Molecular Cross-Talk between Gravity- and Light-Sensing Mechanisms in Euglena gracilis
Abstract
1. Introduction
2. Results
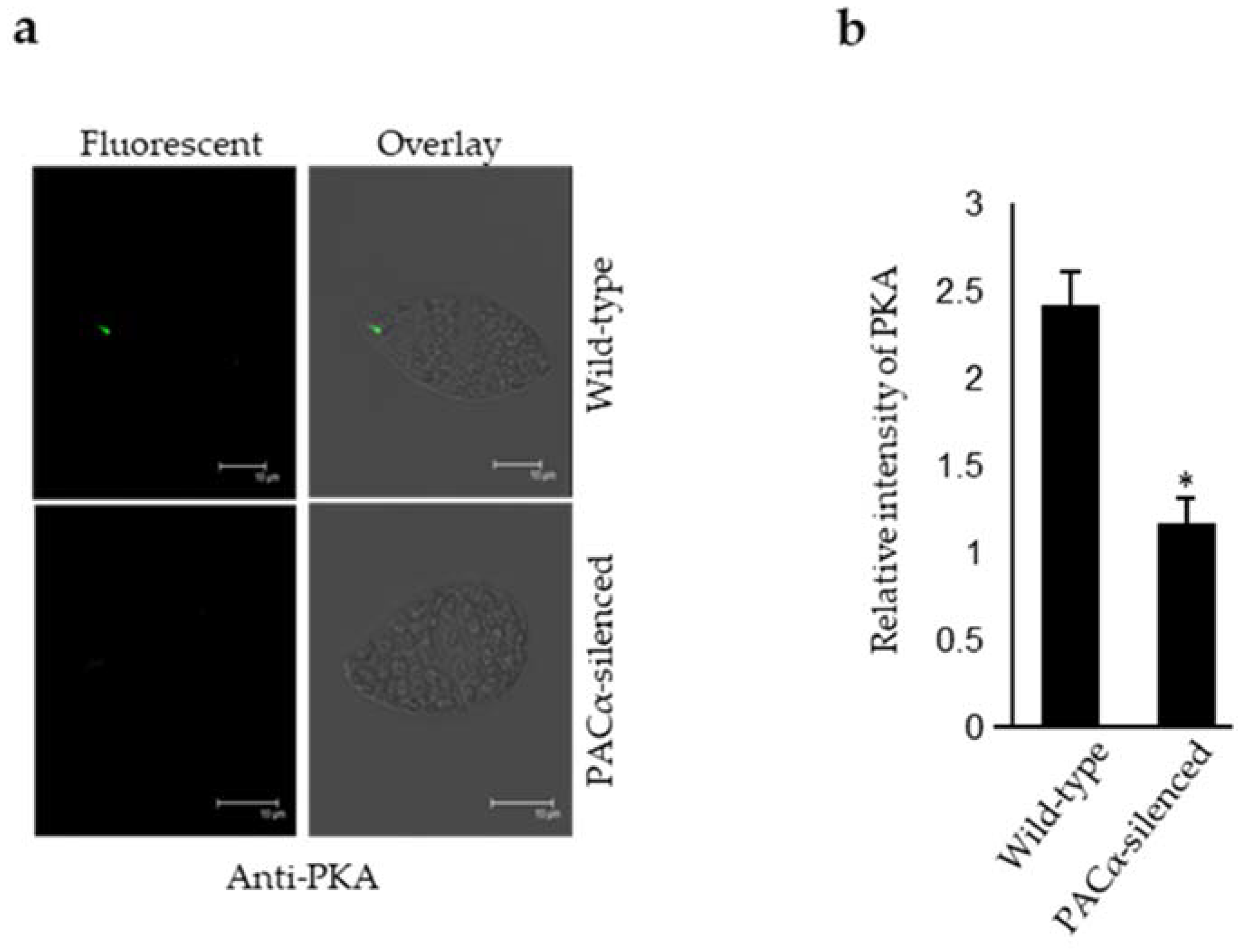
2.1. PKA Resides in the Anterior Region of E. gracilis
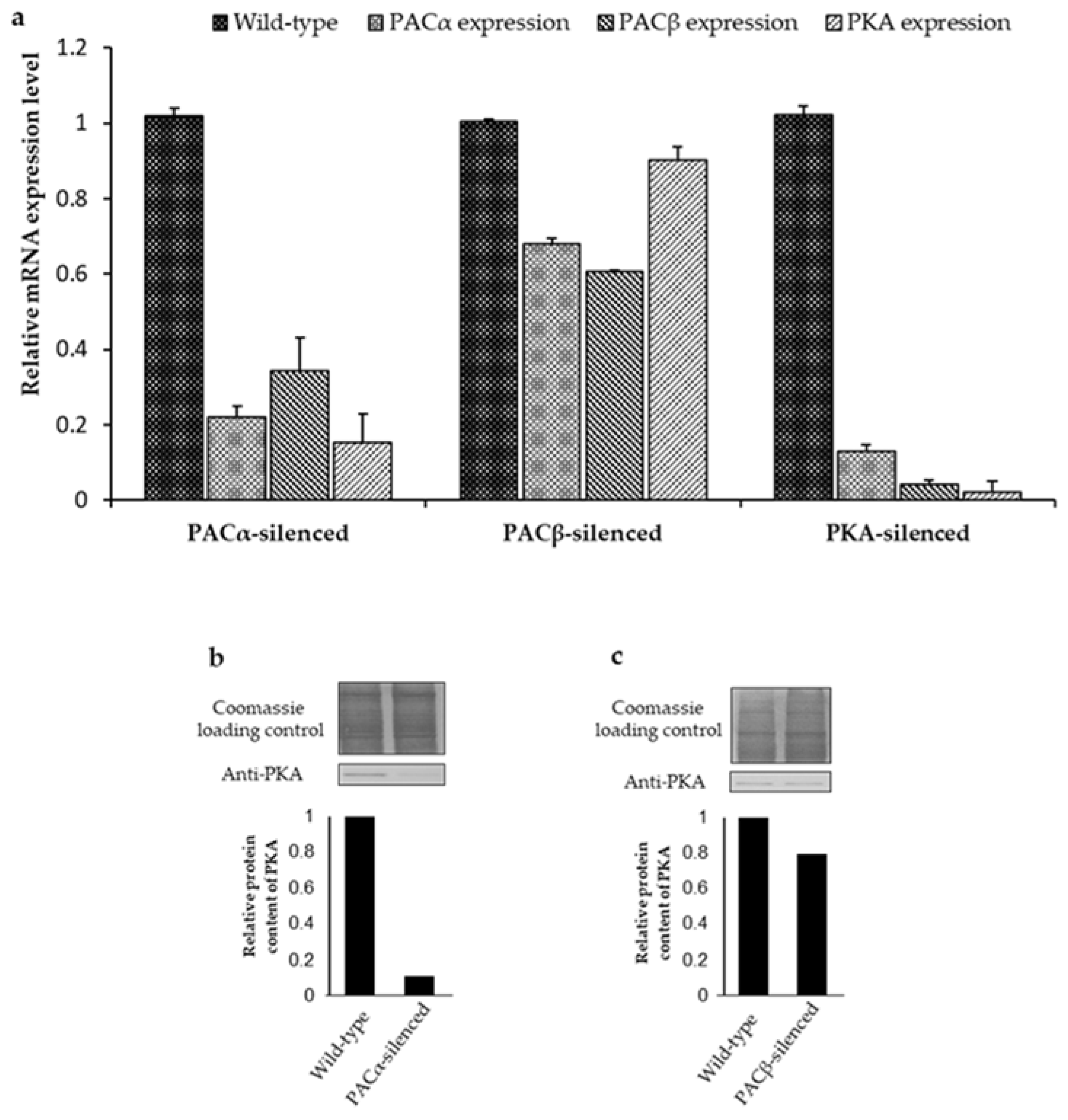
2.2. Interdependency of PKA and PAC Expression Levels
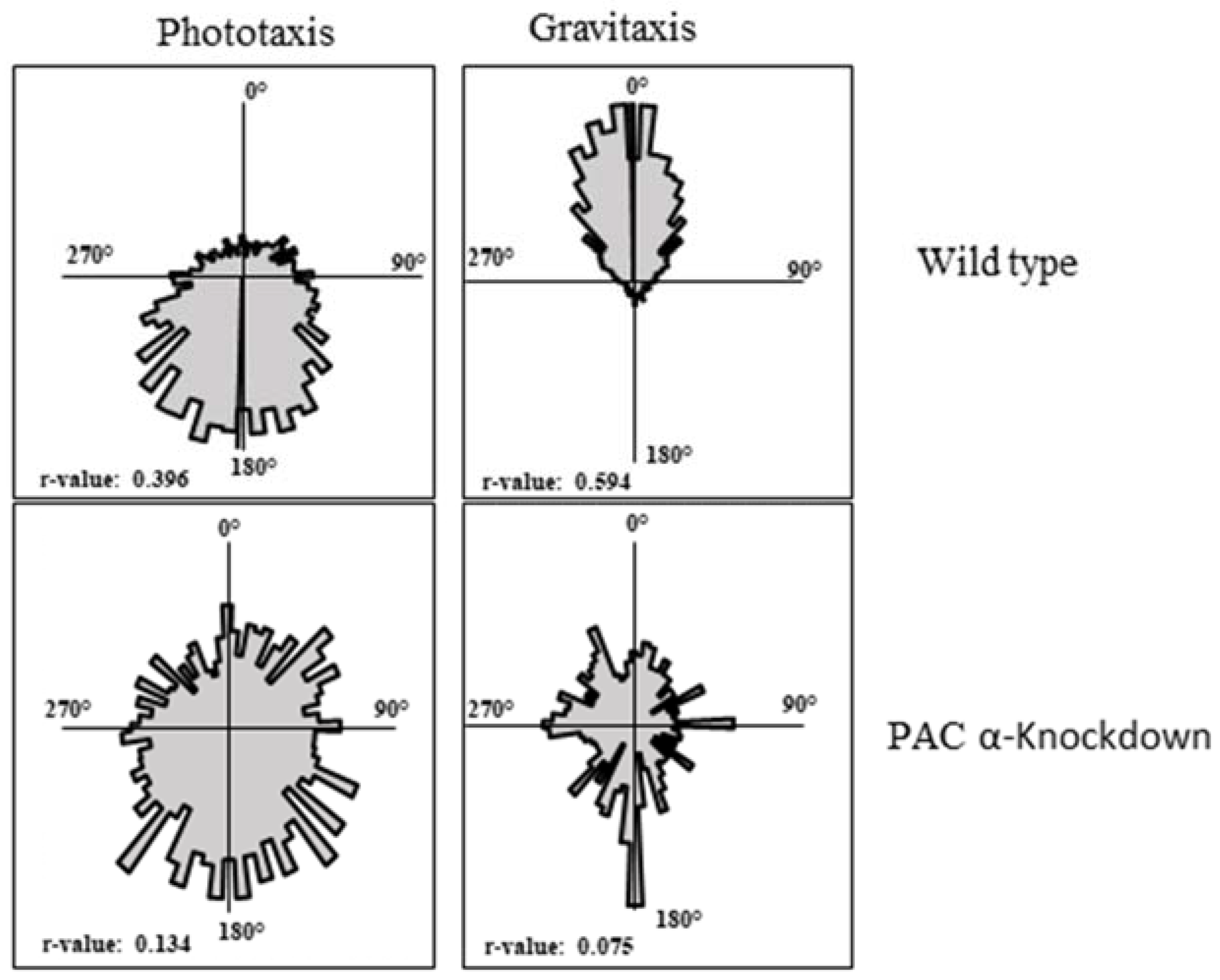
2.3. PACα and PACβ Lead to an Impairment of Gravitaxis
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Growth Conditions
4.2. Motion Analysis
4.3. dsRNA-Mediated Gene Silencing and Expression Analysis
4.4. Biochemistry
4.4.1. Antibodies
4.4.2. Protein Sample Preparation from E. gracilis
4.4.3. SDS PAGE and Immunoblotting
4.4.4. Organelle Fractionation
4.4.5. Indirect-Immunofluorescence Confocal Microscopy
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vandenbrink, J.P.; Kiss, J.Z.; Herranz, R.; Medina, F.J. Light and gravity signals synergize in modulating plant development. Front. Plant Sci. 2014, 5, 563. [Google Scholar] [CrossRef] [PubMed]
- Bostwick, M.; Smith, E.L.; Borba, C.; Newman-Smith, E.; Guleria, I.; Kourakis, M.J.; Smith, W.C. Antagonistic Inhibitory Circuits Integrate Visual and Gravitactic Behaviors. Curr. Biol. 2020, 30, 600–609.e2. [Google Scholar] [CrossRef] [PubMed]
- Lopez, C.; Bachofner, C.; Mercier, M.; Blanke, O. Gravity and observer’s body orientation influence the visual perception of human body postures. J. Vis. 2009, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Häder, D.-P.; Hemmersbach, R. Gravitaxis in Flagellates and Ciliates. In Gravitational Biology I.; Braun, M., Böhmer, M., Häder, D.-P., Hemmersbach, R., Palme, K., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 27–45. ISBN 978-3-319-93893-6. [Google Scholar]
- Kwon, S.-H.; Bae, J.-E.; Lee, S.-H.; Lee, S.-D.; Chae, K.-S. Effects of gravity on positive phototaxis in fruit fly Drosophila melanogaster. Entomol. Res. 2016, 46, 272–277. [Google Scholar] [CrossRef]
- Jékely, G. Evolution of phototaxis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 2795–2808. [Google Scholar] [CrossRef] [PubMed]
- Haga, K.; Iino, M. Asymmetric distribution of auxin correlates with gravitropism and phototropism but not with autostraightening (autotropism) in pea epicotyls. J. Exp. Bot. 2006, 57, 837–847. [Google Scholar] [CrossRef]
- Häder, D.-P.; Lebert, M. Photoorientation in photosynthetic flagellates. Methods Mol. Biol. 2009, 571, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Ebenezer, T.E.; Zoltner, M.; Burrell, A.; Nenarokova, A.; Novák Vanclová, A.M.G.; Prasad, B.; Soukal, P.; Santana-Molina, C.; O’Neill, E.; Nankissoor, N.N.; et al. Transcriptome, proteome and draft genome of Euglena gracilis. BMC Biol. 2019, 17, 11. [Google Scholar] [CrossRef]
- Ebenezer, T.E.; Carrington, M.; Lebert, M.; Kelly, S.; Field, M.C. Euglena gracilis Genome and Transcriptome: Organelles, Nuclear Genome Assembly Strategies and Initial Features. Adv. Exp. Med. Biol. 2017, 979, 125–140. [Google Scholar] [CrossRef]
- Gibbs, S.P. The chloroplasts of Euglena may have evolved from symbiotic green algae. Can. J. Bot. 1978, 56, 2883–2889. [Google Scholar] [CrossRef]
- Porterfield, D.M. Orientation of Motile Unicellular Algae to Oxygen: Oxytaxis in Euglena. Biol. Bull. 1997, 193, 229–230. [Google Scholar] [CrossRef] [PubMed]
- Häder, D.-P.; Richter, P.; Lebert, M. Signal transduction in gravisensing of flagellates. Signal Transduct. 2006, 6, 422–431. [Google Scholar] [CrossRef]
- Wolken, J.J.; Shin, E. Photomotion in Euglena gracilis* I. Photokinesis II. Phototaxis. J. Protozool. 1958, 5, 39–46. [Google Scholar] [CrossRef]
- Häder, D.-P.; Lebert, M. The Photoreceptor for Phototaxis in the Photosynthetic Flagellate Euglena gracilis. Photochem. Photobiol. 1998, 68, 260–265. [Google Scholar] [CrossRef]
- Doughty, M.J.; Diehn, B. Anion sensitivity of motility and step-down photophobic responses of Euglena gracilis. Arch. Microbiol. 1984, 138, 329–332. [Google Scholar] [CrossRef]
- Iseki, M.; Matsunaga, S.; Murakami, A.; Ohno, K.; Shiga, K.; Yoshida, K.; Sugai, M.; Takahashi, T.; Hori, T.; Watanabe, M. A blue-light-activated adenylyl cyclase mediates photoavoidance in Euglena gracilis. Nature 2002, 415, 1047–1051. [Google Scholar] [CrossRef]
- Ntefidou, M.; Iseki, M.; Watanabe, M.; Lebert, M.; Häder, D.-P. Photoactivated adenylyl cyclase controls phototaxis in the flagellate Euglena gracilis. Plant Physiol. 2003, 133, 1517–1521. [Google Scholar] [CrossRef][Green Version]
- Stallwitz, E.; Häder, D.-P. Effects of heavy metals on motility and gravitactic orientation of the flagellate, Euglena gracilis. Eur. J. Protistol. 1994, 30, 18–24. [Google Scholar] [CrossRef]
- Richter, P.; Börnig, A.; Streb, C.; Ntefidou, M.; Lebert, M.; Häder, D.P. Effects of increased salinity on gravitaxis in Euglena gracilis. J. Plant Physiol. 2003, 160, 651–656. [Google Scholar] [CrossRef]
- Daiker, V.; Häder, D.-P.; Richter, P.R.; Lebert, M. The involvement of a protein kinase in phototaxis and gravitaxis of Euglena gracilis. Planta 2011, 233, 1055–1062. [Google Scholar] [CrossRef]
- Daiker, V.; Lebert, M.; Richter, P.; Häder, D.-P. Molecular characterization of a calmodulin involved in the signal transduction chain of gravitaxis in Euglena gracilis. Planta 2010, 231, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Nasir, A.; Le Bail, A.; Daiker, V.; Klima, J.; Richter, P.; Lebert, M. Identification of a flagellar protein implicated in the gravitaxis in the flagellate Euglena gracilis. Sci. Rep. 2018, 8, 7605. [Google Scholar] [CrossRef] [PubMed]
- Häder, D.-P.; Richter, P.R.; Schuster, M.; Daiker, V.; Lebert, M. Molecular analysis of the graviperception signal transduction in the flagellate Euglena gracilis: Involvement of a transient receptor potential-like channel and a calmodulin. Adv. Space Res. 2009, 43, 1179–1184. [Google Scholar] [CrossRef]
- Hader, D.P. Polarotaxis, gravitaxis and vertical phototaxis in the green flagellate, Euglena gracilis. Arch. Microbiol. 1987, 147, 179–183. [Google Scholar] [CrossRef]
- Tahedl, H.; Richter, P.; Lebert, M.; Häder, D.P. cAMP is involved in gravitaxis signal transduction of Euglena gracilis. Microgravity Sci. Technol. 1998, 11, 173–178. [Google Scholar]
- Brown, M.C.; Joaquim, T.R.; Chambers, R.; Onisk, D.V.; Yin, F.; Moriango, J.M.; Xu, Y.; Fancy, D.A.; Crowgey, E.L.; He, Y.; et al. Impact of immunization technology and assay application on antibody performance-a systematic comparative evaluation. PLoS ONE 2011, 6, e28718. [Google Scholar] [CrossRef]
- Mermelstein, C.S.; Rodrigues, A.; Einicker-Lamas, M.; Navarrete, R.D.B.; Farina, M.; Costa, M.L. Distribution of F-actin, α-actinin, tropomyosin, tubulin and organelles in Euglena gracilis by immunofluorescence microscopy. Tissue Cell 1998, 30, 545–553. [Google Scholar] [CrossRef]
- Gualtieri, P.; Barsanti, L.; Rosati, G. Isolation of the photoreceptor (paraflagellar body) of the phototactic flagellate Euglena gracilis. Arch. Microbiol. 1986, 145, 303–305. [Google Scholar] [CrossRef]
- Piccinni, E.; Mammi, M. Motor Apparatus of Euglena gracilis: Ultrastructure of the Basal Portion of the Flagellum and the Paraflagellar Body. Ital. J. Zool. 1978, 45, 405–414. [Google Scholar] [CrossRef][Green Version]
- Michalak, P. Coexpression, coregulation, and cofunctionality of neighboring genes in eukaryotic genomes. Genomics 2008, 91, 243–248. [Google Scholar] [CrossRef]
- Schröder-Lang, S.; Schwärzel, M.; Seifert, R.; Strünker, T.; Kateriya, S.; Looser, J.; Watanabe, M.; Kaupp, U.B.; Hegemann, P.; Nagel, G. Fast manipulation of cellular cAMP level by light in vivo. Nat. Methods 2007, 4, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Berriman, M.; Ghedin, E.; Hertz-Fowler, C.; Blandin, G.; Renauld, H.; Bartholomeu, D.C.; Lennard, N.J.; Caler, E.; Hamlin, N.E.; Haas, B.; et al. The genome of the African trypanosome Trypanosoma brucei. Science 2005, 309, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.N.; Russell, A.G. Clustered organization, polycistronic transcription, and evolution of modification-guide snoRNA genes in Euglena gracilis. Mol. Genet. Genom. 2012, 287, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, A.; Li, X.; Bieberich, C.J. Protein kinase A regulates MYC protein through transcriptional and post-translational mechanisms in a catalytic subunit isoform-specific manner. J. Biol. Chem. 2013, 288, 14158–14169. [Google Scholar] [CrossRef] [PubMed]
- Hardin, P.E. Molecular genetic analysis of circadian timekeeping in Drosophila. Adv. Genet. 2011, 74, 141–173. [Google Scholar] [CrossRef] [PubMed]
- Broughton, S.; Alic, N.; Slack, C.; Bass, T.; Ikeya, T.; Vinti, G.; Tommasi, A.M.; Driege, Y.; Hafen, E.; Partridge, L. Reduction of DILP2 in Drosophila triages a metabolic phenotype from lifespan revealing redundancy and compensation among DILPs. PLoS ONE 2008, 3, e3721. [Google Scholar] [CrossRef]
- Gancedo, J.M. Biological roles of cAMP: Variations on a theme in the different kingdoms of life. Biol. Rev. Camb. Philos. Soc. 2013, 88, 645–668. [Google Scholar] [CrossRef] [PubMed]
- Newton, A.C. Regulation of the ABC kinases by phosphorylation: Protein kinase C as a paradigm. Biochem. J. 2003, 370, 361–371. [Google Scholar] [CrossRef]
- Dodge, K.L.; Khouangsathiene, S.; Kapiloff, M.S.; Mouton, R.; Hill, E.V.; Houslay, M.D.; Langeberg, L.K.; Scott, J.D. mAKAP assembles a protein kinase A/PDE4 phosphodiesterase cAMP signaling module. EMBO J. 2001, 20, 1921–1930. [Google Scholar] [CrossRef]
- Pawson, T.; Scott, J.D. Signaling through scaffold, anchoring, and adaptor proteins. Science 1997, 278, 2075–2080. [Google Scholar] [CrossRef]
- Steinberg, S.F.; Brunton, L.L. Compartmentation of G protein-coupled signaling pathways in cardiac myocytes. Annu. Rev. Pharmacol. Toxicol. 2001, 41, 751–773. [Google Scholar] [CrossRef] [PubMed]
- Hammond, M.; Zoltner, M.; Garrigan, J.; Butterfield, E.; Varga, V.; Lukeš, J.; Field, M.C. The distinctive flagellar proteome of Euglena gracilis illuminates the complexities of protistan flagella adaptation. New Phytol. 2021, 232, 1323–1336. [Google Scholar] [CrossRef] [PubMed]
- Schlösser, U.G. SAG-Sammlung von Algenkulturen at the University of Göttingen Catalogue of Strains 1994. Bot. Acta 1994, 107, 113–186. [Google Scholar] [CrossRef]
- Checcucci, A.; Colombetti, G.; Ferrara, R.; Lenci, F. Action spectra for photoaccumulation of green and colorless Euglena: Evidence for identification of receptor pigments. Photochem. Photobiol. 1976, 23, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Tahedl, H.; Häder, D.P. Automated biomonitoring using real time movement analysis of Euglena gracilis. Ecotoxicol. Environ. Saf. 2001, 48, 161–169. [Google Scholar] [CrossRef]
- Hder, D.-P.; Vogel, K. Simultaneous tracking of flagellates in real time by image analysis. J. Math. Biol. 1991, 30, 63–72. [Google Scholar] [CrossRef]
- Chomczynski, P.; Mackey, K. Short technical reports. Modification of the TRI reagent procedure for isolation of RNA from polysaccharide- and proteoglycan-rich sources. Biotechniques 1995, 19, 942–945. [Google Scholar]
- Ngô, H.; Tschudi, C.; Gull, K.; Ullu, E. Double-stranded RNA induces mRNA degradation in Trypanosoma brucei. Proc. Natl. Acad. Sci. USA 1998, 95, 14687–14692. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Malik, N.; Berrie, A. New stain fixative for proteins separated by gel isoelectric focusing based on coomassie brilliant blue. Anal. Biochem. 1972, 49, 173–176. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasir, A.; Richter, P.R.; Le Bail, A.; Daiker, V.; Stoltze, J.; Prasad, B.; Strauch, S.M.; Lebert, M. Molecular Cross-Talk between Gravity- and Light-Sensing Mechanisms in Euglena gracilis. Int. J. Mol. Sci. 2022, 23, 2776. https://doi.org/10.3390/ijms23052776
Nasir A, Richter PR, Le Bail A, Daiker V, Stoltze J, Prasad B, Strauch SM, Lebert M. Molecular Cross-Talk between Gravity- and Light-Sensing Mechanisms in Euglena gracilis. International Journal of Molecular Sciences. 2022; 23(5):2776. https://doi.org/10.3390/ijms23052776
Chicago/Turabian StyleNasir, Adeel, Peter Rolf Richter, Aude Le Bail, Viktor Daiker, Julia Stoltze, Binod Prasad, Sebastian Michael Strauch, and Michael Lebert. 2022. "Molecular Cross-Talk between Gravity- and Light-Sensing Mechanisms in Euglena gracilis" International Journal of Molecular Sciences 23, no. 5: 2776. https://doi.org/10.3390/ijms23052776
APA StyleNasir, A., Richter, P. R., Le Bail, A., Daiker, V., Stoltze, J., Prasad, B., Strauch, S. M., & Lebert, M. (2022). Molecular Cross-Talk between Gravity- and Light-Sensing Mechanisms in Euglena gracilis. International Journal of Molecular Sciences, 23(5), 2776. https://doi.org/10.3390/ijms23052776






