The miRNA-21-5p Payload in Exosomes from M2 Macrophages Drives Tumor Cell Aggression via PTEN/Akt Signaling in Renal Cell Carcinoma
Abstract
:1. Introduction
2. Results
2.1. M2 Macrophages Promote RCC Migration and Invasion
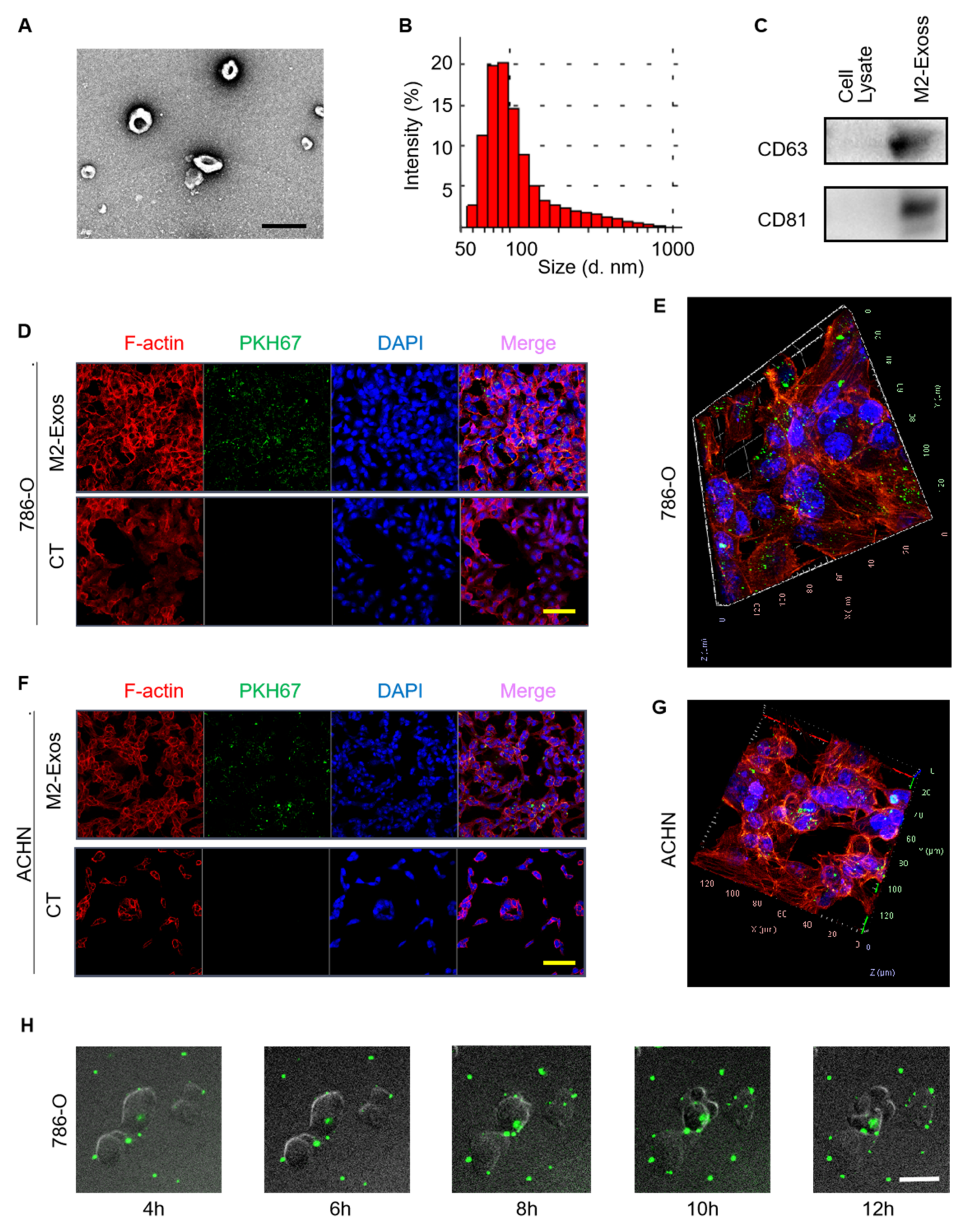
2.2. Characterization and Internalization of M2-Exos
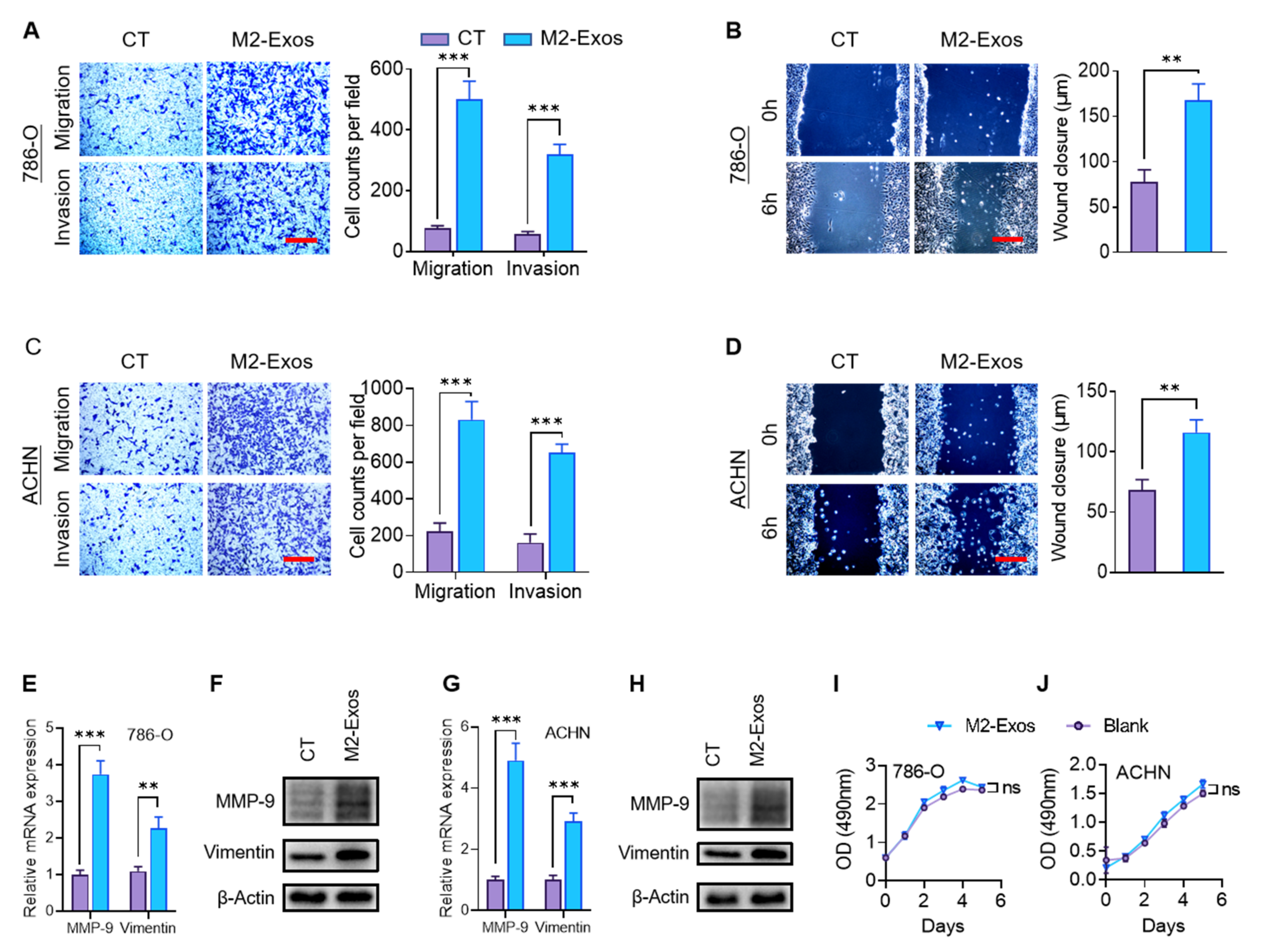
2.3. M2-Exos Promote RCC Migration and Invasion
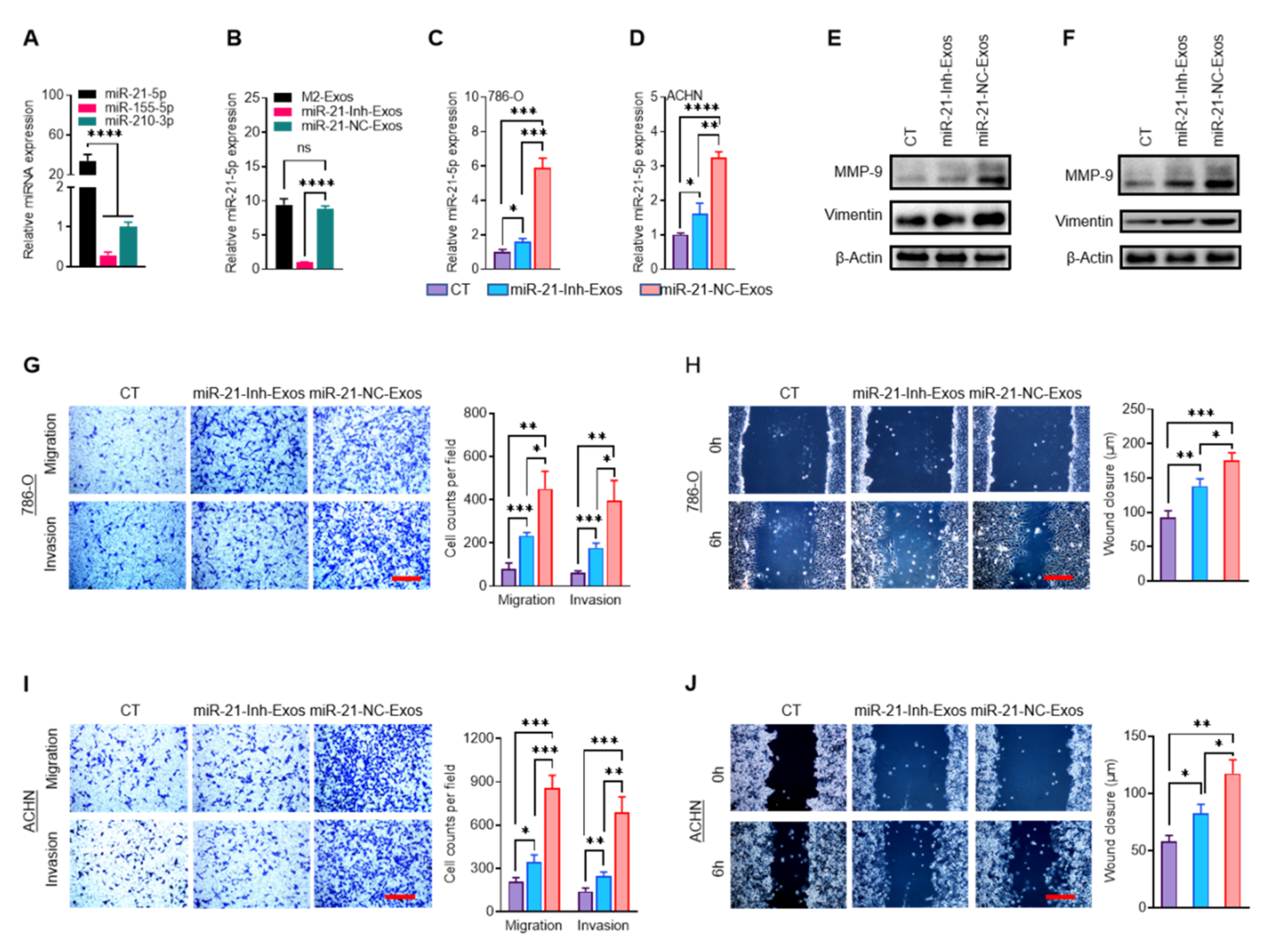
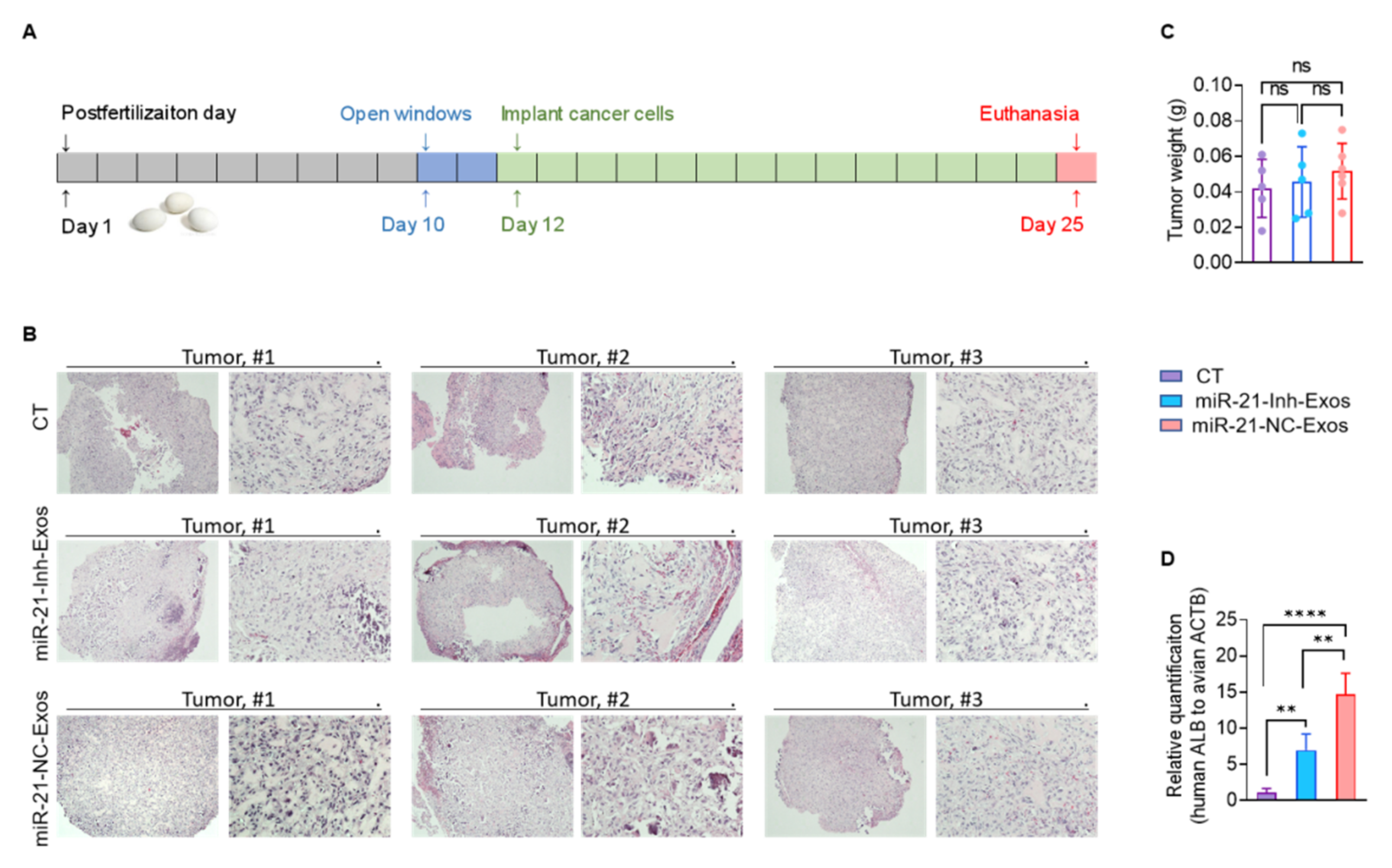
2.4. MiR-21-5p in M2-Exos Directs RCC Aggressive Behaviors In Vitro and Metastasis In Vivo
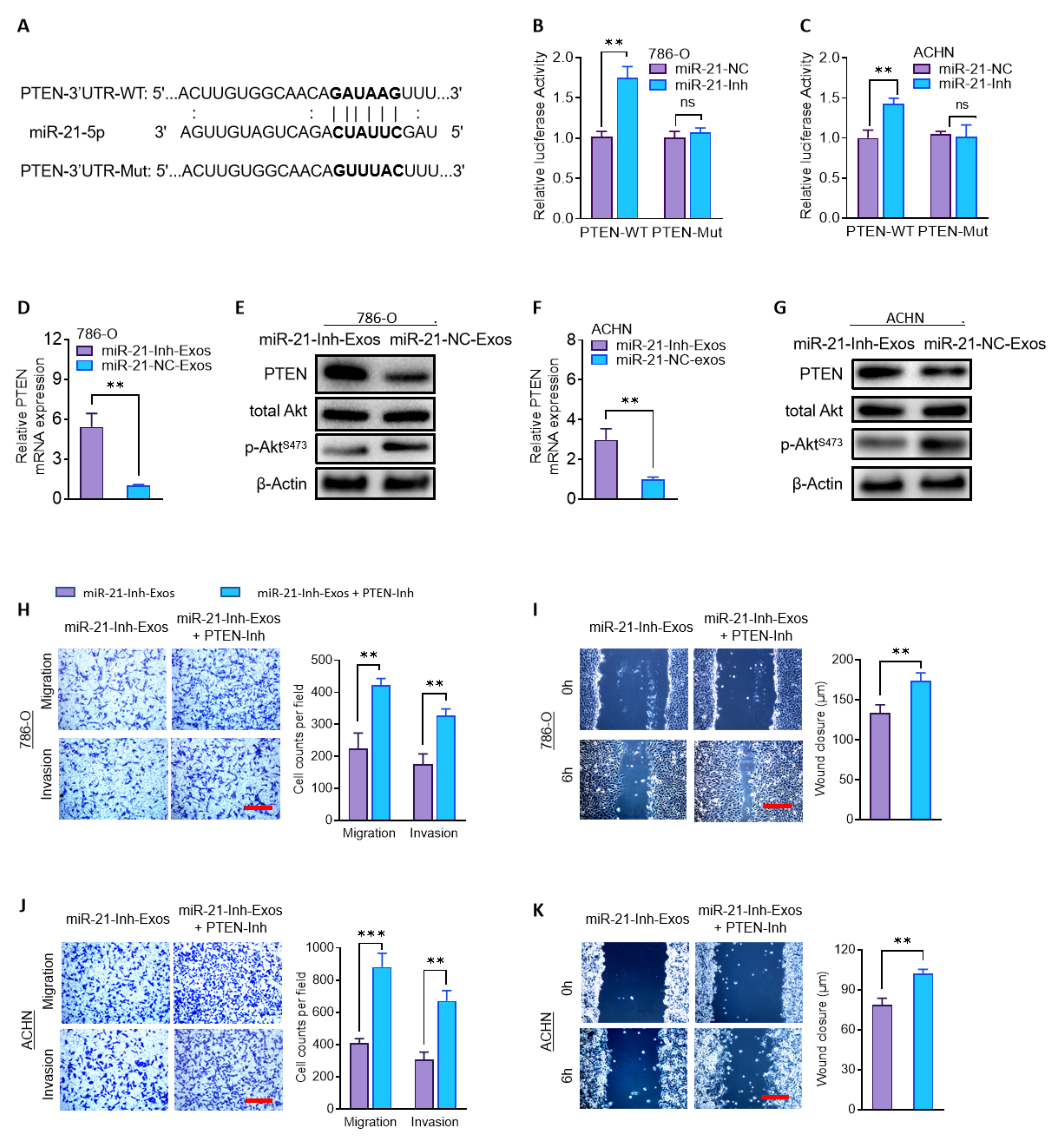
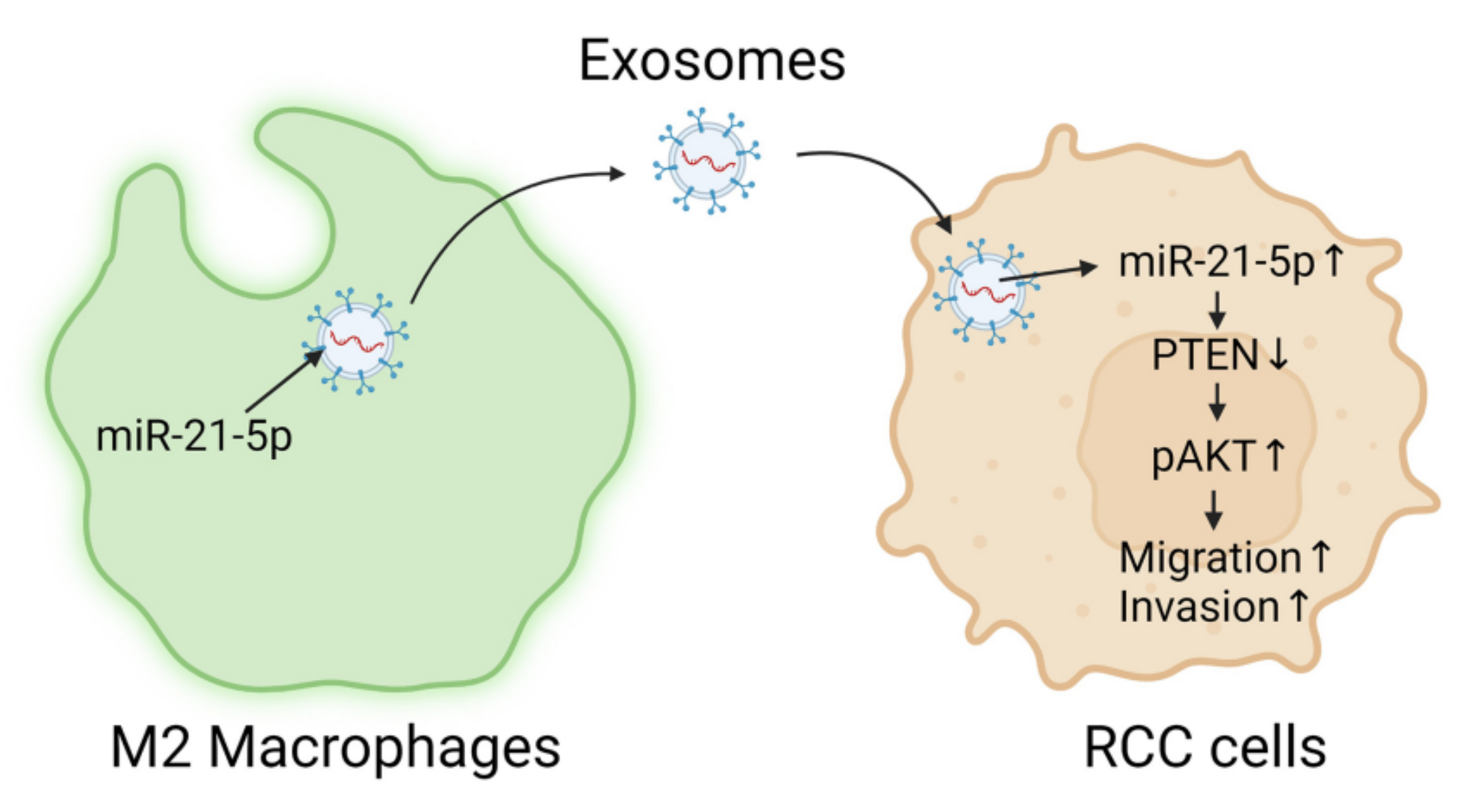
2.5. MiR-21-5p in M2-Exos Promotes RCC Metastasis through PTEN/Akt Signaling
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Exosomes Isolation and TEM
4.3. Internalization of PKH67-Labeled M2-Exos
4.4. RNA Isolation and RT-qPCR
4.5. Western Blotting
4.6. Transwell and Wound-Healing Assays
4.7. Cell Proliferation Assay
4.8. Cell Transfection and miRNA Targeted Luciferase Reporter Assay
4.9. IHC and IF
4.10. CAM Model and Quantitative PCR (qPCR) Analysis of Genomic DNA
4.11. Statistics Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RCC | Renal cell carcinoma |
| ccRCC | clear cell renal cell carcinoma |
| miRNA | microRNA |
| TAMs | Tumor-associated macrophages |
| THP-1-Mφ | Mφ subtype differentiated from THP-1 cell |
| THP-1-M2 | M2 subtype differentiated from THP-1 cell |
| M2-Exos | Exosomes derived from M2 macrophages |
| miR-21-Inh | miR-21-5p inhibitor |
| miR-21-NC | miR-21-5p negative control |
| miR-21-Inh-Exos | Exosomes derived from M2 macrophages transfected with miR-21-Inh |
| miR-21-NC-Exos | Exosomes derived from M2 macrophages transfected with miR-21-NC |
| CAM | Chorioallantoic membrane |
| DLS | Dynamic light scattering |
| DAPI | 4′,6-diamidino-2-phenylindole |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| H&E | Hematoxylin and Eosin |
| IHC | Immunohistochemistry |
| IF | Immunofluorescence |
| 3′UTRs | the 3′-untranslated regions of mRNA |
| qPCR | quantitative PCR |
| RT-qPCR | Reverse transcription-qPCR |
| PTEN | Phosphatase and tensin homolog |
| CD163 | Cluster of differentiation 163 |
| CD206 | Cluster of differentiation 206 |
| HLA-DR | Human leukocyte antigen-DR isotype |
| TNF-α | Tumor necrosis factor alpha |
| MMP-9 | Matrix metalloproteinase 9 |
| EMT | Epithelial-mesenchymal transition |
References
- Capitanio, U.; Bensalah, K.; Bex, A.; Boorjian, S.A.; Bray, F.; Coleman, J.; Gore, J.L.; Sun, M.; Wood, C.; Russo, P. Epidemiology of renal cell carcinoma. Eur. Urol. 2019, 75, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, J.J.; Purdue, M.P.; Signoretti, S.; Swanton, C.; Albiges, L.; Schmidinger, M.; Heng, D.Y.; Larkin, J.; Ficarra, V. Renal cell carcinoma. Nat. Rev. Dis. Primers 2017, 3, 17009. [Google Scholar] [CrossRef] [PubMed]
- Tannir, N.M.; Pal, S.K.; Atkins, M.B. Second-line treatment landscape for renal cell carcinoma: A comprehensive review. Oncologist 2018, 23, 540–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pontes, O.; Oliveira-Pinto, S.; Baltazar, F.; Costa, M. Renal cell carcinoma therapy: Current and new drug candidates. Drug Discov. Today 2022, 27, 304–314. [Google Scholar] [CrossRef]
- Martinez, F.O.; Helming, L.; Gordon, S. Alternative activation of macrophages: An immunologic functional perspective. Annu. Rev. Immunol. 2009, 27, 451–483. [Google Scholar] [CrossRef] [Green Version]
- Mohapatra, S.; Pioppini, C.; Ozpolat, B.; Calin, G.A. Non-coding RNAs regulation of macrophage polarization in cancer. Mol. Cancer. 2021, 20, 24. [Google Scholar] [CrossRef]
- Munir, M.T.; Kay, M.K.; Kang, M.H.; Rahman, M.M.; Al-Harrasi, A.; Choudhury, M.; Moustaid-Moussa, N.; Hussain, F.; Rahman, S.M. Tumor-associated macrophages as multifaceted regulators of breast tumor growth. Int. J. Mol. Sci. 2021, 22, 6526. [Google Scholar] [CrossRef]
- Qian, B.Z.; Pollard, J.W. Macrophage diversity enhances tumor progression and metastasis. Cell 2010, 141, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Condeelis, J.; Pollard, J.W. Macrophages: Obligate partners for tumor cell migration, invasion, and metastasis. Cell 2006, 124, 263–266. [Google Scholar] [CrossRef] [Green Version]
- Pusztai, C.; Yusenko, M.V.; Banyai, D.; Szanto, A.; Kovacs, G. M2 macrophage marker Chitinase 3-Like 2 (CHI3L2) associates with progression of conventional renal cell carcinoma. Anticancer Res. 2019, 39, 6939–6943. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Rai, A.; Chen, M.; Suwakulsiri, W.; Greening, D.W.; Simpson, R.J. Extracellular vesicles in cancer—Implications for future improvements in cancer care. Nat. Rev. Clin. Oncol. 2018, 15, 617–638. [Google Scholar] [CrossRef]
- Lan, J.; Sun, L.; Xu, F.; Liu, L.; Hu, F.; Song, D.; Hou, Z.; Wu, W.; Luo, X.; Wang, J.; et al. M2 macrophage-derived exosomes promote cell migration and invasion in colon cancer. Cancer Res. 2019, 79, 146–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, Z.; Ma, T.; Huang, B.; Lin, L.; Zhou, Y.; Yan, J.; Zou, Y.; Chen, S. Macrophage-derived exosomal microRNA-501-3p promotes progression of pancreatic ductal adenocarcinoma through the TGFBR3-mediated TGF-β signaling pathway. J. Exp. Clin. Cancer Res. 2019, 38, 310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, X.; Qin, C.; Yu, G.; Guo, X.; Cheng, A.; Zhang, H.; Wang, Z. Circular RNA circRAB31 acts as a miR-885-5p sponge to suppress gastric cancer progression via the PTEN/PI3K/AKT pathway. Mol. Ther. Oncolytics 2021, 23, 501–514. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Tang, F.; Min, L.; Hornicek, F.; Duan, Z.; Tu, C. PTEN in osteosarcoma: Recent advances and the therapeutic potential. Biochim. Biophys. Acta Rev. Cancer 2020, 1874, 188405. [Google Scholar] [CrossRef]
- Yang, B.; Feng, X.; Liu, H.; Tong, R.; Wu, J.; Li, C.; Yu, H.; Chen, Y.; Cheng, Q.; Chen, J.; et al. High-metastatic cancer cells derived exosomal miR92a-3p promotes epithelial-mesenchymal transition and metastasis of low-metastatic cancer cells by regulating PTEN/Akt pathway in hepatocellular carcinoma. Oncogene 2020, 39, 6529–6543. [Google Scholar] [CrossRef]
- Wang, X.; Luo, G.; Zhang, K.; Cao, J.; Huang, C.; Jiang, T.; Liu, B.; Su, L.; Qiu, Z. Hypoxic tumor-derived exosomal miR-301a mediates M2 macrophage polarization via PTEN/PI3Kγ to promote pancreatic cancer metastasis. Cancer Res. 2018, 78, 4586–4598. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Zhang, S.; Wang, Q.; Zhang, X. Tumor-recruited M2 macrophages promote gastric and breast cancer metastasis via M2 macrophage-secreted CHI3L1 protein. J. Hematol. Oncol. 2017, 10, 36. [Google Scholar] [CrossRef] [Green Version]
- Kovaleva, O.V.; Samoilova, D.V.; Shitova, M.S.; Gratchev, A. Tumor Associated Macrophages in Kidney Cancer. Anal. Cell. Pathol. 2016, 2016, 9307549. [Google Scholar] [CrossRef] [Green Version]
- Schwende, H.; Fitzke, E.; Ambs, P.; Dieter, P. Differences in the state of differentiation of THP-1 cells induced by phorbol ester and 1,25-dihydroxyvitamin D3. J. Leukoc. Biol. 1996, 59, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Gao, Y.; Ho, C.; Li, L.; Jin, C.; Wang, X.; Zou, C.; Mao, Y.; Wang, X.; Li, Q.; et al. Exosome-delivered CD44v6/C1QBP complex drives pancreatic cancer liver metastasis by promoting fibrotic liver microenvironment. Gut 2021, 71, 568–579. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.; Lv, H.; Lv, G.; Li, T.; Wang, C.; Han, Q.; Yu, L.; Su, B.; Guo, L.; Huang, S.; et al. Tumor-derived exosomal miR-1247-3p induces cancer-associated fibroblast activation to foster lung metastasis of liver cancer. Nat. Commun. 2018, 9, 191. [Google Scholar] [CrossRef] [Green Version]
- Lu, W.; Kang, Y. Epithelial-mesenchymal plasticity in cancer progression and metastasis. Dev. Cell. 2019, 49, 361–374. [Google Scholar] [CrossRef]
- Mittal, V. Epithelial Mesenchymal Transition in Tumor Metastasis. Annu. Rev. Pathol. 2018, 13, 395–412. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Wang, S.; Ni, B.; Xing, S.; Cao, H.; Zhang, Z.; Yu, F.; Zhao, E.; Zhao, G. Hypoxic gastric cancer-derived exosomes promote progression and metastasis via MiR-301a-3p/PHD3/HIF-1α positive feedback loop. Oncogene 2020, 39, 6231–6244. [Google Scholar] [CrossRef]
- Li, Y.Y.; Tao, Y.W.; Gao, S.; Li, P.; Zheng, J.M.; Zhang, S.E.; Liang, J.; Zhang, Y. Cancer-associated fibroblasts contribute to oral cancer cells proliferation and metastasis via exosome-mediated paracrine miR-34a-5p. EBioMedicine 2018, 36, 209–220. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Li, B.; Li, Q.; Wei, S.; He, Z.; Huang, X.; Wang, L.; Xia, Y.; Xu, Z.; Li, Z.; et al. Exosomal miR-21-5p derived from gastric cancer promotes peritoneal metastasis via mesothelial-to-mesenchymal transition. Cell Death Dis. 2018, 9, 854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, S.S.; Zhang, H.P.; Yang, C.Q.; Li, L.N.; Shen, Y.; Zhang, Y.Q. Exosomal miR-155-5p promotes proliferation and migration of gastric cancer cells by inhibiting TP53INP1 expression. Pathol. Res. Pract. 2020, 216, 152986. [Google Scholar] [CrossRef]
- Kia, V.; Paryan, M.; Mortazavi, Y.; Biglari, A.; Mohammadi-Yeganeh, S. Evaluation of exosomal miR-9 and miR-155 targeting PTEN and DUSP14 in highly metastatic breast cancer and their effect on low metastatic cells. J. Cell. Biochem. 2019, 120, 5666–5676. [Google Scholar] [CrossRef]
- Zhang, X.; Sai, B.; Wang, F.; Wang, L.; Wang, Y.; Zheng, L.; Li, G.; Tang, J.; Xiang, J. Hypoxic BMSC-derived exosomal miRNAs promote metastasis of lung cancer cells via STAT3-induced EMT. Mol. Cancer 2019, 18, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; He, J.; Hu, H.; Tu, L.; Sun, Z.; Liu, Y.; Luo, F. Lung CSC-derived exosomal miR-210-3p contributes to a pro-metastatic phenotype in lung cancer by targeting FGFRL1. J. Cell. Mol. Med. 2020, 24, 6324–6339. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D. The chick embryo chorioallantoic membrane (CAM) assay. Reprod. Toxicol. 2017, 70, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Sliwinska, P.; Segura, T.; Iruela-Arispe, M.L. The chicken chorioallantoic membrane model in biology, medicine and bioengineering. Angiogenesis 2014, 17, 779–804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharrow, A.C.; Ishihara, M.; Hu, J.; Kim, I.H.; Wu, L. Using the Chicken Chorioallantoic Membrane In Vivo Model to Study Gynecological and Urological Cancers. J. Vis. Exp. 2020, 2020, e60651. [Google Scholar] [CrossRef]
- Hu, J.; Ishihara, M.; Chin, A.I.; Wu, L. Establishment of xenografts of urological cancers on chicken chorioallantoic membrane (CAM) to study metastasis. Precis. Clin. Med. 2019, 2, 140–151. [Google Scholar] [CrossRef] [Green Version]
- Ishihara, M.; Hu, J.; Zhang, X.; Choi, Y.; Wong, A.; Cano-Ruiz, C.; Zhao, R.; Tan, P.; Tso, J.L.; Wu, L. Comparing metastatic clear cell renal cell carcinoma model established in mouse kidney and on chicken chorioallantoic membrane. J. Vis. Exp. 2020, 2020, e60314. [Google Scholar] [CrossRef]
- Ishihara, M.; Hu, J.; Wong, A.; Cano-Ruiz, C.; Wu, L. Mouse- and patient-derived CAM xenografts for studying metastatic renal cell carcinoma. Enzymes 2019, 46, 59–80. [Google Scholar] [CrossRef] [PubMed]
- Kundekova, B.; Macajova, M.; Meta, M.; Cavarga, I.; Bilcik, B. Chorioallantoic Membrane Models of Various Avian Species: Differences and Applications. Biologyn 2021, 10, 301. [Google Scholar] [CrossRef]
- Nguyen, V.H.L.; Yue, C.; Du, K.Y.; Salem, M.; O’Brien, J.; Peng, C. The role of microRNAs in epithelial ovarian cancer metastasis. Int. J. Mol. Sci. 2020, 21, 7093. [Google Scholar] [CrossRef]
- Li, J.; Li, C.; Li, H.; Zhang, T.; Hao, X.; Chang, J.; Xu, Y. MicroRNA-30a-5p suppresses tumor cell proliferation of human renal cancer via the MTDH/PTEN/AKT pathway. Int. J. Mol. Med. 2018, 41, 1021–1029. [Google Scholar] [CrossRef]
- Song, J.; Yang, P.; Li, X.; Zhu, X.; Liu, M.; Duan, X.; Liu, R. Esophageal cancer-derived extracellular vesicle miR-21-5p contributes to EMT of ESCC cells by disorganizing macrophage polarization. Cancers 2021, 13, 4122. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.; Bi, Q.; Li, Y.; Deng, J.; Wu, N.; Hao, S.; Zhao, M. Long noncoding RNA MEG3 inhibits cell migration and invasion of nonsmall cell lung cancer cells by regulating the miR-21-5p/PTEN axis. Mol. Med. Rep. 2021, 23, 11830. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Lin, J.C.; Hwang, W.L.; Kuo, Y.J.; Chen, H.K.; Tai, S.K.; Lin, C.C.; Yang, M.H. Macrophage-secreted interleukin-35 regulates cancer cell plasticity to facilitate metastatic colonization. Nat. Commun. 2018, 9, 3763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozawa, P.M.M.; Alkhilaiwi, F.; Cavalli, I.J.; Malheiros, D.; de Souza Fonseca Ribeiro, E.M.; Cavalli, L.R. Extracellular vesicles from triple-negative breast cancer cells promote proliferation and drug resistance in non-tumorigenic breast cells. Breast. Cancer. Res. Treat. 2018, 172, 713–723. [Google Scholar] [CrossRef]
- Zheng, P.; Luo, Q.; Wang, W.; Li, J.; Wang, T.; Wang, P.; Chen, L.; Zhang, P.; Chen, H.; Liu, Y.; et al. Tumor-associated macrophages-derived exosomes promote the migration of gastric cancer cells by transfer of functional Apolipoprotein E. Cell. Death Dis. 2018, 9, 434. [Google Scholar] [CrossRef]
- Mi, X.; Xu, R.; Hong, S.; Xu, T.; Zhang, W.; Liu, M. M2 macrophage-derived exosomal lncRNA AFAP1-AS1 and MicroRNA-26a affect cell migration and metastasis in esophageal cancer. Mol. Ther. Nucleic Acids 2020, 22, 779–790. [Google Scholar] [CrossRef]
- Wu, J.; Gao, W.; Tang, Q.; Yu, Y.; You, W.; Wu, Z.; Fan, Y.; Zhang, L.; Wu, C.; Han, G.; et al. M2 macrophage-derived exosomes facilitate HCC metastasis by transferring αMβ2 integrin to tumor cells. Hepatology 2021, 73, 1365–1380. [Google Scholar] [CrossRef]
- Yan, L.; Wang, L.Z.; Xiao, R.; Cao, R.; Pan, B.; Lv, X.Y.; Jiao, H.; Zhuang, Q.; Sun, X.J.; Liu, Y.B. Inhibition of microRNA-21-5p reduces keloid fibroblast autophagy and migration by targeting PTEN after electron beam irradiation. Lab. Investig. 2020, 100, 387–399. [Google Scholar] [CrossRef]
- Jung, M.K.; Mun, J.Y. Sample Preparation and Imaging of Exosomes by Transmission Electron Microscopy. J. Vis. Exp. 2018, 2018, e56482. [Google Scholar] [CrossRef]
- Lyu, T.S.; Ahn, Y.; Im, Y.J.; Kim, S.S.; Lee, K.H.; Kim, J.; Choi, Y.; Lee, D.; Kang, E.; Jin, G.; et al. The characterization of exosomes from fibrosarcoma cell and the useful usage of Dynamic Light Scattering (DLS) for their evaluation. PLoS ONE 2021, 16, e0231994. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Hu, J.; Ishihara, M.; Sharrow, A.C.; Flora, K.; He, Y.; Wu, L. The miRNA-21-5p Payload in Exosomes from M2 Macrophages Drives Tumor Cell Aggression via PTEN/Akt Signaling in Renal Cell Carcinoma. Int. J. Mol. Sci. 2022, 23, 3005. https://doi.org/10.3390/ijms23063005
Zhang Z, Hu J, Ishihara M, Sharrow AC, Flora K, He Y, Wu L. The miRNA-21-5p Payload in Exosomes from M2 Macrophages Drives Tumor Cell Aggression via PTEN/Akt Signaling in Renal Cell Carcinoma. International Journal of Molecular Sciences. 2022; 23(6):3005. https://doi.org/10.3390/ijms23063005
Chicago/Turabian StyleZhang, Zhicheng, Junhui Hu, Moe Ishihara, Allison C. Sharrow, Kailey Flora, Yao He, and Lily Wu. 2022. "The miRNA-21-5p Payload in Exosomes from M2 Macrophages Drives Tumor Cell Aggression via PTEN/Akt Signaling in Renal Cell Carcinoma" International Journal of Molecular Sciences 23, no. 6: 3005. https://doi.org/10.3390/ijms23063005
APA StyleZhang, Z., Hu, J., Ishihara, M., Sharrow, A. C., Flora, K., He, Y., & Wu, L. (2022). The miRNA-21-5p Payload in Exosomes from M2 Macrophages Drives Tumor Cell Aggression via PTEN/Akt Signaling in Renal Cell Carcinoma. International Journal of Molecular Sciences, 23(6), 3005. https://doi.org/10.3390/ijms23063005






