Double-Stranded RNA Enhances Matrix Metalloproteinase-1 and -13 Expressions through TLR3-Dependent Activation of Transglutaminase 2 in Dermal Fibroblasts
Abstract
:1. Introduction
2. Results
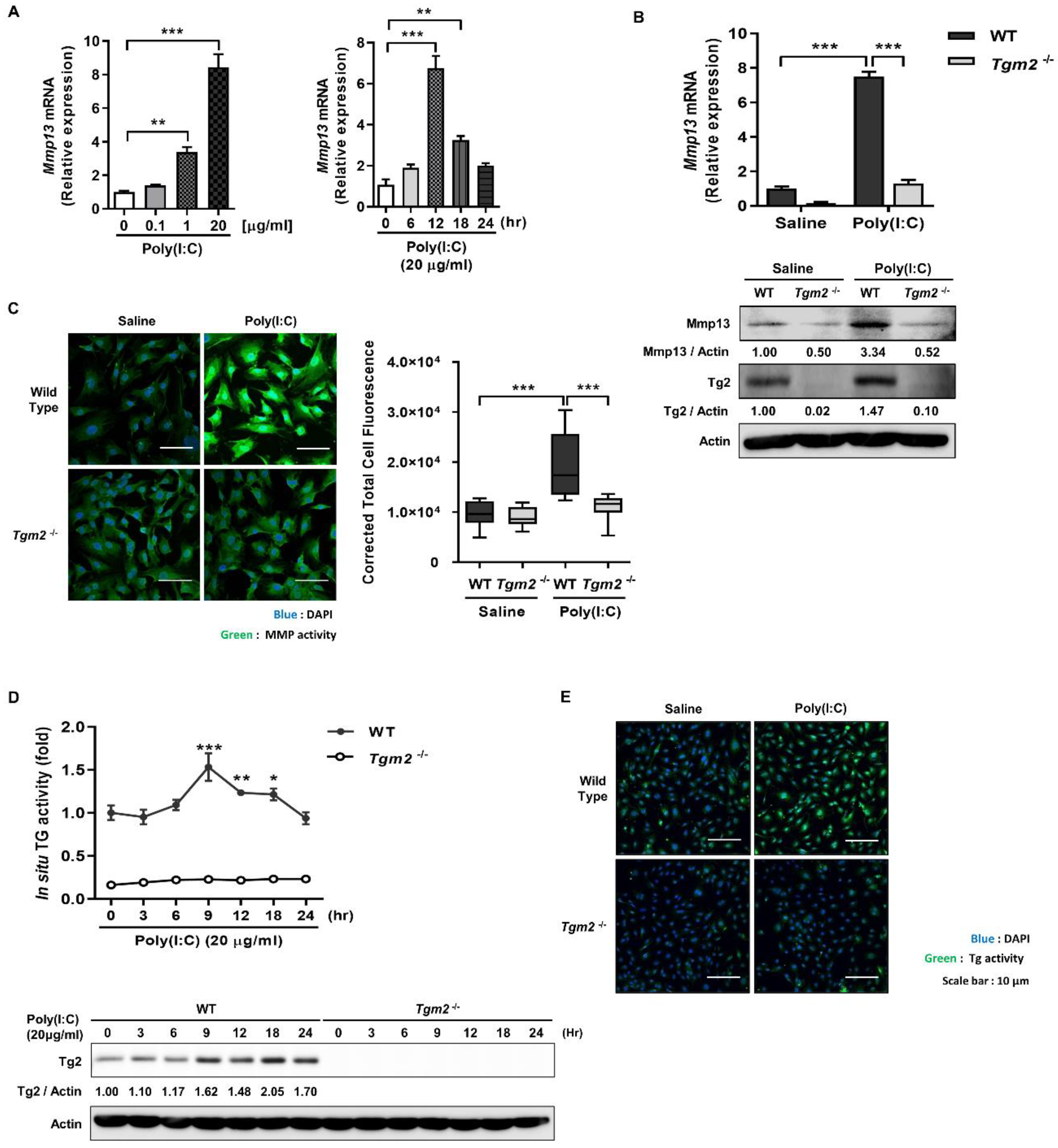
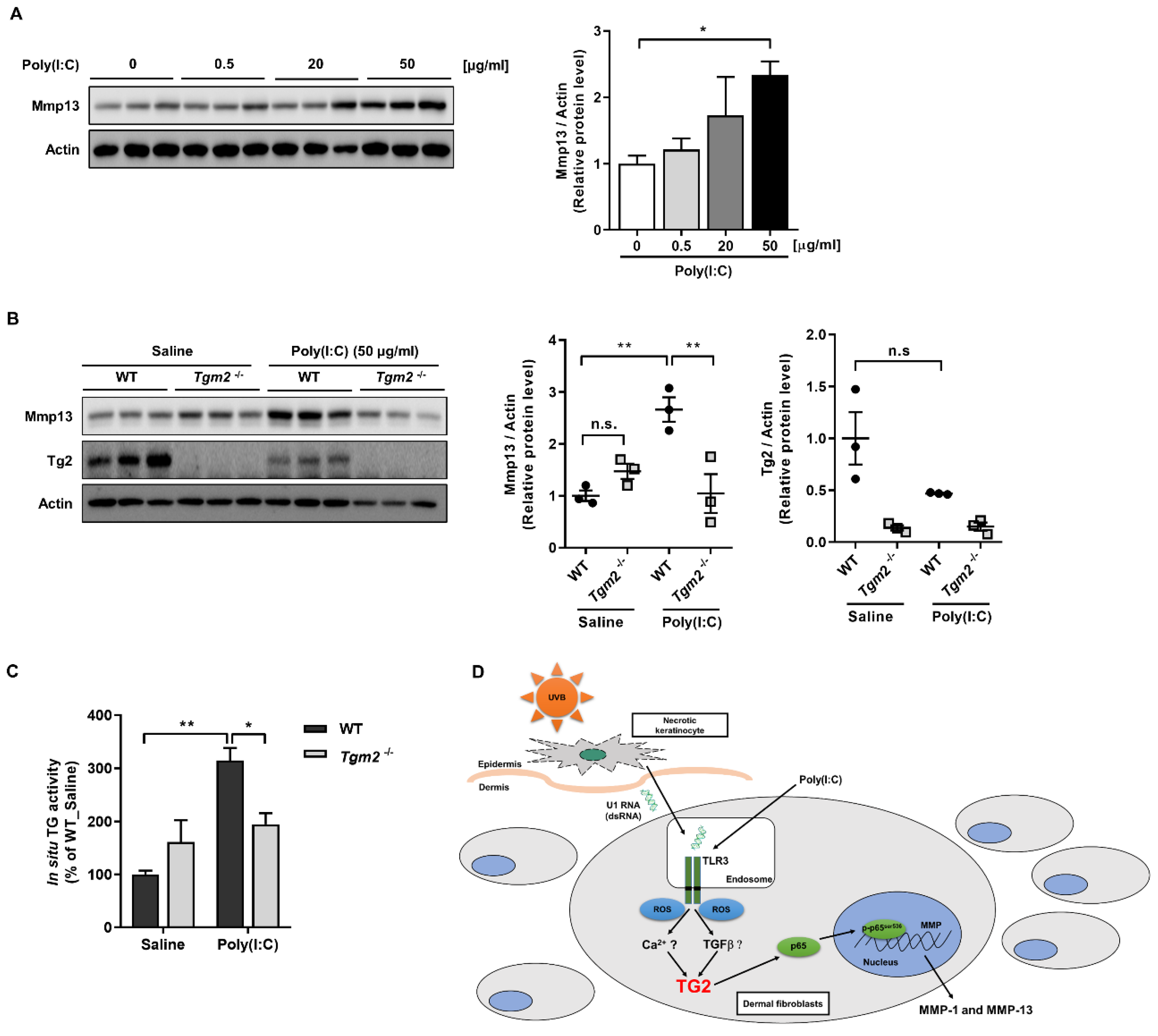
2.1. Poly(I:C) Enhances MMP-1 and -13 Expression through TG2 Activation
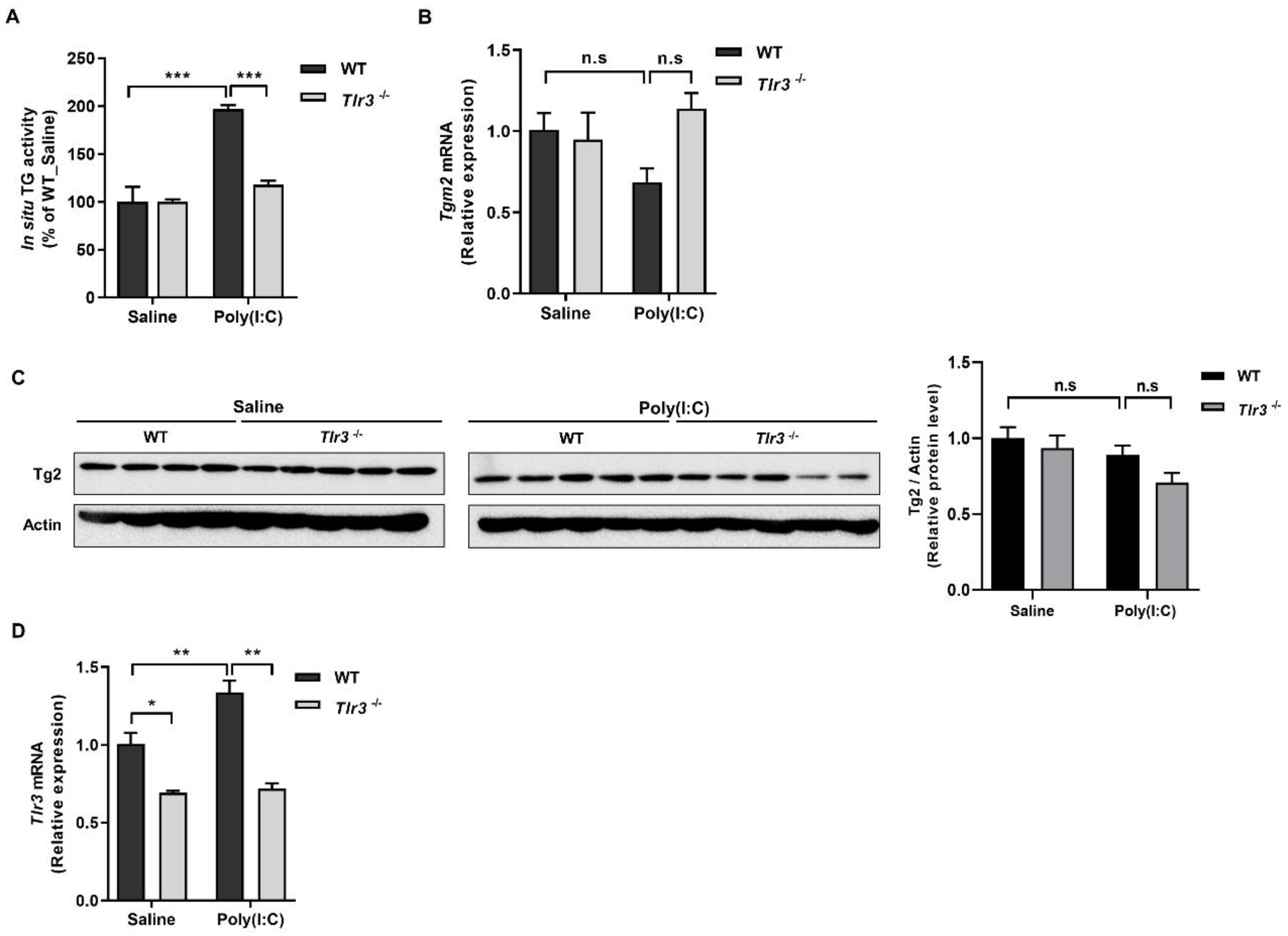
2.2. ROS Generated by Binding of Poly(I:C) with TLR3 Activates TG2
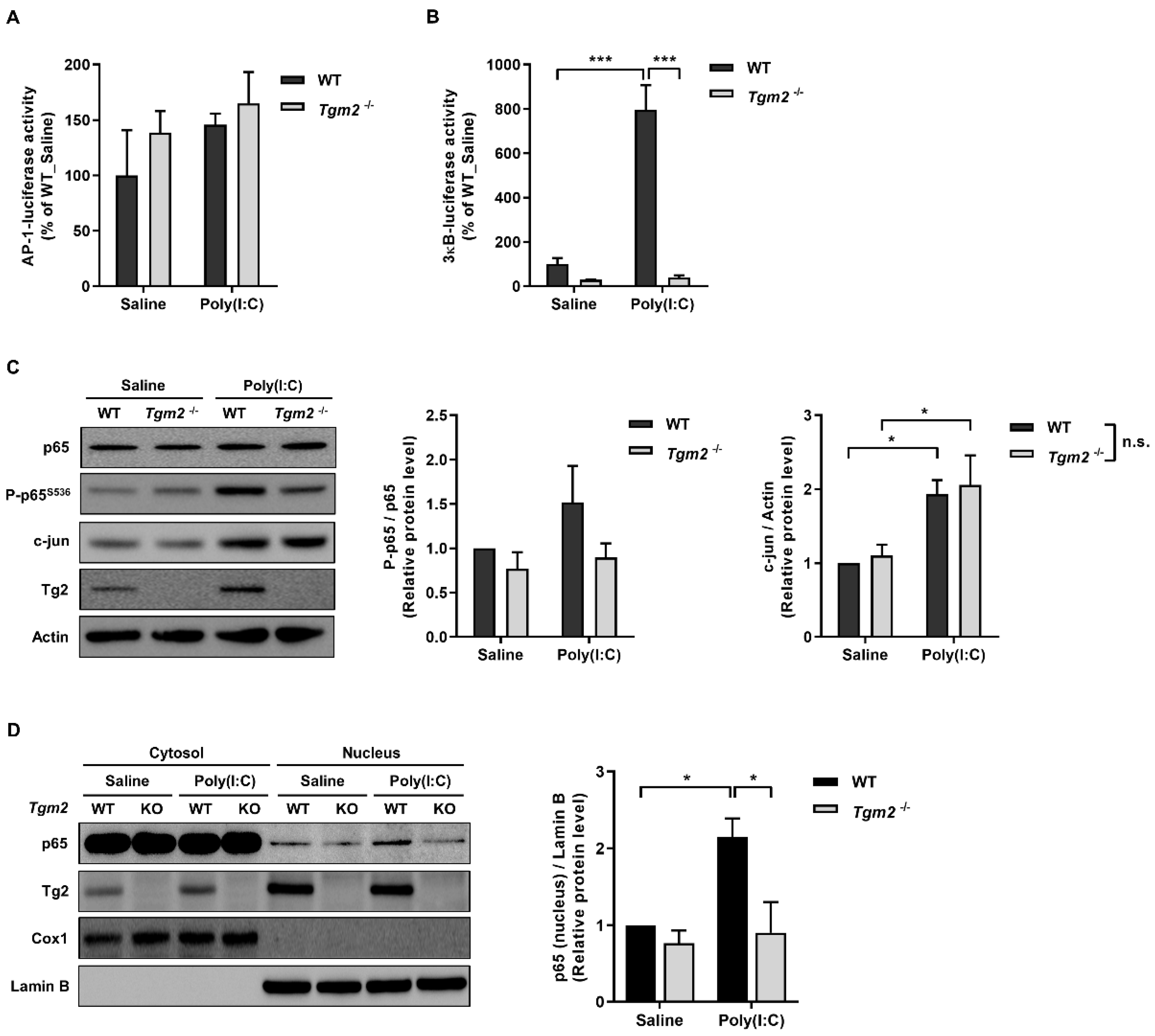
2.3. TG2 Mediates Poly(I:C)-Induced NF-kB Activation
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Poly(I:C) Treatment
4.2. Isolation of Mouse Dermal Fibroblasts and Ex Vivo Mouse Skin Culture
4.3. siRNA Transfection
4.4. Total RNA Extraction and qRT-PCR
4.5. Western Blot Analysis
4.6. In Situ Transglutaminase Assay
4.7. Cell In Situ Zymography
4.8. Measurement of Intracellular ROS
4.9. Luciferase Reporter Assay
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fisher, G.J.; Kang, S.; Varani, J.; Bata-Csorgo, Z.; Wan, Y.; Datta, S.; Voorhees, J.J. Mechanisms of photoaging and chronological skin aging. Arch. Dermatol. 2002, 138, 1462–1470. [Google Scholar] [CrossRef]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of Matrix Metalloproteinases in Photoaging and Photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rittie, L.; Fisher, G.J. Natural and sun-induced aging of human skin. Cold Spring Harb. Perspect. Med. 2015, 5, a015370. [Google Scholar] [CrossRef]
- Hur, S. Double-Stranded RNA Sensors and Modulators in Innate Immunity. Annu. Rev. Immunol. 2019, 37, 349–375. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Sen, G.C. dsRNA-activation of TLR3 and RLR signaling: Gene induction-dependent and independent effects. J. Interferon Cytokine Res. 2014, 34, 427–436. [Google Scholar] [CrossRef] [Green Version]
- Bernard, J.J.; Cowing-Zitron, C.; Nakatsuji, T.; Muehleisen, B.; Muto, J.; Borkowski, A.W.; Martinez, L.; Greidinger, E.L.; Yu, B.D.; Gallo, R.L. Ultraviolet radiation damages self noncoding RNA and is detected by TLR3. Nat. Med. 2012, 18, 1286–1290. [Google Scholar] [CrossRef]
- Yao, C.; Lee, D.H.; Oh, J.H.; Kim, M.K.; Kim, K.H.; Park, C.H.; Chung, J.H. Poly(I:C) induces expressions of MMP-1, -2, and -3 through various signaling pathways including IRF3 in human skin fibroblasts. J. Dermatol. Sci. 2015, 80, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Iismaa, S.E.; Mearns, B.M.; Lorand, L.; Graham, R.M. Transglutaminases and disease: Lessons from genetically engineered mouse models and inherited disorders. Physiol. Rev. 2009, 89, 991–1023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.J.; Lee, J.H.; Lee, K.B.; Shin, J.W.; Kwon, M.A.; Lee, S.; Jeong, E.M.; Cho, S.Y.; Kim, I.G. Transglutaminase 2 crosslinks the glutathione S-transferase tag, impeding protein-protein interactions of the fused protein. Exp. Mol. Med. 2021, 53, 115–124. [Google Scholar] [CrossRef]
- Jeon, J.H.; Choi, K.H.; Cho, S.Y.; Kim, C.W.; Shin, D.M.; Kwon, J.C.; Song, K.Y.; Park, S.C.; Kim, I.G. Transglutaminase 2 inhibits Rb binding of human papillomavirus E7 by incorporating polyamine. EMBO J. 2003, 22, 5273–5282. [Google Scholar] [CrossRef] [Green Version]
- Shin, D.M.; Jeon, J.H.; Kim, C.W.; Cho, S.Y.; Kwon, J.C.; Lee, H.J.; Choi, K.H.; Park, S.C.; Kim, I.G. Cell type-specific activation of intracellular transglutaminase 2 by oxidative stress or ultraviolet irradiation: Implications of transglutaminase 2 in age-related cataractogenesis. J. Biol. Chem. 2004, 279, 15032–15039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, S.Y.; Jeong, E.M.; Lee, J.H.; Kim, H.J.; Lim, J.; Kim, C.W.; Shin, D.M.; Jeon, J.H.; Choi, K.; Kim, I.G. Doxorubicin induces the persistent activation of intracellular transglutaminase 2 that protects from cell death. Mol. Cells 2012, 33, 235–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.J.; Lee, K.B.; Son, Y.H.; Shin, J.; Lee, J.H.; Kim, H.J.; Hong, A.Y.; Bae, H.W.; Kwon, M.A.; Lee, W.J.; et al. Transglutaminase 2 mediates UV-induced skin inflammation by enhancing inflammatory cytokine production. Cell Death Dis. 2017, 8, e3148. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, K.B.; Hong, A.Y.; Son, Y.H.; Lee, D.H.; Jeong, E.M.; Kim, I.G. Transglutaminase 2 mediates UVB-induced matrix metalloproteinase-1 expression by inhibiting nuclear p65 degradation in dermal fibroblasts. Exp. Dermatol. 2021; Early view. [Google Scholar] [CrossRef] [PubMed]
- Jedeszko, C.; Sameni, M.; Olive, M.B.; Moin, K.; Sloane, B.F. Visualizing protease activity in living cells: From two dimensions to four dimensions. Curr. Protoc. Cell Biol. 2008, 39, 4–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Wang, X.; Liu, M.; Hu, Q.; Song, L.; Ye, L.; Zhou, D.; Ho, W. A critical function of toll-like receptor-3 in the induction of anti-human immunodeficiency virus activities in macrophages. Immunology 2010, 131, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Kim, J.J.; Lee, S.J.; Hwang, J.H.; Lee, C.H.; Lee, M.S.; Jo, E.K. TLR3-triggered reactive oxygen species contribute to inflammatory responses by activating signal transducer and activator of transcription-1. J. Immunol. 2013, 190, 6368–6377. [Google Scholar] [CrossRef] [Green Version]
- Liacini, A.; Sylvester, J.; Li, W.Q.; Huang, W.S.; Dehnade, F.; Ahmad, M.; Zafarullah, M. Induction of matrix metalloproteinase-13 gene expression by TNF-alpha is mediated by MAP kinases, AP-1, and NF-kappa B transcription factors in articular chondrocytes. Exp. Cell Res. 2003, 288, 208–217. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Herman, M.; Ciancanelli, M.J.; Perez de Diego, R.; Sancho-Shimizu, V.; Abel, L.; Casanova, J.L. TLR3 immunity to infection in mice and humans. Curr. Opin. Immunol. 2013, 25, 19–33. [Google Scholar] [CrossRef] [Green Version]
- Lichti, U.; Anders, J.; Yuspa, S.H. Isolation and short-term culture of primary keratinocytes, hair follicle populations and dermal cells from newborn mice and keratinocytes from adult mice for in vitro analysis and for grafting to immunodeficient mice. Nat. Protoc. 2008, 3, 799–810. [Google Scholar] [CrossRef]
- Costa, A.; Eberlin, S.; Clerici, S.P.; Abdalla, B.M. In vitro effects of infrared A radiation on the synthesis of MMP-1, catalase, superoxide dismutase and GADD45 alpha protein. Inflamm. Allergy Drug Targets 2015, 14, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.; Little, E.; Quan, H.; Qin, Z.; Voorhees, J.J.; Fisher, G.J. Elevated matrix metalloproteinases and collagen fragmentation in photodamaged human skin: Impact of altered extracellular matrix microenvironment on dermal fibroblast function. J. Investig. Dermatol. 2013, 133, 1362–1366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, C.H.; Lee, M.J.; Ahn, J.; Kim, S.; Kim, H.H.; Kim, K.H.; Eun, H.C.; Chung, J.H. Heat shock-induced matrix metalloproteinase (MMP)-1 and MMP-3 are mediated through ERK and JNK activation and via an autocrine interleukin-6 loop. J. Investig. Dermatol. 2004, 123, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.H.; Lee, M.J.; Lee, S.R.; Kim, K.H.; Cho, K.H.; Eun, H.C.; Chung, J.H. Augmentation of UV-induced skin wrinkling by infrared irradiation in hairless mice. Mech. Ageing Dev. 2005, 126, 1170–1177. [Google Scholar] [CrossRef]
- Jeong, E.M.; Kim, C.W.; Cho, S.Y.; Jang, G.Y.; Shin, D.M.; Jeon, J.H.; Kim, I.G. Degradation of transglutaminase 2 by calcium-mediated ubiquitination responding to high oxidative stress. FEBS Lett. 2009, 583, 648–654. [Google Scholar] [CrossRef] [Green Version]
- Shin, J.W.; Kwon, M.A.; Hwang, J.; Lee, S.J.; Lee, J.H.; Kim, H.J.; Lee, K.B.; Lee, S.J.; Jeong, E.M.; Chung, J.H.; et al. Keratinocyte transglutaminase 2 promotes CCR6(+) gammadeltaT-cell recruitment by upregulating CCL20 in psoriatic inflammation. Cell Death Dis. 2020, 11, 301. [Google Scholar] [CrossRef]
- Oh, K.; Park, H.B.; Byoun, O.J.; Shin, D.M.; Jeong, E.M.; Kim, Y.W.; Kim, Y.S.; Melino, G.; Kim, I.G.; Lee, D.S. Epithelial transglutaminase 2 is needed for T cell interleukin-17 production and subsequent pulmonary inflammation and fibrosis in bleomycin-treated mice. J. Exp. Med. 2011, 208, 1707–1719. [Google Scholar] [CrossRef] [Green Version]
- Kawai, T.; Akira, S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol. Med. 2007, 13, 460–469. [Google Scholar] [CrossRef]
- Chau, T.L.; Gioia, R.; Gatot, J.S.; Patrascu, F.; Carpentier, I.; Chapelle, J.P.; O’Neill, L.; Beyaert, R.; Piette, J.; Chariot, A. Are the IKKs and IKK-related kinases TBK1 and IKK-epsilon similarly activated? Trends Biochem. Sci. 2008, 33, 171–180. [Google Scholar] [CrossRef]
- Wallach-Dayan, S.B.; Izbicki, G.; Cohen, P.Y.; Gerstl-Golan, R.; Fine, A.; Breuer, R. Bleomycin initiates apoptosis of lung epithelial cells by ROS but not by Fas/FasL pathway. Am. J. Physiol. Lung Cell Mol. Physiol. 2006, 290, L790–L796. [Google Scholar] [CrossRef]
- Lee, C.H.; Wu, S.B.; Hong, C.H.; Yu, H.S.; Wei, Y.H. Molecular Mechanisms of UV-Induced Apoptosis and Its Effects on Skin Residential Cells: The Implication in UV-Based Phototherapy. Int. J. Mol. Sci. 2013, 14, 6414–6435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svobodova, A.; Walterova, D.; Vostalova, J. Ultraviolet light induced alteration to the skin. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc 2006, 150, 25–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.X.; Weng, Q.Y.; Fisher, D.E. UV Signaling Pathways within the Skin. J. Investig. Dermatol. 2014, 134, 2080–2085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mauviel, A. Transforming growth factor-beta signaling in skin: Stromal to epithelial cross-talk. J. Investig. Dermatol. 2009, 129, 7–9. [Google Scholar] [CrossRef] [Green Version]
- Horiguchi, M.; Ota, M.; Rifkin, D.B. Matrix control of transforming growth factor-beta function. J. Biochem. 2012, 152, 321–329. [Google Scholar] [CrossRef] [Green Version]
- Verrecchia, F.; Chu, M.L.; Mauviel, A. Identification of novel TGF-beta/Smad gene targets in dermal fibroblasts using a combined cDNA microarray/promoter transactivation approach. J. Biol. Chem. 2001, 276, 17058–17062. [Google Scholar] [CrossRef] [Green Version]
- Shin, D.M.; Jeon, J.H.; Kim, C.W.; Cho, S.Y.; Lee, H.J.; Jang, G.Y.; Jeong, E.M.; Lee, D.S.; Kang, J.H.; Melino, G.; et al. TGF beta mediates activation of transglutaminase 2 in response to oxidative stress that leads to protein aggregation. FASEB J. 2008, 22, 2498–2507. [Google Scholar] [CrossRef]
- Quan, T.H.; He, T.Y.; Kang, S.; Voorhees, J.J.; Fisher, G.J. Solar ultraviolet irradiation reduces collagen in photoaged human skin by blocking transforming growth factor-beta type II receptor/Smad signaling. Am. J. Pathol. 2004, 165, 741–751. [Google Scholar] [CrossRef]
- Popov, Y.; Sverdlov, D.Y.; Sharma, A.K.; Bhaskar, K.R.; Li, S.Y.; Freitag, T.L.; Lee, J.; Dieterich, W.; Melino, G.; Schuppan, D. Tissue Transglutaminase Does Not Affect Fibrotic Matrix Stability or Regression of Liver Fibrosis in Mice. Gastroenterology 2011, 140, 1642–1652. [Google Scholar] [CrossRef] [Green Version]
- Shweke, N.; Boulos, N.; Jouanneau, C.; Vandermeersch, S.; Melino, G.; Dussaule, J.C.; Chatziantoniou, C.; Ronco, P.; Boffa, J.J. Tissue transglutaminase contributes to interstitial renal fibrosis by favoring accumulation of fibrillar collagen through TGF-beta activation and cell infiltration. Am. J. Pathol. 2008, 173, 631–642. [Google Scholar] [CrossRef] [Green Version]
- Johnson, T.S.; Griffin, M.; Thomas, G.L.; Skill, J.; Cox, A.; Yang, B.; Nicholas, B.; Birckbichler, P.J.; MuchanetaKubara, C.; El Nahas, A.M. The role of transglutaminase in the rat subtotal nephrectomy model of renal fibrosis. J. Clin. Investig. 1997, 99, 2950–2960. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Son, Y.H.; Lee, K.B.; Lee, J.H.; Kim, H.J.; Jeong, E.M.; Park, S.C.; Kim, I.G. 4-n-butylresorcinol enhances proteolytic degradation of tyrosinase in B16F10 melanoma cells. Int. J. Cosmet. Sci. 2017, 39, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Jang, G.Y.; Jeon, J.H.; Cho, S.Y.; Shin, D.M.; Kim, C.W.; Jeong, E.M.; Bae, H.C.; Kim, T.W.; Lee, S.H.; Choi, Y.; et al. Transglutaminase 2 suppresses apoptosis by modulating caspase 3 and NF-kappaB activity in hypoxic tumor cells. Oncogene 2010, 29, 356–367. [Google Scholar] [CrossRef] [Green Version]
- Chhabra, A.; Jaiswal, A.; Malhotra, U.; Kohli, S.; Rani, V. Cell in situ zymography: An in vitro cytotechnology for localization of enzyme activity in cell culture. In Vitro Cell. Dev. Biol. Anim. 2012, 48, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Vasanwala, F.H.; Kusam, S.; Toney, L.M.; Dent, A.L. Repression of AP-1 function: A mechanism for the regulation of Blimp-1 expression and B lymphocyte differentiation by the B cell lymphoma-6 protooncogene. J. Immunol. 2002, 169, 1922–1929. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, A.-Y.; Lee, S.-J.; Lee, K.B.; Shin, J.-W.; Jeong, E.M.; Kim, I.-G. Double-Stranded RNA Enhances Matrix Metalloproteinase-1 and -13 Expressions through TLR3-Dependent Activation of Transglutaminase 2 in Dermal Fibroblasts. Int. J. Mol. Sci. 2022, 23, 2709. https://doi.org/10.3390/ijms23052709
Hong A-Y, Lee S-J, Lee KB, Shin J-W, Jeong EM, Kim I-G. Double-Stranded RNA Enhances Matrix Metalloproteinase-1 and -13 Expressions through TLR3-Dependent Activation of Transglutaminase 2 in Dermal Fibroblasts. International Journal of Molecular Sciences. 2022; 23(5):2709. https://doi.org/10.3390/ijms23052709
Chicago/Turabian StyleHong, Ah-Young, Seok-Jin Lee, Ki Baek Lee, Ji-Woong Shin, Eui Man Jeong, and In-Gyu Kim. 2022. "Double-Stranded RNA Enhances Matrix Metalloproteinase-1 and -13 Expressions through TLR3-Dependent Activation of Transglutaminase 2 in Dermal Fibroblasts" International Journal of Molecular Sciences 23, no. 5: 2709. https://doi.org/10.3390/ijms23052709
APA StyleHong, A.-Y., Lee, S.-J., Lee, K. B., Shin, J.-W., Jeong, E. M., & Kim, I.-G. (2022). Double-Stranded RNA Enhances Matrix Metalloproteinase-1 and -13 Expressions through TLR3-Dependent Activation of Transglutaminase 2 in Dermal Fibroblasts. International Journal of Molecular Sciences, 23(5), 2709. https://doi.org/10.3390/ijms23052709





