Abstract
After the serendipitous discovery of cisplatin, a platinum-based drug with chemotherapeutic effects, an incredible amount of research in the area of coordination chemistry has been produced. Other transition metal compounds were studied, and several new relevant metallodrugs have been synthetized in the past few years. This review is focused on coordination compounds with first-row transition metals, namely, copper, cobalt, nickel or manganese, or with zinc, which have potential or effective pharmacological properties. It is known that metal complexes, once bound to organic drugs, can enhance the drugs’ biological activities, such as anticancer, antimicrobial or anti-inflammatory ones. NSAIDs are a class of compounds with anti-inflammatory properties used to treat pain or fever. NSAIDs’ properties can be strongly improved when included in complexes using their compositional N and O donor atoms, which facilitate their coordination to metal ions. This review focuses on the research on this topic and on the promising or effective results that complexes of first-row transition metals and NSAIDs can exhibit.
1. Introduction
In the era of emerging drug resistance, mainly by bacteria, designing potent and successful novel therapeutic agents has become a major concern in the area of bioinorganic chemistry [1,2]. After the serendipitous discovery of the anticancer effects of cisplatin, a platinum-based drug (cis−[Pt(NH3)2Cl2]) which proved to have a strong chemotherapeutic effect, a tremendous amount of research on coordination chemistry-based drugs has been produced. Cisplatin can bind to the purine bases of DNA and is able to cause DNA damage that, in turn, can cause cancer cell apoptosis [3]. However, some adverse side effects, such as toxicity, allergy, gastrointestinal disorders and kidney problems, plus the lack of selectivity and acquired resistance to this compound [4,5,6], led investigators to explore more selective and less toxic substituents. During this endeavor, in the past few years a large number of new metal complexes have been synthetized. Specifically, some first-row transition metals have been preferred, namely, copper, cobalt, manganese, nickel and zinc, not only because they are easily available and cheaper, compared to platinum, but also for their less toxic nature and biocompatibility in living systems [7,8,9]. Additionally, transition metals can play a very important role in metallodrugs’ design due to their cations’ unique coordination environments, charge variation possibilities, Lewis acidic character or redox properties [8,10,11].
Regarding the above-mentioned first-row transition metals, some of their biological/bioinorganic implications deserve to be highlighted. Copper, as Cu(II), is a d9 metal cation structurally and catalytically involved in several biological processes by serving as a cofactor of many metalloproteins [12,13,14] and by promoting numerous enzymatic processes, such as cellular respiration or the biosynthesis of neurotransmitters [7,12,15,16]. In living organisms, copper exists predominantly in the Cu(II) oxidized form—that is, in the cupric form [12]. However, due to its redox ability, copper can also exist in a more reduced form, i.e., Cu(I). Therefore, it can either behave as an antioxidant or a pro-antioxidant species, and can either neutralize or induce the production of reactive oxygen species (ROS) [13,16,17]. Cobalt is another transition metal that is normally found as Co(II) (d7) or Co(III) (d6) forms, even though eventually it can exhibit a wide range of oxidation states from −1 to +4. Co(III) is mainly found in cobalamin (vitamin B12) [18]. Nevertheless, in order to have cobalt ions be biochemically active—i.e., involved in metabolic functions, such as fatty acid and amino acid metabolism [4,17,19] and regulating DNA, albeit indirectly [4,9,20,21]—cobalt needs to adopt Co(II) or Co(III) oxidation states. Manganese is also an essential element, and when as Mn(II), d5, it is associated with various physiological processes, such as development, reproduction and immune functions, energy metabolism and antioxidant defense [22]. Furthermore, it is involved in the synthesis and activation of several enzymes (e.g., transferases, hydrolases and isomerases) by acting as a cofactor [17,23]. In particular, in the central nervous system, the manganese ions act as cofactors for glutamine synthetase (GS) [24], and consequently an extreme exposure to this element is often linked to neurologic pathologies [2,24,25]. Manganese can exist in seven oxidation states (0, II–VII), but the biologically most important is Mn(II), which is inherently stable. In contrast, Mn(III) is unstable under acidic conditions, Mn(V) is unstable under all conditions and Mn(VII) is a strong oxidant species [26,27,28,29]. Nickel, with a d8 electronic configuration, Ni(II), is an essential element, although it still has, to a certain degree, few unclear biological functions [30]. It was originally found in the active center of urease [30,31,32], a non-mammalian enzyme that catalyzes the hydrolysis of urea, but with time, other nickel-dependent and nickel-containing enzymes were discovered [33]. Ni(II) ions are mainly found associated with nucleic acids in humans, since they coordinate with DNA’s nitrogen-containing bases [34]. It is also involved in proteins structurally and functionality [30]. Finally, zinc, another essential element, although not a transition metal by IUPAC definition [35], shows similar chemical properties to its periodic table neighbors, transition metals. As Zn(II), it possesses a 3d10 electronic configuration with all 3d orbitals fully filled. Therefore, even though Zn(II) complexes do not possess ligand field energy stabilization, they show wide coordination flexibility, especially with O donor atoms of amino acids or proteins [17]. Consequently, these characteristics (coordination numbers and structural variety) are critical in what concerns their catalytic roles in metalloenzymes, providing different interaction possibilities with substrates [36]. Zinc possesses a minor plasma pool and has rapid turnover. It is involved in several steps of cellular metabolism and is involved in respiration, immune functions, DNA synthesis and cell division [32,37,38].
2. History and Applications of Metallodrugs
The discovery of cisplatin in the 1960s triggered, firstly, the synthesis of new platinum compounds bearing biological activity, and then the preparation of complexes with other metals [39,40]. More recently, researchers have pursued complexes containing active drugs as ligands, since it has been proved that this approach could be a suitable strategy for developing new and more efficacious pharmacological compounds, together with less toxic effects when compared to the parent drugs [41,42,43,44,45]. Although some metal complexes received attention due to the anticancer activity of cisplatin and its derivatives, in many cases they possess other different and promising biological activities. Consequently, metallodrugs have been found to have innumerous applications, including anti-microbial, anti-inflammatory, anti-viral, anti-arthritic or anti-diabetic, and activities in relation to cardiovascular or gastrointestinal disorders as well [1,46,47,48,49].
The first metallodrugs used in therapy were arsenic-based antimicrobial and antiparasitic agents. In particular, melarsoprol, an arsenic-based drug, is still used against trypanosomiasis [1], but other metal-based drugs with similar properties have emerged and are now commercially available [1,50]. One example is ferrochloroquine, an antimalarial agent, which is now undergoing phase II clinical trials. It is, more precisely, an organometallic compound (see Figure 1) [1,51].
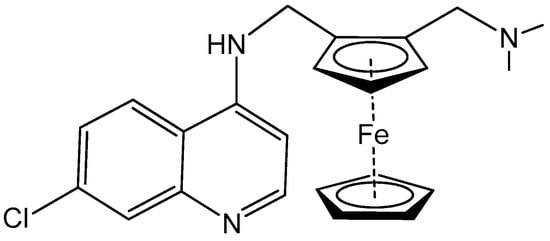
Figure 1.
Ferrochloroquine’s chemical structure.
Taking into account their possible biological activities, new coordination compounds with first row transition metals have been relentlessly investigated. Nowadays, particular attention is directed towards complexes with copper, cobalt, nickel, manganese or zinc, all of them possessing prominent biological effects [46,47,49,52,53].
One of the current focuses regarding these biometal complexes is their potential use as anti-inflammatory agents [7]. The synthesis of transition metal complexes with non-steroidal anti-inflammatory drugs (NSAIDs), used as ligands, started in 1978 with the preparation of an acetylsalicylic acid (aspirin) copper(II) coordination compound [53]. This study was an important breakthrough, since this complex showed a stronger anti-inflammatory response, and less ulcerogenicity and irritation to the digestive tract, than aspirin itself [54]. Since then, other biometal−NSAID complexes have been synthetized and studied in relation to their pharmacological effects. It is in this niche of research that this review is focused.
NSAIDs are a diverse class of compounds with anti-inflammatory properties used to treat pain or fever by inhibiting the two cyclooxygenase (COX) isoenzymes (known as COX-1 and COX-2) and also lipoxygenase (LOX) [17,19,55,56,57]. According to their chemical structures and selectivity, NSAIDs can be separated into different classes. Most of them are nonselective and so inhibit both COX-1 and COX-2. This is the case for (i) acetylated salicylates, (ii) non-acetylated salicylates, (iii) propionic acids, (iv) acetic acids, (v) enolic acids, (vi) anthranilic acids and (vii) naphthylalanine, in which the active agents are acetylsalicylic acid, diflunisial, naproxen or ibuprofen, diclofenac or indomethacin, meloxicam or piroxicam, tolfenamic acid or mefenamic acid and nabumetone, respectively [58]. However, there is a class of NSAIDs, called “coxibs,” whose members known for being selective for COX-2 inhibitors (e.g., celecoxib), and therefore, they have different side effect profiles in the treatment of inflammation [58].
2.1. Biometal−NSAID Complexes: A Few Coordination Topics
Divalent metal ions Cu(II), Co(II), Mn(II), Ni(II) and Zn(II), as mentioned above, can have essential redox and catalytic activities through structural modifications to the molecules (i.e., NSAIDs) they bind. NSAIDs, using N and O donor atoms, are able to easily coordinate to these metal ion centers, and the resulting coordination compounds can show enhanced biological activity compared to their parent NSAIDs [4,7,20,59,60,61]. Combining NSAIDs for treatment is a suitable strategy to modulate the potential effects of some available drugs. In particular, it might be an attractive approach in chemotherapeutics to circumvent multidrug resistance and inflammation-induced metastatic cancers [62,63]. Additionally, the synthesis of new drugs with synergistic biologically active ligands (i.e., the synthesis of metal−NSAID complexes) is an alternative option for modulating the therapeutic efficiency of the referred NSAIDs [63].
Cu(II) complexes with a variety of coordination numbers are common (4, 5 or 6), unlike Cu(I) complexes, which are mainly four coordinated [64]. Nevertheless, the preferred coordination environment of Cu(II) is square planar because of the Jahn–Teller distorting effect of the Cu(II) d9 electronic configuration [65]. Then, the main difference between Cu(II) and Cu(I) quadropoly coordinated compounds is the square-planar geometry of the former compounds against the tetrahedral of the latter. This particular aspect can be extremely important in the electron-transfer efficiency of some metallo-enzymes’ active sites. In most cases, they require distorted tetrahedral geometries, so that almost no energy is spent in structural rearrangements upon electron transfer. Paradigmatic examples are blue, or type 1, copper metalloproteins, which are responsible for carrying out electron transfer in a wide range of biological systems with variable enzymatic architectures [66,67].
Although cobalt complexes can contain cobalt with various oxidation states, as previously stated, Co(III) and Co(II) complexes are the most predominant ones in biological systems [68,69]. Co(III) coordination compounds usually have a low-spin d6 configuration, forming almost exclusively ix coordinate complexes with regular octahedral or distorted octahedral geometries [68]. Co(II) ions, often in high-spin d7 electronic configuration, can generally form four or six coordinate complexes with tetrahedral (distorted) and octahedral geometries [68], respectively, although ive coordinate complexes can also be found [69]. Normally, Co(II) ions easily interact with chelating N and O donor ligands [17,70,71].
Manganese(II) ions prefer to exist in ix coordinate complexes. However, these Mn(II) complexes are often unstable and easily interact with other molecules/ligands which can modify their coordination spheres, with consequences to their interaction modes with specific enzymes [72,73,74].
Complexes of nickel(II), in turn, typically adopt a variety of octahedral, square planar or tetrahedral geometries. However, rarer, ive coordinated compounds may also be formed [75,76].
As mentioned before, despite the lack of ligand field energy stabilization, zinc(II) complexes show coordination flexibility. Various structures of Zn(II) complexes have been observed, although coordination, mainly in tetrahedral geometries, is the most commonly found [77], as it represent the optimal and least strained structure among polyhedral zinc compounds [78].
The NSAIDs, mainly because of their characteristic carboxylic acid functional groups, which are in their anionic (deprotonated) forms at physiological pH, can be used as ligands, as they easily coordinate to metal ions, in a great versatility of coordination modes. Indeed, these drugs are able to coordinate as mono and bidentate modes, or even as bridges originating polynuclear metal complexes [57,79,80]. In Table 1, Table 2, Table 3, Table 4 and Table 5, some examples of these type of coordination modes are given. This review also summarizes the biological activities of some Cu(II), Co(II), Mn(II), Ni(II) and Zn(II) metal coordination compounds with some NSAIDs, which are organized into different groups. However, to the best of our knowledge, copper(II), cobalt(II), nickel(II), manganese(II) and zinc(II) metal complexes with the NSAID naphthylalanine, a non-natural analogue of phenylalanine involved in the inflammatory process [81], are not structurally characterized yet.
2.1.1. Copper(II) Complexes of NSAIDs
Copper(II)−NSAID are the most numerous among the metal−NSAID complexes [82]. The structurally characterized and enumerated Cu(II)−NSAID complexes are mostly mononuclear, with carboxylate groups in bidentate chelating mode—e.g., [Cu(difl)2(py)2] [83], [Cu(nap)(tpy)Cl] [84] or [Cu(dicl)2(temed)] [85], although a monodentate chelating mode can be observed for [Cu(asa)(aroy)(H2O)2] [86], [Cu(Hmel)2(dmf)] [87], [Cu(tolf)2(py)2(MeOH)2] [88] and [Cu(cxb)2Cl2] [89] (see Table 1). With the exception of [Cu(Hmel)2(dmf)] [87] and [Cu(cxb)2Cl2] [89] complexes that are five and four coordinate with distorted square planar and square-pyramidal geometries, respectively, the structurally characterized examples listed in Table 1 are 6-coordinate, exhibiting distorted octahedral geometry.
2.1.2. Cobalt(II) Complexes of NSAIDs
All the reported cobalt(II)−NSAID coordination compounds (Table 2) are mononuclear, with the NSAID carboxylate group being coordinated to the metal ion in a monodentate binding mode as in [Co(asa)(Haroy)(H2O)Cl] [86], [Co(difl)2(MeOH)4] [90], [Co(nap)2(py)2(H2O)2] [91], [Co(dicl)2(py)2(H2O)2] [19] and [Cu(cxb)2Cl2] [89] complexes, with the exception of [Co(Hmel)2(EtOH)2] [92] and [Co(tolf)2(bipyam)] [93] ones, where the carboxylate groups of meloxicam and tolfenamate ligands, respectively, are coordinated in a chelating bidentate mode. The majority of compounds are six coordinate with a distorted octahedral configuration.
2.1.3. Nickel(II) Complexes of NSAIDs
In all the listed mononuclear complexes of Ni(II) ion (Table 3), the carboxylate coordination group is always in a monodentate binding mode, excepting in the [Ni(nap)2(phen)(H2O)] [94] complex. In this last case, the two deprotonated naproxen ligands are coordinated to nickel in two different binding modes: one naproxen ligand is bound to nickel in a bidentate chelating mode, and the other one is coordinated in a monodentate fashion. Again, with the exception of the [Ni(cxb)2Cl2] [89] complex, the reported examples were found to be six coordinate with a distorted octahedral geometry.
2.1.4. Manganese(II) Complexes of NSAIDs
Not a lot of examples with manganese and the specific selected NSAIDs are described in the literature yet. However, for the enumerated cases (see Table 4), it is possible to see a diversity in coordination. The complexes are mononuclear, dinuclear or trinuclear. The structurally characterized mononuclear Mn(II)−NSAID complexes have the NSAIDs’ carboxylate groups bound to the metal ion in a monodentate mode, as in the [Mn(nap)2(py)2(H2O)2] [95] complex, or in a bidentate one, as in [Mn(tolf)2(phen)(H2O)] [96]. The nonlinear trinuclear Mn(II) complex is structurally diverse. The six diclofenac ligands are deprotonated and coordinated to the manganese atoms in three different modes: three of the six diclofenac ligands are in a bidentate binding mode and form µ1,3-bridges between two Mn atoms; two diclofenac ligands are in a tridentate binding mode and form µ1,1-bridges between two Mn atoms; and the sixth diclofenac ligand is monodentately bound to a terminal Mn atom through an oxygen atom. Additionally, the three Mn atoms are six coordinate and exhibit distorted octahedral geometries.
2.1.5. Zinc(II) Complexes of NSAIDs
Similarly to Mn(II)−NSAID complexes, Zn(II)−NSAID complexes present a high diversity of nuclearity: we report in Table 5, mononuclear ([Zn(difl)2(bipy)] [62], [Zn(nap)2(N3)2]Na2 [80] and [Zn(Hmel)2(EtOH)2] [92]); binuclear (Zn2(dicl)4(nic)2 [97]); and trinuclear ([Zn3(tolf)6(CH3OH)2] [98]) cases. In the mononuclear complexes, the carboxylate groups are in either monodentate or chelating bidentate modes. For the binuclear case, one diclofenac molecule is monodently coordinated, while the other is bidently coordinated. Finally, for the centrosymmetric trinuclear complex, the six tolfenamato ligands behave as deprotonated ligands in the bidentate mode, forming six bidentate carboxylate bridges. The central Zn atom is six coordinate with a distorted octahedral geometry. The basal plane of the octahedron is formed by four coordinated carboxylate oxygen atoms of four different tolfenamato bridging ligands, while at the axial positions there are the carboxylate oxygen atoms of the remaining two tolfenamato bridging ligands.

Table 1.
Examples of copper(II) complexes with different NSAIDs.
Table 1.
Examples of copper(II) complexes with different NSAIDs.
| Chemical Formula | NSAID | Chemical Structure | Biological Activity | Ref. | |
|---|---|---|---|---|---|
| NSAID Ligand | NSAID Coordinating Group | ||||
| [Cu(asa)(aroy)(H2O)2] (a) | Aspirin | Acetylated salicylate | 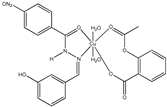 (proposed structure) | Weaker antimicrobial activity comparing to free aspirin. | [86] |
| [Cu(difl)2(py)2] (b) | Diflunisal | Non-acetylated salicylate |  | Moderate to strong DNA and albumin binding. | [83] |
| [Cu(nap)(tpy)Cl] (c) | Naproxen | Propionic acid |  | Hydrolytic DNA cleavage. Moderated cytotoxicity in a human breast cancer cell line (MCF-7). | [84] |
| [Cu(dicl)2(temed)] (d) | Diclofenac | Acetate |  | Significant reversible affinity for BSA, higher than the free NSAID sodium diclofenac. | [85] |
| [Cu(Hmel)2(dmf)] (e) | Meloxicam | Enolic acid |  | Possible beneficial effects as anticancer agent due to its anti-proliferative activity. | [87] |
| [Cu(tolf)2(py)2(MeOH)2] (f) | Tolfenamic acid | Anthranilate |  | Tight binding affinity to BSA and HSA. Scavenging activity (against hydroxyl and superoxide radicals) stronger than free tolfenamic acid. | [88] |
| [Cu(cxb)2Cl2] (g) | Celecoxib | Coxib |  (proposed structure) | Inhibitory activity against Cyclooxygenase II. | [89] |
(a) asa = aspirin and aroy = aroylhydrazone (m-hydroxylbenzaldehyde-4-nitrobenzoylhydrazone); (b) difl = diflunisal and py = pyridine; (c) nap = naproxen and tpy = substituted terpyridine; (d) dicl = deprotonated diclofenac and temed = N,N,N′,N′-tetramethylethylenediamine; (e) Hmel = protonated meloxicam and dmf = dimethylformamide; (f) tolf = tolfenamate and py = pyridine; (g) cxb = celecoxib.

Table 2.
Examples of cobalt(II) complexes with different NSAIDs.
Table 2.
Examples of cobalt(II) complexes with different NSAIDs.
| Chemical Formula | NSAID | Chemical Structure | Biological Activity | Ref. | |
|---|---|---|---|---|---|
| NSAID Ligand | NSAID Coordinating Group | ||||
| [Co(asa)(Haroy)(H2O)Cl] (a) | Aspirin | Acetylated salicylate | 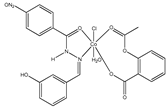 (proposed structure) | Weaker antimicrobial activity comparing to free aspirin. | [86] |
| [Co(difl)2(MeOH)4] (b) | Diflunisal | Non-acetylated salicylate | 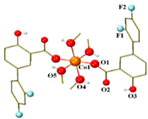 | Radical scavenging ability and DNA and albumin binding. | [90] |
| [Co(nap)2(py)2(H2O)2] (c) | Naproxen | Propionic acid | 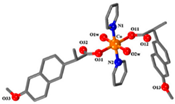 | Good binding affinity to BSA and HSA and to DNA. High scavenging activity against hydroxyl and superoxide radicals. | [91] |
| [Co(dicl)2(py)2(H2O)2] (d) | Diclofenac | Acetate | 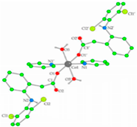 | Antioxidant activity, DNA binding. | [19] |
| [Co(Hmel)2(EtOH)2] (e) | Meloxicam | Enolic acid |  | DNA biding and photocleavage of pUC57 plasmid DNA. | [92] |
| [Co(tolf)2(bipyam)] (f) | Tolfenamic acid | Anthranilate | 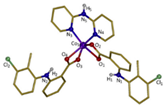 | Good binding affinity to BSA and HSA and higher affinity to bind DNA comparing to free tolfenamic acid. | [93] |
| [Co(cxb)2Cl2] (g) | Celecoxib | Coxib | 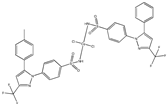 (proposed structure) | Inhibitory activity against cyclooxygenase II | [89] |
(a) asa = aspirin and Haroy = protonated aroylhydrazone (m-hydroxylbenzaldehyde-4-nitrobenzoylhydrazone); (b) difl = diflunisal; (c) np = naproxen and py = pyridine; (d) dicl = deprotonated diclofenac and py = pyridine; (e) Hmel = protonated meloxicam; (f) tolf = tolfenamate and bipyam = 2,2′-bipyridylamine; (g) cxb = celecoxib.

Table 3.
Examples of nickel(II) complexes with different NSAIDs.
Table 3.
Examples of nickel(II) complexes with different NSAIDs.
| Chemical Formula | NSAID | Chemical Structure | Biological Activity | Ref. | |
|---|---|---|---|---|---|
| NSAID Ligand | NSAID Coordinating Group | ||||
| [Ni(asa)(aroy)(H2O)2] (a) | Aspirin | Acetylated salicylate | 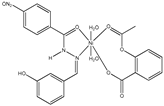 (proposed structure) | Weaker antimicrobial activity comparing to free aspirin. | [86] |
| [Ni(difl)2(MeOH)4] (b) | Diflunisal | Non-acetylated salicylate | 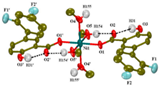 | Albumin and DNA interaction, antioxidant activity. | [31] |
| [Ni(nap)2(phen)(H2O)] (c) | Naproxen | Propionic acid | 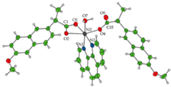 | Significant affinity for BSA and HSA, DNA-binding and antioxidant activity. | [94] |
| [Ni(dicl)(Hdicl)(Hpko)2](dicl) CH3OH•0.6H2O (d) | Diclofenac | Acetate | 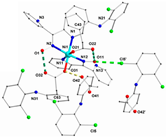 | DNA and albumin binding. | [99] |
| [Ni(Hmel)2(H2O)2]•2H2O (e) | Meloxicam | Enolic acid | 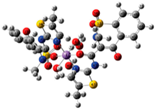 (theoretical structure) | Greater antibacterial activity than free meloxicam. | [100] |
| [Νi(tolf)2(bipy)(CH3OH)2] (f) | Tolfenamic acid | Anthranilate |  | Significant affinity to bind BSA and HSA. Potent scavenging activity of hydroxyl and superoxide radicals. Better DNA binder comparing to free tolfenamic acid. | [101] |
| [Ni(cxb)2Cl2] (g) | Celecoxib | Coxib | 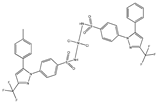 (proposed structure) | Inhibitory activity against cyclooxygenase II. | [89] |
(a) asa = aspirin and aroy = aroylhydrazone (m-hydroxylbenzaldehyde-4-nitrobenzoylhydrazone); (b) difl = diflunisal; (c) np = naproxen and phen = 1,10-phenanthroline; (d) dicl = deprotonated diclofenac and Hpko = protonated 2,20-dipyridylketone oxime; (e) Hmel = protonated meloxicam; (f) tolf = tolfenamate and bipy = 2,20 -bipyridine; (g) cxb = celecoxib.

Table 4.
Examples of manganese(II) complexes with different NSAIDs.
Table 4.
Examples of manganese(II) complexes with different NSAIDs.
| Chemical Formula | NSAID | Chemical Structure | Biological Activity | Ref. | |
|---|---|---|---|---|---|
| NSAID Ligand | NSAID Coordinating Group | ||||
| [{Mn(asa)(nic)}2(H2O)Cl]Cl•2H2O (a) | Aspirin | Acetylated salicylate | 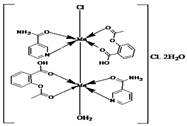 (proposed structure) | Comparing to standard (ascorbic acid) similar antioxidant activities were observed for the Mn(II) complex and both free drugs. | [102] |
| [Mn(nap)2(py)2(H2O)2] (b) | Naproxen | Propionic acid |  | Selective scavenging activity of hydroxyl and superoxide radicals. Binds tighter to CT-DNA than the corresponding free NSAID and exhibits significant affinity to BSA and HSA. | [95] |
| [Mn3(dicl)6(phen)2(MeOH)] (c) | Diclofenac | Acetate | 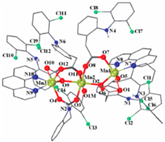 | Higher binding affinities to BSA and HSA comparing to those of free sodium diclofenac. Significant ability to scavenge ABTS and hydroxyl radicals. Potent inhibitory activity of soybean lipoxygenase. | [103] |
| [Mn(Hmel)(Gly)(H2O)2]•5H2O (d) | Meloxicam | Enolic acid | 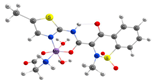 (DFT–optimized geometry) | No antifungal activity against A. niger, but antibacterial activities comparing to amoxycillin/clavulanic and cetaxime (antibacterial agents). | [104] |
| [Mn(tolf)2(phen)(H2O)] (e) | Tolfenamic acid | Anthranilate | 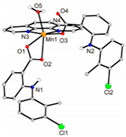 | High scavenging activity against superoxide and hydroxyl radicals. It can also inhibit the activity of soybean lipoxygenase and shows tight binding affinity for BSA and HAS. | [96] |
(a) asa = aspirin and nic = nicotinamide; (b) np = naproxen and py = pyridine; (c) dicl = deprotonated diclofenac and phen = 1,10-phenanthroline; (d) Hmel = protonated meloxicam and gly = glycine; (e) tolf = tolfenamate and phen = 1,10-phenanthroline.

Table 5.
Examples of zinc(II) complexes with different NSAIDs.
Table 5.
Examples of zinc(II) complexes with different NSAIDs.
| Chemical Formula | NSAID | Chemical Structure | Biological Activity | Ref. | |
|---|---|---|---|---|---|
| NSAID Ligand | NSAID Coordinating Group | ||||
| [Zn(asa)2] (a) | Aspirin | Acetylated salicylate | No crystal structure has been published to the best of our knowledge. | After oral administration to rats it caused a decrease in blood glucose levels, and type-2 diabetes-induced damage in rat cardiac tissue was alleviated. This complex also showed a better post-ischemic myocardial dysfunction- preventing effect than free aspirin. | [105,106,107] |
| [Zn(difl)2(bipy)] (b) | Diflunisal | Non-acetylated salicylate | 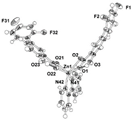 | The complex is a more active radical scavenger and lipooxigenase inhibitor than free diflunisal. The complex also binds strongly to albumins. | [62] |
| [Zn(nap)2(N3)2]Na2 (c) | Naproxen | Propionic acid | 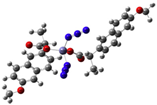 | The complex shows antibacterial activity against Gram-positive (S. aureus) and Gram-negative (E. coli) bacterial strains. | [80] |
| [Zn2(dicl)4(nic)2] (d) | Diclofenac | Acetate | 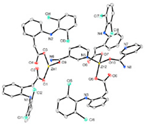 | Improved solubility of the complex in comparision with free NSAID. The complex probably interacts with the grooves of the secondary structure of CT-DNA by electrostatic attraction. | [97] |
| [Zn(Hmel)2(EtOH)2] (e) | Meloxicam | Enolic acid | 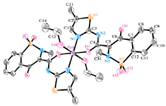 | The complex may interact with DNA through an electrostatic mode and promoted the photo cleavage of a plasmid DNA. | [92] |
| [Zn3(tolf)6(CH3OH)2] (f) | Tolfenamic acid | Anthranilate | 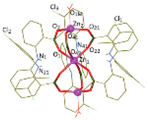 | Good binding constants for BSA and HSA, suggesting a possible release from the serum albumin to the target cell. | [98] |
(a) asa = aspirin; (b) difl = diflunisal and bipy = 2,2′-bipyridine; (c) np = naproxen; (d) dicl = deprotonated diclofenac and nic = nicotinamide; (e) Hmel = protonated meloxicam; (f) tolf = tolfenamate.
As can be seen in Table 1, Table 2, Table 3, Table 4 and Table 5, whatever the metal ion in Cu(II), Co(II), Mn(II), Ni(II) and Zn(II) complexes, the ligand-drugs aspirin, diflunisal, naproxen, diclofenac and tolfenamic acid, are, in their deprotonated forms, coordinated by N and O donor atoms. All form six coordinated structures (regular or distorted octahedral geometries), except for the Cu(II)−meloxicam [87] complex, which has a square pyramidal geometry, as expected. With the ligand celecoxib, four coordinated structures have been proposed for complexes of copper, cobalt and nickel, as well [89]. However, despite a few exceptions, divalent metal−NSAID coordination compounds show a common tendency for octahedral geometries.
In general, all NSAID−metal complexes previously presented show increased biological activity compared to the parent drug, including antitumor, antimicrobial, antioxidant, and interactive activities. This enhancement of the biological activity of the metal complexes can be explained on the basis of Overton’s concept [108] and chelation theory [109,110]. According to the former concept, the cell membrane is selective and thus favors the crossing of lipid-soluble components. Upon chelation, in accordance with chelation theory, the polarity of the metal ion is reduced due to the overlapping with the ligand orbitals and partial sharing of the positive charge with donor groups. Chelation may also increase π−electron delocalization on the chelate ring, and therefore enhance the lipophilicity of the coordination compound, resulting in a better penetration into the cellular lipid membrane and consequent better biological activity [111].
3. Biological Effects of the Metal Complexes
3.1. Anti-Tumor Activity
Epidemiological studies found that there is a strong correlation between inflammation and cancer since there are phenotypical similarities between tumoral and inflammatory cells [112]. Cancer cells can induce tissue and DNA injuries through the secretion of inflammatory signals (cytokines, chemokines, etc.), which promote mutated cells to grow [112]. These cells, in turn, are able to produce more cytokines and recruit new inflammatory cells, creating an inflammatory environment that contributes to angiogenesis, migration and metastasis [112,113]. As metal−NSAID complexes have shown an enhanced affinity for DNA binding, they should be considered for tumor therapy [114]. Some metal−NSAID coordination compounds have shown to be selective for different cell lines, and so these types of “mixed compounds,” i.e., NSAIDs and metal ions, appear to be a very interesting step towards the selective killing of tumor cells [57,114]. According to literature, among the available NSAIDs−metal ion complexes, Cu(II)−NSAID complexes have the best anticancer effects, probably due to their well-known ability to reduce inflammatory processes [115,116]. Nevertheless, Deb and co-workers synthetized and characterized a Zn(II)–naproxen complex and a Zn(II)–mefenamate complex, which proved to have cytotoxic cell killing properties against a breast cancer cell line (MDA-MB-231) [117]. Of note, other metal-based compounds (besides the ones covered by this review) with anti-tumor potential, such as platinum−indomethacin and platinum−tolmetin complexes, have been proved to inhibit the growth of the L929 cancer cell line [118].
In 2012, Sayen and co-workers reported the first diclofenac-based coordination compound synthesized from a wholly aqueous medium. This compound exhibited cytotoxicity against human colon adenocarcinoma cell lines, and the Cu(II) salt and diclofenac have shown no cytotoxic activity per si [119]. More recently, a new Cu(II)−aspirin coordination compound was reported and revealed to be have multiple cellular targets (nucleus, mitochondrion and cyclooxygenase-2) [120]. This complex effectively induces mitochondrial dysfunction and promotes early apoptosis in ovarian cancer cells and also inhibits the expression of cyclooxygenase-2.
3.2. Antimicrobial Activity
Although NSAIDs are commonly used to treat pain, fever and inflammation, few reports suggest that NSAIDs also possess antimicrobial properties [121]. In particular, these drugs can be active against bacteria, viruses and fungi [122]. Their antimicrobial activity may be directly caused by membrane effects, metabolic alterations, DNA intercalation or adhesion suppression. However, indirectly, they can also serve as helper compounds, i.e., by having a synergistic effect, when co administered with other drugs, by inhibiting replication of the plasmids and eliminating them from cells, or stimulating cytokine production from the T-cells, or even potentiating the killing of the phagocytized microbes inside the macrophage [123]. Ibuprofen is one of the most widely used NSAIDs. Its antibacterial and antifungal activities were firstly described by Hersh and co-workers [124] and by Sanyal and co-workers [125], respectively. However, these antimicrobial effects are normally achieved with a high dose (higher than the therapeutic one) [125], which has been a large reason for the synthesis of new coordination compounds bearing this drug in their composition. For example, Abu Ali and co-workers synthetized a Zn(II)−ibuprofen metal complex that showed antibacterial activity against Gram-positive (M. luteus, S. aureus and B. subtilis) and Gram-negative bacteria (E. coli, K. pneumonia and P. mirabilis) [126]. Many other coordination compounds with NSAIDs and different metal ions also show antimicrobial activity. As examples, Table 2 lists a cobalt−naproxen metal complex that exhibited moderate activity against five Gram-positive bacteria, eight Gram-negative bacteria and three fungi. Additionally, Lawal and co-workers reported an example of a Co(II)-aspirin complex that showed a marked inhibitory effect against B. subtilis [127]. Moreover, a Ni(II)−meloxicam [100] complex was found to have stronger antimicrobial activity than free meloxicam (Table 3), and a Mn(II)–meloxicam complex also proved to have better antibacterial activity against E. coli, Coliform, S. aureus, S. typhi, Citrobacter and Listeria compared to the free NSAID (Table 4) ([104]. Additionally, Ashouri and co-workers synthetized novel Co(II) and Mn(II)−diclofenac coordination compounds that showed enhanced inhibitory activity in comparison with free diclofenac and metal salts [128]. It is noteworthy that several NSAIDs have antiviral activity, namely, aspirin, ibuprofen, naproxen, acetaminophen and lornoxicam, being able to potently inhibit the entry of Zika virus into the cells [129]. However, to the best of our knowledge, no studies evaluating the potential of NSAID-based metal complexes as antiviral agents have been done yet.
3.3. Antioxidant Activity
Reactive oxygen (ROS) and reactive nitrogen species (RNS) are normally formed during physiological and metabolic processes (e.g., mitochondrial respiration or inflammation) [130]. However, excessive production of these reactive species plays a critical role in the generation of oxidative stress. It is important to ensure a balance between the pro-oxidant and the antioxidant levels, in order to keep the biological equilibrium of the redox states in the cell [131,132,133]. Otherwise, the ROS/RNS may cause cellular, lipidic or DNA damage [130,134] that may result in many pathological conditions [135]. It is also known that lipid hydroperoxides and the oxygenated products of lipid degradation can contribute to cell proliferation, to signal transduction cascades and to differentiation and apoptosis [136]. Some NSAIDs have shown to be potent scavengers of ROS/RNS [134,137].
From the analysis of the Table 1, Table 2, Table 3, Table 4 and Table 5 (see above), we can see that most of the Cu(II)/Co(II)/Ni(II)/Mn(II)/Zn(II)−NSAID complexes have high antioxidant activity. Cu(II)/Ni(II)−tolfenamic acid complexes [88,101] proved to have strong scavenging activity compared to free tolfenamic acid, and the same behavior was observed for Co(II)/Ni(II)−diflunisal [31,90] and Co(II)/Ni(II)−naproxen [91,94] complexes. Tarushi and co-workers synthetized Zn(II)−diflunisal and Zn(II)−mefenamic acid complexes that proved to have an enhanced scavenging activity, compared with free drug, towards hydroxyl radicals [62,138]. Dimiza and co-workers also characterized Mn(II)−naproxen and Mn(II)−mefenamic acid complexes, which showed selective scavenging activity against hydroxyl and superoxide radicals [95]. Finally, some Zn(II)−tolfenamic acid complexes with low to moderate DPPH radical scavenging activity, but with a high scavenging activity against hydroxyl and superoxide radicals, have been also prepared [139].
4. Interactions with Biomolecules
4.1. Nucleic Acids
Most NSAIDs are found to have chemoprevention effects on different cell lines [140]. Their anticancer effects are proposed to be mainly exerted at the protein level and not at genomic level because NSAIDs have an anionic charge at physiological pH (which does not allow interaction with the polyanionic DNA backbone) [140]. In this context, the coordination of NSAIDs with biologically active metal ions, forming charge neutral complexes that may bind DNA, is a promising approach in order to improve the therapeutic action of NSAIDs or even to reduce their toxicity (such as the hepatotoxicity observed with aspirin) [141,142]. DNA binding can also be effective in antibiotic or antiviral therapeutic agents [92,143]. Shahabadi and co-workers showed that a Pt(II) complex containing ribavirin (an antiviral drug) interacts with DNA, probably via an intercalative mode, and that this complex has a higher affinity to DNA then ribavirin per si [144].
DNA is the primary macromolecule that is targeted by anticancer drugs. Small molecules can induce or suppress cellular interactions related to DNA, thereby changing its structure with inherent consequences for biological mechanisms, such as transcription, replication and repairing processes, which can consequently promote cell death [145,146]. In general, these interaction modes can be divided into two main types: covalent and non-covalent interactions [147]. A covalent bond can be formed when the complex has labile metal−ligand bonds. For example, in the case of cisplatin, the two chloride ligands are replaced by water molecules inside the cell. However, these water molecules are loosely bound to Pt, and a N atom of a nucleobase can displace them and allow the formation of a platinum−DNA covalent bond [94,148]. In a different approach, and in contrast with the DNA covalent interaction described above, metal complexes can interact with DNA in a reversible and non-covalent manner. This type of interaction offers several possibilities to contribute to the bonding, such as hydrogen bonding, π–π stacking and hydrophobic interactions [149]. This non-covalent bonding includes intercalation and groove binding [149,150]. Intercalation is anti-cooperative at adjacent sites, meaning that intercalators can only bind with alternative DNA base pairs. When an intercalator bonds to one DNA base pair, its two neighboring sites may continue unoccupied [151,152]. Groove binding corresponds to the association of the whole or a part of the complex with one of the grooves or with both of them (major and minor groove). This association is carried out by a combination of different parameters, such as electrostatic forces, van der Waals contacts, hydrophobic interactions and hydrogen bonding [153]. Once there are no free energy costs for this kind of binding, groove binders have bigger association constants than mere intercalators [149].
It is noteworthy that these alterations may result in improvements in the metal complexes’ biological activities, i.e., in the metallodrugs efficacy [125,154]. In particular, the interactions of metal−NSAID complexes with DNA, especially with calf-thymus DNA (CT-DNA), are considered of great importance when investigating the potential anticancer and/or anti-inflammatory effects of these coordination compounds, and for that reason, are the subject of many interaction studies [155].
Interactions of Cu(II)/Co(II)/Ni(II)/Mn(II)/Zn(II)−NSAID Complexes with DNA
By means of UV spectroscopy, it is possible to conclude that the bonding strength of the reported complexes (Table 1, Table 2, Table 3, Table 4 and Table 5, see above) to CT-DNA is strong, and in general, these metal−NSAID complexes bond more tightly to DNA than their parent drugs. For instance, the [Cu(nap)(tpy)Cl] [84], [Co(nap)2(py)2(H2O)2] [91], [Ni(nap)2(phen)(H2O)] [94] and [Mn(nap)2(py)2(H2O)2] [95] complexes have higher binding constants (Kb = 2.24 (±0.25) × 105, 3.15 (±0.57) × 104, 1.54 (±0.12) × 105 and 2.29 (±0.13) × 105 M−1, respectively) than the free NSAID naproxen (Kb = 2.67 (±0.22) × 104). In addition, [Cu(nap)(tpy)Cl] [84], [Co(tolf)2(bipyam)] [93], [Ni(dicl)(Hdicl)(Hpko)2](dicl)CH3OH•0.6H2O [99], [Mn(nap)2(py)2(H2O)2] [95] and [Zn(Hmel)2(EtOH)2] [92] exhibit the highest Kb values among the listed Cu(II), Co(II), Ni(II), Mn(II) and Zn(II) complexes, respectively (see Table 6 below).

Table 6.
DNA biding constants (Kb) of Cu(II)/Co(II)/Ni(II)/Mn(II)/Zn(II)−NSAID complexes.
Through cyclic voltametric titration studies and viscosity measurements, it is possible to deduce how the complexes bind to DNA. Looking to the given examples in Table 1, Table 2, Table 3, Table 4 and Table 5, intercalation is the most common bonding mode to DNA. Three exceptions can be seen for [Cu(nap)(tpy)Cl] [84], [Zn2(dicl)4(nic)2] [97] and [Co(Hmel)2(EtOH)2] [92]. In the first two cases, the results demonstrated that the interactions between the complexes and DNA are due to groove binding events, and in the last case the spectroscopic and electrochemical results for Co(II)–meloxicam indicated that the complex can interact with DNA through an electrostatic mode.
Binding studies with the other nucleic acid, RNA, have been less frequent. Recently, this gap has been filled, due to the advantage that RNA offers much more extensive structural diversity than DNA [156]. Therefore, one may expect higher specificity of the compound’s interactions with RNA, through RNA-binding sites [157]. A recent study demonstrated that in the cisplatin treatment of Saccharomyces cerevisiae, the platinum complex accumulates 4 to 20-fold more times in cellular RNA than in genomic DNA [158]. With NSAIDs, Huzaifa and co-workers synthetized novel Cu(II) and Zn(II) coordination compounds, and mefenamic acid was used in their synthesis (although this ligand behaves as a counterion) [157]. These complexes demonstrated their preferential binding to t-RNA, when compared to CT-DNA, supporting the hypothesis that RNA interaction studies may be a new path to explore in the search for the synthesis of new and more efficacious metallodrugs. Regarding the possible antiviral activity of NSAIDs, the study of RNA binding affinity would entail greater importance, since most viruses are RNA viruses. In fact, there are indications that naproxen may bind to RNA groove of the influenza A virus [159], resulting in a novel antiviral drug. However, to the best of our knowledge, no studies to evaluating the interactions between metal−NSAID complexes and RNA viruses have been performed yet.
4.2. Proteins
For decades, metallodrugs research has been focused on DNA binding which, as described above, relies on the damage that metal complexes could inflict on DNA structure. However, it is now known that metallodrugs can exert their effects on other molecules, and proteins have appeared as some of their alternative targets [160]. Of all the molecules present in living organisms, proteins are the most abundant, and they are involved on almost every process. Kinase proteins may be good examples/targets, as their deregulation usually occurs in tumors, which can be crucial for the survival and progression of cancer cells [161]. There are already a few metal complexes that have been designed for the purpose of interacting with the ATP binding sites of kinase proteins and to act as their inhibitors [162,163]. It has been reported that some NSAIDs inhibit telomerase activity, an enzyme that plays a crucial role on a variety of cancers [164,165]. However, to the best of our knowledge, there are no reports of similar assays with metal−NSAID complexes. The same line of reasoning is applied for studies with G-quadruplex structures, which can be very interesting targets for metal complexes as well, since interactions between these structures and metal complexes may block telomerase activity, and consequently inhibit cancer cells to maintain telomere lengths [166,167]. However, no studies have been reported with metal−NSAID complexes, although a manganese coordination compound, a Mn(III)−porphyrin complex, has shown exceptional 10,000-fold selectivity for the telomeric region of duplex DNA [168].
Nevertheless, proteins in cancer cells are not the only target proteins. Host proteins can also act as “self” drug delivery systems. In this field, human serum albumin (HSA) is one of the most interesting systems [169]. HSA is the most abundant protein in blood plasma (60% of the total protein content), and because of its chemical characteristics, it can reversibly bind to drugs. This behavior, already known to occur with some NSAIDs, such as aspirin and ibuprofen, can enhance drugs’ biodistribution and/or bioavailability [160,170]. This issue, that is, drug–protein interactions, has been enlarged to include metallodrug−protein interactions.
Interactions of Cu(II)/Co(II)/Ni(II)/Mn(II)/Zn(II)−NSAID Complexes with Serum Albumins
HSA and its structural homologue bovine serum albumin (BSA) have been the serum albumins used to study interactions with complexes, through quenching studies. From the Scatchard equation and graphs, the binding constants K have been calculated to determine the binding affinities of complexes to serum albumins. Various Cu(II)/Co(II)/Ni(II)/Mn(II)/Zn(II)−NSAID complexes have significant affinity for HSA (and BSA) proteins (see Table 1, Table 2, Table 3, Table 4 and Table 5 above). Among those examples, Cu(II)/Co(II)/Ni(II)/Zn(II)−diflunisal [31,62,83,90], Co(II)/Ni(II)/Mn(II)−naproxen [91,94,95], Cu(II)/Ni(II)/Mn(II)−diclofenac [85,99,103] and Cu(II)/Co(II)/Ni(II)/Zn(II)−tolfenamic acid [88,93,98,101] complexes can be highlighted due to high K values (see Table 7 below), revealing significant affinities for HSA, and tight but reversible binding to these albumin molecules.

Table 7.
HSA biding constants (K) of Cu(II)/Co(II)/Ni(II)/Mn(II)/Zn(II)−NSAID complexes.
It is noteworthy that all K values of the given examples are within an optimal range; i.e., in general they are higher than those of the free corresponding NSAIDs, allowing the binding of the complexes to serum albumins. However, these values are well below the association constant of one of the strongest known non-covalent bonds, the avidin–ligand interaction (K ≈ 1015 M−1), suggesting a possible release from the serum albumin to the target cells [82].
5. Conclusions
During the past 60 years, and after the success of platinum complexes for cancer treatment, metal-based drugs have been studied and nowadays some are commercially available. In particular, metal complexes containing NSAIDs are a group of compounds that have attracted much interest among the scientific community. More specifically, d-block metals and their cations, namely, copper, cobalt, nickel, manganese and zinc are by far the most exploited in NSAID-based metal complexes (metallodrugs).
In this review, the focus has been on the remarkable effects of these metallodrugs, including their wide ranges of biological activities (as anti-tumor, antimicrobial or antioxidant agents), and also on their ability to interact with nucleic acids. Since the pharmacologic effects of NSAIDs can be altered upon coordination to metal ions, it is possible to enhance the biological effects of the drugs and to decrease possible side effects, and eventually it may allow the interaction with new biomolecular targets. In this last case, the present review reports the potential of these coordination compounds as possible candidates for RNA targeting, since this last biomolecule not only plays important roles in cell and molecular biology (e.g., protein synthesis, messenger of genetic information, etc.), but also offers more extensive structural diversity than DNA that could be beneficial for RNA-binding metallodrug therapeutics. However, this review also considered protein targets (HSA, BSA), since binding to these proteins may be also of interest as targets or for the delivery of metallodrugs in chemotherapy. Moving toward a better understanding of these non-DNA molecular targets and their interactions with metallodrugs may be an advantageous path for the further optimization and consequent clinical development of metal−NSAID compounds.
Funding
This work was developed within the scope of the project CICECO-Aveiro Institute of Materials, UIDB/50011/2020, UIDP/50011/2020 and LA/P/0006/2020, financed by national funds through the FCT/MEC (PIDDAC). A.S. gratefully acknowledges FCT for her doctoral grant (2021.08157.BD) The position held by B.J.M.L.F.was finanted by national funds (OE), through FCT—Fundação para a Ciência e a Tecnologia, I.P., in the scope of the framework contract foreseen in numbers 4, 5 and 6 of Article 23, of the Decree-Law 57/2006, of August 29, changed by Law 57/19 July 2017.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mjos, K.D.; Orvig, C. Metallodrugs in Medicinal Inorganic Chemistry. Chem. Rev. 2014, 114, 4540–4563. [Google Scholar] [CrossRef] [PubMed]
- Schrader, S.M.; Vaubourgeix, J.; Nathan, C. Biology of antimicrobial resistance and approaches to combat it. Sci. Transl. Med. 2020, 12, eaaz6992. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Ambika, S.; Manojkumar, Y.; Arunachalam, S.; Gowdhami, B.; Sundaram, K.; Solomon, R.; Akbarsha, M.; Sundararaman, M. Biomolecular Interaction, Anti-Cancer and Anti-Angiogenic Properties of Cobalt(III) Schiff Base Complexes. Sci. Rep. 2019, 9, 2721. [Google Scholar] [CrossRef] [PubMed]
- Kwan Law, B.; Qing Qu, Y.; Mok, S.; Liu, H.; Zeng, W.; Han, Y.; Gordillo-Martinez, F.; Chan, W.; Wong, K.; Wong, V. New perspectives of cobalt tris(bipyridine) system: Anti-cancer effect and its collateral sensitivity towards multidrug-resistant (MDR) cancers. Oncotarget 2017, 8, 55003–55021. [Google Scholar]
- Wojciechowska, A.; Szuster-Ciesielska, A.; Sztandera, M.; Bregier-Jarzebowska, R.; Jarzab, A.; Rojek, T.; Komarnicka, U.; Bojarska-Junak, A.; Jezierska, J. L-argininato copper(II) complexes in solution exert significant selective anticancer and antimicrobial activities. Appl. Organometal. Chem. 2020, 34, e5698. [Google Scholar] [CrossRef]
- Selvaganapathy, M.; Raman, N. Pharmacological Activity of a Few Transition Metal Complexes: A Short Review. J. Chem. Biol. Ther. 2016, 1, 108. [Google Scholar] [CrossRef]
- Ndagi, U.; Mhlongo, N.; Soliman, M.E. Metal complexes in cancer therapy—An update from drug design perspective. Drug Des. Devel. Ther. 2017, 1, 599–616. [Google Scholar] [CrossRef]
- Savithri, K.; Kumar, B.C.V.; Vivek, H.K.; Revanasiddappa, H.D. Synthesis and Characterization of Cobalt(III) and Copper(II) Complexes of 2-((E)-(6-Fluorobenzo[d]thiazol-2-ylimino) methyl)-4-chlorophenol: DNA Binding and Nuclease Studies—SOD and Antimicrobial Activities. Int. J. Spectrosc. 2018, 2018, 8759372. [Google Scholar] [CrossRef]
- Housecroft, C.E.; Sharpe, A.G. Inorganic Chemistry, 4th ed.; Pearson: London, UK, 2012. [Google Scholar]
- Lawrance, G.A. Introduction to Coordination Chemistry, 1st ed.; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Pavelková, M.; Vysloužil, J.; Kubová, K.; Vetchý, D. Biological role of copper as an essential trace element in the human organism. Ces. Slov. Farm. 2018, 67, 143–153. [Google Scholar]
- Osredkar, J.; Sustar, N. Copper and Zinc, Biological Role and Significance of Copper/Zinc Imbalance. J. Clin. Toxicol. 2011, 3, 495–513. [Google Scholar] [CrossRef]
- Agotegaray, M.A.; Boeris, M.A.; Quinzani, O.V. Significant anti-inflammatory properties of a copper(II) fenoprofenate complex compared with its parent drug. Physical and chemical characterization of the complex. J. Braz. Chem. Soc. 2010, 21, 2294–2301. [Google Scholar] [CrossRef]
- Hamamci Alisir, S.; Dege, N.; Tapramaz, R. Synthesis, crystal structures and characterizations of three new copper(II) complexes including anti-inflammatory diclofenac. Acta Crystallogr. Sect. C Struct. Chem. 2019, 75, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Festa, R.A.; Thiele, D.J. Copper: An Essential Metal in Biology. Curr. Biol. 2011, 21, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Krstić, N.S.; Nikolić, R.S.; Stanković, M.N.; Nikolić, N.G.; Đorđević, D.M. Coordination Compounds of M(II) Biometal Ions with Acid- Type Anti-inflammatory Drugs as Ligands—A Review. Trop. J. Pharm. Res. 2015, 14, 337–349. [Google Scholar] [CrossRef]
- Kobayashi, M.; Shimizu, S. Cobalt proteins. Eur. J. Biochem. 1999, 261, 1–9. [Google Scholar] [CrossRef]
- Perontsis, S.; Dimitriou, A.; Fotiadou, P.; Hatzidimitriou, A.G.; Papadopoulos, A.N.; Psomas, G. Cobalt(II) complexes with the non-steroidal anti-inflammatory drug diclofenac and nitrogen-donor ligands. J. Inorg. Biochem. 2019, 196, 110688. [Google Scholar] [CrossRef]
- Parada, J.; Atria, A.; Baggio, R.; Wiese, G.; Lagos, S.; Pavón, A.; Rivas, E.; Navarro, L.; Corsini, G. Antibacterial activity and human cell cytotoxic of cobalt(III) complexes with 1,10-phenanthroline and carbohydrate ligands. J. Chil. Chem. Soc. 2017, 62, 3746–3751. [Google Scholar] [CrossRef][Green Version]
- Shalash, A.M.; Abu Ali, H.I. Synthesis, crystallographic, spectroscopic studies and biological activity of new cobalt(II) complexes with bioactive mixed sulindac and nitrogen-donor ligands. Chem. Cent. J. 2017, 11, 40. [Google Scholar] [CrossRef]
- Horning, K.J.; Caito, S.W.; Tipps, K.G.; Bowman, A.B.; Aschner, M. Manganese is Essential for Neuronal Health. Annu. Rev. Nutr. 2015, 35, 71–108. [Google Scholar] [CrossRef]
- Li, L.; Yang, X. The essential element manganese, oxidative stress, and metabolic diseases: Links and interactions. Oxid. Med. Cell. Longev. 2018, 2018, 7580707. [Google Scholar] [CrossRef] [PubMed]
- Peres, T.V.; Schettinger, M.R.C.; Chen, P.; Carvalho, F.; Avila, D.S.; Bowman, A.; Aschner, M. Manganese-induced neurotoxicity: A review of its behavioral consequences and neuroprotective strategies. BMC Pharmacol. Toxicol. 2016, 17, 57. [Google Scholar] [CrossRef] [PubMed]
- Bouabid, S.; Tinakoua, A.; Lakhdar-Ghazal, N.; Benazzouz, A. Manganese neurotoxicity: Behavioral disorders associated with dysfunctions in the basal ganglia and neurochemical transmission. J. Neurochem. 2016, 136, 677–691. [Google Scholar] [CrossRef] [PubMed]
- Becatti, M.; Bencini, A.; Nistri, S.; Conti, L.; Fabbrini, M.G.; Lucarini, L.; Ghini, V.; Severi, M.; Fiorillo, C.; Giorgi, C.; et al. Different Antioxidant Efficacy of Two MnII-Containing Superoxide Anion Scavengers on Hypoxia/Reoxygenation-Exposed Cardiac Muscle Cells. Sci. Rep. 2019, 9, 10320. [Google Scholar] [CrossRef]
- Armstrong, F.A. Why did Nature choose manganese to make oxygen? Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 1263–1270. [Google Scholar] [CrossRef]
- Zhu, W.; Richards, N.G.J. Biological functions controlled by manganese redox changes in mononuclear Mn-dependent enzymes. Essays Biochem. 2017, 61, 259–270. [Google Scholar]
- Godwin, C.M.; Zehnpfennig, J.R.; Learman, D.R. Biotic and Abiotic Mechanisms of Manganese(II) Oxidation in Lake Erie. Front. Environ. Sci. 2020, 8, 57. [Google Scholar] [CrossRef]
- Kumar, S.; Trivedi, A.V. A Review on Role of Nickel in the Biological System. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 719–727. [Google Scholar] [CrossRef]
- Perontsis, S.; Hatzidimitriou, A.G.; Papadopoulos, A.N.; Psomas, G. Nickel-diflunisal complexes: Synthesis, characterization, in vitro antioxidant activity and interaction with DNA and albumins. J. Inorg. Biochem. 2016, 162, 9–21. [Google Scholar] [CrossRef]
- Zamble, D. Introduction to the Biological Chemistry of Nickel. In The Biological Chemistry of Nickel; RSC Met: London, UK, 2017; pp. 1–11. [Google Scholar]
- Ahn, Y.; Jun, Y. Nickel-Dependent Metalloenzymes. Arch. Biochem. Biophys 2014, 544, 142–152. [Google Scholar]
- Bregadze, V.; Khutsishvili, I.; Melikishvili, S.; Melikishvili, Z. Nickel(II) Ions Interaction with Polynucleotides and DNA of Different GC Composition. arXiv 2009, arXiv:0912.4866. [Google Scholar]
- McNaught, A.D.; Wilkinson, A. IUPAC. Compendium of Chemical Terminology. In Gold Book, 2nd ed.; Blackwell Science Publisher: Oxford, UK, 1997; Volume 1077, p. 2740. [Google Scholar]
- Krężel, A.; Maret, W. The biological inorganic chemistry of zinc ions. Arch. Biochem. Biophys. 2016, 611, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Bonaventura, P.; Benedetti, G.; Albarede, F.; Miossec, P. Zinc and its role in immunity and inflammation. Autoimmun. Rev. 2014, 14, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Khomskii, D.I. Transition Metal Compounds; Cambridge U. Press: Cambridge, UK, 2014. [Google Scholar]
- Tylkowski, B.; Jastrzab, R.; Odani, A. Developments in platinum anticancer drugs. Phys. Sci. Rev. 2019, 3, 160–173. [Google Scholar]
- Makovec, T. Cisplatin and beyond: Molecular mechanisms of action and drug resistance development in cancer chemotherapy. Radiol. Oncol. 2019, 53, 148–158. [Google Scholar] [CrossRef]
- Kovala-Demertzi, D.; Hadjipavlou-Litina, D.; Staninska, M.; Primikiri, A.; Kotoglou, C.; Demertzis, M.A. Anti-oxidant, in vitro, in vivo anti-inflammatory activity and antiproliferative activity of mefenamic acid and its metal complexes with manganese(II), cobalt(II), nickel(II), copper(II) and zinc(II). J. Enzyme Inhib. Med. Chem. 2009, 24, 742–752. [Google Scholar] [CrossRef]
- Leung, C.H.; Lin, S.; Zhong, H.J.; Ma, D.L. Metal complexes as potential modulators of inflammatory and autoimmune responses. Chem. Sci. 2015, 6, 871–884. [Google Scholar] [CrossRef]
- Annunziata, A.; Cucciolito, M.; Esposito, R.; Ferraro, G.; Monti, D.M.; Merlino, A.; Ruffo, F. Five-Coordinate Platinum(II) Compounds as Potential Anticancer Agents. Eur. J. Inorg. Chem. 2020, 2020, 918–929. [Google Scholar] [CrossRef]
- Saddam Hossain, M. Selected Pharmacological Applications of 1stRow Transition Metal Complexes: A review. Clin. Med. Res. 2017, 6, 177–191. [Google Scholar] [CrossRef]
- Anthony, E.J.; Bolitho, E.M.; Bridgewater, H.E.; Carter, O.W.L.; Donnelly, J.M.; Imberti, C.; Lant, E.C.; Lermyte, F.; Needham, R.J; Palau, M.; et al. Metallodrugs are unique: Opportunities and challenges of discovery and development. Chem. Sci. 2020, 11, 12888–12917. [Google Scholar] [CrossRef]
- Bin Ou, Z.; Lu, Y.H.; Lu, Y.M.; Chen, S.; Xiong, Y.H.; Zhou, X.H.; Mao, Z.W.; Le, X.Y. A copper(II) complex with 2-(2’-pyridyl)benzimidazole and L-arginine: Synthesis, structure, antibacterial activities, and DNA interaction. J. Coord. Chem. 2013, 66, 2152–2165. [Google Scholar]
- Raman, N.; Joseph, J.; Velan, A.S.K.; Pothiraj, C. Antifungal Activities of Biorelevant Complexes of Copper(II) with Biosensitive Macrocyclic Ligands. Mycobiology 2006, 34, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, H.; Bhatt, V. The State of Art in Coordination Compounds with Antifungal Activity. J. Chem. Chem. Sci. 2018, 8, 595–605. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Joseph, J. Antifungal Activities of Copper(II) with Biosensitive Macrocyclic Schiff Base Ligands Derived from 4-Aminoantipyrine Derivatives. Mycobiology 2009, 37, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Kumar, G.; Kant, A.; Masram, D.T. Role of Metallodrugs in Medicinal Inorganic Chemistry. In Advances in Metallodrugs: Preparation and Applications in Medicinal Chemistry, 1st ed.; Wiley: Hoboken, NJ, USA, 2020; pp. 71–113. [Google Scholar]
- Pradines, B.; Fusai, T.; Daries, W.; Laloge, V.; Rogier, C.; Millet, P.; Panconi, E.; Kombila, M.; Parzy, D. Ferrocene-chloroquine analogues as antimalarial agents: In vitro activity of ferrochloroquine against 103 Gabonese isolates of Plasmodium falciparum. J. Antimicrob. Chemother. 2001, 48, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Iakovidis, I.; Delimaris, I.; Piperakis, S.M. Copper and Its Complexes in Medicine: A Biochemical Approach. Mol. Biol. Int. 2011, 2011, 594529. [Google Scholar] [CrossRef] [PubMed]
- Weser, U.; Richter, C.; Wendel, A.; Younes, M. Reactivity of antiinflammatory and superoxide dismutase active Cu(II)-salicylates. Bioinorg. Chem. 1978, 8, 201–213. [Google Scholar] [CrossRef]
- Chohan, Z.H.; Iqbal, M.S.; Iqbal, H.S.; Scozzafava, A.; Supuran, C.T. Transition metal acetylsalicylates and their anti-inflammatory activity. J. Enzyme Inhib. Med. Chem. 2002, 17, 87–91. [Google Scholar] [CrossRef]
- Fokunang, C. Overview of non-steroidal anti-inflammatory drugs (nsaids) in resource limited countries. MOJ Toxicol. 2018, 4, 5–13. [Google Scholar] [CrossRef]
- Wongrakpanich, S.; Wongrakpanich, A.; Melhado, K.; Rangaswami, J. A comprehensive review of non-steroidal anti-inflammatory drug use in the elderly. Aging Dis. 2018, 9, 143–150. [Google Scholar] [CrossRef]
- Banti, C.N.; Hadjikakou, S.K. Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) in Metal Complexes and Their Effect at the Cellular Level. Eur. J. Inorg. Chem. 2016, 2016, 3048–3071. [Google Scholar] [CrossRef]
- Ghlichloo, I.; Gerriets, V. Nonsteroidal Anti-inflammatory Drugs (NSAIDs), 1st ed.; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Gałczyńska, K.; Ciepluch, K.; Madej, L.; Kurdziel, K.; Maciejewska, B.; Drulis-Kawa, Z.; Wegierek-Ciuk, A.; Lankoff, A.; Arabski, M. Selective cytotoxicity and antifungal properties of copper(II) and cobalt(II) complexes with imidazole-4-acetate anion or 1-allylimidazole. Sci. Rep. 2019, 9, 9777. [Google Scholar] [CrossRef] [PubMed]
- Gwaram, N.S. Synthesis and characterization of a Schiff base Cobalt(III) complex and assessment of its anti-cancer activity. Chem. Search J. 2017, 8, 56–67. [Google Scholar]
- El-Tabl, A.S.; Mohamed Abd El-Waheed, M.; Wahba, M.A.; Abd El-Halim Abou El-Fadl, N. Synthesis, characterization, and anticancer activity of new metal complexes derived from 2-hydroxy-3-(hydroxyimino)-4-oxopentan-2-ylidene)benzohydrazide. Bioinorg. Chem. Appl. 2015, 2015, 126023. [Google Scholar] [CrossRef]
- Tarushi, A.; Kakoulidou, C.; Raptopoulou, C.P.; Psycharis, V.; Kessissoglou, D.P.; Zoi, I.; Papadopoulos, A.N.; Psomas, G. Zinc complexes of diflunisal: Synthesis, characterization, structure, antioxidant activity, and in vitro and in silico study of the interaction with DNA and albumins. J. Inorg. Biochem. 2017, 170, 85–97. [Google Scholar] [CrossRef]
- Srivastava, P.; Singh, K.; Verma, M.; Sivakumar, S.; Patra, A.K. Photoactive platinum(II) complexes of nonsteroidal anti-inflammatory drug naproxen: Interaction with biological targets, antioxidant activity and cytotoxicity. Eur. J. Med. Chem. 2018, 144, 243–254. [Google Scholar] [CrossRef]
- Crichton, R.R. Biological Inorganic Chemistry—A New Introduction to Molecular Structure and Function, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Fraústo da Silva, J.J.R.; Williams, R.J.P. The Biological Chemistry of the Elements: The Inorganic Chemistry of Life, 2nd ed.; Oxford University Press: New York, NY, USA, 2001. [Google Scholar]
- Bertini, I.; Gray, H.B.; Stiefel, E.; Valentine, J. Biological Inorganic Chemistry: Structure and Reactivity, 3rd ed.; University Science Books: Herndon, VA, USA, 2007. [Google Scholar]
- Solomon, E.I.; Hadt, R.G. Recent advances in understanding blue copper proteins. Coord. Chem. Rev. 2011, 255, 774–789. [Google Scholar] [CrossRef]
- Blackman, A.G. Cobalt: Inorganic & Coordination Chemistry. In Encyclopedia of Inorganic Chemistry, 1st ed.; Wiley: Hoboken, NJ, USA, 2006. [Google Scholar]
- Renfrew, A.K.; O’Neill, E.S.; Hambley, T.W.; New, E.J. Harnessing the properties of cobalt coordination complexes for biological application. Coord. Chem. Rev. 2018, 375, 221–233. [Google Scholar] [CrossRef]
- Chang, E.L.; Simmers, C.; Knight, D.A. Cobalt complexes as antiviral and antibacterial agents. Pharmaceuticals 2010, 3, 1711–1728. [Google Scholar] [CrossRef]
- Vlasiou, M. Synthesis and Structure of Cobalt Coordinated Molecules with Anticancer Activity: Recent Advances and Some General Considerations. EC Chem. 2015, 2, 35–47. [Google Scholar]
- Cieslik, P.; Comba, P.; Dittmar, B.; Ndiaye, D.; Tóth, É.; Velmurugan, G.; Wadepohl, H. Exceptional Manganese(II) Stability and Manganese(II)/Zinc(II) Selectivity with Rigid Polydentate Ligands. Angew. Chemie. Int. Ed. 2022, 61, e20211558. [Google Scholar] [CrossRef] [PubMed]
- Crowley, J.D.; Traynor, D.A.; Weatherburn, D.C. Enzymes and proteins containing manganese: An overview. In Metal Ions in Biological Systems, 1st ed.; CRC Press: Boca Raton, FL, USA, 2000; Volume 37, pp. 209–278. [Google Scholar]
- Charles Dismukes, G. Manganese enzymes with binuclear active sites. Chem. Rev. 1996, 96, 2909–2926. [Google Scholar] [CrossRef] [PubMed]
- Eaton, D.R.; Zaw, K. Geometry of Nickel(II) Complexes. J. Am. Chem. Soc. 1972, 94, 4394–4395. [Google Scholar] [CrossRef]
- Rasyda, Y.A.; Rahardjo, S.B.; Nurdiyah, F. Synthesis and Characterization Complex Nickel(II) with Diphenylamine. IOP Conf. Ser. Mater. Sci. Eng. 2019, 578, 1–6. [Google Scholar] [CrossRef]
- Maret, W. New perspectives of zinc coordination environments in proteins. J. Inorg. Biochem. 2012, 111, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Dudev, T.; Lim, C. Tetrahedral vs octahedral zinc complexes with ligands of biological interest: A DFT/CDM study. J. Am. Chem. Soc. 2000, 122, 11146–11153. [Google Scholar] [CrossRef]
- Gouda, A.A.; Kotb El-Sayed, M.I.; Amin, A.S.; El Sheikh, R. Spectrophotometric and spectrofluorometric methods for the determination of non-steroidal anti-inflammatory drugs: A review. Arab. J. Chem. 2013, 6, 145–163. [Google Scholar] [CrossRef]
- Chiniforoshan, H.; Tabrizi, L.; Hadizade, M.; Sabzalian, M.R.; Chermahini, A.N.; Rezapour, M. Anti-inflammatory drugs interacting with Zn(II) metal ion based on thiocyanate and azide ligands: Synthesis, spectroscopic studies, DFT calculations and antibacterial assays. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 128, 183–190. [Google Scholar] [CrossRef]
- La Manna, S.; Di Natale, C.; Florio, D.; Marasco, D. Peptides as therapeutic agents for inflammatory-related diseases. Int. J. Mol. Sci. 2018, 19, 2714. [Google Scholar] [CrossRef]
- Psomas, G.; Kessissoglou, D.P. Quinolones and non-steroidal anti-inflammatory drugs interacting with copper(II), nickel(II), cobalt(II) and zinc(II): Structural features, biological evaluation and perspectives. Dalton Trans. 2013, 42, 6252–6276. [Google Scholar] [CrossRef]
- Fountoulaki, S.; Perdih, F.; Turel, I.; Kessissoglou, D.P.; Psomas, G. Non-steroidal anti-inflammatory drug diflunisal interacting with Cu(II). Structure and biological features. J. Inorg. Biochem. 2011, 105, 1645–1655. [Google Scholar] [CrossRef] [PubMed]
- Mahendiran, D.; Gurumoorthy, P.; Gunasekaran, K.; Senthil Kumar, R.; Rahiman, A.K. Structural modeling, in vitro antiproliferative activity, and the effect of substituents on the DNA fastening and scission actions of heteroleptic copper(II) complexes with terpyridines and naproxen. New J. Chem. 2015, 39, 7895–7911. [Google Scholar] [CrossRef]
- Kumar, S.; Pal, R.; Venugopalan, P.; Ferretti, V.; Perontsis, S.; Psomas, G. Copper(II) diclofenac complexes: Synthesis, structural studies and interaction with albumins and calf-thymus DNA. J. Inorg. Biochem. 2018, 187, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Folorunso Akinyele, O.; Oluwatola Akinnusi, T.; Adekunle Ajayeoba, T.; Olaolu Ayeni, A.; Moyosore Durosinmi, L. Synthesis, Characterization and Antimicrobial Activities of Cobalt(II), Nickel(II) and Copper(II) Complexes of Aroylhydrazone Mixed with Aspirin. Sci. J. Chem. 2019, 7, 67–71. [Google Scholar] [CrossRef]
- Cini, R.; Tamasi, G.; Defazio, S.; Hursthouse, M.B. Unusual coordinating behavior by three non-steroidal anti-inflammatory drugs from the oxicam family towards copper(II). Synthesis, X-ray structure for copper(II)-isoxicam, -meloxicam and -cinnoxicam-derivative complexes, and cytotoxic activity for a copper(II)-piroxicam complex. J. Inorg. Biochem. 2007, 101, 1140–1152. [Google Scholar]
- Tarushi, A.; Perontsis, S.; Hatzidimitriou, A.G.; Papadopoulos, A.N.; Kessissoglou, D.P.; Psomas, G. Copper(II) complexes with the non-steroidal anti-inflammatory drug tolfenamic acid: Structure and biological features. J. Inorg. Biochem. 2015, 149, 68–79. [Google Scholar] [CrossRef]
- Vadivel, E.; Korgaonkar, K.U. Synthesis, Characterization and Docking Studies of Metal (II) Complexes of Anti-inflammatory Drug Celecoxib. J. Chem. Pharm. Res. 2018, 10, 137–141. [Google Scholar]
- Tsiliou, S.; Kefala, L.-A.; Hatzidimitriou, A.G.; Kessissoglou, D.P.; Perdih, F.; Papadopoulos, A.N.; Turel, I.; Psomas, G. Cobalt(II) complexes with non-steroidal anti-inflammatory drugs and α-diimines. J. Inorg. Biochem. 2016, 160, 125–139. [Google Scholar] [CrossRef]
- Dimiza, F.; Papadopoulos, A.N.; Tangoulis, V.; Psycharis, V.; Raptopoulou, C.P.; Kessissoglou, D.P.; Psomas, G. Biological evaluation of cobalt(II) complexes with non-steroidal anti-inflammatory drug naproxen. J. Inorg. Biochem. 2012, 107, 54–64. [Google Scholar] [CrossRef]
- Sanatkar, T.H.; Hadadzadeh, H.; Simpson, J.; Jannesari, Z. The meloxicam complexes of Co(II) and Zn(II): Synthesis, crystal structures, photocleavage and in vitro DNA-binding. J. Mol. Struct. 2013, 1049, 336–344. [Google Scholar] [CrossRef]
- Tsiliou, S.; Kefala, L.A.; Perdih, F.; Turel, I.; Kessissoglou, D.P.; Psomas, G. Cobalt(II) complexes with non-steroidal anti-inflammatory drug tolfenamic acid: Structure and biological evaluation. Eur. J. Med. Chem. 2012, 48, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Totta, X.; Hatzidimitriou, A.G.; Papadopoulos, A.N.; Psomas, G. Nickel(II)-naproxen mixed-ligand complexes: Synthesis, structure, antioxidant activity and interaction with albumins and calf-thymus DNA. N. J. Chem. 2017, 41, 4478–4492. [Google Scholar] [CrossRef]
- Dimiza, F.; Raptopoulou, C.P.; Psycharis, V.; Papadopoulos, A.N.; Psomas, G. Manganese(II) complexes with the non-steroidal anti-inflammatory drugs naproxen and mefenamic acid: Synthesis, structure, antioxidant capacity, and interaction with albumins and DNA. New J. Chem. 2018, 42, 16666–16681. [Google Scholar] [CrossRef]
- Zampakou, M.; Rizeq, N.; Tangoulis, V.; Papadopoulos, A.N.; Perdih, F.; Turel, I.; Psomas, G. Manganese(II) complexes with the non-steroidal anti-inflammatory drug tolfenamic acid: Structure and biological perspectives. Inorg. Chem. 2014, 53, 2040–2052. [Google Scholar] [CrossRef]
- dos Santos, P.R.; Pich, C.T.; Back, D.; Smiderle, F.; Dumas, F.; Moura, S. Synthesis, chemical characterization and DNA interaction study of new diclofenac and ibuprofen zinc (II)-nicotinamide ternary complexes as cyclooxygenase inhibitor prototypes. J. Inorg. Biochem. 2020, 206, 111046–111057. [Google Scholar] [CrossRef]
- Tarushi, A.; Totta, X.; Raptopoulou, C.P.; Psycharis, V.; Psomas, G.; Kessissoglou, D.P. Structural features of mono- and tri-nuclear Zn(ii) complexes with a non-steroidal anti-inflammatory drug as ligand. Dalton Trans. 2012, 41, 7082–7091. [Google Scholar] [CrossRef]
- Kyropoulou, M.; Raptopoulou, C.P.; Psycharis, V.; Psomas, G. Ni(II) complexes with non-steroidal anti-inflammatory drug diclofenac: Structure and interaction with DNA and albumins. Polyhedron 2013, 61, 126–136. [Google Scholar] [CrossRef]
- Franzé, J.A.; Carvalho, T.F.; Gaglieri, C.; Caires, F.J.; Bannach, G.; Castro, R.C.; Treu-Filho, O.; Ionashiro, M.; Mendes, R.A. Synthesis, characterization, thermal and spectroscopic studies and bioactivity of complexes of meloxicam with some bivalent transition metals. J. Therm. Anal. Calorim. 2017, 127, 1393–1405. [Google Scholar] [CrossRef]
- Totta, X.; Hatzidimitriou, A.G.; Papadopoulos, A.N.; Psomas, G. Nickel(II) complexes of the non-steroidal anti-inflammatory drug tolfenamic acid: Synthesis, structure, antioxidant activity and interaction with albumins and calf-thymus DNA. Polyhedron 2016, 117, 172–183. [Google Scholar] [CrossRef]
- Osowole, A.; Odutemu, A.E. Synthesis, Physicochemical and Antioxidant Properties of Some Metal(II) Complexes of Mixed Drugs, Aspirin and Nicotinamide. Lett. Health Biol. Sci. 2016, 2, 1–6. [Google Scholar] [CrossRef][Green Version]
- Zampakou, M.; Tangoulis, V.; Raptopoulou, C.P.; Psycharis, V.; Papadopoulos, A.N.; Psomas, G. Structurally diverse manganese(II)-diclofenac complexes showing enhanced antioxidant activity and affinity to serum albumins in comparison to sodium diclofenac. Eur. J. Inorg. Chem. 2015, 2015, 2285–2294. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Sadeek, S.A.; Zordok, W.A.; Mohamed, A.A. Meloxicam and study of their antimicrobial effects against phyto- and human pathogens. Molecules 2021, 26, 1480. [Google Scholar] [CrossRef] [PubMed]
- Singla, A.K.; Wadhwa, H. Zinc-aspirin complex: Synthesis, physicochemical and biological evaluation. Int. J. Pharm. 1994, 108, 173–185. [Google Scholar] [CrossRef]
- Korkmaz-Icöz, S.; Atmanli, A.; Li, S.; Radovits, T.; Hegedus, P.; Barnucz, E.; Hirschberg, K.; Loganathan, S.; Yoshikawa, Y.; Yasui, H.; et al. Superiority of zinc complex of acetylsalicylic acid to acetylsalicylic acid in preventing postischemic myocardial dysfunction. Exp. Biol. Med. 2015, 240, 1247–1255. [Google Scholar] [CrossRef]
- Korkmaz-Icöz, S.; Al Said, S.; Radovits, T.; Li, S.; Brune, M.; Hegedus, P.; Atmanli, A.; Ruppert, M.; Brlecic, P.; Lehmann, L.H.; et al. Oral treatment with a zinc complex of acetylsalicylic acid prevents diabetic cardiomyopathy in a rat model of type-2 diabetes: Activation of the Akt pathway. Cardiovasc. Diabetol. 2016, 15, 1–16. [Google Scholar] [CrossRef]
- Kleinzeller, A. Charles Ernest Overton’s Concept of a Cell Membrane. In Membrane Permeability, 1st ed.; Deamer, D.W., Kleinzeller, A., Fambrough, D.M., Eds.; Academic Press: Cambridge, MA, USA, 1999; Volume 48, pp. 1–22. [Google Scholar]
- Raman, N.; Kulandaisamy, A.; Thangaraja, C.; Manisankar, P.; Viswanathan, S.; Vedhi, C. Synthesis, structural characterisation and electrochemical and antibacterial studies of Schiff base copper complexes. Transit. Met. Chem. 2004, 29, 129–135. [Google Scholar] [CrossRef]
- Al-Amiery, A.A.; Kadhum, A.A.H.; Mohamad, A.B. Antifungal and antioxidant activities of pyrrolidone thiosemicarbazone complexes. Bioinorg. Chem. Appl. 2012, 2012, 1–7. [Google Scholar] [CrossRef]
- Panchal, P.K.; Pansuriya, P.B.; Patel, M.N. In-vitro biological evaluation of some ONS and NS donor Schiff’s bases and their metal complexes. J. Enzyme Inhib. Med. Chem. 2006, 21, 453–458. [Google Scholar] [CrossRef]
- Zappavigna, S.; Cossu, A.M.; Grimaldi, A.; Bocchetii, M.; Ferraro, G.A.; Nicoletii, G.F.; Filosa, R.; Caraglia, M. Anti-inflammatory drugs as anticancer agents. Int. J. Mol. Sci. 2020, 21, 2605. [Google Scholar] [CrossRef]
- Philip, M.; Rowley, D.A.; Schreiber, H. Inflammation as a tumor promoter in cancer induction. Semin. Cancer Biol. 2004, 14, 433–439. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, F.; Shang, L. Advances in antitumor effects of NSAIDs. Cancer Manag. Res. 2018, 10, 4631–4640. [Google Scholar] [CrossRef] [PubMed]
- Weder, J.E.; Dillon, C.T.; Hambley, T.W.; Kennedy, B.J.; Lay, P.A.; Biffin, J.R.; Regtop, H.L.; Davies, N.M. Copper complexes of non-steroidal anti-inflammatory drugs: An opportunity yet to be realized. Coord. Chem. Rev. 2002, 232, 95–126. [Google Scholar] [CrossRef]
- Roy, S.; Banerjee, R.; Sarkar, M. Direct binding of Cu(II)-complexes of oxicam NSAIDs with DNA backbone. J. Inorg. Biochem. 2006, 100, 1320–1331. [Google Scholar] [CrossRef] [PubMed]
- Deb, J.; Lakshman, T.R.; Ghosh, I.; Jana, S.S.; Paine, T.K. Mechanistic studies of in vitro anti-proliferative and anti-inflammatory activities of the Zn(II)–NSAID complexes of 1,10-phenanthroline5,6-dione in MDA-MB-231 cells. Dalton Trans. 2020, 49, 11375–11384. [Google Scholar] [CrossRef]
- Dendrinou-Samara, C.; Tsotsou, G.; Ekateriniadou, L.V.; Kortsaris, A.H.; Raptopoulou, C.P.; Terzis, A.; Kyriakidis, D.A.; Kessissoglou, D.P. Anti-inflammatory drugs interacting with Zn(II), Cd(II) and Pt(II) metal ions. J. Inorg. Biochem. 1998, 71, 171–179. [Google Scholar] [CrossRef]
- Sayen, S.; Carlier, A.; Tarpin, M.; Guillon, E. A novel copper(II) mononuclear complex with the non-steroidal anti-inflammatory drug diclofenac: Structural characterization and biological activity. J. Inorg. Biochem. 2013, 120, 39–43. [Google Scholar] [CrossRef]
- Shi, X.; Fang, H.; Guo, Y.; Yuan, H.; Guo, Z.; Wang, X. Anticancer copper complex with nucleus, mitochondrion and cyclooxygenase-2 as multiple targets. J. Inorg. Biochem. 2019, 190, 38–44. [Google Scholar] [CrossRef]
- Yin, Z.; Wang, Y.; Whittell, L.; Jergic, S.; Liu, M.; Harry, E.; Dizon, N.E.; Kelso, M.J.; Beck, J.L.; Oakley, A.J. DNA replication is the target for the antibacterial effects of nonsteroidal anti-inflammatory drugs. Chem. Biol. 2014, 21, 481–487. [Google Scholar] [CrossRef]
- Lagadinou, M.; Onisor, M.O.; Rigas, A.; Musetescu, D.-V.; Gkentzi, D.; Assimakopoulos, S.F.; Panos, G.; Marangos, M. Antimicrobial properties on non-antibiotic drugs in the era of increased bacterial resistance. Antibiotics 2020, 9, 107. [Google Scholar] [CrossRef]
- Obad, J.; Šušković, J.; Kos, B. Antimicrobial activity of ibuprofen: New perspectives on an ‘old’ non-antibiotic drug. Eur. J. Pharm. Sci. 2015, 71, 93–98. [Google Scholar] [CrossRef]
- Hersh, E.V.; Hammond, B.F.; Fleury, A.A. Antimicrobial activity of flurbiprofen and ibuprofen in vitro against six common periodontal pathogens. J. Clin. Dent. 1991, 3, 1–5. [Google Scholar]
- Sanyal, A.K.; Roy, D.; Chowdhury, B.; Banerjee, A.B. Ibuprofen, a unique anti-inflammatory compound with antifungal activity against dermatophytes. Lett. Appl. Microbiol. 1993, 17, 109–111. [Google Scholar] [CrossRef]
- Abu Ali, H.; Omar, S.N.; Darawsheh, M.D.; Fares, H. Synthesis characterization and antimicrobial activity of zinc(II) ibuprofen complexes with nitrogen-based ligands. J. Coord. Chem. 2016, 69, 1110–1122. [Google Scholar] [CrossRef]
- Lawal, A.; Obaleye, J. Synthesis, characterization and antibacterial activity of aspirin and paracetamolmetal complexes. Biokemistri 2010, 19, 9–15. [Google Scholar] [CrossRef]
- Ashouri, F.; Reza, A.; Molaeian, S.; Fall, M.A.; Butcher, R.J. The novel cobalt and manganese polymeric complex with the non- steroidal anti-inflammatory drug diclofenac: Synthesis, characterization and antibacterial studies. J. Mol. Struct. 2020, 1204, 127483. [Google Scholar] [CrossRef]
- Pan, T.; Peng, Z.; Tan, L.; Zou, F.; Zhou, N.; Liu, B.; Liang, L.; Chen, C.; Liu, J.; Wu, L.; et al. Nonsteroidal Anti-inflammatory Drugs Potently Inhibit the Replication of Zika Viruses by Inducing the Degradation of AXL. J. Virol. 2018, 92, e01018-18. [Google Scholar] [CrossRef]
- Mahmood, K.-A.S.; Ahmed, J.H.; Jawad, A.M. Non-Steroidal Anti-Inflammatory Drugs (Nsaids), Free Radicals and Reactive Oxygen Species (Ros): A Review of Literature. Med. J. Basrah Univ. 2009, 27, 46–53. [Google Scholar] [CrossRef][Green Version]
- Lorente, L.; Martín, M.M.; Abreu-González, P.; Domínguez-Rodriguez, A.; Labarta, L.; Díaz, C.; Solé-Violán, J.; Ferreres, J.; Cabrera, J.; Igeño, J.C.; et al. Sustained high serum malondialdehyde levels are associated with severity and mortality in septic patients. Crit. Care 2013, 17, R290. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Kadam, C.; Abhang, S.A. Evaluation of serum levels of reduced glutathione, glutathione-s-transferase and nitric oxide in breast cancer patients undergoing adjuvant chemotherapy. Int. J. Curr. Res. 2014, 5, 51–57. [Google Scholar]
- Costa, D.; Gomes, A.; Lima, J.L.F.C.; Fernandes, E. Singlet oxygen scavenging activity of non-steroidal anti-inflammatory drugs. Redox Rep. 2008, 13, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Končič, M.; Rajič, Z.; Petrič, N.; Zorc, B. Antioxidant activity of NSAID hydroxamic acids. Acta Pharm. 2009, 59, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Alajmi, M.F.; Rehman, T.; Amir, S.; Khan, R.A. Copper(II) complexes as potential anticancer and Nonsteroidal anti- inflammatory agents: In vitro and in vivo studies. Sci. Rep. 2019, 9, 5237–5254. [Google Scholar] [CrossRef] [PubMed]
- Mouithys-Mickalad, A.M.L.; Zheng, S.X.; Deby-Dupont, G.P.; Deby, C.M.; Lamy, M.M.; Reginster, J.Y.; Henrotin, Y.E. In vitro study of the antioxidant properties of non steroidal anti-inflammatory drugs by chemiluminescence and electron spin resonance (ESR). Free Radic. Res. 2000, 33, 607–621. [Google Scholar] [CrossRef]
- Tarushi, A.; Karaflow, Z.; Kljun, J.; Turel, I.; Psomas, G.; Papadopoulos, A.N.; Kessissoglou, D.P. Antioxidant capacity and DNA-interaction studies of zinc complexes with a non-steroidal anti-inflammatory drug, mefenamic acid. J. Inorg. Biochem. 2013, 128, 85–96. [Google Scholar] [CrossRef]
- Tarushi, A.; Totta, X.; Papadopoulos, A.; Kljun, J.; Turel, I.; Kessissoglou, D.P.; Psomas, G. Antioxidant activity and interaction with DNA and albumins of zinc-tolfenamato complexes. Crystal structure of [Zn(tolfenamato) 2(2,2′-dipyridylketoneoxime)2]. Eur. J. Med. Chem. 2014, 74, 187–198. [Google Scholar] [CrossRef]
- Goswami, S.; Ray, S.; Sarkar, M. Spectroscopic studies on the interaction of DNA with the copper complexes of NSAIDs lornoxicam and isoxicam. Int. J. Biol. Macromol. 2016, 93, 47–56. [Google Scholar] [CrossRef]
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda: Maryland, USA. 2012. Available online: www.livertox.nih.gov (accessed on 23 January 2022).
- Laster, J.; Satoskar, R. Aspirin-Induced Acute Liver Injury. ACG Case Rep. J. 2014, 2, 48–49. [Google Scholar] [CrossRef]
- Gibson, D. Drug-DNA interactions and novel drug design. Pharmacogenomics J. 2002, 2, 275–276. [Google Scholar] [CrossRef]
- Shahabadi, N.; Maghsudi, M.; Mahdavi, M.; Pourfoulad, M. Interaction of calf thymus dna with the antiviral drug lamivudine. DNA Cell Biol. 2012, 31, 122–127. [Google Scholar] [CrossRef]
- Packianathan, S.; Arun, T.; Raman, N. DNA interaction and efficient antimicrobial activities of 4N chelating metal complexes. J. Photochem. Photobiol. B Biol. 2015, 148, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Anitha, P.; Chitrapriya, N.; Jang, Y.J.; Viswanathamurthi, P. Synthesis, characterization, DNA interaction, antioxidant and anticancer activity of new ruthenium(II) complexes of thiosemicarbazone/semicarbazone bearing 9,10-phenanthrenequinone. J. Photochem. Photobiol. B Biol. 2013, 129, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Pages, B.J.; Ang, D.L.; Wright, E.P.; Aldrich-Wright, J.R. Metal complex interactions with DNA. Dalton Trans. 2015, 44, 3505–3526. [Google Scholar] [CrossRef] [PubMed]
- Goodsell, D.S. The Molecular Perspective: Cisplatin. Oncologist 2006, 11, 316–317. [Google Scholar] [CrossRef]
- Erxleben, A. Investigation of Non-covalent Interactions of Metal Complexes with DNA in Cell-free Systems. Bioorganometallic Chem. Mech. 2017, 71, 102–111. [Google Scholar] [CrossRef]
- Komor, A.C.; Barton, J.K. The Path for Metal Complexes to a DNA Target. Chem. Commun. 2013, 49, 3617–3630. [Google Scholar] [CrossRef]
- Yousuf, M.; Youn, I.S.; Yun, J.; Rasheed, L.; Valero, R.; Shi, G.; Kim, K.S. Violation of DNA neighbor exclusion principle in RNA recognition. Chem. Sci. 2016, 7, 3581–3588. [Google Scholar] [CrossRef]
- Blackburn, G.M.; Gait, J.M.; Loakes, D.; Williams, M.D. Nucleic Acids in Chemistry and Biology, 3rd ed.; RSC Publishing: London, UK, 2006. [Google Scholar]
- Smith, J.A.; Keene, F.R.; Li, F.; Collins, J.G. Noncovalent DNA Binding of Metal Complexes. In Comprehensive Inorganic Chemistry II, 2nd ed.; Reedijk, J., Poeppelmeier, K., Eds.; Elsevier: Oxford, UK, 2013; Volume 3, pp. 709–750. [Google Scholar]
- Li, S.; Cooper, V.R.; Thonhauser, T.; Lundqvist, B.I.; Langreth, D.C. Stacking interactions and DNA intercalation. J. Phys. Chem. B 2009, 113, 11166–11172. [Google Scholar] [CrossRef]
- Thamilarasan, V.; Sengottuvelan, N.; Stalin, N.; Srinivasan, P.; Chakkaravarthi, G. Synthesis, interactions, molecular structure, biological properties and molecular docking studies on Mn, Co, Zn complexes containing acetylacetone and pyridine ligands with DNA duplex. J. Photochem. Photobiol. B Biol. 2016, 160, 110–120. [Google Scholar] [CrossRef]
- Alberti, E.; Zampakou, M.; Donghi, D. Covalent and non-covalent binding of metal complexes to RNA. J. Inorg. Biochem. 2016, 163, 278–291. [Google Scholar] [CrossRef]
- Khan, H.Y.; Tabassum, S.; Arjmand, F. Evaluation of cytotoxic potential of structurally well-characterized RNA targeted ionic non-steroidal anti-inflammatory (NSAID) Cu(II) & Zn(II) DACH-mefenamato drug conjugates against human cancer cell lines. RSC Adv. 2020, 10, 166–178. [Google Scholar]
- Hostetter, A.A.; Osborn, M.F.; DeRose, V.J. RNA-Pt adducts following cisplatin treatment of Saccharomyces cerevisiae. ACS Chem. Biol. 2012, 7, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Lejal, N.; Tarus, B.; Bouguyon, E.; Chenavas, S.; Bertho, N.; Delmas, B.; Ruigrok, R.W.H.; Di Primo, C.; Slama-Schwok, A. Structure-based discovery of the novel antiviral properties of naproxen against the nucleoprotein of influenza a virus. Antimicrob. Agents Chemother. 2013, 57, 2231–2242. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.P.; Holtkamp, H.U.; Hartinger, C.G. Antitumor Metallodrugs that Target Proteins. Met. Ions Life Sci. 2018, 18, 351–386. [Google Scholar]
- Cicenas, J.; Zalyte, E.; Bairoch, A.; Gaudet, P. Kinases and Cancer. Cancers 2018, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- Filippakopoulos, P.; Kräling, K.; Celik, M.A.; Harms, K. Structurally Sophisticated Octahedral Metal Complexes as Highly Selective Protein Kinase Inhibitors. J. Am. Chem. Soc. 2011, 133, 5976–5986. [Google Scholar]
- Kunick, C.; Ott, I. Metal Complexes as Protein Kinase Inhibitors. Bioorganometallic Chem. 2010, 49, 5226–5227. [Google Scholar] [CrossRef]
- He, H.; Xia, H.; Wang, J.D.; Gu, Q.; Lin, M.C.M; Zou, B.; Lam, S.K.; Chan, A.O.O.; Yuen, M.F.; Kung, H.F.; et al. Inhibition of human telomerase reverse transcriptase by nonsteroidal antiinflammatory drugs in colon carcinoma. Cancer 2006, 106, 1243–1249. [Google Scholar] [CrossRef]
- Rana, C.; Piplani, H.; Vaish, V.; Nehru, B.; Sanyal, S.N. Downregulation of telomerase activity by diclofenac and curcumin is associated with cell cycle arrest and induction of apoptosis in colon cancer. Tumor Biol. 2015, 36, 5999–6010. [Google Scholar] [CrossRef]
- Bruijnincx, P.; Sadler, P. New trends for metal complexes with anticancer activity. Chem. Biol. 2008, 12, 197–206. [Google Scholar] [CrossRef]
- Xu, Y.; Ishizuka, T.; Yang, J.; Ito, K.; Katada, H.; Komiyama, M.; Hayashi, T. Oligonucleotide Models of Telomeric DNA and RNA Form a Hybrid G-quadruplex Structure as a Potential Component of Telomeres. J. Biol. Chem. 2012, 287, 41787–41796. [Google Scholar] [CrossRef] [PubMed]
- Dixon, I.M.; Lopez, F.; Tejera, A.M.; Estève, J.-P.; Blasco, M.A.; Pratviel, G.; Meunier, B. A G-quadruplex ligand with 10000-fold selectivity over duplex DNA. J. Am. Chem. Soc. 2007, 129, 1502–1503. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.T.; Kuhlmann, M.; Hvam, M.L.; Howard, K.A. Albumin-based drug delivery: Harnessing nature to cure disease. Mol. Cell. Ther. 2016, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Topala, T.; Bodoki, A.; Oprean, L.; Oprean, R. Bovine Serum Albumin Interactions with Metal Complexes. Clujul Med. 2014, 87, 215–219. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).