The Defensive Role of Endogenous H2S in Brassica rapa against Mercury-Selenium Combined Stress
Abstract
:1. Introduction
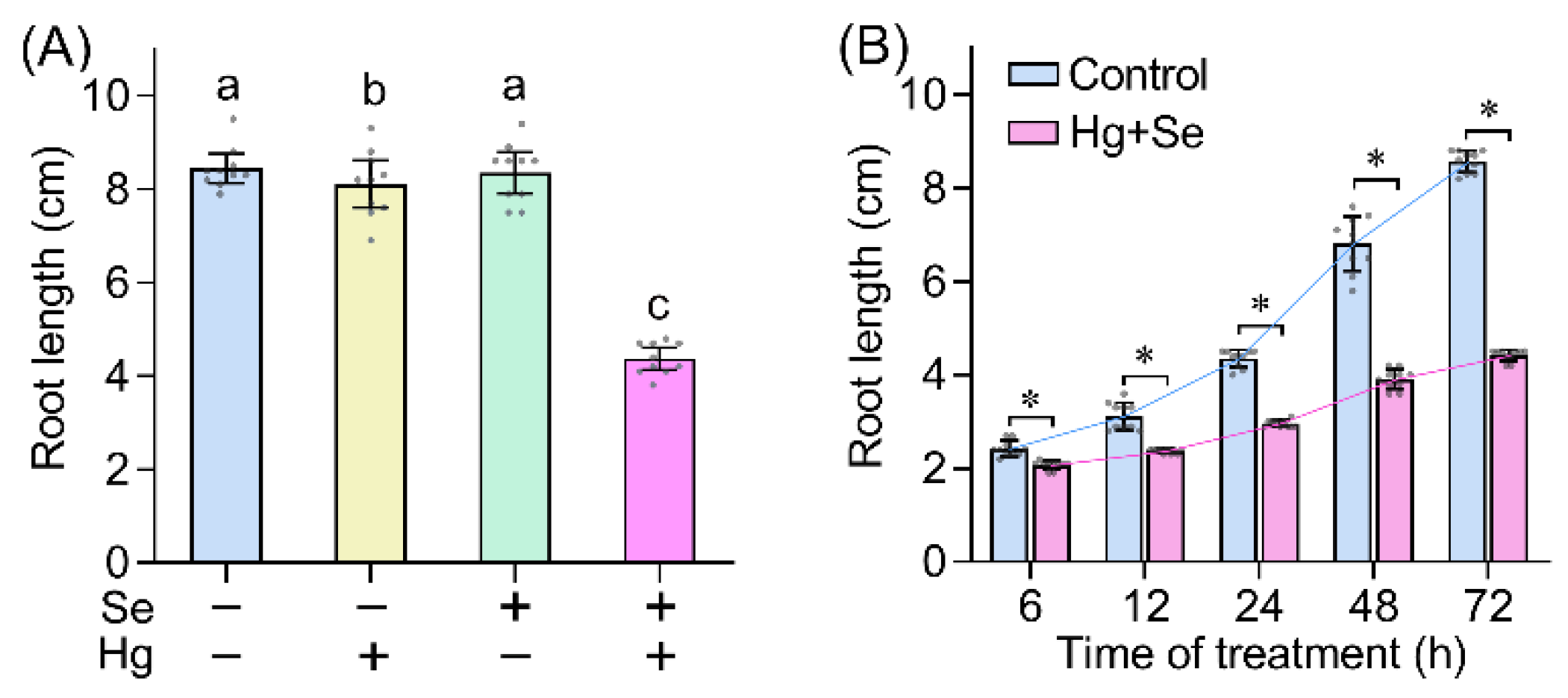
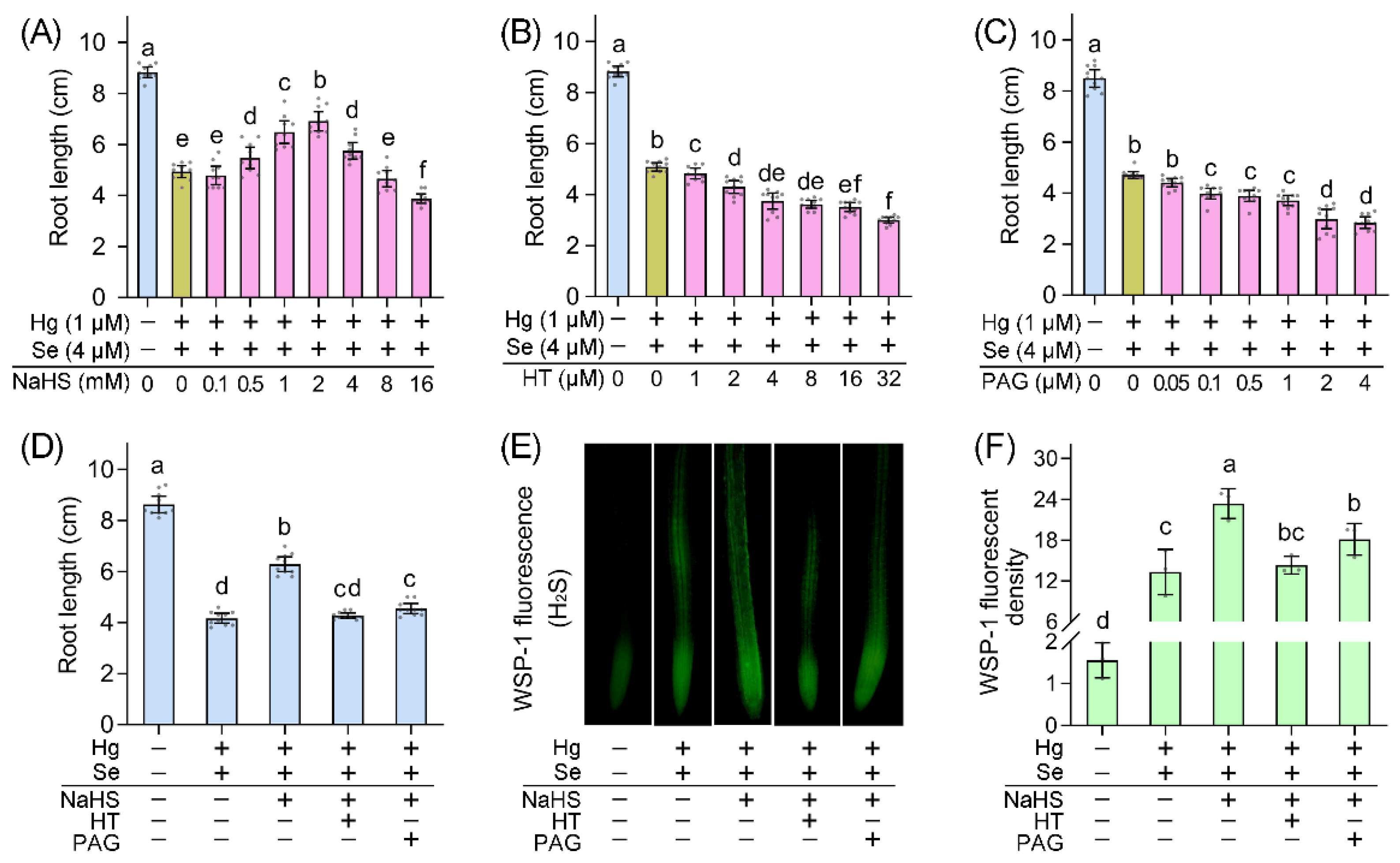
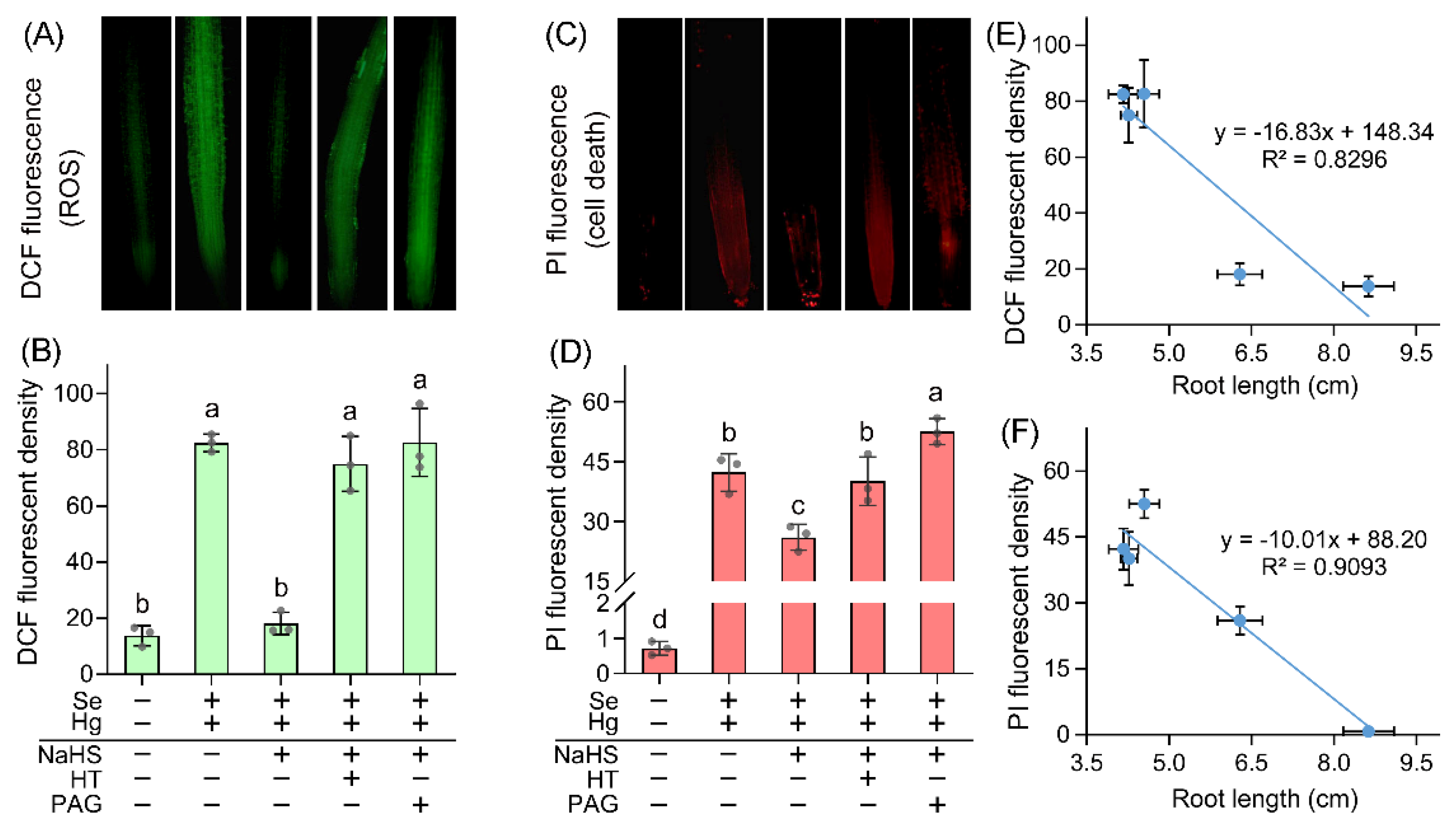
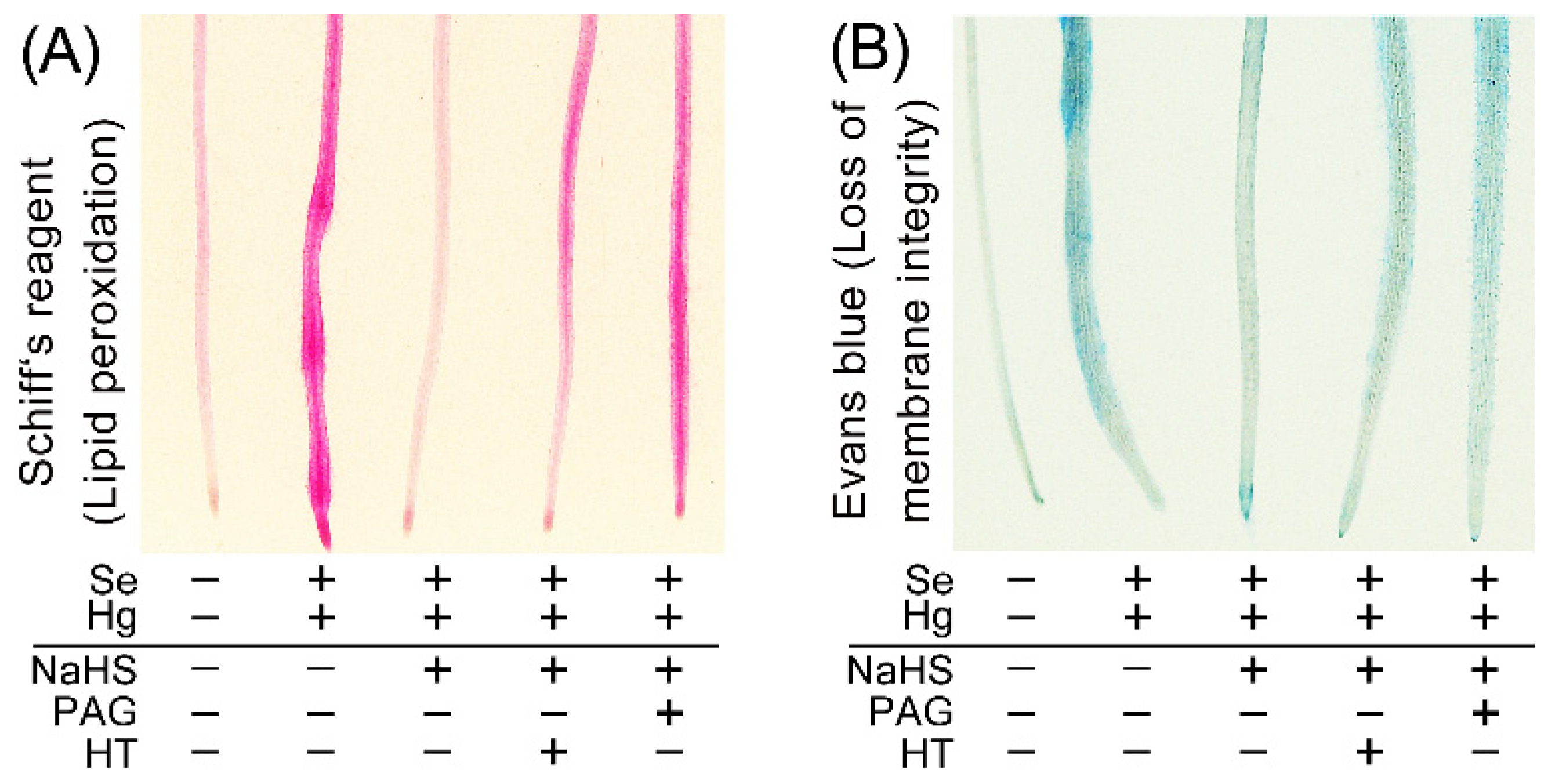
2. Results and Discussion
3. Materials and Methods
3.1. Plant Culture, Treatment, and Chemicals
3.2. Evaluation of Endogenous H2S Level in Root Tip
3.3. Evaluation of Total ROS Level in Root Tip
3.4. Evaluation of Cell Death in Root Tip
3.5. Evaluation of Oxidative Injury in Root Tip
3.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Gworek, B.; Dmuchowski, W.; Baczewska-Dąbrowska, A.H. Mercury in the terrestrial environment: A review. Environ. Sci. Eur. 2020, 32, 128. [Google Scholar] [CrossRef]
- Zhou, Z.S.; Huang, S.Q.; Guo, K.; Mehta, S.K.; Zhang, P.C.; Yang, Z.M. Metabolic adaptations to mercury-induced oxidative stress in roots of Medicago sativa L. J. Inorg. Biochem. 2007, 101, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.S.; Wang, S.J.; Yang, Z.M. Biological detection and analysis of mercury toxicity to alfalfa (Medicago sativa) plants. Chemosphere 2008, 70, 1500–1509. [Google Scholar] [CrossRef]
- Chen, J.; Yang, Z.M. Mercury toxicity, molecular response and tolerance in higher plants. Biometals 2012, 25, 847–857. [Google Scholar] [CrossRef]
- Tran, T.A.T.; Dinh, Q.T.; Zhou, F.; Zhai, H.; Xue, M.; Du, Z.; Bañuelos, G.S.; Liang, D. Mechanisms underlying mercury detoxification in soil–plant systems after selenium application: A review. Environ. Sci. Pollut. Res. 2021, 28, 46852–46876. [Google Scholar] [CrossRef]
- Tran, T.A.T.; Zhou, F.; Yang, W.; Wang, M.; Dinh, Q.T.; Wang, D.; Liang, D. Detoxification of mercury in soil by selenite and related mechanisms. Ecotoxicol. Environ. Saf. 2018, 159, 77–84. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Raza, A.; Hawrylak-Nowak, B.; Matraszek-Gawron, R.; Nahar, K.; Fujita, M. Selenium Toxicity in Plants and Environment: Biogeochemistry and Remediation Possibilities. Plants 2020, 9, 1711. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, X.; Jiang, C.; Li, Q.; Liu, Y.; Gu, C.; Shang, L.; Li, P.; Lin, Y.; Larssen, T. Understanding the paradox of selenium contamination in mercury mining areas: High soil content and low accumulation in rice. Environ. Pollut. 2014, 188, 27–36. [Google Scholar] [CrossRef]
- Bian, Z.-W.; Chen, J.; Li, H.; Liu, D.-D.; Yang, L.-F.; Zhu, Y.-L.; Zhu, W.-L.; Liu, W.; Ying, Z.-Z. The phytotoxic effects of selenium–mercury interactions on root growth in Brassica rapa (LvLing). Hortic. Environ. Biotechnol. 2016, 57, 232–240. [Google Scholar] [CrossRef]
- Arif, Y.; Hayat, S.; Yusuf, M.; Bajguz, A. Hydrogen sulfide: A versatile gaseous molecule in plants. Plant Physiol. Biochem. 2021, 158, 372–384. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, M.; Zhou, H.; Zhao, D.; Gotor, C.; Romero, L.C.; Shen, J.; Ge, Z.; Zhang, Z.; Shen, W.; et al. Hydrogen sulfide, a signaling molecule in plant stress responses. J. Integr. Plant Biol. 2021, 63, 146–160. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Z.; Jing, T.; Liu, Z.; Zhang, L.; Jin, Z.; Liu, D.; Pei, Y. H2S acting as a downstream signaling molecule of SA regulates Cd tolerance in Arabidopsis. Plant Soil 2015, 393, 137–146. [Google Scholar] [CrossRef]
- Chen, J.; Wang, W.-H.; Wu, F.-H.; You, C.-Y.; Liu, T.-W.; Dong, X.-J.; He, J.-X.; Zheng, H.-L. Hydrogen sulfide alleviates aluminum toxicity in barley seedlings. Plant Soil 2013, 362, 301–318. [Google Scholar] [CrossRef]
- Fang, H.; Liu, Z.; Jin, Z.; Zhang, L.; Liu, D.; Pei, Y. An emphasis of hydrogen sulfide-cysteine cycle on enhancing the tolerance to chromium stress in Arabidopsis. Environ. Pollut. 2016, 213, 870–877. [Google Scholar] [CrossRef]
- Bharwana, S.A.; Ali, S.; Farooq, M.A.; Ali, B.; Iqbal, N.; Abbas, F.; Ahmad, M.S. Hydrogen sulfide ameliorates lead-induced morphological, photosynthetic, oxidative damages and biochemical changes in cotton. Environ. Sci. Pollut. Res. 2014, 21, 717–731. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, M.; Jiang, M. Hydrogen sulfide alleviates mercury toxicity by sequestering it in roots or regulating reactive oxygen species productions in rice seedlings. Plant Physiol. Biochem. 2017, 111, 179–192. [Google Scholar] [CrossRef]
- Chen, Y.; Mo, H.Z.; Zheng, M.Y.; Xian, M.; Qi, Z.Q.; Li, Y.Q.; Hu, L.B.; Chen, J.; Yang, L.F. Selenium inhibits root elongation by repressing the generation of endogenous hydrogen sulfide in Brassica rapa. PLoS ONE 2014, 9, e110904. [Google Scholar] [CrossRef]
- Mei, L.; Zhu, Y.; Zhang, X.; Zhou, X.; Zhong, Z.; Li, H.; Li, Y.; Li, X.; Daud, M.K.; Chen, J.; et al. Mercury-Induced Phytotoxicity and Responses in Upland Cotton (Gossypium hirsutum L.) Seedlings. Plants 2021, 10, 1494. [Google Scholar] [CrossRef]
- Kolbert, Z.; Lehotai, N.; Molnar, A.; Feigl, G. “The roots” of selenium toxicity: A new concept. Plant Signal. Behav. 2016, 11, e1241935. [Google Scholar] [CrossRef] [Green Version]
- Ryan, P.R.; Delhaize, E.; Watt, M.; Richardson, A.E. Plant roots: Understanding structure and function in an ocean of complexity. Ann. Bot. 2016, 118, 555–559. [Google Scholar] [CrossRef] [Green Version]
- Christou, A.; Manganaris, G.A.; Papadopoulos, I.; Fotopoulos, V. Hydrogen sulfide induces systemic tolerance to salinity and non-ionic osmotic stress in strawberry plants through modification of reactive species biosynthesis and transcriptional regulation of multiple defence pathways. J. Exp. Bot. 2013, 64, 1953–1966. [Google Scholar] [CrossRef]
- Zhang, L.; Pei, Y.; Wang, H.; Jin, Z.; Liu, Z.; Qiao, Z.; Fang, H.; Zhang, Y. Hydrogen sulfide alleviates cadmium-induced cell death through restraining ROS accumulation in roots of Brassica rapa L. ssp. pekinensis. Oxidative Med. Cell. Longev. 2015, 2015, 804603. [Google Scholar] [CrossRef] [Green Version]
- Fang, H.; Jing, T.; Liu, Z.; Zhang, L.; Jin, Z.; Pei, Y. Hydrogen sulfide interacts with calcium signaling to enhance the chromium tolerance in Setaria italica. Cell Calcium 2014, 56, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Lai, D.; Mao, Y.; Zhou, H.; Li, F.; Wu, M.; Zhang, J.; He, Z.; Cui, W.; Xie, Y. Endogenous hydrogen sulfide enhances salt tolerance by coupling the reestablishment of redox homeostasis and preventing salt-induced K+ loss in seedlings of Medicago sativa. Plant Sci. 2014, 225, 117–129. [Google Scholar] [CrossRef]
- Cui, W.; Chen, H.; Zhu, K.; Jin, Q.; Xie, Y.; Cui, J.; Xia, Y.; Zhang, J.; Shen, W. Cadmium-induced hydrogen sulfide synthesis is involved in cadmium tolerance in Medicago sativa by reestablishment of reduced (Homo)glutathione and reactive oxygen species homeostases. PLoS ONE 2014, 9, e109669. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ye, Y.-K.; Wang, S.-H.; Luo, J.-P.; Tang, J.; Ma, D.-F. Hydrogen sulfide counteracts chlorophyll loss in sweetpotato seedling leaves and alleviates oxidative damage against osmotic stress. Plant Growth Regul. 2009, 58, 243–250. [Google Scholar] [CrossRef]
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The significance of reactive oxygen species and antioxidant defense system in plants: A concise overview. Front. Plant Sci. 2021, 11, 552969. [Google Scholar] [CrossRef]
- Foreman, J.; Demidchik, V.; Bothwell, J.H.; Mylona, P.; Miedema, H.; Torres, M.A.; Linstead, P.; Costa, S.; Brownlee, C.; Jones, J.D.; et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 2003, 422, 442–446. [Google Scholar] [CrossRef]
- Huang, H.; Ullah, F.; Zhou, D.-X.; Yi, M.; Zhao, Y. Mechanisms of ROS regulation of plant development and stress responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, J.; Liu, J.; Liu, T.; Xue, S. Hydrogen sulfide (H2S) signaling in plant development and stress responses. aBIOTECH 2021, 2, 32–63. [Google Scholar] [CrossRef] [PubMed]
- Mostofa, M.G.; Rahman, A.; Ansary, M.M.U.; Watanabe, A.; Fujita, M.; Tran, L.-S.P. Hydrogen sulfide modulates cadmium-induced physiological and biochemical responses to alleviate cadmium toxicity in rice. Sci. Rep. 2015, 5, 14078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gémes, K.; Kim, Y.J.; Park, K.Y.; Moschou, P.N.; Andronis, E.; Valassaki, C.; Roussis, A.; Roubelakis-Angelakis, K.A. An NADPH-oxidase/polyamine oxidase feedback loop controls oxidative burst under salinity. Plant Physiol. 2016, 172, 1418–1431. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.; Jia, D.; Liu, T. Polyamine oxidases play various roles in plant development and abiotic stress tolerance. Plants 2019, 8, 184. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Ye, X.; Yang, K.; Shi, Z.; Wang, N.; Yang, L.; Chen, J. Characterization, expression, and functional analysis of polyamine oxidases and their role in selenium-induced hydrogen peroxide production in Brassica rapa. J. Sci. Food Agric. 2019, 99, 4082–4093. [Google Scholar] [CrossRef]
- Chen, Y.; Mo, H.Z.; Hu, L.; Li, Y.; Chen, J.; Yang, L. The endogenous nitric oxide mediates selenium-induced phytotoxicity by promoting ROS generation in Brassica rapa. PLoS ONE 2014, 9, e110901. [Google Scholar] [CrossRef]
- Montero-Palmero, M.B.; Martín-Barranco, A.; Escobar, C.; Hernández, L.E. Early transcriptional responses to mercury: A role for ethylene in mercury-induced stress. New Phytol. 2014, 201, 116–130. [Google Scholar] [CrossRef]
- Zhang, Z.; Jin, S.; Teng, X.; Duan, X.; Chen, Y.; Wu, Y. Hydrogen sulfide attenuates cardiac injury in takotsubo cardiomyopathy by alleviating oxidative stress. Nitric Oxide 2017, 67, 10–25. [Google Scholar] [CrossRef]
- Yu, X.Z.; Chu, Y.P.; Zhang, H.; Lin, Y.J.; Tian, P. Jasmonic acid and hydrogen sulfide modulate transcriptional and enzymatic changes of plasma membrane NADPH oxidases (NOXs) and decrease oxidative damage in Oryza sativa L. during thiocyanate exposure. Ecotoxicology 2021, 30, 1511–1520. [Google Scholar] [CrossRef]
- Natasha; Shahid, M.; Khalid, S.; Bibi, I.; Bundschuh, J.; Niazi, N.K.; Dumat, C. A critical review of mercury speciation, bioavailability, toxicity and detoxification in soil-plant environment: Ecotoxicology and health risk assessment. Sci. Total Environ. 2020, 711, 134749. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, J.; Shen, J.; Zhou, H.; Zhao, D.; Gotor, C.; Romero, L.C.; Fu, L.; Li, Z.; Yang, J.; et al. Hydrogen sulfide-linked persulfidation of ABI4 controls ABA responses through the transactivation of MAPKKK18 in Arabidopsis. Mol. Plant 2021, 14, 921–936. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, X.; Jia, H.; Li, F.; Ma, Y.; Liesche, J.; Liao, M.; Ding, X.; Liu, C.; Chen, Y.; et al. Persulfidation-induced structural change in SnRK2.6 establishes intramolecular interaction between phosphorylation and persulfidation. Mol. Plant 2021, 14, 1814–1830. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Li, H.; Huang, S.; Wang, C.; Sun, W.-J.; Mo, H.-Z.; Shi, Z.Q.; Chen, J. Eugenol confers cadmium tolerance via intensifying endogenous hydrogen sulfide signaling in Brassica rapa. J. Agric. Food Chem. 2018, 66, 9914–9922. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Pan, J.; Li, S.; Zhao, Y.; Wu, L.Y.; Berkman, C.E.; Whorton, A.R.; Xian, M. Capture and visualization of hydrogen sulfide by a fluorescent probe. Angew. Chem. 2011, 123, 10511–10513. [Google Scholar] [CrossRef]
- Li, Y.J.; Chen, J.; Xian, M.; Zhou, L.G.; Han, F.X.; Gan, L.J.; Shi, Z.Q. In site bioimaging of hydrogen sulfide uncovers its pivotal role in regulating nitric oxide-induced lateral root formation. PLoS ONE 2014, 9, e90340. [Google Scholar] [CrossRef]
- Kellermeier, F.; Chardon, F.; Amtmann, A. Natural variation of Arabidopsis root architecture reveals complementing adaptive strategies to potassium starvation. Plant Physiol. 2013, 161, 1421–1432. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.S.; Yang, Z.M. Nitric oxide reduces aluminum toxicity by preventing oxidative stress in the roots of Cassia tora L. Plant Cell Physiol. 2005, 46, 1915–1923. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, Y.; Kobayashi, Y.; Matsumoto, H. Lipid peroxidation is an early symptom triggered by aluminum, but not the primary cause of elongation inhibition in pea roots. Plant Physiol. 2001, 125, 199–208. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Yang, H.; Bian, Z.; Lu, H.; Zhang, L.; Chen, J. The Defensive Role of Endogenous H2S in Brassica rapa against Mercury-Selenium Combined Stress. Int. J. Mol. Sci. 2022, 23, 2854. https://doi.org/10.3390/ijms23052854
Yang L, Yang H, Bian Z, Lu H, Zhang L, Chen J. The Defensive Role of Endogenous H2S in Brassica rapa against Mercury-Selenium Combined Stress. International Journal of Molecular Sciences. 2022; 23(5):2854. https://doi.org/10.3390/ijms23052854
Chicago/Turabian StyleYang, Lifei, Huimin Yang, Zhiwei Bian, Haiyan Lu, Li Zhang, and Jian Chen. 2022. "The Defensive Role of Endogenous H2S in Brassica rapa against Mercury-Selenium Combined Stress" International Journal of Molecular Sciences 23, no. 5: 2854. https://doi.org/10.3390/ijms23052854
APA StyleYang, L., Yang, H., Bian, Z., Lu, H., Zhang, L., & Chen, J. (2022). The Defensive Role of Endogenous H2S in Brassica rapa against Mercury-Selenium Combined Stress. International Journal of Molecular Sciences, 23(5), 2854. https://doi.org/10.3390/ijms23052854







