Long-Term Changes in Ovarian Follicles of Gilts Exposed Neonatally to Methoxychlor: Effects on Oocyte-Derived Factors, Anti-Müllerian Hormone, Follicle-Stimulating Hormone, and Cognate Receptors
Abstract
1. Introduction
2. Results
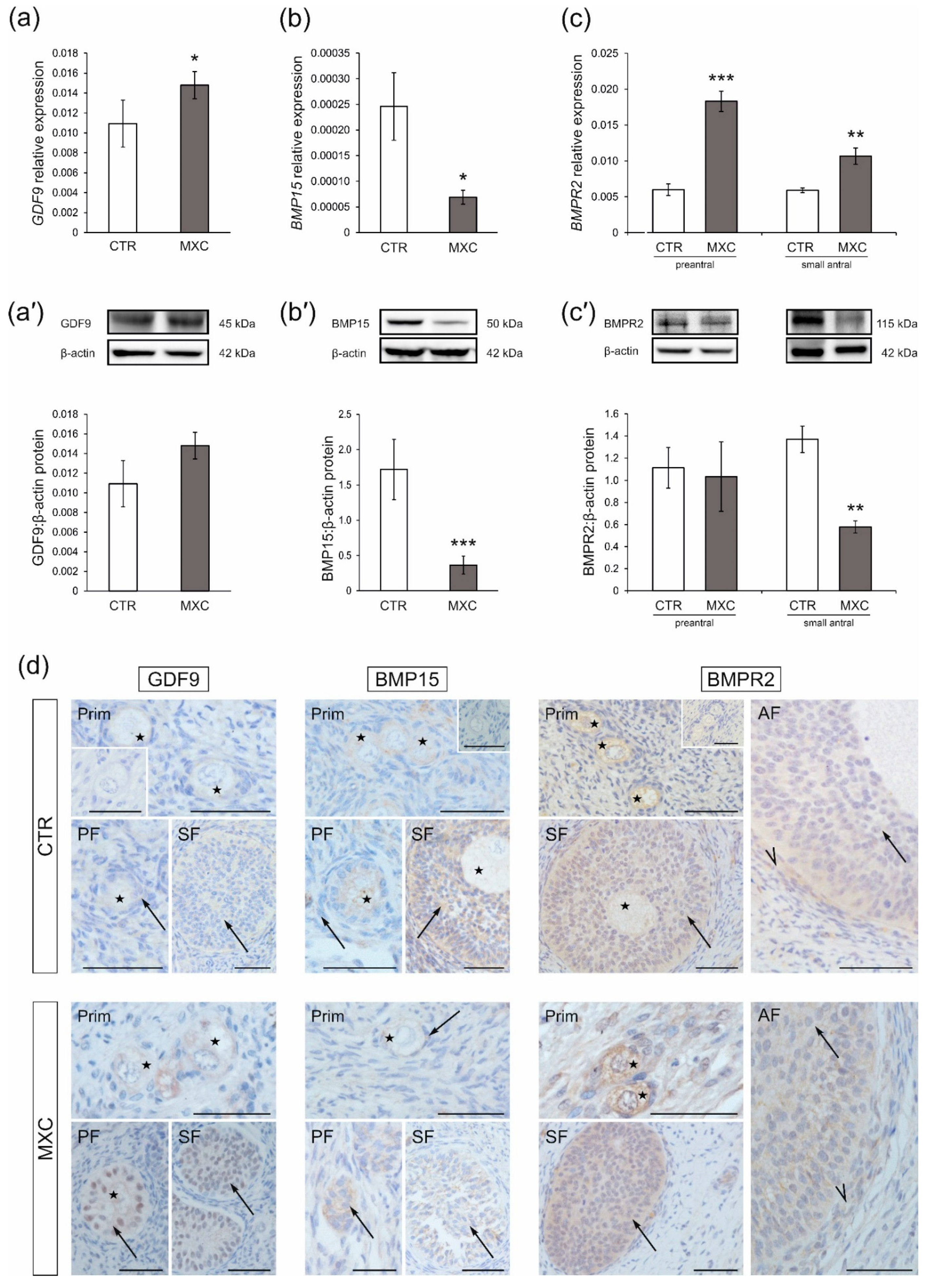
2.1. Effect of Neonatal Exposure to MXC on GDF9 and BMP15 Expression in Preantral Follicles of Gilts
2.2. Effect of Neonatal Exposure to MXC on BMPR2 Expression in Preantral and Small Antral Follicles of Gilts
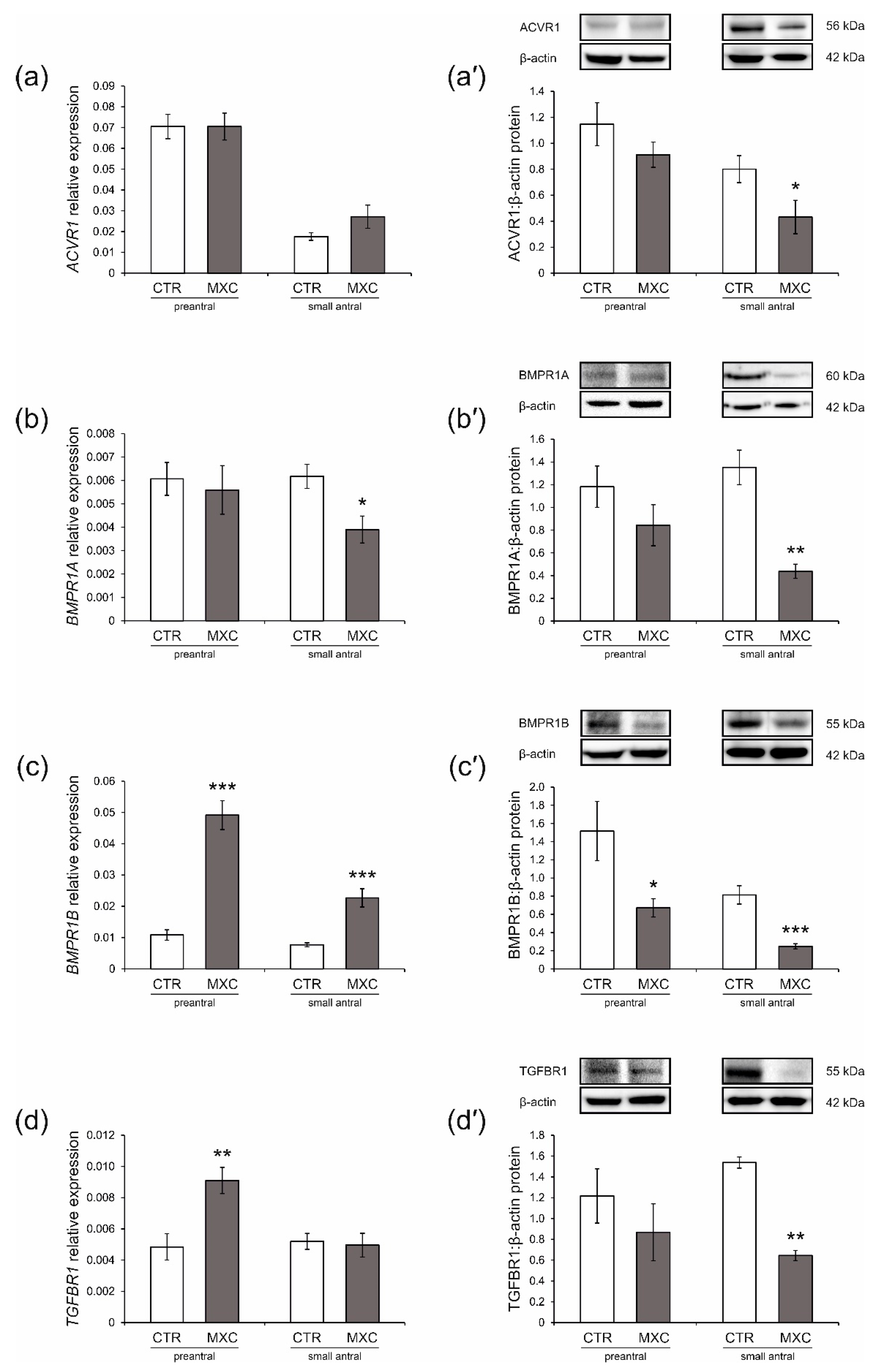
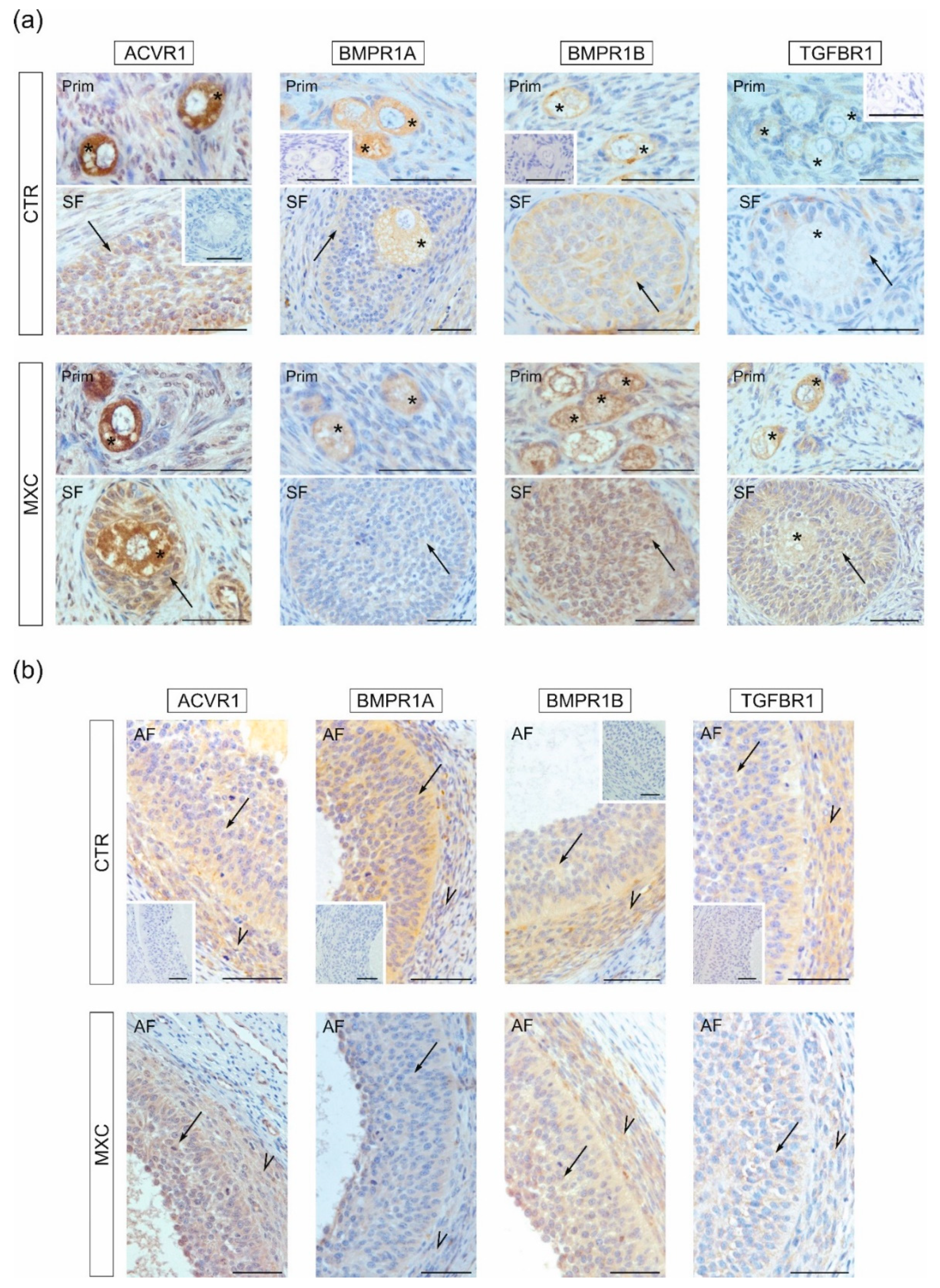
2.3. Effect of Neonatal Exposure to MXC on ACVR1, BMPR1A, BMPR1B, TGFBR1 Expression in Preantral and Small Antral Follicles of Gilts
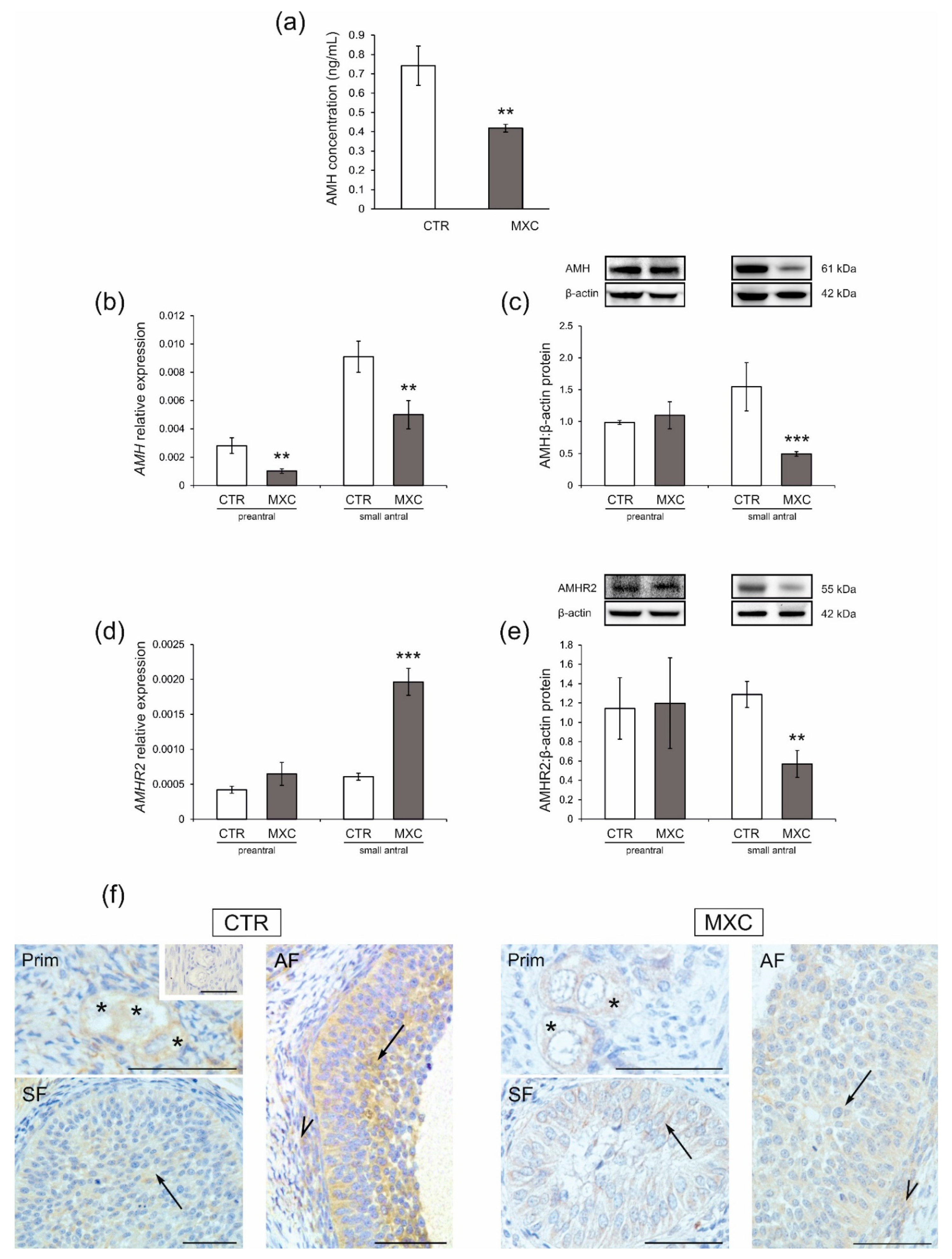
2.4. Effect of Neonatal Exposure to MXC on Plasma AMH Concentration and the Expression of AMHR2 in Preantral and Small Antral Follicles of Gilts
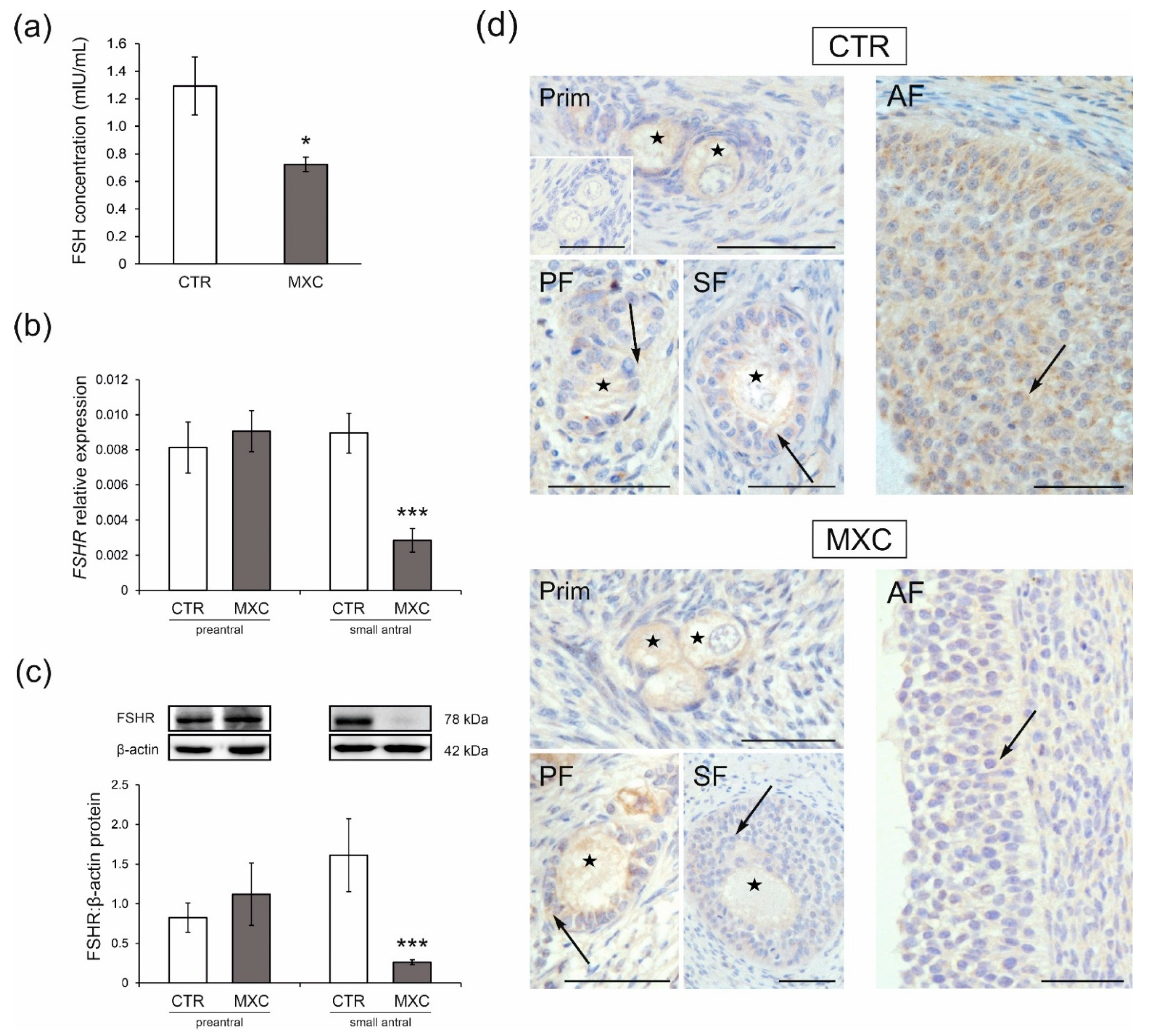
2.5. Effect of Neonatal Exposure to MXC on Plasma FSH Concentration and the Expression of FSHR in Preantral and Small Antral Follicles of Gilts
3. Discussion
4. Materials and Methods
4.1. Animals and Tissue Preparation
4.2. Hormone Assays
4.3. Real-Time PCR
4.4. Western Blot
4.5. Immunohistochemistry
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hwang, K.-A.; Choi, K.-C. Endocrine-disrupting chemicals with estrogenicity posing the risk of cancer progression in estrogen-responsive organs. Adv. Mol. Toxicol. 2015, 9, 1–33. [Google Scholar]
- WHO (World Health Organization). Global Insecticide Use for Vector-Borne Disease Control: A 10-Year Assessment, 2000–2009, 5th ed.; WHO/HTM/NTD/VEM/WHOPES/2011; WHO (World Health Organization): Geneva, Switzerland, 2011; Available online: http://whqlibdoc.who.int/publications/2011/9789241502153_eng.pdf (accessed on 10 January 2012).
- Persistent Organic Pollutants Review Committee. Methoxychlor: Draft Risk Profile. Available online: https://echa.europa.eu/documents/10162/b65a738e-b50f-64e5-cbb0-f2711d49c25e (accessed on 31 August 2021).
- Gaido, K.W.; Maness, S.C.; McDonnell, D.P.; Dehal, S.S.; Kupfer, D.; Safe, S. Interaction of methoxychlor and related compounds with estrogen receptor alpha and beta, and androgen receptor: Structure-activity studies. Mol. Pharmacol. 2000, 58, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Amstislavsky, S.Y.; Amstislavskaya, T.G.; Amstislavsky, V.S.; Tibeikina, M.A.; Osipov, K.V.; Eroschenko, V.P. Reproductive abnormalities in adult male mice following preimplantation exposures to estradiol or pesticide methoxychlor. Reprod. Toxicol. 2006, 21, 154–159. [Google Scholar] [CrossRef]
- Patel, S.; Zhou, C.; Rattan, S.; Flaws, J.A. Effects of endocrine-disrupting chemicals on the Ovary. Biol. Reprod. 2015, 93, 20. [Google Scholar] [CrossRef] [PubMed]
- Armenti, A.E.; Zama, A.M.; Passantino, L.; Uzumcu, M. Developmental methoxychlor exposure affects multiple reproductive parameters and ovarian folliculogenesis and gene expression in adult rats. Toxicol. Appl. Pharmacol. 2008, 233, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Eppig, J.J. Oocyte control of ovarian follicular development and function in mammals. Reproduction 2001, 122, 829–838. [Google Scholar] [CrossRef]
- Kidder, G.M.; Vanderhyden, B.C. Bidirectional communication between oocytes and follicle cells: Ensuring oocyte developmental competence. Can. J. Physiol. Pharmacol. 2010, 88, 399–413. [Google Scholar] [CrossRef]
- Sanfins, A.; Rodrigues, P.; Albertini, D.F. GDF-9 and BMP-15 direct the follicle symphony. J. Assist. Reprod. Genet. 2018, 35, 1741–1750. [Google Scholar] [CrossRef]
- De Castro, F.C.; Cruz, M.H.; Leal, C.L. Role of growth differentiation factor 9 and bone morphogenetic protein 15 in ovarian function and their importance in mammalian female fertility—A review. Asian-Australas. J. Anim. Sci. 2016, 29, 1065–1074. [Google Scholar] [CrossRef]
- Durlinger, A.L.; Gruijters, M.J.; Kramer, P.; Karels, B.; Kumar, T.R.; Matzuk, M.M.; Rose, U.M.; de Jong, F.H.; Uilenbroek, J.T.; Grootegoed, J.A.; et al. Anti-Müllerian hormone attenuates the effects of FSH on follicle development in the mouse ovary. Endocrinology 2001, 142, 4891–4899. [Google Scholar] [CrossRef]
- Durlinger, A.L.; Gruijters, M.J.; Kramer, P.; Karels, B.; Ingraham, H.A.; Nachtigal, M.W.; Uilenbroek, J.T.; Grootegoed, J.A.; Themmen, A.P. Anti-Müllerian hormone inhibits initiation of primordial follicle growth in the mouse ovary. Endocrinology 2002, 143, 1076–1084. [Google Scholar] [CrossRef]
- Salmon, N.A.; Handyside, A.H.; Joyce, I.M. Oocyte regulation of anti-Müllerian hormone expression in granulosa cells during ovarian follicle development in mice. Dev. Biol. 2004, 266, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Vitt, U.A.; Mazerbourg, S.; Klein, C.; Hsueh, A.J. Bone morphogenetic protein receptor type II is a receptor for growth differentiation factor-9. Biol. Reprod. 2002, 67, 473–480. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mazerbourg, S.; Klein, C.; Roh, J.; Kaivo-Oja, N.; Mottershead, D.G.; Korchynskyi, O.; Ritvos, O.; Hsueh, A.J. Growth differentiation factor-9 signaling is mediated by the type I receptor, activin receptor-like kinase 5. Mol. Endocrinol. 2004, 18, 653–665. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Li, Q.; Wigglesworth, K.; Rangarajan, A.; Kattamuri, C.; Peterson, R.T.; Eppig, J.J.; Thompson, T.B.; Matzuk, M.M. Growth differentiation factor 9:bone morphogenetic protein 15 heterodimers are potent regulators of ovarian functions. Proc. Natl. Acad. Sci. USA 2013, 110, E776–E785. [Google Scholar] [CrossRef] [PubMed]
- Di Clemente, N.; Josso, N.; Gouédard, L.; Belville, C. Components of the anti-Müllerian hormone signaling pathway in gonads. Mol. Cell. Endocrinol. 2003, 211, 9–14. [Google Scholar] [CrossRef]
- Gruijters, M.J.; Visser, J.A.; Durlinger, A.L.; Themmen, A.P. Anti-Müllerian hormone and its role in ovarian function. Mol. Cell. Endocrinol. 2003, 211, 85–90. [Google Scholar] [CrossRef]
- Knapczyk-Stwora, K.; Grzesiak, M.; Witek, P.; Duda, M.; Koziorowski, M.; Slomczynska, M. Neonatal exposure to agonists and antagonists of sex steroid receptors induces changes in the expression of oocyte-derived growth factors and their receptors in ovarian follicles in gilts. Theriogenology 2019, 134, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Knapczyk-Stwora, K.; Grzesiak, M.; Witek, P.; Duda, M.; Koziorowski, M.; Slomczynska, M. Neonatal exposure to agonists and antagonists of sex steroid receptors affects AMH and FSH plasma level and their receptors expression in the adult pig ovary. Animals 2020, 10, 12. [Google Scholar] [CrossRef]
- Monniaux, D.; Clément, F.; Dalbiès-Tran, R.; Estienne, A.; Fabre, S.; Mansanet, C.; Monget, P. The ovarian reserve of primordial follicles and the dynamic reserve of antral growing follicles: What is the link? Biol. Reprod. 2014, 90, 85. [Google Scholar] [CrossRef]
- Witek, P.; Enguita, F.J.; Grzesiak, M.; Costa, M.C.; Gabriel, A.; Koziorowski, M.; Slomczynska, M.; Knapczyk-Stwora, K. Effects of neonatal exposure to methoxychlor on corpus luteum in gilts: A transcriptomic analysis. Mol. Reprod. Dev. 2021, 88, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, F.; McTavish, K.J.; Shimasaki, S. Integral role of GDF-9 and BMP-15 in ovarian function. Mol. Reprod. Dev. 2011, 78, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Vitt, U.A.; McGee, E.A.; Hayashi, M.; Hsueh, A.J. In vivo treatment with GDF-9 stimulates primordial and primary follicle progression and theca cell marker CYP17 in ovaries of immature rats. Endocrinology 2000, 141, 3814–3820. [Google Scholar] [CrossRef]
- Abdel-Ghani, M.A.; El-Sherry, T.M.; Abdelhafeez, H.H. Effect of growth differentiation factor-9 (GDF-9) on the progression of buffalo follicles in vitrified-warmed ovarian tissues. Reprod. Domest. Anim. 2016, 51, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Patiño, L.C.; Walton, K.L.; Mueller, T.D.; Johnson, K.E.; Stocker, W.; Richani, D.; Agapiou, D.; Gilchrist, R.B.; Laissue, P.; Harrison, C.A. BMP15 mutations associated with Primary Ovarian Insufficiency reduce expression, activity, or synergy with GDF9. J. Clin. Endocrinol. Metab. 2017, 102, 1009–1019. [Google Scholar] [PubMed]
- De Sousa Abreu, R.; Penalva, L.O.; Marcotte, E.M.; Vogel, C. Global signatures of protein and mRNA expression levels. Mol. Biosyst. 2009, 5, 1512–1526. [Google Scholar] [CrossRef] [PubMed]
- Vogel, C.; Marcotte, E.M. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet. 2012, 13, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.E.; LaPolt, P.S.; Yoon, B.S.; Chen, J.Y.; Lu, J.K.; Lyons, K.M. The type I BMP receptor BmprIB is essential for female reproductive function. Proc. Natl. Acad. Sci. USA 2001, 98, 7994–7999. [Google Scholar] [CrossRef] [PubMed]
- Durlinger, A.L.; Kramer, P.; Karels, B.; de Jong, F.H.; Uilenbroek, J.T.; Grootegoed, J.A.; Themmen, A.P. Control of primordial follicle recruitment by anti-Müllerian hormone in the mouse ovary. Endocrinology 1999, 140, 5789–5796. [Google Scholar] [CrossRef]
- Uzumcu, M.; Kuhn, P.E.; Marano, J.E.; Armenti, A.E.; Passantino, L. Early postnatal methoxychlor exposure inhibits folliculogenesis and stimulates anti-Mullerian hormone production in the rat ovary. J. Endocrinol. 2006, 191, 549–558. [Google Scholar] [CrossRef]
- Knapczyk-Stwora, K.; Grzesiak, M.; Ciereszko, R.E.; Czaja, E.; Koziorowski, M.; Slomczynska, M. The impact of sex steroid agonists and antagonists on folliculogenesis in the neonatal porcine ovary via cell proliferation and apoptosis. Theriogenology 2018, 113, 19–26. [Google Scholar] [CrossRef]
- Fujibe, Y.; Baba, T.; Nagao, S.; Adachi, S.; Ikeda, K.; Morishita, M.; Kuno, Y.; Suzuki, M.; Mizuuchi, M.; Honnma, H.; et al. Androgen potentiates the expression of FSH receptor and supports preantral follicle development in mice. J. Ovarian. Res. 2019, 12, 31. [Google Scholar] [CrossRef] [PubMed]
- Dewailly, D.; Robin, G.; Peigne, M.; Decanter, C.; Pigny, P.; Catteau-Jonard, S. Interactions between androgens, FSH, anti-Müllerian hormone and estradiol during folliculogenesis in the human normal and polycystic ovary. Hum. Reprod. Update 2016, 22, 709–724. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, K.; Okazaki, S.; Nishimura, S.; Nakamura, H.; Kitamura, Y.; Hatayama, K.; Nakamura, A.; Tsuda, T.; Katsumata, T.; Nishikawa, A.; et al. A repeated 28-day oral dose toxicity study of methoxychlor in rats, based on the ‘enhanced OECD test guideline 407’ for screening endocrine-disrupting chemicals. Arch. Toxicol. 2001, 75, 513–521. [Google Scholar] [CrossRef]
- Tomic, D.; Frech, M.S.; Babus, J.K.; Gupta, R.K.; Furth, P.A.; Koos, R.D.; Flaws, J.A. Methoxychlor induces atresia of antral follicles in ERalpha-overexpressing mice. Toxicol. Sci. 2006, 93, 196–204. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gervásio, C.G.; Bernuci, M.P.; Silva-de-Sá, M.F.; Rosa-E-Silva, A.C. The role of androgen hormones in early follicular development. ISRN Obstet. Gynecol. 2014, 2014, 818010. [Google Scholar] [CrossRef] [PubMed]
- Britt, K.L.; Findlay, J.K. Estrogen actions in the ovary revisited. J. Endocrinol. 2002, 175, 269–276. [Google Scholar] [CrossRef]
- Jayawardana, B.C.; Shimizu, T.; Nishimoto, H.; Kaneko, E.; Tetsuka, M.; Miyamoto, A. Hormonal regulation of expression of growth differentiation factor-9 receptor type I and II genes in the bovine ovarian follicle. Reproduction 2006, 131, 545–553. [Google Scholar] [CrossRef]
- Hunzicker-Dunn, M.; Maizels, E.T. FSH signaling pathways in immature granulosa cells that regulate target gene expression: Branching out from protein kinase A. Cell. Signal. 2006, 18, 1351–1359. [Google Scholar] [CrossRef]
- Almeida, F.R.C.L.; Costermans, N.G.J.; Soede, N.M.; Bunschoten, A.; Keijer, J.; Kemp, B.; Teerds, K.J. Presence of anti-Müllerian hormone (AMH) during follicular development in the porcine ovary. PLoS ONE 2018, 13, e0197894. [Google Scholar] [CrossRef]
- Abbott, D.H.; Padmanabhan, V.; Dumesic, D.A. Contributions of androgen and estrogen to fetal programming of ovarian dysfunction. Reprod. Biol. Endocrinol. 2006, 4, 17. [Google Scholar] [CrossRef]
- Quinn, R.L.; Shuttleworth, G.; Hunter, M.G. Immunohistochemical localization of the bone morphogenetic protein receptors in the porcine ovary. J. Anat. 2004, 205, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Ye, L.; Li, H.; Ruge, F.; Yang, Y.; Jiang, W.G. Growth differentiation factor-9 expression is inversely correlated with an aggressive behaviour in human bladder cancer cells. Int. J. Mol. Med. 2012, 29, 428–434. [Google Scholar] [PubMed]
- Du, P.; Ye, L.; Yang, Y.; Jiang, W.G. Reduced expression of growth and differentiation factor-9 (GDF9) is associated with aggressive behaviour of human clear-cell renal cell carcinoma and poor patient survival. Anticancer Res. 2014, 34, 6515–6520. [Google Scholar] [PubMed]
- Durlej, M.; Knapczyk-Stwora, K.; Duda, M.; Galas, J.; Slomczynska, M. The expression of FSH receptor (FSHR) in the neonatal porcine ovary and its regulation by flutamide. Reprod. Domest. Anim. 2011, 46, 377–384. [Google Scholar] [CrossRef]
- Zhao, S.; Fernald, R.D. Comprehensive algorithm for quantitative real-time polymerase chain reaction. J. Comput. Biol. 2005, 12, 1047–1064. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
| Antibody | Dilution Used for | Species | Supplier | |
|---|---|---|---|---|
| WB | IHC | |||
| Anti-ACVR1 | 1:1000 | 1:200 | goat polyclonal | Acris Antibodies GmbH, Herford, Germany (AP22507PU-N, Lot no. 19042) |
| Anti-AMH | 1:1000 | - | rabbit polyclonal | Acris Antibodies GmbH, Herford, Germany (TA336233, Lot no. OC22813) |
| Anti-AMHR2 | 1:1000 | 1:100 | rabbit polyclonal | LifeSpan BioSciences Inc., Seattle, WA, USA (LS-B11943, Lot no. 44994) |
| Anti-BMP15 | 1:500 | 1:50 | rabbit polyclonal | Biorbyt Ltd., Cambridge, UK (orb377952, Lot no. CQ2185) |
| Anti-BMPR1A | 1:1000 | 1:100 | rabbit polyclonal | Kindly provided by Prof. C. H. Heldin (Ludwig Institute for Cancer Research Ltd., Uppsala, Sweden) |
| Anti-BMPR1B | 1:1000 | 1:100 | rabbit polyclonal | Kindly provided by Prof. C. H. Heldin (Ludwig Institute for Cancer Research Ltd., Uppsala, Sweden) |
| Anti-BMPR2 | 1:1000 | 1:100 | rabbit polyclonal | Kindly provided by Prof. C. H. Heldin (Ludwig Institute for Cancer Research Ltd., Uppsala, Sweden) |
| Anti-FSHR | 1:1000 | 1:100 | rabbit polyclonal | Bioss Antibodies, Woburn, MA, USA (BS-0895R, Lot no. AF12207065) |
| Anti-GDF9 | 1:1000 | 1:500 | rabbit polyclonal | Abcam Cambridge, UK (ab93892, Lot no. GR269496-1) |
| Anti-TGFBR1 | 1:1000 | 1:50 | rabbit polyclonal | Abgent San Diego, CA, USA (AP7822c, Lot no. SA110808BQ) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Witek, P.; Grzesiak, M.; Koziorowski, M.; Slomczynska, M.; Knapczyk-Stwora, K. Long-Term Changes in Ovarian Follicles of Gilts Exposed Neonatally to Methoxychlor: Effects on Oocyte-Derived Factors, Anti-Müllerian Hormone, Follicle-Stimulating Hormone, and Cognate Receptors. Int. J. Mol. Sci. 2022, 23, 2780. https://doi.org/10.3390/ijms23052780
Witek P, Grzesiak M, Koziorowski M, Slomczynska M, Knapczyk-Stwora K. Long-Term Changes in Ovarian Follicles of Gilts Exposed Neonatally to Methoxychlor: Effects on Oocyte-Derived Factors, Anti-Müllerian Hormone, Follicle-Stimulating Hormone, and Cognate Receptors. International Journal of Molecular Sciences. 2022; 23(5):2780. https://doi.org/10.3390/ijms23052780
Chicago/Turabian StyleWitek, Patrycja, Małgorzata Grzesiak, Marek Koziorowski, Maria Slomczynska, and Katarzyna Knapczyk-Stwora. 2022. "Long-Term Changes in Ovarian Follicles of Gilts Exposed Neonatally to Methoxychlor: Effects on Oocyte-Derived Factors, Anti-Müllerian Hormone, Follicle-Stimulating Hormone, and Cognate Receptors" International Journal of Molecular Sciences 23, no. 5: 2780. https://doi.org/10.3390/ijms23052780
APA StyleWitek, P., Grzesiak, M., Koziorowski, M., Slomczynska, M., & Knapczyk-Stwora, K. (2022). Long-Term Changes in Ovarian Follicles of Gilts Exposed Neonatally to Methoxychlor: Effects on Oocyte-Derived Factors, Anti-Müllerian Hormone, Follicle-Stimulating Hormone, and Cognate Receptors. International Journal of Molecular Sciences, 23(5), 2780. https://doi.org/10.3390/ijms23052780






