Protective Effects of Melatonin and Misoprostol against Experimentally Induced Increases in Intestinal Permeability in Rats
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Chemicals and Drugs
4.2. Study Formulations
4.3. Animals, Anesthesia, and Surgery
4.4. Perfusion Study
4.5. Determination of Blood-to-Lumen Jejunal Mucosal 51Cr-EDTA Clearance
4.6. Histology
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schoultz, I.; Keita, Å.V. The Intestinal Barrier and Current Techniques for the Assessment of Gut Permeability. Cells 2020, 9, 1909. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Leblond, C.P. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine I. Columnar cell. Am. J. Anat. 1974, 141, 461–479. [Google Scholar] [CrossRef] [PubMed]
- Marchiando, A.M.; Shen, L.; Graham, W.; Edelblum, K.L.; Duckworth, C.; Guan, Y.; Montrose, M.H.; Turner, J.R.; Watson, A.J. The Epithelial Barrier Is Maintained by In Vivo Tight Junction Expansion during Pathologic Intestinal Epithelial Shedding. Gastroenterology 2011, 140, 1208–1218.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Itallie, C.M.; Anderson, J.M. Claudins and Epithelial Paracellular Transport. Annu. Rev. Physiol. 2006, 68, 403–429. [Google Scholar] [CrossRef]
- Dey, I.; Bradbury, N.A. Chapter Ten—Physiology of the Gut: Experimental Models for Investigating Intestinal Fluid and Electrolyte Transport. In Current Topics in Membranes; Levitane, I., Delpire, E., Rasgado-Flores, H., Eds.; Cell Volume Regulation; Academic Press: Cambridge, MA, USA, 2018; Volume 81, pp. 337–381. [Google Scholar]
- Buckley, A.; Turner, J.R. Cell Biology of Tight Junction Barrier Regulation and Mucosal Disease. Cold Spring Harb. Perspect. Biol. 2018, 10, a029314. [Google Scholar] [CrossRef]
- Camilleri, M. Leaky gut: Mechanisms, measurement and clinical implications in humans. Gut 2019, 68, 1516–1526. [Google Scholar] [CrossRef]
- Camilleri, M.; Gorman, H. Intestinal permeability and irritable bowel syndrome. Neurogastroenterol. Motil. 2007, 19, 545–552. [Google Scholar] [CrossRef]
- Sangild, P.T.; Shen, R.L.; Pontoppidan, P.E.L.; Rathe, M. Animal models of chemotherapy-induced mucositis: Translational relevance and challenges. Am. J. Physiol. Liver Physiol. 2018, 314, G231–G246. [Google Scholar] [CrossRef]
- Odenwald, M.A.; Turner, M.A.O.J.R. The intestinal epithelial barrier: A therapeutic target? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 9–21. [Google Scholar] [CrossRef]
- Bubenik, G.A. Review: Gastrointestinal Melatonin: Localization, Function, and Clinical Relevance. Dig. Dis. Sci. 2002, 47, 2336–2348. [Google Scholar] [CrossRef]
- Sommansson, A.; Saudi, W.S.W.; Nylander, O.; Sjöblom, M. Melatonin inhibits alcohol-induced increases in duodenal mucosal permeability in rats in vivo. Am. J. Physiol. Liver Physiol. 2013, 305, G95–G105. [Google Scholar] [CrossRef] [PubMed]
- Monobe, M.; Hino, M.; Sumi, M.; Uzawa, A.; Hirayama, R.; Ando, K.; Kojima, S. Protective effects of melatonin on γ-ray induced intestinal damage. Int. J. Radiat. Biol. 2005, 81, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Johansson, C.; Aly, A.; Befrits, R.; Smedfors, B.; Uribe, A. Protection of the Gastroduodenal Mucosa by Prostaglandins. Scand. J. Gastroenterol. 1985, 20, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Davies, N.M.; Longstreth, J.; Jamali, F. Misoprostol Therapeutics Revisited. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2001, 21, 60–73. [Google Scholar] [CrossRef]
- Graham, D.; Agrawal, N.; Roth, S. Prevention of Nsaid-Induced Gastric Ulcer with Misoprostol: Multicentre, Double-Blind, Placebo-Controlled Trial. Lancet 1988, 332, 1277–1280. [Google Scholar] [CrossRef]
- Maher, S.; Brayden, D.J. Formulation strategies to improve the efficacy of intestinal permeation enhancers. Adv. Drug Deliv. Rev. 2021, 177, 113925. [Google Scholar] [CrossRef]
- Dahlgren, D.; Roos, C.; Lundqvist, A.; Tannergren, C.; Sjöblom, M.; Sjögren, E.; Lennernäs, H. Effect of absorption-modifying excipients, hypotonicity, and enteric neural activity in an in vivo model for small intestinal transport. Int. J. Pharm. 2018, 549, 239–248. [Google Scholar] [CrossRef]
- Dahlgren, D.; Cano-Cebrián, M.-J.; Hellström, P.M.; Wanders, A.; Sjöblom, M.; Lennernäs, H. Prevention of Rat Intestinal Injury with a Drug Combination of Melatonin and Misoprostol. Int. J. Mol. Sci. 2020, 21, 6771. [Google Scholar] [CrossRef]
- Nylander, O.; Sababí, M.; Bark, J. Characterization of51Cr-EDTA as a marker of duodenal mucosal permeability. Acta Physiol. Scand. 1991, 143, 117–126. [Google Scholar] [CrossRef]
- Sommansson, A.; Nylander, O.; Sjöblom, M. Melatonin decreases duodenal epithelial paracellular permeability via a nicotinic receptor-dependent pathway in rats in vivo. J. Pineal Res. 2012, 54, 282–291. [Google Scholar] [CrossRef]
- Bubenik, G.A.; Brown, G.M. Pinealectomy Reduces Melatonin Levels in the Serum but Not in the Gastrointestinal Tract of Rats. Neurosignals 1997, 6, 40–44. [Google Scholar] [CrossRef]
- Sjöblom, M.; Flemström, G. Central nervous alpha1-adrenoceptor stimulation induces duodenal luminal release of melatonin. J. Pineal Res. 2004, 36, 103–108. [Google Scholar] [CrossRef]
- Siah, K.T.H.; Wong, R.K.M.; Ho, K.Y. Melatonin for the treatment of irritable bowel syndrome. World J. Gastroenterol. 2014, 20, 2492–2498. [Google Scholar] [CrossRef]
- Gao, T.; Wang, T.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Melatonin-mediated MT2 attenuates colitis induced by dextran sodium sulfate via PI3K/AKT/Nrf2/SIRT1/RORα/NF-κB signaling pathways. Int. Immunopharmacol. 2021, 96, 107779. [Google Scholar] [CrossRef]
- Rakhimova, O.Y.; Rakhimova, O.Y. Use of melatonin in combined treatment for inflammatory bowel diseases. Ter. Arkhiv 2010, 82, 64–68. [Google Scholar]
- Pandi-Perumal, S.R.; Trakht, I.; Srinivasan, V.; Spence, D.W.; Maestroni, G.J.; Zisapel, N.; Cardinali, D.P. Physiological effects of melatonin: Role of melatonin receptors and signal transduction pathways. Prog. Neurobiol. 2008, 85, 335–353. [Google Scholar] [CrossRef]
- Peters, K.; Dahlgren, D.; Lennernäs, H.; Sjöblom, M. Melatonin-Activated Receptor Signaling Pathways Mediate Protective Effects on Surfactant-Induced Increase in Jejunal Mucosal Permeability in Rats. Int. J. Mol. Sci. 2021, 22, 10762. [Google Scholar] [CrossRef]
- Musa, A.E.; Shabeeb, D.; Alhilfi, H.S.Q. Protective Effect of Melatonin against Radiotherapy-Induced Small Intestinal Oxidative Stress: Biochemical Evaluation. Medicina 2019, 55, 308. [Google Scholar] [CrossRef] [Green Version]
- Najafi, M.; Cheki, M.; Hassanzadeh, G.; Amini, P.; Shabeeb, D.; Musa, A.E. Protection from Radiation-induced Damage in Rat’s Ileum and Colon by Combined Regimens of Melatonin and Metformin: A Histopathological Study. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2020, 19, 180–189. [Google Scholar] [CrossRef]
- Sezen, O.; Erdemci, B.; Calik, M.; Koc, M. The role of melatonin in preventing radiation-induced intestinal injury. J. BUON 2021, 26, 626–633. [Google Scholar]
- Reiter, R.J.; Mayo, J.C.; Tan, D.-X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Mannino, G.; Caradonna, F.; Cruciata, I.; Lauria, A.; Perrone, A.; Gentile, C. Melatonin reduces inflammatory response in human intestinal epithelial cells stimulated by interleukin-1β. J. Pineal Res. 2019, 67, e12598. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yuan, X.-Q.; Zhang, X.-M. Melatonin reduces inflammation in intestinal cells, organoids and intestinal explants. Inflammopharmacology 2021, 29, 1555–1564. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, Y.; Narumiya, S. Prostaglandin E Receptors. J. Biol. Chem. 2007, 282, 11613–11617. [Google Scholar] [CrossRef] [Green Version]
- Abramovitz, M.; Adam, M.; Boie, Y.; Carrière, M.-C.; Denis, D.; Godbout, C.; Lamontagne, S.; Rochette, C.; Sawyer, N.; Tremblay, N.M.; et al. The utilization of recombinant prostanoid receptors to determine the affinities and selectivities of prostaglandins and related analogs. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2000, 1483, 285–293. [Google Scholar] [CrossRef]
- Field, J.T.; Martens, M.D.; Mughal, W.; Hai, Y.; Chapman, D.; Hatch, G.M.; Ivanco, T.L.; Diehl-Jones, W.; Gordon, J.W. Misoprostol regulates Bnip3 repression and alternative splicing to control cellular calcium homeostasis during hypoxic stress. Cell Death Discov. 2018, 4, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.; Verne, G.N. Intestinal Hyperpermeability: A Gateway to Multi-Organ Failure? Available online: https://www.jci.org/articles/view/124366/pdf (accessed on 29 August 2021).
- Anderberg, E.K.; Artursson, P. Epithelial Transport of Drugs in Cell Culture. VIII: Effects of Sodium Dodecyl Sulfate on Cell Membrane and Tight Junction Permeability in Human Intestinal Epithelial (Caco-2) Cells. J. Pharm. Sci. 1993, 82, 392–398. [Google Scholar] [CrossRef]
- Swenson, E.S.; Milisen, W.B.; Curatolo, W. Intestinal Permeability Enhancement: Efficacy, Acute Local Toxicity, and Reversibility. Pharm. Res. 1994, 11, 1132–1142. [Google Scholar] [CrossRef]
- Dahlgren, D.; Roos, C.; Lundqvist, A.; Tannergren, C.; Sjöblom, M.; Sjögren, E.; Lennernäs, H. Time-dependent effects on small intestinal transport by absorption-modifying excipients. Eur. J. Pharm. Biopharm. 2018, 132, 19–28. [Google Scholar] [CrossRef]
- Lichtenberg, D.; Robson, R.J.; Dennis, E.A. Solubilization of phospholipids by detergents structural and kinetic aspects. Biochim. Biophys. Acta (BBA) Rev. Biomembr. 1983, 737, 285–304. [Google Scholar] [CrossRef]
- Anosov, A.; Smirnova, E.; Korepanova, E.; Shogenov, I. The effects of SDS at subsolubilizing concentrations on the planar lipid bilayer permeability: Two kinds of current fluctuations. Chem. Phys. Lipids 2019, 218, 10–15. [Google Scholar] [CrossRef]
- Narkar, Y.; Burnette, R.; Bleher, R.; Albrecht, R.; Kandela, A.; Robinson, J.R. Evaluation of Mucosal Damage and Recovery in the Gastrointestinal Tract of Rats by a Penetration Enhancer. Pharm. Res. 2008, 25, 25–38. [Google Scholar] [CrossRef]
- Sjöblom, M. The Duodenal Mucosal Bicarbonate Secretion. Upsala J. Med Sci. 2005, 110, 115–150. [Google Scholar] [CrossRef]
- Dahlgren, D.; Roos, C.; Peters, K.; Lundqvist, A.; Tannergren, C.; Sjögren, E.; Sjöblom, M.; Lennernäs, H. Evaluation of drug permeability calculation based on luminal disappearance and plasma appearance in the rat single-pass intestinal perfusion model. Eur. J. Pharm. Biopharm. 2019, 142, 31–37. [Google Scholar] [CrossRef]
- Fihn, B.-M.; Sjöqvist, A.; Jodal, M. Effect of cholera toxin on passive transepithelial transport of 51 Cr-ethylenediaminetetraacetic acid and 14 C-mannitol in rat jejunum. Acta Physiol. Scand. 2001, 171, 153–160. [Google Scholar] [CrossRef]
- Sedin, J.; Sjöblom, M.; Nylander, O. Prevention of duodenal ileus reveals functional differences in the duodenal response to luminal hypertonicity in Sprague-Dawley and Dark Agouti rats. Acta Physiol. 2014, 210, 573–589. [Google Scholar] [CrossRef]
- Sedin, J.; Sjöblom, M.; Nylander, O. The selective cyclooxygenase-2 inhibitor parecoxib markedly improves the ability of the duodenum to regulate luminal hypertonicity in anaesthetized rats. Acta Physiol. 2012, 205, 433–451. [Google Scholar] [CrossRef]
- Pihl, L.; Nylander, O. Products of cyclooxygenase-2 depress duodenal function in rats subjected to abdominal surgery. Acta Physiol. 2006, 186, 279–290. [Google Scholar] [CrossRef]
- Dahlgren, D.; Sjöblom, M.; Hellström, P.M.; Lennernäs, H. Chemotherapeutics-Induced Intestinal Mucositis: Pathophysiology and Potential Treatment Strategies. Front. Pharmacol. 2021, 12, 681417. [Google Scholar] [CrossRef]
- George, R.P.; Barker, T.; Lymn, K.A.; Bigatton, D.A.; Howarth, G.S.; Whittaker, A.L. A Judgement Bias Test to Assess Affective State and Potential Therapeutics in a Rat Model of Chemotherapy-Induced Mucositis. Sci. Rep. 2018, 8, 8193. [Google Scholar] [CrossRef]
- Van Sebille, Y.Z.A.; Stansborough, R.; Wardill, H.; Bateman, E.; Gibson, R.; Keefe, D.M. Management of Mucositis During Chemotherapy: From Pathophysiology to Pragmatic Therapeutics. Curr. Oncol. Rep. 2015, 17, 50. [Google Scholar] [CrossRef]
- Dahlgren, D.; Roos, C.; Lundqvist, A.; Tannergren, C.; Langguth, P.; Sjöblom, M.; Sjögren, E.; Lennernäs, H. Preclinical Effect of Absorption Modifying Excipients on Rat Intestinal Transport of Model Compounds and the Mucosal Barrier Marker 51Cr-EDTA. Mol. Pharm. 2017, 14, 4243–4251. [Google Scholar] [CrossRef]
- Nylander, O.; Kvietys, P.; Granger, D.N. Effects of hydrochloric acid on duodenal and jejunal mucosal permeability in the rat. Am. J. Physiol.-Gastrointest. Liver Physiol. 1989, 257, G653–G660. [Google Scholar] [CrossRef] [PubMed]
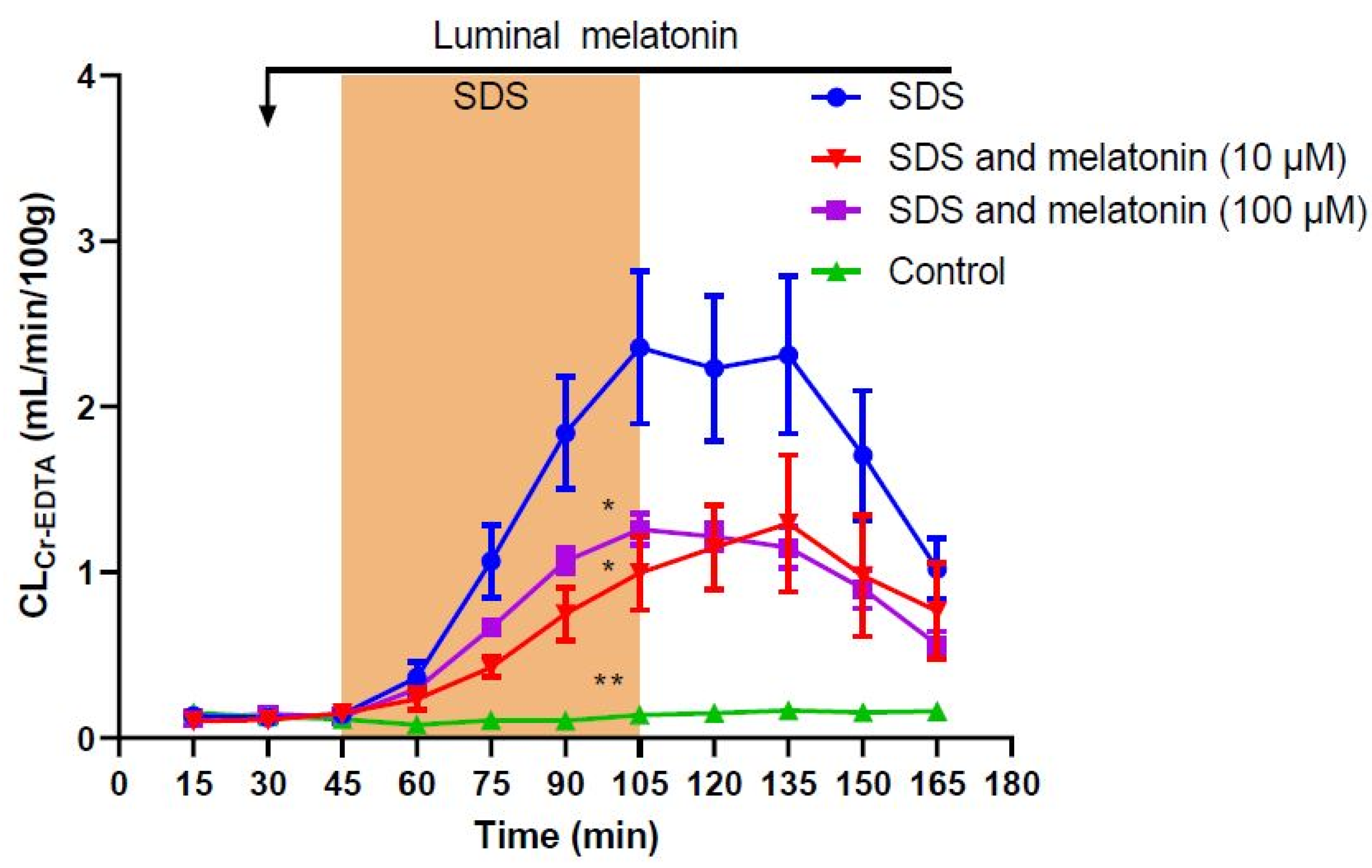
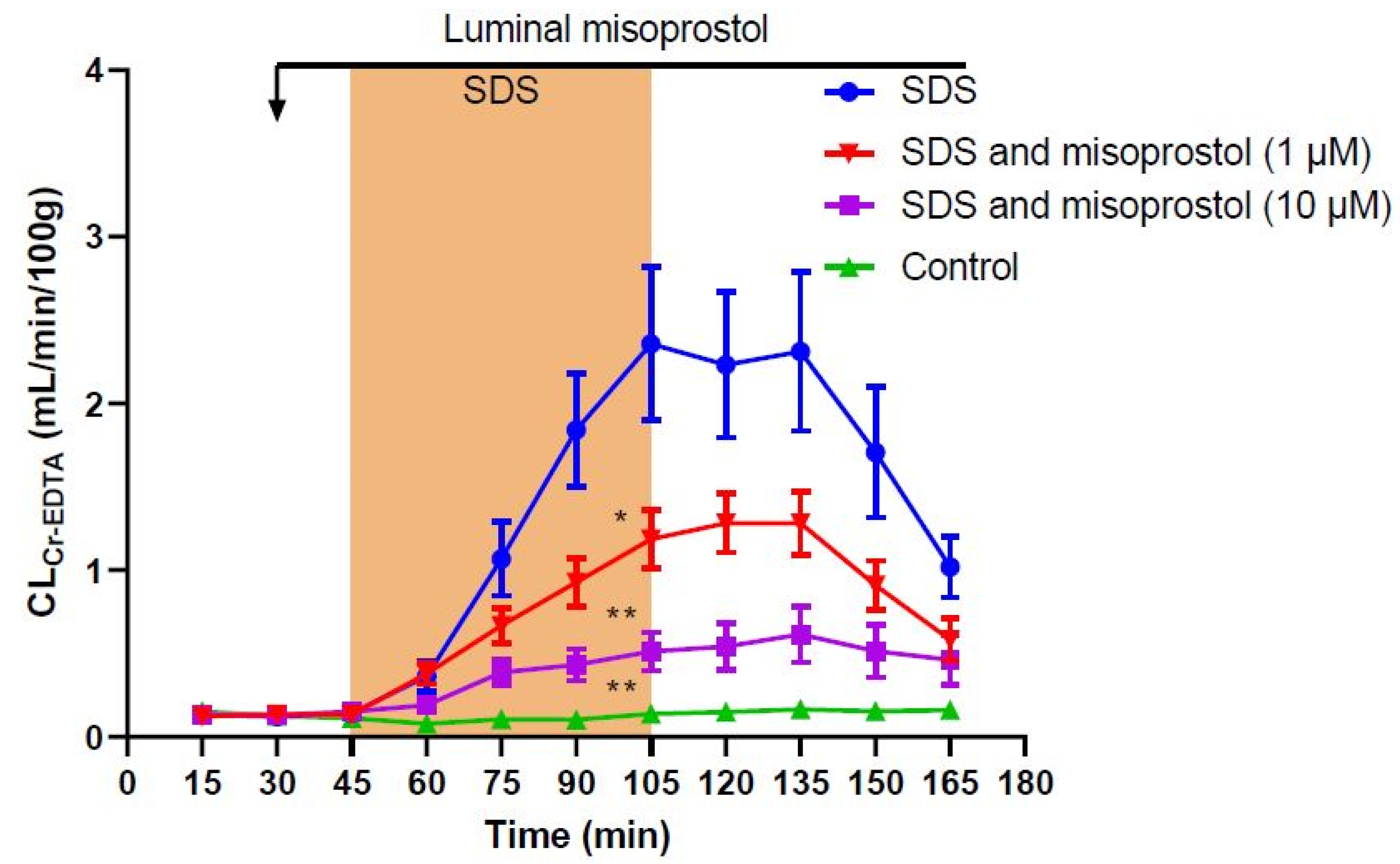
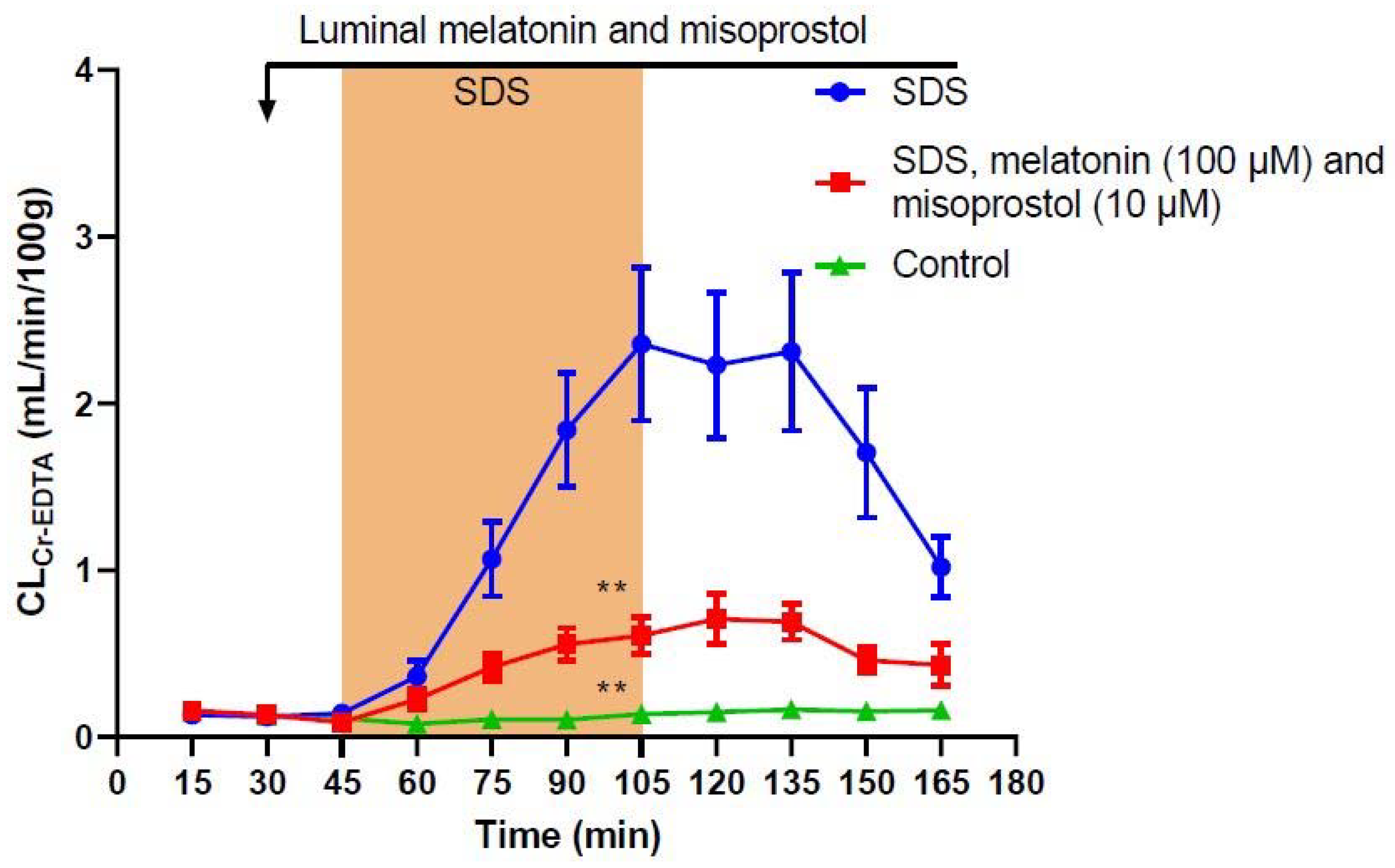


| Treatment | Total CLCr-EDTA (mL/100 g) | p-Value (Difference from SDS) |
|---|---|---|
| Control | 14.0 ± 2.0 | 0.0050 |
| SDS | 204.0 ± 22.8 | - |
| SDS + melatonin 10 µM | 91.4 ± 18.2 | 0.025 |
| SDS + melatonin 100 µM | 100.3 ± 5.7 | 0.048 |
| SDS + misoprostol 1 µM | 102.0 ± 10.6 | 0.032 |
| SDS + misoprostol 10 µM | 50.0 ± 8.6 | 0.0068 |
| SDS + melatonin 100 µM and misoprostol 10 µM | 56.6 ± 7.3 | 0.0077 |
| Treatment | Villi | Edema | Inflammation | Mucus | Apoptosis |
|---|---|---|---|---|---|
| Control | Normal | Minor effect | Normal | Normal | Normal |
| SDS | Nd | Nd | Nd | Nd | Nd |
| SDS + melatonin 100 µM | Nd | Nd | Nd | Nd | Nd |
| SDS + misoprostol 10 µM | Nd | Nd | Nd | Nd | Nd |
| SDS + melatonin 100 µM and misoprostol 10 µM | Nd | Nd | Nd | Nd | Nd |
| Parameters | Normal | Minor Effect | Major Effect |
|---|---|---|---|
| Villus length and width | Normal height and width | Slightly shortened and widened | Strongly shortened and widened |
| Edema | No edema | Some small edema | Many and/or larger edema |
| Mucus distribution | Even distribution of mucus over the entire villi | Mucus lacking in small areas | Mucus lacking in large areas |
| Signs of inflammation | Absence of neutrophils | Infiltration of some neutrophils | Infiltration of a large number of neutrophils |
| Apoptosis | Normal number of apoptotic cells | Slightly increased number of apoptotic cells | Strongly increased number of apoptotic cells |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peters, K.; Dahlgren, D.; Egerszegi, P.P.; Lennernäs, H.; Sjöblom, M. Protective Effects of Melatonin and Misoprostol against Experimentally Induced Increases in Intestinal Permeability in Rats. Int. J. Mol. Sci. 2022, 23, 2912. https://doi.org/10.3390/ijms23062912
Peters K, Dahlgren D, Egerszegi PP, Lennernäs H, Sjöblom M. Protective Effects of Melatonin and Misoprostol against Experimentally Induced Increases in Intestinal Permeability in Rats. International Journal of Molecular Sciences. 2022; 23(6):2912. https://doi.org/10.3390/ijms23062912
Chicago/Turabian StylePeters, Karsten, David Dahlgren, Péter Pál Egerszegi, Hans Lennernäs, and Markus Sjöblom. 2022. "Protective Effects of Melatonin and Misoprostol against Experimentally Induced Increases in Intestinal Permeability in Rats" International Journal of Molecular Sciences 23, no. 6: 2912. https://doi.org/10.3390/ijms23062912
APA StylePeters, K., Dahlgren, D., Egerszegi, P. P., Lennernäs, H., & Sjöblom, M. (2022). Protective Effects of Melatonin and Misoprostol against Experimentally Induced Increases in Intestinal Permeability in Rats. International Journal of Molecular Sciences, 23(6), 2912. https://doi.org/10.3390/ijms23062912






