Gene-Delivery Ability of New Hydrogenated and Partially Fluorinated Gemini bispyridinium Surfactants with Six Methylene Spacers
Abstract
:1. Introduction
2. Results
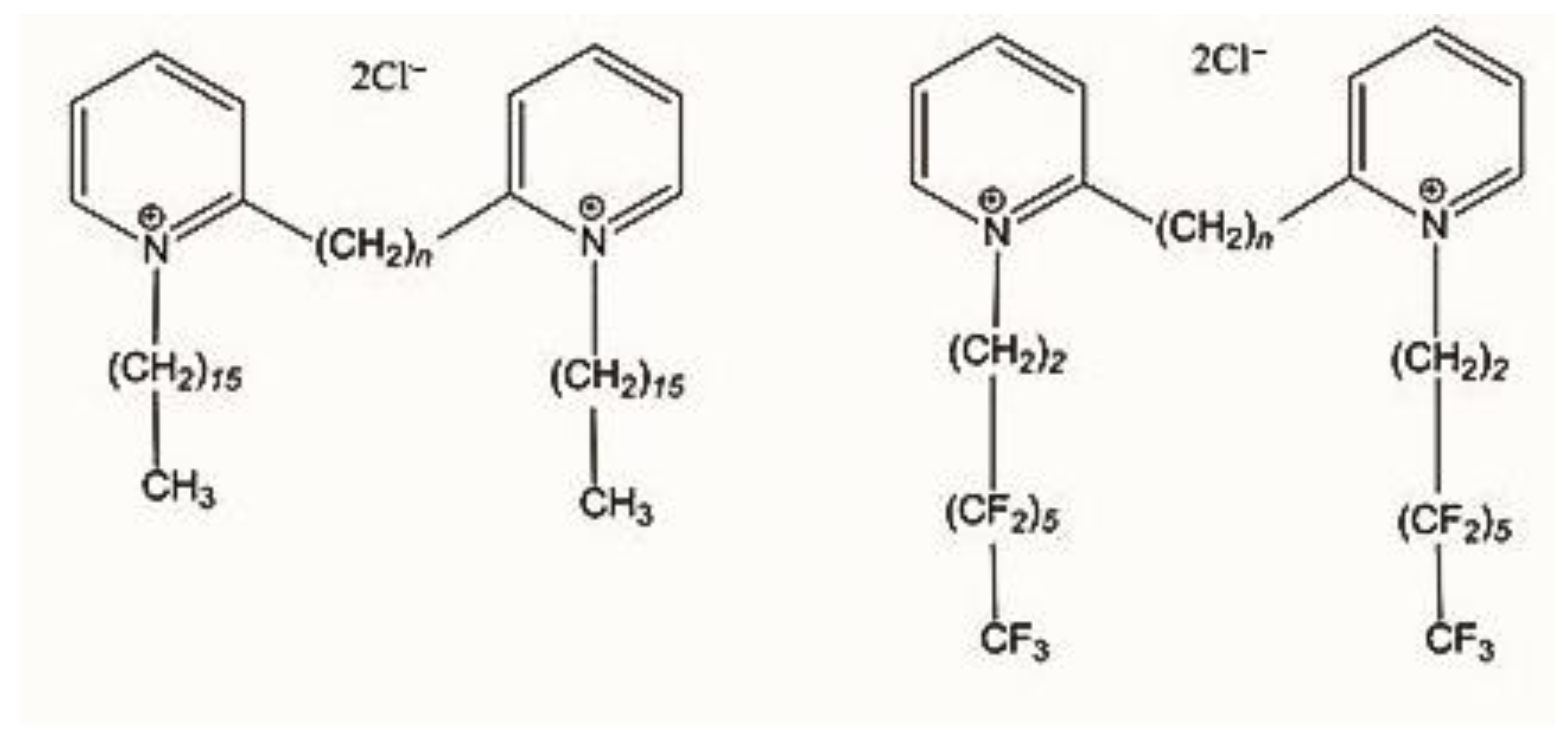
2.1. Synthesis
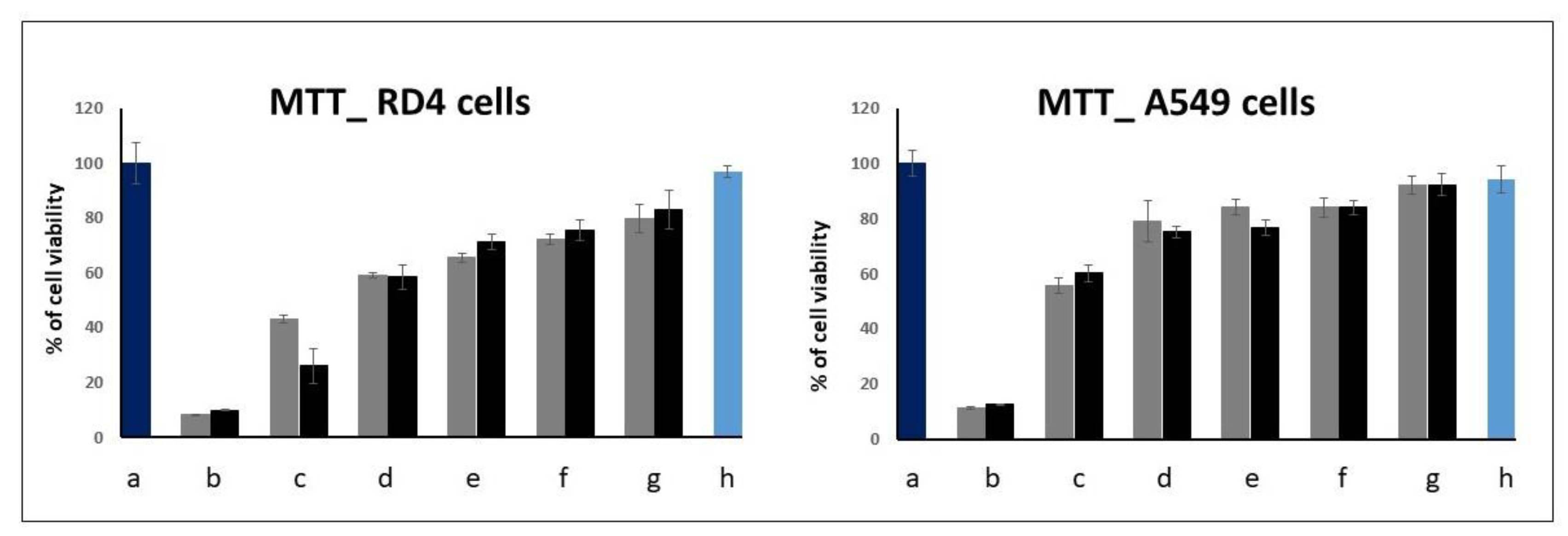
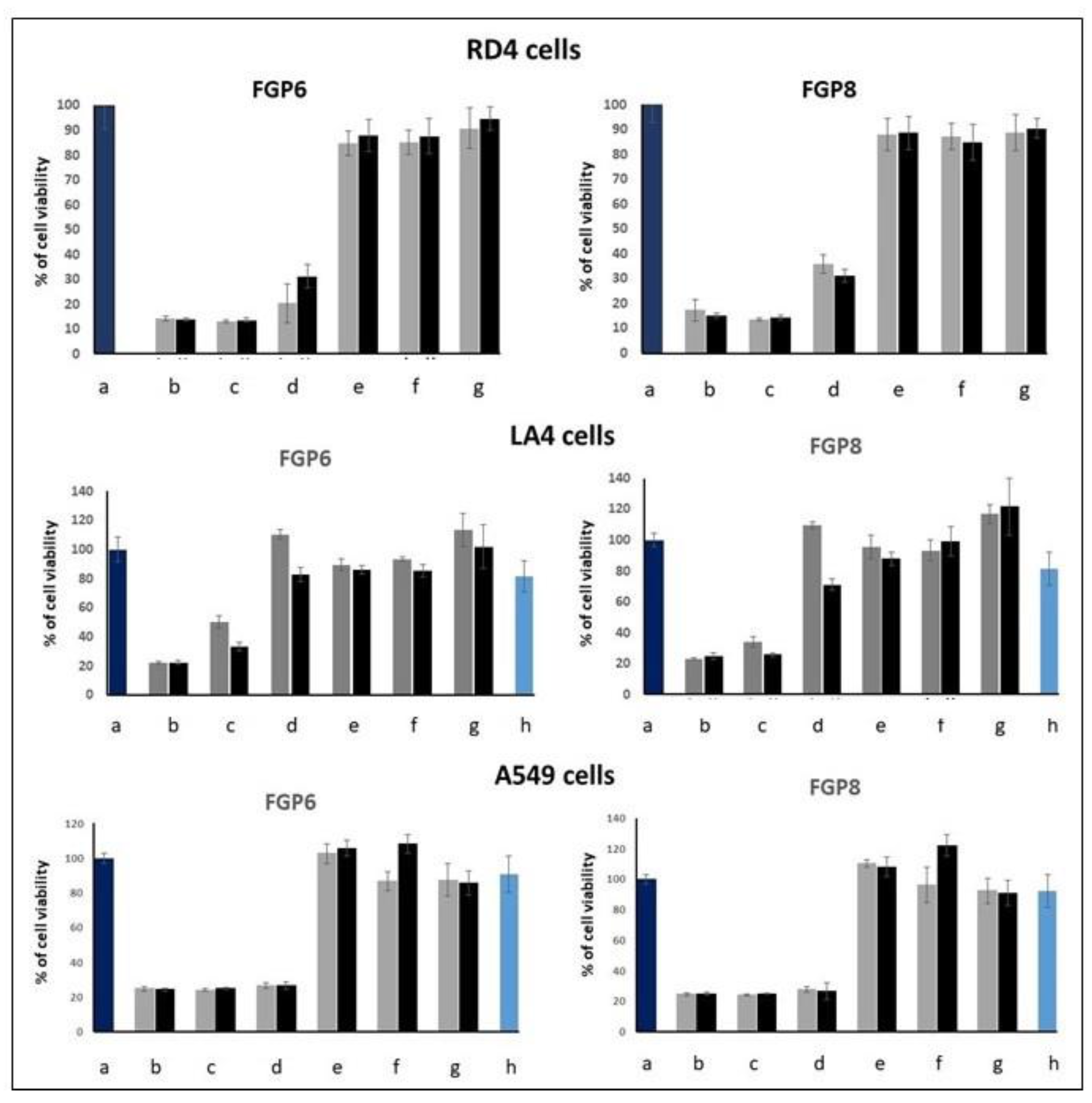
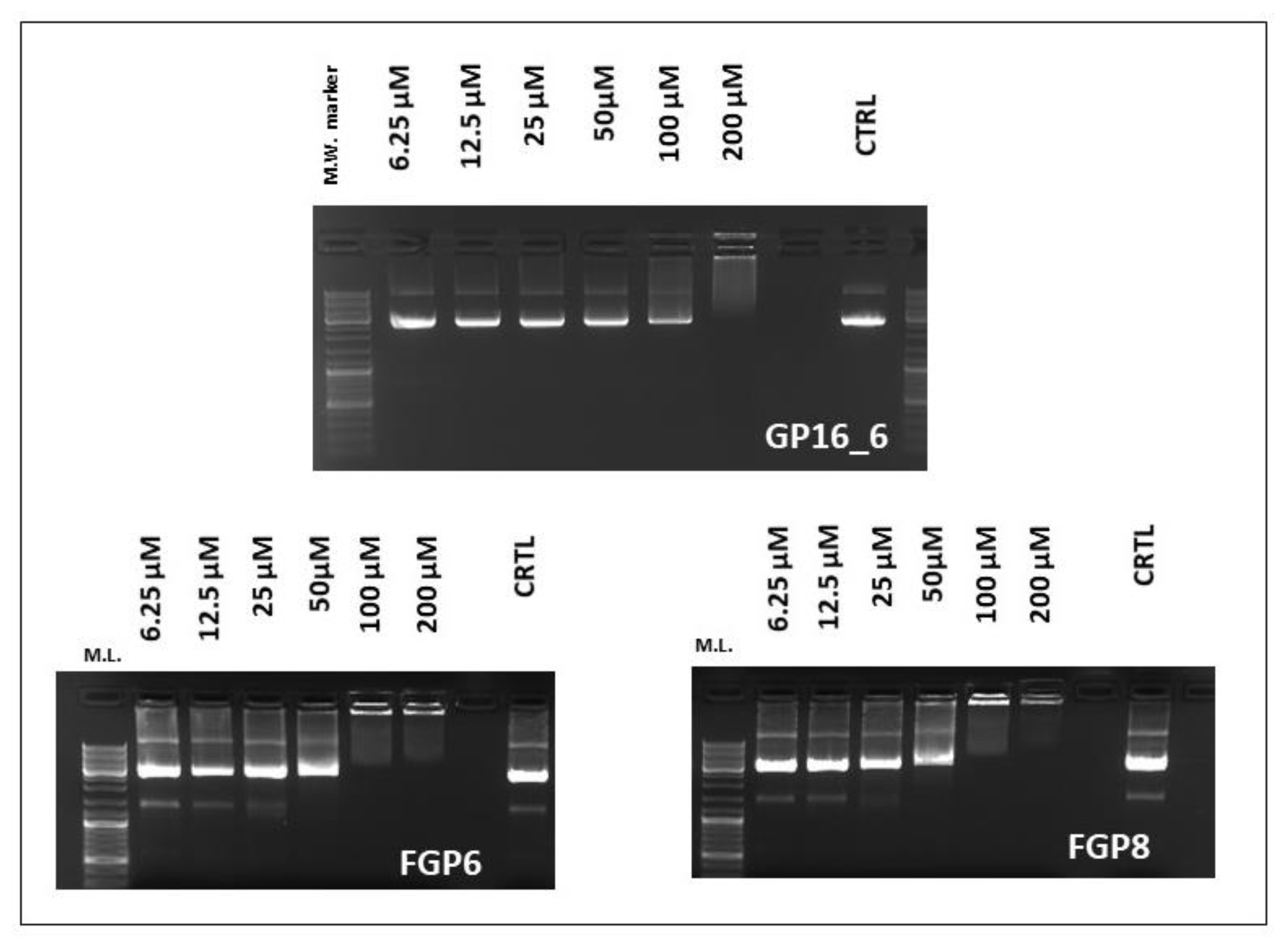
2.2. EMSA and MTT Assays
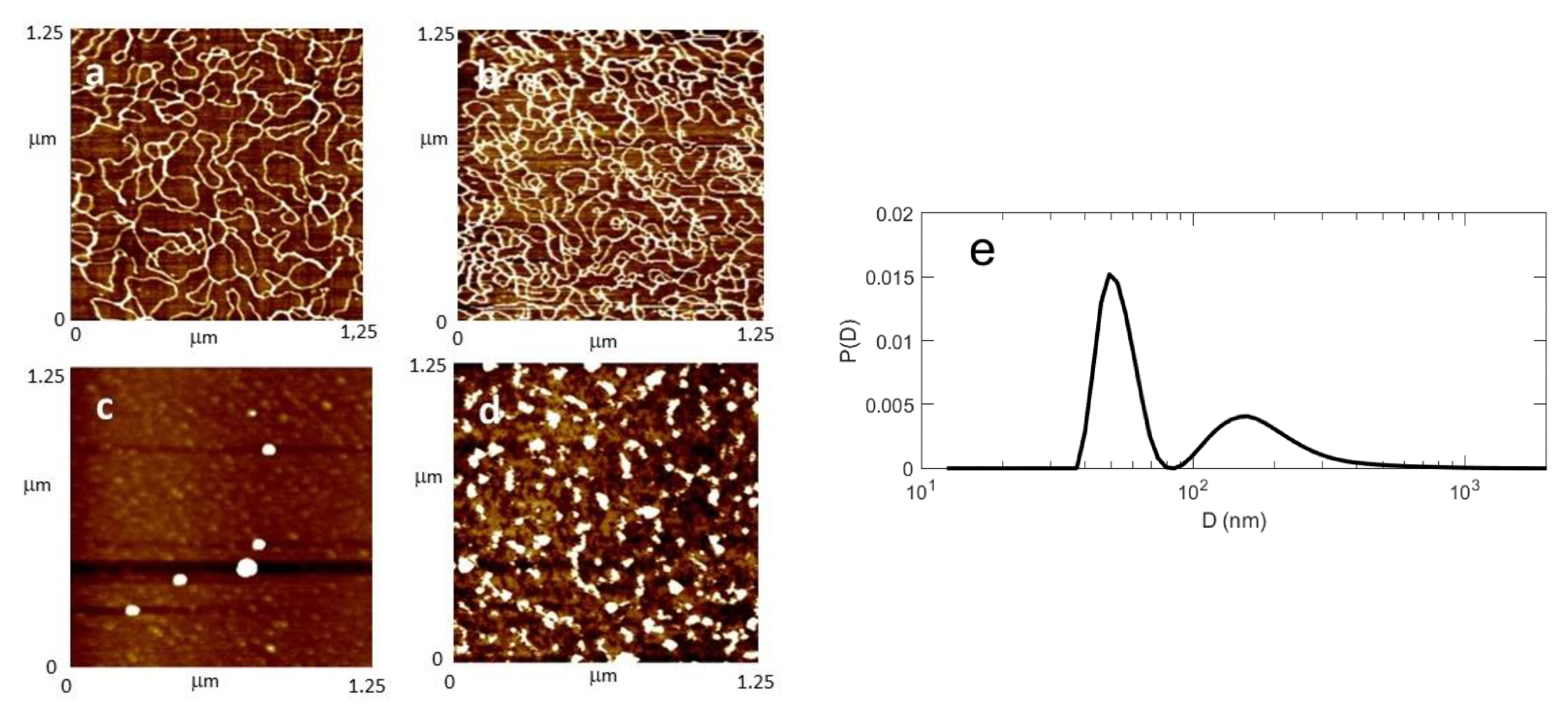
2.3. AFM and DLS
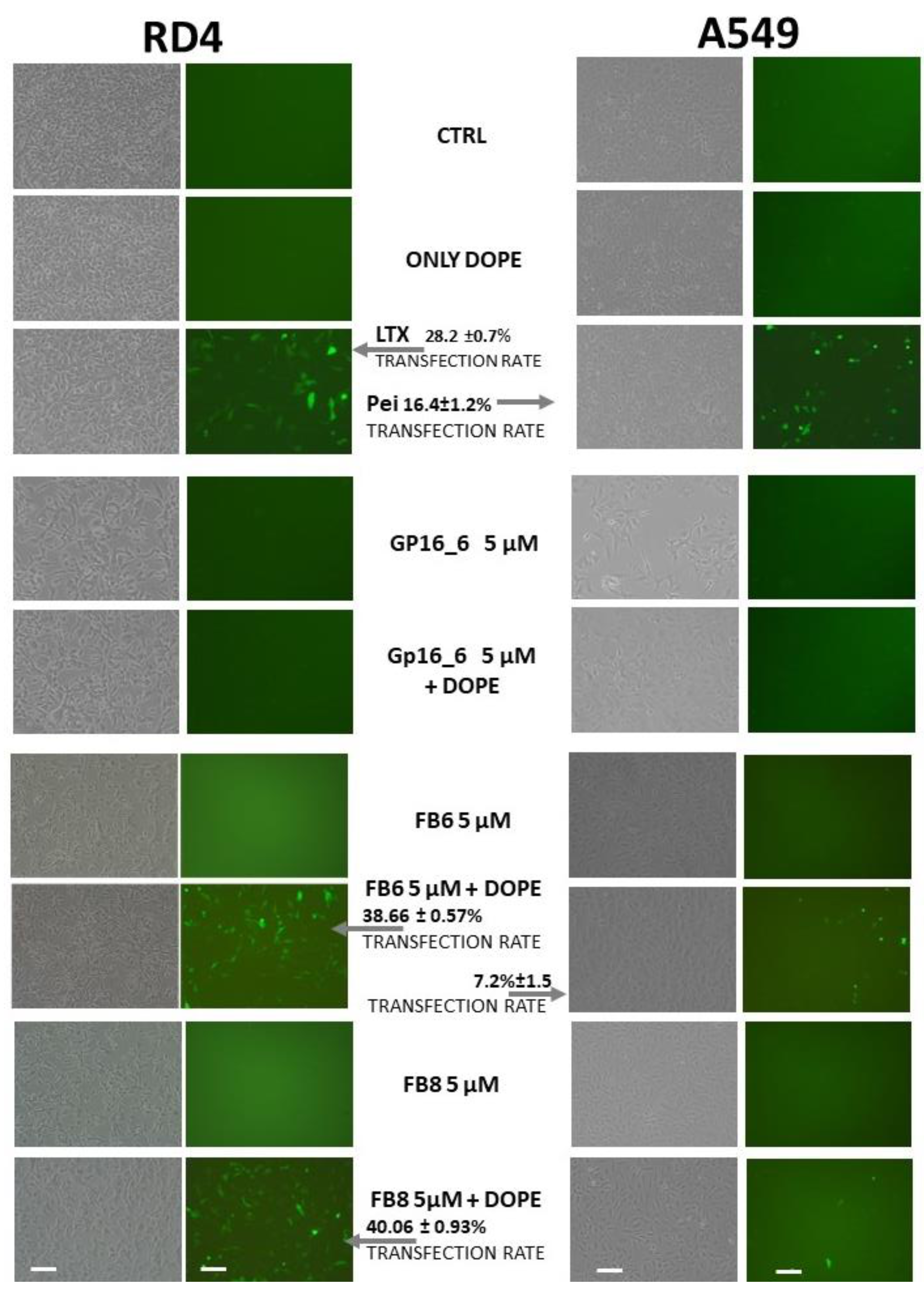
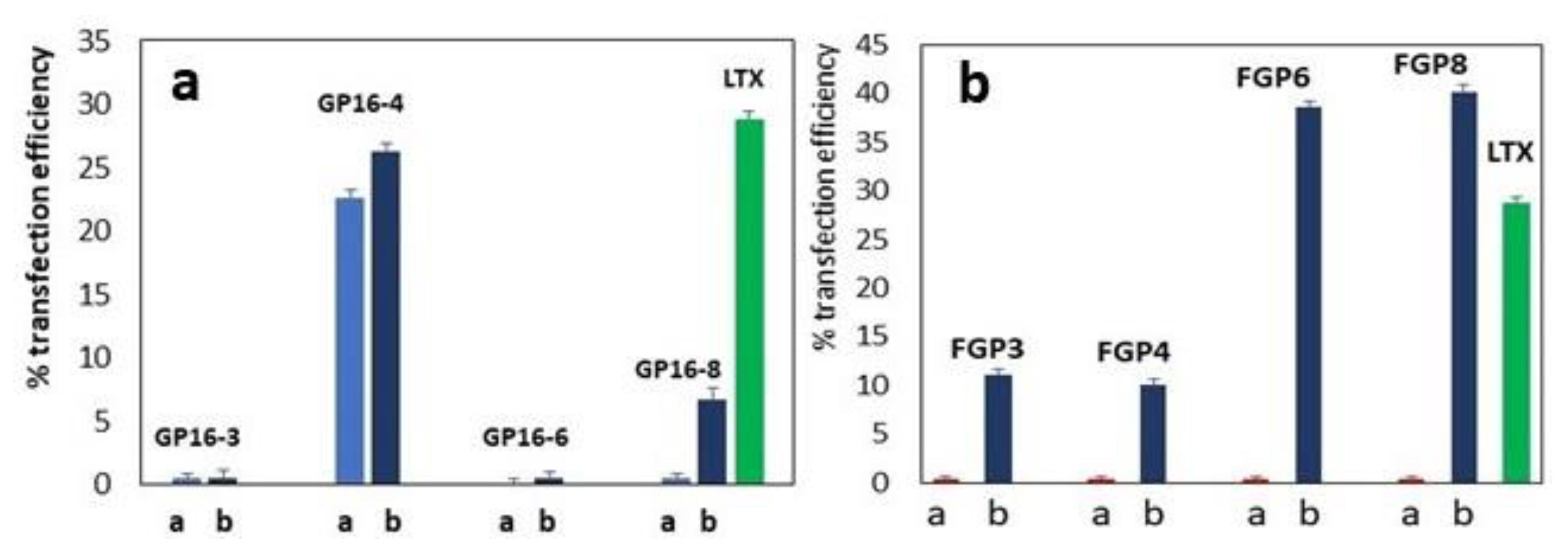
2.4. Transient Transfection Experiments
3. Materials and Methods
3.1. Compounds
3.1.1. Chemical Synthesis
Synthesis of the 1,6-Bis(2-pyridyl)hexane
Synthesis of 1,1′-Bis(3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluorooctyl)-2,2′-hexamethylenebis (Pyridinium) Chloride
Synthesis of 1,1′-Bis-hexadecyl-2,2′-hexamethylenebispyridinium Chloride (GP16_6)
3.2. DNA Preparation and Storage
3.3. Cell Culture
3.4. Electrophoresis Mobility Shift Assay (EMSA)
3.5. MTT Proliferation Assay
3.6. Transient Transfection Assay
3.7. Sample Preparation and Atomic Force Microscopy (AFM) Imaging
3.8. Dynamic Light Scattering (DLS)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gomes-Silva, D.; Ramos, C.A. Cancer immunotherapy using CAR-T cells: From the research bench to the assembly line. Biotechnol. J. 2018, 13, 1700097–1700105. [Google Scholar] [CrossRef] [PubMed]
- June, C.H.; O’Connor, R.S.; Kawalekar, O.U.; Ghassemi, S.; Milone, M.C. CAR T cell immunotherapy for human cancer. Science 2018, 359, 1361–1365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, D.; Arora, S.; Singh, J.; Layek, B. A review of the tortuous path of nonviral gene delivery and recent progress, International. J. Biol. Macromol. 2021, 183, 2055–2073. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.D.; Rhodes, D.G.; Burgess, D.J. DNA-based Therapeutics and DNA Delivery Systems: A Comprehensive Review. AAPS J. 2005, 7, E61–E77. [Google Scholar] [CrossRef] [Green Version]
- Ginn, S.L.; Amaya, A.K.; Alexander, I.E.; Edelstein, M.; Abedi, M.R. Gene therapy clinical trial worldwide to 2017: An update. J. Gene Med. 2018, 20, 1–16. [Google Scholar] [CrossRef]
- O’Callaghan, K.P.; Blatz, A.M.; Offit, P.A. Developing a SARS-CoV-2 Vaccine at Warp Speed. JAMA 2020, 324, 437–438. [Google Scholar] [CrossRef]
- Kyriakidis, N.C.; López-Cortés, A.; González, E.V.; Grimaldos, A.B.; Prado, O.E. SARS-CoV-2 vaccines strategies: A comprehensive review of phase 3 candidates. NPJ Vaccines 2021, 6, 28–45. [Google Scholar] [CrossRef]
- Pushparajah, D.; Jimenez, S.; Wong, S.; Alattas, H.; Nafissi, N.; Slavcev, R.A. Advances in gene-based vaccine platforms to address the COVID-19 pandemic. Adv. Drug Deliv. Rev. 2021, 170, 113–141. [Google Scholar] [CrossRef]
- Dong, Y.; Dai, T.T.; Wei, Y.; Zhang, L.; Zheng, M.; Zhou, F. A systematic review of SARS-CoV-2 vaccine candidates, Signal Transduction and Targeted. Therapy 2020, 5, 237. [Google Scholar]
- Chandra, S.P.; Veni, P.; Diksha, S.; Shobha, U.; Mukesh, S. Vaccination strategies to combat novel corona virus SARS-CoV-2. Life Sci. 2020, 256, 117956–117965. [Google Scholar]
- Krammer, F. SARS-CoV-2 vaccines in development. Nature 2020, 586, 516–527. [Google Scholar] [CrossRef]
- Liu, L.; Wang, P.; Nair, M.S.; Yu, J.; Rapp, M.; Wang, Q.; Luo, Y.; Chan, W.J.F.; Sahi, V.; Figueroa, A.; et al. Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike. Nature 2020, 584, 450–456. [Google Scholar] [CrossRef]
- Okba, N.M.A.; Müller, M.A.; Li, W.; Wang, C.; Geurtsvan Kessel, C.H.; Corman, V.M.; Lamers, M.M.; Sikkema, R.S.; de Bruin, E.; Chandler, F.D.; et al. Severe acute respiratory syndrome coronavirus 2-specific antibody responses in coronavirus disease patients. Emerg. Infect. Dis. 2020, 26, 1478–1488. [Google Scholar] [CrossRef]
- Fisicaro, E.; Compari, C.; Duce, E.; Donofrio, G.; Różycka-Roszak, B.; Wozniak, E. Biologically Active Bisquaternary Ammonium Chlorides: Physico-Chemical Properties of Long Chain Amphiphiles and Their Evaluation as Non-Viral Vectors for Gene Delivery. Biochim. Biophys. Acta Gen. Subj. 2005, 1722, 224–233. [Google Scholar] [CrossRef]
- Quagliotto, P.; Viscardi, G.; Barolo, C.; Barni, E.; Bellinvia, S.; Fisicaro, E.; Compari, C. Gemini Pyridinium Surfactants: Synthesis and Conductimetric Study of a Novel Class of Amphiphiles. J. Org. Chem. 2003, 68, 7651–7660. [Google Scholar] [CrossRef]
- Fisicaro, E.; Compari, C.; Biemmi, M.; Duce, E.; Peroni, M.; Barbero, N.; Viscardi, G.; Quagliotto, P. The Unusual Behaviour of the Aqueous Solutions of Gemini Bispyridinium Surfactants: Apparent and Partial Molar Enthalpies of the Dimethanesulfonates. J. Phys. Chem. B 2008, 112, 12312–12317. [Google Scholar] [CrossRef]
- Fisicaro, E.; Compari, C.; Bacciottini, F.; Barbero, N.; Viscardi, G.; Quagliotto, P. Is the Counterion Responsible for the Unusual Thermodynamic Behavior of the Aqueous Solutions of Gemini Bispyridinium Surfactants? Colloids Surf. A Physicochem. Eng. Asp. 2014, 443, 249–254. [Google Scholar] [CrossRef]
- Fisicaro, E.; Compari, C.; Bacciottini, F.; Contardi, L.; Barbero, N.; Viscardi, G.; Quagliotto, P.; Donofrio, G.; Różycka-Roszak, B.; Misiak, P.; et al. Nonviral Gene-Delivery: Gemini Bispyridinium Surfactant-Based DNA Nanoparticles. J. Phys. Chem. B 2014, 118, 13183–13191. [Google Scholar] [CrossRef]
- Barbero, N.; Magistris, C.; Quagliotto, P.; Bonandini, L.; Barolo, C.; Buscaino, R.; Compari, C.; Contardi, L.; Fisicaro, E.; Viscardi, G. Synthesis and Physico-Chemical Characterization of Long Alkyl Chain Gemini Pyridinium Surfactants for Gene Delivery. Chem. Plus. Chem. 2015, 80, 952–962. [Google Scholar]
- Quagliotto, P.; Barolo, C.; Barbero, N.; Barni, E.; Compari, C.; Fisicaro, E.; Viscardi, G. Synthesis and Characterization of Highly Fluorinated Gemini Pyridinium Surfactants. Eur. J. Org. Chem. 2009, 19, 3167–3177. [Google Scholar] [CrossRef]
- Fisicaro, E.; Compari, C.; Bacciottini, F.; Contardi, L.; Pongiluppi, E.; Barbero, N.; Viscardi, G.; Quagliotto, P.; Donofrio, G.; Krafft, M.P. Nonviral Gene-Delivery by Highly Fluorinated Gemini Bispyridinium Surfactants-Based DNA Nanoparticles. J. Colloid Interface Sci. 2017, 487, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Rosen, M.J.; Tracy, D.J.J. Gemini surfactants. Surfactants Deterg. 1998, 1, 547–554. [Google Scholar] [CrossRef]
- Menger, F.M.; Keiper, J.S. Gemini surfactants. Angew. Chem. Int. Ed. 2000, 39, 1906–1920. [Google Scholar] [CrossRef]
- Zana, R. Novel Surfactants-Preparation, Applications, and Biodegradability. Dimeric (Gemini) Surfactants. In Surfactant Science Series; Holmberg, K., Ed.; Dekker, M., Inc.: New York, NY, USA, 1998; Volume 74, 241p. [Google Scholar]
- Fisicaro, E. Gemini surfactants: Chemico-physical and biological properties. Cell. Mol. Biol. Lett. 1997, 2, 45–63 and references therein. [Google Scholar]
- Zana, R.; Xia, J. Gemini Surfactants, Interfacial and Solution Phase Behavior, and Applications; Marcel Dekker, Inc.: New York, NY, USA, 2004. [Google Scholar]
- Rosen, M.J. Geminis: A new generation of surfactants. Chemtech 1993, 23, 30–33. [Google Scholar]
- Devínsky, F.; Pisárčik, M.; Lukáč, M. Cationic Amphiphiles—Self-Assembling Systems for Biomedicine and Biopharmacy; Nova Science Publishers, Inc.: New York, NY, USA, 2017. [Google Scholar]
- Machlouf, A.; Hajdu, I.; Badea, I. Gemini surfactant-based system for drug and gene delivery. In Organic Materials as Smart Nanocarriers for Drug Delivery; Grumezescu, A.M., Ed.; William Andrew Publishing: Oxford, UK, 2018; pp. 561–600. [Google Scholar]
- Elsabahy, M.; Badea, I.; Verrall, R.; Donkuru, M.C.D.; Foldvari, M. Dicationic gemini nanoparticle design for gene therapy. In Organic Nanomaterials: Synthesis, Characterization, and Device Applications; Torres, T., Bottari, G., Eds.; Wiley: Hoboken, NJ, USA, 2013; pp. 509–528. [Google Scholar]
- Sharma, V.D.; Ilies, M.A. Heterocyclic Cationic Gemini Surfactants: A Comparative Overview of Their Synthesis, Self-assembling, Physicochemical, and Biological Properties. Med. Res. Rev. 2014, 34, 1–44. [Google Scholar] [CrossRef]
- Bell, P.C.; Bergsma, M.; Dolbnya, I.P.; Bras, W.; Stuart, M.C.A.; Rowan, A.E.; Feiters, M.C.; Engberts, J.B.F.N. Transfection mediated by gemini surfactants: Engineered escape from the endosomal compartment. J. Am. Chem. Soc. 2003, 125, 1551–1558. [Google Scholar] [CrossRef] [Green Version]
- Kirby, A.J.; Camilleri, P.; Engberts, J.B.F.N.; Feiters, M.C.; Nolte, R.J.M.; Soderman, O.; Bergsma, M.; Bell, P.C.; Fielden, M.L.; Garcia Rodriguez, C.L.; et al. Gemini surfactants: New synthetic vectors for gene transfection. Angew. Chem. Int. Ed. 2003, 42, 1448–1457. [Google Scholar] [CrossRef]
- Ahmed, T.; Kamel, A.O.; Wettig, S.D. Interactions between DNA and Gemini surfactant: Impact on gene therapy: Part I. Nanomedicine 2016, 11, 289–306. [Google Scholar] [CrossRef]
- Badea, I.; Shaterian, N.; Foldvari, M. Topical Gene Delivery in Mice Using Gemini Surfactant—Lipid Nanoparticles with and without Tape Electrode Electroporation. Drug Deliv. Lett. 2011, 1, 62–66. [Google Scholar]
- Yang, P.; Singh, J.; Wettig, S.; Foldvari, M.; Verrall, R.; Badea, I. Enhanced Gene Expression in Epithelial Cells Transfected with Amino Acid-substituted Gemini Nanoparticles. Eur. J. Pharm. Biopharm. 2010, 75, 311–320. [Google Scholar] [CrossRef]
- Sing, J.; Michel, D.; Chitanda, J.M.; Verral, R.; Badea, I. Evaluation of Cellular Uptake and Iintracellular Trafficking as Determining Factors of Gene Expression for Amino Acid-Substituted Gemini Surfactant-Based DNA Nanoparticles. J. Nanobiotechnol. 2012, 10, 7. [Google Scholar] [CrossRef] [Green Version]
- Donkuru, M.D.; Badea, I.; Wettig, S.; Verrall, R.; Elsabahy, M.; Foldvari, M. Advancing Nonviral Gene Delivery: Lipid- and Surfactant-Based Nanoparticle Design Strategies. Nanomedicine 2010, 5, 1103–1127. [Google Scholar] [CrossRef]
- Sharma, V.D.; Lees, J.; Hoffman, N.E.; Brailoiu, E.; Madesh, M.; Wunder, S.L.; Ilies, M.A. Modulation of Pyridinium Cationic Lipid-DNA Complex Properties by Pyridinium Gemini Surfactants and Its Impact on Lipoplex Transfection Properties. Mol. Pharm. 2014, 11, 545–559. [Google Scholar] [CrossRef] [Green Version]
- Boussif, O.; Gaucheron, J.; Boulanger, C.; Santaella, C.; Kolbe, H.V.J.; Vierling, P. Enhanced in Vitro and in Vivo Cationic Lipid-Mediated Gene Delivery with a Fluorinated Glycerophosphoethanolamine Helper Lipid. J. Gene Med. 2001, 3, 109–114. [Google Scholar] [CrossRef]
- Boulanger, C.; Di Giorgio, C.; Gaucheron, J.; Santaella, C. Transfection with Fluorinated Lipoplexes Based on New Fluorinated Cationic Lipids and in the Presence of a Bile Salt Surfactant. Bioconjug. Chem. 2004, 15, 901–908. [Google Scholar] [CrossRef]
- Gaucheron, J.; Santaella, C.; Vierling, P. Improved in Vitro Gene Transfer Mediated by Fluorinated Lipoplexes in the Presence of a Bile Salt Surfactants. J. Gen. Med. 2001, 3, 338–344. [Google Scholar] [CrossRef]
- Palchetti, S.; Pozzi, D.; Marchini, C.; Amici, A.; Andreani, C.; Bartolacci, C.; Digiacomo, L.; Gambini, V.; Cardarelli, F.; Di Rienzo, C.; et al. Manipulation of lipoplex concentration at the cell surface boosts transfection efficiency in hard-to-transfect cells. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 681–691. [Google Scholar] [CrossRef]
- Silva, J.P.N.; Oliveira, A.C.N.; Lúcio, M.; Gomes, A.C.; Coutinho, P.J.G.; Real Oliveira, M.E.C.D. Tunable pDNA/DODAB:MO lipoplexes: The effect of incubation temperature on pDNA/DODAB:MO lipoplexes structure and transfection efficiency. Colloids Surf. B Biointerfaces 2014, 121, 371–379. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.; Wettig, S.D.; Badea, I.; Foldvari, M.; Verrall, R.E. Investigation of complexes formed by interaction of cationic gemini surfactants with deoxyribonucleic acid. Phys. Chem. Chem. Phys. 2007, 9, 1616–1628. [Google Scholar] [CrossRef]
- Li, X.; Turanek, J.; Knoetigova, P.; Kudlackova, H.; Masek, J.; Pennington, D.B.; Rankin, S.E.; Knutson, B.L.; Lehmler, H.J. Synthesis and Biocompatibility Evaluation of Fluorinated, Single-tailed Glucopyranoside Surfactants. N. J. Chem. 2008, 32, 2169–2179, Erratum in N. J. Chem. 2009, 33, 2491–2493. [Google Scholar] [CrossRef]
- Abe, M. Synthesis and applications of surfactants containing fluorine. Curr. Opin. Colloid Interface Sci. 1999, 4, 354–356. [Google Scholar] [CrossRef]
- Kissa, E. Fluorinated Surfactants; Marcel Dekker, Inc.: New York, NY, USA, 1994. [Google Scholar]
- Riess, J.G. Highly Fluorinated Amphiphilic Molecules and Self-Assemblies with Biomedical Potential. Curr. Opin. Colloid Sci. 2009, 14, 294–304. [Google Scholar] [CrossRef]
- Fisicaro, E.; Ghiozzi, A.; Pelizzetti, E.; Viscardi, G.; Quagliotto, P. Effect of the Counterion on Thermodynamic Properties of Aqueous Micellar Solutions of 1-(3, 3, 4, 4, 5, 5, 6, 6, 6-nonafluorohexyl) Pyridinium Halides. Part I: Apparent and Partial Molar Enthalpies and Volumes at 298 K. J. Colloid Interface Sci. 1996, 182, 549–557. [Google Scholar] [CrossRef]
- Fisicaro, E.; Viscardi, G.; Quagliotto, P.; Trossarelli, L. Thermodynamic Properties of Aqueous Micellar Solutions of N-(1H,1H,2H,2H Perfluoro-Octyl) Pyridinium Chloride and N-(1H,1H,2H,2H Perfluoro-Decyl) Pyridinium Chloride. Colloids Surf. A Physicochem. Eng. Asp. 1994, 84, 59–70. [Google Scholar] [CrossRef]
- Jennings, K.H.; Marshall, I.C.B.; Wilkinson, M.J.; Kremer, A.; Kirby, A.J.; Camilleri, P. Aggregation Properties of a Novel Class of Cationic Gemini Surfactants Correlate with Their Efficiency as Gene Transfection Agent. Langmuir 2002, 18, 2426–2429. [Google Scholar] [CrossRef]
- Liu, F.; Yang, J.; Huang, L.; Liu, D. Effect of Non-ionic Surfactants on the Formation of DNA/Emulsion Complexes and Emulsion-mediated Gene Transfer. Pharm. Res. 1996, 13, 1642–1646. [Google Scholar] [CrossRef]
- Hara, T.; Liu, F.; Liu, D.; Huang, L. Emulsion Formulations as a Vector for Gene Delivery in vitro and vivo. Adv. Drug. Deliv. Rev. 1997, 24, 265–271. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Massa, M.; Rivara, M.; Donofrio, G.; Cristofolini, L.; Peracchia, E.; Compari, C.; Bacciottini, F.; Orsi, D.; Franceschi, V.; Fisicaro, E. Gene-Delivery Ability of New Hydrogenated and Partially Fluorinated Gemini bispyridinium Surfactants with Six Methylene Spacers. Int. J. Mol. Sci. 2022, 23, 3062. https://doi.org/10.3390/ijms23063062
Massa M, Rivara M, Donofrio G, Cristofolini L, Peracchia E, Compari C, Bacciottini F, Orsi D, Franceschi V, Fisicaro E. Gene-Delivery Ability of New Hydrogenated and Partially Fluorinated Gemini bispyridinium Surfactants with Six Methylene Spacers. International Journal of Molecular Sciences. 2022; 23(6):3062. https://doi.org/10.3390/ijms23063062
Chicago/Turabian StyleMassa, Michele, Mirko Rivara, Gaetano Donofrio, Luigi Cristofolini, Erica Peracchia, Carlotta Compari, Franco Bacciottini, Davide Orsi, Valentina Franceschi, and Emilia Fisicaro. 2022. "Gene-Delivery Ability of New Hydrogenated and Partially Fluorinated Gemini bispyridinium Surfactants with Six Methylene Spacers" International Journal of Molecular Sciences 23, no. 6: 3062. https://doi.org/10.3390/ijms23063062
APA StyleMassa, M., Rivara, M., Donofrio, G., Cristofolini, L., Peracchia, E., Compari, C., Bacciottini, F., Orsi, D., Franceschi, V., & Fisicaro, E. (2022). Gene-Delivery Ability of New Hydrogenated and Partially Fluorinated Gemini bispyridinium Surfactants with Six Methylene Spacers. International Journal of Molecular Sciences, 23(6), 3062. https://doi.org/10.3390/ijms23063062






