A New Role of Acute Phase Proteins: Local Production Is an Ancient, General Stress-Response System of Mammalian Cells
Abstract
:1. Introduction
2. Main
2.1. Common Pathways of Renal Fibrosis and Cancer Progression
2.2. The Heat Shock Response and the Acute Phase Response
2.3. Breast Cancer
2.4. Modulated Electro-Hyperthermia (mEHT)
2.5. Acute (AKI) and Chronic Kidney Disease (CKD)
2.6. The Potential of Degradomic Analysis
3. Previous Findings
4. Known Roles of Major Acute-Phase Proteins Detected by NGS in Different Models
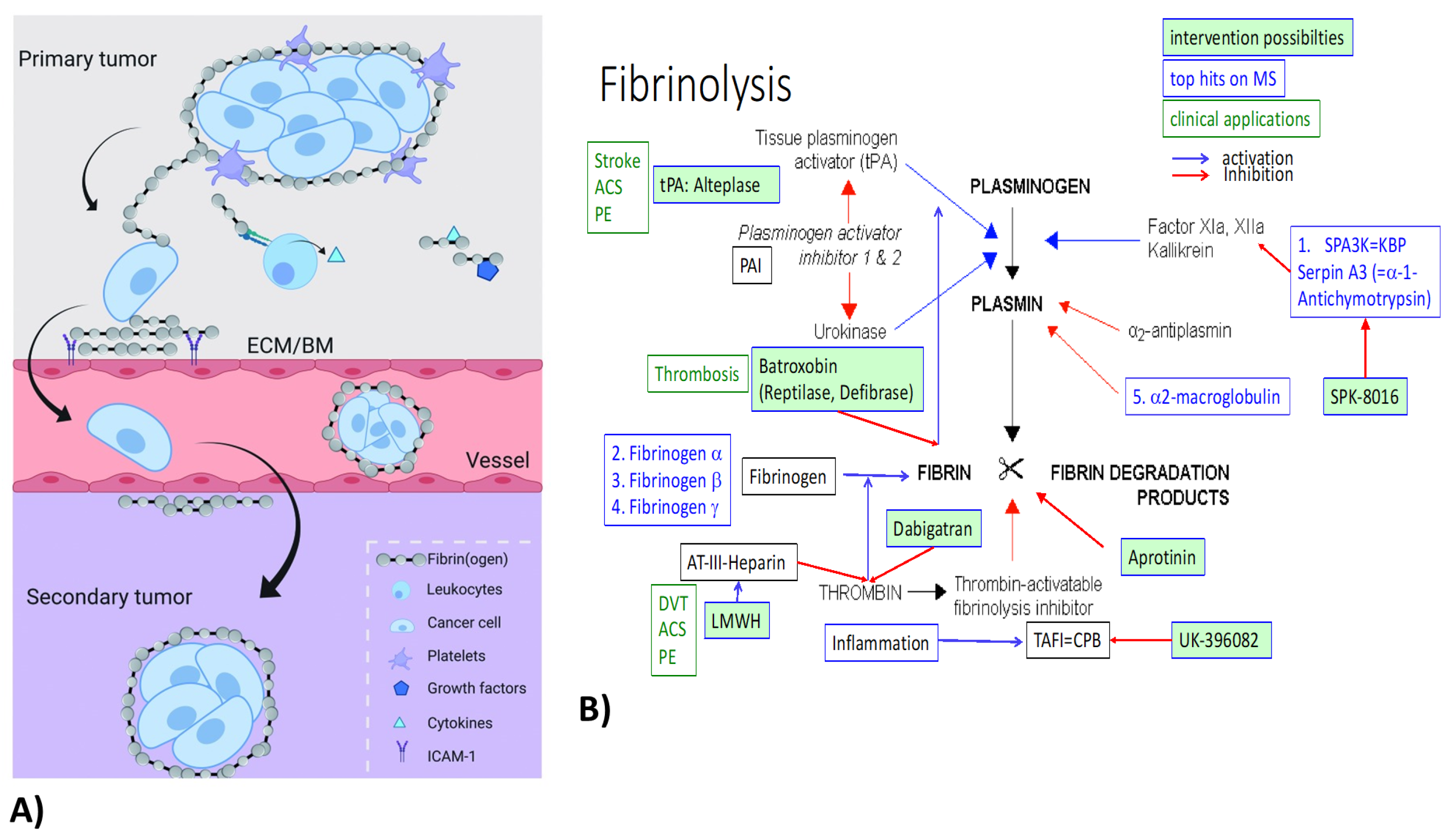
4.1. Fibrinogens
4.2. Haptoglobin
4.3. Protease Inhibitors (Serpins, ITI)
4.3.1. Serpins
4.3.2. Inter-Alpha-Trypsin Inhibitor (ITI)
4.4. Alpha-2-Macroglobulin (a2-MG)
4.5. Complement Factors (C4B)
5. Summary and Future Perspectives
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| A1M | alpha-1-microglobulin |
| a2-MG | alpha-2-macroglobulin |
| AMBP | bikunin precursor |
| ACS | acute coronary syndrome |
| AKI | acute kidney injury |
| APP | acute-phase proteins |
| APR | acute-phase response |
| AT-III | antithrombin-3 |
| CKD | chronic kidney disease |
| CPB | carboxypeptidase-B |
| CRC | colorectal cancer |
| DVT | deep vein thrombosis |
| ECM | extracellular matrix |
| EMF | focused electromagnetic field |
| FN | fibrin(ogen) |
| GS | glomerular sclerosis |
| HIF-1 | hypoxia-inducible factor-1 |
| Hp | haptoglobin |
| HSR | heat shock response |
| IalphaI/ITI | inter-alpha-trypsin inhibitor |
| IL | interleukin |
| LMWH | low-molecular-weight heparin |
| LPS | endotoxin/lipopolysaccharide |
| mEHT | modulated electro-hyperthermia |
| MS | mass spectrometry |
| NGS | next-generation sequencing |
| NSCLC | non-small-cell lung cancer |
| PAI | plasminogen activator inhibitor |
| PCR | polymerase chain reaction |
| PE | pulmonary embolism |
| P | properidin |
| PTX3 | pentraxin-related gene |
| RT-PCR | real-time polymerase chain reaction |
| SERPIN | serine protease inhibitor |
| SPA3K | serine protease inhibitor (Serpin)-A3 = alpha-1-anitchymotrypsin |
| SPK | Spa3K inhibitor |
| TAFI | thrombin-activatable fibrinolysis inhibitor |
| TEC | tubular epithelial cell |
| TGF-beta | transforming growth factor beta |
| TME | tumor microenvironment |
| TNBC | triple-negative breast cancer |
| TNF-alpha | tumor necrosis factor-alpha |
| tPA | tissue plasminogen activator |
| TSL | thermo-sensitive liposome |
References
- Marcos-Perez, D.; Sanchez-Flores, M.; Proietti, S.; Bonassi, S.; Costa, S.; Teixeira, J.J.; Fernández-Tajes, J.; Pasaro, E.; Laffon, B.; Valdiglesias, V. Association of inflammatory mediators with frailty status in older adults: Results from a systematic review and meta-analysis. Geroscience 2020, 42, 1451–1473. [Google Scholar] [CrossRef] [PubMed]
- Gasparics, A.; Kokeny, G.; Fintha, A.; Bencs, R.; Mozes, M.M.; Agoston, E.I.; Buday, A.; Ivics, Z.; Hamar, P.; Gyorffy, B.; et al. Alterations in SCAI Expression during Cell Plasticity, Fibrosis and Cancer. Pathol. Oncol. Res. 2018, 24, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Fintha, A.; Gasparics, A.; Fang, L.; Erdei, Z.; Hamar, P.; Mozes, M.M.; Kokeny, G.; Rosivall, L.; Sebe, A. Characterization and role of SCAI during renal fibrosis and epithelial-to-mesenchymal transition. Am. J. Pathol. 2013, 182, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Hamar, P.; Song, E.; Kokeny, G.; Chen, A.; Ouyang, N.; Lieberman, J. Small interfering RNA targeting Fas protects mice against renal ischemia-reperfusion injury. Proc. Natl. Acad. Sci. USA 2004, 101, 14883–14888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, L.L.; Xu, G.G.; Zhao, Q.; Zhou, L.L.; Wang, D.; Chen, W.W. The association between hypoxia inducible factor 1 subunit alpha gene rs2057482 polymorphism and cancer risk: A meta-analysis. BMC Cancer 2019, 19, 1123. [Google Scholar] [CrossRef] [PubMed]
- Danics, L.; Schvarcz, C.C.; Viana, P.; Vancsik, T.; Krenacs, T.; Benyo, Z.; Kaucsar, T.; Hamar, P. Exhaustion of Protective Heat Shock Response Induces Significant Tumor Damage by Apoptosis after Modulated Electro-Hyperthermia Treatment of Triple Negative Breast Cancer Isografts in Mice. Cancers 2020, 12, 2581. [Google Scholar] [CrossRef] [PubMed]
- Gomez, C.C. Role of heat shock proteins in aging and chronic inflammatory diseases. Geroscience 2021, 43, 2515–2532. [Google Scholar] [CrossRef] [PubMed]
- Smith-Sonneborn, J. Telomerase Biology Associations Offer Keys to Cancer and Aging Therapeutics. Curr. Aging Sci. 2020, 13, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Gabay, C.; Kushner, I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 1999, 340, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Moshage, H. Cytokines and the hepatic acute phase response. J. Pathol. 1997, 181, 257–266. [Google Scholar] [CrossRef]
- Schrodl, W.; Buchler, R.; Wendler, S.; Reinhold, P.; Muckova, P.; Reindl, J.; Rhode, H. Acute phase proteins as promising biomarkers: Perspectives and limitations for human and veterinary medicine. Proteomics Clin. Appl. 2016, 10, 1077–1092. [Google Scholar] [CrossRef] [PubMed]
- Schvarcz, C.C.; Danics, L.; Krenacs, T.; Viana, P.; Beres, R.; Vancsik, T.; Nagy, A.; Gyenesei, A.; Kun, J.; Fonovic, M.; et al. Modulated Electro-Hyperthermia Induces a Prominent Local Stress Response and Growth Inhibition in Mouse Breast Cancer Isografts. Cancers 2021, 13, 1744. [Google Scholar] [CrossRef] [PubMed]
- Roka, B.; Tod, P.; Kaucsar, T.; Vizovisek, M.; Vidmar, R.; Turk, B.; Fonovic, M.; Szenasi, G.; Hamar, P. The Acute Phase Response Is a Prominent Renal Proteome Change in Sepsis in Mice. Int. J. Mol. Sci. 2019, 21, 200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bukosza, E.E.; Kornauth, C.; Hummel, K.; Schachner, H.; Huttary, N.; Krieger, S.; Nobauer, K.; Oszwald, A.; Razzazi Fazeli, E.; Kratochwill, K.; et al. ECM Characterization Reveals a Massive Activation of Acute Phase Response during FSGS. Int. J. Mol. Sci. 2020, 21, 2095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WCRF International, Worldwide Cancer Data. Available online: https://www.wcrf.org/dietandcancer/worldwide-cancer-data/ (accessed on 10 November 2014).
- Motamed, H.H.; Shariati, M.; Ahmadi, R.; Khatamsaz, S.; Mokhtari, M. The apoptotic effects of progesterone on breast cancer (MCF-7) and human osteosarcoma (MG-636) cells. Physiol. Int. 2020, 107, 406–418. [Google Scholar] [CrossRef] [PubMed]
- Danics, L.; Schvarcz, C.C.; Zolcsak, Z.; Benyo, Z.; Kaucsar, T.; Hamar, P. Modulated electro hyperthermia inhibits tumor progression in a triple negative mouse breast cancer model. Oncothermia J. 2018, 24, 442–454. [Google Scholar]
- Tod, P.; Bukosza, E.E.; Roka, B.; Kaucsar, T.; Fintha, A.; Krenacs, T.; Szenasi, G.; Hamar, P. Post-Ischemic Renal Fibrosis Progression Is Halted by Delayed Contralateral Nephrectomy: The Involvement of Macrophage Activation. Int. J. Mol. Sci. 2020, 21, 3825. [Google Scholar] [CrossRef] [PubMed]
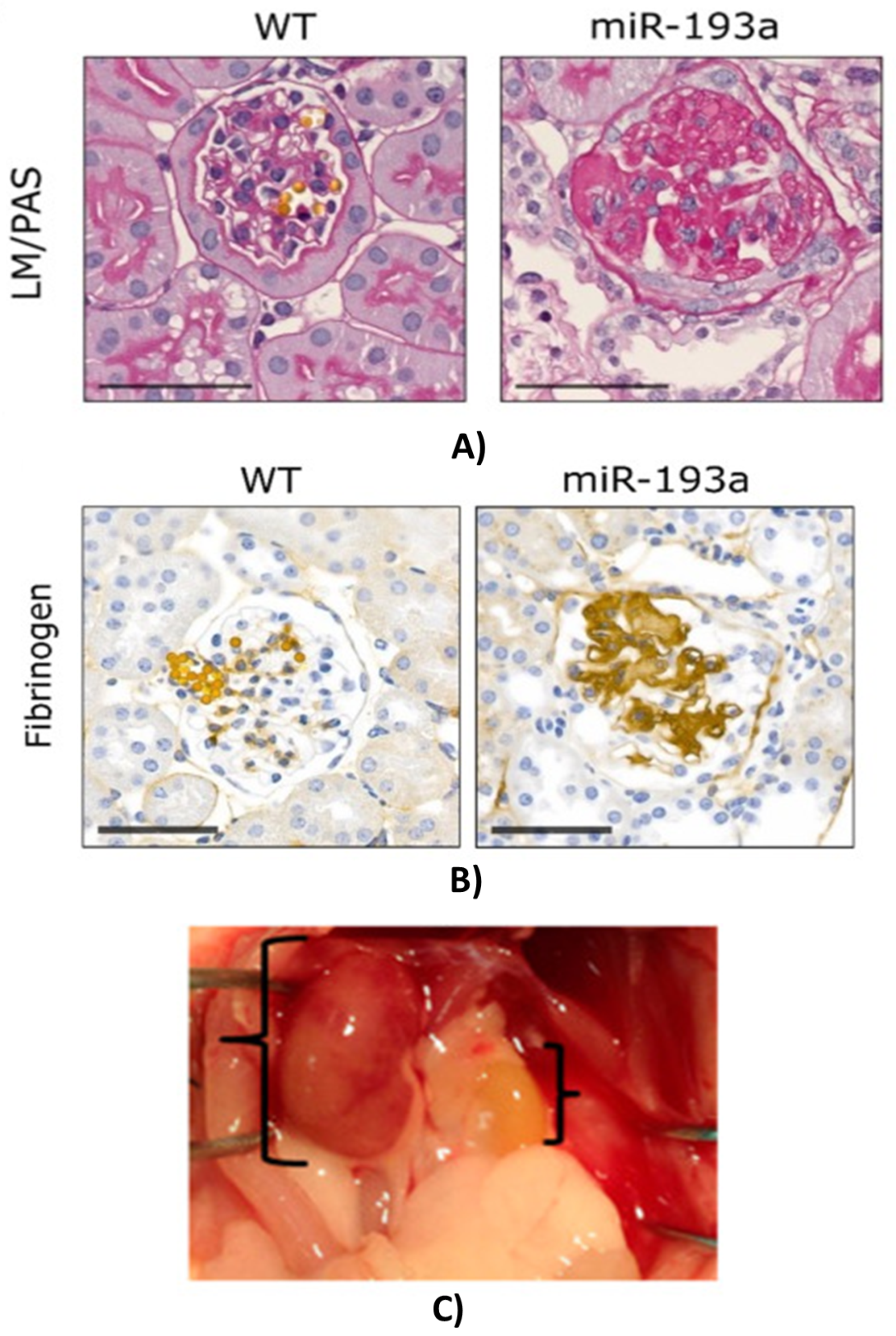
- Gebeshuber, C.C.; Kornauth, C.; Dong, L.; Sierig, R.; Seibler, J.; Reiss, M.; Tauber, S.; Bilban, M.; Wang, S.; Kain, R.; et al. Focal segmental glomerulosclerosis is induced by microRNA-193a and its downregulation of WT1. Nat. Med. 2013, 19, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Davalieva, K.; Kiprijanovska, S.; Komina, S.; Petrusevska, G.; Zografska, N.N.; Polenakovic, M. Proteomics analysis of urine reveals acute phase response proteins as candidate diagnostic biomarkers for prostate cancer. Proteome Sci. 2015, 13, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sirolli, V.; Pieroni, L.; Di Liberato, L.; Urbani, A.; Bonomini, M. Urinary Peptidomic Biomarkers in Kidney Diseases. Int. J. Mol. Sci. 2019, 21, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grozdanić, M.; Vidmar, R.; Vizovišek, M.; Fonović, M. Degradomics in Biomarker Discovery. Proteom.—Clin. Appl. 2019, 13, 1800138. [Google Scholar] [CrossRef] [PubMed]
- Vizovišek, M.; Vidmar, R.; Fonović, M. FPPS: Fast Profiling of Protease Specificity. Methods Mol. Biol. 2017, 1574, 183–195. [Google Scholar] [PubMed]
- Vidmar, R.; Vizovišek, M.; Turk, D.; Turk, B.; Fonović, M. Protease cleavage site fingerprinting by label-free in-gel degradomics reveals pH-dependent specificity switch of legumain. Embo J. 2017, 36, 2455–2465. [Google Scholar] [CrossRef] [PubMed]
- Sobotič, B.; Vizovišek, M.; Vidmar, R.; Van Damme, P.; Gocheva, V.; Joyce, J.J.; Gevaert, K.; Turk, V.; Turk, B.; Fonović, M. Proteomic Identification of Cysteine Cathepsin Substrates Shed from the Surface of Cancer Cells. Mol. Cell Proteom. 2015, 14, 2213–2228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vilar, R.; Fish, R.R.; Casini, A.; Neerman-Arbez, M. Fibrin(ogen) in human disease: Both friend and foe. Haematologica 2020, 105, 284–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simpson-Haidaris, P.P.; Rybarczyk, B. Tumors and fibrinogen. The role of fibrinogen as an extracellular matrix protein. Ann. N. Y. Acad. Sci. 2001, 936, 406–425. [Google Scholar] [CrossRef] [PubMed]
- Degen, J.J.; Drew, A.A.; Palumbo, J.J.; Kombrinck, K.K.; Bezerra, J.J.; Danton, M.M.; Holmback, K.; Suh, T.T. Genetic manipulation of fibrinogen and fibrinolysis in mice. Ann. N. Y. Acad. Sci. 2001, 936, 276–290. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Topete, D.; Iwaki, T.; Ploplis, V.V.; Castellino, F.F. Delayed inflammatory responses to endotoxin in fibrinogen-deficient mice. J. Pathol. 2006, 210, 325–333. [Google Scholar] [CrossRef]
- Sorensen, I.; Susnik, N.; Inhester, T.; Degen, J.J.; Melk, A.; Haller, H.; Schmitt, R. Fibrinogen, acting as a mitogen for tubulointerstitial fibroblasts, promotes renal fibrosis. Kidney Int. 2011, 80, 1035–1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petzelbauer, P.; Zacharowski, P.P.; Miyazaki, Y.; Friedl, P.; Wickenhauser, G.; Castellino, F.F.; Groger, M.; Wolff, K.; Zacharowski, K. The fibrin-derived peptide Bbeta15-42 protects the myocardium against ische-mia-reperfusion injury. Nat. Med. 2005, 11, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Rybarczyk, B.B.; Simpson-Haidaris, P.P. Fibrinogen assembly, secretion, and deposition into extracellular matrix by MCF-7 human breast carcinoma cells. Cancer Res. 2000, 60, 2033–2039. [Google Scholar]
- Baskin, J.J.; Pui, C.C.; Reiss, U.; Wilimas, J.J.; Metzger, M.M.; Ribeiro, R.R.; Howard, S.S. Management of occlusion and thrombosis associated with long-term indwelling central venous catheters. Lancet 2009, 374, 159–169. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Outes, A.; Terleira-Fernandez, A.A.; Calvo-Rojas, G.; Suarez-Gea, M.M.; Vargas-Castrillon, E. Dabigatran, Rivaroxaban, or Apixaban versus Warfarin in Patients with Nonvalvular Atrial Fibrillation: A Systematic Review and Meta-Analysis of Subgroups. Thrombosis 2013, 2013, 640723. [Google Scholar] [CrossRef]
- Spark Therapeutics, I. Available online: https://sparktx.com/pipelines/spk-8016-hemophilia-a-inhibitor-market/ (accessed on 6 November 2018).
- Pfizer, A. Study In Healthy People of Multiple Doses of UK-396,082 Given By Mouth, to Investigate the Safety, Toleration and Time Course of Blood Concentration of UK-396,082 and Its Effects. 2010. Available online: https://clinicaltrials.gov/ct2/show/NCT01091532 (accessed on 24 March 2010).
- Saeed, S.S. Denning-Kendall, A. McDonald-Gibson, W.J. Collier, H.O.J. Human haptoglobin: An endogenous inhibitor of prostaglandin synthase. In Inflammation: Mechanisms and Treatment; Springer: Dordrecht, The Netherlands, 1980; Volume 4, pp. 285–300. [Google Scholar]
- NCBI HP Haptoglobin [Homo Sapiens (Human)]. Available online: https://www.ncbi.nlm.nih.gov/gene/3240 (accessed on 27 February 2022).
- Cid, M.M.; Grant, D.D.; Hoffman, G.G.; Auerbach, R.; Fauci, A.A.; Kleinman, H.H. Identification of haptoglobin as an angiogenic factor in sera from patients with systemic vasculitis. J. Clin. Investig. 1993, 91, 977–985. [Google Scholar] [CrossRef] [Green Version]
- Quaye, I.I. Haptoglobin, inflammation and disease. Trans. R Soc. Trop. Med. Hyg. 2008, 102, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Snellman, O.; Sylvén, B. Haptoglobin acting as a Natural Inhibitor of Cathepsin B Activity. Nature 1967, 216, 1033. [Google Scholar] [CrossRef] [PubMed]
- Tveitaras, M.M.; Selheim, F.; Sortland, K.; Reed, R.R.; Stuhr, L. Protein expression profiling of plasma and lungs at different stages of metastatic development in a human triple negative breast cancer xenograft model. PLoS ONE 2019, 14, e0215909. [Google Scholar] [CrossRef]
- Trayhurn, P.; Wood, I.I. Adipokines: Inflammation and the pleiotropic role of white adipose tissue. Br. J. Nutr. 2004, 92, 347–355. [Google Scholar] [CrossRef] [Green Version]
- Smeets, M.M.; Fontijn, J.; Kavelaars, A.; Pasterkamp, G.; De Kleijn, D.D. The acute phase protein haptoglobin is locally expressed in arthritic and oncological tissues. Int. J. Exp. Pathol. 2003, 84, 69–74. [Google Scholar] [CrossRef]
- Zager, R.R.; Vijayan, A.; Johnson, A.A. Proximal tubule haptoglobin gene activation is an integral component of the acute kidney injury “stress response”. Am. J. Physiol. Renal. Physiol. 2012, 303, F139–F148. [Google Scholar] [CrossRef] [Green Version]
- Abdullah, M.; Schultz, H.; Kahler, D.; Branscheid, D.; Dalhoff, K.; Zabel, P.; Vollmer, E.; Goldmann, T. Expression of the acute phase protein haptoglobin in human lung cancer and tumor-free lung tissues. Pathol. Res. Pract. 2009, 205, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Marino-Crespo, O.; Cuevas-Alvarez, E.; Harding, A.A.; Murdoch, C.; Fernandez-Briera, A.; Gil-Martin, E. Haptoglobin expression in human colorectal cancer. Histol. Histopathol. 2019, 34, 953–963. [Google Scholar] [PubMed]
- Nabli, H.; Tuller, E.; Sharpe-Timms, K.K. Haptoglobin expression in endometrioid adenocarcinoma of the uterus. Reprod Sci. 2010, 17, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Bax, H.H.; Scotto, D.; Souri, E.E.; Sollie, S.; Harris, R.R.; Hammar, N.; Walldius, G.; Winship, A.; Ghosh, S.; et al. Immune mediator expression signatures are associated with improved outcome in ovarian carcinoma. Oncoimmunology 2019, 8, e1593811. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, U.; Reddy, O.; Mukherjee, G. Elevated serum haptoglobin is associated with clinical outcome in triple-negative breast cancer patients. Asian Pac. J. Cancer Prev. 2012, 13, 4541–4544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huber-Lang, M.; Ekdahl, K.K.; Wiegner, R.; Fromell, K.; Nilsson, B. Auxiliary activation of the complement system and its importance for the pathophysiology of clinical conditions. Semin Immunopathol. 2018, 40, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Tsalamandris, S.; Oikonomou, E.; Latsios, G.; Tousoulis, D. Anti-Inflammatory Treatment. In Coronary Artery Disease from Biology to Clinical Practice; Tousoulis, D., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 237–271. [Google Scholar]
- Heit, B.; Kim, H.; Cosio, G.; Castano, D.; Collins, R.; Lowell, C.C.; Kain, K.K.; Trimble, W.W.; Grinstein, S. Multimolecular signaling complexes enable Syk-mediated signaling of CD36 internalization. Dev. Cell 2013, 24, 372–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vicuna, L.; Strochlic, D.D.; Latremoliere, A.; Bali, K.K.; Simonetti, M.; Husainie, D.; Prokosch, S.; Riva, P.; Griffin, R.R.; Njoo, C.; et al. The serine protease inhibitor SerpinA3N attenuates neuropathic pain by inhibiting T cell-derived leukocyte elastase. Nat. Med. 2015, 21, 518–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, G.G.; Smirnova, I.I.; Akaaboune, M.; Hantai, D.; Festoff, B.B. Serine proteases and their serpin inhibitors in Alzheimer’s disease. Biomed Pharmacother 1994, 48, 296–304. [Google Scholar] [CrossRef]
- Wolf, G.; Kalluri, R.; Ziyadeh, F.F.; Neilson, E.E.; Stahl, R.R. Angiotensin II induces alpha3(IV) collagen expression in cultured murine proximal tubular cells. Proc. Assoc. Am. Physicians 1999, 111, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Vecchi, M.; Confalonieri, S.; Nuciforo, P.; Vigano, M.M.; Capra, M.; Bianchi, M.; Nicosia, D.; Bianchi, F.; Galimberti, V.; Viale, G.; et al. Breast cancer metastases are molecularly distinct from their primary tumors. Oncogene 2008, 27, 2148–2158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chelbi, S.S.; Wilson, M.M.; Veillard, A.A.; Ingles, S.S.; Zhang, J.; Mondon, F.; Gascoin-Lachambre, G.; Doridot, L.; Mignot, T.T.; Rebourcet, R.; et al. Genetic and epigenetic mechanisms collaborate to control SERPINA3 expression and its association with placental diseases. Hum. Mol. Genet 2012, 21, 1968–1978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jinawath, N.; Vasoontara, C.; Jinawath, A.; Fang, X.; Zhao, K.; Yap, K.K.; Guo, T.; Lee, C.C.; Wang, W.; Balgley, B.B.; et al. Oncoproteomic analysis reveals co-upregulation of RELA and STAT5 in carboplatin resistant ovarian carcinoma. PLoS ONE 2010, 5, e11198. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.M.; Rizzo, N.; Liguori, C.; Zucca, G.; Faa, G. Alpha-1-antichymotrypsin immunoreactivity in papillary carcinoma of the thyroid gland. Histopathology 1998, 33, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.Y.; Platt, M.M.; Fu, H.; Gui, Y.; Wang, Y.; Gonzalez-Juarbe, N.; Zhou, D.; Yu, Y. Global Proteome and Phosphoproteome Characterization of Sepsis-induced Kidney Injury. Mol. Cell Proteom. 2020, 19, 2030–2047. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Navarro, A.; Mejia-Vilet, J.J.; Perez-Villalva, R.; Carrillo-Perez, D.D.; Marquina-Castillo, B.; Gamba, G.; Bobadilla, N.N. SerpinA3 in the Early Recognition of Acute Kidney Injury to Chronic Kidney Disease (CKD) transition in the rat and its Potentiality in the Recognition of Patients with CKD. Sci. Rep. 2019, 9, 10350. [Google Scholar] [CrossRef] [PubMed]
- Bergwik, J.; Kristiansson, A.; Welinder, C.; Goransson, O.; Hansson, S.S.; Gram, M.; Erlandsson, L.; Akerstrom, B. Knockout of the radical scavenger alpha1-microglobulin in mice results in defective bikunin synthesis, endoplasmic reticulum stress and increased body weight. Free Radic. Biol. Med. 2021, 162, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Goulding, D.D.; Nikolova, V.V.; Mishra, L.; Zhuo, L.; Kimata, K.; McBride, S.S.; Moy, S.S.; Harry, G.G.; Garantziotis, S. Inter-alpha-inhibitor deficiency in the mouse is associated with alterations in anxiety-like behavior, exploration and social approach. Genes Brain Behav. 2019, 18, e12505. [Google Scholar] [CrossRef] [PubMed]
- Lord, M.M.; Melrose, J.; Day, A.A.; Whitelock, J.J. The Inter-alpha-Trypsin Inhibitor Family: Versatile Molecules in Biology and Pathology. J. Histochem. Cytochem. 2020, 68, 907–927. [Google Scholar] [CrossRef]
- Jiang, X.; Bai, X.X.; Li, B.; Li, Y.; Xia, K.; Wang, M.; Li, S.; Wu, H. Plasma Inter-Alpha-Trypsin Inhibitor Heavy Chains H3 and H4 Serve as Novel Diagnostic Biomarkers in Human Colorectal Cancer. Dis. Markers 2019, 2019, 5069614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- UniProt UniProtKB—Q14624 (ITIH4 HUMAN). Available online: https://www.uniprot.org/uniprot/Q14624 (accessed on 23 February 2022).
- Wessels, M.R.; Butko, P.; Ma, M.; Warren, H.B.; Lage, A.L.; Carroll, M.C. Studies of group B streptococcal infection in mice deficient in complement component C3 or C4 demonstrate an essential role for complement in both innate and acquired immunity. Proc. Natl. Acad. Sci. USA 1995, 92, 11490–11494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schubart, A.; Anderson, K.; Mainolfi, N.; Sellner, H.; Ehara, T.; Adams, C.M.; Mac Sweeney, A.; Liao, S.M.; Crowley, M.; Littlewood-Evans, A.; et al. Small-molecule factor B inhibitor for the treatment of complement-mediated diseases. Proc. Natl. Acad. Sci. USA 2019, 116, 7926–7931. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamar, P. A New Role of Acute Phase Proteins: Local Production Is an Ancient, General Stress-Response System of Mammalian Cells. Int. J. Mol. Sci. 2022, 23, 2972. https://doi.org/10.3390/ijms23062972
Hamar P. A New Role of Acute Phase Proteins: Local Production Is an Ancient, General Stress-Response System of Mammalian Cells. International Journal of Molecular Sciences. 2022; 23(6):2972. https://doi.org/10.3390/ijms23062972
Chicago/Turabian StyleHamar, Péter. 2022. "A New Role of Acute Phase Proteins: Local Production Is an Ancient, General Stress-Response System of Mammalian Cells" International Journal of Molecular Sciences 23, no. 6: 2972. https://doi.org/10.3390/ijms23062972
APA StyleHamar, P. (2022). A New Role of Acute Phase Proteins: Local Production Is an Ancient, General Stress-Response System of Mammalian Cells. International Journal of Molecular Sciences, 23(6), 2972. https://doi.org/10.3390/ijms23062972





