The Effects of Light and the Circadian System on Rhythmic Brain Function
Abstract
1. Introduction
2. The Role of Light and the Circadian Clock for Rhythmic Brain Function
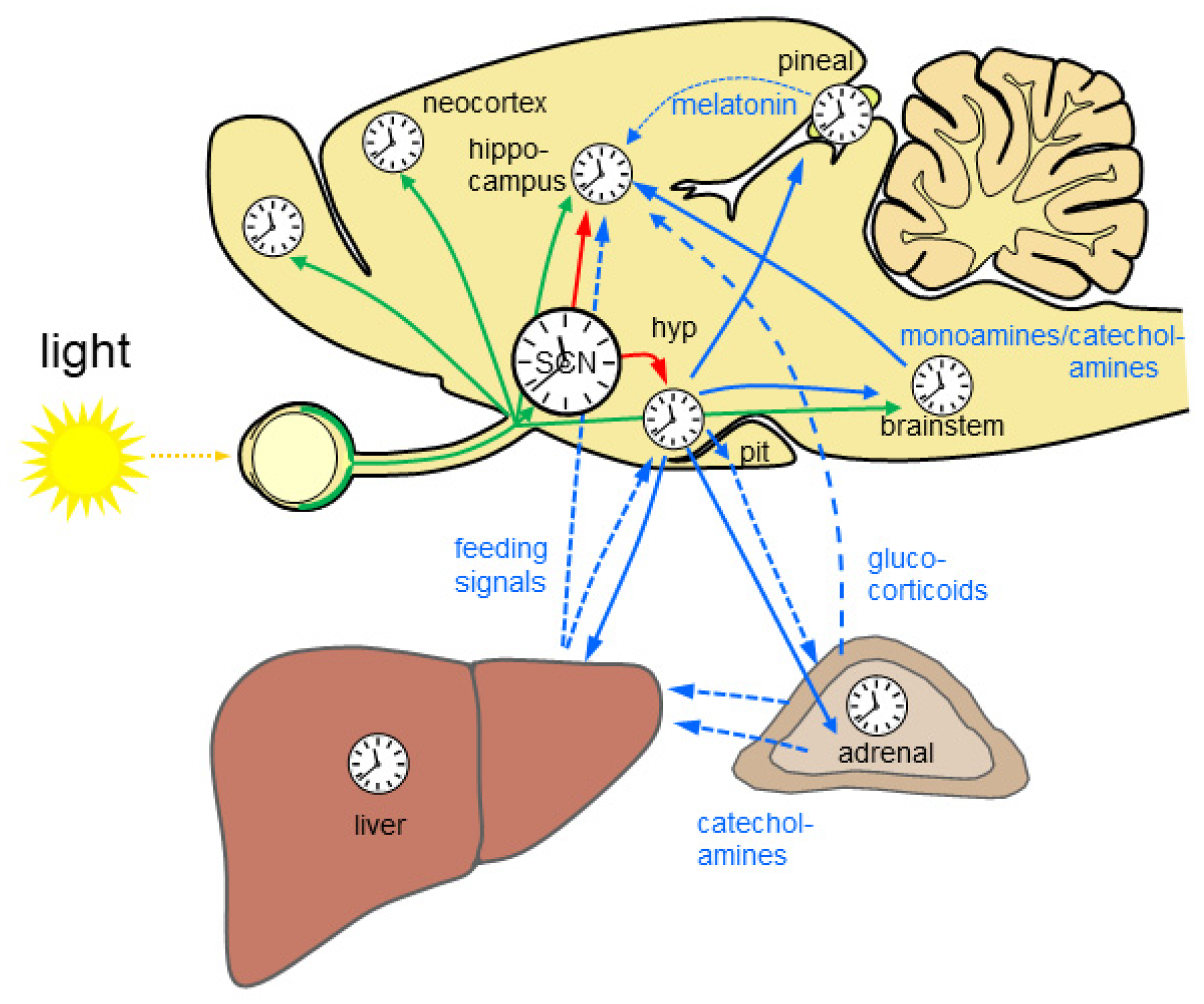
2.1. The Mammalian Circadian System
2.2. Light Input into the Circadian System—Entrainment and Masking
2.3. The Brain Molecular Clockwork
2.4. Rhythmic Gene and Protein Expression in the Brain
Sleep Deprivation, Epilepsy, and Glucocorticoids Affect Gene and Protein Expression in the Brain
2.5. Circadian and Light-Driven Brain Function
2.5.1. Rhythmic Hormone Release
2.5.2. Rhythms in Food Intake
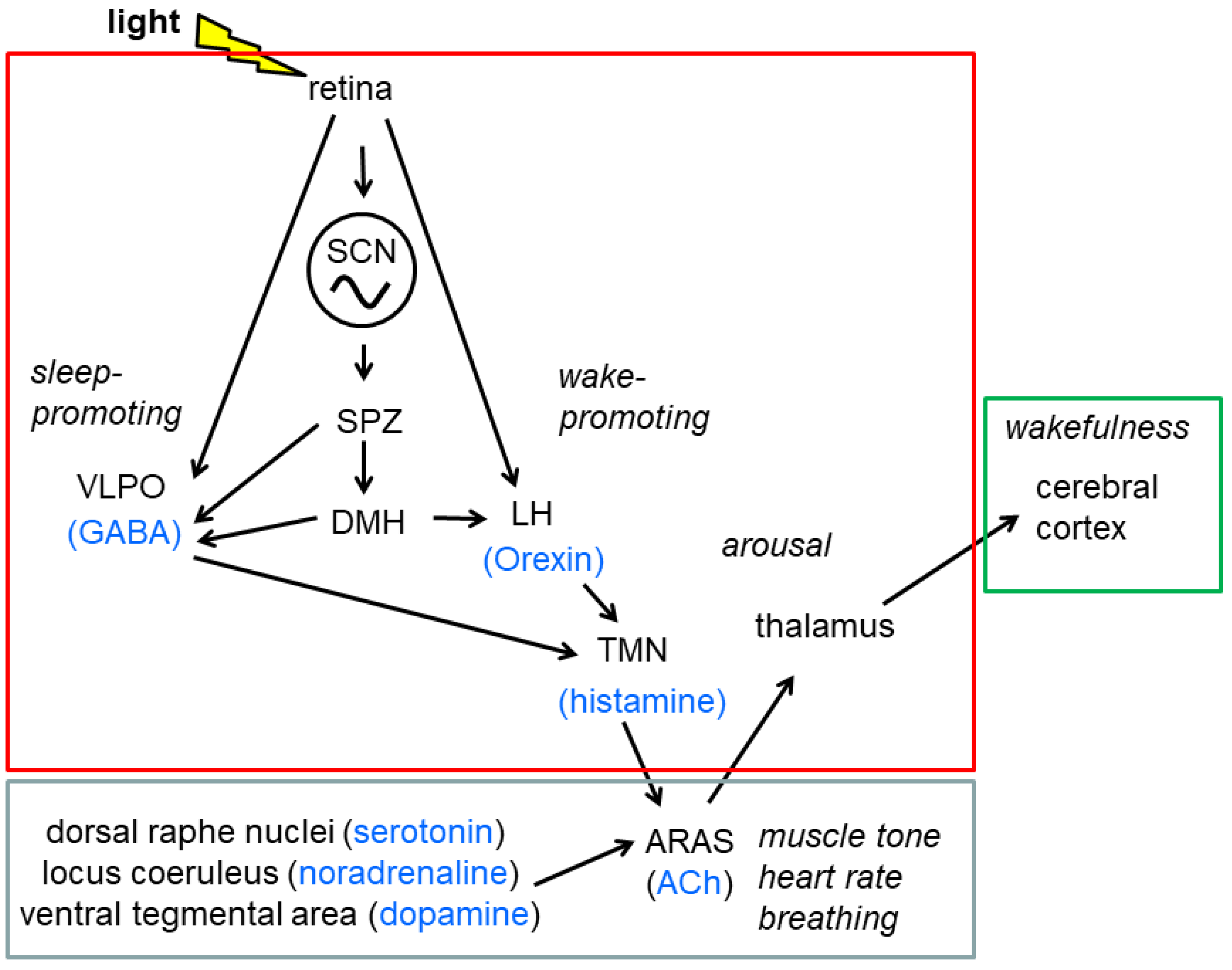
2.5.3. The Sleep Wake Cycle
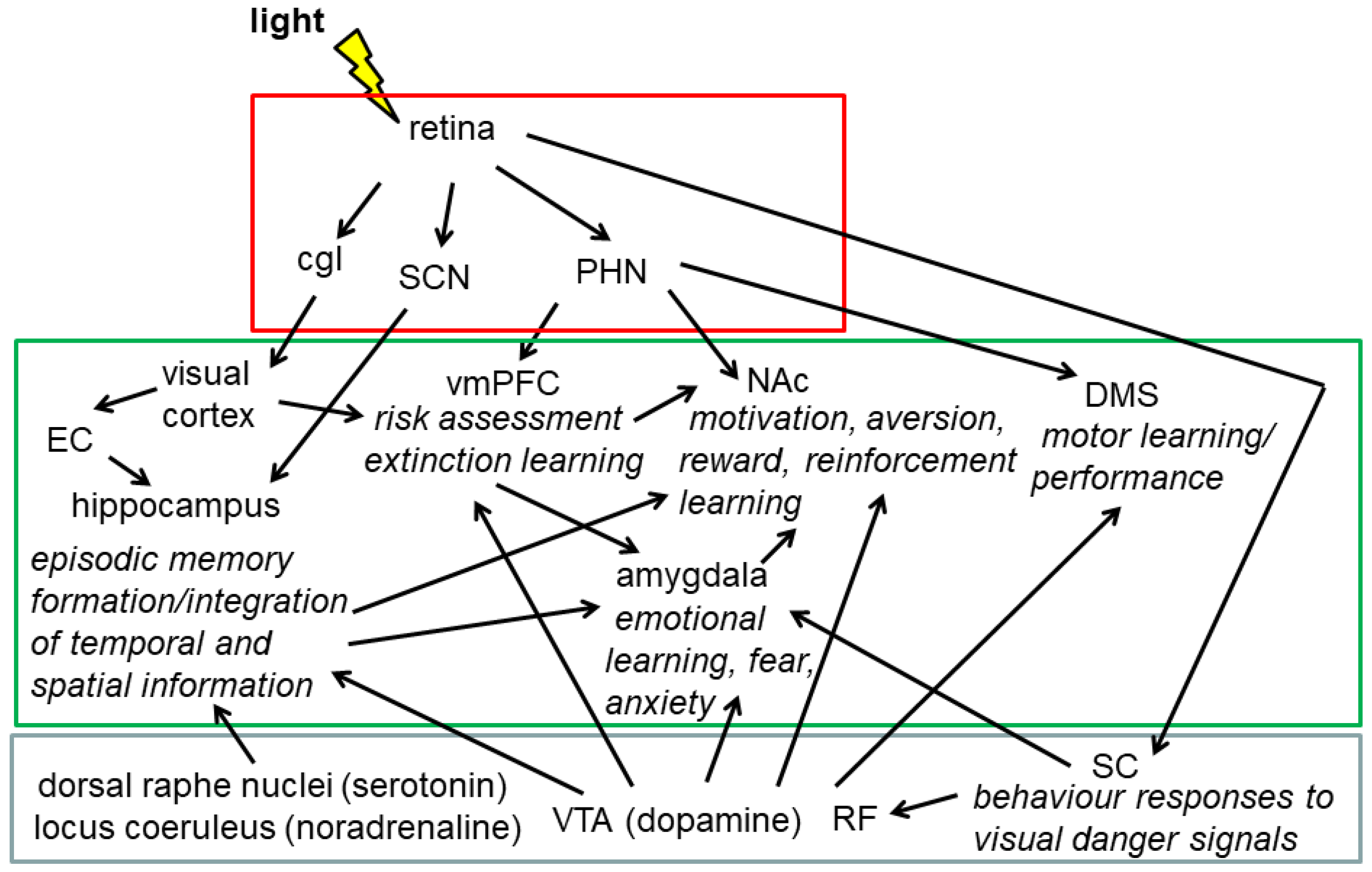
2.5.4. Cognitive Performance and Emotion-Related Behaviour
2.6. Neuropathological Conditions and Circadian Misalignment
3. Summary
4. Conclusions and Outlook
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davies, W.I.L.; Collin, S.P.; Hunt, D.M. Molecular ecology and adaptation of visual photopigments in craniates. Mol. Ecol. 2012, 21, 3121–3158. [Google Scholar] [CrossRef]
- Maor, R.; Dayan, T.; Ferguson-Gow, H.; Jones, K.E. Temporal niche expansion in mammals from a nocturnal ancestor after dinosaur extinction. Nat. Ecol. Evol. 2017, 1, 1889–1895. [Google Scholar] [CrossRef] [PubMed]
- Ankel-Simons, F.; Rasmussen, D. Diurnality, nocturnality, and the evolution of primate visual systems. Am. J. Phys. Anthr. 2008, 137, 100–117. [Google Scholar] [CrossRef]
- Verra, D.M.; Sajdak, B.; Merriman, D.; Hicks, D. Diurnal rodents as pertinent animal models of human retinal physiology and pathology. Prog. Retin. Eye Res. 2020, 74, 100776. [Google Scholar] [CrossRef] [PubMed]
- Reppert, S.M.; Weaver, D.R. Coordination of circadian timing in mammals. Nature 2002, 418, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Buijs, R.M.; La Fleur, S.E.; Wortel, J.; Van Heyningen, C.; Zuiddam, L.; Mettenleiter, T.C.; Kalsbeek, A.; Nagai, K.; Niijima, A. The suprachiasmatic nucleus balances sympathetic and parasympathetic output to peripheral organs through separate preautonomic neurons. J. Comp. Neurol. 2003, 464, 36–48. [Google Scholar] [CrossRef]
- Kalsbeek, A.; Palm, I.F.; La Fleur, S.E.; Scheer, F.; Perreau-Lenz, S.; Ruiter, M.; Kreier, F.; Cailotto, C.; Buijs, R.M. SCN Outputs and the Hypothalamic Balance of Life. J. Biol. Rhythm. 2006, 21, 458–469. [Google Scholar] [CrossRef]
- Balsalobre, A.; Brown, S.A.; Marcacci, L.; Tronche, F.; Kellendonk, C.; Reichardt, H.M.; Schütz, G.; Schibler, U. Resetting of Circadian Time in Peripheral Tissues by Glucocorticoid Signaling. Science 2000, 289, 2344–2347. [Google Scholar] [CrossRef]
- Pasquier, D.A.; Reinoso-Suarez, F. The topographic organization of hypothalamic and brain stem projections to the hippocampus. Brain Res. Bull. 1978, 3, 373–389. [Google Scholar] [CrossRef]
- Korf, H.-W.; von Gall, C. Circadian Physiology. In Neuroscience in the 21st Century; Pfaff, D.W., Volkow, N.D., Eds.; Springer Science+Business Media: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Fernandez, D.C.; Fogerson, P.M.; Ospri, L.L.; Thomsen, M.B.; Layne, R.M.; Severin, D.; Zhan, J.; Singer, J.H.; Kirkwood, A.; Zhao, H.; et al. Light Affects Mood and Learning through Distinct Retina-Brain Pathways. Cell 2018, 175, 71–84.e18. [Google Scholar] [CrossRef] [PubMed]
- Mrosovsky, N. Masking: History, Definitions, and Measurement. Chronol. Int. 1999, 16, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Aschoff, J. Exogenous and Endogenous Components in Circadian Rhythms. Cold Spring Harb. Symp. Quant. Biol. 1960, 25, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.; Philp, A.R.; Stone, E.M. Visual function testing: A quantifiable visually guided behavior in mice. Vis. Res. 2008, 48, 346–352. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Crawley, J.; Goodwin, F.K. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol. Biochem. Behav. 1980, 13, 167–170. [Google Scholar] [CrossRef]
- Bourin, M.; Hascoët, M. The mouse light/dark box test. Eur. J. Pharmacol. 2003, 463, 55–65. [Google Scholar] [CrossRef]
- Lucas, R.J.; Hattar, S.; Takao, M.; Berson, D.M.; Foster, R.G.; Yau, K.-W. Diminished Pupillary Light Reflex at High Irradiances in Melanopsin-Knockout Mice. Science 2003, 299, 245–247. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Sato, T.K.; Castrucci, A.M.; Rollag, M.D.; DeGrip, W.J.; Hogenesch, J.B.; Provencio, I.; Kay, S.A. Melanopsin (Opn4) Requirement for Normal Light-Induced Circadian Phase Shifting. Science 2002, 298, 2213–2216. [Google Scholar] [CrossRef]
- Ruby, N.F.; Brennan, T.J.; Xie, X.; Cao, V.; Franken, P.; Heller, H.C.; O’Hara, B.F. Role of Melanopsin in Circadian Responses to Light. Science 2002, 298, 2211–2213. [Google Scholar] [CrossRef]
- Ecker, J.L.; Dumitrescu, O.N.; Wong, K.; Alam, N.M.; Chen, S.-K.; LeGates, T.; Renna, J.M.; Prusky, G.T.; Berson, D.M.; Hattar, S. Melanopsin-Expressing Retinal Ganglion-Cell Photoreceptors: Cellular Diversity and Role in Pattern Vision. Neuron 2010, 67, 49–60. [Google Scholar] [CrossRef]
- Hattar, S.; Lucas, R.J.; Mrosovsky, N.; Thompson, S.; Douglas, R.H.; Hankins, M.W.; Lem, J.; Biel, M.; Hofmann, F.; Foster, R.G.; et al. Melanopsin and rod–cone photoreceptive systems account for all major accessory visual functions in mice. Nature 2003, 424, 75–81. [Google Scholar] [CrossRef]
- Peirson, S.N.; Brown, L.; Pothecary, C.A.; Benson, L.A.; Fisk, A.S. Light and the laboratory mouse. J. Neurosci. Methods 2018, 300, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Aschoff, J.; Von Goetz, C. Masking of circadian activity rhythms in hamsters by darkness. J. Comp. Physiol. A Sens. Neural Behav. Physiol. 1988, 162, 559–562. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, M.; Rauch, A.; Korf, H.; Von Gall, C. The Endogenous Melatonin (MT) Signal Facilitates Reentrainment of the Circadian System to Light-Induced Phase Advances by Acting Upon MT2 Receptors. Chronobiol. Int. 2012, 29, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.B.; Field, M.D.; Maywood, E.S.; Hastings, M.H. Differential Resynchronisation of Circadian Clock Gene Expression within the Suprachiasmatic Nuclei of Mice Subjected to Experimental Jet Lag. J. Neurosci. 2002, 22, 7326–7330. [Google Scholar] [CrossRef]
- Wever, R.A. Phase shifts of human circadian rhythms due to shifts of artificial Zeitgebers. Chronobiologia 1980, 7, 303–327. [Google Scholar] [PubMed]
- Gillette, M.U.; Mitchell, J.W. Signaling in the suprachiasmatic nucleus: Selectively responsive and integrative. Cell Tissue Res. 2002, 309, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Ginty, D.D.; Kornhauser, J.M.; Thompson, M.A.; Bading, H.; Mayo, K.E.; Takahashi, J.S.; Greenberg, M.E. Regulation of CREB Phosphorylation in the Suprachiasmatic Nucleus by Light and a Circadian Clock. Science 1993, 260, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kornhauser, J.; Zee, P.; Mayo, K.; Takahashi, J.; Turek, F. Effects of aging on light-induced phase-shifting of circadian behavioral rhythms, Fos expression and creb phosphorylation in the hamster suprachiasmatic nucleus. Neuroscience 1996, 70, 951–961. [Google Scholar] [CrossRef]
- Duffield, G.E.; Robles-Murguia, M.; Hou, T.Y.; McDonald, K.A. Targeted Disruption of the Inhibitor of DNA Binding 4 (Id4) Gene Alters Photic Entrainment of the Circadian Clock. Int. J. Mol. Sci. 2021, 22, 9632. [Google Scholar] [CrossRef] [PubMed]
- Gau, D.; Lemberger, T.; von Gall, C.; Kretz, O.; Le Minh, N.; Gass, P.; Schmid, W.; Schibler, U.; Korf, H.; Schütz, G. Phosphorylation of CREB Ser142 Regulates Light-Induced Phase Shifts of the Circadian Clock. Neuron 2002, 34, 245–253. [Google Scholar] [CrossRef]
- Pittendrigh, C.S.; Daan, S. A functional analysis of circadian pacemakers in nocturnal rodents. J. Comp. Physiol. 1976, 106, 223–252. [Google Scholar] [CrossRef]
- Edelstein, K.; Amir, S. The Role of the Intergeniculate Leaflet in Entrainment of Circadian Rhythms to a Skeleton Photoperiod. J. Neurosci. 1999, 19, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Inouye, S.T.; Kawamura, H. Persistence of circadian rhythmicity in a mammalian hypothalamic “island” containing the suprachiasmatic nucleus. Proc. Natl. Acad. Sci. USA 1979, 76, 5962–5966. [Google Scholar] [CrossRef]
- Reick, M.; Garcia, J.A.; Dudley, C.; McKnight, S.L. NPAS2: An Analog of Clock Operative in the Mammalian Forebrain. Science 2001, 293, 506–509. [Google Scholar] [CrossRef] [PubMed]
- Green, C.B. Circadian Posttranscriptional Regulatory Mechanisms in Mammals. Cold Spring Harb. Perspect. Biol. 2018, 10, a030692. [Google Scholar] [CrossRef]
- Koike, N.; Yoo, S.-H.; Huang, H.-C.; Kumar, V.; Lee, C.; Kim, T.-K.; Takahashi, J.S. Transcriptional Architecture and Chromatin Landscape of the Core Circadian Clock in Mammals. Science 2012, 338, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, S.; Ishida, Y.; Inouye, S.-I.T. Circadian rhythms of adenosine triphosphate contents in the suprachiasmatic nucleus, anterior hypothalamic area and caudate putamen of the rat—Negative correlation with electrical activity. Brain Res. 1994, 664, 237–240. [Google Scholar] [CrossRef]
- Colwell, C.S. Linking neural activity and molecular oscillations in the SCN. Nat. Rev. Neurosci. 2011, 12, 553–569. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, W.J.; Gainer, H. Suprachiasmatic Nucleus: Use of 14 C-Labeled Deoxyglucose Uptake as a Functional Marker. Science 1977, 197, 1089–1091. [Google Scholar] [CrossRef]
- Musiek, E.; Lim, M.M.; Yang, G.; Bauer, A.Q.; Qi, L.; Lee, Y.; Roh, J.H.; Ortiz-Gonzalez, X.; Dearborn, J.; Culver, J.P.; et al. Circadian clock proteins regulate neuronal redox homeostasis and neurodegeneration. J. Clin. Investig. 2013, 123, 5389–5400. [Google Scholar] [CrossRef]
- Chung, S.; Lee, E.J.; Yun, S.; Choe, H.K.; Park, S.B.; Son, H.J.; Kim, K.S.; Dluzen, D.E.; Lee, I.; Hwang, O.; et al. Impact of circadian nuclear receptor REV-ERBalpha on midbrain dopamine production and mood regulation. Cell 2014, 157, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, S.; Fukushima, H.; Hosoda, H.; Serita, T.; Ishikawa, R.; Rokukawa, T.; Kawahara-Miki, R.; Zhang, Y.; Ohta, M.; Okada, S.; et al. Hippocampal clock regulates memory retrieval via Dopamine and PKA-induced GluA1 phosphorylation. Nat. Commun. 2019, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Brancaccio, M.; Patton, A.; Chesham, J.E.; Maywood, E.S.; Hastings, M.H. Astrocytes Control Circadian Timekeeping in the Suprachiasmatic Nucleus via Glutamatergic Signaling. Neuron 2017, 93, 1420–1435.e5. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.A.H.; Stahr, A.; Ingenwerth, M.; Theis, M.; Steinhäuser, C.; Von Gall, C. Connexin30 and Connexin43 show a time-of-day dependent expression in the mouse suprachiasmatic nucleus and modulate rhythmic locomotor activity in the context of chronodisruption. Cell Commun. Signal. 2019, 17, 61. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Yang, T.; Cui, S.; Chen, G. Connexin Hemichannels in Astrocytes: Role in CNS Disorders. Front. Mol. Neurosci. 2019, 12, 23. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Herzog, E.D.; Yamazaki, S.; Straume, M.; Tei, H.; Sakaki, Y.; Menaker, M.; Block, G.D. Circadian Rhythms in Isolated Brain Regions. J. Neurosci. 2002, 22, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.M.-C.; Dragich, J.M.; Kudo, T.; Odom, I.H.; Welsh, D.K.; O’Dell, T.J.; Colwell, C.S. Expression of the Circadian Clock Gene Period2 in the Hippocampus: Possible Implications for Synaptic Plasticity and Learned Behaviour. ASN Neuro 2009, 1, AN20090020. [Google Scholar] [CrossRef]
- Schmal, C.; Herzog, E.D.; Herzel, H. Measuring Relative Coupling Strength in Circadian Systems. J. Biol. Rhythm. 2018, 33, 84–98. [Google Scholar] [CrossRef]
- Bunger, M.K.; Wilsbacher, L.D.; Moran, S.M.; Clendenin, C.; Radcliffe, L.A.; Hogenesch, J.B.; Simon, M.C.; Takahashi, J.S.; Bradfield, C.A. Mop3 Is an Essential Component of the Master Circadian Pacemaker in Mammals. Cell 2000, 103, 1009–1017. [Google Scholar] [CrossRef]
- Van Der Horst, G.T.J.; Muijtjens, M.; Kobayashi, K.; Takano, R.; Kanno, S.-I.; Takao, M.; De Wit, J.; Verkerk, A.; Eker, A.P.M.; Van Leenen, D.; et al. Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature 1999, 398, 627–630. [Google Scholar] [CrossRef]
- Van Gelder, R.N.; Gibler, T.M.; Tu, D.; Embry, K.; Selby, C.P.; Thompson, C.L.; Sancar, A. Pleiotropic effects of cryptochromes 1 and 2 on free-running and light-entrained murine circadian rhythms. J. Neurogenet. 2002, 16, 181–203. [Google Scholar] [CrossRef] [PubMed]
- Selby, C.P.; Thompson, C.; Schmitz, T.M.; Van Gelder, R.; Sancar, A. Functional redundancy of cryptochromes and classical photoreceptors for nonvisual ocular photoreception in mice. Proc. Natl. Acad. Sci. USA 2000, 97, 14697–14702. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, M.; Müller, C.M.; Mordel, J.; Meissl, H.; Ansari, N.; Deller, T.; Korf, H.-W.; Von Gall, C. The Mammalian Molecular Clockwork Controls Rhythmic Expression of Its Own Input Pathway Components. J. Neurosci. 2009, 29, 6114–6123. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, M.; Ingenwerth, M.; Sager, M.; von Gall, C.; Ali, A. Does a Red House Affect Rhythms in Mice with a Corrupted Circadian System? Int. J. Mol. Sci. 2021, 22, 2288. [Google Scholar] [CrossRef]
- Kondratova, A.A.; Dubrovsky, Y.V.; Antoch, M.P.; Kondratov, R.V. Circadian clock proteins control adaptation to novel environment and memory formation. Aging 2010, 2, 285–297. [Google Scholar] [CrossRef]
- Wardlaw, S.M.; Phan, T.X.; Saraf, A.; Chen, X.; Storm, D.R. Genetic disruption of the core circadian clock impairs hippocampus-dependent memory. Learn. Mem. 2014, 21, 417–423. [Google Scholar] [CrossRef]
- Ali, A.; Schwarz-Herzke, B.; Stahr, A.; Prozorovski, T.; Aktas, O.; Von Gall, C. Premature aging of the hippocampal neurogenic niche in adult Bmal1- deficient mice. Aging 2015, 7, 435–449. [Google Scholar] [CrossRef]
- Laposky, A.; Easton, A.; Dugovic, C.; Walisser, J.; Bradfield, C.; Turek, F. Deletion of the mammalian circadian clock gene BMAL1/Mop3 alters baseline sleep architecture and the response to sleep deprivation. Sleep 2005, 28, 395–409. [Google Scholar] [CrossRef]
- Zhang, R.; Lahens, N.F.; Ballance, H.I.; Hughes, M.E.; Hogenesch, J.B.; Pfeffer, M.; Maronde, E.; Bonig, H. A circadian gene expression atlas in mammals: Implications for biology and medicine. Proc. Natl. Acad. Sci. USA 2014, 111, 16219–16224. [Google Scholar] [CrossRef]
- Panda, S.; Antoch, M.P.; Miller, B.H.; Su, A.I.; Schook, A.B.; Straume, M.; Schultz, P.G.; Kay, S.A.; Takahashi, J.; Hogenesch, J.B. Coordinated Transcription of Key Pathways in the Mouse by the Circadian Clock. Cell 2002, 109, 307–320. [Google Scholar] [CrossRef]
- Akhtar, R.; Reddy, A.B.; Maywood, E.S.; Clayton, J.D.; King, V.M.; Smith, A.G.; Gant, T.; Hastings, M.H.; Kyriacou, C.P. Circadian Cycling of the Mouse Liver Transcriptome, as Revealed by cDNA Microarray, Is Driven by the Suprachiasmatic Nucleus. Curr. Biol. 2002, 12, 540–550. [Google Scholar] [CrossRef]
- Duffield, G.E.; Best, J.D.; Meurers, B.H.; Bittner, A.; Loros, J.J.; Dunlap, J.C. Circadian Programs of Transcriptional Activation, Signaling, and Protein Turnover Revealed by Microarray Analysis of Mammalian Cells. Curr. Biol. 2002, 12, 551–557. [Google Scholar] [CrossRef]
- Storch, K.-F.; Paz, C.; Signorovitch, J.; Raviola, E.; Pawlyk, B.; Li, T.; Weitz, C.J. Intrinsic Circadian Clock of the Mammalian Retina: Importance for Retinal Processing of Visual Information. Cell 2007, 130, 730–741. [Google Scholar] [CrossRef] [PubMed]
- Noya, S.B.; Colameo, D.; Brüning, F.; Spinnler, A.; Mircsof, D.; Opitz, L.; Mann, M.; Tyagarajan, S.K.; Robles, M.S.; Brown, S.A. The forebrain synaptic transcriptome is organized by clocks but its proteome is driven by sleep. Science 2019, 366, eaav2642. [Google Scholar] [CrossRef] [PubMed]
- Brüning, F.; Noya, S.B.; Bange, T.; Koutsouli, S.; Rudolph, J.D.; Tyagarajan, S.K.; Cox, J.; Mann, M.; Brown, S.A.; Robles, M.S. Sleep-wake cycles drive daily dynamics of synaptic phosphorylation. Science 2019, 366, eaav3617. [Google Scholar] [CrossRef] [PubMed]
- Wisor, J.P.; O’Hara, B.F.; Terao, A.; Selby, C.P.; Kilduff, T.S.; Sancar, A.; Edgar, D.M.; Franken, P. A role for cryptochromes in sleep regulation. BMC Neurosci. 2002, 3, 20. [Google Scholar] [CrossRef]
- Maret, S.; Dorsaz, S.; Gurcel, L.; Pradervand, S.; Petit, B.; Pfister, C.; Hagenbuchle, O.; O’Hara, B.F.; Franken, P.; Tafti, M. Homer1a is a core brain molecular correlate of sleep loss. Proc. Natl. Acad. Sci. USA 2007, 104, 20090–20095. [Google Scholar] [CrossRef]
- Nollet, M.; Wisden, W.; Franks, N.P. Sleep deprivation and stress: A reciprocal relationship. Interface Focus 2020, 10, 20190092. [Google Scholar] [CrossRef]
- Mongrain, V.; Hernandez, S.A.; Pradervand, S.; Dorsaz, S.; Curie, T.; Hagiwara, G.; Gip, P.; Heller, H.C.; Franken, P. Separating the Contribution of Glucocorticoids and Wakefulness to the Molecular and Electrophysiological Correlates of Sleep Homeostasis. Sleep 2010, 33, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Debski, K.J.; Ceglia, N.; Ghestem, A.; Ivanov, A.I.; Brancati, G.E.; Bröer, S.; Bot, A.M.; Müller, J.A.; Schoch, S.; Becker, A.; et al. The circadian dynamics of the hippocampal transcriptome and proteome is altered in experimental temporal lobe epilepsy. Sci. Adv. 2020, 6, eaat5979. [Google Scholar] [CrossRef]
- Cano-López, I.; Gonzalez-Bono, E. Cortisol levels and seizures in adults with epilepsy: A systematic review. Neurosci. Biobehav. Rev. 2019, 103, 216–229. [Google Scholar] [CrossRef]
- Goto, M.; Oshima, I.; Tomita, T.; Ebihara, S. Melatonin Content of the Pineal Gland in Different Mouse Strains. J. Pineal Res. 1989, 7, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Ishida, A.; Mutoh, T.; Ueyama, T.; Bando, H.; Masubuchi, S.; Nakahara, D.; Tsujimoto, G.; Okamura, H. Light activates the adrenal gland: Timing of gene expression and glucocorticoid release. Cell Metab. 2005, 2, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Krontira, A.; Cruceanu, C.; Binder, E.B. Glucocorticoids as Mediators of Adverse Outcomes of Prenatal Stress. Trends Neurosci. 2020, 43, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Oster, H.; Challet, E.; Ott, V.; Arvat, E.; de Kloet, R.; Dijk, D.-J.; Lightman, S.; Vgontzas, A.; Van Cauter, E. The Functional and Clinical Significance of the 24-Hour Rhythm of Circulating Glucocorticoids. Endocr. Rev. 2016, 38, 3–45. [Google Scholar] [CrossRef]
- Gilhooley, M.; Pinnock, S.; Herbert, J. Rhythmic expression of per1 in the dentate gyrus is suppressed by corticosterone: Implications for neurogenesis. Neurosci. Lett. 2011, 489, 177–181. [Google Scholar] [CrossRef]
- Malek, Z.S.; Sage, D.; Pévet, P.; Raison, S. Daily Rhythm of Tryptophan Hydroxylase-2 Messenger Ribonucleic Acid within Raphe Neurons Is Induced by Corticoid Daily Surge and Modulated by Enhanced Locomotor Activity. Endocrinology 2007, 148, 5165–5172. [Google Scholar] [CrossRef]
- Lamont, E.W.; Robinson, B.; Stewart, J.; Amir, S. The central and basolateral nuclei of the amygdala exhibit opposite diurnal rhythms of expression of the clock protein Period2. Proc. Natl. Acad. Sci. USA 2005, 102, 4180–4184. [Google Scholar] [CrossRef] [PubMed]
- McCauley, J.P.; Petroccione, M.A.; D’Brant, L.Y.; Todd, G.C.; Affinnih, N.; Wisnoski, J.J.; Zahid, S.; Shree, S.; Sousa, A.A.; De Guzman, R.M.; et al. Circadian Modulation of Neurons and Astrocytes Controls Synaptic Plasticity in Hippocampal Area CA1. Cell Rep. 2020, 33, 108255. [Google Scholar] [CrossRef]
- Challet, E. The circadian regulation of food intake. Nat. Rev. Endocrinol. 2019, 15, 393–405. [Google Scholar] [CrossRef]
- Gonzalez, A.; Jensen, L.T.; Iordanidou, P.; Strom, M.; Fugger, L.; Burdakov, D. Inhibitory Interplay between Orexin Neurons and Eating. Curr. Biol. 2016, 26, 2486–2491. [Google Scholar] [CrossRef]
- Nagai, K.; Nishio, T.; Nakagawa, H.; Nakamura, S.; Fukuda, Y. Effect of bilateral lesions of the suprachiasmatic nuclei on the circadian rhythm of food-intake. Brain Res. 1978, 142, 384–389. [Google Scholar] [CrossRef]
- Stephan, F.K. The “other” circadian system: Food as a Zeitgeber. J. Biol. Rhythm. 2002, 17, 284–292. [Google Scholar] [CrossRef]
- Pendergast, J.S.; Yamazaki, S. The Mysterious Food-Entrainable Oscillator: Insights from Mutant and Engineered Mouse Models. J. Biol. Rhythm. 2018, 33, 458–474. [Google Scholar] [CrossRef]
- Bray, M.S.; Ratcliffe, W.F.; Grenett, M.H.; Brewer, R.A.; Gamble, K.; Young, M.E. Quantitative analysis of light-phase restricted feeding reveals metabolic dyssynchrony in mice. Int. J. Obes. 2013, 37, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, C.; Shikata, N.; Seki, S.; Koyama, N.; Noguchi, Y. Early nocturnal meal skipping alters the peripheral clock and increases lipogenesis in mice. Nutr. Metab. 2012, 9, 78. [Google Scholar] [CrossRef] [PubMed]
- Borbely, A.A.; Daan, S.; Wirz-Justice, A.; Deboer, T. The two-process model of sleep regulation: A reappraisal. J. Sleep Res. 2016, 25, 131–143. [Google Scholar] [CrossRef]
- Scammell, T.E.; Arrigoni, E.; Lipton, J.O. Neural Circuitry of Wakefulness and Sleep. Neuron 2017, 93, 747–765. [Google Scholar] [CrossRef]
- Gais, S.; Mölle, M.; Helms, K.; Born, J. Learning-Dependent Increases in Sleep Spindle Density. J. Neurosci. 2002, 22, 6830–6834. [Google Scholar] [CrossRef]
- Altevogt, B.M.; Colten, H.R. Sleep physiology. In Sleep Disorders and Sleep Deprivation: Un Unmet Public Health Problem; Academic Press: New York, NY, USA, 2006. [Google Scholar]
- Peigneux, P.; Laureys, S.; Fuchs, S.; Collette, F.; Perrin, F.; Reggers, J.; Phillips, C.; Degueldre, C.; Del Fiore, G.; Aerts, J.; et al. Are Spatial Memories Strengthened in the Human Hippocampus during Slow Wave Sleep? Neuron 2004, 44, 535–545. [Google Scholar] [CrossRef]
- Vyazovskiy, V.V. Sleep, recovery, and metaregulation: Explaining the benefits of sleep. Nat. Sci. Sleep 2015, 7, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Tononi, G.; Cirelli, C. Sleep and the Price of Plasticity: From Synaptic and Cellular Homeostasis to Memory Consolidation and Integration. Neuron 2014, 81, 12–34. [Google Scholar] [CrossRef]
- de Vivo, L.; Bellesi, M.; Marshall, W.; Bushong, E.A.; Ellisman, M.H.; Tononi, G.; Cirelli, C. Ultrastructural evidence for synaptic scaling across the wake/sleep cycle. Science 2017, 355, 507–510. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Kang, H.; Xu, Q.; Chen, M.J.; Liao, Y.; Thiyagarajan, M.; O’Donnell, J.; Christensen, D.J.; Nicholson, C.; Iliff, J.J.; et al. Sleep Drives Metabolite Clearance from the Adult Brain. Science 2013, 342, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Hablitz, L.M.; Vinitsky, H.S.; Sun, Q.; Stæger, F.F.; Sigurdsson, B.; Mortensen, K.N.; Lilius, T.O.; Nedergaard, M. Increased glymphatic influx is correlated with high EEG delta power and low heart rate in mice under anesthesia. Sci. Adv. 2019, 5, eaav5447. [Google Scholar] [CrossRef]
- Nedergaard, M.; Goldman, S.A. Glymphatic failure as a final common pathway to dementia. Science 2020, 370, 50–56. [Google Scholar] [CrossRef]
- Alhola, P.; Polo-Kantola, P. Sleep deprivation: Impact on cognitive performance. Neuropsychiatr. Dis. Treat. 2007, 3, 553–567. [Google Scholar]
- Van Someren, E.J.W. Brain mechanisms of insomnia: New perspectives on causes and consequences. Physiol. Rev. 2021, 101, 995–1046. [Google Scholar] [CrossRef]
- Fisk, A.S.; Tam, S.K.E.; Brown, L.A.; Vyazovskiy, V.V.; Bannerman, D.M.; Peirson, S.N. Light and Cognition: Roles for Circadian Rhythms, Sleep, and Arousal. Front. Neurol. 2018, 9, 56. [Google Scholar] [CrossRef]
- Pilorz, V.; Tam, S.K.E.; Hughes, S.; Pothecary, C.A.; Jagannath, A.; Hankins, M.W.; Bannerman, D.M.; Lightman, S.L.; Vyazovskiy, V.V.; Nolan, P.M.; et al. Melanopsin Regulates Both Sleep-Promoting and Arousal-Promoting Responses to Light. PLoS Biol. 2016, 14, e1002482. [Google Scholar] [CrossRef]
- Saper, C.B.; Scammell, T.E.; Lu, J. Hypothalamic regulation of sleep and circadian rhythms. Nature 2005, 437, 1257–1263. [Google Scholar] [CrossRef]
- Chung, S.; Weber, F.; Zhong, P.; Tan, C.L.; Nguyen, T.N.; Beier, K.; Hörmann, N.; Chang, W.-C.; Zhang, Z.; Do, J.P.; et al. Identification of preoptic sleep neurons using retrograde labelling and gene profiling. Nature 2017, 545, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, K.S.; Sergeeva, O.A.; Haas, H.L.; Selbach, O. Orexins/hypocretins and aminergic systems. Acta Physiol. 2010, 198, 263–275. [Google Scholar] [CrossRef]
- Date, Y.; Ueta, Y.; Yamashita, H.; Yamaguchi, H.; Matsukura, S.; Kangawa, K.; Sakurai, T.; Yanagisawa, M.; Nakazato, M. Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc. Natl. Acad. Sci. USA 1999, 96, 748–753. [Google Scholar] [CrossRef]
- Peyron, C.; Tighe, D.K.; Pol, A.N.V.D.; de Lecea, L.; Heller, H.C.; Sutcliffe, J.G.; Kilduff, T. Neurons Containing Hypocretin (Orexin) Project to Multiple Neuronal Systems. J. Neurosci. 1998, 18, 9996–10015. [Google Scholar] [CrossRef]
- Mieda, M.; Williams, S.C.; Sinton, C.M.; Richardson, J.A.; Sakurai, T.; Yanagisawa, M. Orexin Neurons Function in an Efferent Pathway of a Food-Entrainable Circadian Oscillator in Eliciting Food-Anticipatory Activity and Wakefulness. J. Neurosci. 2004, 24, 10493–10501. [Google Scholar] [CrossRef] [PubMed]
- Porkka-Heiskanen, T.; Strecker, R.E.; Thakkar, M.; Bjørkum, A.A.; Greene, R.W.; McCarley, R.W. Adenosine: A Mediator of the Sleep-Inducing Effects of Prolonged Wakefulness. Science 1997, 276, 1265–1268. [Google Scholar] [CrossRef]
- Nawrot, P.; Jordan, S.; Eastwood, J.; Rotstein, J.; Hugenholtz, A.; Feeley, M. Effects of caffeine on human health. Food Addit. Contam. 2003, 20, 1–30. [Google Scholar] [CrossRef]
- Peng, W.; Wu, Z.; Song, K.; Zhang, S.; Li, Y.; Xu, M. Regulation of sleep homeostasis mediator adenosine by basal forebrain glutamatergic neurons. Science 2020, 369, 6508. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, Y.-H.; Chou, T.C.; Gaus, S.E.; Elmquist, J.K.; Shiromani, P.; Saper, C.B. Contrasting Effects of Ibotenate Lesions of the Paraventricular Nucleus and Subparaventricular Zone on Sleep–Wake Cycle and Temperature Regulation. J. Neurosci. 2001, 21, 4864–4874. [Google Scholar] [CrossRef]
- Muindi, F.; Zeitzer, J.M.; Heller, H.C. Retino-hypothalamic regulation of light-induced murine sleep. Front. Syst. Neurosci. 2014, 8, 135. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shiromani, P.J.; Xu, M.; Winston, E.M.; Shiromani, S.N.; Gerashchenko, D.; Weaver, D.R. Sleep rhythmicity and homeostasis in mice with targeted disruption of mPeriod genes. Am. J. Physiol. Integr. Comp. Physiol. 2004, 287, R47–R57. [Google Scholar] [CrossRef] [PubMed]
- Czeisler, C.A.; Gooley, J.J. Sleep and Circadian Rhythms in Humans. Cold Spring Harb. Symp. Quant. Biol. 2007, 72, 579–597. [Google Scholar] [CrossRef] [PubMed]
- Kyriacou, C.P.; Hastings, M.H. Circadian clocks: Genes, sleep, and cognition. Trends Cogn. Sci. 2010, 14, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Wright, K.P.; Hull, J.T.; Hughes, R.J.; Ronda, J.M.; Czeisler, C.A. Sleep and Wakefulness Out of Phase with Internal Biological Time Impairs Learning in Humans. J. Cogn. Neurosci. 2006, 18, 508–521. [Google Scholar] [CrossRef] [PubMed]
- Abel, T.; Lattal, K. Molecular mechanisms of memory acquisition, consolidation and retrieval. Curr. Opin. Neurobiol. 2001, 11, 180–187. [Google Scholar] [CrossRef]
- Shimizu, K.; Kobayashi, Y.; Nakatsuji, E.; Yamazaki, M.; Shimba, S.; Sakimura, K.; Fukada, Y. SCOP/PHLPP1beta mediates circadian regulation of long-term recognition memory. Nat. Commun. 2016, 7, 12926. [Google Scholar] [CrossRef]
- Roedel, A.; Storch, C.; Holsboer, F.; Ohl, F. Effects of light or dark phase testing on behavioural and cognitive performance in DBA mice. Lab. Anim. 2006, 40, 371–381. [Google Scholar] [CrossRef]
- Richetto, J.; Polesel, M.; Weber-Stadlbauer, U. Effects of light and dark phase testing on the investigation of behavioural paradigms in mice: Relevance for behavioural neuroscience. Pharmacol. Biochem. Behav. 2019, 178, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Moser, E.I.; Kropff, E.; Moser, M.B. Place cells, grid cells, and the brain’s spatial representation system. Annu. Rev. Neurosci. 2008, 31, 69–89. [Google Scholar] [CrossRef]
- Fanselow, M.S.; Dong, H.-W. Are the Dorsal and Ventral Hippocampus Functionally Distinct Structures? Neuron 2010, 65, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Jilg, A.; Lesny, S.; Peruzki, N.; Schwegler, H.; Selbach, O.; Dehghani, F.; Stehle, J.H. Temporal dynamics of mouse hippocampal clock gene expression support memory processing. Hippocampus 2009, 20, 377–388. [Google Scholar] [CrossRef]
- Van der Zee, E.A.; Havekes, R.; Barf, R.P.; Hut, R.A.; Nijholt, I.M.; Jacobs, E.H.; Gerkema, M.P. Circadian Time-Place Learning in Mice Depends on Cry Genes. Curr. Biol. 2008, 18, 844–848. [Google Scholar] [CrossRef] [PubMed]
- Kwapis, J.L.; Alaghband, Y.; Kramár, E.A.; López, A.J.; Ciernia, A.V.; White, A.O.; Shu, G.; Rhee, D.; Michael, C.M.; Montellier, E.; et al. Epigenetic regulation of the circadian gene Per1 contributes to age-related changes in hippocampal memory. Nat. Commun. 2018, 9, 1–14. [Google Scholar] [CrossRef]
- Toda, T.; Parylak, S.L.; Linker, S.B.; Gage, F.H. The role of adult hippocampal neurogenesis in brain health and disease. Mol. Psychiatry 2019, 24, 67–87. [Google Scholar] [CrossRef]
- Kempermann, G.; Song, H.; Gage, F.H. Neurogenesis in the Adult Hippocampus. Cold Spring Harb. Perspect Biol. 2015, 7, a018812. [Google Scholar] [CrossRef]
- Gonçalves, J.T.; Schafer, S.T.; Gage, F.H. Adult Neurogenesis in the Hippocampus: From Stem Cells to Behavior. Cell 2016, 167, 897–914. [Google Scholar] [CrossRef]
- Ali, A.A.; von Gall, C. Adult Neurogenesis under Control of the Circadian System. Cells 2022, 11, 764. [Google Scholar] [CrossRef]
- Lommen, J.; Detken, J.; Harr, K.; von Gall, C.; Ali, A. Analysis of Spatial and Temporal Distribution of Purinergic P2 Receptors in the Mouse Hippocampus. Int. J. Mol. Sci. 2021, 22, 8078. [Google Scholar] [CrossRef]
- Lommen, J.; Stahr, A.; Ingenwerth, M.; Ali, A.A.H.; Von Gall, C. Time-of-day-dependent expression of purinergic receptors in mouse suprachiasmatic nucleus. Cell Tissue Res. 2017, 369, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.A.H.; Schwarz-Herzke, B.; Rollenhagen, A.; Anstötz, M.; Holub, M.; Lübke, J.; Rose, C.R.; Schnittler, H.; Von Gall, C. Bmal1-deficiency affects glial synaptic coverage of the hippocampal mossy fiber synapse and the actin cytoskeleton in astrocytes. Glia 2019, 68, 947–962. [Google Scholar] [CrossRef]
- Fields, R.D.; Burnstock, G. Purinergic signalling in neuron–glia interactions. Nat. Rev. Neurosci. 2006, 7, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.G.; Hagan, J.J.; Rawlins, J.N. Allocentric spatial learning by hippocampectomised rats: A further test of the “spatial mapping” and “working memory” theories of hippocampal function. Q. J. Exp. Psychol. B 1986, 38, 365–395. [Google Scholar]
- Lonze, B.; Ginty, D.D. Function and Regulation of CREB Family Transcription Factors in the Nervous System. Neuron 2002, 35, 605–623. [Google Scholar] [CrossRef]
- Athos, J.; Impey, S.; Pineda, V.V.; Chen, X.; Storm, D.R. Hippocampal CRE-mediated gene expression is required for contextual memory formation. Nat. Neurosci. 2002, 5, 1119–1120. [Google Scholar] [CrossRef]
- Schafe, G.E.; Atkins, C.; Swank, M.W.; Bauer, E.P.; Sweatt, J.D.; LeDoux, J.E. Activation of ERK/MAP Kinase in the Amygdala Is Required for Memory Consolidation of Pavlovian Fear Conditioning. J. Neurosci. 2000, 20, 8177–8187. [Google Scholar] [CrossRef]
- Eckel-Mahan, K.; Phan, T.; Han, S.; Wang, H.; Chan, G.C.-K.; Scheiner, Z.S.; Storm, D.R. Circadian oscillation of hippocampal MAPK activity and cAMP: Implications for memory persistence. Nat. Neurosci. 2008, 11, 1074–1082. [Google Scholar] [CrossRef]
- Gerstner, J.R.; Yin, J.C.P. Circadian rhythms and memory formation. Nat. Rev. Neurosci. 2010, 11, 577–588. [Google Scholar] [CrossRef]
- Craig, L.A.; McDonald, R.J. Chronic disruption of circadian rhythms impairs hippocampal memory in the rat. Brain Res. Bull. 2008, 76, 141–151. [Google Scholar] [CrossRef]
- Gibson, E.M.; Wang, C.; Tjho, S.; Khattar, N.; Kriegsfeld, L.J. Experimental ‘Jet Lag’ Inhibits Adult Neurogenesis and Produces Long-Term Cognitive Deficits in Female Hamsters. PLoS ONE 2010, 5, e15267. [Google Scholar] [CrossRef]
- Li, Y.; Chen, X.; Dzakpasu, R.; Conant, K. Dopamine-dependent effects on basal and glutamate stimulated network dynamics in cultured hippocampal neurons. J. Neurochem. 2017, 140, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, J.D.; Huganir, R.L. The Cell Biology of Synaptic Plasticity: AMPA Receptor Trafficking. Annu. Rev. Cell Dev. Biol. 2007, 23, 613–643. [Google Scholar] [CrossRef] [PubMed]
- Korshunov, K.S.; Blakemore, L.J.; Trombley, P.Q. Dopamine: A Modulator of Circadian Rhythms in the Central Nervous System. Front. Cell. Neurosci. 2017, 11, 91. [Google Scholar] [CrossRef]
- Kim, J.; Jang, S.; Choe, H.K.; Chung, S.; Son, G.H.; Kim, A.K. Implications of Circadian Rhythm in Dopamine and Mood Regulation. Mol. Cells 2017, 40, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Radwan, B.; Liu, H.; Chaudhury, D. The role of dopamine in mood disorders and the associated changes in circadian rhythms and sleep-wake cycle. Brain Res. 2019, 1713, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Grippo, R.; Purohit, A.M.; Zhang, Q.; Zweifel, L.; Güler, A.D. Direct Midbrain Dopamine Input to the Suprachiasmatic Nucleus Accelerates Circadian Entrainment. Curr. Biol. 2017, 27, 2465–2475.e3. [Google Scholar] [CrossRef]
- Kalen, P.; Rosegren, E.; Lindvall, O.; Bjorklund, A. Hippocampal Noradrenaline and Serotonin Release over 24 Hours as Measured by the Dialysis Technique in Freely Moving Rats: Correlation to Behavioural Activity State, Effect of Handling and Tail-Pinch. Eur. J. Neurosci. 1989, 1, 181–188. [Google Scholar] [CrossRef]
- Maity, S.; Rah, S.; Sonenberg, N.; Gkogkas, C.G.; Nguyen, P.V. Norepinephrine triggers metaplasticity of LTP by increasing translation of specific mRNAs. Learn. Mem. 2015, 22, 499–508. [Google Scholar] [CrossRef]
- Normann, C.; Clark, K. Selective modulation of Ca2+ influx pathways by 5-HT regulates synaptic long-term plasticity in the hippocampus. Brain Res. 2005, 1037, 187–193. [Google Scholar] [CrossRef]
- Ovsepian, S.V. Differential cholinergic modulation of synaptic encoding and gain control mechanisms in rat hippocampus. Neurosci. Res. 2008, 61, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.P.; Pliássova, A.; Cunha, R.A. The physiological effects of caffeine on synaptic transmission and plasticity in the mouse hippocampus selectively depend on adenosine A1 and A2A receptors. Biochem. Pharmacol. 2019, 166, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, T.; Endo, Y.; Arita, J.; Kimura, F. Acetylcholine release in the rat hippocampus as measured by the microdialysis method correlates with motor activity and exhibits a diurnal variation. Neuroscience 1991, 44, 607–612. [Google Scholar] [CrossRef]
- Smith, J.W.; Evans, A.T.; Costall, B.; Smythe, J.W. Thyroid hormones, brain function and cognition: A brief review. Neurosci. Biobehav. Rev. 2002, 26, 45–60. [Google Scholar] [CrossRef]
- Shan, L.-L.; Guo, H.; Song, N.-N.; Jia, Z.-P.; Hu, X.-T.; Huang, J.-F.; Ding, Y.-Q.; Richter-Levin, G.; Zhou, Q.-X.; Xu, L. Light exposure before learning improves memory consolidation at night. Sci. Rep. 2015, 5, 15578. [Google Scholar] [CrossRef]
- Duda, M.; Domagalik, A.; Orlowska-Feuer, P.; Krzysztynska-Kuleta, O.; Beldzik, E.; Smyk, M.K.; Stachurska, A.; Oginska, H.; Jeczmien-Lazur, J.S.; Fafrowicz, M.; et al. Melanopsin: From a small molecule to brain functions. Neurosci. Biobehav. Rev. 2020, 113, 190–203. [Google Scholar] [CrossRef]
- Lo, L.; Anderson, D.J. A Cre-Dependent, Anterograde Transsynaptic Viral Tracer for Mapping Output Pathways of Genetically Marked Neurons. Neuron 2011, 72, 938–950. [Google Scholar] [CrossRef]
- Milosavljevic, N.; Cehajic-Kapetanovic, J.; Procyk, C.; Lucas, R. Chemogenetic Activation of Melanopsin Retinal Ganglion Cells Induces Signatures of Arousal and/or Anxiety in Mice. Curr. Biol. 2016, 26, 2358–2363. [Google Scholar] [CrossRef]
- Zhang, C.; Truong, K.K.; Zhou, Q.-Y. Efferent Projections of Prokineticin 2 Expressing Neurons in the Mouse Suprachiasmatic Nucleus. PLoS ONE 2009, 4, e7151. [Google Scholar] [CrossRef]
- Swanson, L.W.; Cowan, W.M. The connections of the septal region in the rat. J. Comp. Neurol. 1979, 186, 621–655. [Google Scholar] [CrossRef]
- Quirk, G.J.; Russo, G.K.; Barron, J.L.; Lebron, K. The Role of Ventromedial Prefrontal Cortex in the Recovery of Extinguished Fear. J. Neurosci. 2000, 20, 6225–6231. [Google Scholar] [CrossRef]
- Cataldi, S.; Stanley, A.T.; Miniaci, M.C.; Sulzer, D. Interpreting the role of the striatum during multiple phases of motor learning. FEBS J. 2021. [Google Scholar] [CrossRef]
- Oishi, Y.; Xu, Q.; Wang, L.; Zhang, B.J.; Takahashi, K.; Takata, Y.; Luo, Y.J.; Cherasse, Y.; Schiffmann, S.N.; de Kerchove d’Exaerde, A.; et al. Slow-wave sleep is controlled by a subset of nucleus accumbens core neurons in mice. Nat. Commun. 2017, 8, 734. [Google Scholar] [CrossRef]
- Penders, T.M.; Stanciu, C.N.; Schoemann, A.M.; Ninan, P.T.; Bloch, R.; Saeed, S.A. Bright Light Therapy as Augmentation of Pharmacotherapy for Treatment of Depression: A Systematic Review and Meta-Analysis. Prim Care Companion CNS Disord. 2016, 18, 26717. [Google Scholar] [CrossRef]
- Bedrosian, T.A.; Nelson, R.J. Influence of the modern light environment on mood. Mol. Psychiatry 2013, 18, 751–757. [Google Scholar] [CrossRef]
- An, K.; Zhao, H.; Miao, Y.; Xu, Q.; Li, Y.-F.; Ma, Y.-Q.; Shi, Y.-M.; Shen, J.-W.; Meng, J.-J.; Yao, Y.-G.; et al. A circadian rhythm-gated subcortical pathway for nighttime-light-induced depressive-like behaviors in mice. Nat. Neurosci. 2020, 23, 1–12. [Google Scholar] [CrossRef]
- Chen, B.; May, P.J. The feedback circuit connecting the superior colliculus and central mesencephalic reticular formation: A direct morphological demonstration. Exp. Brain Res. 2000, 131, 10–21. [Google Scholar] [CrossRef]
- Kragel, P.A.; Čeko, M.; Theriault, J.; Chen, D.; Satpute, A.B.; Wald, L.W.; Lindquist, M.A.; Barrett, L.F.; Wager, T.D. A human colliculus-pulvinar-amygdala pathway encodes negative emotion. Neuron 2021, 109, 2404–2412.e5. [Google Scholar] [CrossRef]
- Touitou, Y.; Reinberg, A.; Touitou, D. Association between light at night, melatonin secretion, sleep deprivation, and the internal clock: Health impacts and mechanisms of circadian disruption. Life Sci. 2017, 173, 94–106. [Google Scholar] [CrossRef]
- Reschke, L.; McCarthy, R.; Herzog, E.D.; Fay, J.C.; Jungheim, E.S.; England, S.K. Chronodisruption: An untimely cause of preterm birth? Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 52, 60–67. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.-X.; Korkmaz, A.; Erren, T.C.; Piekarski, C.; Tamura, H.; Manchester, L.C. Light at Night, Chronodisruption, Melatonin Suppression, and Cancer Risk: A Review. Crit. Rev. Oncog. 2007, 13, 303–328. [Google Scholar] [CrossRef]
- Menéndez-Velázquez, A.; Morales, D.; García-Delgado, A.B. Light Pollution and Circadian Misalignment: A Healthy, Blue-Free, White Light-Emitting Diode to Avoid Chronodisruption. Int. J. Environ. Res. Public Health 2022, 19, 1849. [Google Scholar] [CrossRef] [PubMed]
- Roth, T. Shift work disorder: Overview and diagnosis. J. Clin. Psychiatry 2012, 73, e09. [Google Scholar] [CrossRef] [PubMed]
- Lamont, E.W.; Legault-Coutu, D.; Cermakian, N.; Boivin, D.B. The role of circadian clock genes in mental disorders. Dialog-Clin. Neurosci. 2007, 9, 333–342. [Google Scholar]
- McClung, C.A. Circadian genes, rhythms and the biology of mood disorders. Pharmacol. Ther. 2007, 114, 222–232. [Google Scholar] [CrossRef]
- Musiek, E.S. Circadian Rhythms in AD Pathogenesis: A Critical Appraisal. Curr. Sleep Med. Rep. 2017, 3, 85–92. [Google Scholar] [CrossRef]
- Musiek, E.S.; Holtzman, D.M. Mechanisms linking circadian clocks, sleep, and neurodegeneration. Science 2016, 354, 1004–1008. [Google Scholar] [CrossRef]
- Vloeberghs, E.; Van Dam, D.; Engelborghs, S.; Nagels, G.; Staufenbiel, M.; De Deyn, P.P. Altered circadian locomotor activity in APP23 mice: A model for BPSD disturbances. Eur. J. Neurosci. 2004, 20, 2757–2766. [Google Scholar] [CrossRef]
- Pfeffer, M.; Plenzig, S.; Gispert, S.; Wada, K.; Korf, H.-W.; Von Gall, C. Disturbed sleep/wake rhythms and neuronal cell loss in lateral hypothalamus and retina of mice with a spontaneous deletion in the ubiquitin carboxyl-terminal hydrolase L1 gene. Neurobiol. Aging 2012, 33, 393–403. [Google Scholar] [CrossRef]
- Pfeffer, M.; Zimmermann, Z.; Gispert, S.; Auburger, G.; Korf, H.-W.; Von Gall, C. Impaired Photic Entrainment of Spontaneous Locomotor Activity in Mice Overexpressing Human Mutant α-Synuclein. Int. J. Mol. Sci. 2018, 19, 1651. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
von Gall, C. The Effects of Light and the Circadian System on Rhythmic Brain Function. Int. J. Mol. Sci. 2022, 23, 2778. https://doi.org/10.3390/ijms23052778
von Gall C. The Effects of Light and the Circadian System on Rhythmic Brain Function. International Journal of Molecular Sciences. 2022; 23(5):2778. https://doi.org/10.3390/ijms23052778
Chicago/Turabian Stylevon Gall, Charlotte. 2022. "The Effects of Light and the Circadian System on Rhythmic Brain Function" International Journal of Molecular Sciences 23, no. 5: 2778. https://doi.org/10.3390/ijms23052778
APA Stylevon Gall, C. (2022). The Effects of Light and the Circadian System on Rhythmic Brain Function. International Journal of Molecular Sciences, 23(5), 2778. https://doi.org/10.3390/ijms23052778





