High Doses of Pesticides Induce mtDNA Damage in Intact Mitochondria of Potato In Vitro and Do Not Impact on mtDNA Integrity of Mitochondria of Shoots and Tubers under In Vivo Exposure
Abstract
1. Introduction
2. Results
2.1. Optimization of the Method for Estimating of the mtDNA Damage Amount
2.2. Pesticides Effect on the Amount of mtDNA Damage In Vitro
2.3. Effect of Pesticides on Seed Germination and Potato Growth under Controlled Conditions
2.4. Effect of Pesticides on the Amount of mtDNA Damage in Potato Shoots
2.5. Effect of Pesticides on the Potato Growth and the Weight of the Crop in the Field Experiment
2.6. Effect of Pesticides on the Amount of mtDNA Damage in Potato Tubers
2.7. Effect of Pesticides on Enzyme Activity in Potato Tubers
2.8. Effect of Pesticides on the Rate of H2O2 Production in Potato Tubers
3. Discussion
3.1. Identification of mtDNA Regions That Are More Prone to Oxidative Damage
3.2. Effect of Metribuzin and Imidacloprid on Potato mtDNA In Vitro
3.3. Effect of Metribuzin and Imidacloprid on Potato mtDNA In Vivo
4. Materials and Methods
4.1. Chemicals
4.2. Optimization of the Method for Measuring of the Number of mtDNA Damage
4.3. Designs of Experiments
- Control: seeds were not treated with pesticides.
- Imidacloprid+/−: seeds were treated with insecticide before planting.
- Imidacloprid−/+: seeds were not treated with an insecticide before planting, and treated during the vegetation.
- Imidacloprid+/+: seeds were treated with an insecticide both before planting and during the vegetation.
- Metribuzin: seeds were treated with a metribuzin before planting.
4.4. Mitochondria Isolation
4.5. DNA Isolation
4.6. Measuring of the mtDNA Damage Amount
4.7. Assays of Enzyme Activity
4.8. Assays of H2O2 Rate Production
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Jong, H. Impact of the Potato on Society. Am. J. Potato Res. 2016, 93, 415–429. [Google Scholar] [CrossRef]
- FAOSTAT. [Electronic Resource]. Available online: https://www.fao.org/faostat/ru/#home (accessed on 19 January 2022).
- Warren, G.F. Spectacular Increases in Crop Yields in the United States in the Twentieth Century. Weed Technol. 1998, 12, 752–760. [Google Scholar] [CrossRef]
- R4P Network. Trends and Challenges in Pesticide Resistance Detection. Trends Plant Sci. 2016, 21, 834–853.
- Mahmood, I.; Imadi, S.R.; Shazadi, K.; Gul, A.; Hakeem, K. Effects of Pesticides on Environment. Plant Soil Microbes 2016, 253–269. [Google Scholar]
- Mineau, P.; Whiteside, M. Pesticide acute toxicity is a better correlate of U.S. grassland bird declines than agricultural intensification. PLoS ONE 2013, 8, e57457. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Lim, W.; Song, G. Mediation of oxidative stress toxicity induced by pyrethroid pesticides in fish. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2020, 234, 108758. [Google Scholar] [CrossRef] [PubMed]
- Martyniuk, C.J.; Mehinto, A.C.; Denslow, N.D. Organochlorine pesticides: Agrochemicals with potent endocrine-disrupting properties in fish. Mol. Cell. Endocrinol. 2020, 507, 110764. [Google Scholar] [CrossRef] [PubMed]
- Syromyatnikov, M.; Gureev, A.; Mikhaylov, E.; Parshin, P.; Popov, V. Pesticides effect on the level of mtDNA damage in bublebees heads (Bombus terrestris L.). Periódico Tchê Química 2020, 17, 394–402. [Google Scholar] [CrossRef]
- Syromyatnikov, M.Y.; Gureev, A.P.; Starkova, N.N.; Savinkova, O.V.; Starkov, A.A.; Lopatin, A.V.; Popov, V.N. Method for detection of mtDNA damages for evaluating of pesticides toxicity for bumblebees (Bombus terrestris L.). Pestic. Biochem. Physiol. 2020, 169, 104675. [Google Scholar] [CrossRef] [PubMed]
- Hahn, M.; Geisthardt, M.; Brühl, C.A. Effects of herbicide-treated host plants on the development of Mamestra brassicae L. caterpillars. Environ. Toxicol. Chem. 2014, 33, 2633–2638. [Google Scholar] [CrossRef] [PubMed]
- Bohnenblust, E.W.; Vaudo, A.D.; Egan, J.F.; Mortensen, D.A.; Tooker, J.F. Effects of the herbicide dicamba on nontarget plants and pollinator visitation. Environ. Toxicol. Chem. 2016, 35, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Kabir, E.; Jahan, S.A. Exposure to pesticides and the associated human health effects. Sci. Total Environ. 2017, 575, 525–535. [Google Scholar] [CrossRef]
- Jaga, K.; Dharmani, C. The epidemiology of pesticide exposure and cancer: A review. Rev. Environ. Health 2005, 20, 15–38. [Google Scholar] [CrossRef] [PubMed]
- Aloizou, A.M.; Siokas, V.; Sapouni, E.M.; Sita, N.; Liampas, I.; Brotis, A.G.; Rakitskii, V.N.; Burykina, T.I.; Aschner, M.; Bogdanos, D.P.; et al. Parkinson’s disease and pesticides: Are microRNAs the missing link? Sci. Total Environ. 2020, 744, 140591. [Google Scholar] [CrossRef] [PubMed]
- Sherer, T.B.; Richardson, J.R.; Testa, C.M.; Seo, B.B.; Panov, A.V.; Yagi, T.; Matsuno-Yagi, A.; Miller, G.W.; Greenamyre, J.T. Mechanism of toxicity of pesticides acting at complex I: Relevance to environmental etiologies of Parkinson’s disease. J. Neurochem. 2007, 100, 1469–1479. [Google Scholar] [CrossRef] [PubMed]
- Lümmen, P. Mitochondrial Electron Transport Complexes as Biochemical Target Sites for Insecticides and Acaricids. In Insecticides Design Using Advanced Technologies; Springer: Berlin/Heidelberg, Germany, 2007; pp. 197–215. [Google Scholar]
- Song, C.; Scharf, M.E. Mitochondrial impacts of insecticidal formate esters in insecticide-resistant and insecticide-susceptible Drosophila melanogaster. Pest Manag. Sci. 2009, 65, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Suvetha, L.; Ramesh, M.; Saravanan, M. Influence of cypermethrin toxicity on ionic regulation and gill Na+/K+-ATPase activity of a freshwater teleost fish Cyprinus carpio. Environ. Toxicol. Pharmacol. 2010, 29, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Burchfield, S.L.; Bailey, D.C.; Todt, C.E.; Denney, R.D.; Negga, R.; Fitsanakis, V.A. Acute exposure to a glyphosate-containing herbicide formulation inhibits Complex II and increases hydrogen peroxide in the model organism Caenorhabditis elegans. Environ. Toxicol. Pharmacol. 2019, 66, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Reid, R.C.; Minteer, S.D. A Paper-based Mitochondrial Electrochemical Biosensor for Pesticide Detection. Electroanalysis 2015, 28, 854–859. [Google Scholar] [CrossRef]
- Li, Q.; Kobayashi, M.; Kawada, T. Carbamate pesticide-induced apoptosis in human T lymphocytes. Int. J. Environ. Res. Public Health 2015, 12, 3633–3645. [Google Scholar] [CrossRef]
- Abendroth, J.; Martin, A.; Roeth, F. Plant Response to Combinations of Mesotrione and Photosystem II Inhibitors. Weed Technol. 2017, 20, 267–274. [Google Scholar] [CrossRef]
- Zong, X.; Zhang, J.; Zhu, J.; Zhang, L.; Jiang, L.; Yin, Y.; Guo, H. Effects of polysterene microplastic on uptake and toxicity of copper and cadmium in hydroponic wheat seedlings (Triticum aestivum L.). Ecotoxicol. Environ. Saf. 2021, 217, 112217. [Google Scholar] [CrossRef]
- Jabłońska-Trypuć, A. Pesticides as Inducers of Oxidative Stress. React. Oxyg. Species 2017, 854–859. [Google Scholar] [CrossRef]
- Wang, C.Y.; Zhao, Z.B. Somatic mtDNA mutations in lung tissues of pesticide-exposed fruit growers. Toxicology 2012, 291, 51–55. [Google Scholar] [CrossRef]
- Schertl, P.; Braun, H.P. Respiratory electron transfer pathways in plant mitochondria. Front. Plant Sci. 2014, 5, 163. [Google Scholar] [CrossRef]
- Braun, H.P. The Oxidative Phosphorylation system of the mitochondria in plants. Mitochondrion 2020, 53, 66–75. [Google Scholar] [CrossRef]
- Formaggioni, A.; Luchetti, A.; Plazzi, F. Mitochondrial Genomic Landscape: A Portrait of the Mitochondrial Genome 40 Years after the First Complete Sequence. Life 2021, 11, 663. [Google Scholar] [CrossRef]
- Varré, J.S.; D’Agostino, N.; Touzet, P.; Gallina, S.; Tamburino, R.; Cantarella, C.; Ubrig, E.; Cardi, T.; Drouard, L.; Gualberto, J.M.; et al. Complete Sequence, Multichromosomal Architecture and Transcriptome Analysis of the Solanum tuberosum Mitochondrial Genome. Int. J. Mol. Sci. 2019, 20, 4788. [Google Scholar] [CrossRef]
- Vitkalova, I.Y.; Gureev, A.P.; Shaforostova, E.A.; Boyko, O.N.; Igamberdiev, A.U.; Popov, V.N. The effect of pesticides on the mtDNA integrity and bioenergetic properties of potato mitochondria. Pestic. Biochem. Physiol. 2021, 172, 104764. [Google Scholar] [CrossRef]
- Kumar, A.; Verma, A. Accidental human poisoning with a neonicotinoid insecticide, imidacloprid: A rare case report from rural India with a brief review of literature. Egypt. J. Forensic Sci. 2013, 3, 123–126. [Google Scholar] [CrossRef]
- Pesticides.ru [Electronic Resource]. Available online: https://www.pesticidy.ru/active_substance/imidacloprid (accessed on 19 January 2022).
- Saleh, S.M.; Alminderej, F.M.; Ali, R.; Abdallah, O.I. Optical sensor film for metribuzin pesticide detection. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 229, 117971. [Google Scholar] [CrossRef] [PubMed]
- Pesticides.ru [Electronic Resource]. Available online: https://www.pesticidy.ru/active_substance/metribuzin (accessed on 19 January 2022).
- Gureev, A.P.; Sitnikov, V.V.; Pogorelov, D.I.; Vitkalova, I.Y.; Igamberdiev, A.U.; Popov, V.N. The effect of pesticides on the NADH supported mitochondrial respiration of permeabilized potato mitochondria. Pest Biochem. Phys. 2022, 181, 105056. [Google Scholar] [CrossRef]
- Yakes, F.M.; Van Houten, B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc. Natl. Acad. Sci. USA 1997, 94, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Rong, Z.; Tu, P.; Xu, P.; Sun, Y.; Yu, F.; Tu, N. The Mitochondrial Response to DNA Damage. Front. Cell Dev. Biol. 2021, 9, 669379. [Google Scholar] [CrossRef] [PubMed]
- Pickrell, A.M.; Pinto, M.; Hida, A.; Moraes, C.T. Striatal dysfunctions associated with mitochondrial DNA damage in dopaminergic neurons in a mouse model of Parkinson’s disease. J. Neurosci. 2011, 31, 17649–17658. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.; Pickrell, A.M.; Fukui, H.; Moraes, C.T. Mitochondrial DNA damage in a mouse model of Alzheimer’s disease decreases amyloid beta plaque formation. Neurobiol. Aging. 2013, 34, 2399–2407. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.; Calvert, P.A.; Mercer, J.R.; Harrison, J.; Baker, L.; Figg, N.L.; Kumar, S.; Wang, J.C.; Hurst, L.A.; Obaid, D.R.; et al. Mitochondrial DNA damage can promote atherosclerosis independently of reactive oxygen species through effects on smooth muscle cells and monocytes and correlates with higher-risk plaques in humans. Circulation 2013, 128, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Pachouri, U.C.; Khaidem, D.C.; Kundu, A.; Chopra, C.; Singh, P. Mitochondrial DNA Damage and Diseases. F1000Research 2015, 4, 176. [Google Scholar] [CrossRef] [PubMed]
- Sanders, L.H.; Howlett, E.H.; McCoy, J.; Greenamyre, J.T. Mitochondrial DNA damage as a peripheral biomarker for mitochondrial toxin exposure in rats. Toxicol. Sci. 2014, 142, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Furda, A.M.; Bess, A.S.; Meyer, J.N.; Van Houten, B. Analysis of DNA damage and repair in nuclear and mitochondrial DNA of animal cells using quantitative PCR. Methods Mol. Biol. 2012, 920, 111–132. [Google Scholar] [PubMed]
- Gureev, A.P.; Shaforostova, E.A.; Starkov, A.A.; Popov, V.N. Simplified qPCR method for detecting excessive mtDNA damage induced by exogenous factors. Toxicology 2017, 382, 67–74. [Google Scholar] [CrossRef]
- Mambo, E.; Gao, X.; Cohen, Y.; Guo, Z.; Talalay, P.; Sidransky, D. Electrophile and oxidant damage of mitochondrial DNA leading to rapid evolution of homoplasmic mutations. Proc. Natl. Acad. Sci. USA 2003, 100, 1838–1843. [Google Scholar] [CrossRef]
- Rothfuss, O.; Gasser, T.; Patenge, N. Analysis of differential DNA damage in the mitochondrial genome employing a semi-long run real-time PCR approach. Nucleic Acids Res. 2010, 38, e24. [Google Scholar] [CrossRef]
- Morley, S.A.; Ahmad, N.; Nielsen, B.L. Plant Organelle Genome Replication. Plants 2019, 8, 358. [Google Scholar] [CrossRef] [PubMed]
- Henle, E.S.; Han, Z.; Tang, N.; Rai, P.; Luo, Y.; Linn, S. Sequence-specific DNA cleavage by Fe2+-mediated fenton reactions has possible biological implications. J. Biol. Chem. 1999, 274, 962–971. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.; Cole, T.D.; Wemmer, D.E.; Linn, S. Localization of Fe2+ at an RTGR sequence within a DNA duplex explains preferential cleavage by Fe2+ and H2O2. J. Mol. Biol. 2001, 312, 1089–1101. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, Y.; Lim, S.; Jo, K. Single-molecule visualization of ROS-induced DNA damage in large DNA molecules. Analyst 2016, 141, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Gureev, A.P.; Shaforostova, E.A.; Laver, D.A.; Khorolskaya, V.G.; Syromyatnikov, M.Y.; Popov, V.N. Methylene blue elicits non-genotoxic H2O2 production and protects brain mitochondria from rotenone toxicity. J. Appl. Biomed. 2019, 17, 107–114. [Google Scholar] [CrossRef] [PubMed]
- El-Maarouf-Bouteau, H.; Mazuy, C.; Corbineau, F.; Bailly, C. DNA alteration and programmed cell death during ageing of sunflower seed. J. Exp. Bot. 2011, 62, 5003–5011. [Google Scholar] [CrossRef]
- Michelini, L.G.; Benevento, C.E.; Rossato, F.A.; Siqueira-Santos, E.S.; Castilho, R.F. Effects of partial inhibition of respiratory complex I on H2O2 production by isolated brain mitochondria in different respiratory states. Neurochem. Res. 2014, 39, 2419–2430. [Google Scholar] [CrossRef]
- Boerth, D.W.; Eder, E.; Stanks, J.R.; Wanek, P.; Wacker, M.; Gaulitz, S.; Skypeck, D.; Pandolfo, D.; Yashin, M. DNA adducts as biomarkers for oxidative and genotoxic stress from pesticides in crop plants. J. Agric. Food Chem. 2008, 56, 6751–6760. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.M.; Reinke, R.F.; Coombes, N.E.; Helliwell, S.; Mo, J. Influence of imidacloprid seed treatments on rice germination and early seedling growth. Pest Manag. Sci. 2008, 64, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Popov, V.N.; Simonian, R.A.; Skulachev, V.P.; Starkov, A.A. Inhibition of the alternative oxidase stimulates H2O2 production in plant mitochondria. FEBS Lett. 1997, 415, 87–90. [Google Scholar] [CrossRef]
- Vanlerberghe, G.C. Alternative oxidase: A mitochondrial respiratory pathway to maintain metabolic and signaling homeostasis during abiotic and biotic stress in plants. Int. J. Mol. Sci. 2013, 14, 6805–6847. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kumar, V.; Shahzad, B.; Ramakrishnan, M.; Singh Sidhu, G.P.; Bali, A.S.; Handa, N.; Kapoor, D.; Yadav, P.; Khanna, K.; et al. Photosynthetic Response of Plants Under Different Abiotic Stresses: A Review. J. Plant Growth Regul. 2019, 39, 509–531. [Google Scholar] [CrossRef]
- Bocharov, S.S.; Fomin, N.A. The influence of humic preparations on the adaptation of potatoes to pesticide load. Niva Volga Reg. 2013, 4, 1–8. (In Russian) [Google Scholar]
- Shakir, S.K.; Kanwal, M.; Murad, W.; ur Rehman, Z.; ur Rehman, S.; Daud, M.K.; Azizullah, A. Effect of some commonly used pesticides on seed germination, biomass production and photosynthetic pigments in tomato (Lycopersicon esculentum). Ecotoxicology 2016, 25, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.E.; Kanan, H.O.; Inanaga, S.; Ma, Y.Q.; Sugimoto, Y. Impact of pesticide seed treatments on aphid control and yield of wheat in the Sudan. Crop Prot. 2001, 20, 929–934. [Google Scholar] [CrossRef]
- Kesseler, A.; Diolez, P.; Brinkmann, K.; Brand, M.D. Characterisation of the control of respiration in potato tuber mitochondria using the top-down approach of metabolic control analysis. Eur. J. Biochem. 1992, 210, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, H.A.; Borges, R.; Centeno, D.C. Activity of alternative oxidase and plant uncoupling mitochondrial protein in potato tubers stored at low temperature or submitted to artificial aging. Braz. J. Plant Physiol. 2004, 16, 69–76. [Google Scholar] [CrossRef][Green Version]
- Bizerra, P.F.V.; Guimarães, A.R.J.S.; Maioli, M.A.; Mingatto, F.E. Imidacloprid affects rat liver mitochondrial bioenergetics by inhibiting FoF1-ATP synthase activity. J. Toxicol. Environ. Health Part A 2018, 81, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Rego, A.C.; Vesce, S.; Nicholls, D.G. The mechanism of mitochondrial membrane potential retention following release of cytochrome c in apoptotic GT1-7 neural cells. Cell Death Differ. 2001, 8, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Korshunov, S.S.; Skulachev, V.P.; Starkov, A.A. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 1997, 416, 15–18. [Google Scholar] [CrossRef]
- Moeder, W.; Del Pozo, O.; Navarre, D.A.; Martin, G.B.; Klessig, D.F. Aconitase plays a role in regulating resistance to oxidative stress and cell death in Arabidopsis and Nicotiana benthamiana. Plant Mol. Biol. 2007, 63, 273–287. [Google Scholar] [CrossRef] [PubMed]
- Munkvold, G.P.; McGee, D.C.; Iles, A. Effects of imidacloprid seed treatment of corn on foliar feeding and Erwinia stewartii transmission by the corn flea beetle. Plant Dis. 1996, 80, 749–774. [Google Scholar] [CrossRef]
- Sloderbeck, P.E.; Witt, M.D.; Buschman, L.L. Effects of imidacloprid seed treatment on green bug (Homoptera: Aphididae) infestations on three sorghum hybrids. Southwest. Entomol. 1996, 21, 181–187. [Google Scholar]
- Vicente, J.A.; Peixoto, F.; Lopes, M.L.; Madeira, V.M. Differential sensitivities of plant and animal mitochondria to the herbicide paraquat. J. Biochem. Mol. Toxicol. 2001, 15, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Yang, H. Metabolism and detoxification of pesticides in plants. Sci. Total Environ. 2021, 790, 148034. [Google Scholar] [CrossRef] [PubMed]
- Eprintsev, A.T.; Fedorin, D.N.; Cherkasskikh, M.V.; Igamberdiev, A.U. Effect of Salt Stress on the Expression and Promoter Methylation of the Genes Encoding the Mitochondrial and Cytosolic Forms of Aconitase and Fumarase in Maize. Int. J. Mol. Sci. 2021, 22, 6012. [Google Scholar] [CrossRef] [PubMed]
- Popov, V.N.; Eprintsev, A.T.; Fedorin, D.N.; Igamberdiev, A.U. Succinate dehydrogenase in Arabidopsis thaliana is regulated by light via phytochrome A. FEBS Lett. 2010, 584, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Lindvall, O.; Barry, D.I.; Kikvadze, I.; Brundin, P.; Bolwig, T.G.; Björklund, A. Intracerebral grafting of fetal noradrenergic locus coeruleus neurons: Evidence for seizure suppression in the kindling model of epilepsy. Prog. Brain Res. 1988, 78, 79–86. [Google Scholar] [PubMed]
- Fenske, R.A.; Day, E.W., Jr. Assessment of Exposure for Pesticide Handlers in Agricultural, Residential and Institutional Environments; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2005. [Google Scholar]
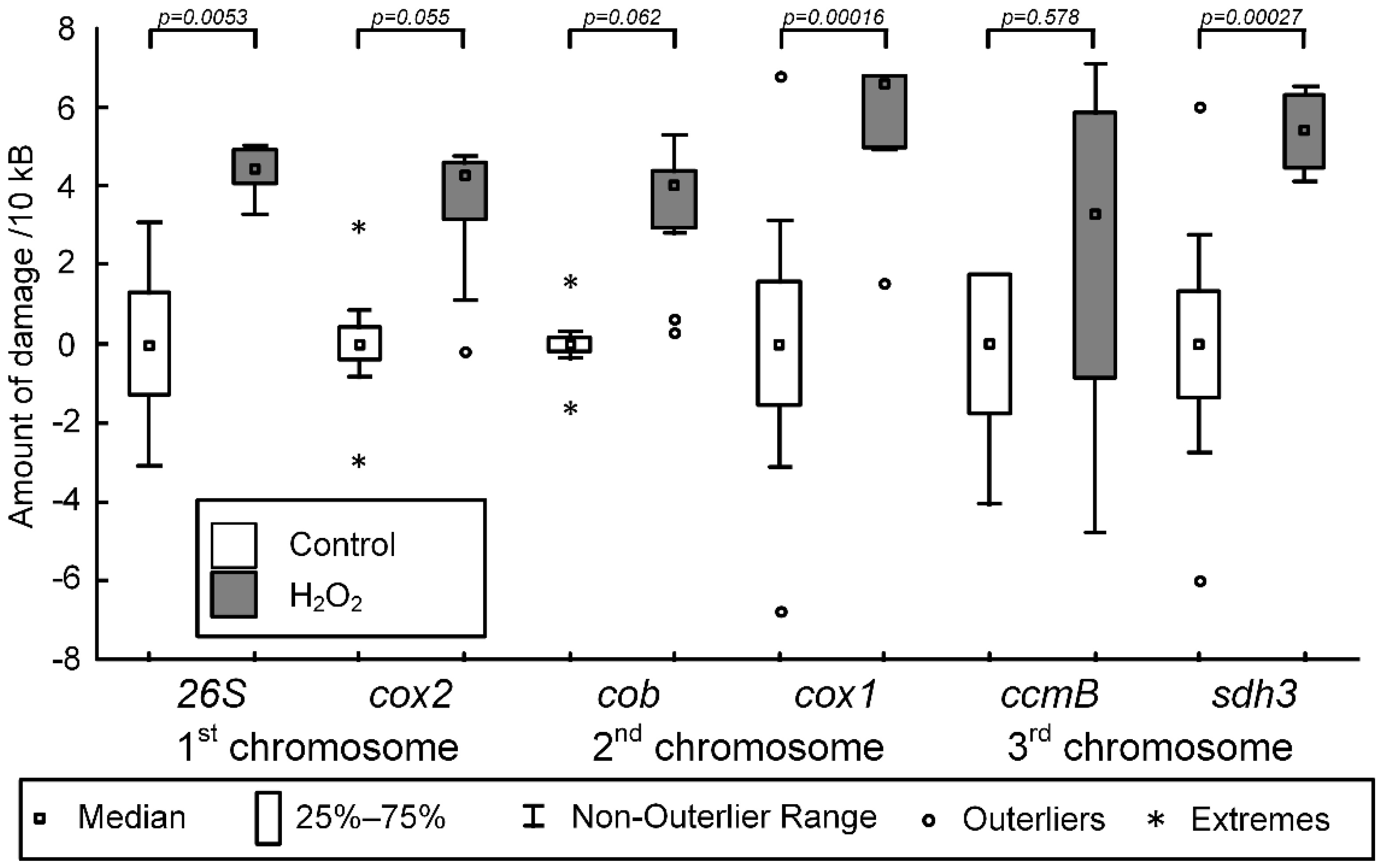
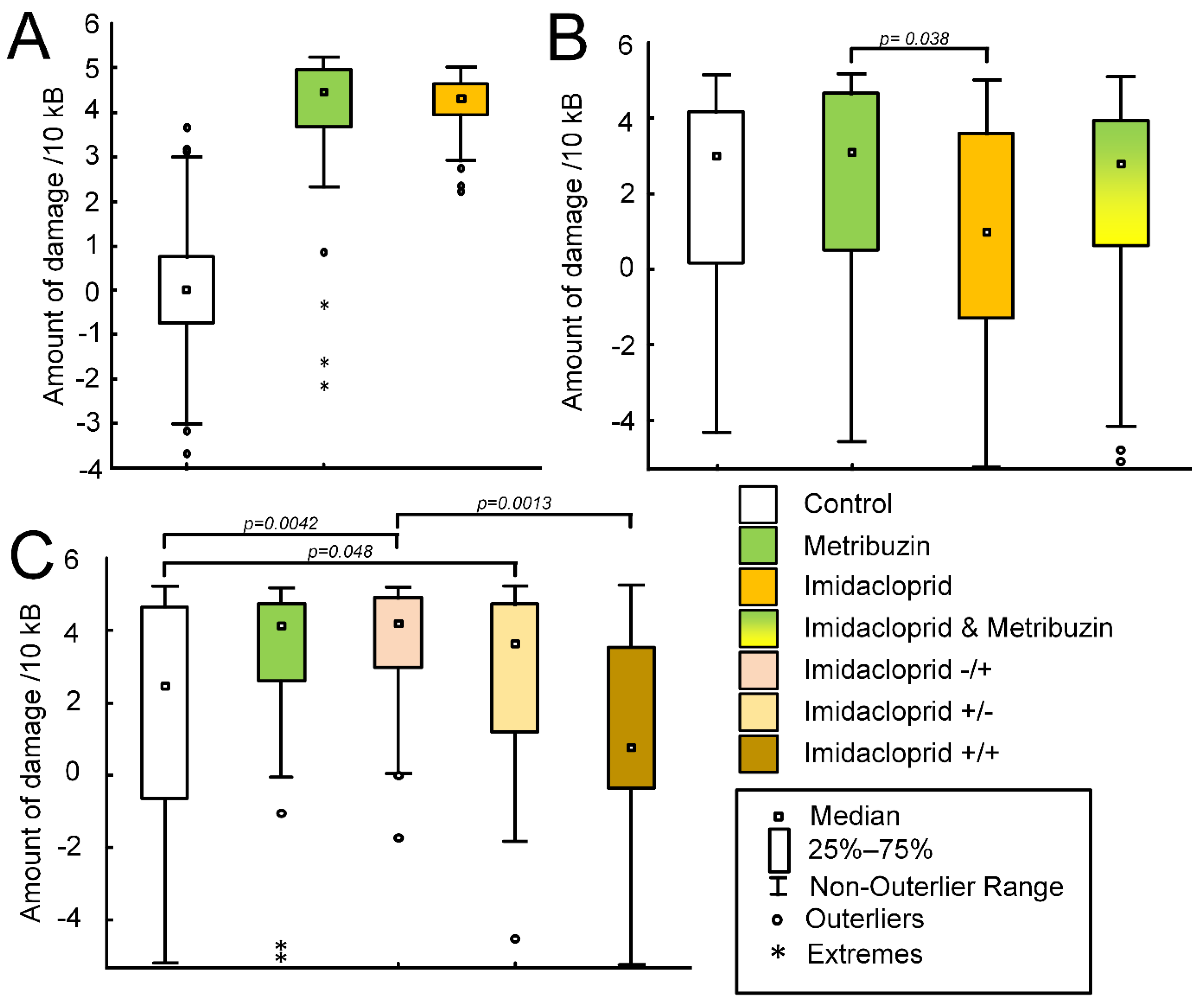

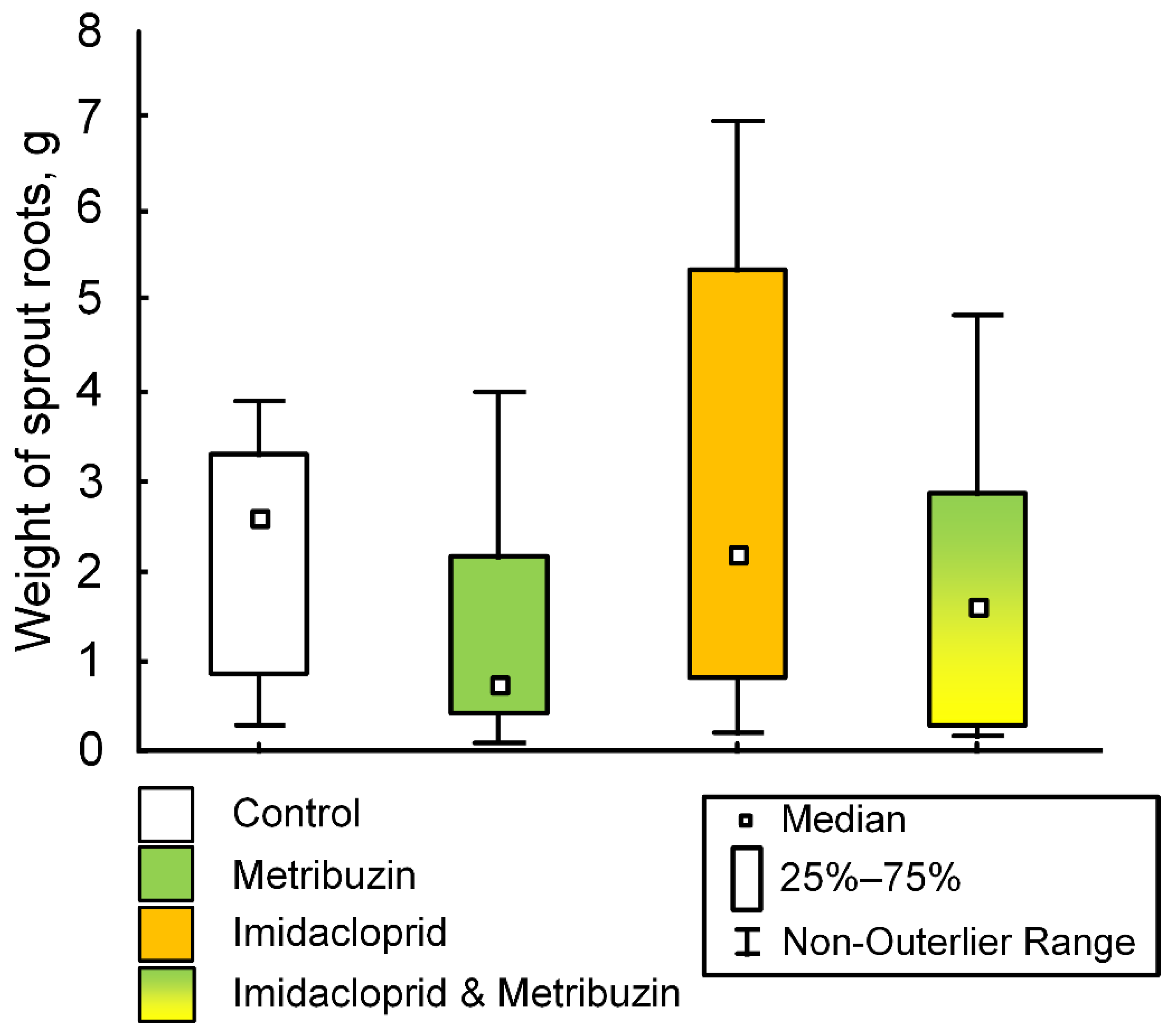


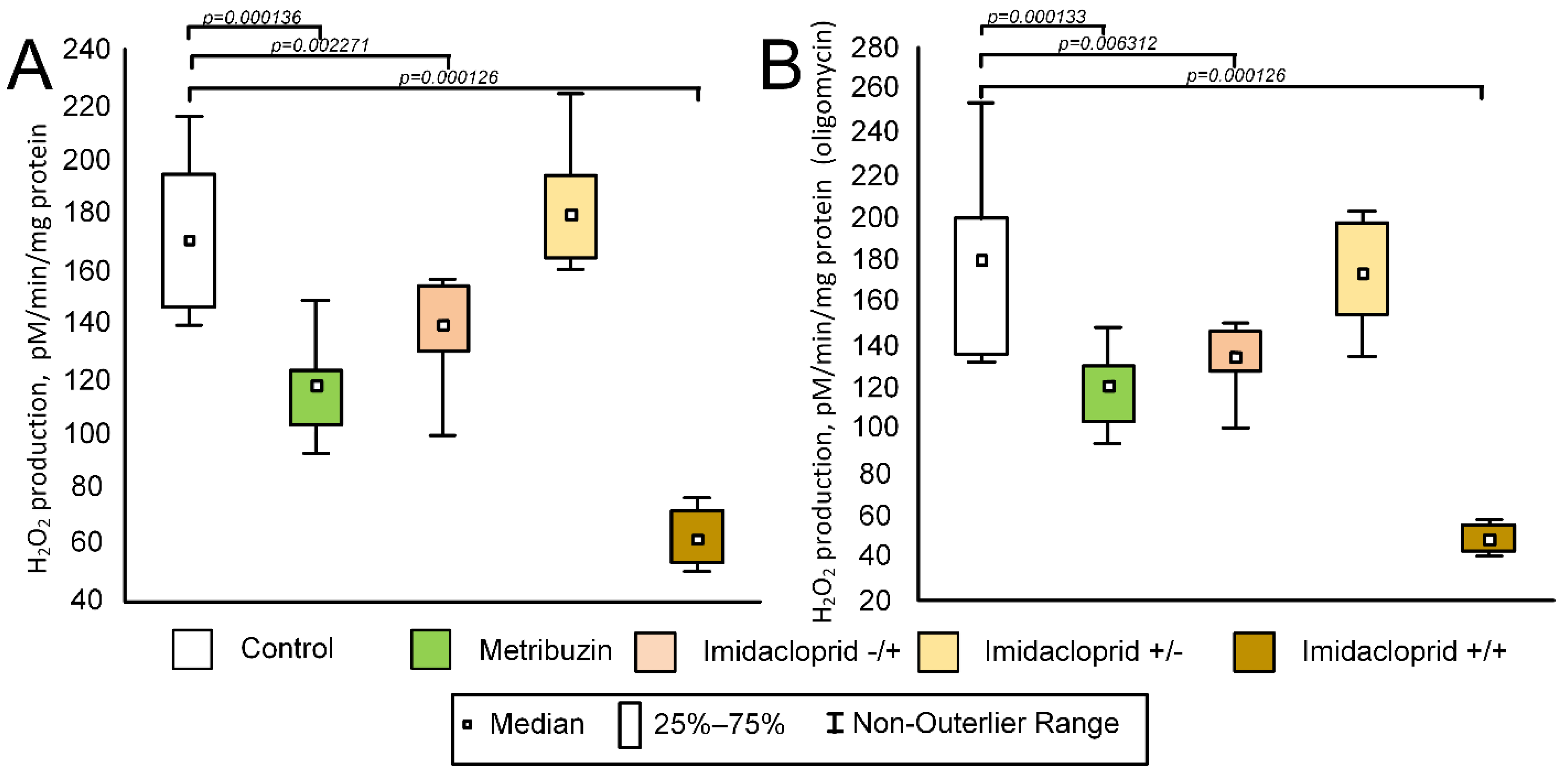

| mtDNA Fragment | Control | Metribuzin | Imidacloprid |
|---|---|---|---|
| 26s | 0.00 (−1.57; 1.57) | 4.35 (3.56; 4.48) 11 | 4.26 (3.95; 4.59) 22 |
| F (2,17) = 20.6552, p = 0.00003 | |||
| Cox2 | 0.00 (−1.59; 1.59) | 4.58 (3.58; 4.77) 1 | 4.56 (4.14; 4.70) 22 |
| F (2,17) = 10.1543, p = 0.0013 | |||
| Cob | 0.00 (−1.83; 1.83) | 4.01 (2.65; 4.28) | 4.39 (4.15; 4.58) 22 |
| F (2,17) = 6.1623, p = 0.0097 | |||
| Cox1 | 0.00 (−0.75; 0.75) | 4.8 (4.28; 5.03) 111 | 4.55 (4.03; 4.78) 22 |
| F (2,17) = 39.88, p = 0.00001 | |||
| ccmB | 0.00 (−1.5;1.5) | 4.72 (3.89; 5.23) 111 | 4.22 (3.63; 4.38) 22 |
| F (2,17) = 17.0728, p = 0.00009 | |||
| Sdh3 | 0.00 (−1.11:1.11) | 4.96 (3.84; 5.10) 1 | 4.24 (3.80; 4.51) 22 |
| F (2,17) = 6.5538, p = 0.0078 | |||
| mtDNA Fragment | Control | Metribuzin | Imidacloprid | Metribuzin–Imidacloprid |
|---|---|---|---|---|
| 26s | 4.34 (2.04; 4.81) | 1.64 (0.11; 2.71) 111 | 1.21 (−1.31; 3.22) 22 | 1.96 (−0.88; 2.99) 333 |
| F (3,116) = 3.99, p = 0.0095 | ||||
| cox2 | 3.42 (1.71; 4.21) | 2.86 (0.92; 3.62) | 3.28 (2.28; 3.87) | 2.69 (0.40; 3.62) |
| F (3,120) = 1.108, p = 0.35 | ||||
| cob | 2.025 (−0.67; 3.66) | 4.43 (3.29; 4.73) | 1.53 (−0.99; 3.59) 44 | 2.75 (−0.42; 4.18) |
| F (3,116) = 3.99, p = 0.0095 | ||||
| cox1 | −1.49 (−4.10; 3.56) | 1.40 (−3.80; 4.8) | −2.28 (−4.35; 4.85) | 3.76 (−2.81; 4.76) |
| F (3,108) = 1.232, p = 0.3 | ||||
| ccmB | 3.45 (−1.22; 4.49) | 4.2 (1.43; 4.79) | −5.11 (−5.23; 3.95) 22444555 | 4.43 (1.09; 4.83) 666 |
| F (3,104) = 7.2946, p = 0.0002 | ||||
| sdh3 | 4.80 (2.48; 4.97) | 4.76 (4.60; 5.10) 1 | 4.49 (2.59; 4.80) | 4.65 (0.84; 4.89) 5 |
| F (3,108) = 3.2145, p = 0.026 | ||||
| mtDNA Fragment | Control | Metribuzine | Imidacloprid−/+ | Imidacloprid+/− | Imidacloprid+/+ |
|---|---|---|---|---|---|
| 26s | 3.26 (−4.89; 5.00) | 4.43 (2.88; 5.00) | 4.16 (3.68; 4.45) | 1.54 (−0.92; 3.00) 888 | 1.31 (−1.17; 3.18) 99 |
| F (4,123) = 6.22, p = 0.0001 | |||||
| cox2 | 3.59 (−4.67; 4.73) | 4.70 (4.00; 4.79) | 4.29 (3.52; 4.77) | 3.10 (0.00; 4.69) | 3.74 (−0.12; 4.72) |
| F (4,123) = 2.37, p = 0.056 | |||||
| cob | 4.72 (4.68; 4.73) | 4.70 (−4.70; 4.73) | 2.56 (1.25; 4.66) | 4.13 (3.17; 4.73) | 4.73 (4.68; 4.74) 7 |
| F (4,91) = 4.58, p = 0.0021 | |||||
| cox1 | 1.69 (−4.78; 4.31) | 2.77 (−5.03; 4.92) | 4.93 (3.73; 5.01) 22 | 4.90 (4.50; 5.00) 33 | −0.33 (−3.25; 2.79) |
| F (4,115) = 8.77, p = 0.00001 | |||||
| ccmB | 4.61 (−3.62; 5.17) | 4.60 (3.15; 5.07) | 4.82 (3.44; 5.10) | 4.71 (1.72; 5.13) | 0.00 (−4.78; 4.45) 777999*** |
| F (4,123) = 7.10, p = 0.00004 | |||||
| sdh3 | 4.35 (−4.45; 4.93) | 4.05 (3.40; 4.40) | 3.77 (2.25; 4.56) | 4.71 (1.72; 5.13) | −3.84 (−5.07; 1.99) 4777999*** |
| F (4,23) = 11.89, p = 0.00001 | |||||
| Chromosome | Gene | Sequence(5′-3′) | PCR-Product | Fragment Length (bp) | Percent of Gene Coverage |
|---|---|---|---|---|---|
| 1 | 26s Forward | CATCCGCCCCAGATAAACTA | |||
| 26s Reverse Long | GTACCGTGAGGGAAAGGTGA | 33993-35978 | 1986 | 57 | |
| 26s Reverse Short | ATAGGTGGGAGGTGGTGACA | 33993-34126 | 134 | 4 | |
| cox2 Forward | CGTACAGGGAAGGTGGAAGA | ||||
| cox2 Reverse Long | TTTAAACGACCCGGTACAGC | 212390-214469 | 2080 | 96 | |
| cox2 Reverse Short | CACAAGGAGCAATTGTGAGG | 212390-212510 | 121 | 5 | |
| 2 | cob Forward | TTGACATCCGATCCAACCTA | |||
| cob Reverse Long | AATGTAACTCCCGAAGGA | 33662-35769 | 2108 | 88 | |
| cob Reverse Short | CCATGCCATTCTTCGTAGTA | 33662-33854 | 193 | 16 | |
| cox1 Forward | ATAATCTGGAATGCGACGTG | ||||
| cox1 Reverse Long | TAAACCCAATGGGAACCAAA | 76578-78565 | 1988 | 83 | |
| cox1 Reverse Short | GGACATACCCTGAAACTTTA | 76578-76699 | 122 | 8 | |
| 3 | ccmB Forward | AACGCCCTTAATGCTAGGTT | |||
| ccmB Reverse Long | CCCCCTCCCGCTATTACTAC | 10037-11945 | 1909 | 85 | |
| ccmB Reverse Short | TTTGGCAAGCAATAAGCACT | 10037-10201 | 165 | 27 | |
| sdh3 Forward | CCCTATCTCCTCATCTTCCT | ||||
| sdh3 Reverse Long | GTGGCTCGTCCGTGATAACT | 35468-37421 | 1936 | 74 | |
| sdh3 Reverse Short | AGGTGAAGCAAATCAAACCT | 35468-35634 | 149 | 46 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alimova, A.A.; Sitnikov, V.V.; Pogorelov, D.I.; Boyko, O.N.; Vitkalova, I.Y.; Gureev, A.P.; Popov, V.N. High Doses of Pesticides Induce mtDNA Damage in Intact Mitochondria of Potato In Vitro and Do Not Impact on mtDNA Integrity of Mitochondria of Shoots and Tubers under In Vivo Exposure. Int. J. Mol. Sci. 2022, 23, 2970. https://doi.org/10.3390/ijms23062970
Alimova AA, Sitnikov VV, Pogorelov DI, Boyko ON, Vitkalova IY, Gureev AP, Popov VN. High Doses of Pesticides Induce mtDNA Damage in Intact Mitochondria of Potato In Vitro and Do Not Impact on mtDNA Integrity of Mitochondria of Shoots and Tubers under In Vivo Exposure. International Journal of Molecular Sciences. 2022; 23(6):2970. https://doi.org/10.3390/ijms23062970
Chicago/Turabian StyleAlimova, Alina A., Vadim V. Sitnikov, Daniil I. Pogorelov, Olga N. Boyko, Inna Y. Vitkalova, Artem P. Gureev, and Vasily N. Popov. 2022. "High Doses of Pesticides Induce mtDNA Damage in Intact Mitochondria of Potato In Vitro and Do Not Impact on mtDNA Integrity of Mitochondria of Shoots and Tubers under In Vivo Exposure" International Journal of Molecular Sciences 23, no. 6: 2970. https://doi.org/10.3390/ijms23062970
APA StyleAlimova, A. A., Sitnikov, V. V., Pogorelov, D. I., Boyko, O. N., Vitkalova, I. Y., Gureev, A. P., & Popov, V. N. (2022). High Doses of Pesticides Induce mtDNA Damage in Intact Mitochondria of Potato In Vitro and Do Not Impact on mtDNA Integrity of Mitochondria of Shoots and Tubers under In Vivo Exposure. International Journal of Molecular Sciences, 23(6), 2970. https://doi.org/10.3390/ijms23062970







