Pediococcus acidilactici CECT9879 (pA1c) Counteracts the Effect of a High-Glucose Exposure in C. elegans by Affecting the Insulin Signaling Pathway (IIS)
Abstract
1. Introduction
2. Results and Discussion
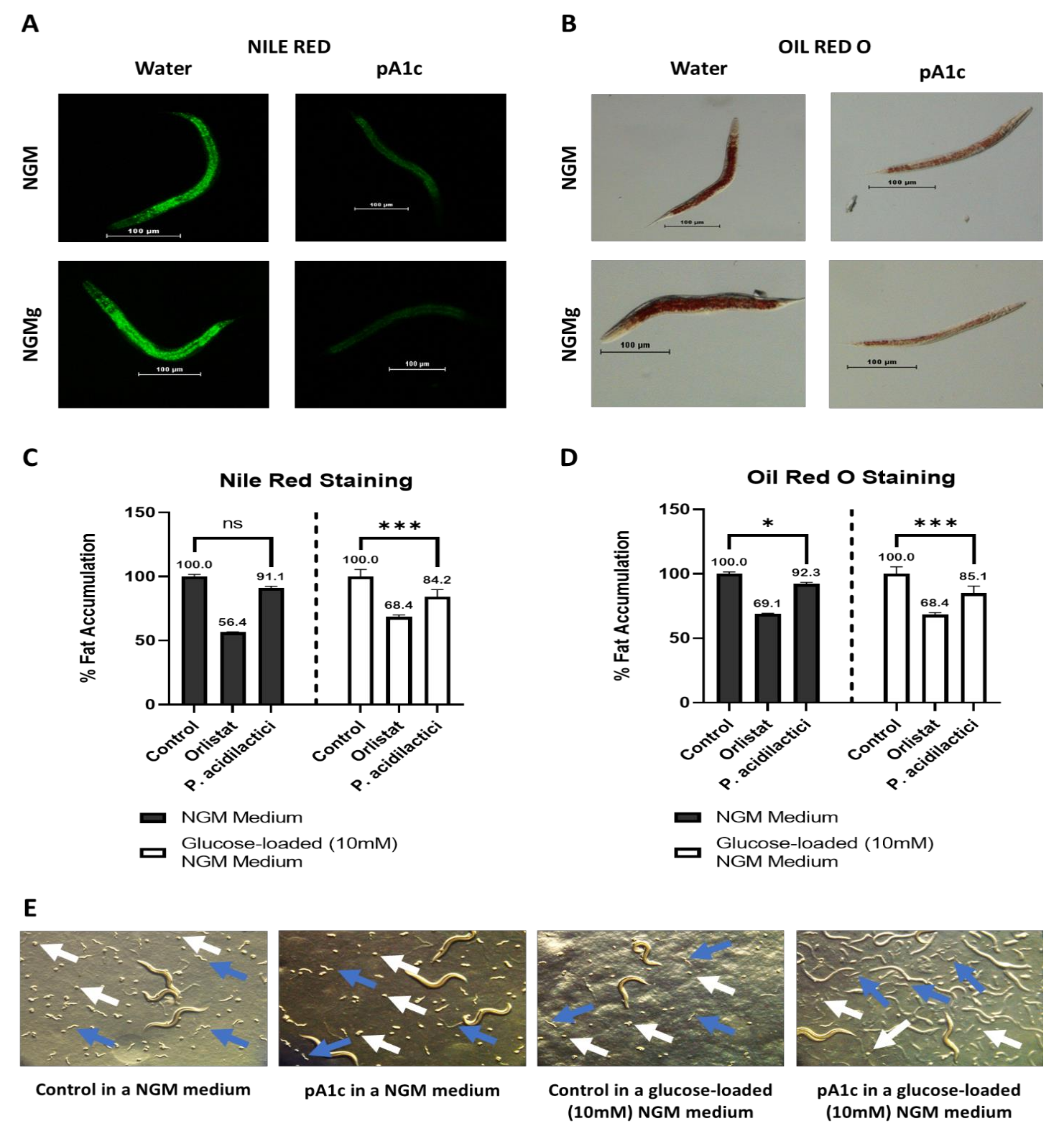
2.1. pA1c Reduces Fat Accumulation in C. elegans
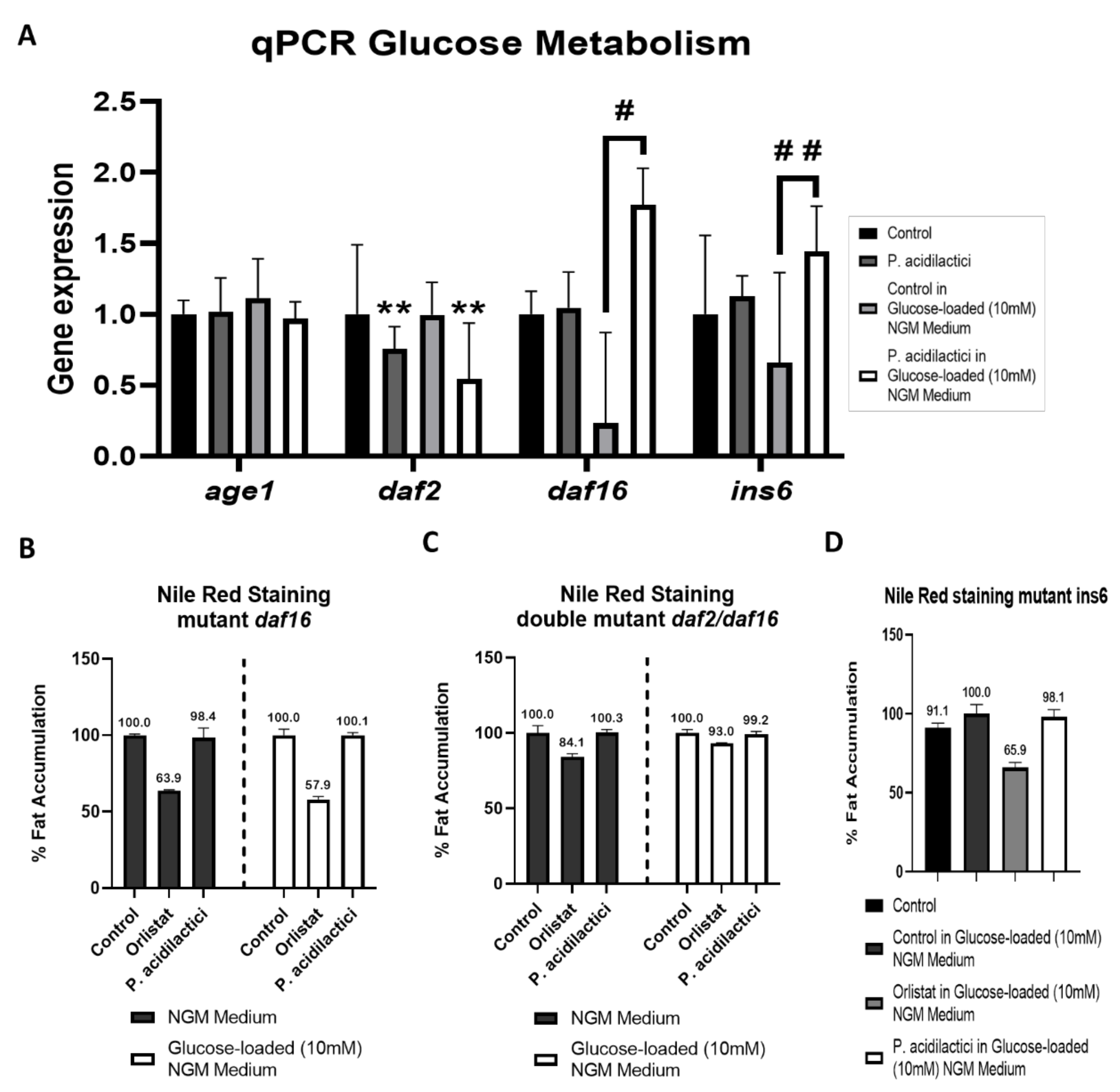
2.2. pA1c Modulates the Insulin Signaling Pathway in C. elegans
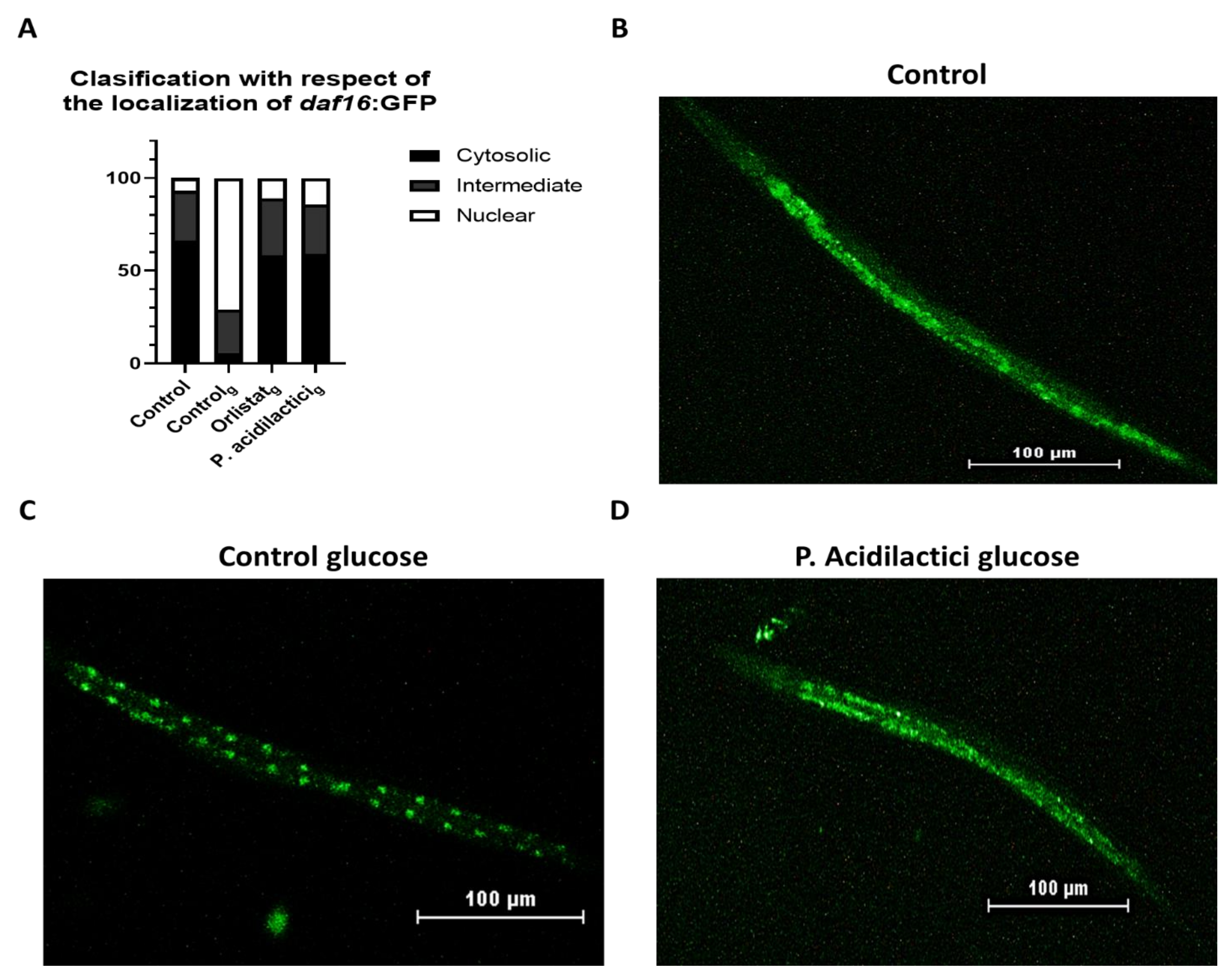
2.3. pA1c Inhibits the High-Glucose-Induced Nuclear Translocation of Daf-16
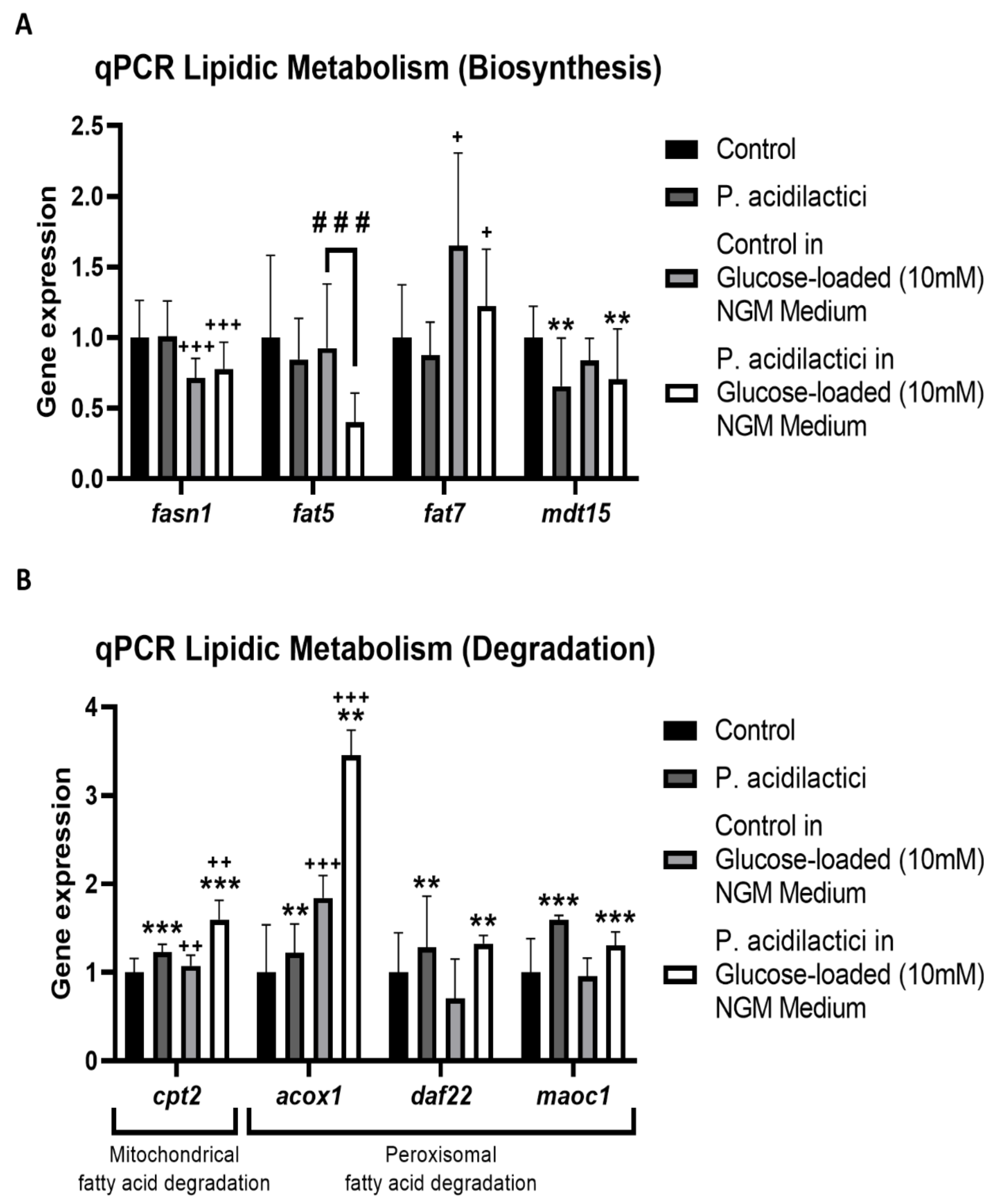
2.4. pA1c Modulates the Fatty Acid Metabolic Pathway in C. elegans
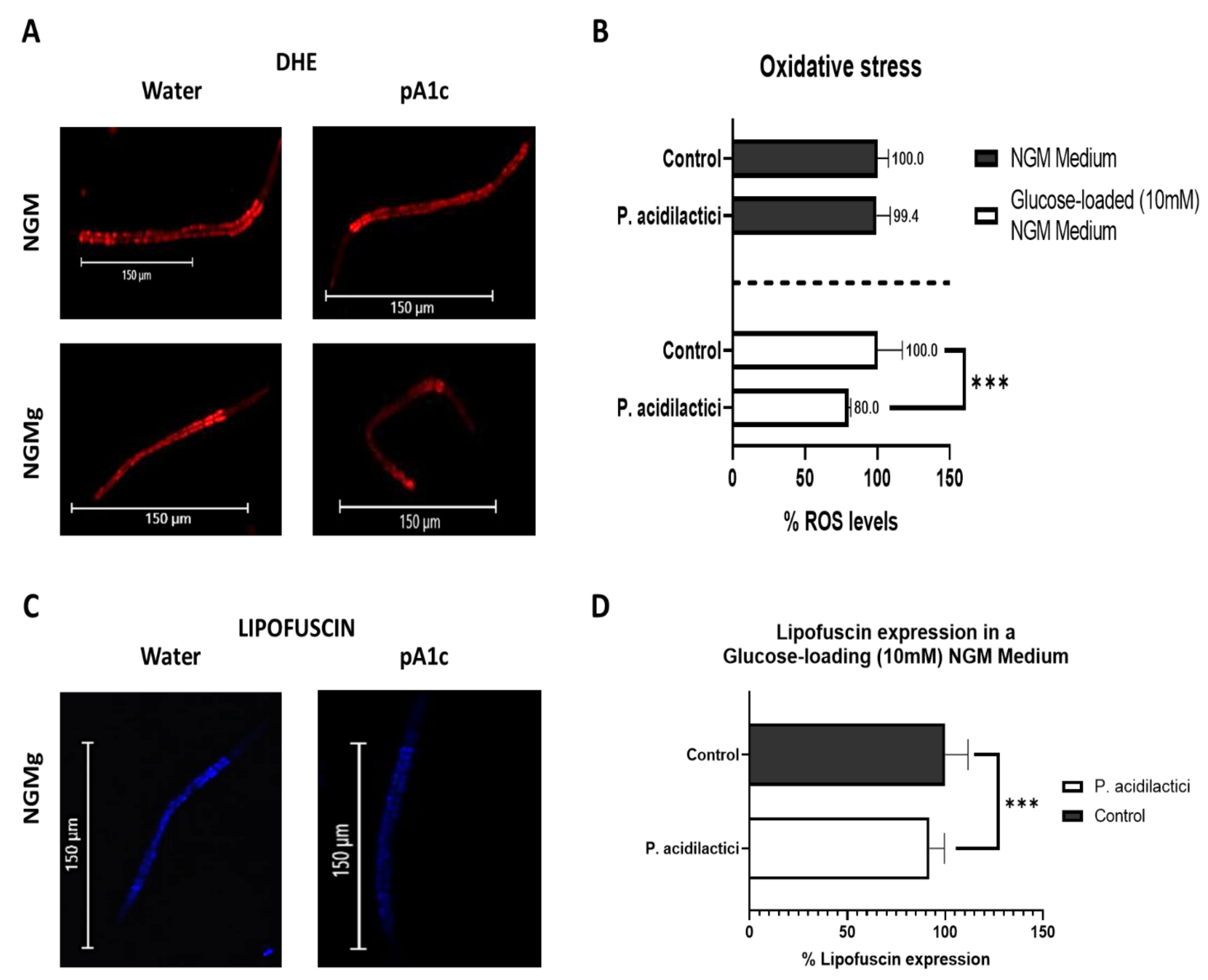
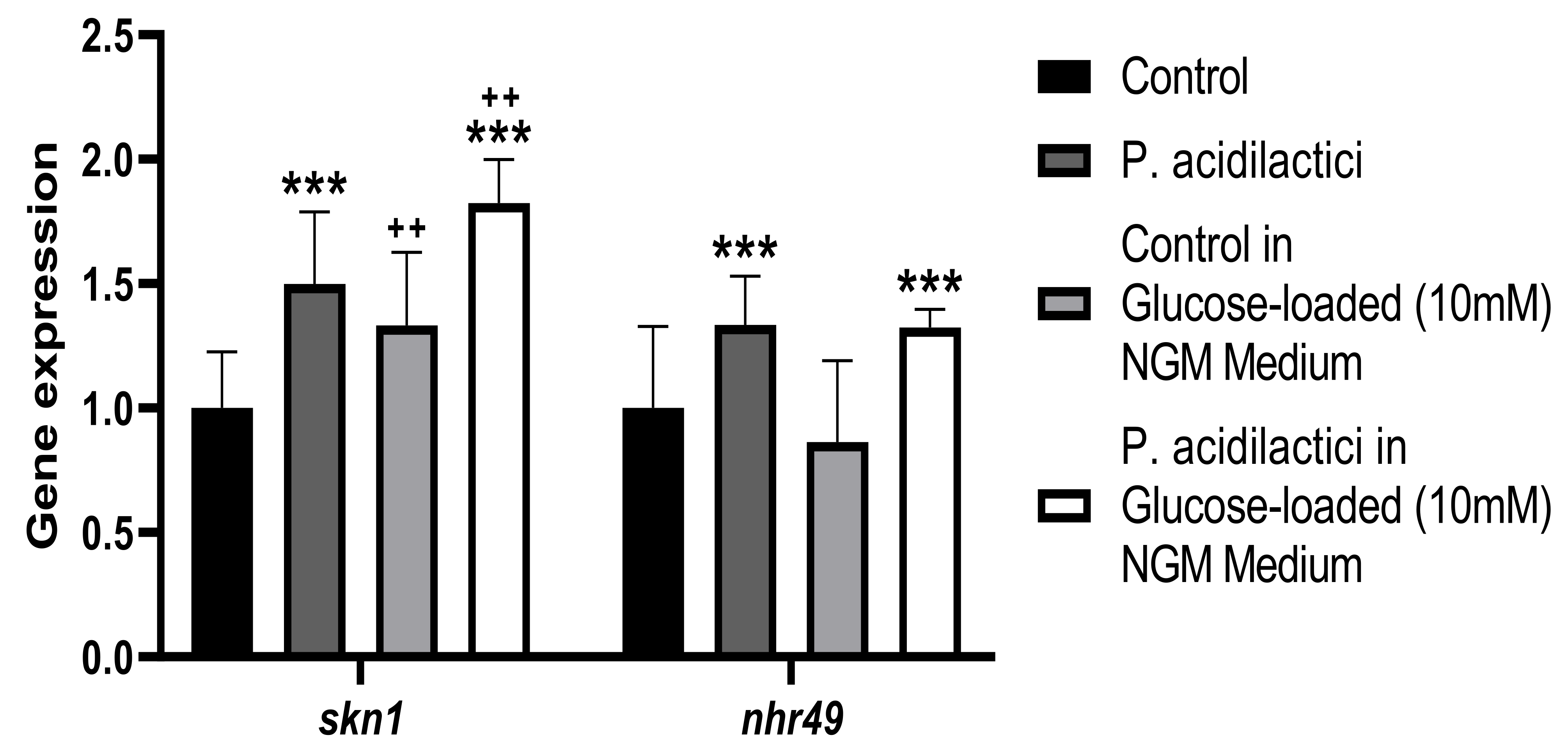
2.5. Supplementation with pA1c Enhanced Stress Oxidative Response by Reducing Reactive Oxygen Species (ROS), Improved Aging and Increased Lifespan in C. elegans
3. Matherial and Methods
3.1. Strains and Culture
3.2. Experimental Design
3.3. Nile Red Staining
3.4. Oil Red O (ORO) Staining
3.5. DHE Staining
3.6. C. elegans Aging Visualization
3.7. Daf-16:GFP Assay
3.8. Image Acquisition and Quantification
3.9. Lifespan Analysis
3.10. Egg Lying
3.11. RNA Extraction and a Quantitative PCR Analyses
3.12. Statistical Analysis
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Mayoral, L.P.C.; Andrade, G.M.; Mayoral, E.P.C.; Huerta, T.H.; Canseco, S.P.; Rodal Canales, F.J.; Cabrera-Fuentes, H.A.; Cruz, M.M.; Pérez Santiago, A.D.; Alpuche, J.J.; et al. Obesity subtypes, related biomarkers & heterogeneity. Indian J. Med. Res. 2020, 151, 11. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A. Evaluation of obesity. Who are the obese? Postgrad. Med. 2003, 114, 19–38. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Sánchez, A.; Madrigal-Santillán, E.; Bautista, M.; Esquivel-Soto, J.; Morales-González, Á.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sánchez-Rivera, G.; Valadez-Vega, C.; Morales-González, J.A. Inflammation, Oxidative Stress, and Obesity. Int. J. Mol. Sci. 2011, 12, 3117. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, R.; Azevedo, I. Chronic Inflammation in Obesity and the Metabolic Syndrome. Mediat. Inflamm. 2010, 2010, 289645. [Google Scholar] [CrossRef] [PubMed]
- Aranaz, P.; Navarro-Herrera, D.; Zabala, M.; Romo-Hualde, A.; López-Yoldi, M.; Vizmanos, J.L.; Milagro, F.I.; González-Navarro, C.J. Phenolic Compounds Reduce the Fat Content in Caenorhabditis elegans by Affecting Lipogenesis, Lipolysis, and Different Stress Responses. Pharmaceuticals 2020, 13, 355. [Google Scholar] [CrossRef] [PubMed]
- Ortega, F.B.; Lavie, C.J.; Blair, S.N. Obesity and Cardiovascular Disease. Circ. Res. 2016, 118, 1752–1770. [Google Scholar] [CrossRef]
- Jokinen, E. Obesity and cardiovascular disease. Minerva Pediatr. 2015, 67, 25–32. [Google Scholar] [PubMed]
- Koliaki, C.; Liatis, S.; Kokkinos, A. Obesity and cardiovascular disease: Revisiting an old relationship. Metabolism 2019, 92, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Piché, M.E.; Tchernof, A.; Després, J.P. Obesity Phenotypes, Diabetes and Cardiovascular Diseases. Circ. Res. 2020, 126, 1477–1500. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.G.; Cook, D.A.; Clark, M.M.; Bardia, A.; Levine, J.A. Treatment of Obesity. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2007; Volume 82, pp. 93–102. [Google Scholar] [CrossRef]
- Camilleri, M.; Acosta, A. Combination Therapies for Obesity. Metab. Syndr. Relat. Disord. 2018, 16, 390. [Google Scholar] [CrossRef] [PubMed]
- Gérard, P. Gut microbiota and obesity. Cell. Mol. Life Sci. 2015, 73, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.C.; Hoffmann, C.; Mota, J.F. The human gut microbiota: Metabolism and perspective in obesity. Gut Microbes 2018, 9, 308. [Google Scholar] [CrossRef] [PubMed]
- Saad, M.J.A.; Santos, A.; Prada, P.O. Linking gut microbiota and inflammation to obesity and insulin resistance. Physiology 2016, 31, 283–293. [Google Scholar] [CrossRef]
- Abenavoli, L.; Scarpellini, E.; Colica, C.; Boccuto, L.; Salehi, B.; Sharifi-Rad, J.; Aiello, V.; Romano, B.; De Lorenzo, A.; Izzo, A.A.; et al. Gut Microbiota and Obesity: A Role for Probiotics. Nutrients 2019, 11, 2690. [Google Scholar] [CrossRef] [PubMed]
- Fontané, L.; Benaiges, D.; Goday, A.; Llauradó, G.; Pedro-Botet, J. Influence of the microbiota and probiotics in obesity. Clínica Investig. Arterioscler. 2018, 30, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Cerdó, T.; García-Santos, J.A.; Bermúdez, M.G.; Campoy, C. The Role of Probiotics and Prebiotics in the Prevention and Treatment of Obesity. Nutrients 2019, 11, 635. [Google Scholar] [CrossRef] [PubMed]
- Mazloom, K.; Siddiqi, I.; Covasa, M. Probiotics: How Effective Are They in the Fight against Obesity? Nutrients 2019, 11, 258. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, G.A.; Ashrafi, K. Insights and challenges in using C. elegans for investigation of fat metabolism. Crit. Rev. Biochem. Mol. Biol. 2015, 50, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Mullaney, B.C.; Ashrafi, K.C. elegans Fat Storage and Metabolic Regulation. Biochim. Biophys. Acta 2009, 1791, 474. [Google Scholar] [CrossRef]
- Franco-Juárez, B.; Gómez-Manzo, S.; Hernández-Ochoa, B.; Cárdenas-Rodríguez, N.; Arreguin-Espinosa, R.; de la Cruz, V.P.; Ortega-Cuellar, D. Effects of High Dietary Carbohydrate and Lipid Intake on the Lifespan of C. elegans. Cells 2021, 10, 2359. [Google Scholar] [CrossRef] [PubMed]
- Cabello-Olmo, M.; Oneca, M.; Pajares, M.J.; Jiménez, M.; Ayo, J.; Encío, I.J.; Barajas, M.; Araña, M. Antidiabetic Effects of Pediococcus acidilactici pA1c on HFD-Induced Mice. Nutrients 2022, 14, 692. [Google Scholar] [CrossRef]
- Escorcia, W.; Ruter, D.L.; Nhan, J.; Curran, S.P. Quantification of Lipid Abundance and Evaluation of Lipid Distribution in Caenorhabditis elegans by Nile Red and Oil Red O Staining. J. Vis. Exp. 2018, 2018, 57352. [Google Scholar] [CrossRef] [PubMed]
- Roselli, M.; Schifano, E.; Guantario, B.; Zinno, P.; Uccelletti, D.; Devirgiliis, C. Caenorhabditis elegans and Probiotics Interactions from a Prolongevity Perspective. Int. J. Mol. Sci. 2019, 20, 5020. [Google Scholar] [CrossRef]
- Martorell, P.; Llopis, S.; González, N.; Montón, F.; Ortiz, P.; Genovés, S.; Ramón, D. Caenorhabditis elegans as a Model to Study the Effectiveness and Metabolic Targets of Dietary Supplements Used for Obesity Treatment: The Specific Case of a Conjugated Linoleic Acid Mixture (Tonalin). J. Agric. Food Chem. 2012, 60, 11071–11079. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Christensen, K.B.; Olsen, L.C.B.; Christensen, L.P.; Grevsen, K.; Færgeman, N.J.; Kristiansen, K.; Young, J.F.; Oksbjerg, N. Bioactive Components from Flowers of Sambucus nigra L. Increase Glucose Uptake in Primary Porcine Myotube Cultures and Reduce Fat Accumulation in Caenorhabditis elegans. J. Agric. Food Chem. 2013, 61, 11033–11040. [Google Scholar] [CrossRef]
- Lin, Y.; Yang, N.; Bao, B.; Wang, L.; Chen, J.; Liu, J. Luteolin reduces fat storage in Caenorhabditis elegans by promoting the central serotonin pathway. Food Funct. 2020, 11, 730–740. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Wei, Z.; Luo, H.; Yang, Y.; Wu, Z.; Gan, L.; Yang, X. Inhibition of Fat Accumulation by Hesperidin in Caenorhabditis elegans. J. Agric. Food Chem. 2016, 64, 5207–5214. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Shen, P.; Chang, A.L.; Qi, W.; Kim, K.H.; Kim, D.; Park, Y. trans-Trismethoxy resveratrol decreased fat accumulation dependent on fat-6 and fat-7 in Caenorhabditis elegans. Food Funct. 2019, 10, 4966–4974. [Google Scholar] [CrossRef]
- Shen, P.; Zhang, R.; McClements, D.J.; Park, Y. Nanoemulsion-based delivery systems for testing nutraceutical efficacy using Caenorhabditis elegans: Demonstration of curcumin bioaccumulation and body-fat reduction. Food Res. Int. 2019, 120, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Herrera, D.; Aranaz, P.; Eder-Azanza, L.; Zabala, M.; Hurtado, C.; Romo-Hualde, A.; Martínez, J.A.; González-Navarro, C.J.; Vizmanos, J.L. Dihomo-gamma-linolenic acid induces fat loss in C. elegans in an omega-3-independent manner by promoting peroxisomal fatty acid β-oxidation. Food Funct. 2018, 9, 1621–1637. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Herrera, D.; Aranaz, P.; Eder-Azanza, L.; Zabala, M.; Romo-Hualde, A.; Hurtado, C.; Calavia, D.; López-Yoldi, M.; Martínez, J.A.; González-Navarro, C.J.; et al. Borago officinalis seed oil (BSO), a natural source of omega-6 fatty acids, attenuates fat accumulation by activating peroxisomal beta-oxidation both in C. elegans and in diet-induced obese rats. Food Funct. 2018, 9, 4340–4351. [Google Scholar] [CrossRef]
- Silva, Á.; Gonzalez, N.; Terrén, A.; García, A.; Martinez-Blanch, J.F.; Illescas, V.; Morales, J.; Maroto, M.; Genovés, S.; Ramón, D.; et al. An Infant Milk Formula Supplemented with Heat-Treated Probiotic Bifidobacterium animalis subsp. lactis CECT 8145, Reduces Fat Deposition in C. elegans and Augments Acetate and Lactate in a Fermented Infant Slurry. Foods 2020, 9, 652. [Google Scholar] [CrossRef] [PubMed]
- Martorell, P.; Llopis, S.; González, N.; Chenoll, E.; López-Carreras, N.; Aleixandre, A.; Chen, Y.; Karoly, E.D.; Ramón, D.; Genovés, S. Probiotic strain Bifidobacterium animalis subsp. lactis CECT 8145 reduces fat content and modulates lipid metabolism and antioxidant response in Caenorhabditis elegans. J. Agric. Food Chem. 2016, 64, 3462–3472. [Google Scholar] [CrossRef] [PubMed]
- Balaguer, F.; Enrique, M.; Llopis, S.; Barrena, M.; Navarro, V.; Álvarez, B.; Chenoll, E.; Ramón, D.; Tortajada, M.; Martorell, P. Lipoteichoic acid from Bifidobacterium animalis subsp. lactis BPL1: A novel postbiotic that reduces fat deposition via IGF-1 pathway. Microb. Biotechnol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.T.; Hu, P.J. Insulin/insulin-like growth factor signaling in C. elegans. WormBook 2013, 1–43. [Google Scholar] [CrossRef]
- Das, D.; Arur, S. Conserved insulin signaling in the regulation of oocyte growth, development, and maturation. Mol. Reprod. Dev. 2017, 84, 444–459. [Google Scholar] [CrossRef]
- Kenyon, C.; Chang, J.; Gensch, E.; Rudner, A.; Tabtiang, R. A C. elegans mutant that lives twice as long as wild type. Nature 1993, 366, 461–464. [Google Scholar] [CrossRef]
- Dillin, A.; Crawford, D.K.; Kenyon, C. Timing requirements for insulin/IGF-1 signaling in C. elegans. Science 2002, 298, 830–834. [Google Scholar] [CrossRef]
- Ayyadevara, S.; Alla, R.; Thaden, J.J.; Shmookler Reis, R.J. Remarkable longevity and stress resistance of nematode PI3K-null mutants. Aging Cell 2008, 7, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, K.; Chang, F.Y.; Watts, J.L.; Fraser, A.G.; Kamath, R.S.; Ahringer, J.; Ruvkun, G. Genome-wide RNAi analysis of Caenorhabditis elegans fat regulatory genes. Nature 2003, 421, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Perez, C.L.; Van Gilst, M.R. A 13C isotope labeling strategy reveals the influence of insulin signaling on lipogenesis in C. elegans. Cell Metab. 2008, 8, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Horikawa, M.; Sakamoto, K. Polyunsaturated fatty acids are involved in regulatory mechanism of fatty acid homeostasis via daf-2/insulin signaling in Caenorhabditis elegans. Mol. Cell. Endocrinol. 2010, 323, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.; Choi, E.; Lee, D.; Jeong, D.E.; Jang, S.K.; Lee, S.J. Heat shock factor 1 mediates the longevity conferred by inhibition of TOR and insulin/IGF-1 signaling pathways in C. elegans. Aging Cell 2013, 12, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Ayuda-Durán, B.; González-Manzano, S.; Miranda-Vizuete, A.; Dueñas, M.; Santos-Buelga, C.; González-Paramás, A.M. Epicatechin modulates stress-resistance in C. elegans via insulin/IGF-1 signaling pathway. PLoS ONE 2019, 14, e0199483. [Google Scholar] [CrossRef]
- Scott, B.A.; Avidan, M.S.; Crowder, C.M. Regulation of hypoxic death in C. elegans by the insulin/IGF receptor homolog DAF-2. Science 2002, 296, 2388–2391. [Google Scholar] [CrossRef]
- Kaletsky, R.; Lakhina, V.; Arey, R.; Williams, A.; Landis, J.; Ashraf, J.; Murphy, C.T. The C. elegans adult neuronal IIS/FOXO transcriptome reveals adult phenotype regulators. Nature 2016, 529, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Greenway, F.L. Caenorhabditis elegans as a model for obesity research. Int. J. Obes. 2012, 36, 186–194. [Google Scholar] [CrossRef]
- Shen, P.; Yue, Y.; Zheng, J.; Park, Y. Caenorhabditis elegans: A Convenient In Vivo Model for Assessing the Impact of Food Bioactive Compounds on Obesity, Aging, and Alzheimer’s Disease. Annu. Rev. Food Sci. Technol. 2018, 9, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Tissenbaum, H.A. DAF-16: FOXO in the Context of C. elegans. Curr. Top. Dev. Biol. 2018, 127, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Son, H.G.; Jung, Y.; Lee, S.J.V. The role of dietary carbohydrates in organismal aging. Cell. Mol. Life Sci. 2017, 74, 1793–1803. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Murphy, C.T.; Kenyon, C. Glucose Shortens the Lifespan of Caenorhabditis elegans by Down-Regulating Aquaporin Gene Expression. Cell Metab. 2009, 10, 379. [Google Scholar] [CrossRef] [PubMed]
- Henderson, S.T.; Johnson, T.E. daf-16 integrates developmental and environmental inputs to mediate aging in the nematode Caenorhabditis elegans. Curr. Biol. 2001, 11, 1975–1980. [Google Scholar] [CrossRef]
- Chauhan, A.P.; Chaubey, M.G.; Patel, S.N.; Madamwar, D.; Singh, N.K. Extension of life span and stress tolerance modulated by DAF-16 in Caenorhabditis elegans under the treatment of Moringa oleifera extract. 3 Biotech 2020, 10, 504. [Google Scholar] [CrossRef] [PubMed]
- Franco-Juárez, B.; Mejía-Martínez, F.; Moreno-Arriola, E.; Hernández-Vázquez, A.; Gómez-Manzo, S.; Marcial-Quino, J.; Arreguín-Espinosa, R.; Velázquez-Arellano, A.; Ortega-Cuellar, D. A high glucose diet induces autophagy in a HLH-30/TFEB-dependent manner and impairs the normal lifespan of C. elegans. Aging 2018, 10, 2657–2667. [Google Scholar] [CrossRef] [PubMed]
- Buranasin, P.; Mizutani, K.; Iwasaki, K.; Mahasarakham, C.P.N.; Kido, D.; Takeda, K.; Izumi, Y. High glucose-induced oxidative stress impairs proliferation and migration of human gingival fibroblasts. PLoS ONE 2018, 13, e0201855. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.; Montalbano, A.; Wood, M.P.; Schisa, J.A. Biphasic adaptation to osmotic stress in the C. elegans germ line. Am. J. Physiol. Cell Physiol. 2017, 312, C741–C748. [Google Scholar] [CrossRef] [PubMed]
- Watts, J.L.; Ristow, M. Lipid and Carbohydrate Metabolism in Caenorhabditis elegans. Genetics 2017, 207, 413. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Ikita, K.; Ashida, T.; Motoyama, Y.; Yamaguchi, Y.; Satouchi, K. Effects of growth temperature on the fatty acid composition of the free-living nematode Caenorhabditis elegans. Lipids 1996, 31, 1173–1178. [Google Scholar] [CrossRef]
- Watts, J.L.; Browse, J. Genetic dissection of polyunsaturated fatty acid synthesis in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2002, 99, 5854–5859. [Google Scholar] [CrossRef]
- Shen, P.; Yue, Y.; Kim, K.H.; Park, Y. Piceatannol Reduces Fat Accumulation in Caenorhabditis elegans. J. Med. Food 2017, 20, 887–894. [Google Scholar] [CrossRef]
- Yue, Y.; Hao, G.; Cho, J.; Park, Y. Curcumin reduced fat accumulation in Caenorhabditis elegans. Curr. Res. Food Sci. 2021, 4, 551–556. [Google Scholar] [CrossRef]
- Ling, Y.; Teng, L.L.; Hua, J.; Li, D.S.; Luo, S.H.; Liu, Y.C.; Liu, Y.; Li, S.H. Leucosceptroid B from glandular trichomes of Leucosceptrum canum reduces fat accumulation in Caenorhabditis elegans through suppressing unsaturated fatty acid biosynthesis. Chin. J. Nat. Med. 2019, 17, 892–899. [Google Scholar] [CrossRef]
- Taubert, S.; Van Gilst, M.R.; Hansen, M.; Yamamoto, K.R. A Mediator subunit, MDT-15, integrates regulation of fatty acid metabolism by NHR-49-dependent and -independent pathways in C. elegans. Genes Dev. 2006, 20, 1137–1149. [Google Scholar] [CrossRef] [PubMed]
- Farias-Pereira, R.; Savarese, J.; Yue, Y.; Lee, S.H.; Park, Y. Fat-lowering effects of isorhamnetin are via NHR-49-dependent pathway in Caenorhabditis elegans. Curr. Res. Food Sci. 2019, 2, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef] [PubMed]
- Marsova, M.; Poluektova, E.; Odorskaya, M.; Ambaryan, A.; Revishchin, A.; Pavlova, G.; Danilenko, V. Protective effects of Lactobacillus fermentum U-21 against paraquat-induced oxidative stress in Caenorhabditis elegans and mouse models. World J. Microbiol. Biotechnol. 2020, 36, 104. [Google Scholar] [CrossRef] [PubMed]
- Achuthan, A.A.; Duary, R.K.; Madathil, A.; Panwar, H.; Kumar, H.; Batish, V.K.; Grover, S. Antioxidative potential of lactobacilli isolated from the gut of Indian people. Mol. Biol. Rep. 2012, 39, 7887–7897. [Google Scholar] [CrossRef] [PubMed]
- Grompone, G.; Martorell, P.; Llopis, S.; González, N.; Genovés, S.; Mulet, A.P.; Fernández-Calero, T.; Tiscornia, I.; Bollati-Fogolín, M.; Chambaud, I.; et al. Anti-inflammatory Lactobacillus rhamnosus CNCM I-3690 strain protects against oxidative stress and increases lifespan in Caenorhabditis elegans. PLoS ONE 2012, 7, e52493. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, T.; Sakamoto, K. Killed Bifidobacterium longum enhanced stress tolerance and prolonged life span of Caenorhabditis elegans via DAF-16. Br. J. Nutr. 2018, 120, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Zia, A.; Farkhondeh, T.; Pourbagher-Shahri, A.M.; Samarghandian, S. The role of curcumin in aging and senescence: Molecular mechanisms. Biomed. Pharmacother. 2021, 134, 111119. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Rezaizadehnajafi, L.; Wink, M. Influence of resveratrol on oxidative stress resistance and life span in Caenorhabditis elegans. J. Pharm. Pharmacol. 2013, 65, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Ye, K.; Ji, C.B.; Lu, X.W.; Ni, Y.H.; Gao, C.L.; Chen, X.H.; Zhao, Y.P.; Gu, G.X.; Guo, X.R. Resveratrol attenuates radiation damage in Caenorhabditis elegans by preventing oxidative stress. J. Radiat. Res. 2010, 51, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Fischer, N.; Büchter, C.; Koch, K.; Albert, S.; Csuk, R.; Wätjen, W. The resveratrol derivatives trans-3,5-dimethoxy-4-fluoro-4’-hydroxystilbene and trans-2,4’,5-trihydroxystilbene decrease oxidative stress and prolong lifespan in Caenorhabditis elegans. J. Pharm. Pharmacol. 2017, 69, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.; Yue, Y.; Park, Y. A living model for obesity and aging research: Caenorhabditis elegans. Crit. Rev. Food Sci. Nutr. 2017, 58, 741–754. [Google Scholar] [CrossRef] [PubMed]
- Tosato, M.; Zamboni, V.; Ferrini, A.; Cesari, M. The aging process and potential interventions to extend life expectancy. Clin. Interv. Aging 2007, 2, 401. [Google Scholar]
- Chen, C.; Zhou, M.; Ge, Y.; Wang, X. SIRT1 and aging related signaling pathways. Mech. Ageing Dev. 2020, 187, 111215. [Google Scholar] [CrossRef] [PubMed]
- Niccoli, T.; Partridge, L. Ageing as a risk factor for disease. Curr. Biol. 2012, 22, R741–R752. [Google Scholar] [CrossRef]
- Kwon, G.; Lee, J.; Koh, J.H.; Lim, Y.H. Lifespan Extension of Caenorhabditis elegans by Butyricicoccus pullicaecorum and Megasphaera elsdenii with Probiotic Potential. Curr. Microbiol. 2018, 75, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Kwon, G.; Lee, J.; Lim, Y.H. Dairy Propionibacterium extends the mean lifespan of Caenorhabditis elegans via activation of the innate immune system. Sci. Rep. 2016, 6, 31713. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Hamazaki, Y.; Sun, S.; Nishikawa, Y.; Kage-Nakadai, E. Clostridium butyricum MIYAIRI 588 Increases the Lifespan and Multiple-Stress Resistance of Caenorhabditis elegans. Nutrients 2018, 10, 1921. [Google Scholar] [CrossRef]
- Shaikhulova, S.; Fakhrullina, G.; Nigamatzyanova, L.; Akhatova, F.; Fakhrullin, R. Worms eat oil: Alcanivorax borkumensis hydrocarbonoclastic bacteria colonise Caenorhabditis elegans nematodes intestines as a first step towards oil spills zooremediation. Sci. Total Environ. 2021, 761, 143209. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Roxo, M.; Cheng, X.; Zhang, S.; Cheng, H.; Wink, M. Pro-oxidant and lifespan extension effects of caffeine and related methylxanthines in Caenorhabditis elegans. Food Chem. X 2019, 1, 100005. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Zhao, X.; Zhang, J.; Li, M.; Qi, Y.; Zhou, L. Calycosin promotes lifespan in Caenorhabditis elegans through insulin signaling pathway via daf-16, age-1 and daf-2. J. Biosci. Bioeng. 2017, 124, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Weimer, S.; Priebs, J.; Kuhlow, D.; Groth, M.; Priebe, S.; Mansfeld, J.; Merry, T.L.; Dubuis, S.; Laube, B.; Pfeiffer, A.F.; et al. D-Glucosamine supplementation extends life span of nematodes and of ageing mice. Nat. Commun. 2014, 5, 3563. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, G.; Mohankumar, A.; Kalaiselvi, D.; Nivitha, S.; Murugesh, E.; Shanmughavel, P.; Sundararaj, P. Diosgenin a phytosterol substitute for cholesterol, prolongs the lifespan and mitigates glucose toxicity via DAF-16/FOXO and GST-4 in Caenorhabditis elegans. Biomed. Pharmacother. 2017, 95, 1693–1703. [Google Scholar] [CrossRef] [PubMed]
- Zečić, A.; Braeckman, B.P. DAF-16/FoxO in Caenorhabditis elegans and Its Role in Metabolic Remodeling. Cells 2020, 9, 109. [Google Scholar] [CrossRef]
- Wang, H.; Liu, J.; Li, T.; Liu, R.H. Blueberry extract promotes longevity and stress tolerance via DAF-16 in Caenorhabditis elegans. Food Funct. 2018, 9, 5273–5282. [Google Scholar] [CrossRef]
- Qi, W.; Gutierrez, G.E.; Gao, X.; Dixon, H.; McDonough, J.A.; Marini, A.M.; Fisher, A.L. The ω-3 fatty acid α-linolenic acid extends Caenorhabditis elegans lifespan via NHR-49/PPARα and oxidation to oxylipins. Aging Cell 2017, 16, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- Brandstädt, S.; Schmeisser, K.; Zarse, K.; Ristow, M. Lipid-lowering fibrates extend C. elegans lifespan in a NHR-49/PPARalpha-dependent manner. Aging 2013, 5, 270–275. [Google Scholar] [CrossRef][Green Version]
- Blackwell, T.K.; Steinbaugh, M.J.; Hourihan, J.M.; Ewald, C.Y.; Isik, M. SKN-1/Nrf, stress responses, and aging in Caenorhabditis elegans. Free Radic. Biol. Med. 2015, 88, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Fang, E.F.; Waltz, T.B.; Kassahun, H.; Lu, Q.; Kerr, J.S.; Morevati, M.; Fivenson, E.M.; Wollman, B.N.; Marosi, K.; Wilson, M.A.; et al. Tomatidine enhances lifespan and healthspan in C. elegans through mitophagy induction via the SKN-1/Nrf2 pathway. Sci. Rep. 2017, 7, 46208. [Google Scholar] [CrossRef] [PubMed]
- Koch, K.; Weldle, N.; Baier, S.; Büchter, C.; Wätjen, W. Hibiscus sabdariffa L. extract prolongs lifespan and protects against amyloid-β toxicity in Caenorhabditis elegans: Involvement of the FoxO and Nrf2 orthologues DAF-16 and SKN-1. Eur. J. Nutr. 2020, 59, 137–150. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yavorov-Dayliev, D.; Milagro, F.I.; Ayo, J.; Oneca, M.; Aranaz, P. Pediococcus acidilactici CECT9879 (pA1c) Counteracts the Effect of a High-Glucose Exposure in C. elegans by Affecting the Insulin Signaling Pathway (IIS). Int. J. Mol. Sci. 2022, 23, 2689. https://doi.org/10.3390/ijms23052689
Yavorov-Dayliev D, Milagro FI, Ayo J, Oneca M, Aranaz P. Pediococcus acidilactici CECT9879 (pA1c) Counteracts the Effect of a High-Glucose Exposure in C. elegans by Affecting the Insulin Signaling Pathway (IIS). International Journal of Molecular Sciences. 2022; 23(5):2689. https://doi.org/10.3390/ijms23052689
Chicago/Turabian StyleYavorov-Dayliev, Deyan, Fermín I. Milagro, Josune Ayo, María Oneca, and Paula Aranaz. 2022. "Pediococcus acidilactici CECT9879 (pA1c) Counteracts the Effect of a High-Glucose Exposure in C. elegans by Affecting the Insulin Signaling Pathway (IIS)" International Journal of Molecular Sciences 23, no. 5: 2689. https://doi.org/10.3390/ijms23052689
APA StyleYavorov-Dayliev, D., Milagro, F. I., Ayo, J., Oneca, M., & Aranaz, P. (2022). Pediococcus acidilactici CECT9879 (pA1c) Counteracts the Effect of a High-Glucose Exposure in C. elegans by Affecting the Insulin Signaling Pathway (IIS). International Journal of Molecular Sciences, 23(5), 2689. https://doi.org/10.3390/ijms23052689






