1. Introduction
The application of the assisted reproductive technologies (ART) has been constantly improving over the last four decades since the first IVF-assisted birth in 1978 [
1]. While the number of ART cycles steadily increase worldwide, live birth rates vary significantly over different regions—from 12–51% for fresh embryos to 18–57% for cryopreserved ones [
2].
One of the ways to increase ART efficiency is double embryo transfer [
3,
4,
5,
6], but this approach has well-known risks of multiple pregnancies, associated with different complications for infant and mother health [
7,
8], which have tendency to increase with age [
9,
10]. Over the past two decades, there has been a shift towards the elective single embryo transfer (eSET) protocols [
11]. While this approach mitigates risks associated with multi-pregnancy, it requires developing an accurate strategy of embryo quality assessment, selection, and transfer. Initially, the question was raised of when the embryo should be transferred. Centers of Disease Control and Prevention (CDC) in the USA reported that in 2013, most of the embryos were transferred on day 3 at the cleavage stage or day 5 at the blastocyst stage, but transfers on day 1, 2, 4, and 6 have been reported as well [
12]. A meta-analysis including 1654 patients has shown significantly higher clinical pregnancy and live birth rates among embryos transferred at the blastocyst stage compared to those transferred at cleavage state. The 5th-day blastocysts have better synchronization with an IVF stimulated endometrium and the high implantation potential [
13]. One of the most obvious and widely used methods of embryo quality assessment utilizes this correlation and is based on morphological criteria, which considers blastocoel size, form, and shape of trophectoderm and inner cell mass for the evaluation of embryo development and viability [
14]. Currently, the morphology-based grading system of embryo quality assessment is also enhanced by morphokinetics analysis using time-lapse light microscopy [
15,
16,
17] and deep machine learning methods [
18]. However, embryo morphological phenotype evaluation alone is insufficient for accurate prediction of successful implantation in the situation of choice between multiple embryos of “excellent” (“A”) grade [
19,
20]. Moreover, aneuploid embryos can form morphologically high-scoring blastocysts or even reach the term of delivery. Despite the existing correlation between karyotype and morphology [
21], classic grading or time-lapse microscopy is not an alternative to genetic analysis for accurate assessment of embryo karyotype [
22,
23,
24,
25]. At the same time, genetic abnormalities highly interfere with implantation and physiological development [
26]. That is why methods of preimplantation genetic testing for aneuploidies (PGT-A) are generally used to check the karyotype of selected embryos before the transfer [
27,
28,
29]. However, this invasive method can result in decrease of pregnancy rates if biopsies are performed at the cleavage stage [
30,
31]. No such effects were found after blastocysts biopsies, but the technical complexity of PGT-A using NGS or array hybridization makes it applicable only for frozen-thawed embryos to avoid day 6 fresh transfers, which were reported to be associated with lower success rates [
32]. The high cost of PGT-A procedures can also be considered as a limiting factor for broad method application.
Limitations of morphology-based and genetic classification methods stimulated the search for new approaches for the non-invasive biochemical or molecular evaluation of embryo viability and quality. One of the promising non-invasive approaches includes measurements of biochemical intermediates, such as glucose, lactate, pyruvate, amino acids, b-HCG, or other proteins in the spent embryo culture medium [
33,
34,
35,
36]. Recently, several spectroscopy methods (Raman spectroscopy, NMR spectroscopy, IR spectroscopy) have been used to study the embryonic metabolome [
37,
38,
39] as well as gas chromatography coupled with mass spectrometry [
40]. The embryo metabolome might reflect the dynamic changes of cell activity regulated by the expression of certain sets of genes and their target proteins, affecting the molecular phenotype of the embryo and its implantation potential. The metabolic activity of the embryo can be assessed by examining the exometabolome of the spent culture medium, which is formed by dynamic nutrient intake and secretion of metabolites. Thus, exometabolomics of spent embryo culture medium (SECM) might provide useful insights on the functional features of embryonic development and overall embryo viability to improve the success rates of the eSET protocol.
One of the most advanced and powerful methods of metabolites profiling is the combination of high-performance liquid chromatography followed by mass spectrometry (HPLC-MS). This approach is widely approved in modern omics research, but it is still rarely used for human embryo culture media analysis. In this study, the HPLC-MS method was used to evaluate the differences in the metabolomic profiles of culture media from human embryos of different morphological classes and karyotypes. Metabolite content of paired SECM on the 3rd and 5th day of cultivation was also studied to show dynamic changes and check the reproducibility of results on different LC-MS systems. Finally, changes in the metabolomic profiles of SECM from embryos with different implantation outcomes after transfer were analyzed.
3. Discussion
The elective single-embryo transfer has been shown in several studies to be the recommended method compared to multiple embryo transfer, since it demonstrates a similar birth rate and is devoid of disadvantages associated with multiple pregnancies [
41,
42,
43]. However, this method requires a comprehensive assessment of the viability and implantation potential of a single embryo, as there is no room for error. It should be noted that currently there are no reliable criteria for choosing the best candidate from two euploid embryos with excellent morphological qualities. To address these issues, routine morphology analysis, even with time-lapse microscopy, should be supported by biochemical or molecular methods. One of such methods is metabolomic profiling which allows taking of a “snapshot” of current metabolites composition which might correlate with the embryo functional condition and implantation potential. While direct measurement of embryo metabolism is complicated, spent culture media exometabolome may provide crucial information on embryo development based on different nutrient consumption and metabolites excretion to media by embryos of different morphological quality or the same quality but different implantation potential.
In this study, metabolite profiles of spent culture media were obtained in two cultivation protocols: five days of continuous cultivation or five days of discontinuous cultivation with a complete refreshment of the media on day 3. The media samples were grouped into four classes according to embryo morphological grade and their quality for transfer (
Table 1). This classification was based on the experience of ART specialists of our clinic and literature data. Gardner et al. showed that embryos with ≥3AA grades had the highest probability to give clinical pregnancy [
44]. Additionally, in a recent article Zhao et al. 2018, based on Gardner’s classification, divided the embryos according to morphological characteristics into four classes: excellent (3–6, AA), good (3–6 AB, BA), medium (3–6 BB, AC, CA), poor (1–6, combinations of B and C). It was shown that embryos of excellent and good quality have a close probability of clinical pregnancy (65 and 59%, respectively), while in the middle and poor classes these parameters decreased (50 and 33%, respectively) [
45]. In our study, we assigned some of the embryos of good quality (mentioned above) to an excellent class. We assumed that embryos with similar efficiency should have similar metabolic features; however, the number of cells in the embryo may also affect the quantitative parameters of metabolites in culture media. Unfortunately, the exact number of cells in each morphological group of human embryos is unknown (at least we could not find such data). This is apparently due to the lack of non-invasive evaluation methods and the inability to use many human embryos in experiments. Therefore, we conditionally divided the samples available at the time of the study, as indicated in
Table 1.
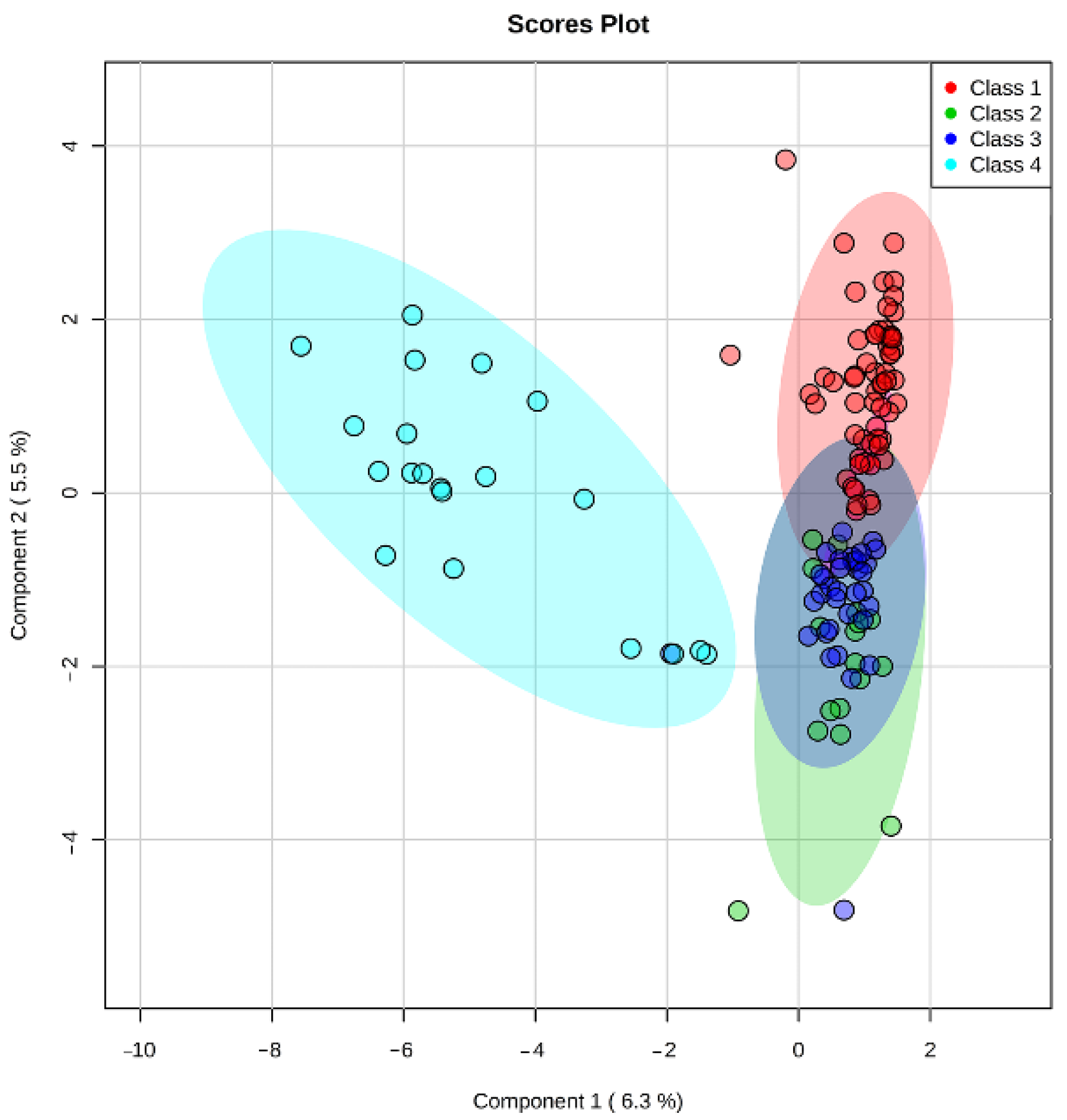
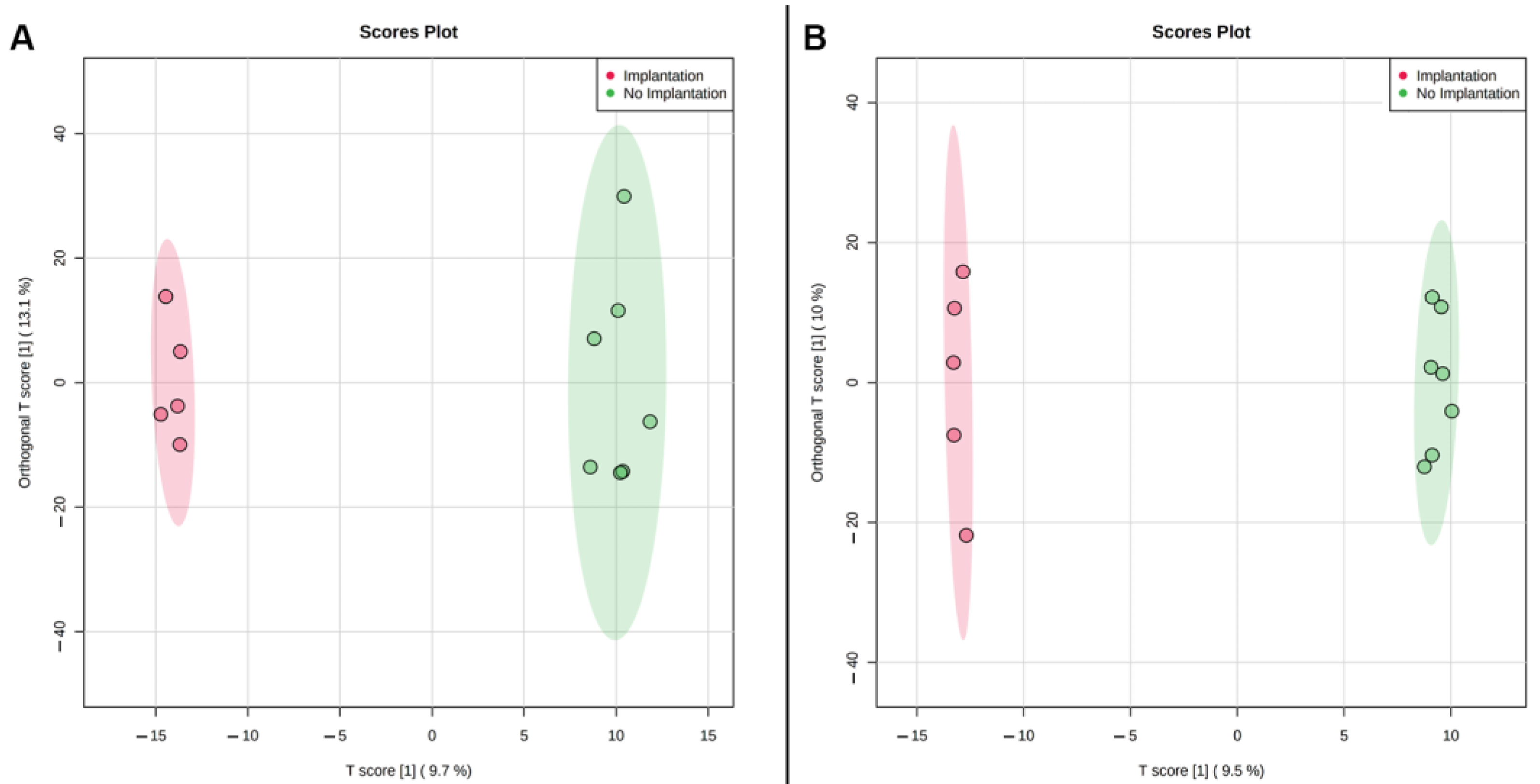
Multivariate PLS-DA statistics were used for intergroup (
Figure 1) and intragroup (
Figure 2) comparative analysis. Intergroup PLS-DA for the samples obtained in continuous protocol showed clustering of ‘excellent’ (Class 1) and ‘poor’ (Class 4) groups of embryos from ‘good’ (Class 2) and ‘fair’ (Class 3) groups that were mostly overlapped. It can also be seen that Class 1 and Class 3 tend to separate while Class 2 overlaps with them. A possible explanation is that Class 2 includes groups of embryos that have a similar number of cells to both Class 1 and Class 3. Since the morphological evaluation by the embryologist does not allow for an accurate cell count of each embryo, this circumstance may also contribute to the observed intersections of clusters of similar morphological groups. This result might indicate that a correlation exists between the embryo’s developmental stage and its metabolic activity. Class 1 embryo is expected to have normal phenotype and thus have optimal metabolic activity, and metabolic rates of Class 2 and 3 embryos may be variable due to different possible origins of their “fair” phenotype and lower cell number, while Class 4 embryo should have the lowest metabolic rates due to significant retardation or the arrest of the development. It may be proposed that in embryos with the smaller cell numbers there is a shift in gene expression leading to the formation of ATP-dependent ion gradients necessary for blastocele formation that takes a large percentage of the embryo’s energy to create and maintain the blastocoel cavity [
46]. In these embryos, this process may follow in expense of the others, such as cell division. Hence the embryo must possess some degree of metabolic competency to arrange its full-fledged development.
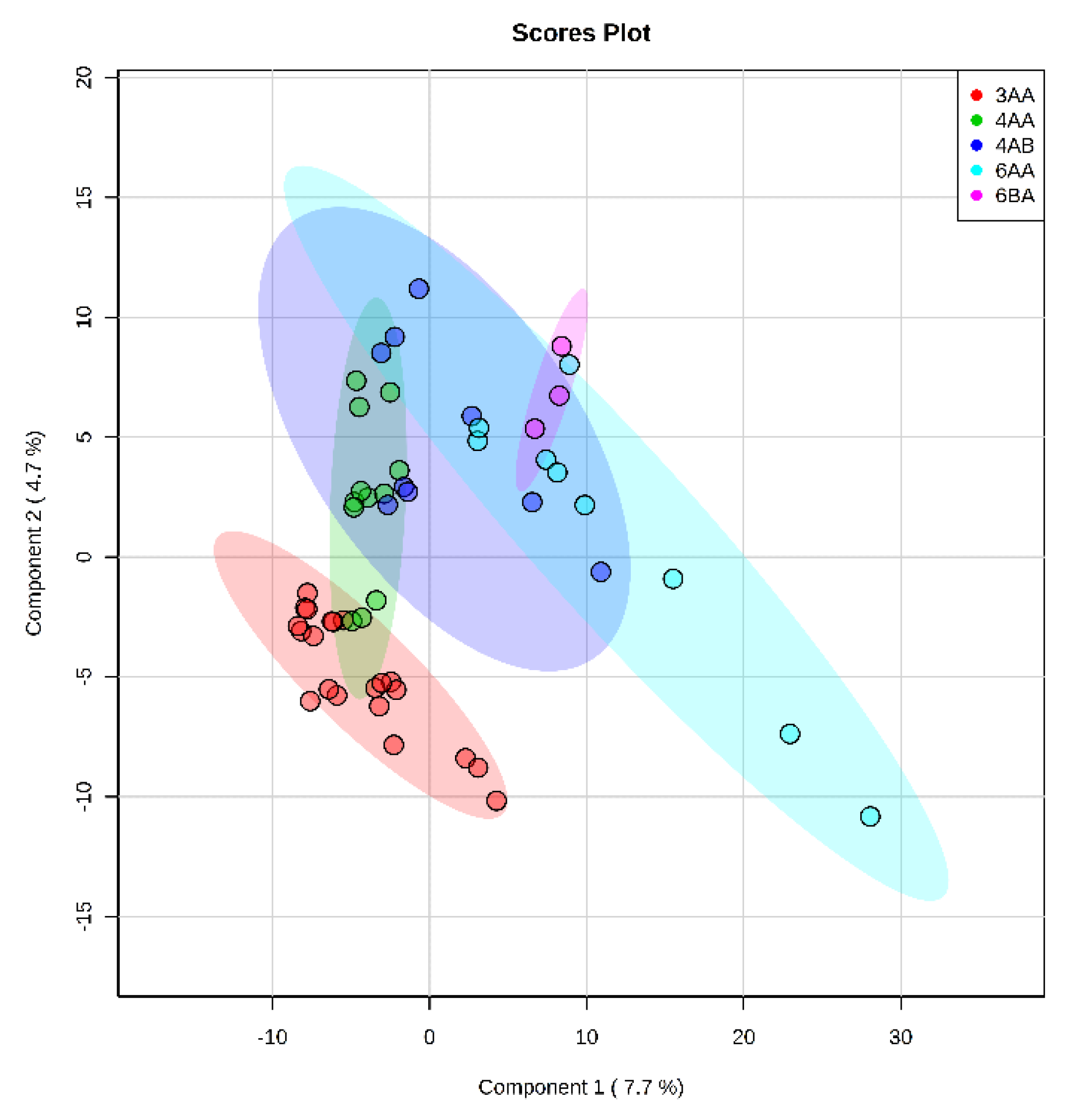
Intragroup comparison made for Class 1 embryos showed that metabolite profiles within the class are quite similar for different grades (
Figure 2). One possible explanation may be that any significant metabolic profile shift would significantly influence the development with a consequent class change. Thus, in Class 1, minor variability is observed, with a slight clustering of type 3AA embryos, which may be associated with a smaller number of cells in this group. Additionally, in 3AA embryos, the metabolic pathways responsible for expansion are just beginning to activate compared to more developed ones. Detected differences between morphological groups indicate the need for careful selection of samples according to the specified criteria since they can have a significant impact on the results of the metabolomic analysis.
The metabolomic profiling of SECM has been done previously by a range of spectroscopic methods including Fourier Transform infrared spectroscopy (FT-IR), near-infrared spectroscopy (NIR), Raman spectroscopy [
47,
48] and NMR [
39]. These studies showed differences in metabolism ratios which correlating with embryo viability and implantation potential [
39,
49,
50,
51,
52]. Seli et al. used NIR spectroscopy and found that viability indices calculated on metabolic profiles of day 2–3 SECM are independent of embryo morphology and correlate with reproductive potential of embryos [
53]. This work received considerable interest and discussion. Subsequent randomized control studies of spent embryo culture media on day 2 and day 5 by NIR spectroscopy in adjunct to morphology did not confirm any significant improvement of ongoing pregnancy rates compared to classic morphology-based criteria [
54]. Another randomized control study of day 3 SECM with NIR spectroscopy also failed to find any improvement in transfer outcome by this combined approach [
55]. Evaluation of the technical design of the experiment revealed that such results were obtained due to limitations of the existing equipment. Additionally, the signal-to-noise ratio threshold was not strong enough for correct viability predictions and noise filtration, which impaired method robustness and reproducibility [
56,
57].
Raman spectroscopy, while being a powerful non-destructive analysis method with high specificity, has relatively low sensitivity, which can limit its usage for analysis of complex biological fluids, such as serum plasma or spent culture media [
58]. Nutrient consumption and metabolite excretion rates even between excellent, fully developed blastocyst and poor-quality morula can differ by a narrow margin, which requires highly sensitive analysis methods. Some highly active compounds, such as hormones or mediators, usually have extremely small concentrations, and their detection is also complicated. Mass-spectrometry coupled with gas or liquid chromatography is an effective and versatile method of analysis, that provides an alternative for spectroscopy-based metabolomic profiling. Cortezzi et al. used direct MS analysis, without chromatographic separation, of the day 3 culture medium with known implantation outcome to predict their implantation potential. The spectra were obtained in negative-ion mode and analyzed by PLS-DA to test the performance of the statistical model on implantation potential prediction. The model correctly identified 100% of positive implantations and 70% of negative ones. The culture media from embryos with positive or negative outcome showed specific biochemical signatures which contribute the most to the difference between groups, though no identification was provided for the detected ions [
59]. While untargeted metabolomics of spent culture media is good for biomarkers search, there is often a need not to discover new, but thoroughly identify and quantify existing biomarkers found by biochemical or molecular methods. Targeted free fatty acid LC-MS profiling revealed a statistically significant decrease of docosahexaenoic acid and an insignificant decrease of other essential free fatty acids in day 6 spent culture from morphologically “good” embryos compared to “poor” ones. Authors suggested targeted metabolomic profiling as a powerful method for non-invasive assessment of embryo quality [
60].
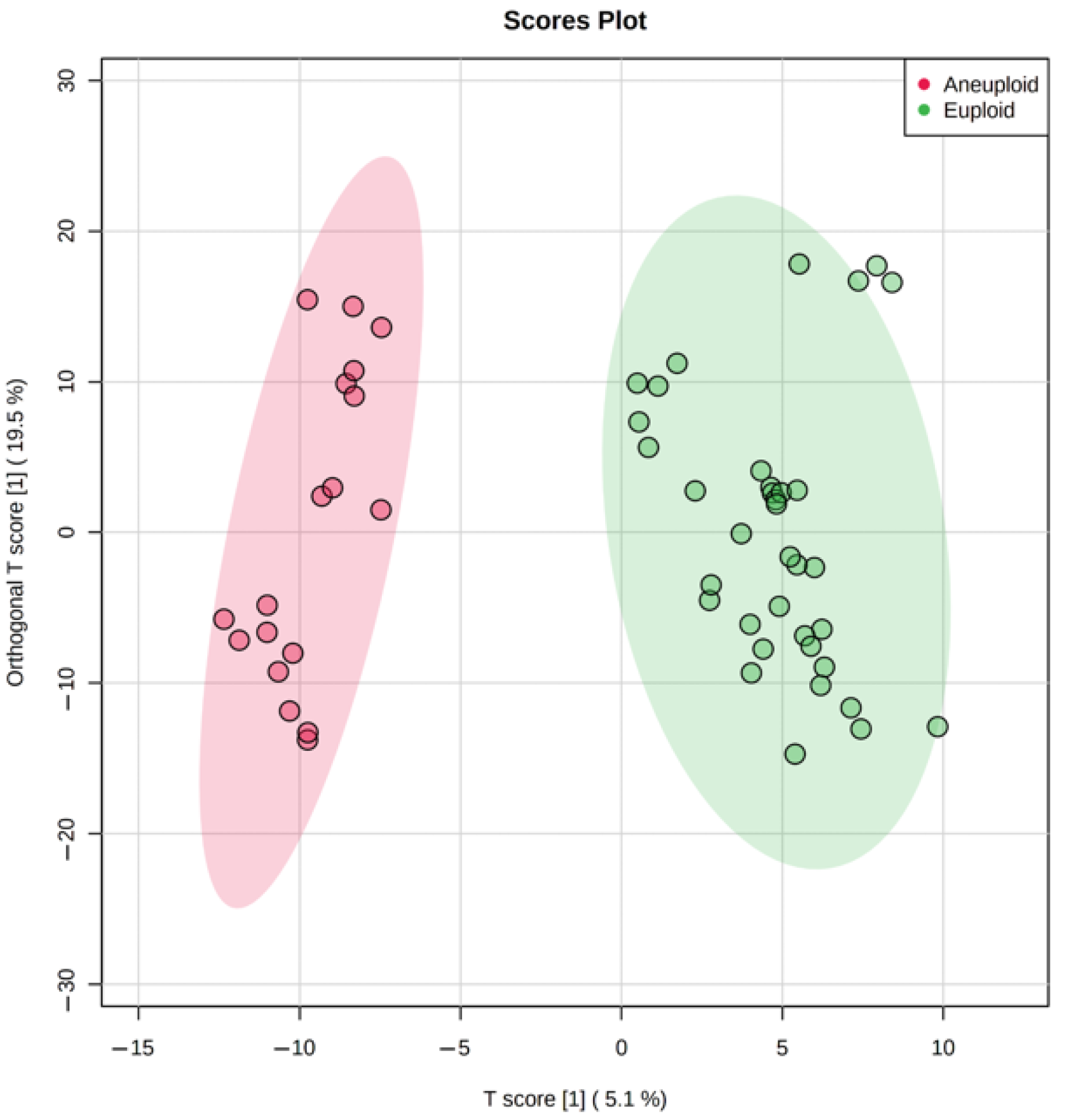
The genetic analysis revealed a substantial amount of Class 1 and 2 embryos with an abnormal karyotype (
Table 1), hence we tested metabolomic profiling as a possible non-invasive alternative for PGT-A. Multivariate statistics (OPLS-DA) has divided culture media samples of the aneuploid group from the normal group within Class 1 (
Figure 3), demonstrating the potential influence of the karyotype on metabolite content. Univariate statistics revealed 23 molecular ions differing between these groups (the fold change > 2). Interestingly, 22 of 23 had increased abundance, probably due to decreased uptake of medium components. Most of these 23 ions were not identified by HMDB search within 10 ppm mass accuracy. It can be assumed that with significant changes in the metabolism of aneuploid embryos, they would not have reached the appropriate stage of development and would not have shown the best phenotype. This may explain the fact that we were not able to detect pronounced changes among the known components of the nutrient media and their possible metabolites. However, multivariate statistics have revealed sets of ions, the cumulative change in the content of which has led to the clustering of groups depending on the karyotype. This suggests that aneuploid embryos may have specific differences in metabolism, and certain molecular signatures can be used to detect aneuploidies.
For the evaluation of the overall metabolic activity of embryos with different karyotypes, all significantly different molecular ions defined by multivariate statistics were mapped to the HMDB database, and metabolites identified were enriched by KEGG biochemical pathways database. Amino acids and fatty acids metabolism pathways were among the most enriched ones, which is fully in accordance with the known importance of amino acid turnover for normal embryo development [
61]. Aneuploidies are known to have a devastating effect on embryonal development, affecting all levels of the organization, and only rare karyotype abnormalities like trisomy of chromosome 21 are tolerable enough to give live birth but associated with multiple organ pathologies and disorders [
62,
63].
Due to the incident nature of the karyotype abnormalities, we combined all types of aneuploidies including monosomy, trisomy, and their combinations in one group and analyzed them against euploid embryos. However, the multivariate statistical analysis still shows excellent clustering of euploid and aneuploid embryos, though the contribution of single metabolites might have been smoothed by sample heterogeneity (
Figure 3). Further studies with samples grouping by individual types of aneuploidies will likely reveal specific features in the composition of metabolites that are characteristic of each karyotype abnormality. Research conducted by Sanchez-Ribas et al. confirms this assumption [
36]. It was shown that several potential metabolites can distinguish trisomy 21 embryos from normal ones although authors had to group ‘pure’ trisomy 21 samples with those having trisomy 21 in combination with other aneuploidies due to extremely rare occurrence of ‘pure’ trisomy 21 [
36]. The preliminary results from another study suggest that metabolomics markers may be sensitive to trisomy 18 and may be able to distinguish trisomy 18 from trisomy 21 [
64]. Raman spectroscopy and multivariate statistics were recently used to detect aneuploid embryos. The method showed results consistent with PGT-A testing performed by next-generation sequencing, achieving 95.9% of correct detections of either euploidy or aneuploidy [
65]. Significant differences in Raman spectra associated with small RNA and lipids were also observed. Thus, metabolomic profiling coupled with targeted metabolomics could be a feasible method for the non-invasive detection of aneuploidies.
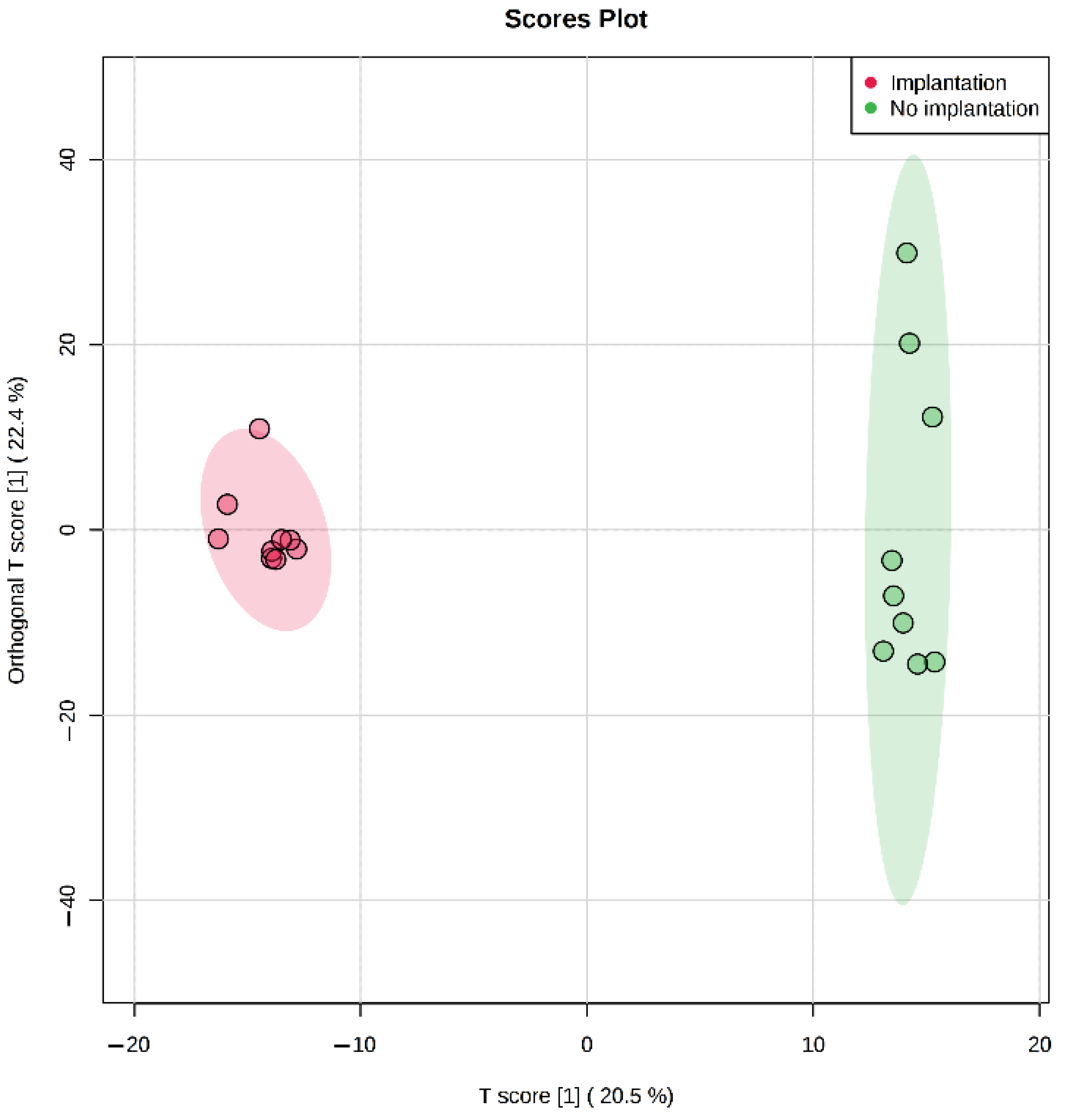
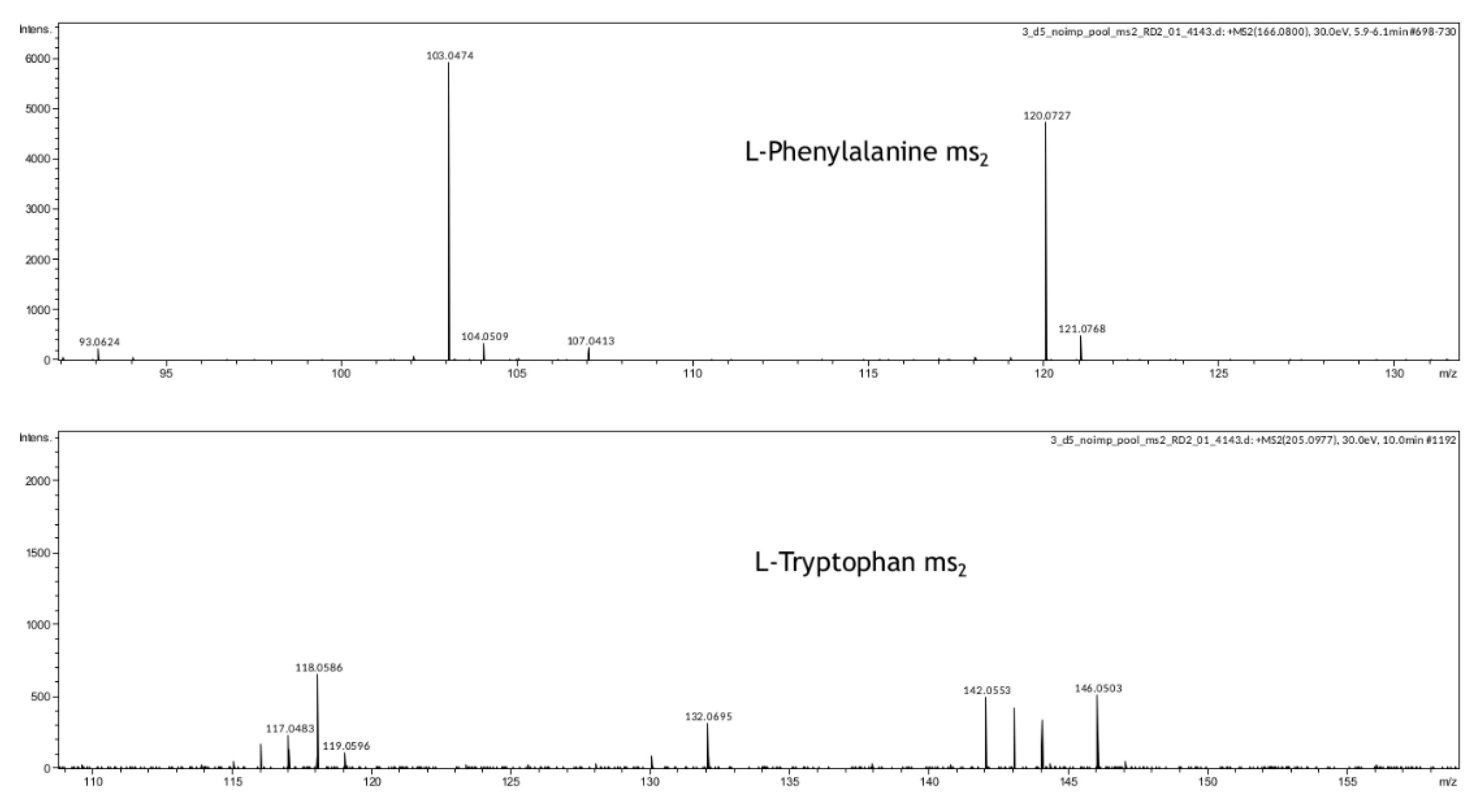
In our study, we have also demonstrated that metabolomic profiling of spent culture media of day 5 embryos from the same morphological class can be used to assess their implantation potential. After five days of incubation molecular ions corresponding to phenylalanine, valine, proline, and tryptophan were found to be decreased in spent culture media from embryos with successful implantation, changes of other amino acid derivatives were observed as well (
Table 4 and
Table S3). Proline, valine, and phenylalanine identifications were confirmed using isotope-labeled standards (
Table S3, Figure S1), phenylalanine and tryptophan were further confirmed by MSn analysis (
Figure 5). This result is in accordance with previous reports of the amino acid turnover rate as a predictor of embryo viability [
34]. It was also shown that differences in the composition of the culture media of the implanted embryos (Class 1) can be detected on the 3rd day of cultivation, as well as on the 5th day after media replacement. In spent culture media of days 3 and 5, 46 and 29 statistically differing molecular ions were found by univariate statistics. No amino acids were identified among the changing features except glutamine. That may be explained by the fact that culture media was refreshed on day 3 in this study, which might negate all accumulated composition differences observed after 5 days of cultivation in continuous protocol. It can be concluded that differences between embryos with distinct implantation potential can be seen starting at least from day 3, which may allow the application of culture medium analysis in different transfer protocols for both fresh and cryopreserved embryos.
Pathway enrichment of metabolites marked as important by multivariate statistics was performed for all implantation outcomes and karyotype comparisons. The most enriched pathways for compounds contributing to karyotype differences were related to the pentose phosphate pathway, vitamin B6, and amino acid metabolism pathways. Glucose-dependent nucleotide synthesis by the pentose phosphate pathway was found to control activation pathway for trophectoderm specific gene transcription during mammalian embryogenesis [
66]. Amino acids turnover and high levels of vitamins A and B6 were previously reported to be associated with aneuploidies, embryo quality, and morphokinetics [
61,
67]. The enrichment of amino acid and vitamin B6 metabolism pathways, as well as lipid-associated pathways, were also observed (
Table 5,
Table 6 and
Table 7) in culture media of embryos with successful or unsuccessful implantation. Drabkova et al. showed that there were not any significant changes in media amino acid composition in day 3 SECM. Additionally, positive correlations with glutamine and negative with glutamate concentration were reported [
68]. Our findings also show increased glutamine depletion in day 3 medium from embryos successfully implanted later (
Table S4). Amino acids are the key components of a variety of pathways, even those not directly involved into amino acid biosynthesis or degradation.
Earlier studies of lipid effects on embryo quality revealed a significant impact of fatty acids levels on embryo development [
69,
70]. Fatty acid derivatives such as eicosanoids may be produced on the blastocyst–endometrium interface and seem to be one of the key factors of the decidualization and implantation process [
71]. Our results indicate that lipid metabolism may play significant role during embryo development and specific lipid compound sets should be investigated as potential biomarkers for karyotype and embryo assessment.
In a recent study, Harden S.L. et al. investigated the exometabolome of decidualizing human stromal cells in culture and concluded that the metabolic footprints of cellular microenvironment generated by different decidual subpopulations encode spatiotemporal information that may be important for their differentiation and optimal embryo implantation. It has been shown that in all investigated timepoints of decidualization the most enriched metabolic pathways of stromal cells exometabolome are phenylalanine, tyrosine and tryptophan biosynthesis, valine, leucine, and isoleucine biosynthesis, glycerophospholipid metabolism, linoleic acid metabolism, purine and pyrimidine metabolism [
72]. It is interesting to note, that the similar pathways were enriched after analysis of day 5 human embryo exometabolome in our study. It is intriguing to propose that these pathways and their secreted metabolites are necessary for the embryonal and endometrial cells to form complementary microenvironment that guide the initial steps of the implantation. Together, these findings support the importance of found molecular signatures as possible markers of successful implantation. Thus, metabolomic profiling of spent culture media may be a promising method in combination with morphological analysis as it provides additional information on embryo quality. Even two morphologically identical embryos may have different metabolic activity and thus implantation potential, or class 2 embryo may have worth morphology but far better metabolic rate. This fact may be crucial for successful eSET, especially with increasing age, when the number of available embryos is limited.
It should be noted that not all successful implants develop into a full-fledged pregnancy and result in delivery. This may be due to different factors such as incorrect localization of the implanted embryo, abnormalities of the placenta development, and fetus gestation [
73]. Therefore, after embryo transfer long-term monitoring and detailed analysis of pregnancy outcomes are necessary for further method development. Another limitation of the method may be the small volume of the probe, which makes precise identification and quantification of low abundant metabolites a challenging task. The method we used showed good results in aneuploidies detection, but we pulled all aneuploidy types to one group because of the rare occurrence of such cases. A longer collection period and more test samples for each type of aneuploidy are required for method refinement and validation. Additionally, we understand that our data was obtained using one type of the commercial culture media provided on the market for human embryo cultivation, thus any differences in composition and nutrient ratio (which are known to exist and often not evident due to unavailable media formulations) may lead to the specific metabolic shifts that in turn will change features of metabolomic profile. Thus, to obtain the set of “universal” biomarkers of embryo quality it seems reasonable to evaluate the difference between metabolomic profiles according to corresponding culture conditions.
In conclusion, our data suggest that the application of HPLC-MS for metabolomic analysis can be used as a powerful, sensitive, and versatile method that may provide valuable data both for embryo karyotype and implantation potential assessment. Further implementation of targeted metabolomics in combination with time-lapse morphology analysis may significantly improve the positive outcome ratio after embryo transfer.