Exploiting Radiation Induction of Antigens in Cancer: Targeted Drug Delivery
Abstract
:1. Introduction
2. Radiation-Inducible Antigens
- Cancer-specific;
- Accessible to antibody binding;
- Not shed/remained tethered to cancer;
- Over-expressed in many cancer subtypes;
- Endocytosis of the antibody/antigen complex is not an essential criterion for prioritization of inducible antigens, but it is ideal for antibody–drug conjugates.
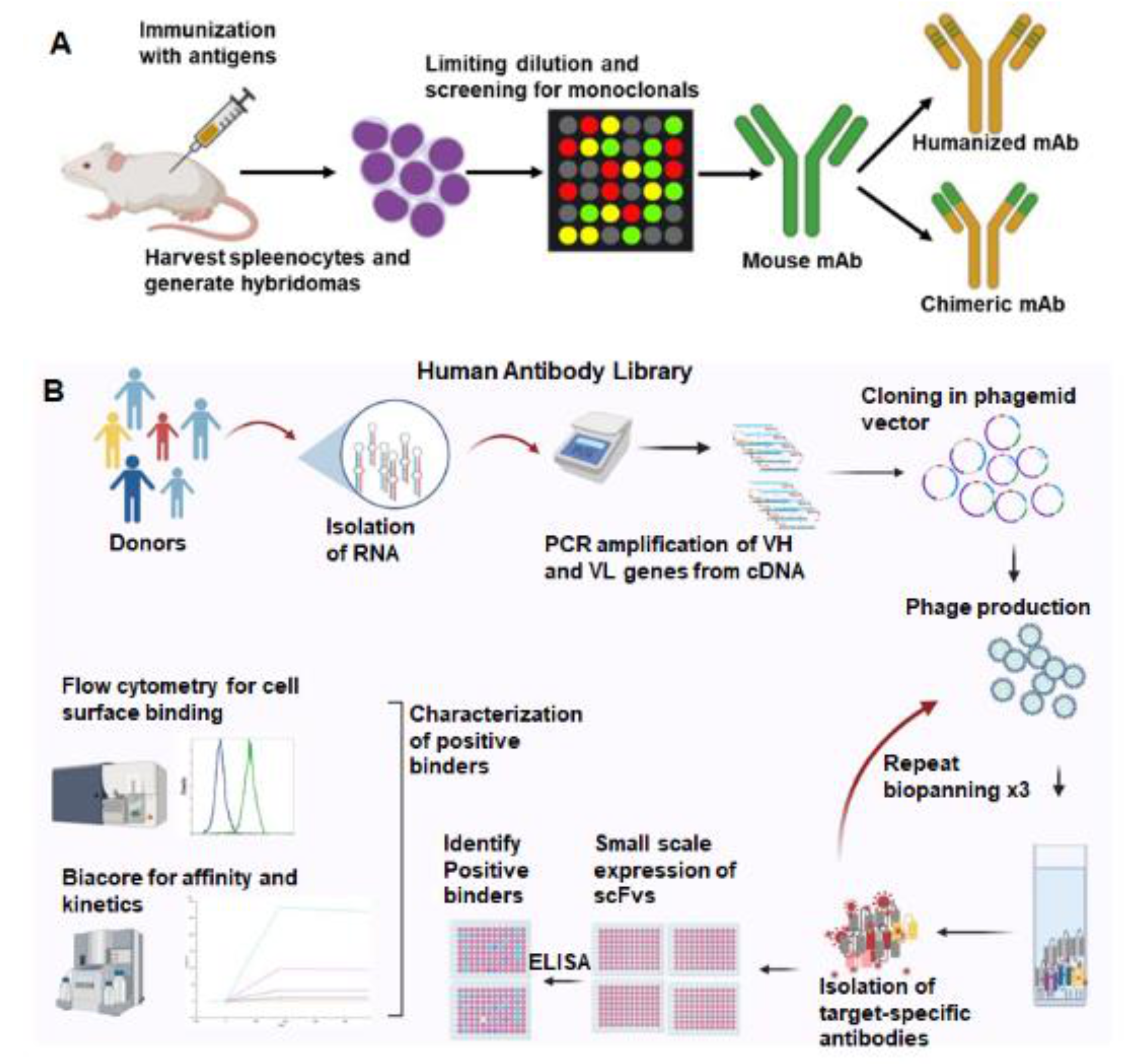
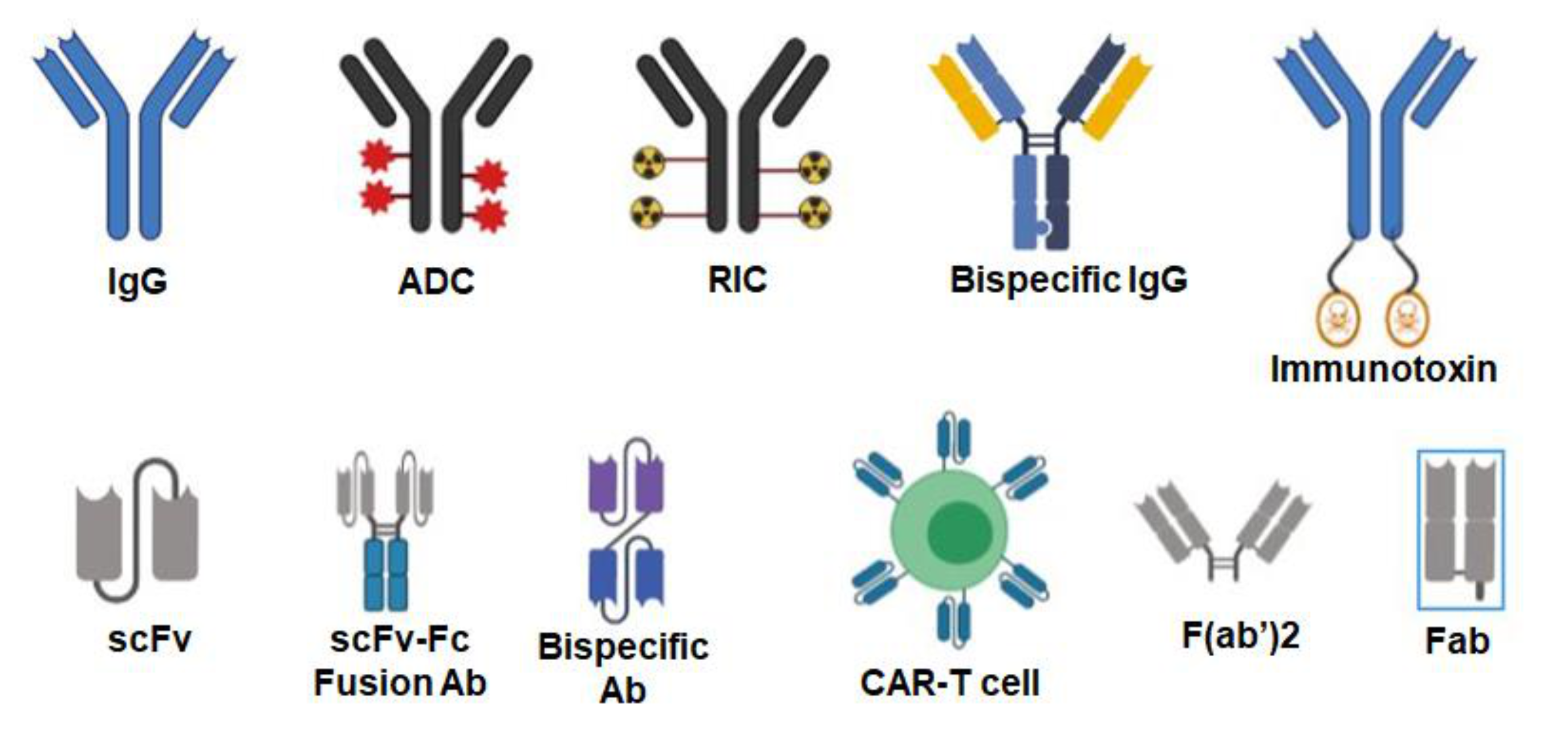
3. Antibodies Targeted to Inducible Cancer Antigens
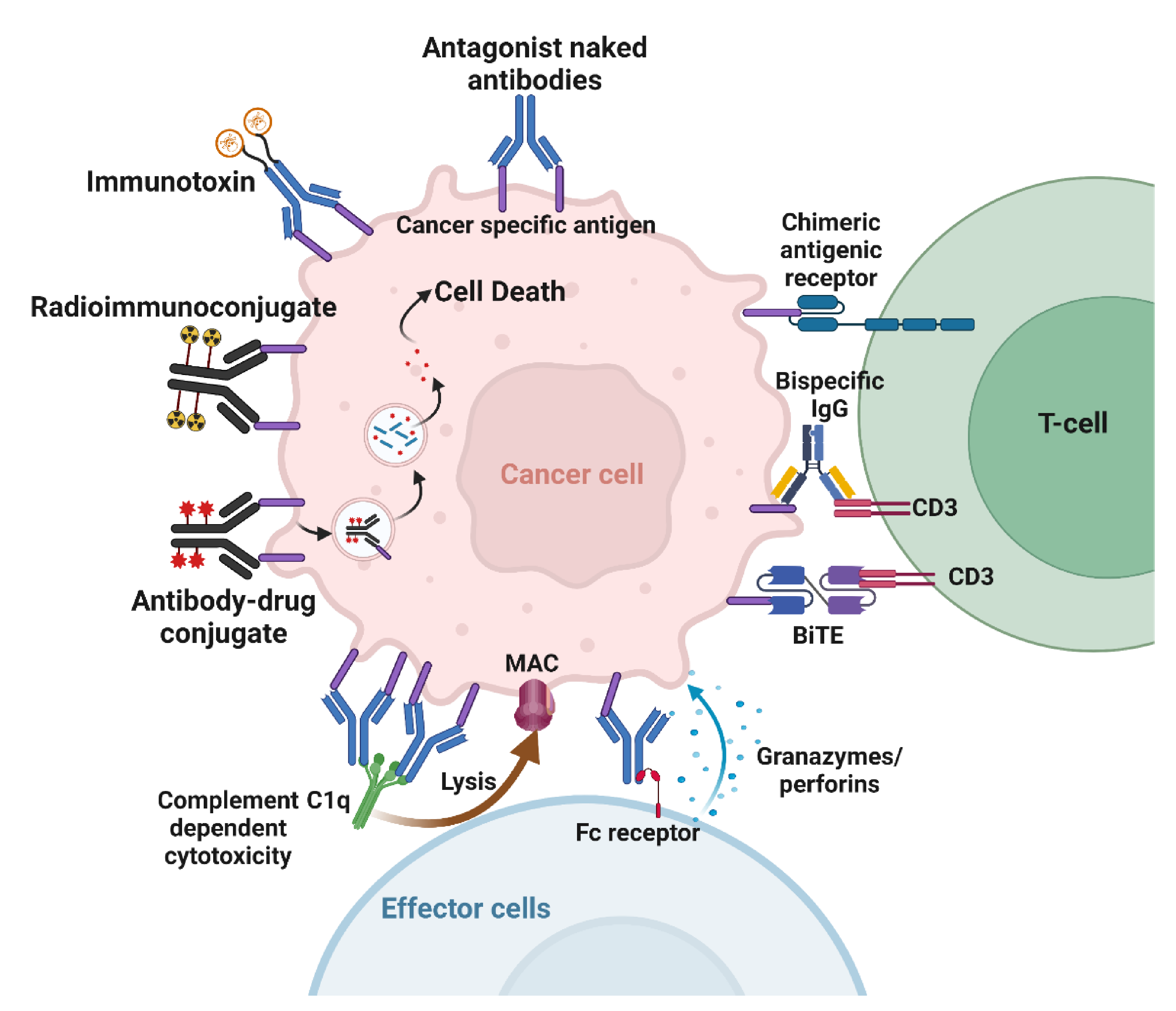
4. Mechanisms of Action of Cancer Therapeutic Antibodies
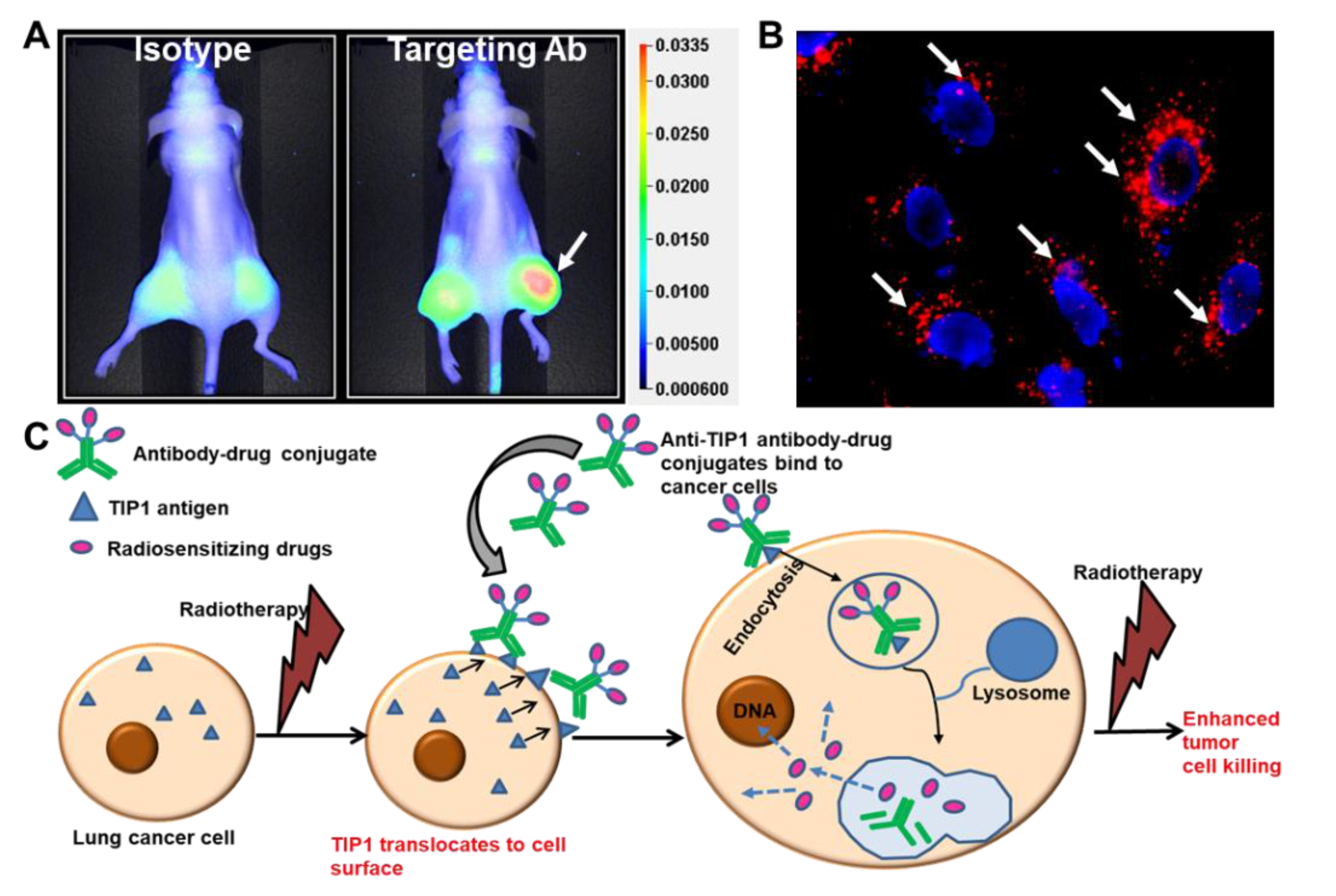
5. Radiosensitizing Therapeutic Antibodies
6. Antibody–Drug Conjugates
7. Planned Clinical Trials
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Gray, J.E.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; Cho, B.C.; et al. Three-Year Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC-Update from PACIFIC. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2020, 15, 288–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonner, J.A.; Harari, P.M.; Giralt, J.; Cohen, R.B.; Jones, C.U.; Sur, R.K.; Raben, D.; Baselga, J.; Spencer, S.A.; Zhu, J.; et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010, 11, 21–28. [Google Scholar] [CrossRef]
- Lewis, C.D.; Singh, A.K.; Hsu, F.F.; Thotala, D.; Hallahan, D.E.; Kapoor, V. Targeting a Radiosensitizing Antibody-Drug Conjugate to a Radiation-Inducible Antigen. Clin Cancer Res. 2021, 27, 3224–3233. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.R.; Yang, H.C.; Savariar, E.N.; Aguilera, J.; Crisp, J.L.; Jones, K.A.; Whitney, M.A.; Lippman, S.M.; Cohen, E.E.W.; Tsien, R.Y.; et al. Anti-tubulin drugs conjugated to anti-ErbB antibodies selectively radiosensitize. Nat. Commun. 2016, 7, 13019. [Google Scholar] [CrossRef]
- Hingorani, D.V.; Doan, M.K.; Camargo, M.F.; Aguilera, J.; Song, S.M.; Pizzo, D.; Scanderbeg, D.J.; Cohen, E.E.W.; Lowy, A.M.; Adams, S.R.; et al. Precision Chemoradiotherapy for HER2 Tumors Using Antibody Conjugates of an Auristatin Derivative with Reduced Cell Permeability. Mol. Cancer Ther. 2020, 19, 157–167. [Google Scholar] [CrossRef] [Green Version]
- Tsao, L.C.; Force, J.; Hartman, Z.C. Mechanisms of Therapeutic Antitumor Monoclonal Antibodies. Cancer Res. 2021, 81, 4641–4651. [Google Scholar] [CrossRef]
- Gasser, M.; Waaga-Gasser, A.M. Therapeutic Antibodies in Cancer Therapy. Adv. Exp. Med. Biol. 2016, 917, 95–120. [Google Scholar]
- Silvestri, M.; Cristaudo, A.; Morrone, A.; Messina, C.; Bennardo, L.; Nistico, S.P.; Mariano, M.; Cameli, N. Emerging Skin Toxicities in Patients with Breast Cancer Treated with New Cyclin-Dependent Kinase 4/6 Inhibitors: A Systematic Review. Drug Saf. 2021, 44, 725–732. [Google Scholar] [CrossRef]
- Bennardo, L.; Passante, M.; Cameli, N.; Cristaudo, A.; Patruno, C.; Nistico, S.P.; Silvestri, M. Skin Manifestations after Ionizing Radiation Exposure: A Systematic Review. Bioengineering 2021, 8, 153. [Google Scholar] [CrossRef]
- Hallahan, D.; Geng, L.; Qu, S.; Scarfone, C.; Giorgio, T.; Donnelly, E.; Gao, X.; Clanton, J. Integrin-mediated targeting of drug delivery to irradiated tumor blood vessels. Cancer Cell 2003, 3, 63–74. [Google Scholar] [CrossRef] [Green Version]
- Hariri, G.; Zhang, Y.; Fu, A.; Han, Z.; Brechbiel, M.; Tantawy, M.N.; Peterson, T.; Mernaugh, R.; Hallahan, D. Radiation-Guided P-Selectin Antibody Targeted to Lung Cancer. Ann. Biomed. Eng. 2008, 36, 821–830. [Google Scholar] [CrossRef] [Green Version]
- Shamay, Y.; Elkabets, M.; Li, H.; Shah, J.; Brook, S.; Wang, F.; Adler, F.; Baut, E.; Scaltriti, M.; Jena, P.V.; et al. P-selectin is a nanotherapeutic delivery target in the tumor microenvironment. Sci. Transl. Med. 2016, 8, 345ra87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elfiky, A.A.; Baghdady, A.M.; Ali, S.A.; Ahmed, M.I. GRP78 targeting: Hitting two birds with a stone. Life Sci. 2020, 260, 118317. [Google Scholar] [CrossRef] [PubMed]
- Dadey, D.Y.A.; Kapoor, V.; Khudanyan, A.; Thotala, D.; Hallahan, D.E. PERK Regulates Glioblastoma Sensitivity to ER Stress Although Promoting Radiation Resistance. Mol. Cancer Res. 2018, 16, 1447–1453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dadey, D.Y.A.; Kapoor, V.; Hoye, K.; Khudanyan, A.; Collins, A.; Thotala, D.; Hallahan, D.E. Antibody Targeting GRP78 Enhances the Efficacy of Radiation Therapy in Human Glioblastoma and Non-Small Cell Lung Cancer Cell Lines and Tumor Models. Clin. Cancer Res. 2017, 23, 2556–2564. [Google Scholar] [CrossRef] [Green Version]
- Dadey, D.Y.; Kapoor, V.; Khudanyan, A.; Urano, F.; Kim, A.H.; Thotala, D.; Hallahan, D.E. The ATF6 pathway of the ER stress response contributes to enhanced viability in glioblastoma. Oncotarget 2016, 7, 2080–2092. [Google Scholar] [CrossRef] [Green Version]
- Jaboin, J.J.; Han, Z.; Hallahan, D.E. Using in vivo biopanning for the development of radiation-guided drug delivery systems. Methods Mol. Biol. 2009, 542, 285–300. [Google Scholar]
- Ferraro, D.J.; Bhave, S.R.; Kotipatruni, R.P.; Hunn, J.C.; Wildman, S.A.; Hong, C.; Dadey, D.Y.A.; Muhoro, L.K.; Jaboin, J.J.; Thotala, D.; et al. High-throughput identification of putative receptors for cancer-binding peptides using biopanning and microarray analysis. Integr. Biol. 2013, 5, 342–350. [Google Scholar] [CrossRef] [Green Version]
- Han, Z.; Fu, A.; Wang, H.; Diaz, R.; Geng, L.; Onishko, H.; Hallahan, D.E. Noninvasive assessment of cancer response to therapy. Nat. Med. 2008, 14, 343–349. [Google Scholar] [CrossRef]
- Passarella, R.J.; Zhou, L.; Phillips, J.G.; Wu, H.; Hallahan, D.E.; Diaz, R. Recombinant peptides as biomarkers for tumor response to molecular targeted therapy. Clin. Cancer Res. 2009, 15, 6421–6429. [Google Scholar] [CrossRef] [Green Version]
- Hallahan, D.E.; Virudachalam, S. Accumulation of P-selectin in the lumen of irradiated blood vessels. Radiat. Res. 1999, 152, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Hallahan, D.; Clark, E.T.; Kuchibhotla, J.; Gewertz, B.L.; Collins, T. E-selectin gene induction by ionizing radiation is independent of cytokine induction. Biochem. Biophys. Res. Commun. 1995, 217, 784–795. [Google Scholar] [CrossRef] [PubMed]
- Hallahan, D.E.; Mauceri, H.J.; Seung, L.P.; Dunphy, E.J.; Wayne, J.D.; Hanna, N.N.; Toledano, A.; Hellman, S.; Kufe, D.W.; Weichselbalum, R.R. Spatial and temporal control of gene therapy using ionizing radiation. Nat. Med. 1995, 1, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Hallahan, D.; Kuchibhotla, J.; Wyble, C. Cell adhesion molecules mediate radiation-induced leukocyte adhesion to the vascular endothelium. Cancer Res. 1996, 56, 5150–5155. [Google Scholar] [PubMed]
- Lonberg, N. Human antibodies from transgenic animals. Nat. Biotechnol. 2005, 23, 1117–1125. [Google Scholar] [CrossRef]
- Alfaleh, M.A.; Alsaab, H.O.; Mahmoud, A.B.; Alkayyal, A.A.; Jones, M.L.; Mahler, S.M.; Hashem, A.M. Phage Display Derived Monoclonal Antibodies: From Bench to Bedside. Front Immunol. 2020, 11, 1986. [Google Scholar] [CrossRef]
- Patel, D.; Bassi, R.; Hooper, A.; Prewett, M.; Hicklin, D.J.; Kang, X. Anti-epidermal growth factor receptor monoclonal antibody cetuximab inhibits EGFR/HER-2 heterodimerization and activation. Int. J. Oncol. 2009, 34, 25–32. [Google Scholar]
- Dassonville, O.; Bozec, A.; Fischel, J.L.; Milano, G. EGFR targeting therapies: Monoclonal antibodies versus tyrosine kinase inhibitors. Similarities and differences. Crit. Rev. Oncol. Hematol. 2007, 62, 53–61. [Google Scholar] [CrossRef]
- Imai, K.; Takaoka, A. Comparing antibody and small-molecule therapies for cancer. Nat. Rev. Cancer 2006, 6, 714–727. [Google Scholar] [CrossRef]
- Markovic, A.; Chung, C.H. Current role of EGF receptor monoclonal antibodies and tyrosine kinase inhibitors in the management of head and neck squamous cell carcinoma. Expert Rev. Anticancer Ther. 2012, 12, 1149–1159. [Google Scholar] [CrossRef] [Green Version]
- Gul, N.; Babes, L.; Siegmund, K.; Korthouwer, R.; Bogels, M.; Braster, R.; Vidarsson, G.; ten Hagen, T.L.M.; Kubes, P.; van Egmond, M. Macrophages eliminate circulating tumor cells after monoclonal antibody therapy. J. Clin. Investig. 2014, 124, 812–823. [Google Scholar] [CrossRef] [PubMed]
- Gogesch, P.; Dudek, S.; van Zandbergen, G.; Waibler, Z.; Anzaghe, M. The Role of Fc Receptors on the Effectiveness of Therapeutic Monoclonal Antibodies. Int. J. Mol. Sci. 2021, 22, 8947. [Google Scholar] [CrossRef] [PubMed]
- Hassan, R.; Williams-Gould, J.; Steinberg, S.M.; Liewehr, D.J.; Yokokawa, J.; Tsang, K.Y.; Surawski, R.J.; Scott, T.; Camphausen, K.; Xin, X.; et al. Tumor-directed radiation and the immunotoxin SS1P in the treatment of mesothelin-expressing tumor xenografts. Clin. Cancer Res. 2006, 12, 4983–4988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poty, S.; Carter, L.M.; Mandleywala, K.; Membreno, R.; Abdel-Atti, D.; Ragupathi, A.; Scholz, W.W.; Zeglis, B.M.; Lewis, J.S. Leveraging Bioorthogonal Click Chemistry to Improve (225)Ac-Radioimmunotherapy of Pancreatic Ductal Adenocarcinoma. Clin. Cancer Res. 2019, 25, 868–880. [Google Scholar] [CrossRef] [Green Version]
- Nelson, B.J.B.; Andersson, J.D.; Wuest, F. Targeted Alpha Therapy: Progress in Radionuclide Production, Radiochemistry, and Applications. Pharmaceutics 2020, 13, 49. [Google Scholar] [CrossRef]
- Yan, H.; Kapoor, V.; Nguyen, K.; Akers, W.J.; Li, H.; Scott, J.; Laforest, R.; Rogers, B.; Thotala, D.; Thotala, D. Anti-tax interacting protein-1 (TIP-1) monoclonal antibody targets human cancers. Oncotarget 2016, 7, 43352–43362. [Google Scholar] [CrossRef] [Green Version]
- Cirrone, G.A.P.; Manti, L.; Margarone, D.; Petringa, G.; Giuffrida, L.; Minopoli, A.; Picciotto, A.; Russo, G.; Cammarata, F.P.; Pisciotta, P.; et al. First experimental proof of Proton Boron Capture Therapy (PBCT) to enhance protontherapy effectiveness. Sci. Rep. 2018, 8, 141. [Google Scholar] [CrossRef] [Green Version]
- Barth, R.F.; Mi, P.; Yang, W. Boron delivery agents for neutron capture therapy of cancer. Cancer Commun. 2018, 38, 35. [Google Scholar] [CrossRef] [Green Version]
- Cimas, F.J.; Niza, E.; Juan, A.; Noblejas-Lopez, M.D.M.; Bravo, I.; Lara-Sanchez, A.; Alonso-Moreno, C.; Ocaña, A. Controlled Delivery of BET-PROTACs: In Vitro Evaluation of MZ1-Loaded Polymeric Antibody Conjugated Nanoparticles in Breast Cancer. Pharmaceutics 2020, 12, 986. [Google Scholar] [CrossRef]
- Juan, A.; Cimas, F.J.; Bravo, I.; Pandiella, A.; Ocana, A.; Alonso-Moreno, C. An Overview of Antibody Conjugated Polymeric Nanoparticles for Breast Cancer Therapy. Pharmaceutics 2020, 12, 802. [Google Scholar] [CrossRef]
- Juan, A.; Cimas, F.J.; Bravo, I.; Pandiella, A.; Ocana, A.; Alonso-Moreno, C. Antibody Conjugation of Nanoparticles as Therapeutics for Breast Cancer Treatment. Int. J. Mol. Sci. 2020, 21, 6018. [Google Scholar] [CrossRef] [PubMed]
- Suurs, F.V.; Lub-de Hooge, M.N.; de Vries, E.G.E.; de Groot, D.J.A. A review of bispecific antibodies and antibody constructs in oncology and clinical challenges. Pharmacol. Ther. 2019, 201, 103–119. [Google Scholar] [CrossRef] [PubMed]
- Green, D.J.; O’Steen, S.; Lin, Y.; Comstock, M.L.; Kenoyer, A.L.; Hamlin, D.K.; Wilbur, D.S.; Fisher, D.R.; Nartea, M.; Hylarides, M.D.; et al. CD38-bispecific antibody pretargeted radioimmunotherapy for multiple myeloma and other B-cell malignancies. Blood 2018, 131, 611–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, D.J.; Frayo, S.L.; Lin, Y.; Hamlin, D.K.; Fisher, D.R.; Frost, S.H.; Kenoyer, A.L.; Hylarides, M.D.; Gopal, A.K.; Gooley, A.T.; et al. Comparative Analysis of Bispecific Antibody and Streptavidin-Targeted Radioimmunotherapy for B-cell Cancers. Cancer Res. 2016, 76, 6669–6679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, A.J.; Campian, J.L.; Hui, C.; Rudra, S.; Rao, Y.J.; Thotala, D.; Hallahan, D.; Huang, J. Impact of concurrent versus adjuvant chemotherapy on the severity and duration of lymphopenia in glioma patients treated with radiation therapy. J. Neurooncol. 2018, 136, 403–411. [Google Scholar] [CrossRef]
- Kleinberg, L.; Sloan, L.; Grossman, S.; Lim, M. Radiotherapy, Lymphopenia, and Host Immune Capacity in Glioblastoma: A Potentially Actionable Toxicity Associated With Reduced Efficacy of Radiotherapy. Neurosurgery 2019, 85, 441–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasche, L.; Menoret, E.; Dubljevic, V.; Menu, E.; Vanderkerken, K.; Lapa, C.; Steinbrunn, T.; Chatterjee, M.; Knop, S.; Düll, J.; et al. A GRP78-Directed Monoclonal Antibody Recaptures Response in Refractory Multiple Myeloma with Extramedullary Involvement. Clin. Cancer Res. 2016, 22, 4341–4349. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Han, M.; Whetsell, W.; Jr Wang, J.; Rich, J.; Hallahan, D.; Han, Z. Tax-interacting protein 1 coordinates the spatiotemporal activation of Rho GTPases and regulates the infiltrative growth of human glioblastoma. Oncogene 2014, 33, 1558–1569. [Google Scholar] [CrossRef] [Green Version]
- Mohanty, S.; Ovee, M.; Banerjee, M. PDZ Domain Recognition: Insight from Human Tax-Interacting Protein 1 (TIP-1) Interaction with Target Proteins. Biology 2015, 4, 88–103. [Google Scholar] [CrossRef] [Green Version]
- Khongorzul, P.; Ling, C.J.; Khan, F.U.; Ihsan, A.U.; Zhang, J. Antibody–Drug Conjugates: A Comprehensive Review. Molecular Cancer Res. 2020, 18, 3–19. [Google Scholar] [CrossRef] [Green Version]
- Coats, S.; Williams, M.; Kebble, B.; Dixit, R.; Tseng, L.; Yao, N.-S.; Tice, D.A.; Soria, J.-C. Antibody–Drug Conjugates: Future Directions in Clinical and Translational Strategies to Improve the Therapeutic Index. Clin. Cancer Res. 2019, 25, 5441–5448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drago, J.Z.; Modi, S.; Chandarlapaty, S. Unlocking the potential of antibody–drug conjugates for cancer therapy. Nat. Rev. Clin. Oncol. 2021, 18, 327–344. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.; Goetsch, L.; Dumontet, C.; Corvaïa, N. Strategies and challenges for the next generation of antibody–drug conjugates. Nat. Rev. Drug Discov. 2017, 16, 315–337. [Google Scholar] [CrossRef] [PubMed]
- Lyon, R. Drawing lessons from the clinical development of antibody-drug conjugates. Drug Discov. Today Technol. 2018, 30, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Bourillon, L.; Bourgier, C.; Gaborit, N.; Garambois, V.; Lles, E.; Zampieri, A.; Ogier, C.; Jarlier, M.; Radosevic-Robin, N.; Orsetti, B.; et al. An auristatin-based antibody-drug conjugate targeting HER3 enhances the radiation response in pancreatic cancer. Int. J. Cancer 2019, 145, 1838–1851. [Google Scholar] [CrossRef]
- Buckel, L.; Savariar, E.N.; Crisp, J.L.; Jones, K.A.; Hicks, A.M.; Scanderbeg, D.J.; Nguyen, Q.T.; Sicklick, J.K.; Lowy, A.M.; Tsien, R.Y.; et al. Tumor radiosensitization by monomethyl auristatin E: Mechanism of action and targeted delivery. Cancer Res. 2015, 75, 1376–1387. [Google Scholar] [CrossRef] [Green Version]
- Reardon, D.A.; Lassman, A.B.; van den Bent, M.; Kumthekar, P.; Merrell, R.; Scott, A.M.; Fichtel, L.; Sulman, E.P.; Gomez, E.; Fischer, J.; et al. Efficacy and safety results of ABT-414 in combination with radiation and temozolomide in newly diagnosed glioblastoma. Neuro Oncol. 2017, 19, 965–975. [Google Scholar] [CrossRef] [Green Version]
- Bensch, F.; Van Der Veen, E.L.; Lub-De Hooge, M.N.; Jorritsma-Smit, A.; Boellaard, R.; Kok, I.C.; Oosting, S.F.; Schröder, C.P.; Hiltermann, T.J.N.; Van Der Wekken, A.J.; et al. 89Zr-atezolizumab imaging as a non-invasive approach to assess clinical response to PD-L1 blockade in cancer. Nat. Med. 2018, 24, 1852–1868. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapoor, V.; Singh, A.K.; Lewis, C.D.; Deore, S.; Hallahan, D.E. Exploiting Radiation Induction of Antigens in Cancer: Targeted Drug Delivery. Int. J. Mol. Sci. 2022, 23, 3041. https://doi.org/10.3390/ijms23063041
Kapoor V, Singh AK, Lewis CD, Deore S, Hallahan DE. Exploiting Radiation Induction of Antigens in Cancer: Targeted Drug Delivery. International Journal of Molecular Sciences. 2022; 23(6):3041. https://doi.org/10.3390/ijms23063041
Chicago/Turabian StyleKapoor, Vaishali, Abhay K. Singh, Calvin D. Lewis, Sapna Deore, and Dennis E. Hallahan. 2022. "Exploiting Radiation Induction of Antigens in Cancer: Targeted Drug Delivery" International Journal of Molecular Sciences 23, no. 6: 3041. https://doi.org/10.3390/ijms23063041
APA StyleKapoor, V., Singh, A. K., Lewis, C. D., Deore, S., & Hallahan, D. E. (2022). Exploiting Radiation Induction of Antigens in Cancer: Targeted Drug Delivery. International Journal of Molecular Sciences, 23(6), 3041. https://doi.org/10.3390/ijms23063041






