The Role of Endothelial Progenitor Cells in Atherosclerosis and Impact of Anti-Lipemic Treatments on Endothelial Repair
Abstract
1. Introduction
2. Endothelium Biology and Function
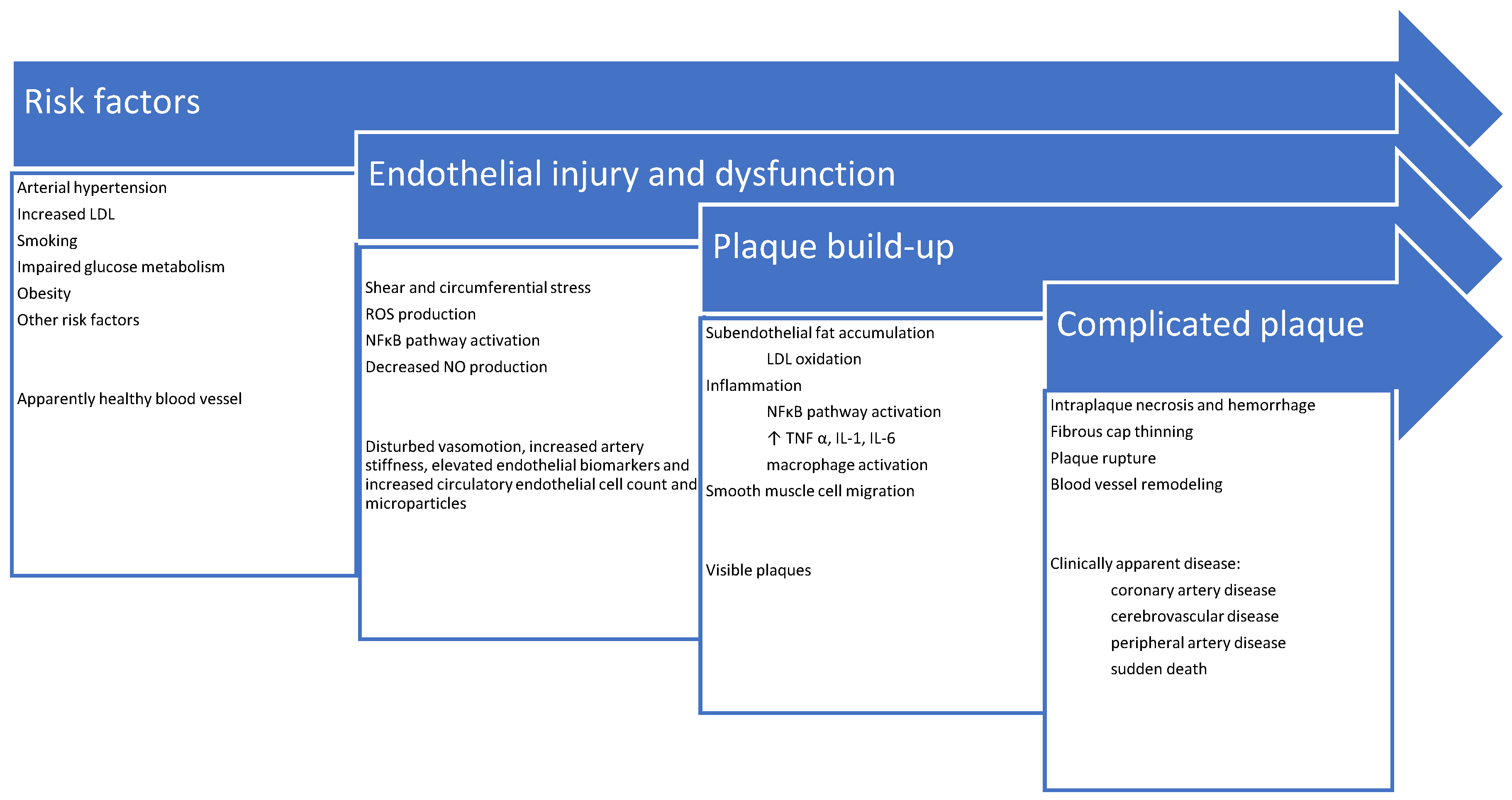
3. Pathophysiology of Atherosclerosis
4. Endothelial Repair
5. Endothelial Repair in Patients with Lipid Disorders
6. Standard Treatment for Lipid Disorders: Statins, Ezetimibe and Fibrates
7. Novel Treatments for Lipid Disorders: Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) Modulating Agents and Angiopoietin-like Proteins (Angtpl3) Inhibitors
8. Plasma Apheresis
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Milutinović, A.; Šuput, D.; Zorc-Pleskovič, R. Pathogenesis of Atherosclerosis in the Tunica Intima, Media, and Adventitia of Coronary Arteries: An Updated Review. Bosn. J. Basic Med. Sci. 2020, 20, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Rosenblit, P.D. Extreme Atherosclerotic Cardiovascular Disease (ASCVD) Risk Recognition. Curr. Diab. Rep. 2019, 19, 61. [Google Scholar] [CrossRef] [PubMed]
- Silveira Rossi, J.L.; Barbalho, S.M.; Reverete de Araujo, R.; Bechara, M.D.; Sloan, K.P.; Sloan, L.A. Metabolic Syndrome and Cardiovascular Diseases: Going beyond Traditional Risk Factors. Diabetes Metab. Res. Rev. 2021, e3502. [Google Scholar] [CrossRef] [PubMed]
- Polovina, M.M.; Potpara, T.S. Endothelial Dysfunction in Metabolic and Vascular Disorders. Postgrad. Med. 2014, 126, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Golledge, J. Update on the Pathophysiology and Medical Treatment of Peripheral Artery Disease. Nat. Rev. Cardiol. 2022, 19, 663–669. [Google Scholar] [CrossRef]
- Fonarow, G.C.; Kosiborod, M.N.; Rane, P.B.; Nunna, S.; Villa, G.; Habib, M.; Arellano, J.; Mues, K.E.; Sun, K.; Wade, R.L. Patient Characteristics and Acute Cardiovascular Event Rates among Patients with Very High-Risk and Non-Very High-Risk Atherosclerotic Cardiovascular Disease. Clin. Cardiol. 2021, 44, 1457–1466. [Google Scholar] [CrossRef]
- Aplin, M.; Andersen, A.; Brandes, A.; Dominguez, H.; Dahl, J.S.; Damgaard, D.; Iversen, H.K.; Iversen, K.K.; Nielsen, E.; Risum, N.; et al. Assessment of Patients with a Suspected Cardioembolic Is-chemic Stroke. A National Consensus Statement. Scand. Cardiovasc. J. 2021, 55, 315–325. [Google Scholar] [CrossRef]
- Feig, J.E. Regression of Atherosclerosis: Insights from Animal and Clinical Studies. Ann. Glob. Health 2014, 80, 13–23. [Google Scholar] [CrossRef]
- Chopra, H.; Hung, M.K.; Kwong, D.L.; Zhang, C.F.; Pow, E.H.N. Insights into Endothelial Progenitor Cells: Origin, Classification, Potentials, and Prospects. Stem Cells Int. 2018, 2018, 9847015. [Google Scholar] [CrossRef]
- Yang, J.-X.; Pan, Y.-Y.; Wang, X.-X.; Qiu, Y.-G.; Mao, W. Endothelial Progenitor Cells in Age-Related Vascular Remodeling. Cell Transpl. 2018, 27, 786–795. [Google Scholar] [CrossRef]
- Rajendran, P.; Rengarajan, T.; Thangavel, J.; Nishigaki, Y.; Sakthisekaran, D.; Sethi, G.; Nishigaki, I. The Vascular Endothelium and Human Diseases. Int. J. Biol. Sci. 2013, 9, 1057. [Google Scholar] [CrossRef] [PubMed]
- Fortini, F.; Vieceli Dalla Sega, F.; Marracino, L.; Severi, P.; Rapezzi, C.; Rizzo, P.; Ferrari, R. Well-Known and Novel Players in Endothelial Dysfunction: Updates on a Notch(Ed) Landscape. Biomedicines 2021, 9, 997. [Google Scholar] [CrossRef] [PubMed]
- Sancheti, S.; Shah, P.; Phalgune, D.S. Correlation of Endothelial Dysfunction Measured by Flow-Mediated Vasodilatation to Severity of Coronary Artery Disease. Indian Heart J. 2018, 70, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.F. An Investigation of the Underlying Mechanisms of Arterial Vasomotion. Ph.D. Thesis, University of Oxford, Oxford, UK, 2020. [Google Scholar]
- Cyr, A.R.; Huckaby, L.V.; Shiva, S.S.; Zuckerbraun, B.S. Nitric Oxide and Endothelial Dysfunction. Crit. Care Clin. 2020, 36, 307–321. [Google Scholar] [CrossRef]
- Kwaifa, I.K.; Bahari, H.; Yong, Y.K.; Noor, S.M. Endothelial Dysfunction in Obesity-Induced Inflammation: Molecular Mechanisms and Clinical Implications. Biomolecules 2020, 10, 291. [Google Scholar] [CrossRef]
- Schmidt, K.; De Wit, C. Endothelium-Derived Hyperpolarizing Factor and Myoendothelial Coupling: The In Vivo Perspective. Front. Physiol. 2020, 11, 602930. [Google Scholar] [CrossRef]
- Altabas, V.; Altabas, K.; Kirigin, L. Endothelial Progenitor Cells (EPCs) in Ageing and Age-Related Diseases: How Currently Available Treatment Modalities Affect EPC Biology, Atherosclerosis, and Cardiovascular Outcomes. Mech. Ageing Dev. 2016, 159, 49–62. [Google Scholar] [CrossRef]
- Grover, S.P.; Mackman, N. Tissue Factor in Atherosclerosis and Atherothrombosis. Atherosclerosis 2020, 307, 80–86. [Google Scholar] [CrossRef]
- Poston, R.N. Atherosclerosis: Integration of Its Pathogenesis as a Self-Perpetuating Propagating Inflammation: A Review. Cardiovasc. Endocrinol. Metab. 2019, 8, 51–61. [Google Scholar] [CrossRef]
- Haverich, A. A Surgeon’s View on the Pathogenesis of Atherosclerosis. Circulation 2017, 135, 205–207. [Google Scholar] [CrossRef]
- Subbotin, V.M. Excessive Intimal Hyperplasia in Human Coronary Arteries before Intimal Lipid Depositions Is the Initiation of Coronary Atherosclerosis and Constitutes a Therapeutic Target. Drug Discov. Today 2016, 21, 1578–1595. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, F.; Kramer, M.C.A.; Woudstra, P.; Yahagi, K.; Ladich, E.; Finn, A.V.; De Winter, R.J.; Kolodgie, F.D.; Wight, T.N.; Davis, H.R.; et al. Natural Progression of Atherosclerosis from Pathologic Intimal Thickening to Late Fibroatheroma in Human Coronary Arteries: A Pathology Study. Atherosclerosis 2015, 241, 772–782. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. Effects of Shear Stress on Endothelial Cells: Go with the Flow. Acta Physiol. 2017, 219, 382–408. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.-Z.; Jiménez, J.M.; Ou, K.; McCormick, M.E.; Zhang, L.-D.; Davies, P.F. Hemodynamic Disturbed Flow Induces Differential DNA Methylation of Endothelial Kruppel-Like Factor 4 Promoter In Vitro and In Vivo. Circ. Res. 2014, 115, 32–43. [Google Scholar] [CrossRef]
- Staarmann, B.; Smith, M.; Prestigiacomo, C.J. Shear Stress and Aneurysms: A Review. Neurosurg. Focus 2019, 47, E2. [Google Scholar] [CrossRef]
- Zhong, S.; Li, L.; Shen, X.; Li, Q.; Xu, W.; Wang, X.; Tao, Y.; Yin, H. An Update on Lipid Oxidation and Inflammation in Cardiovascular Diseases. Free Radic. Biol. Med. 2019, 144, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammation during the Life Cycle of the Atherosclerotic Plaque. Cardiovasc. Res. 2021, 117, 2525–2536. [Google Scholar] [CrossRef]
- Basu, D.; Hu, Y.; Huggins, L.-A.; Mullick, A.E.; Graham, M.J.; Wietecha, T.; Barnhart, S.; Mogul, A.; Pfeiffer, K.; Zirlik, A.; et al. Novel Reversible Model of Atherosclerosis and Regression Using Oligonucleotide Regulation of the LDL Receptor. Circ. Res. 2018, 122, 560–567. [Google Scholar] [CrossRef]
- Sorokin, V.; Vickneson, K.; Kofidis, T.; Woo, C.C.; Lin, X.Y.; Foo, R.; Shanahan, C.M. Role of Vascular Smooth Muscle Cell Plasticity and Interactions in Vessel Wall Inflammation. Front. Immunol. 2020, 11, 599415. [Google Scholar] [CrossRef]
- Katsuumi, G.; Shimizu, I.; Yoshida, Y.; Minamino, T. Vascular Senescence in Cardiovascular and Metabolic Diseases. Front. Cardiovasc. Med. 2018, 5, 18. [Google Scholar] [CrossRef]
- Erbel, C.; Wolf, A.; Lasitschka, F.; Linden, F.; Domschke, G.; Akhavanpoor, M.; Doesch, A.O.; Katus, H.A.; Gleissner, C.A. Prevalence of M4 Macrophages within Human Coronary Atherosclerotic Plaques Is Associated with Features of Plaque Instability. Int. J. Cardiol. 2015, 186, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Shioi, A.; Ikari, Y. Plaque Calcification During Atherosclerosis Progression and Regression. J. Atheroscler. Thromb. 2018, 25, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Canet-Soulas, E.; Bessueille, L.; Mechtouff, L.; Magne, D. The Elusive Origin of Atherosclerotic Plaque Calcification. Front. Cell Dev. Biol. 2021, 9, 390. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Z.; Zhang, L.; Wang, Y. Roles of Cells from the Arterial Vessel Wall in Atherosclerosis. Mediat. Inflamm. 2017, 2017, 8135934. [Google Scholar] [CrossRef] [PubMed]
- Yousufuddin, M.; Young, N. Aging and Ischemic Stroke. Aging 2019, 11, 2542–2544. [Google Scholar] [CrossRef] [PubMed]
- White, S.J.; Newby, A.C.; Johnson, T.W. Endothelial Erosion of Plaques as a Substrate for Coronary Thrombosis. Thromb. Haemost. 2016, 115, 509–519. [Google Scholar]
- Gunawardena, T.; Merinopoulos, I.; Wickramarachchi, U.; Vassiliou, V.; Eccleshall, S. Endothelial Dysfunction and Coronary Vasoreactivity—A Review of the History, Physiology, Diagnostic Techniques, and Clinical Relevance. Curr. Cardiol. Rev. 2021, 17, 85–100. [Google Scholar] [CrossRef]
- Rocha, H.N.; Garcia, V.P.; Batista, G.M.; Silva, G.M.; Mattos, J.D.; Campos, M.O.; Nóbrega, A.C.; Fernandes, I.A.; Rocha, N.G. Disturbed Blood Flow Induces Endothelial Apoptosis without Mobilizing Repair Mechanisms in Hypertension. Life Sci. 2018, 209, 103–110. [Google Scholar] [CrossRef]
- Altabas, V. Diabetes, Endothelial Dysfunction, and Vascular Repair: What Should a Diabetologist Keep His Eye On? Int. J. Endocrinol. 2015, 2015, 848272. [Google Scholar] [CrossRef]
- Zhang, M.; Rehman, J.; Malik, A.B. Endothelial Progenitor Cells and Vascular Repair. Curr. Opin. Hematol. 2014, 21, 224. [Google Scholar] [CrossRef]
- Basile, D.P.; Yoder, M.C. Circulating and Tissue Resident Endothelial Progenitor Cells. J. Cell. Physiol. 2014, 229, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Morrone, D.; Picoi, M.E.L.; Felice, F.; De Martino, A.; Scatena, C.; Spontoni, P.; Naccarato, A.G.; Di Stefano, R.; Bortolotti, U.; Dal Monte, M. Endothelial Progenitor Cells: An Appraisal of Relevant Data from Bench to Bedside. Int. J. Mol. Sci. 2021, 22, 12874. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Kang, L.; Wang, Z.; Chen, A.; Zhao, Q.; Li, H. 17β-Estradiol Promotes Recovery after Myocardial Infarction by Enhancing Homing and Angiogenic Capacity of Bone Marrow-Derived Endothelial Progenitor Cells through ERα-SDF-1/CXCR4 Crosstalking. Acta Biochim. Biophys. Sin. 2018, 50, 1247–1256. [Google Scholar] [CrossRef]
- Naito, T.; Shun, M.; Nishimura, H.; Gibo, T.; Tosaka, M.; Kawashima, M.; Ando, A.; Ogawa, T.; Sanaka, T.; Nitta, K. Pleiotropic Effect of Erythropoiesis-Stimulating Agents on Circulating Endothelial Progenitor Cells in Dialysis Patients. Clin. Exp. Nephrol. 2021, 25, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Mogharbel, B.F.; Abdelwahid, E.; Irioda, A.C.; Francisco, J.C.; Simeoni, R.B.; De Souza, D.; De Souza, C.M.C.O.; Beltrame, M.P.; Ferreira, R.J.; Guarita-Souza, L.C.; et al. Bone Marrow-Derived Stem Cell Populations Are Differentially Regulated by Thyroid or/and Ovarian Hormone Loss. Int. J. Mol. Sci. 2017, 18, 2139. [Google Scholar] [CrossRef]
- Balistreri, C.R.; Buffa, S.; Pisano, C.; Lio, D.; Ruvolo, G.; Mazzesi, G. Are Endothelial Progenitor Cells the Real Solution for Cardiovascular Diseases? Focus on Controversies and Perspectives. BioMed Res. Int. 2015, 2015, 835934. [Google Scholar] [CrossRef] [PubMed]
- Mobarrez, F.; Antoniewicz, L.; Bosson, J.A.; Kuhl, J.; Pisetsky, D.S.; Lundbäck, M. The Effects of Smoking on Levels of Endothelial Progenitor Cells and Microparticles in the Blood of Healthy Volunteers. PLoS ONE 2014, 9, e90314. [Google Scholar] [CrossRef]
- Berezin, A.E. Endothelial Progenitor Cells Dysfunction and Impaired Tissue Reparation: The Missed Link in Diabetes Mellitus Development. Diabetes Metab. Syndr. Clin. Res. Rev. 2017, 11, 215–220. [Google Scholar] [CrossRef]
- Seijkens, T.; Hoeksema, M.A.; Beckers, L.; Smeets, E.; Meiler, S.; Levels, J.; Tjwa, M.; De Winther, M.P.; Lutgens, E. Hypercholesterolemia-Induced Priming of Hematopoietic Stem and Progenitor Cells Aggravates Atherosclerosis. FASEB J. 2014, 28, 2202–2213. [Google Scholar] [CrossRef]
- Mandraffino, G.; Imbalzano, E.; Sardo, M.A.; D’ascola, A.; Mamone, F.; Gullo, A.L.; Alibrandi, A.; Loddo, S.; Mormina, E.; David, A. Circulating Progenitor Cells in Hypertensive Patients with Different Degrees of Cardiovascular Involvement. J. Hum. Hypertens. 2014, 28, 543–550. [Google Scholar] [CrossRef]
- Massot, A.; Navarro-Sobrino, M.; Penalba, A.; Arenillas, J.F.; Giralt, D.; Ribo, M.; Molina, C.A.; Alvarez-Sabín, J.; Montaner, J.; Rosell, A. Decreased Levels of Angiogenic Growth Factors in Intracranial Atherosclerotic Disease despite Severity-Related Increase in Endothelial Progenitor Cell Counts. Cerebrovasc. Dis. 2013, 35, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Ji, K.; Qian, L.; Nan, J.; Xue, Y.; Zhang, S.; Wang, G.; Yin, R.; Zhu, Y.; Wang, L.; Ma, J.; et al. Ox-LDL Induces Dysfunction of Endothelial Progenitor Cells via Activation of NF-B. BioMed Res. Int. 2015, 2015, 175291. [Google Scholar] [CrossRef] [PubMed]
- Hermsdorff, H.H.M.; Barbosa, K.B.; Volp, A.C.P.; Puchau, B.; Bressan, J.; Zulet, M.Á.; Martínez, J.A. Gender-Specific Relationships between Plasma Oxidized Low-Density Lipoprotein Cholesterol, Total Antioxidant Capacity, and Central Adiposity Indicators. Eur. J. Prev. Cardiol. 2014, 21, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, X.; Bu, H.; Hu, K.; Si, Z.; Chen, L.; Chen, Y.; Yang, L.; Jiang, Y.; Xu, Y.; Zhao, P.; et al. Differences in the Reaction of Hyperlipidemia on Different Endothelial Progenitor Cells Based on Sex. Biomed. Rep. 2021, 15, 64. [Google Scholar] [CrossRef] [PubMed]
- Schwertani, A.; Choi, H.Y.; Genest, J. HDLs and the Pathogenesis of Atherosclerosis. Curr. Opin. Cardiol. 2018, 33, 311–316. [Google Scholar] [CrossRef]
- Lucchesi, D.; Popa, S.G.; Sancho, V.; Giusti, L.; Garofolo, M.; Daniele, G.; Pucci, L.; Miccoli, R.; Penno, G.; Del Prato, S. Influence of High Density Lipoprotein Cholesterol Levels on Circulating Monocytic Angiogenic Cells Functions in Individuals with Type 2 Diabetes Mellitus. Cardiovasc. Diabetol. 2018, 17, 78. [Google Scholar] [CrossRef]
- Shih, C.-M.; Lin, F.-Y.; Yeh, J.-S.; Lin, Y.-W.; Loh, S.-H.; Tsao, N.-W.; Nakagami, H.; Morishita, R.; Sawamura, T.; Li, C.-Y.; et al. Dysfunctional High Density Lipoprotein Failed to Rescue the Function of Oxidized Low Density Lipoprotein-Treated Endothelial Progenitor Cells: A Novel Index for the Prediction of HDL Functionality. Transl. Res. 2019, 205, 17–32. [Google Scholar] [CrossRef]
- Peterson, S.J.; Shapiro, J.I.; Thompson, E.; Singh, S.; Liu, L.; Weingarten, J.A.; O’Hanlon, K.; Bialczak, A.; Bhesania, S.R.; Abraham, N.G. Oxidized HDL, Adipokines, and Endothelial Dysfunction: A Potential Biomarker Profile for Cardiovascular Risk in Women with Obesity. Obesity 2019, 27, 87–93. [Google Scholar] [CrossRef]
- Ren, Z.; Guo, J.; Xiao, X.; Huang, J.; Li, M.; Cai, R.; Zeng, H. The Effect of Sex Differences on Endothelial Function and Circulating Endothelial Progenitor Cells in Hypertriglyceridemia. Cardiol. Res. Pract. 2020, 2020, 2132918. [Google Scholar] [CrossRef]
- Wils, J.; Favre, J.; Bellien, J. Modulating Putative Endothelial Progenitor Cells for the Treatment of Endothelial Dysfunction and Cardiovascular Complications in Diabetes. Pharmacol. Ther. 2017, 170, 98–115. [Google Scholar] [CrossRef]
- Chia, P.Y.; Teo, A.; Yeo, T.W. Overview of the Assessment of Endothelial Function in Humans. Front. Med. 2020, 7, 542567. [Google Scholar] [CrossRef] [PubMed]
- Maruhashi, T.; Kihara, Y.; Higashi, Y. Assessment of Endothelium-Independent Vasodilation: From Methodology to Clinical Perspectives. J. Hypertens. 2018, 36, 1460–1467. [Google Scholar] [CrossRef] [PubMed]
- De Macedo, A.R.; Machado, J.C.; Luz, L.M.S.; Da Nobrega, A.C.L.; De Souza, M.N. A Novel Model to Simulate Venous Occlusion Plethysmography Data and to Estimate Arterial and Venous Parameters. Res. Biomed. Eng. 2020, 36, 463–473. [Google Scholar] [CrossRef]
- Masi, S.; Rizzoni, D.; Taddei, S.; Widmer, R.J.; Montezano, A.C.; Lüscher, T.F.; Schiffrin, E.L.; Touyz, R.M.; Paneni, F.; Lerman, A. Assessment and Pathophysiology of Microvascular Disease: Recent Progress and Clinical Implications. Eur. Heart J. 2021, 42, 2590–2604. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. 2018 Practice Guidelines for the Management of Arterial Hypertension of the European Society of Cardiology and the European Society of Hypertension. Blood Press. 2018, 27, 314–340. [Google Scholar] [CrossRef] [PubMed]
- Bochenek, M.L.; Schäfer, K. Role of Endothelial Cells in Acute and Chronic Thrombosis. Hämostaseologie 2019, 39, 128–139. [Google Scholar] [CrossRef]
- Gendron, N.; Smadja, D.M. Circulating Endothelial Cells: A New Biomarker of Endothelial Dysfunction in Hematological Diseases. Ann. Biol. Clin. 2016, 74, 395–404. [Google Scholar] [CrossRef]
- Chen, S.; Sun, Y.; Neoh, K.H.; Chen, A.; Li, W.; Yang, X.; Han, R.P. Microfluidic Assay of Circulating Endothelial Cells in Coronary Artery Disease Patients with Angina Pectoris. PLoS ONE 2017, 12, e0181249. [Google Scholar]
- Vítková, V.; Živný, J.; Janota, J. Endothelial Cell-Derived Microvesicles: Potential Mediators and Biomarkers of Pathologic Processes. Biomark. Med. 2018, 12, 161–175. [Google Scholar] [CrossRef]
- Byrne, P.; Cullinan, J.; Smith, A.; Smith, S.M. Statins for the Primary Prevention of Cardiovascular Disease: An Overview of Systematic Reviews. BMJ Open 2019, 9, e023085. [Google Scholar] [CrossRef]
- Virani, S.S.; Smith, S.C., Jr.; Stone, N.J.; Grundy, S.M. Secondary Prevention for Atherosclerotic Cardiovascular Disease: Comparing Recent US and European Guidelines on Dyslipidemia. Circulation 2020, 141, 1121–1123. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, M.B.; Nordestgaard, B.G.; Afzal, S.; Falk, E. ACC/AHA Guidelines Superior to ESC/EAS Guidelines for Primary Prevention with Statins in Non-Diabetic Europeans: The Copenhagen General Population Study. Eur. Heart J. 2017, 38, 586–594. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gorabi, A.M.; Kiaie, N.; Hajighasemi, S.; Banach, M.; Penson, P.E.; Jamialahmadi, T.; Sahebkar, A. Statin-Induced Nitric Oxide Signaling: Mechanisms and Therapeutic Implications. J. Clin. Med. 2019, 8, 2051. [Google Scholar] [CrossRef]
- Sandhu, K.; Mamas, M.; Butler, R. Endothelial Progenitor Cells: Exploring the Pleiotropic Effects of Statins. World J. Cardiol. 2017, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ricottini, E.; Madonna, R.; Grieco, D.; Zoccoli, A.; Stampachiacchiere, B.; Patti, G.; Tonini, G.; De Caterina, R.; Di Sciascio, G. Effect of High-Dose Atorvastatin Reload on the Release of Endothelial Progenitor Cells in Patients on Long-Term Statin Treatment Who Underwent Percutaneous Coronary Intervention (from the ARMYDA-EPC Study). Am. J. Cardiol. 2016, 117, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Eisen, A.; Leshem-Lev, D.; Yavin, H.; Orvin, K.; Mager, A.; Rechavia, E.; Bental, T.; Dadush, O.; Battler, A.; Kornowski, R. Effect of High Dose Statin Pretreatment on Endothelial Progenitor Cells after Percutaneous Coronary Intervention (HIPOCRATES Study). Cardiovasc. Drugs Ther. 2015, 29, 129–135. [Google Scholar] [CrossRef]
- Hibbert, B.; Ma, X.; Pourdjabbar, A.; Simard, T.; Rayner, K.; Sun, J.; Chen, Y.-X.; Filion, L.; O’Brien, E.R. Pre-Procedural Atorvastatin Mobilizes Endothelial Progenitor Cells: Clues to the Salutary Effects of Statins on Healing of Stented Human Arteries. PLoS ONE 2011, 6, e16413. [Google Scholar] [CrossRef]
- Oikonomou, E.; Siasos, G.; Zaromitidou, M.; Hatzis, G.; Mourouzis, K.; Chrysohoou, C.; Zisimos, K.; Mazaris, S.; Tourikis, P.; Athanasiou, D. Atorvastatin Treatment Improves Endothelial Function through Endothelial Progenitor Cells Mobilization in Ischemic Heart Failure Patients. Atherosclerosis 2015, 238, 159–164. [Google Scholar] [CrossRef]
- Ansheles, A.A.; Rvacheva, A.V.; Sergienko, I.V. Effect of Atorvastatin Therapy on the Level of CD34+ CD133+ CD309+ Endothelial Progenitor Cells in Patients with Coronary Heart Disease. Bull. Exp. Biol. Med. 2017, 163, 133–136. [Google Scholar] [CrossRef]
- Ye, H.; He, F.; Fei, X.; Lou, Y.; Wang, S.; Yang, R.; Hu, Y.; Chen, X. High-Dose Atorvastatin Reloading before Percutaneous Coronary Intervention Increased Circulating Endothelial Progenitor Cells and Reduced Inflammatory Cytokine Expression during the Perioperative Period. J. Cardiovasc. Pharmacol. Ther. 2014, 19, 290–295. [Google Scholar] [CrossRef]
- Huang, B.; Cheng, Y.; Xie, Q.; Lin, G.; Wu, Y.; Feng, Y.; Gao, J.; Xu, D. Effect of 40 Mg versus 10 Mg of Atorvastatin on Oxidized Low-Density Lipoprotein, High-Sensitivity C-Reactive Protein, Circulating Endothelial-Derived Microparticles, and Endothelial Progenitor Cells in Patients with Ischemic Cardiomyopathy. Clin. Cardiol. 2012, 35, 125–130. [Google Scholar] [CrossRef]
- Niu, H.; Wei, Z.; Zhang, Y.; He, J.; Jia, D. Atorvastatin Improves Coronary Flow and Endothelial Function in Patients with Coronary Slow Flow. Exp. Ther. Med. 2018, 15, 904–908. [Google Scholar] [CrossRef] [PubMed]
- Baran, Ç.; Durdu, S.; Dalva, K.; Zaim, Ç.; Dogan, A.; Ocakoglu, G.; Gürman, G.; Arslan, Ö.; Akar, A.R. Effects of Preoperative Short Term Use of Atorvastatin on Endothelial Progenitor Cells after Coronary Surgery: A Randomized, Controlled Trial. Stem Cell Rev. Rep. 2012, 8, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Spadaccio, C.; Pollari, F.; Casacalenda, A.; Alfano, G.; Genovese, J.; Covino, E.; Chello, M. Atorvastatin Increases the Number of Endothelial Progenitor Cells after Cardiac Surgery: A Randomized Control Study. J. Cardiovasc. Pharmacol. 2010, 55, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.M.; Rutella, S.; Giannico, M.B.; Perfetti, M.; Zaccone, V.; Brugaletta, S.; Garramone, B.; Niccoli, G.; Porto, I.; Liuzzo, G.; et al. Effect of Intensive vs. Standard Statin Therapy on Endothelial Progenitor Cells and Left Ventricular Function in Patients with Acute Myocardial Infarction: Statins for Regeneration after Acute Myocardial Infarction and PCI (STRAP) Trial. Int. J. Cardiol. 2008, 130, 457–462. [Google Scholar] [CrossRef]
- Liu, H.; Qi, X.; Long-le Ma, D.-K.Y.; Wang, L. Atorvastatin Improves Endothelial Progenitor Cell Function and Reduces Pulmonary Hypertension in Patients with Chronic Pulmonary Heart Disease. Exp. Clin. Cardiol. 2013, 18, e40. [Google Scholar]
- Sobrino, T.; Blanco, M.; Pérez-Mato, M.; Rodríguez-Yáñez, M.; Castillo, J. Increased Levels of Circulating Endothelial Progenitor Cells in Patients with Ischaemic Stroke Treated with Statins during Acute Phase. Eur. J. Neurol. 2012, 19, 1539–1546. [Google Scholar] [CrossRef]
- Chantzichristos, V.G.; Agouridis, A.P.; Moutzouri, E.; Stellos, K.; Elisaf, M.S.; Tselepis, A.D. Effect of Rosuvastatin or Its Combination with Omega-3 Fatty Acids on Circulating CD34+ Progenitor Cells and on Endothelial Colony Formation in Patients with Mixed Dyslipidaemia. Atherosclerosis 2016, 251, 240–247. [Google Scholar] [CrossRef]
- Tousoulis, D.; Andreou, I.; Tsiatas, M.; Miliou, A.; Tentolouris, C.; Siasos, G.; Papageorgiou, N.; Papadimitriou, C.A.; Dimopoulos, M.-A.; Stefanadis, C. Effects of Rosuvastatin and Allopurinol on Circulating Endothelial Progenitor Cells in Patients with Congestive Heart Failure: The Impact of Inflammatory Process and Oxidative Stress. Atherosclerosis 2011, 214, 151–157. [Google Scholar] [CrossRef]
- Erbs, S.; Beck, E.B.; Linke, A.; Adams, V. High-Dose Rosuvastatin in Chronic Heart Failure Promotes Vasculogenesis, Corrects Endothelial Function, and Improves Cardiac Remodeling—Results from a Randomized, Double-Blind, and Placebo-Controlled Study. Int. J. Cardiol. 2011, 146, 56–63. [Google Scholar] [CrossRef]
- Pirro, M.; Schillaci, G.; Romagno, P.F.; Mannarino, M.R.; Bagaglia, F.; Razzi, R.; Pasqualini, L.; Vaudo, G.; Mannarino, E. Influence of Short-Term Rosuvastatin Therapy on Endothelial Progenitor Cells and Endothelial Function. J. Cardiovasc. Pharmacol. Ther. 2009, 14, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Pesaro, A.E.P.; Serrano, C.V., Jr.; Katz, M.; Marti, L.; Fernandes, J.L.; Parra, P.R.; Campos, A.H. Increasing Doses of Simvastatin versus Combined Ezetimibe/Simvastatin: Effect on Circulating Endothelial Progenitor Cells. J. Cardiovasc. Pharmacol. Ther. 2013, 18, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Westerweel, P.E.; Visseren, F.L.; Hajer, G.R.; Olijhoek, J.K.; Hoefer, I.E.; De Bree, P.; Rafii, S.; Doevendans, P.A.; Verhaar, M.C. Endothelial Progenitor Cell Levels in Obese Men with the Metabolic Syndrome and the Effect of Simvastatin Monotherapy vs. Simvastatin/Ezetimibe Combination Therapy. Eur. Heart J. 2008, 29, 2808–2817. [Google Scholar] [CrossRef] [PubMed]
- Paradisi, G.; Bracaglia, M.; Basile, F.; Di’Ipolito, S.; Di Nicuolo, F. Effect of Pravastatin on Endothelial Function and Endothelial Progenitor Cells in Healthy Postmenopausal Women. Clin. Exp. Obstet. Gynecol. 2012, 39, 153–159. [Google Scholar] [PubMed]
- Higashi, Y.; Matsuoka, H.; Umei, H.; Sugano, R.; Fujii, Y.; Soga, J.; Kihara, Y.; Chayama, K.; Imaizumi, T. Endothelial Function in Subjects with Isolated Low HDL Cholesterol: Role of Nitric Oxide and Circulating Progenitor Cells. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E202–E209. [Google Scholar] [CrossRef]
- Lin, L.-Y.; Huang, C.-C.; Chen, J.-S. Effects of Pitavastatin versus Atorvastatin on the Peripheral Endothelial Progenitor Cells and Vascular Endothelial Growth Factor in High-Risk Patients: A Pilot Prospective, Double-Blind, Randomized Study. Cardiovasc. Diabetol. 2014, 13, 111. [Google Scholar] [CrossRef]
- Chiang, K.-H.; Cheng, W.-L.; Shih, C.-M.; Lin, Y.-W.; Tsao, N.-W.; Kao, Y.-T.; Lin, C.-T.; Wu, S.-C.; Huang, C.-Y.; Lin, F.-Y. Statins, HMG-CoA Reductase Inhibitors, Improve Neovascularization by Increasing the Expression Density of CXCR4 in Endothelial Progenitor Cells. PLoS ONE 2015, 10, e0136405. [Google Scholar] [CrossRef][Green Version]
- Cerda, A.; Fajardo, C.M.; Basso, R.G.; Hirata, M.H.; Hirata, R.D.C. Role of MicroRNAs 221/222 on Statin Induced Nitric Oxide Release in Human Endothelial Cells. Arq. Bras. Cardiol. 2014, 104, 195–201. [Google Scholar] [CrossRef]
- António, N.; Fernandes, R.; Soares, A.; Soares, F.; Lopes, A.; Carvalheiro, T.; Paiva, A.; Pêgo, G.M.; Providência, L.A.; Gonçalves, L.; et al. Impact of Prior Chronic Statin Therapy and High-Intensity Statin Therapy at Discharge on Circulating Endothelial Progenitor Cell Levels in Patients with Acute Myocardial Infarction: A Prospective Observational Study. Eur. J. Clin. Pharm. 2014, 70, 1181–1193. [Google Scholar] [CrossRef]
- Ricottini, E.; Madonna, R.; Patti, G.; Grieco, D.; Zoccoli, A.; Stampachiacchiere, B.; D’Ambrosio, A.; Tonini, G.; Caterina, R.D.; Sciascio, G.D. Benefit of Atorvastatin Reload on Endothelial Progenitor Cells in Patients on Chronic Statin Treatment Undergoing PCI. J. Am. Coll. Cardiol. 2013, 61, E1635. [Google Scholar] [CrossRef][Green Version]
- Di Sciascio, G.; Patti, G.; Pasceri, V.; Gaspardone, A.; Colonna, G.; Montinaro, A. Efficacy of Atorvastatin Reload in Patients on Chronic Statin Therapy Undergoing Percutaneous Coronary Intervention: Results of the ARMYDA-RECAPTURE (Atorvastatin for Reduction of Myocardial Damage During Angioplasty) Randomized Trial. J. Am. Coll. Cardiol. 2009, 54, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Newman, C.B.; Preiss, D.; Tobert, J.A.; Jacobson, T.A.; Page, R.L., II; Goldstein, L.B.; Chin, C.; Tannock, L.R.; Miller, M.; Raghuveer, G.; et al. Statin Safety and As-sociated Adverse Events: A Scientific Statement from the American Heart Association. Arter. Thromb. Vasc. Biol. 2019, 39, e38–e81. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Liu, W.; Zeng, J.; Meng, J.; Jiang, H.; Wang, J.; Xing, D. Niemann-Pick C1-Like 1 Inhibitors for Reducing Cholesterol Absorption. Eur. J. Med. Chem. 2022, 230, 114111. [Google Scholar] [CrossRef] [PubMed]
- Lins, L.C.A.; França, C.N.; Fonseca, F.A.H.; Barbosa, S.P.M.; Matos, L.N.; Aguirre, A.C.; Bianco, H.T.; Do Amaral, J.B.; Izar, M.C. Effects of Ezetimibe on Endothelial Progenitor Cells and Microparticles in High-Risk Patients. Cell Biochem. Biophys. 2014, 70, 687–696. [Google Scholar] [CrossRef]
- Huang, W.-P.; Yin, W.-H.; Chen, J.-S.; Huang, P.-H.; Chen, J.-W.; Lin, S.-J. Fenofibrate Reverses Dysfunction of EPCs Caused by Chronic Heart Failure. J. Cardiovasc. Transl. Res. 2020, 13, 158–170. [Google Scholar] [CrossRef]
- Bardolia, C.; Amin, N.S.; Turgeon, J. Emerging Non-Statin Treatment Options for Lowering Low-Density Lipoprotein Cholesterol. Front. Cardiovasc. Med. 2021, 8, 789931. [Google Scholar] [CrossRef]
- Tang, Y.; Li, S.-L.; Hu, J.-H.; Sun, K.-J.; Liu, L.-L.; Xu, D.-Y. Research Progress on Alternative Non-Classical Mechanisms of PCSK9 in Atherosclerosis in Patients with and without Diabetes. Cardiovasc. Diabetol. 2020, 19, 33. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, R.; Garg, J.; Shah, N.; Sumner, A. PCSK9 Inhibitors: A New Era of Lipid Lowering Therapy. World J. Cardiol. 2017, 9, 76–91. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Puri, R.; Anderson, T.; Ballantyne, C.M.; Cho, L.; Kastelein, J.J.P.; Koenig, W.; Somaratne, R.; Kassahun, H.; Yang, J.; et al. Effect of Evolocumab on Progression of Coronary Disease in Statin-Treated Patients: The GLAGOV Randomized Clinical Trial. JAMA 2016, 316, 2373–2384. [Google Scholar] [CrossRef]
- Lepor, N.E.; Sun, J.; Canton, G.; Contreras, L.; Hippe, D.S.; Isquith, D.A.; Balu, N.; Kedan, I.; Simonini, A.A.; Yuan, C. Regression in Carotid Plaque Lipid Content and Neovasculature with PCSK9 Inhibition: A Time Course Study. Atherosclerosis 2021, 327, 31–38. [Google Scholar] [CrossRef]
- Schwartz, G.G.; Steg, P.G.; Szarek, M.; Bhatt, D.L.; Bittner, V.A.; Diaz, R.; Edelberg, J.M.; Goodman, S.G.; Hanotin, C.; Harrington, R.A.; et al. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N. Engl. J. Med. 2018, 379, 2097–2107. [Google Scholar] [CrossRef] [PubMed]
- Sabatine, M.S.; De Ferrari, G.M.; Giugliano, R.P.; Huber, K.; Lewis, B.S.; Ferreira, J.; Kuder, J.F.; Murphy, S.A.; Wiviott, S.D.; Kurtz, C.E. Clinical Benefit of Evolocumab by Severity and Extent of Coronary Artery Disease: Analysis from FOURIER. Circulation 2018, 138, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Tripaldi, R.; Lanuti, P.; Simeone, P.G.; Liani, R.; Bologna, G.; Ciotti, S.; Simeone, P.; Di Castelnuovo, A.; Marchisio, M.; Cipollone, F. Endogenous PCSK9 May Influence Circulating CD45 Neg/CD34 Bright and CD45 Neg/CD34 Bright/CD146 Neg Cells in Patients with Type 2 Diabetes Mellitus. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zadok, O.I.B.; Mager, A.; Leshem-Lev, D.; Lev, E.; Kornowski, R.; Eisen, A. The Effect of Proprotein Convertase Subtilisin Kexin Type 9 Inhibitors on Circulating Endothelial Progenitor Cells in Patients with Cardiovascular Disease. Cardiovasc. Drugs Ther. 2021, 36, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Guedeney, P.; Giustino, G.; Sorrentino, S.; Claessen, B.E.; Camaj, A.; Kalkman, D.N.; Vogel, B.; Sartori, S.; De Rosa, S.; Baber, U. Efficacy and Safety of Alirocumab and Evolocumab: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Eur. Heart J. 2019, 43, e17–e25. [Google Scholar] [CrossRef] [PubMed]
- Santulli, G. Angiopoietin-Like Proteins: A Comprehensive Look. Front. Endocrinol. 2014, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Oike, Y.; Tian, Z.; Miyata, K.; Morinaga, J.; Endo, M.; Kadomatsu, T. ANGPTL2—A New Causal Player in Accelerating Heart Disease Development in the Aging. Circ. J. 2017, 81, 1379–1385. [Google Scholar] [CrossRef]
- Singh, A.K.; Chaube, B.; Zhang, X.; Sun, J.; Citrin, K.M.; Canfrán-Duque, A.; Aryal, B.; Rotllan, N.; Varela, L.; Lee, R.G. Hepatocyte-Specific Suppression of ANGPTL4 Improves Obesity-Associated Diabetes and Mitigates Atherosclerosis in Mice. J. Clin. Investig. 2021, 131, e140989. [Google Scholar] [CrossRef]
- Robciuc, M.R.; Maranghi, M.; Lahikainen, A.; Rader, D.; Bensadoun, A.; Öörni, K.; Metso, J.; Minicocci, I.; Ciociola, E.; Ceci, F. Angptl3 Deficiency Is Associated with Increased Insulin Sensitivity, Lipoprotein Lipase Activity, and Decreased Serum Free Fatty Acids. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1706–1713. [Google Scholar] [CrossRef]
- Dewey, F.E.; Gusarova, V.; Dunbar, R.L.; O’Dushlaine, C.; Schurmann, C.; Gottesman, O.; McCarthy, S.; Van Hout, C.V.; Bruse, S.; Dansky, H.M. Genetic and Pharmacologic Inactivation of ANGPTL3 and Cardiovascular Disease. N. Engl. J. Med. 2017, 377, 211–221. [Google Scholar] [CrossRef]
- Graham, M.J.; Lee, R.G.; Brandt, T.A.; Tai, L.-J.; Fu, W.; Peralta, R.; Yu, R.; Hurh, E.; Paz, E.; McEvoy, B.W. Cardiovascular and Metabolic Effects of ANGPTL3 Antisense Oligonucleotides. N. Engl. J. Med. 2017, 377, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Quagliarini, F.; Wang, Y.; Kozlitina, J.; Grishin, N.V.; Hyde, R.; Boerwinkle, E.; Valenzuela, D.M.; Murphy, A.J.; Cohen, J.C.; Hobbs, H.H. Atypical Angiopoietin-like Protein That Regulates ANGPTL3. Proc. Natl. Acad. Sci. USA 2012, 109, 19751–19756. [Google Scholar] [CrossRef] [PubMed]
- Schinzari, F.; Vizioli, G.; Campia, U.; Tesauro, M.; Cardillo, C. Variable Changes of Circulating ANGPTL3 and ANGPTL4 in Different Obese Phenotypes: Relationship with Vasodilator Dysfunction. Biomedicines 2021, 9, 1037. [Google Scholar] [CrossRef] [PubMed]
- Raal, F.J.; Rosenson, R.S.; Reeskamp, L.F.; Hovingh, G.K.; Kastelein, J.J.; Rubba, P.; Ali, S.; Banerjee, P.; Chan, K.-C.; Gipe, D.A. Evinacumab for Homozygous Familial Hypercholesterolemia. N. Engl. J. Med. 2020, 383, 711–720. [Google Scholar] [CrossRef]
- Camenisch, G.; Pisabarro, M.T.; Sherman, D.; Kowalski, J.; Nagel, M.; Hass, P.; Xie, M.-H.; Gurney, A.; Bodary, S.; Liang, X.H.; et al. ANGPTL3 Stimulates Endothelial Cell Adhesion and Migration via Integrin Alpha Vbeta 3 and Induces Blood Vessel Formation In Vivo. J. Biol. Chem. 2002, 277, 17281–17290. [Google Scholar] [CrossRef]
- Caiado, F.; Dias, S. Endothelial Progenitor Cells and Integrins: Adhesive Needs. Fibrogenes. Tissue Repair 2012, 5, 4. [Google Scholar] [CrossRef]
- Luo, F.; Wu, P.; Chen, J.; Guo, Y.; Wang, J.; Li, X.; Fang, Z. ANGPTL3 Possibly Promotes Cardiac Angiogenesis through Improving Proangiogenic Ability of Endothelial Progenitor Cells after Myocardial Infarction. Lipids Health Dis. 2018, 17, 1–3. [Google Scholar] [CrossRef]
- Lui, M.; Garberich, R.; Strauss, C.; Davin, T.; Knickelbine, T. Usefulness of Lipid Apheresis in the Treatment of Familial Hypercholesterolemia. J. Lipids 2014, 2014, 864317. [Google Scholar] [CrossRef]
- Thompson, G.; Parhofer, K.G. Current Role of Lipoprotein Apheresis. Curr. Atheroscler. Rep. 2019, 21, 26. [Google Scholar] [CrossRef]
- Wang, A.; Richhariya, A.; Gandra, S.R.; Calimlim, B.; Kim, L.; Quek, R.G.; Nordyke, R.J.; Toth, P.P. Systematic Review of Low-Density Lipoprotein Cholesterol Apheresis for the Treatment of Familial Hypercholesterolemia. J. Am. Heart Assoc. 2016, 5, e003294. [Google Scholar] [CrossRef]
- Tavori, H.; Giunzioni, I.; Linton, M.F.; Fazio, S. Loss of Plasma Proprotein Convertase Subtilisin/Kexin 9 (PCSK9) after Lipoprotein Apheresis. Circ. Res. 2013, 113, 1290–1295. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, M.; Hiramori, K.; Imaizumi, T.; Kitabatake, A.; Hishida, H.; Nomura, M.; Fujii, T.; Sakuma, I.; Fukami, K.; Honda, T.; et al. Intravascular Ultrasound Evaluation of Coronary Plaque Regression by Low Density Lipoprotein-Apheresis in Familial Hypercholesterolemia: The Low Density Lipoprotein-Apheresis Coronary Morphology and Reserve Trial (LACMART). J. Am. Coll. Cardiol. 2002, 40, 220–227. [Google Scholar] [CrossRef]
- Banerjee, S.; Luo, P.; Reda, D.J.; Latif, F.; Hastings, J.L.; Armstrong, E.J.; Bagai, J.; Abu-Fadel, M.; Baskar, A.; Kamath, P. Plaque Regression and Endothelial Progenitor Cell Mobilization with Intensive Lipid Elimination Regimen (PREMIER). Circ. Cardiovasc. Interv. 2020, 13, e008933. [Google Scholar] [CrossRef] [PubMed]
- Mörtzell Henriksson, M.; Newman, E.; Witt, V.; Derfler, K.; Leitner, G.; Eloot, S.; Dhondt, A.; Deeren, D.; Rock, G.; Ptak, J.; et al. Adverse Events in Apheresis: An Update of the WAA Registry Data. Transfus. Apher. Sci. 2016, 54, 2–15. [Google Scholar] [CrossRef] [PubMed]
| Substance | Study Population | Major Findings in Study Groups |
|---|---|---|
| Atorvastatin reloading with 80 mg [76,77] | 53 patients on long-term statin treatment who underwent percutaneous coronary interventions (PCI) | ↑ EPC count ↑ EPC-CFU |
| Atorvastatin 80 mg vs. atorvastatin 10 mg preloading [78] | 20 statin-naïve male patients undergoing angiography | 3.5-fold increase in EPC levels in the 80 mg group |
| Atorvastatin 40 mg vs. atorvastatin 10 mg [79] | 26 patients with ischemic heart failure | ↑ EPC ↑ FMD ↓ TNF-α |
| Atorvastatin 40 mg vs. atorvastatin 10 mg vs. placebo [80] | 58 patients with coronary heart disease | ↑ EPC in both atorvastatin groups ↓ VEGF and CRP |
| Atorvastatin 80 mg reloading vs. 40 mg vs. no statin [81] | 45 patients undergoing coronary angioplasty | ↑ early EPCs in 80 mg group ↓ increase in cardiac troponin |
| Atorvastatin 40 mg vs. 10 mg [82] | 100 patients with ischemic cardiomyopathy | ↑ EPC ↓ hsCRP, oxLDL |
| Atorvastatin 40 mg vs. control group [83] | 108 patients with coronary slow flow | ↑ EPCs ↑ EPC adhesion, migration and proliferation ↑ NO ↓ hs-CRP, ET-1 and IL-6 |
| Atorvastatin 40 mg vs. placebo [84] | 60 consecutive patients who underwent isolated, first-time CABG | ↑ early EPCs ↓ hsCRP Less atrial fibrillation |
| Atorvastatin 20 mg vs. placebo [85] | 50 patients undergoing elective coronary surgery | ↑ EPCs |
| Atorvastatin 80 mg vs. atorvastatin 20 mg [86] | 40 ST-segment elevation myocardial infarction (STEMI) patients undergoing PCI | ↑ EPCs |
| Atorvastatin 20 mg vs. placebo [87] | 68 patients with chronic pulmonary heart disease | ↑ EPCs |
| Atorvastatin 20 mg vs. no statin [88] | 48 patients with a first-time non-lacunar ischaemic stroke | ↑ EPC increment EPC increment ≥4 CFU-EC predicted favorable clinical outcome |
| Rosuvastatin 40 mg [89] | 26 patients with mixed dyslipidaemia | ↑ EPC count ↑ EPC-CFU |
| Rosuvastatin 10 mg vs. placebo [90] | 60 patients with systolic heart failure | ↑ EPC FMD, VEGF, fibrinogen, MMP-9, IL-6, IL-1β, oxLDL, PerOx, NT-proBNP, and uric acid levels did not correlate with EPC level |
| Rosuvastatin 40 mg vs. placebo [91] | 42 patients with chronic heart failure (CHF) | ↑ EPC ↑ FMD |
| Rosuvastatin 10 mg vs. no treatment [92] | 32 hypercholesterolemic patients | ↑ EPC ↑ FMD |
| Simvastatin 80 mg vs. simvastatin 20/10 mg ezetimibe [93] | 68 patients with coronary artery disease | no effect on EPC |
| Simvastatin 80 mg mono-treatment with combination treatment of 10 mg simvastatin and 10 mg ezetimibe [94] | 19 obese men with the metabolic syndrome | ↑ EPCs regardless of study group |
| Pravastatin 40 mg vs. placebo [95] | 20 healthy postmenopausal women | ↑ EPC-CFU |
| Pravastatin 10 mg vs. placebo [96] | 29 patients with isolated low HDL cholesterol | ↑ EPC ↑ FMD |
| Pitavastatin 2 mg vs. atorvastatin 10 mg [97] | 26 patients at high cardiovascular risk | ↑ EPC ↑ eNOS expression ↑ adhesion ability of early EPCs ↑ migration and tube formation capacities of late EPCs |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altabas, V.; Biloš, L.S.K. The Role of Endothelial Progenitor Cells in Atherosclerosis and Impact of Anti-Lipemic Treatments on Endothelial Repair. Int. J. Mol. Sci. 2022, 23, 2663. https://doi.org/10.3390/ijms23052663
Altabas V, Biloš LSK. The Role of Endothelial Progenitor Cells in Atherosclerosis and Impact of Anti-Lipemic Treatments on Endothelial Repair. International Journal of Molecular Sciences. 2022; 23(5):2663. https://doi.org/10.3390/ijms23052663
Chicago/Turabian StyleAltabas, Velimir, and Lora Stanka Kirigin Biloš. 2022. "The Role of Endothelial Progenitor Cells in Atherosclerosis and Impact of Anti-Lipemic Treatments on Endothelial Repair" International Journal of Molecular Sciences 23, no. 5: 2663. https://doi.org/10.3390/ijms23052663
APA StyleAltabas, V., & Biloš, L. S. K. (2022). The Role of Endothelial Progenitor Cells in Atherosclerosis and Impact of Anti-Lipemic Treatments on Endothelial Repair. International Journal of Molecular Sciences, 23(5), 2663. https://doi.org/10.3390/ijms23052663






