [Green Version]
- Little, T.J.; Cvijanovic, N.; Dipatrizio, N.V.; Argueta, D.A.; Rayner, C.K.; Feinle-Bisset, C.; Young, R.L. Endocannabinoids and cannabinoid receptors as regulators of endocrine functions and tissue metabolism: Plasma endocannabinoid levels in lean, overweight, and obese humans: Relationships to intestinal permeability markers, inflammation, and incretin secret. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E489–E495. [Google Scholar] [CrossRef]
- Sipe, J.C.; Scott, T.M.; Murray, S.; Harismendy, O.; Simon, G.M.; Cravatt, B.F.; Waalen, J. Biomarkers of endocannabinoid system activation in severe obesity. PLoS ONE 2010, 5, e8792. [Google Scholar] [CrossRef] [Green Version]
- Quercioli, A.; Pataky, Z.; Vincenti, G.; Makoundou, V.; Di Marzo, V.; Montecucco, F.; Carballo, S.; Thomas, A.; Staub, C.; Steffens, S.; et al. Elevated endocannabinoid plasma levels are associated with coronary circulatory dysfunction in obesity. Eur. Heart J. 2011, 32, 1369–1378. [Google Scholar] [CrossRef] [Green Version]
- Argueta, D.A.; DiPatrizio, N.V. Peripheral endocannabinoid signaling controls hyperphagia in western diet-induced obesity. Physiol. Behav. 2017, 171, 32–39. [Google Scholar] [CrossRef] [Green Version]
- Charytoniuk, T.; Drygalski, K.; Konstantynowicz-Nowicka, K.; Berk, K.; Chabowski, A. Alternative treatment methods attenuate the development of NAFLD: A review of resveratrol molecular mechanisms and clinical trials. Nutrition 2017, 34, 108–117. [Google Scholar] [CrossRef]
- Auguet, T.; Berlanga, A.; Guiu-Jurado, E.; Terra, X.; Martinez, S.; Aguilar, C.; Filiu, E.; Alibalic, A.; Sabench, F.; Hernández, M.; et al. Endocannabinoid receptors gene expression in morbidly obese women with nonalcoholic fatty liver disease. BioMed Res. Int. 2014, 2014, 502542. [Google Scholar] [CrossRef]
- Purohit, V.; Rapaka, R.; Shurtleff, D. Role of cannabinoids in the development of fatty liver (steatosis). AAPS J. 2010, 12, 233–237. [Google Scholar] [CrossRef] [Green Version]
- Alswat, K.A. The role of endocannabinoids system in fatty liver disease and therapeutic potentials. Saudi J. Gastroenterol. 2013, 19, 144–151. [Google Scholar] [CrossRef]
- Jorgačević, B.; Vučević, D.; Samardžić, J.; Mladenović, D.; Vesković, M.; Vukićević, D.; Ješić, R.; Radosavljević, T. The Effect of CB1 Antagonism on Hepatic Oxidative/Nitrosative Stress and Inflammation in Nonalcoholic Fatty Liver Disease. Curr. Med. Chem. 2020, 28, 169–180. [Google Scholar] [CrossRef]
- Chang, E.; Kim, D.H.; Yang, H.; Lee, D.H.; Bae, S.H.; Park, C.Y. CB1 receptor blockade ameliorates hepatic fat infiltration and inflammation and increases Nrf2-AMPK pathway in a rat model of severely uncontrolled diabetes. PLoS ONE 2018, 13, e0206152. [Google Scholar] [CrossRef]
- Irungbam, K.; Churin, Y.; Matono, T.; Weglage, J.; Ocker, M.; Glebe, D.; Hardt, M.; Koeppel, A.; Roderfeld, M.; Roeb, E. Cannabinoid receptor 1 knockout alleviates hepatic steatosis by downregulating perilipin 2. Lab. Investig. 2020, 100, 454–465. [Google Scholar] [CrossRef]
- Shi, D.; Zhan, X.; Yu, X.; Jia, M.; Zhang, Y.; Yao, J.; Hu, X.; Bao, Z. Inhibiting CB1 receptors improves lipogenesis in an in vitro non-alcoholic fatty liver disease model. Lipids Health Dis. 2014, 13, 173. [Google Scholar] [CrossRef] [Green Version]
- Deveaux, V.; Cadoudal, T.; Ichigotani, Y.; Teixeira-Clerc, F.; Louvet, A.; Manin, S.; Van Nhieu, J.T.; Belot, M.P.; Zimmer, A.; Even, P.; et al. Cannabinoid CB2 receptor potentiates obesity-associated inflammation, insulin resistance and hepatic steatosis. PLoS ONE 2009, 4, e5844. [Google Scholar] [CrossRef]
- Julien, B.; Grenard, P.; Teixeira-Clerc, F.; Van Nhieu, J.T.; Li, L.; Karsak, M.; Zimmer, A.; Mallat, A.; Lotersztajn, S. Antifibrogenic role of the cannabinoid receptor CB2 in the liver. Gastroenterology 2005, 128, 742–755. [Google Scholar] [CrossRef]
- Groop, L.C. Insulin resistance: The fundamental trigger of type 2 diabetes. Diabetes Obes. Metab. 1999, 1, 1–7. [Google Scholar] [CrossRef]
- Ndisang, J.F.; Vannacci, A.; Rastogi, S. Insulin Resistance, Type 1 and Type 2 Diabetes, and Related Complications 2017. J. Diabetes Res. 2017, 2017, 1478294. [Google Scholar] [CrossRef]
- Nagappan, A.; Shin, J.; Jung, M.H. Role of cannabinoid receptor type 1 in insulin resistance and its biological implications. Int. J. Mol. Sci. 2019, 20, 2109. [Google Scholar] [CrossRef] [Green Version]
- Jourdan, T.; Demizieux, L.; Gresti, J.; Djaouti, L.; Gaba, L.; Vergès, B.; Degrace, P. Antagonism of peripheral hepatic cannabinoid receptor-1 improves liver lipid metabolism in mice: Evidence from cultured explants. Hepatology 2012, 55, 790–799. [Google Scholar] [CrossRef]
- Lipina, C.; Vaanholt, L.M.; Davidova, A.; Mitchell, S.E.; Storey-Gordon, E.; Hambly, C.; Irving, A.J.; Speakman, J.R.; Hundal, H.S. CB1 receptor blockade counters age-induced insulin resistance and metabolic dysfunction. Aging Cell 2016, 15, 325–335. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Zhou, L.; Xiong, K.; Godlewski, G.; Mukhopadhyay, B.; Tam, J.; Yin, S.; Gao, P.; Shan, X.; Pickel, J.; et al. Hepatic cannabinoid receptor-1 mediates diet-induced insulin resistance via inhibition of insulin signaling and clearance in mice. Gastroenterology 2012, 142, 1218–1228. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Godlewski, G.; Jourdan, T.; Liu, Z.; Cinar, R.; Xiong, K.; Kunos, G. Cannabinoid-1 Receptor Antagonism Improves Glycemic Control and Increases Energy Expenditure Through Sirtuin-1/Mechanistic Target of Rapamycin Complex 2 and 5′Adenosine Monophosphate–Activated Protein Kinase Signaling. Hepatology 2019, 69, 1535–1548. [Google Scholar] [CrossRef]
- Jourdan, T.; Nicoloro, S.M.; Zhou, Z.; Shen, Y.; Liu, J.; Coffey, N.J.; Cinar, R.; Godlewski, G.; Gao, B.; Aouadi, M.; et al. Decreasing CB1 receptor signaling in Kupffer cells improves insulin sensitivity in obese mice. Mol. Metab. 2017, 6, 1517–1528. [Google Scholar] [CrossRef]
- Lopez-Rodriguez, A.B.; Mela, V.; Acaz-Fonseca, E.; Garcia-Segura, L.M.; Viveros, M.P. CB2 cannabinoid receptor is involved in the anti-inflammatory effects of leptin in a model of traumatic brain injury. Exp. Neurol. 2016, 279, 274–282. [Google Scholar] [CrossRef]
- Ashton, J.; Glass, M. The Cannabinoid CB2 Receptor as a Target for Inflammation-Dependent Neurodegeneration. Curr. Neuropharmacol. 2007, 5, 73–80. [Google Scholar] [CrossRef] [Green Version]
- Javed, H.; Azimullah, S.; Haque, M.E.; Ojha, S.K. Cannabinoid type 2 (CB2) receptors activation protects against oxidative stress and neuroinflammation associated dopaminergic neurodegeneration in rotenone model of parkinson’s disease. Front. Neurosci. 2016, 10. [Google Scholar] [CrossRef] [Green Version]
- Verty, A.N.A.; Stefanidis, A.; McAinch, A.J.; Hryciw, D.H.; Oldfield, B. Anti-obesity effect of the CB2 receptor agonist JWH-015 in diet-induced obese mice. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [Green Version]
- Oka, S.; Wakui, J.; Ikeda, S.; Yanagimoto, S.; Kishimoto, S.; Gokoh, M.; Nasui, M.; Sugiura, T. Involvement of the Cannabinoid CB2 Receptor and Its Endogenous Ligand 2-Arachidonoylglycerol in Oxazolone-Induced Contact Dermatitis in Mice. J. Immunol. 2006, 177, 8796–8805. [Google Scholar] [CrossRef]
- Maestroni, G.J.M. The endogenous cannabinoid 2-arachidonoyl glycerol as in vivo chemoattractant for dendritic cells and adjuvant for Th1 response to a soluble protein. FASEB J. 2004, 18, 1914–1916. [Google Scholar] [CrossRef] [Green Version]
- Turcotte, C.; Chouinard, F.; Lefebvre, J.S.; Flamand, N. Regulation of inflammation by cannabinoids, the endocannabinoids 2-arachidonoyl-glycerol and arachidonoyl-ethanolamide, and their metabolites. J. Leukoc. Biol. 2015, 97, 1049–1070. [Google Scholar] [CrossRef]
- Mendizabal-Zubiaga, J.; Melser, S.; Bénard, G.; Ramos, A.; Reguero, L.; Arrabal, S.; Elezgarai, I.; Gerrikagoitia, I.; Suarez, J.; De Fonseca, F.R.; et al. Cannabinoid CB1 receptors are localized in striated muscle mitochondria and regulate mitochondrial respiration. Front. Physiol. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Cavuoto, P.; McAinch, A.J.; Hatzinikolas, G.; Cameron-Smith, D.; Wittert, G.A. Effects of cannabinoid receptors on skeletal muscle oxidative pathways. Mol. Cell. Endocrinol. 2007, 267, 63–69. [Google Scholar] [CrossRef]
- Chanda, D.; Kim, D.K.; Li, T.; Kim, Y.H.; Koo, S.H.; Lee, C.H.; Chiang, J.Y.L.; Choi, H.S. Cannabinoid Receptor Type 1 (CB1R) signaling regulates hepatic gluconeogenesis via induction of endoplasmic reticulum-bound transcription factor cAMP-responsive element-binding protein H (CREBH) in primary hepatocytes. J. Biol. Chem. 2011, 286, 27971–27979. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, Y.; Shimano, H. CREBH regulates systemic glucose and lipid metabolism. Int. J. Mol. Sci. 2018, 19, 1396. [Google Scholar] [CrossRef] [Green Version]
- Jourdan, T.; Godlewski, G.; Cinar, R.; Bertola, A.; Szanda, G.; Liu, J.; Tam, J.; Han, T.; Mukhopadhyay, B.; Skarulis, M.C.; et al. Activation of the Nlrp3 inflammasome in infiltrating macrophages by endocannabinoids mediates beta cell loss in type 2 diabetes. Nat. Med. 2013, 19, 1132–1140. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Lee, K.J.; Kim, J.S.; Rho, J.G.; Shin, J.J.; Song, W.K.; Lee, E.K.; Egan, J.M.; Kim, W. Cannabinoids regulate Bcl-2 and cyclin D2 expression in pancreatic β cells. PLoS ONE 2016, 11, e0150981. [Google Scholar] [CrossRef]
- Zywno, H.; Bzdega, W.; Kolakowski, A.; Kurzyna, P.; Harasim-Symbor, E.; Sztolsztener, K.; Chabowski, A.; Konstantynowicz-Nowicka, K. The influence of coumestrol on sphingolipid signaling pathway and insulin resistance development in primary rat hepatocytes. Biomolecules 2021, 11, 268. [Google Scholar] [CrossRef]
- Brozinick, J.T.; Hawkins, E.; Hoang Bui, H.; Kuo, M.S.; Tan, B.; Kievit, P.; Grove, K. Plasma sphingolipids are biomarkers of metabolic syndrome in non-human primates maintained on a Western-style diet. Int. J. Obes. 2013, 37, 1064–1070. [Google Scholar] [CrossRef] [Green Version]
- Cinar, R.; Godlewski, G.; Liu, J.; Tam, J.; Jourdan, T.; Mukhopadhyay, B.; Harvey-White, J.; Kunos, G. Hepatic cannabinoid-1 receptors mediate diet-induced insulin resistance by increasing de novo synthesis of long-chain ceramides. Hepatology 2014, 59, 143–153. [Google Scholar] [CrossRef] [Green Version]
- Jakicic, J.M.; Davis, K.K. Obesity and physical activity. Psychiatr. Clin. N. Am. 2011, 34, 829–840. [Google Scholar] [CrossRef]
- Charytoniuk, T.; Zywno, H.; Konstantynowicz-Nowicka, K.; Berk, K.; Bzdega, W.; Chabowski, A. Can physical activity support the endocannabinoid system in the preventive and therapeutic approach to neurological disorders? Int. J. Mol. Sci. 2020, 21, 4221. [Google Scholar] [CrossRef]
- Sparling, P.B.; Giu, A.; Piomelli, D.; Rosskopf, L.; Ca, A.D. Exercise activates the endocannabinoid system. Neuroreport 2003, 14, 1–3. [Google Scholar] [CrossRef]
- Raichlen, D.A.; Foster, A.D.; Seillier, A.; Giuffrida, A.; Gerdeman, G.L. Exercise-induced endocannabinoid signaling is modulated by intensity. Eur. J. Appl. Physiol. 2013, 113, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Feuerecker, M.; Hauer, D.; Toth, R.; Demetz, F.; Hölzl, J.; Thiel, M.; Kaufmann, I.; Schelling, G.; Choukèr, A. Effects of exercise stress on the endocannabinoid system in humans under field conditions. Eur. J. Appl. Physiol. 2012, 112, 2777–2781. [Google Scholar] [CrossRef] [PubMed]
- Raichlen, D.A.; Foster, A.D.; Gerdeman, G.L.; Seillier, A.; Giuffrida, A. Wired to run: Exercise-induced endocannabinoid signaling in humans and cursorial mammals with implications for the “runner’s high”. J. Exp. Biol. 2012, 215, 1331–1336. [Google Scholar] [CrossRef] [Green Version]
- Hill, M.N.; Titterness, A.K.; Morrish, A.C.; Carrier, E.J.; Lee, T.T.Y.; Gil-Mohapel, J.; Gorzalka, B.B.; Hillard, C.J.; Christie, B.R. Endogenous cannabinoid signaling is required for voluntary exercise-induced enhancement of progenitor cell proliferation in the hippocampus. Hippocampus 2010, 20, 513–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heyman, E.; Gamelin, F.; Goekint, M.; Piscitelli, F. Intense exercise increases circulating endocannabinoid and BDNF levels in humans. Possible implications for reward and depression. Psychoneuroendocrinology 2012, 37, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Cedernaes, J.; Fanelli, F.; Fazzini, A.; Pagotto, U.; Broman, J.E.; Vogel, H.; Dickson, S.L.; Schiöth, H.B.; Benedict, C. Sleep restriction alters plasma endocannabinoids concentrations before but not after exercise in humans. Psychoneuroendocrinology 2016, 74, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V.; Silvestri, C. Lifestyle and metabolic syndrome: Contribution of the endocannabinoidome. Nutrients 2019, 11, 1956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brellenthin, A.G.; Crombie, K.M.; Hillard, C.J.; Koltyn, K.F. Endocannabinoid and mood responses to exercise in adults with varying activity levels. Med. Sci. Sports Exerc. 2017, 49, 1688–1696. [Google Scholar] [CrossRef]
- Thompson, Z.; Argueta, D.; Garland, T.; DiPatrizio, N. Circulating levels of endocannabinoids respond acutely to voluntary exercise, are altered in mice selectively bred for high voluntary wheel running, and differ between the sexes. Physiol. Behav. 2017, 170, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Crombie, K.M.; Brellenthin, A.G.; Hillard, C.J.; Koltyn, K.F. Endocannabinoid and opioid system interactions in exercise-induced hypoalgesia. Pain Med. 2018, 19, 118–123. [Google Scholar] [CrossRef]
- Moosavi Sohroforouzani, A.; Shakerian, S.; Ghanbarzadeh, M.; Alaei, H. Treadmill exercise improves LPS-induced memory impairments via endocannabinoid receptors and cyclooxygenase enzymes. Behav. Brain Res. 2020, 380, 112440. [Google Scholar] [CrossRef]
- Ferreira-Vieira, T.H.; Bastos, C.P.; Pereira, G.S.; Moreira, F.A.; Massensini, A.R. A role for the endocannabinoid system in exercise-induced spatial memory enhancement in mice. Hippocampus 2014, 24, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, R.S.; Sorgi, C.A.; Peti, A.P.F.; Veras, F.P.; Faccioli, L.H.; Galdino, G. Involvement of Spinal Cannabinoid CB2 Receptors in Exercise-Induced Antinociception. Neuroscience 2019, 418, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Gomes da Silva, S.; Araujo, B.H.S.; Cossa, A.C.; Scorza, F.A.; Cavalheiro, E.A.; Naffah-Mazzacoratti, M.d.G.; Arida, R.M. Physical exercise in adolescence changes CB1 cannabinoid receptor expression in the rat brain. Neurochem. Int. 2010, 57, 492–496. [Google Scholar] [CrossRef]
- Richey, J.M.; Woolcott, O. Re-visiting the Endocannabinoid System and Its Therapeutic Potential in Obesity and Associated Diseases. Curr. Diab. Rep. 2017, 17. [Google Scholar] [CrossRef]
- Dietrich, A.; McDaniel, W.F. Endocannabinoids and exercise. Br. J. Sports Med. 2004, 38, 536–541. [Google Scholar] [CrossRef]
- Schönke, M.; Martinez-Tellez, B.; Rensen, P.C. Role of the endocannabinoid system in the regulation of the skeletal muscle response to exercise. Curr. Opin. Pharmacol. 2020, 52, 52–60. [Google Scholar] [CrossRef]
- Gamelin, F.X.; Aucouturier, J.; Iannotti, F.A.; Piscitelli, F.; Mazzarella, E.; Aveta, T.; Leriche, M.; Dupont, E.; Cieniewski-Bernard, C.; Leclair, E.; et al. Exercise training and high-fat diet elicit endocannabinoid system modifications in the rat hypothalamus and hippocampus. J. Physiol. Biochem. 2016, 73, 335–347. [Google Scholar] [CrossRef]
- Di Marzo, V.; Côté, M.; Matias, I.; Lemieux, I.; Arsenault, B.J.; Cartier, A.; Piscitelli, F.; Petrosino, S.; Alméras, N.; Després, J.P. Changes in plasma endocannabinoid levels in viscerally obese men following a 1 year lifestyle modification programme and waist circumference reduction: Associations with changes in metabolic risk factors. Diabetologia 2009, 52, 213–217. [Google Scholar] [CrossRef] [Green Version]
- You, T.; Disanzo, B.L.; Wang, X.; Yang, R.; Gong, D. Adipose tissue endocannabinoid system gene expression: Depot differences and effects of diet and exercise. Lipids Health Dis. 2011, 10, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Aranda, F.; Sauchelli, S.; Pastor, A.; Gonzalez, M.L.; De La Torre, R.; Granero, R.; Jiménez-Murcia, S.; Baños, R.; Botella, C.; Fernández-Real, J.M.; et al. Moderate-vigorous physical activity across body mass index in females: Moderating effect of endocannabinoids and temperament. PLoS ONE 2014, 9, e104534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, Z.C.; Liu, D.Y.; Zhang, L.L.; Shen, C.Y.; Ma, Q.L.; Cao, T.B.; Wang, L.J.; Nie, H.; Zidek, W.; Tepel, M.; et al. Exercise reduces adipose tissue via cannabinoid receptor type 1 which is regulated by peroxisome proliferator-activated receptor-δ. Biochem. Biophys. Res. Commun. 2007, 354, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Ju, F.; Zhu, X.; Zhao, G.; Yang, Q. Effect of diet and physical exercise on endocannabinoid system and energy homeostasis in obese mice. Int. J. Clin. Exp. Med. 2021, 14, 1445–1454. [Google Scholar]
| Subjects | Performed Activity | Main Outcomes | Reference |
|---|---|---|---|
| Viscerally obese men (n = 49) | 1-year lifestyle modification program including regular physical activity and healthy diet | ECS: ↓ plasma AEA and 2-AG Metabolic effects: ↓ body weight and waist circumference ↓ visceral adipose tissue ↑ HDL3−C | [109] |
| Overweight or obese women (n = 30) | 20 weeks of moderate (HRmax = 45–50%) or intense aerobic exercises (HRmax = 70–75%) combined with caloric restriction | ECS: ↑ cb1r gluteal adipose tissue gene expression ↓ faah abdominal adipose tissue gene expression Metabolic effects: ↓ body weight and waist circumference ↓ glucose ↓ insulin | [110] |
| Obese women (n = 77) | 6 days of normal, daytime physical activity and 6 days of moderate–vigorous physical activity | ECS: ↓ plasma 2-AG ↑ plasma AEA and OEA (only for moderate–vigorous physical activity) Metabolic effects: ↓ BMI and waist circumference (only for moderate–vigorous physical activity) | [111] |
| Subjects | Performed Activity | Main Outcomes | Reference |
|---|---|---|---|
| Male Wistar rats fed with HFD | 1 h of swimming, 3 times a week for 6 months | ECS: ↓ expression of CB1R in VAT and SAT ↑ expression of PPARδ in VAT Metabolic effects: ↓ body weight ↓ visceral adipose tissue percentage ↓ blood pressure | [112] |
| C57Bl/6J male mice fed with HFD | 1 h of treadmill running, 6 times a week for 6 weeks | ECS: ↓ plasma AEA and 2-AG ↓ CB1R and CB2R expression in the brain ↓ CB2R expression in the epididymal fat ↓ MAGL, DAGL- α and β, FAAH, and NAPE-PLD expression in the brain and epididymal fat Metabolic effects: ↓ body weight ↓ body fat percentage ↓ LDL-C ↓ TG ↑ HDL-C | [113] |
| Male Wistar rats fed with HFD | 1 h of treadmill running, 5 times a week for 12 weeks (70–80% MAV) | ECS: no effect on AEA and 2-AG in SAT and VAT ↑ DAGL-α and FAAH expression in SAT ↑ cb1r and trpv1 gene expression in SAT Metabolic effects: ↓ body weight ↓ fasting plasma glucose | [10] |
| Male Wistar rats fed with HFD | 1 h of treadmill running 5 times a week for 12 weeks (70–80% MAV) | ECS: ↑ cb1r and trpv1 gene expression in hippocampus ↑ DAGL-α expression in hippocampus ↑ faah gene expression in hippocampus ↓ napepld gene expression in hippocampus Metabolic effects:↓ body weight ↓ fasting plasma glucose | [108] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charytoniuk, T.; Zywno, H.; Berk, K.; Bzdega, W.; Kolakowski, A.; Chabowski, A.; Konstantynowicz-Nowicka, K. The Endocannabinoid System and Physical Activity—A Robust Duo in the Novel Therapeutic Approach against Metabolic Disorders. Int. J. Mol. Sci. 2022, 23, 3083. https://doi.org/10.3390/ijms23063083
Charytoniuk T, Zywno H, Berk K, Bzdega W, Kolakowski A, Chabowski A, Konstantynowicz-Nowicka K. The Endocannabinoid System and Physical Activity—A Robust Duo in the Novel Therapeutic Approach against Metabolic Disorders. International Journal of Molecular Sciences. 2022; 23(6):3083. https://doi.org/10.3390/ijms23063083
Chicago/Turabian StyleCharytoniuk, Tomasz, Hubert Zywno, Klaudia Berk, Wiktor Bzdega, Adrian Kolakowski, Adrian Chabowski, and Karolina Konstantynowicz-Nowicka. 2022. "The Endocannabinoid System and Physical Activity—A Robust Duo in the Novel Therapeutic Approach against Metabolic Disorders" International Journal of Molecular Sciences 23, no. 6: 3083. https://doi.org/10.3390/ijms23063083
APA StyleCharytoniuk, T., Zywno, H., Berk, K., Bzdega, W., Kolakowski, A., Chabowski, A., & Konstantynowicz-Nowicka, K. (2022). The Endocannabinoid System and Physical Activity—A Robust Duo in the Novel Therapeutic Approach against Metabolic Disorders. International Journal of Molecular Sciences, 23(6), 3083. https://doi.org/10.3390/ijms23063083






