Abstract
Circadian rhythms are essential for the survival of all organisms, enabling them to predict daily changes in the environment and time their behaviour appropriately. The molecular basis of such rhythms is the circadian clock, a self-sustaining molecular oscillator comprising a transcriptional–translational feedback loop. This must be continually readjusted to remain in alignment with the external world through a process termed entrainment, in which the phase of the master circadian clock in the suprachiasmatic nuclei (SCN) is adjusted in response to external time cues. In mammals, the primary time cue, or “zeitgeber”, is light, which inputs directly to the SCN where it is integrated with additional non-photic zeitgebers. The molecular mechanisms underlying photic entrainment are complex, comprising a number of regulatory factors. This review will outline the photoreception pathways mediating photic entrainment, and our current understanding of the molecular pathways that drive it in the SCN.
1. Introduction
The lives of most organisms on Earth, from bacteria to humans, are governed by the daily environmental changes that occur across the day/night cycle. In response, life has evolved an internal timing system, the circadian clock, to anticipate these changes and fine-tune physiology and behaviour to the varied demands of day and night.
The core circadian clock consists of an autoregulatory transcriptional-translational feedback loop (TTFL) whereby the transcription factors CLOCK and BMAL1 heterodimerise to drive expression of Per and Cry genes via E-box response elements [1]. PER and CRY proteins, within a protein complex, then feedback to suppress their transcription by inhibiting CLOCK:BMAL1 activity (Figure 1). The degradation of PER and CRY reverses this inhibition and the TTFL restarts. The result is a near 24 hour (h) oscillation in PER/CRY production and breakdown. It is now known that this core TTFL is at the centre of a complex network of additional feedback loops, which interact to regulate the precision and stability of the circadian clock (reviewed in [2]).
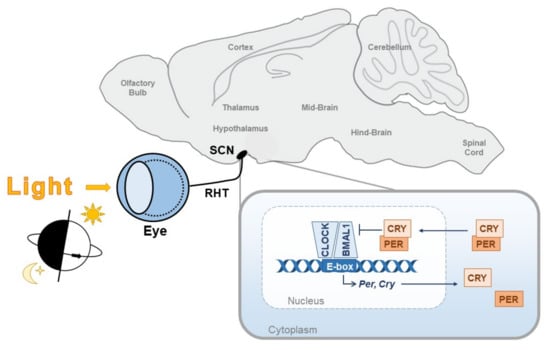
Figure 1.
The circadian clock. The core circadian clock consists of a molecular transcriptional-translational feedback loop in which the transcription factors, CLOCK and BMAL1, heterodimerise and induce expression of the core clock genes, Per and Cry, via E-box response elements. PER and CRY then feedback onto CLOCK and BMAL1 by inhibiting their transcriptional activity. This feedback loop cycles with a period of around 24 h, therefore it must be continually readjusted to be aligned with the external world. The primary time cue for this is the daily light/dark cycle. Light information is transmitted via the retinohypothalamic tract (RHT) directly to the master clock in the suprachiasmatic nucleus (SCN).
In humans and mice, the endogenous circadian clock cycles with a period slightly greater or less than 24 h, respectively. As a result, the clock must be continually readjusted to remain in alignment with the external world. This is achieved through a process termed entrainment using external time cues called “zeitgebers”. In mammals, entrainment begins by adjusting the phase of the master clock in the suprachiasmatic nuclei (SCN). The SCN in turn entrains the peripheral clocks throughout the body into a global alignment of the circadian system. In mammals, including humans, the primary zeitgeber is the changing light environment at dawn and dusk, which is transmitted directly to the SCN [3,4] (Figure 1). Within the SCN, light information is integrated with signals from a range of other non-photic zeitgebers including food, temperature and sleep, to align the biological and environmental day [5].
Such an alignment allows organisms to deliver the correct materials, in the correct concentration to the correct organ systems at the optimal time of day. This “fine-tuning” of biology is essential for survival. Without entrainment of the circadian system all “fine-tuning” is lost and physiology and behaviour drifts into chaos, termed “internal desynchrony”. Despite the critical importance of entrainment, the mechanisms that drive these pathways remain only partially understood. This review will outline our current understanding of the molecular mechanisms underlying photic entrainment, and discuss recent advances in how light signals are integrated with other non-photic zeitgebers.
2. Phase Shifting of the Clock
Light is a powerful zeitgeber which can shift the phase of the circadian clock. However, the effects of light vary depending on the time of light exposure. Light detected during the twilight hours of dawn and dusk has the greatest impact, whilst light delivered during daytime has very little effect [6]. A key point is that the effects of light at dawn vs. dusk have opposite effects. Light exposure during the late night (dawn) will result in a phase advance of activity onset, therefore the animal will start its activity earlier the following day. Whereas light administered during the early night (dusk) will cause a phase delay in activity [7,8]. Such differential effects of light are represented as the “phase response curve” (PRC), an example of which is illustrated in Figure 2. Similar differential responses (PRCs) are seen across all organisms, including in both nocturnal and diurnal mammals. Although the size of the delays and advances vary between species, in all PRCs delays result from dusk light exposure, whilst advances occur after dawn light exposure. Overall entrainment is achieved by the summation of phase delays and phase advances.
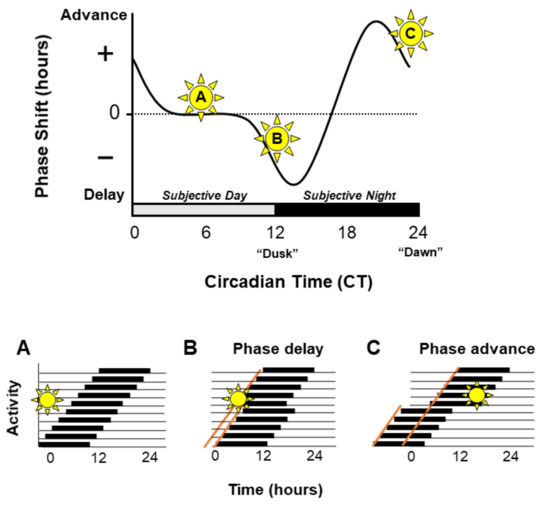
Figure 2.
The phase response curve. The phase response curve demonstrates the effect of light exposure at different times of the circadian cycle on the phase of the circadian clock. Light delivered during the subjective day, the ‘dead-zone’ will have no effect on the phase of the clock (A). Light exposure during the early subjective night will lead to delays in the phase of the clock (B). Whereas light exposure at the end of the subjective night will lead to phase advances (C). This is demonstrated by representative actograms showing free running rest/activity rhythms (panels A–C); black bars represent periods of activity and black lines indicate rest.
3. Photoreception for Entrainment
In mammals, light input to the SCN is provided exclusively by the retina via the retinohypothalamic tract (RHT). Photoentrainment is abolished in blinded (enucleated) mammals [9,10,11], and in contrast to other vertebrates, mammals appear to lack extra-retinal photoreceptors [12]. However, the primary photoreceptors responsible for photoentrainment are not the rods and cones but a ‘third’ class of photoreceptor based upon a small number of photosensitive retinal ganglion cells (pRGCs) that utilise the photopigment melanopsin (OPN4) [13]. This was demonstrated by studies showing that circadian responses to light are intact in mice lacking rod and cone photoreceptors [14,15], but are impaired following ablation of the melanopsin gene, Opn4 [16,17]. Nonetheless, it is only in the absence of all three photopigments (rod, cone and melanopsin) that circadian entrainment is completely abolished [18,19], demonstrating that rods and cones can contribute to photoentrainment in the absence of melanopsin.
For example, transgenic mouse studies have demonstrated that rod photoreceptors can partially mediate entrainment under scotopic (very dim) light levels [20,21]. This finding is consistent with the observation that rods drive electrical responses in light-sensitive SCN neurons under low light conditions [22]. In addition, studies have also shown that UV light detected by UV-sensitive cone photoreceptors (S-cones) will induce electrical responses in the SCN, along with phase-shifts in circadian activity rhythms [23,24]. Significantly, this input is sufficient for entrainment in the absence of melanopsin and rod photoreceptor signalling [25]. Finally, photoentrainment will occur following stimulation of the green-light sensitive M-cones. However, patterns of entrainment are not identical following OPN4, S-cone and M-cone stimulation, suggesting that different photoreceptor classes are “tuned” to the different features of the twilight transition. Indeed, recent evidence suggests that input from cones signals information on the spectral composition (colour) of light to the SCN, through activation of colour-sensitive neurons which can drive circadian phase and modulate entrainment [26,27]. This is thought to contribute to the detection of twilight transitions, during which there is a shift in the spectral environment to shorter wavelengths [28,29], which may act to support circadian entrainment under unreliable light intensity conditions. It is worth stressing that the melanopsin-expressing pRGCs are required to relay the rod and cone input to the SCN. If the pRGCs are specifically ablated in mice with intact rod and cone photoreceptors, photoentrainment is lost [30,31]. As a result, under normal circumstances, photoentrainment is achieved as a result of an integrated light input from all three photoreceptor classes, each encoding different aspects of the light environment at twilight.
4. Photosensitive Retinal Ganglion Cells (pRGCs)
The pRGCs represent a diverse population of cells consisting of five known subtypes (M1-M5) and a recently discovered sixth (M6), classified based on their anatomical and morphological differences [32,33]. They also exhibit distinct electrophysiological properties and mediate different light responses in addition to photoentrainment, including pupil constriction, sleep induction, masking, alertness and mood [12]. This appears to be achieved through diverse axonal projections to the SCN and multiple other brain targets. However, currently, the functional roles of each subtype remain poorly defined. A further complexity within these subpopulations has been demonstrated recently by the identification of distinct gene expression profiles by single cell transcriptomics [34]. Despite this diversity, retrograde tracing has identified the pRGC subtypes that mediate entrainment, with the majority of projections to the SCN attributed to M1 cells and a small proportion to M2 cells [35]. These subtypes vary in their expression of the two isoforms of melanopsin; M1 cells express both the short (OPN4S) and long (OPN4L) isoform, whereas M2 cells express only OPN4L [36,37]. In line with this, both isoforms have been shown to be required for photoentrainment [38]. Notably, it is a specific subset of just 200 M1 pRGCs that innervate the SCN, which are molecularly distinct to those projecting to other brain regions as they lack Brn3b expression [39]. Furthermore, gene expression patterns suggest that there are molecularly diverse subpopulations within this subset of M1 cells [34]. Further studies are required to determine whether these “molecular subpopulations” are sufficiently stable to constitute robust subdivisions of M1 pRGCs.
5. Molecular Photoentrainment of the Suprachiasmatic Nuclei (SCN)
Retinal innervation of the SCN from the pRGCs is via the monosynaptic pathway of the RHT, the primary neurotransmitters of which are glutamate and pituitary adenylate cyclase-activating polypeptide (PACAP) [40]. Light-induced glutamate and PACAP release results in a rise in Ca2+ and cAMP levels, which in turn initiate a suite of kinase-based signalling cascades involving protein kinase A (PKA), protein kinase C (PKC), protein kinase G (PKG), mitogen-activated protein kinase (MAPK) and Ca2+/calmodulin-dependent protein kinase II (CaMKII) [41]. The primary mechanism for entrainment is considered to be the activation of the transcription factor cAMP response element-binding protein (CREB), through phosphorylation at Ser133 and Ser142 [42,43], which then modulates the transcription of the clock genes Per1/Per2 (Figure 3). The light-induced upregulation of Per1/2 adjusts the TTFL which shifts the phase of the clock into alignment with the external light/dark cycle. The phase at which light-induced Per1/2 upregulation occurs will determine the direction of the phase shift. As outlined above, dawn light exposure will lead to a phase advance in the clock, whereas dusk light exposure will lead to a phase delay [7,44]. Whilst the molecular pathways underlying both delays and advances are broadly similar and rely on cAMP-CREB transcription, there are key differences. Per1/Per2 expression within the SCN is rhythmic, overall both genes are elevated during the day and low at night. Light at dawn acutely increases Per1 transcription thus advancing the onset of the rhythm in Per1, whilst light at dusk increases Per2 stability, thus delaying the offset of the Per2 rhythm [45]. Furthermore, Ca2+ release by ryanodine receptors only correlates with phase delays [46], whilst the increase in cyclin GMP (cGMP) and activation of cGMP-dependent kinase (PKG) characterises phase advances [47,48]. Accordingly, sildenafil, which inhibits cGMP-specific phosphodiesterase 5, enhances photic phase advances in hamsters [49].
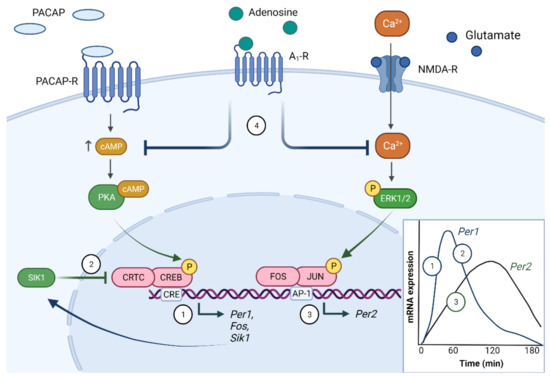
Figure 3.
Molecular photoentrainment of the SCN. The light-induced release of glutamate and pituitary adenylate cyclase-activating polypeptide (PACAP) from the RHT nerve terminals leads to a rise in intracellular Ca2+ and cAMP levels in the SCN. These trigger a cascade of events including activation of protein kinase A (PKA), which activates the transcription factor cAMP response element-binding protein (CREB), together with co-activators such as CREB-regulated transcription coactivator 1 (CRTC1). This leads to the upregulation of CRE-driven genes, including the core clock component, Per1 (1). In addition, Sik1 is upregulated, which feedbacks on the CREB pathway by phosphorylation of CRTC1. This deactivates CRTC1 leading to a decline in CREB-induced gene transcription and therefore a decline in Per1 expression (2). In parallel, the activation of ERK1/2 by Ca2+ influx leads to the upregulation of the immediate-early transcription factors JUN and FOS. These heterodimerise to form AP-1, which drives Per2 transcription leading to an increase in Per2 expression (3). Adenosine, which accumulates in the extracellular space during wakefulness, modulates these light-activated signalling pathways. Adenosine predominantly signals through the Gi (inhibitory) coupled A1 receptor in the SCN, which results in a decrease in intracellular cAMP and Ca2+ levels, and therefore a downregulation of the subsequent signalling events (4). Figure created with BioRender.com.
The light-regulated transcriptome comprises hundreds of genes, including immediate early genes (IEGs) and CREB target genes [50,51]. Undoubtedly, the most important of these are Per1 and Per2, but other elements of this transcriptome also play an important role. IEGs are part of the transcriptional network controlling Per1/2 transcription. Per1 is rapidly upregulated within 30 min of light exposure, whereas Per2 induction is slower with a timeframe of several hours [44]. Recent evidence suggests this is due to the induction of Per1 by CREB, and the induction of Per2 by a parallel pathway involving the activation of ERK1/2 by Ca2+ influx, leading to upregulation of the immediate-early transcription factors JUN and FOS [52]. These then heterodimerise to form AP-1, which drives Per2 transcription and other genes containing AP-1 response elements (Figure 3). In addition, other light-regulated IEGs have also been shown to regulate photic input; Npas4 is upregulated by light within the SCN and regulates the expression of multiple genes including Per1. Npas4−/− animals show unstable photic entrainment and reduced phase-shifting in response to light [53]. In addition, the light-regulated transcriptome also includes multiple kinases and phosphatases, such as Sik1 and Dusp4 [54], which are part of the cascade regulating light responses within the SCN, and thus photic entrainment. It is clear that whilst the role of a notable fraction of the light-regulated transcriptome can be linked to photoentrainment, the functional role of much of this light-regulated transcriptome remains unknown.
6. Gating the Light Sensitivity of the Clock
The molecular light responses of the SCN are regulated throughout the day/night cycle such that the SCN responds to resetting signals in a time-dependent manner. Central to this is the gating of the light-induced phosphorylation of CREB, which is limited to nocturnal hours and, therefore, light exposure during the subjective day has little or no effect upon Per1/2 expression [42,55]. The signalling pathways mediating this are currently poorly understood, however vasoactive intestinal peptide (VIP) is thought to be necessary for photic gating [56], and recent evidence has demonstrated that it acts via DUSP4, a negative regulator of ERK1/2 [54]. VIP-expressing neurons receive direct innervation from the retina, and VIP signalling is necessary for maintaining the synchronisation of circadian oscillations across the SCN network [57,58]. Interestingly, another negative regulator of ERK1/2, the Ras-like G-protein DEXRAS1, has also been shown to be necessary for photic gating. Transgenic mouse studies found that loss of DEXRAS1 altered the phase-dependent light sensitivity of the clock, with daytime light pulses inducing phase shifts in activity [59,60]. Therefore, inhibitors of ERK1/2 appear to be important for blocking light-induced responses of the SCN during the day. Overall, this gating of the SCN response to light acts to limit the impact of transient fluctuations in the light environment on the clock, which may otherwise result in desynchronization with the light/dark cycle.
The effect of light on the circadian system is not equal at all times, it varies with both time of day (see discussion on the PRC above) and physiological state. The shape of the PRC varies with the temporal niche occupied by the organism, nocturnal animals typically display large delays at dusk, but smaller advances at dawn, whereas the reverse is true for diurnal animals [6]. Furthermore, sleep history impacts photic responses; sleep deprivation reduces both light-induced electrical activity within the SCN [61] and the size of resulting phase shifts in mice [62,63] and humans [64], although exceptions exist; sleep deprivation potentiates photic phase shifts in the diurnal rodent, Arvicanthus ansorgie [65]. The molecular mechanisms underlying these observations can in part be attributed to adenosine signalling. Adenosine, as a by-product of ATP metabolism, accumulates in the extracellular space in a manner correlating with wake time [66,67]. It then activates signalling from adenosine receptors, which are predominantly the A1 (Gi-coupled) and A2A (Gs-coupled) receptors in the SCN. Such G protein-coupled receptor (GPCR) signalling converges on the same pathways that are activated by light, specifically cAMP-CREB and Ca2+-ERK1/2-AP-1, thus resulting in Per1/2 upregulation. As the A1 receptor is the dominant form within the SCN, elevated adenosine, as occurs following sleep deprivation, inhibits the effect of light on the circadian system (Figure 3), whilst adenosine A1/A2A antagonists enhance photic responses [52]. Caffeine, which has A1/A2A antagonism properties, both enhances photic shifts in humans and counters the effects of sleep deprivation on the clock in mice [52,63,68,69]. This pathway provides a molecular framework by which the major drives that control sleep/wake transitions interact, namely the circadian (Process C) and homeostatic (Process S) drives [70,71], thus allowing sleep history to shape photic entrainment processes [52].
7. Buffering Photoentrainment
Another key feature of photoentrainment is that re-entrainment to a shifted light/dark cycle is slow, taking several days. It is typically limited to one hour per day in most mammals [72]. This mechanism acts to limit the size of phase shifts to “buffer” the circadian system from extreme shifts which may result in internal desynchronization. This buffering is thought to be due to the constrained induction of Per1 by light. After Per1 mRNA is induced by light it peaks at 1 h before returning to baseline after 3 h [73]. This suggests that CREB-mediated transcription is curtailed shortly after its induction by light. This inhibition has been shown to be driven by the CRTC1-SIK1 pathway, whereby light induces transcription of CREB-regulated transcription coactivator 1 (CRTC1), which then co-activates CREB inducing expression of Per1 and Sik1 [50]. SIK1 then phosphorylates CRTC1 leading to its deactivation and, therefore, the decline in Per1 transcription (Figure 3). The net result is that SIK1 acts as a “brake” on the induction of Per1. This has been demonstrated at a behavioural level through RNAi knockdown of Sik1 in the SCN of mice. Following a 6 h advance of the light/dark cycle there was a rapid re-entrainment in Sik1 knockdown mice compared to controls [50]. This rapid re-entrainment is also observed in transgenic mice expressing a catalytically inactive version of SIK1 [74].
In addition, the activation of Per1 expression by CLOCK:BMAL1 binding at the E-box response element is also subject to modulation to limit light-induced Per1 expression. Transgenic mouse studies suggest that the transcriptional repressor, ID2, interacts with CLOCK:BMAL1 to limit Per1 induction and circadian responses to light; in the absence of Id2, mice show rapid re-entrainment to phase delays which is accompanied by elevated light-induced Per1 expression [75,76]. ID2 is thought to act by sequestering CLOCK and BMAL1 to the cytoplasm [77]. Interestingly, recent evidence suggests that another member of the same family, ID4, is also involved in photic entrainment but with opposing effects to ID2 [78].
The regulation of PER stability is another target for buffering the effects of light on the clock. For example, there is evidence that another member of the SIK family, SIK3, may participate in this process. In vitro experiments have shown that phosphorylation of PER2 by SIK3 regulates the abundance of PER2 by promoting its degradation [79]. Transgenic mouse studies suggest that this is important at the behavioural level, with Sik3−/− mice exhibiting significant phase delays and lengthened circadian periods. This suggests that the two SIK isoforms regulate circadian entrainment via distinct substrates and signalling pathways. However, different SIK mouse models yield conflicting results; gain of function mutants of Sik1 [80] or Sik3 [81] do not show deficits in circadian behaviour, however gain of function kinase models cannot fully capture a kinase’s function. Such models cannot describe how each kinase is endogenously regulated, and the context in which they are activated, as they are “always on”.
Moreover, the SIK family of kinases is also now known to regulate sleep through a pathway involving phosphorylation of synaptic proteins [82]. Mice with a gain of function mutation in Sik3 have a constitutively elevated sleep need, associated with hyperphosphorylation of proteins at the synapse [81,82]. Such protein phosphorylation is also seen following sleep deprivation [83], suggesting that the action of SIK on synaptic proteins provides a molecular substrate for sleep. The regulation of SIK1 by light appears also to regulate a similar set of substrates, thus leading to the induction sleep in mice [74]. Thus, it appears that the SIK family of kinases play an important role in transducing both environmental and physiological signals to both the sleep and circadian systems in parallel in order to adjust sleep/wake timing.
PER stability is also modulated by the key clock component casein kinase 1 (CK1), which phosphorylates PER1, PER2 and PER3 to target these proteins for degradation [84,85]. This action is thought to underpin the role of CK1 as a regulator of the speed of the TTFL [86,87]. Furthermore, lack of CK1ε leads to faster re-entrainment following both phase advances and delays of the light/dark cycle [88]. This suggests that CK1ε limits the light-induced accumulation of PER in the SCN, which in turn limits the size of behavioural phase shifts.
In addition to the cell autonomous molecular clockwork, light can directly affect circuit level properties of the SCN. Much of the robustness of SCN rhythms is attributed to the tight coupling between the cells of the SCN through the neurotransmitters arginine vasopressin (AVP), VIP and GABA. These are expressed by discrete cell types within the SCN, with AVP restricted largely to the shell region and VIP to the core, where input from the RHT is localised. Light input activates VIP neurons, which in turn activate other regions including the AVP neurons and dorsomedial hypothalamus, thus regulating circadian rhythms of rest and activity [54,89]. The ablation of vasopressin V1a and V1b receptors or their blockade resulted in reduced synchrony amongst the SCN cells and more rapid entrainment to a shifted light/dark cycle [90]. Whilst AVP/VIP-mediated coupling confers great stability on SCN rhythms, it is also believed to confer rigidity, or inertia to resetting. The temporary loss of this coupling would allow the individual neurons to unlock their phase coupling and, therefore, achieve greater shifts, as illustrated by the studies above.
8. Concluding Remarks
It is clear that the molecular basis of photic entrainment in the SCN is complex. Whilst the classical cAMP-CREB-PER pathway plays a central role, recent advances have identified additional key signalling pathways and regulators that act in parallel. Collectively, these pathways regulate the phase-shifting effects of light on the clock, thus making circadian entrainment a gradual and carefully controlled process. Significantly, major progress has been made in our understanding of the interaction between sleep and circadian entrainment, which has revealed molecular cross-talk between these two processes and further strengthened their close bidirectional relationship.
Author Contributions
Conceptualization, A.A., A.J. and R.G.F.; writing—original draft preparation, A.A. and A.J.; writing—review and editing, A.A., A.J. and R.G.F. Figures, A.A. and R.G.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by BB/N01992X/1 David Phillips fellowship from the BBSRC to AJ and WT106174/Z/14/ZMA from the Wellcome Trust to RGF. The APC charges were paid by Circadian Therapeutics Ltd.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Figure 3 was created with BioRender.com.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Reppert, S.M.; Weaver, D.R. Coordination of circadian timing in mammals. Nature 2002, 418, 935–941. [Google Scholar] [CrossRef]
- Cox, K.H.; Takahashi, J.S. Circadian clock genes and the transcriptional architecture of the clock mechanism. J. Mol. Endocrinol. 2019, 63, R93–R102. [Google Scholar] [CrossRef] [PubMed]
- Roenneberg, T.; Kumar, C.J.; Merrow, M. The human circadian clock entrains to sun time. Curr. Biol. 2007, 17, R44. [Google Scholar] [CrossRef] [PubMed]
- Foster, R.G. Shedding light on the biological clock. Neuron 1998, 20, 829–832. [Google Scholar] [CrossRef][Green Version]
- Challet, E.; Pevet, P. Interactions between photic and nonphotic stimuli to synchronize the master circadian clock in mammals. Front. Biosci. 2003, 8, 246–257. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Daan, S.; Pittendrigh, C.S. A Functional analysis of circadian pacemakers in nocturnal rodents—II. The variability of phase response curves. J. Comp. Physiol. 1976, 106, 253–266. [Google Scholar] [CrossRef]
- De Coursey, P.J. Daily light sensitivity rhythm in a rodent. Science 1960, 131, 33–35. [Google Scholar] [CrossRef]
- Khalsa, S.B.S.; Jewett, M.E.; Cajochen, C.; Czeisler, C.A. A phase response curve to single bright light pulses in human subjects. J. Physiol. 2003, 549, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Foster, R.G.; Provencio, I.; Hudson, D.; Fiske, S.; De Grip, W.; Menaker, M. Circadian photoreception in the retinally degenerate mouse (rd/rd). J. Comp. Physiol. A 1991, 169, 39–50. [Google Scholar] [CrossRef]
- Nelson, R.J.; Zucker, I. Absence of extraocular photoreception in diurnal and nocturnal rodents exposed to direct sunlight. Comp. Biochem. Physiol. 1981, 69, 145–148. [Google Scholar] [CrossRef]
- Czeisler, C.A.; Shanahan, T.L.; Klerman, E.B.; Martens, H.; Brotman, D.J.; Emens, J.S.; Klein, T.; Rizzo, J.F. Suppression of Melatonin Secretion in Some Blind Patients by Exposure to Bright Light. N. Engl. J. Med. 1995, 332, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Foster, R.G.; Hughes, S.; Peirson, S.N. Circadian photoentrainment in mice and humans. Biology 2020, 9, 180. [Google Scholar] [CrossRef]
- Hattar, S.; Liao, H.W.; Takao, M.; Berson, D.M.; Yau, K.W. Melanopsin-containing retinal ganglion cells: Architecture, projections, and intrinsic photosensitivity. Science 2002, 295, 1065–1070. [Google Scholar] [CrossRef]
- Freedman, M.S.; Lucas, R.J.; Soni, B.; Von Schantz, M.; Muñoz, M.; David-Gray, Z.; Foster, R. Regulation of mammalian circadian behavior by non-rod, non-cone, ocular photoreceptors. Science 1999, 284, 502–504. [Google Scholar] [CrossRef] [PubMed]
- Lucas, R.J.; Freedman, M.S.; Muñoz, M.; Garcia-Fernández, J.M.; Foster, R.G. Regulation of the mammalian pineal by non-rod, non-cone, ocular photoreceptors. Science 1999, 284, 505–507. [Google Scholar] [CrossRef]
- Panda, S.; Sato, T.K.; Castrucci, A.M.; Rollag, M.D.; DeGrip, W.J.; Hogenesch, J.B.; Provencio, I.; Kay, S.A. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science 2002, 298, 2213–2216. [Google Scholar] [CrossRef] [PubMed]
- Ruby, N.F.; Brennan, T.J.; Xie, X.; Cao, V.; Franken, P.; Heller, H.C.; O’Hara, B.F. Role of melanopsin in circadian responses to light. Science 2002, 298, 2211–2213. [Google Scholar] [CrossRef]
- Panda, S.; Provencio, I.; Tu, D.C.; Pires, S.S.; Rollag, M.D.; Maria Castrucci, A.; Pletcher, M.T.; Sato, T.K.; Wiltshire, T.; Andahazy, M.; et al. Melanopsin Is Required for Non-Image-Forming Photic Responses in Blind Mice. Curr. Opin. Neurobiol. 1997, 78, 1. [Google Scholar] [CrossRef]
- Hattar, S.; Lucas, R.J.; Mrosovsky, N.; Thompson, S.; Douglas, R.H.; Hankins, M.W.; Lem, J.; Biel, M.; Hofmann, F.; Foster, R.G.; et al. Melanopsin and rod—cone photoreceptive systems account for all major accessory visual functions in mice. Nature 2003, 424, 76–81. [Google Scholar] [CrossRef]
- Lall, G.S.; Revell, V.L.; Momiji, H.; Al Enezi, J.; Altimus, C.M.; Güler, A.D.; Aguilar, C.; Cameron, M.A.; Allender, S.; Hankins, M.W.; et al. Distinct contributions of rod, cone, and melanopsin photoreceptors to encoding irradiance. Neuron 2010, 66, 417–428. [Google Scholar] [CrossRef]
- Altimus, C.M.; Güler, A.D.; Alam, N.M.; Arman, A.C.; Prusky, G.T.; Sampath, A.P.; Hattar, S. Rod photoreceptors drive circadian photoentrainment across a wide range of light intensities. Nat. Neurosci. 2010, 13, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Aggelopoulos, N.C.; Meissl, H. Responses of neurones of the rat suprachiasmatic nucleus to retinal illumination under photopic and scotopic conditions. J. Physiol. 2000, 523, 211–222. [Google Scholar] [CrossRef]
- Van Oosterhout, F.; Fisher, S.P.; Van Diepen, H.C.; Watson, T.S.; Houben, T.; Vanderleest, H.T.; Thompson, S.; Peirson, S.N.; Foster, R.G.; Meijer, J.H. Ultraviolet light provides a major input to non-image-forming light detection in mice. Curr. Biol. 2012, 22, 1397–1402. [Google Scholar] [CrossRef]
- Van Diepen, H.C.; Ramkisoensing, A.; Peirson, S.N.; Foster, R.G.; Meijer, J.H. Irradiance encoding in the suprachiasmatic nuclei by rod and cone photoreceptors. FASEB J. 2013, 27, 4204–4212. [Google Scholar] [CrossRef] [PubMed]
- Van Diepen, H.C.; Schoonderwoerd, R.A.; Ramkisoensing, A.; Janse, J.A.M.; Hattar, S.; Meijer, J.H. Distinct contribution of cone photoreceptor subtypes to the mammalian biological clock. Proc. Natl. Acad. Sci. USA 2021, 118, e2024500118. [Google Scholar] [CrossRef]
- Mouland, J.W.; Martial, F.; Watson, A.; Lucas, R.J.; Brown, T.M. Cones Support Alignment to an Inconsistent World by Suppressing Mouse Circadian Responses to the Blue Colors Associated with Twilight. Curr. Biol. 2019, 29, 4260–4267.e4. [Google Scholar] [CrossRef]
- Walmsley, L.; Hanna, L.; Mouland, J.; Martial, F.; West, A.; Smedley, A.R.; Bechtold, D.A.; Webb, A.R.; Lucas, R.J.; Brown, T.M. Colour As a Signal for Entraining the Mammalian Circadian Clock. PLoS Biol. 2015, 13, e1002127. [Google Scholar] [CrossRef]
- Spitschan, M.; Aguirre, G.K.; Brainard, D.H.; Sweeney, A.M. Variation of outdoor illumination as a function of solar elevation and light pollution. Sci. Rep. 2016, 6, 26756. [Google Scholar] [CrossRef]
- Foster, R.G.; Helfrich-Förster, C. The regulation of circadian clocks by light in fruitflies and mice. Phil. Trans. R. Soc. Lond. B 2001, 356, 1779–1789. [Google Scholar] [CrossRef] [PubMed]
- Hatori, M.; Le, H.; Vollmers, C.; Keding, S.R.; Tanaka, N.; Schmedt, C.; Jegla, T.; Panda, S. Inducible ablation of melanopsin-expressing retinal ganglion cells reveals their central role in non-image forming visual responses. PLoS ONE 2008, 3, e2451. [Google Scholar] [CrossRef]
- Güler, A.D.; Ecker, J.L.; Lall, G.S.; Haq, S.; Altimus, C.M.; Liao, H.W.; Barnard, A.R.; Cahill, H.; Badea, T.C.; Zhao, H.; et al. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature 2008, 453, 102–105. [Google Scholar] [CrossRef]
- Schmidt, T.M.; Chen, S.K.; Hattar, S. Intrinsically photosensitive retinal ganglion cells: Many subtypes, diverse functions. Trends Neurosci. 2011, 34, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Quattrochi, L.E.; Stabio, M.E.; Kim, I.; Ilardi, M.C.; Michelle Fogerson, P.; Leyrer, M.L.; Berson, D.M. The M6 cell: A small-field bistratified photosensitive retinal ganglion cell. J. Comp. Neurol. 2019, 527, 297–311. [Google Scholar] [CrossRef]
- Berg, D.J.; Kartheiser, K.; Leyrer, M.; Saali, A.; Berson, D.M. Transcriptomic signatures of postnatal and adult intrinsically photosensitive ganglion cells. eNeuro 2019, 6, 1–30. [Google Scholar] [CrossRef]
- Baver, S.B.; Pickard, G.E.; Sollars, P.J.; Pickard, G.E. Two types of melanopsin retinal ganglion cell differentially innervate the hypothalamic suprachiasmatic nucleus and the olivary pretectal nucleus. Eur. J. Neurosci. 2008, 27, 1763–1770. [Google Scholar] [CrossRef]
- Pires, S.S.; Hughes, S.; Turton, M.; Melyan, Z.; Peirson, S.N.; Zheng, L.; Kosmaoglou, M.; Bellingham, J.; Cheetham, M.E.; Lucas, R.J.; et al. Differential expression of two distinct functional isoforms of melanopsin (Opn4) in the mammalian retina. J. Neurosci. 2009, 29, 12332–12342. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.; Welsh, L.; Katti, C.; González-Menéndez, I.; Turton, M.; Halford, S.; Sekaran, S.; Peirson, S.N.; Hankins, M.W.; Foster, R.G. Differential Expression of Melanopsin Isoforms Opn4L and Opn4S during Postnatal Development of the Mouse Retina. PLoS ONE 2012, 7, 34531. [Google Scholar] [CrossRef]
- Jagannath, A.; Hughes, S.; Abdelgany, A.; Pothecary, C.A.; Di Pretoro, S.; Pires, S.S.; Vachtsevanos, A.; Pilorz, V.; Brown, L.A.; Hossbach, M.; et al. Isoforms of Melanopsin Mediate Different Behavioral Responses to Light. Curr. Biol. 2015, 25, 2430–2434. [Google Scholar] [CrossRef]
- Chen, S.K.; Badea, T.C.; Hattar, S. Photoentrainment and pupillary light reflex are mediated by distinct populations of ipRGCs. Nature 2011, 476, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Hannibal, J.; Møller, M.; Ottersen, O.P.; Fahrenkrug, J. PACAP and glutamate are co-stored in the retinohypothalamic tract. J. Comp. Neurol. 2000, 418, 147–155. [Google Scholar] [CrossRef]
- Meijer, J.H.; Schwartz, W.J. In search of the pathways for light-induced pacemaker resetting in the suprachiasmatic nucleus. J. Biol. Rhythm. 2003, 18, 235–249. [Google Scholar] [CrossRef]
- Ginty, D.D.; Kornhauser, J.M.; Thompson, M.A.; Bading, H.; Mayo, K.E.; Takahashi, J.S.; Greenberg, M.E. Regulation of CREB phosphorylation in the suprachiasmatic nucleus by light and a circadian clock. Science 1993, 260, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Gau, D.; Lemberger, T.; Von Gall, C.; Kretz, O.; Le Minh, N.; Gass, P.; Schmid, W.; Schibler, U.; Korf, H.W.; Schütz, G. Phosphorylation of CREB Ser142 regulates light-induced phase shifts of the circadian clock. Neuron 2002, 34, 245–253. [Google Scholar] [CrossRef]
- Shearman, L.P.; Zylka, M.J.; Weaver, D.R.; Kolakowski, L.F.; Reppert, S.M. Two period homologs: Circadian expression and photic regulation in the suprachiasmatic nuclei. Neuron 1997, 19, 1261–1269. [Google Scholar] [CrossRef]
- Schwartz, W.J.; Tavakoli-Nezhad, M.; Lambert, C.M.; Weaver, D.R.; De La Iglesia, H.O. Distinct patterns of Period gene expression in the suprachiasmatic nucleus underlie circadian clock photoentrainment by advances or delays. Proc. Natl. Acad. Sci. USA 2011, 108, 17219–17224. [Google Scholar] [CrossRef]
- Ding, J.M.; Buchanan, G.F.; Tischkau, S.A.; Chen, D.; Kuriashkina, L.; Faiman, L.E.; Alster, J.M.; McPherson, P.S.; Campbell, K.P.; Gillette, M.U. A neuronal ryanodine receptor mediates light-induced phase delays of the circadian clock. Nature 1998, 394, 381–384. [Google Scholar] [CrossRef]
- Mathur, A.; Golombek, D.A.; Ralph, M.R. cGMP-dependent protein kinase inhibitors block light-induced phase advances of circadian rhythms in vivo. Am. J. Physiol. 1996, 270, R1031–R1036. [Google Scholar] [CrossRef]
- Weber, E.T.; Gannon, R.L.; Rea, M.A. cGMP-dependent protein kinase inhibitor blocks light-induced phase advances of circadian rhythms in vivo. Neurosci. Lett. 1995, 197, 227–230. [Google Scholar] [CrossRef]
- Agostino, P.V.; Plano, S.A.; Golombek, D.A. Sildenafil accelerates reentrainment of circadian rhythms after advancing light schedules. Proc. Natl. Acad. Sci. USA 2007, 104, 9834–9839. [Google Scholar] [CrossRef]
- Jagannath, A.; Butler, R.; Godinho, S.I.H.; Couch, Y.; Brown, L.A.; Vasudevan, S.R.; Flanagan, K.C.; Anthony, D.; Churchill, G.C.; Wood, M.J.A.; et al. The CRTC1-SIK1 pathway regulates entrainment of the circadian clock. Cell 2013, 154, 1100–1111. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.E.; Viswanathan, N.; Kuhlman, S.; Davis, F.C.; Weitz, C.J. A screen for genes induced in the suprachiasmatic nucleus by light. Science 1998, 279, 1544–1547. [Google Scholar] [CrossRef] [PubMed]
- Jagannath, A.; Varga, N.; Dallmann, R.; Rando, G.; Gosselin, P.; Ebrahimjee, F.; Taylor, L.; Mosneagu, D.; Stefaniak, J.; Walsh, S.; et al. Adenosine integrates light and sleep signalling for the regulation of circadian timing in mice. Nat. Commun. 2021, 12, 2113. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Berto, S.; Kulkarni, A.; Jeong, B.; Joseph, C.; Cox, K.H.; Greenberg, M.E.; Kim, T.-K.; Konopka, G.; Takahashi, J.S. NPAS4 regulates the transcriptional response of the suprachiasmatic nucleus to light and circadian behavior. Neuron 2021, 109, 3268–3282.e6. [Google Scholar] [CrossRef]
- Hamnett, R.; Crosby, P.; Chesham, J.E.; Hastings, M.H. Vasoactive intestinal peptide controls the suprachiasmatic circadian clock network via ERK1/2 and DUSP4 signalling. Nat. Commun. 2019, 10, 542. [Google Scholar] [CrossRef]
- Obrietan, K.; Impey, S.; Smith, D.; Athos, J.; Storm, D.R. Circadian regulation of cAMP response element-mediated gene expression in the suprachiasmatic nuclei. J. Biol. Chem. 1999, 274, 17748–17756. [Google Scholar] [CrossRef]
- Hughes, A.T.; Fahey, B.; Cutler, D.J.; Coogan, A.N.; Piggins, H.D. Aberrant Gating of Photic Input to the Suprachiasmatic Circadian Pacemaker of Mice Lacking the VPAC2 Receptor. J. Neurosci. 2004, 24, 3522–3526. [Google Scholar] [CrossRef]
- Aton, S.J.; Colwell, C.S.; Harmar, A.J.; Waschek, J.; Herzog, E.D. Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nat. Neurosci. 2005, 8, 476–483. [Google Scholar] [CrossRef]
- Maywood, E.S.; Reddy, A.B.; Wong, G.K.Y.; O’Neill, J.S.; O’Brien, J.A.; McMahon, D.G.; Harmar, A.J.; Okamura, H.; Hastings, M.H. Synchronization and maintenance of timekeeping in suprachiasmatic circadian clock cells by neuropeptidergic signaling. Curr. Biol. 2006, 16, 599–605. [Google Scholar] [CrossRef]
- Cheng, H.Y.M.; Dziema, H.; Papp, J.; Mathur, D.P.; Koletar, M.; Ralph, M.R.; Penninger, J.M.; Obrietan, K. The molecular gatekeeper Dexras1 sculpts the photic responsiveness of the mammalian circadian clock. J. Neurosci. 2006, 26, 12984–12995. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.Y.M.; Obrietan, K.; Cain, S.W.; Lee, B.Y.; Agostino, P.V.; Joza, N.A.; Harrington, M.E.; Ralph, M.R.; Penninger, J.M. Dexras1 potentiates photic and suppresses nonphotic responses of the circadian clock. Neuron 2004, 43, 715–728. [Google Scholar] [CrossRef] [PubMed]
- Deboer, T.; Détári, L.; Meijer, J.H. Long term effects of sleep deprivation on the mammalian circadian pacemaker. Sleep 2007, 30, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Mistlberger, R.E.; Landry, G.J.; Marchant, E.G. Sleep deprivation can attenuate light-induced phase shifts of circadian rhythms in hamsters. Neurosci. Lett. 1997, 238, 5–8. [Google Scholar] [CrossRef]
- Van Diepen, H.C.; Lucassen, E.A.; Yasenkov, R.; Groenen, I.; Ijzerman, A.P.; Meijer, J.H.; Deboer, T. Caffeine increases light responsiveness of the mouse circadian pacemaker. Eur. J. Neurosci. 2014, 40, 3504–3511. [Google Scholar] [CrossRef]
- Burgess, H.J. Partial sleep deprivation reduces phase advances to light in humans. J. Biol. Rhythm. 2010, 25, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Jha, P.K.; Bouâouda, H.; Gourmelen, S.; Dumont, S.; Fuchs, F.; Goumon, Y.; Bourgin, P.; Kalsbeek, A.; Challet, E. Sleep deprivation and caffeine treatment potentiate photic resetting of the master circadian clock in a diurnal rodent. J. Neurosci. 2017, 37, 4343–4358. [Google Scholar] [CrossRef]
- Greene, R.W.; Bjorness, T.E.; Suzuki, A. The adenosine-mediated, neuronal-glial, homeostatic sleep response. Curr. Opin. Neurobiol. 2017, 44, 236–242. [Google Scholar] [CrossRef]
- Porkka-Heiskanen, T.; Strecker, R.E.; Thakkar, M.; Bjorkum, A.A.; Greene, R.W.; McCarley, R.W. Adenosine: A mediator of the sleep-inducing effects of prolonged wakefulness. Science 1997, 276, 1265–1268. [Google Scholar] [CrossRef] [PubMed]
- Oike, H.; Kobori, M.; Suzuki, T.; Ishida, N. Caffeine lengthens circadian rhythms in mice. Biochem. Biophys. Res. Commun. 2011, 410, 654–658. [Google Scholar] [CrossRef]
- Burke, T.M.; Markwald, R.R.; McHill, A.W.; Chinoy, E.D.; Snider, J.A.; Bessman, S.C.; Jung, C.M.; O’Neill, J.S.; Wright, K.P. Effects of caffeine on the human circadian clock in vivo and in vitro. Sci. Transl. Med. 2015, 7, 305ra146. [Google Scholar] [CrossRef] [PubMed]
- Borbély, A.A.; Daan, S.; Wirz-Justice, A.; Deboer, T. The two-process model of sleep regulation: A reappraisal. J. Sleep Res. 2016, 25, 131–143. [Google Scholar] [CrossRef]
- Borbély, A. A two process model of sleep regulation. Hum. Neurobiol. 1982, 1, 195–204. [Google Scholar]
- Aschoff, J. Biological Rhythms; Plenum Press: New York, NY, USA, 1981. [Google Scholar]
- Shigeyoshi, Y.; Taguchi, K.; Yamamoto, S.; Takekida, S.; Yan, L.; Tei, H.; Moriya, T.; Shibata, S.; Loros, J.J.; Dunlap, J.C.; et al. Light-induced resetting of a mammalian circadian clock is associated with rapid induction of the mPer1 transcript. Cell 1997, 91, 1043–1053. [Google Scholar] [CrossRef]
- Taylor, L.; Palumaa, T.; Reardon, P.K.; Walsh, S.; Johnson, B.H.; Liberatori, S.; Hasan, S.; Clark, K.; Cohen, P.; Vasudevan, S.; et al. Light regulated SIK1 remodels the synaptic phosphoproteome to induce sleep. bioRxiv 2021. [Google Scholar] [CrossRef]
- Duffield, G.E.; Watson, N.P.; Mantani, A.; Peirson, S.N.; Robles-Murguia, M.; Loros, J.J.; Israel, M.A.; Dunlap, J.C. A Role for Id2 in Regulating Photic Entrainment of the Mammalian Circadian System. Curr. Biol. 2009, 19, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Duffield, G.E.; Han, S.; Hou, T.Y.; de la Iglesia, H.O.; McDonald, K.A.; Mecklenburg, K.L.; Robles-Murguia, M. Inhibitor of DNA binding 2 (Id2) Regulates Photic Entrainment Responses in Mice: Differential Responses of the Id2-/- Mouse Circadian System Are Dependent on Circadian Phase and on Duration and Intensity of Light. J. Biol. Rhythm. 2020, 35, 555–575. [Google Scholar] [CrossRef]
- Ward, S.M.; Fernando, S.J.; Hou, T.Y.; Duffield, G.E. The transcriptional repressor ID2 can interact with the canonical clock components CLOCK and BMAL1 and mediate inhibitory effects on mPer1 expression. J. Biol. Chem. 2010, 285, 38987–39000. [Google Scholar] [CrossRef]
- Duffield, G.E.; Robles-Murguia, M.; Hou, T.Y.; McDonald, K.A. Targeted Disruption of the Inhibitor of DNA Binding 4 (Id4) Gene Alters Photic Entrainment of the Circadian Clock. Int. J. Mol. Sci. 2021, 22, 9632. [Google Scholar] [CrossRef]
- Hayasaka, N.; Hirano, A.; Miyoshi, Y.; Tokuda, I.T.; Yoshitane, H.; Matsuda, J.; Fukada, Y. Salt-inducible kinase 3 regulates the mammalian circadian clock by destabilizing per2 protein. Elife 2017, 6, e24779. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Miyoshi, C.; Fujiyama, T.; Kakizaki, M.; Ikkyu, A.; Honda, T.; Choi, J.; Asano, F.; Mizuno, S.; Takahashi, S.; et al. Loss of the conserved PKA sites of SIK1 and SIK2 increases sleep need. Sci. Rep. 2020, 10, 8676. [Google Scholar] [CrossRef] [PubMed]
- Funato, H.; Miyoshi, C.; Fujiyama, T.; Kanda, T.; Sato, M.; Wang, Z.; Ma, J.; Nakane, S.; Tomita, J.; Ikkyu, A.; et al. Forward-genetics analysis of sleep in randomly mutagenized mice. Nature 2016, 539, 378–383. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, J.; Miyoshi, C.; Li, Y.; Sato, M.; Ogawa, Y.; Lou, T.; Ma, C.; Gao, X.; Lee, C.; et al. Quantitative phosphoproteomic analysis of the molecular substrates of sleep need. Nature 2018, 558, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Brüning, F.; Noya, S.B.; Bange, T.; Koutsouli, S.; Rudolph, J.D.; Tyagarajan, S.K.; Cox, J.; Mann, M.; Brown, S.A.; Robles, M.S. Sleep-wake cycles drive daily dynamics of synaptic phosphorylation. Science 2019, 366, eaav3617. [Google Scholar] [CrossRef]
- Akashi, M.; Tsuchiya, Y.; Yoshino, T.; Nishida, E. Control of Intracellular Dynamics of Mammalian Period Proteins by Casein Kinase I ε (CKIε) and CKI in Cultured Cells. Mol. Cell. Biol. 2002, 22, 1693–1703. [Google Scholar] [CrossRef]
- Eide, E.J.; Woolf, M.F.; Kang, H.; Woolf, P.; Hurst, W.; Camacho, F.; Vielhaber, E.L.; Giovanni, A.; Virshup, D.M. Control of Mammalian Circadian Rhythm by CKIε-Regulated Proteasome-Mediated PER2 Degradation. Mol. Cell. Biol. 2005, 25, 2795–2807. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.J.; Logunova, L.; Maywood, E.S.; Gallego, M.; Lebiecki, J.; Brown, T.M.; Sládek, M.; Semikhodskii, A.S.; Glossop, N.R.J.; Piggins, H.D.; et al. Setting Clock Speed in Mammals: The CK1ε tau Mutation in Mice Accelerates Circadian Pacemakers by Selectively Destabilizing PERIOD Proteins. Neuron 2008, 58, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Etchegaray, J.-P.; Machida, K.K.; Noton, E.; Constance, C.M.; Dallmann, R.; Di Napoli, M.N.; DeBruyne, J.P.; Lambert, C.M.; Yu, E.A.; Reppert, S.M.; et al. Casein Kinase 1 Delta Regulates the Pace of the Mammalian Circadian Clock. Mol. Cell. Biol. 2009, 29, 3853–3866. [Google Scholar] [CrossRef] [PubMed]
- Pilorz, V.; Cunningham, P.S.; Jackson, A.; West, A.C.; Wager, T.T.; Loudon, A.S.I.; Bechtold, D.A. A novel mechanism controlling resetting speed of the circadian clock to environmental stimuli. Curr. Biol. 2014, 24, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Todd, W.D.; Venner, A.; Anaclet, C.; Broadhurst, R.Y.; De Luca, R.; Bandaru, S.S.; Issokson, L.; Hablitz, L.M.; Cravetchi, O.; Arrigoni, E.; et al. Suprachiasmatic VIP neurons are required for normal circadian rhythmicity and comprised of molecularly distinct subpopulations. Nat. Commun. 2020, 11, 4410. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Suzuki, T.; Mizoro, Y.; Kori, H.; Okada, K.; Chen, Y.; Fustin, J.M.; Yamazaki, F.; Mizuguchi, N.; Zhang, J.; et al. Mice genetically deficient in vasopressin V1a and V1b receptors are resistant to jet lag. Science 2013, 342, 85–90. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).