Modulatory Effects of Fractalkine on Inflammatory Response and Iron Metabolism of Lipopolysaccharide and Lipoteichoic Acid-Activated THP-1 Macrophages
Abstract
1. Introduction
2. Results
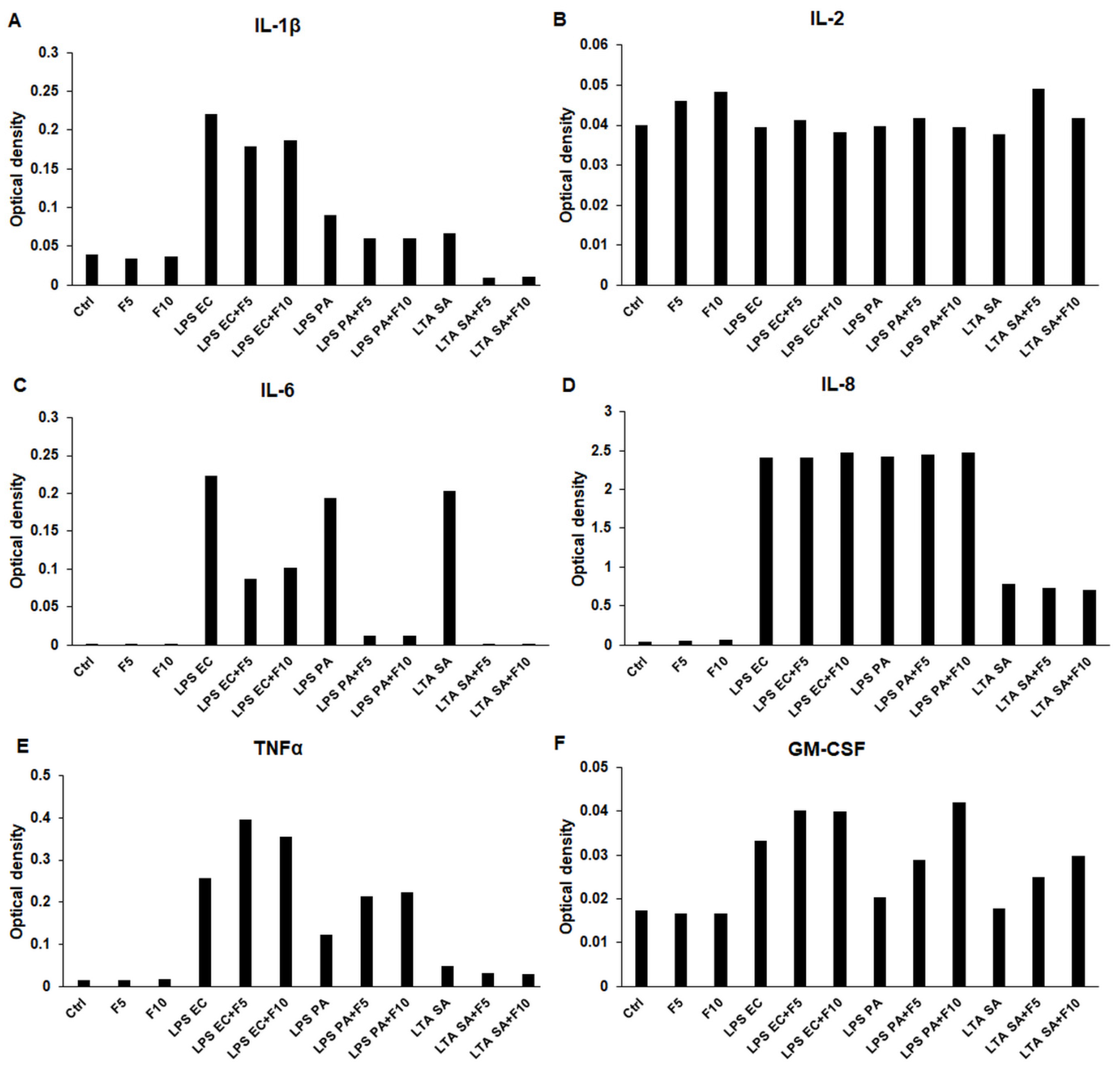
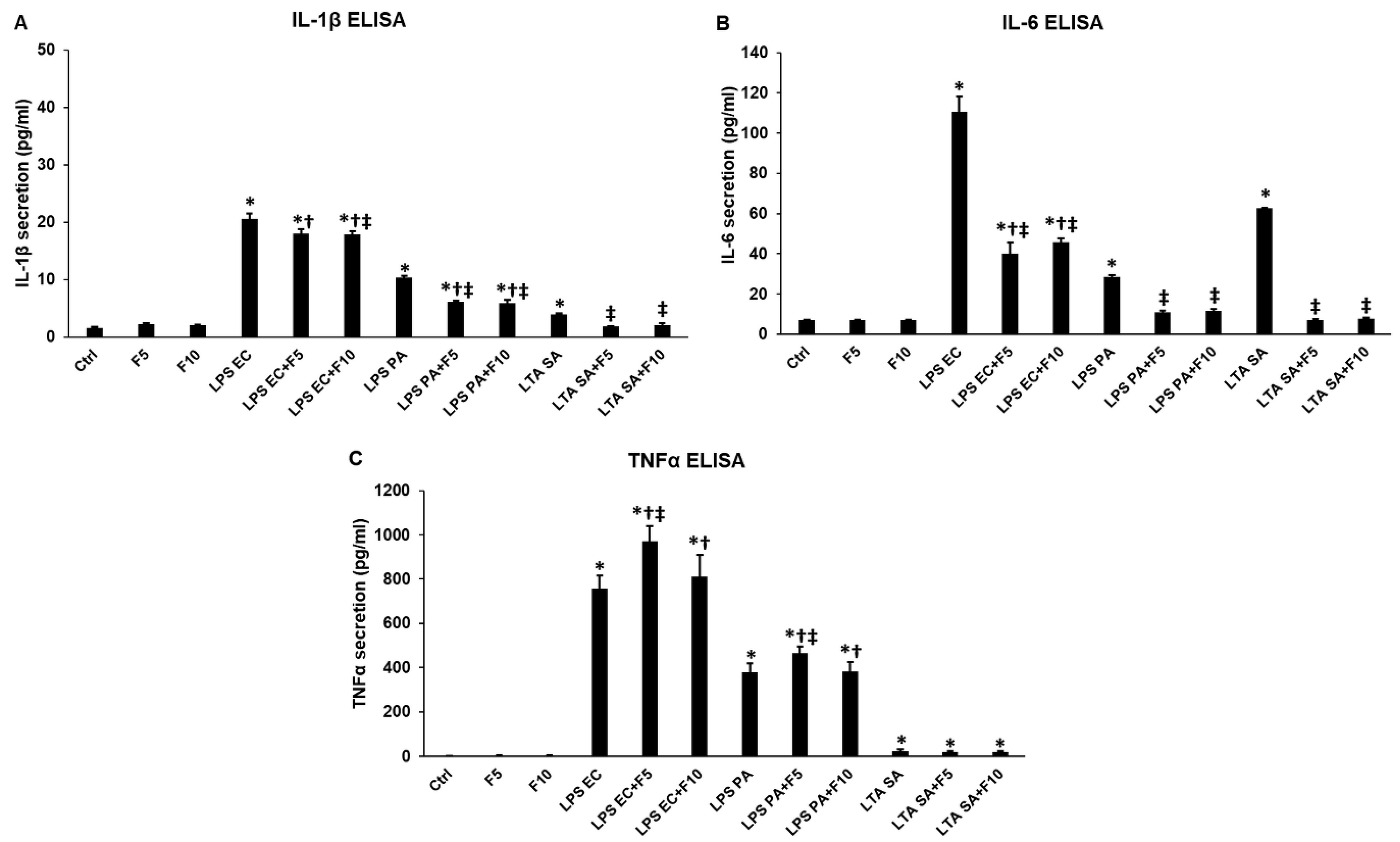
2.1. Effects of Fractalkine on the Pro-Inflammatory Cytokine Expression of LPS or LTA Activated THP-1 Cells
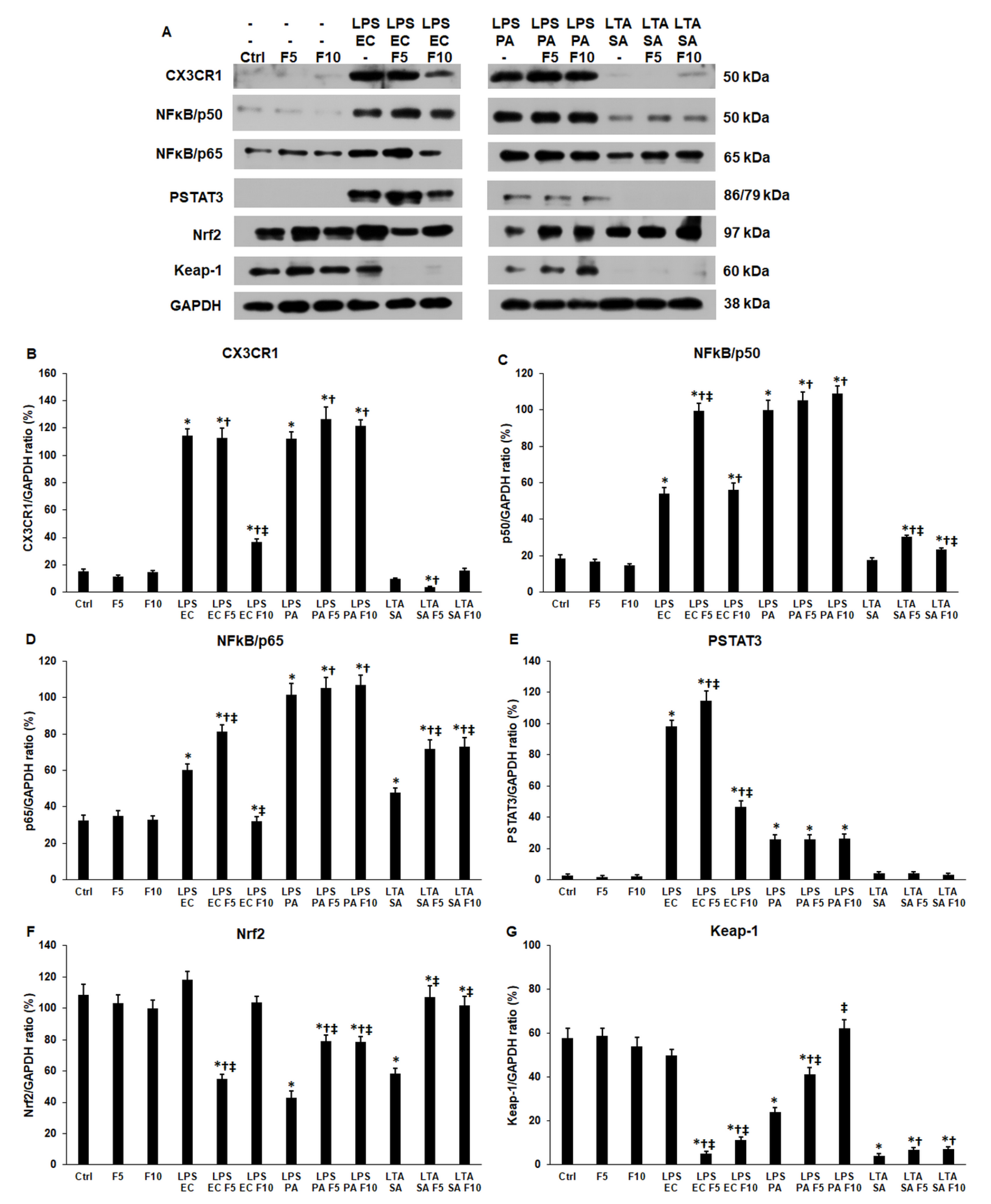
2.2. Fractalkine Affects Differently the Expression of CX3CR1 Receptor and the Downstream Signalling Pathways in LPS or LTA Treated THP-1 Cells
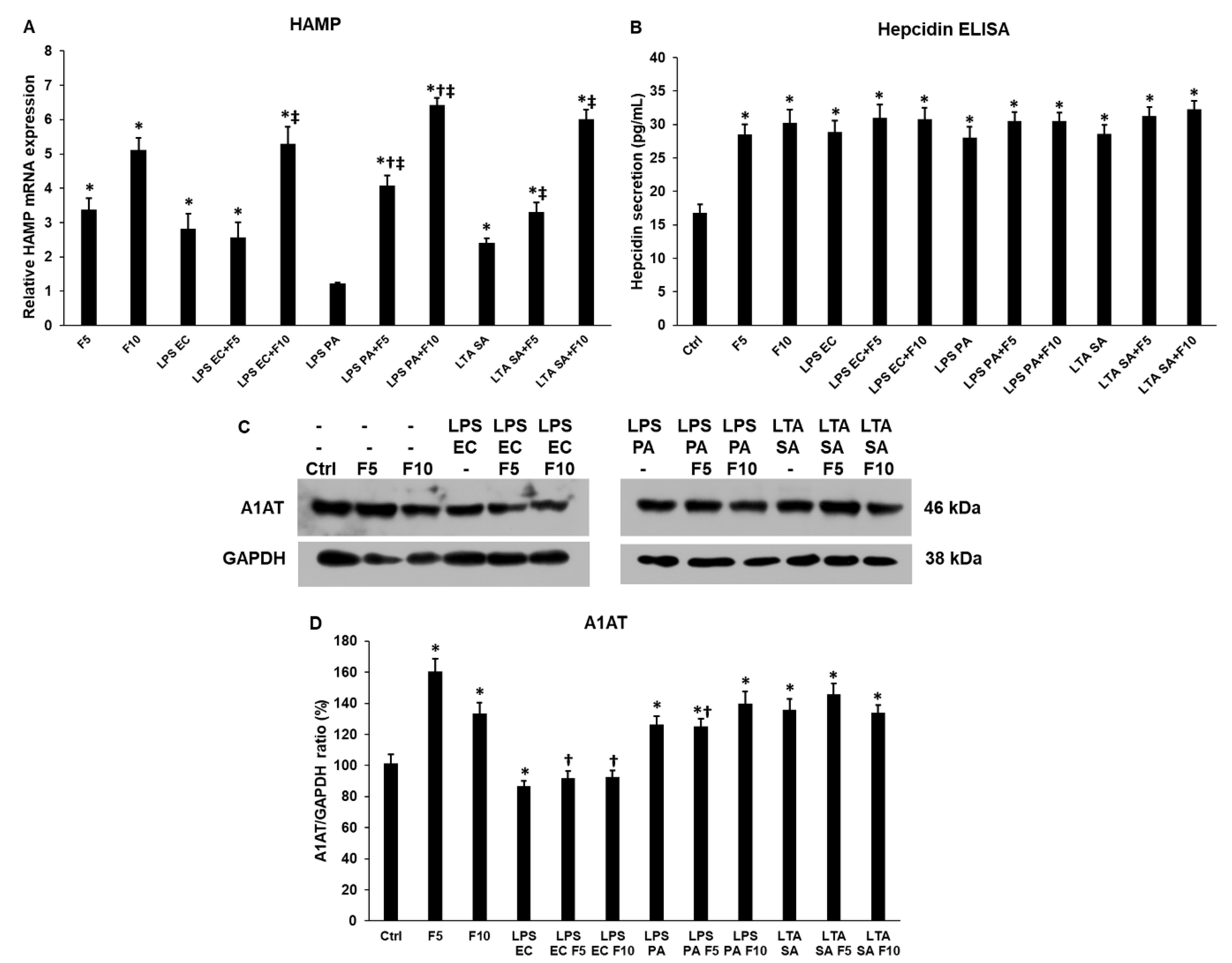
2.3. Fractalkine Increases HAMP mRNA Expression but Not Hepcidin Secretion of THP-1 Cells
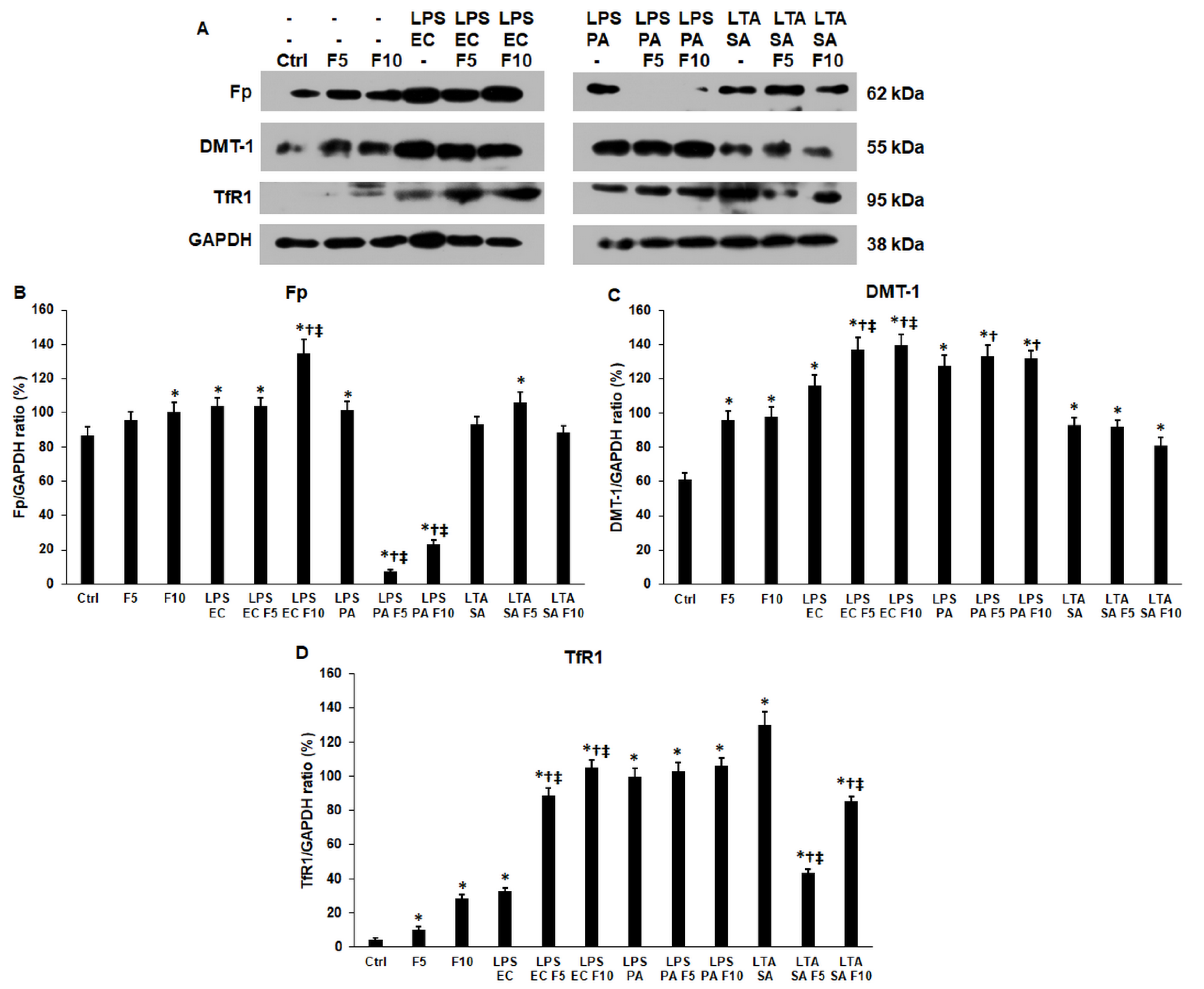
2.4. Fractalkine Modifies the Expression of Iron Transporter Proteins of the LPS and LTA Treated THP-1 Cells
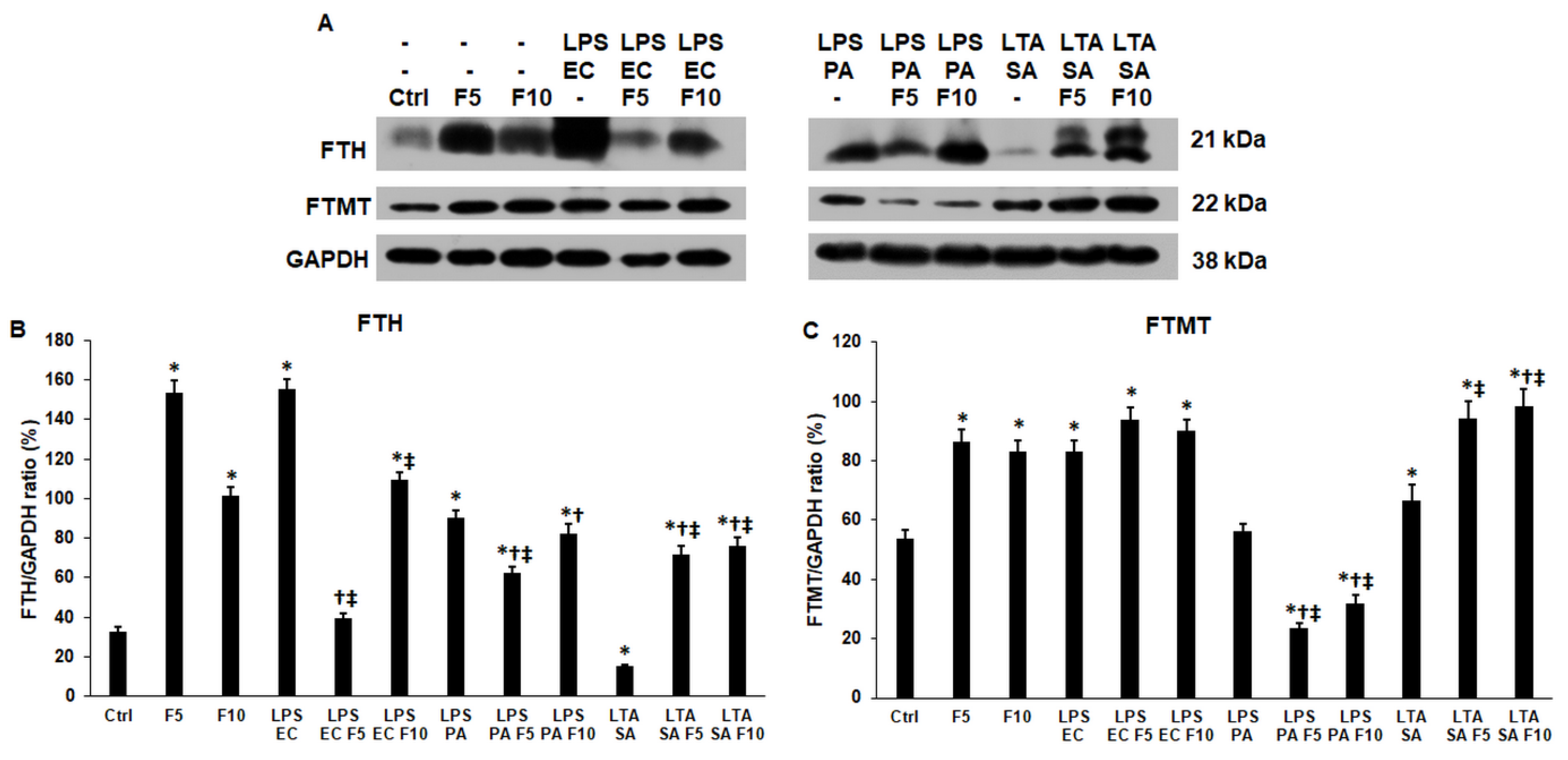
2.5. Fractalkine Affects Differently the Expression of the Cytosolic and Mitochondrial Iron Storage Proteins of the LPS and LTA Treated THP-1 Cells
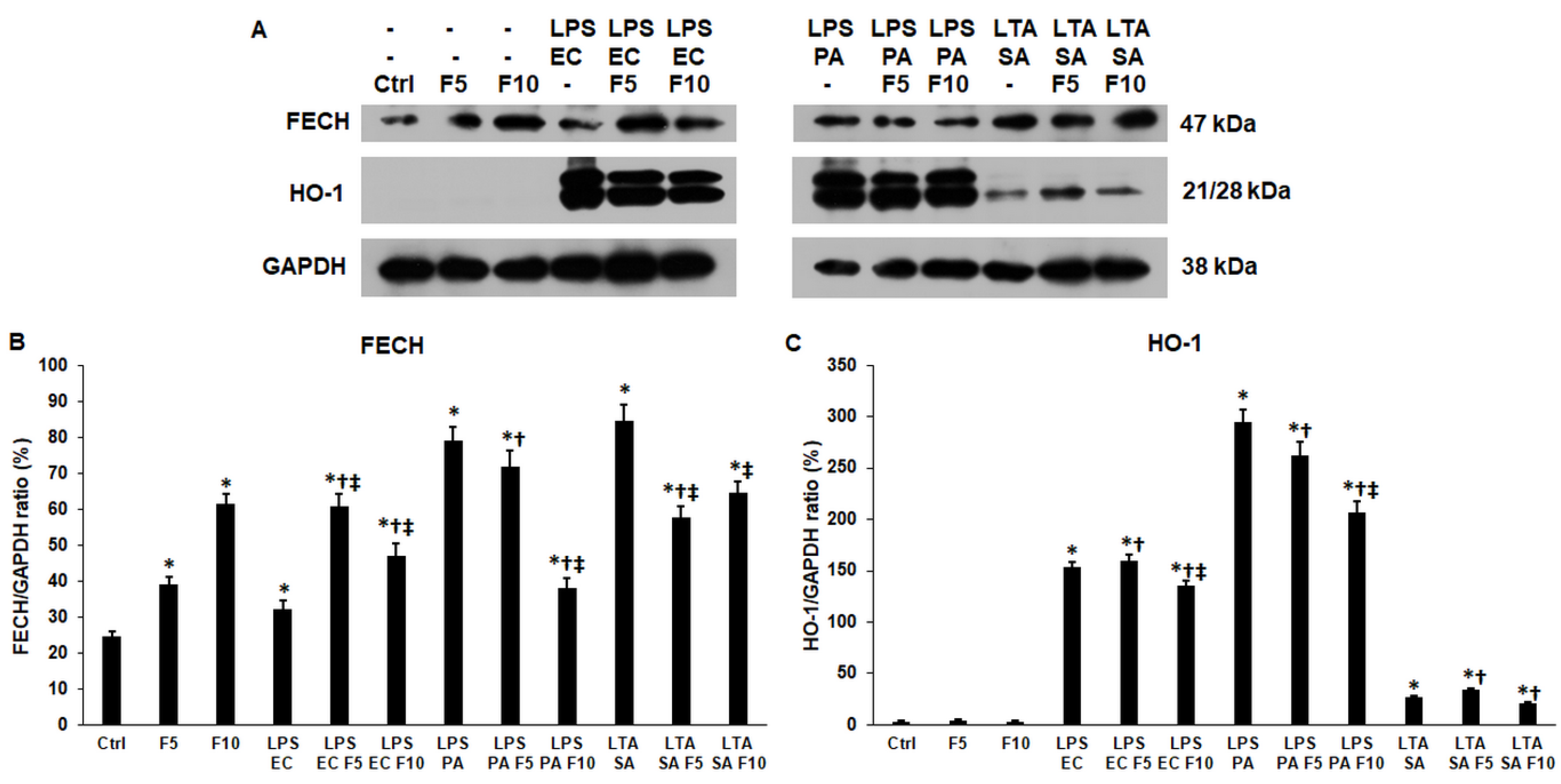
2.6. Fractalkine Alters the Levels of the Enzymes Responsible for the Heme and Iron Sulfur Cluster Syntheses of LPS and LTA Treated THP-1 Cells
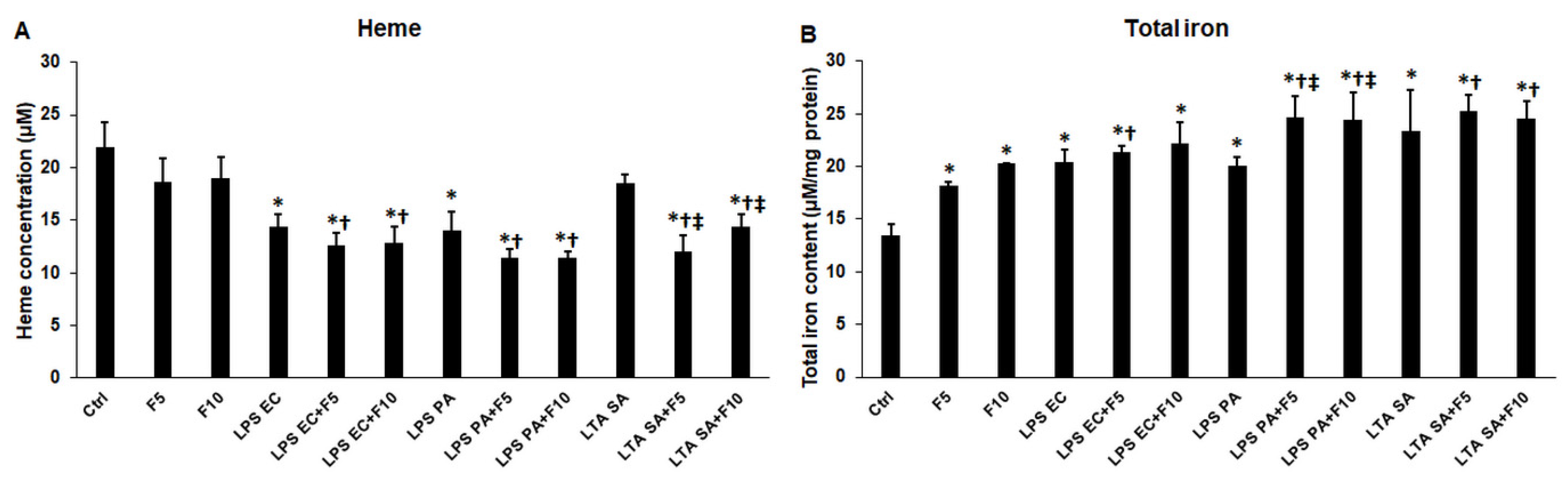
2.7. Fractalkine Changes the Heme Concentration and Iron Content of the Activated THP-1 Cells
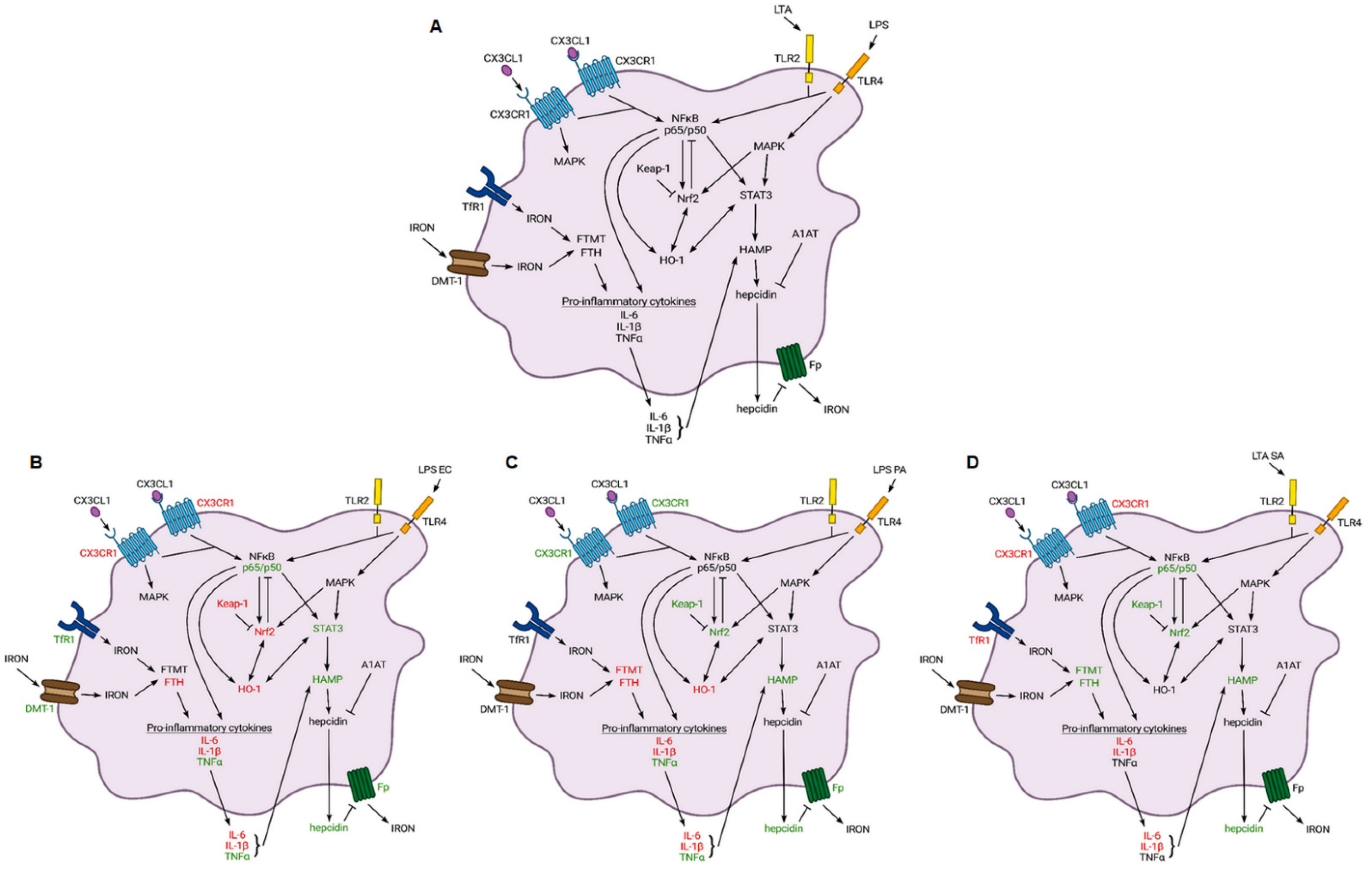
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Treatments
4.2. ELISArray
4.3. Enzyme-Linked Immunosorbent Assay (ELISA)
4.4. Real Time PCR
4.5. Western Blot
4.6. Intracellular Total Iron and Heme Measurements
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage Activation and Polarization: Nomenclature and Experimental Guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Bosco, M.C. Macrophage Polarization: Reaching across the Aisle? J. Allergy Clin. Immunol. 2019, 143, 1348–1350. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Gordon, S. The M1 and M2 Paradigm of Macrophage Activation: Time for Reassessment. F1000Prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef]
- Tarique, A.A.; Logan, J.; Thomas, E.; Holt, P.G.; Sly, P.D.; Fantino, E. Phenotypic, Functional, and Plasticity Features of Classical and Alternatively Activated Human Macrophages. Am. J. Respir. Cell Mol. Biol. 2015, 53, 676–688. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.S.; Sim, J.R.; Yun, C.H.; Han, S.H. Lipoteichoic Acids as a Major Virulence Factor Causing Inflammatory Responses via Toll-like Receptor 2. Arch. Pharm. Res. 2016, 39, 1519–1529. [Google Scholar] [CrossRef]
- El-Zayat, S.R.; Sibaii, H.; Mannaa, F.A. Toll-like Receptors Activation, Signaling, and Targeting: An Overview. Bull. Natl. Res. Cent. 2019, 43, 187. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kawai, T. Toll-like Receptor Signaling Pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef]
- Pandur, E.; Tamási, K.; Pap, R.; Varga, E.; Miseta, A.; Sipos, K. Fractalkine Induces Hepcidin Expression of BV-2 Microglia and Causes Iron Accumulation in SH-SY5Y Cells. Cell. Mol. Neurobiol. 2019, 39, 985–1001. [Google Scholar] [CrossRef]
- White, G.E.; Greaves, D.R. Fractalkine: A Survivor’s Guide Chemokines as Antiapoptotic Mediators. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 589–594. [Google Scholar] [CrossRef]
- Jones, B.A.; Beamer, M.; Ahmed, S. Fractalkine/CX3CL1: A Potential New Target for Inflammatory Diseases. Mol. Interv. 2010, 10, 263–270. [Google Scholar] [CrossRef]
- Umehara, H.; Bloom, E.T.; Okazaki, T.; Nagano, Y.; Yoshie, O.; Imai, T. Fractalkine in Vascular Biology: From Basic Research to Clinical Disease. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Imaizumi, T.; Yoshida, H.; Satoh, K. Regulation of CX3CL1/Fractalkine Expression in Endothelial Cells. J. Atheroscler. Thromb. 2004, 11, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Hatori, K.; Nagai, A.; Heisel, R.; Ryu, J.K.; Kim, S.U. Fractalkine and Fractalkine Receptors in Human Neurons and Glial Cells. J. Neurosci. Res. 2002, 69, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Pandur, E.; Pap, R.; Montskó, G.; Jánosa, G.; Sipos, K.; Kovács, G.L. Fractalkine Enhances Endometrial Receptivity and Activates Iron Transport towards Trophoblast Cells in an in Vitro Co-Culture System of HEC-1A and JEG-3 Cells. Exp. Cell Res. 2021, 403, 112583. [Google Scholar] [CrossRef] [PubMed]
- Pap, R.; Montskó, G.; Jánosa, G.; Sipos, K.; Kovács, G.L.; Pandur, E. Fractalkine Regulates HEC-1A/JEG-3 Interaction by Influencing the Expression of Implantation-Related Genes in an in Vitro Co-Culture Model. Int. J. Mol. Sci. 2020, 21, 3175. [Google Scholar] [CrossRef] [PubMed]
- Arnoux, I.; Audinat, E. Fractalkine Signaling and Microglia Functions in the Developing Brain. Neural Plast. 2015, 2015, 689404. [Google Scholar] [CrossRef]
- Winter, A.N.; Subbarayan, M.S.; Grimmig, B.; Weesner, J.A.; Moss, L.; Peters, M.; Weeber, E.; Nash, K.; Bickford, P.C. Two Forms of CX3CL1 Display Differential Activity and Rescue Cognitive Deficits in CX3CL1 Knockout Mice. J. Neuroinflamm. 2020, 17, 157. [Google Scholar] [CrossRef]
- Schäfer, A.; Schulz, C.; Eigenthaler, M.; Fraccarollo, D.; Kobsar, A.; Gawaz, M.; Ertl, G.; Walter, U.; Bauersachs, J. Novel Role of the Membrane-Bound Chemokine Fractalkine in Platelet Activation and Adhesion. Blood 2004, 103, 407–412. [Google Scholar] [CrossRef]
- Cardona, A.E.; Pioro, E.P.; Sasse, M.E.; Kostenko, V.; Cardona, S.M.; Dijkstra, I.M.; Huang, D.R.; Kidd, G.; Dombrowski, S.; Dutta, R.; et al. Control of Microglial Neurotoxicity by the Fractalkine Receptor. Nat. Neurosci. 2006, 9, 917–924. [Google Scholar] [CrossRef]
- Lee, M.; Lee, Y.; Song, J.; Lee, J.; Chang, S.Y. Tissue-Specific Role of CX3CR1 Expressing Immune Cells and Their Relationships with Human Disease. Immune Netw. 2018, 18, e5. [Google Scholar] [CrossRef]
- Burgess, M.; Wicks, K.; Gardasevic, M.; Mace, K.A. Cx3CR1 Expression Identifies Distinct Macrophage Populations That Contribute Differentially to Inflammation and Repair. ImmunoHorizons 2019, 3, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Q.; Ou, J.; Zhang, S.; Ming, Y. Crosstalk between the CX3CL1/CX3CR1 Axis and Inflammatory Signaling Pathways in Tissue Injury. Curr. Protein Pept. Sci. 2019, 20, 844–854. [Google Scholar] [CrossRef]
- Rivas-Fuentes, S.; Salgado-Aguayo, A.; Arratia-Quijada, J.; Gorocica-Rosete, P. Regulation and Biological Functions of the CX3CL1-CX3CR1 Axis and Its Relevance in Solid Cancer: A Mini-Review. J. Cancer 2021, 12, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T. Macrophages and Systemic Iron Homeostasis. J. Innate Immun. 2012, 4, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Cairo, G.; Recalcati, S.; Mantovani, A.; Locati, M. Iron Trafficking and Metabolism in Macrophages: Contribution to the Polarized Phenotype. Trends Immunol. 2011, 32, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Wlaschek, M.; Singh, K.; Sindrilaru, A.; Crisan, D.; Scharffetter-Kochanek, K. Iron and Iron-Dependent Reactive Oxygen Species in the Regulation of Macrophages and Fibroblasts in Non-Healing Chronic Wounds. Free Radic. Biol. Med. 2019, 133, 262–275. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Geng, J.; Gao, J.; Zhao, H.; Li, J.; Shi, Y.; Yang, B.; Xiao, C.; Linghu, Y.; Sun, X.; et al. Macrophage Achieves Self-Protection against Oxidative Stress-Induced Ageing through the Mst-Nrf2 Axis. Nat. Commun. 2019, 10, 755. [Google Scholar] [CrossRef] [PubMed]
- Herb, M.; Schramm, M. Functions of ROS in Macrophages and Antimicrobial Immunity. Antioxidants 2021, 10, 313. [Google Scholar] [CrossRef]
- Kowdley, K.V.; Gochanour, E.M.; Sundaram, V.; Shah, R.A.; Handa, P. Hepcidin Signaling in Health and Disease: Ironing Out the Details. Hepatol. Commun. 2021, 5, 723–735. [Google Scholar] [CrossRef]
- Hundhausen, C.; Misztela, D.; Berkhout, T.A.; Broadway, N.; Saftig, P.; Reiss, K.; Hartmann, D.; Fahrenholz, F.; Postina, R.; Matthews, V.; et al. The Disintegrin-like Metalloproteinase ADAM10 Is Involved in Constitutive Cleavage of CX3CL1 (Fractalkine) and Regulates CX3CL1-Mediated Cell-Cell Adhesion. Blood 2003, 102, 1186–1195. [Google Scholar] [CrossRef]
- Garton, K.J.; Gough, P.J.; Blobel, C.P.; Murphy, G.; Greaves, D.R.; Dempsey, P.J.; Raines, E.W. Tumor Necrosis Factor-α-Converting Enzyme (ADAM17) Mediates the Cleavage and Shedding of Fractalkine (CX3CL1). J. Biol. Chem. 2001, 276, 37993–38001. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wei, Q.; Hu, Y.; Wang, C. Role of Fractalkine in Promoting Inflammation in Sepsis-Induced Multiple Organ Dysfunction. Infect. Genet. Evol. 2020, 85, 104569. [Google Scholar] [CrossRef] [PubMed]
- Thome, A.D.; Standaert, D.G.; Harms, A.S. Fractalkine Signaling Regulates the Inflammatory Response in an α-Synuclein Model of Parkinson Disease. PLoS ONE 2015, 10, e0140566. [Google Scholar] [CrossRef]
- Ferretti, E.; Pistoia, V.; Corcione, A. Role of Fractalkine/CX3CL1 and Its Receptor in the Pathogenesis of Inflammatory and Malignant Diseases with Emphasis on B Cell Malignancies. Mediators Inflamm. 2014, 2014, 480941. [Google Scholar] [CrossRef] [PubMed]
- Pandur, E.; Varga, E.; Tamási, K.; Pap, R.; Nagy, J.; Sipos, K. Effect of Inflammatory Mediators Lipopolysaccharide and Lipoteichoic Acid on Iron Metabolism of Differentiated SH-SY5Y Cells Alters in the Presence of BV-2 Microglia. Int. J. Mol. Sci. 2019, 20, 17. [Google Scholar] [CrossRef]
- Pandur, E.; Tamási, K.; Pap, R.; Jánosa, G.; Sipos, K. Distinct Effects of Escherichia Coli, Pseudomonas Aeruginosa and Staphylococcus Aureus Cell Wall Component-Induced Inflammation on the Iron Metabolism of THP-1 Cells. Int. J. Mol. Sci. 2021, 22, 1497. [Google Scholar] [CrossRef]
- Plitnick, L.M.; Jordan, R.A.; Banas, J.A.; Jelley-Gibbs, D.M.; Walsh, M.C.; Preissler, M.T.; Gosselin, E.J. Lipoteichoic Acid Inhibits Interleukin-2 (IL-2) Function by Direct Binding to IL-2. Clin. Diagn. Lab. Immunol. 2001, 8, 972–979. [Google Scholar] [CrossRef][Green Version]
- Costalonga, M.; Zell, T. Lipopolysaccharide Enhances in Vivo Interleukin-2 Production and Proliferation by Naive Antigen-Specific CD4 T Cells via a Toll-like Receptor 4-Dependent Mechanism. Immunology 2007, 122, 124–130. [Google Scholar] [CrossRef]
- Hamilton, J.A. GM-CSF-Dependent Inflammatory Pathways. Front. Immunol. 2019, 10, 2055. [Google Scholar] [CrossRef]
- Cornut, M.; Bourdonnay, E.; Henry, T. Transcriptional Regulation of Inflammasomes. Int. J. Mol. Sci. 2020, 21, 8087. [Google Scholar] [CrossRef]
- Li, W.; Khor, T.O.; Xu, C.; Shen, G.; Jeong, W.S.; Yu, S.; Kong, A.N. Activation of Nrf2-Antioxidant Signaling Attenuates NFκB-Inflammatory Response and Elicits Apoptosis. Biochem. Pharmacol. 2008, 76, 1485–1489. [Google Scholar] [CrossRef] [PubMed]
- Sangkhae, V.; Nemeth, E. Regulation of the Iron Homeostatic Hormone Hepcidin. Adv. Nutr. 2017, 8, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Kanamori, Y.; Murakami, M.; Sugiyama, M.; Hashimoto, O.; Matsui, T.; Funaba, M. Hepcidin and IL-1β. Vitam. Horm. 2019, 110, 143–156. [Google Scholar] [PubMed]
- Pereira, I.M.; Marote, A.; Salgado, A.J.; Silva, N.A. Filling the Gap: Neural Stem Cells as a Promising Therapy for Spinal Cord Injury. Pharmaceuticals 2019, 12, 65. [Google Scholar] [CrossRef]
- Kagan, P.; Sultan, M.; Tachlytski, I.; Safran, M.; Ben-Ari, Z. Both MAPK and STAT3 Signal Transduction Pathways Are Necessary for IL-6-Dependent Hepatic Stellate Cells Activation. PLoS ONE 2017, 12, e0176173. [Google Scholar] [CrossRef]
- Gagliardo, B.; Kubat, N.; Faye, A.; Jaouen, M.; Durel, B.; Deschemin, J.-C.; Canonne-Hergaux, F.; Sari, M.-A.; Vaulont, S. Pro-Hepcidin Is Unable to Degrade the Iron Exporter Ferroportin Unless Maturated by a Furin-Dependent Process. J. Hepatol. 2009, 50, 394–401. [Google Scholar] [CrossRef]
- Pandur, E.; Nagy, J.; Poór, V.S.; Sarnyai, Á.; Huszár, A.; Miseta, A.; Sipos, K. α-1 Antitrypsin Binds Preprohepcidin Intracellularly and Prohepcidin in the Serum. FEBS J. 2009, 276, 2012–2021. [Google Scholar] [CrossRef]
- Aschemeyer, S.; Qiao, B.; Stefanova, D.; Valore, E.V.; Sek, A.C.; Alex Ruwe, T.; Vieth, K.R.; Jung, G.; Casu, C.; Rivella, S.; et al. Structure-Function Analysis of Ferroportin Defines the Binding Site and an Alternative Mechanism of Action of Hepcidin. Blood 2018, 131, 899–910. [Google Scholar] [CrossRef]
- Billesbølle, C.B.; Azumaya, C.M.; Kretsch, R.C.; Powers, A.S.; Gonen, S.; Schneider, S.; Arvedson, T.; Dror, R.O.; Cheng, Y.; Manglik, A. Structure of Hepcidin-Bound Ferroportin Reveals Iron Homeostatic Mechanisms. Nature 2020, 586, 807–811. [Google Scholar] [CrossRef]
- Moreira, A.C.; Mesquita, G.; Gomes, M.S. Ferritin: An Inflammatory Player Keeping Iron at the Core of Pathogen-Host Interactions. Microorganisms 2020, 8, 589. [Google Scholar] [CrossRef]
- Mesquita, G.; Silva, T.; Gomes, A.C.; Oliveira, P.F.; Alves, M.G.; Fernandes, R.; Almeida, A.A.; Moreira, A.C.; Gomes, M.S. H-Ferritin Is Essential for Macrophages’ Capacity to Store or Detoxify Exogenously Added Iron. Sci. Rep. 2020, 10, 3061. [Google Scholar] [CrossRef] [PubMed]
- Zarjou, A.; Black, L.M.; McCullough, K.R.; Hull, T.D.; Esman, S.K.; Boddu, R.; Varambally, S.; Chandrashekar, D.S.; Feng, W.; Arosio, P.; et al. Ferritin Light Chain Confers Protection against Sepsis-Induced Inflammation and Organ Injury. Front. Immunol. 2019, 10, 131. [Google Scholar] [CrossRef] [PubMed]
- Bourdon, E.; Kang, D.K.; Ghosh, M.C.; Drake, S.K.; Wey, J.; Levine, R.L.; Rouault, T.A. The Role of Endogenous Heme Synthesis and Degradation Domain Cysteines in Cellular Iron-Dependent Degradation of IRP2. Blood Cells Mol. Dis. 2003, 31, 247–255. [Google Scholar] [CrossRef]
- Hull, T.D.; Agarwal, A.; George, J.F. The Mononuclear Phagocyte System in Homeostasis and Disease: A Role for Heme Oxygenase-1. Antioxid. Redox Signal. 2014, 20, 1770–1788. [Google Scholar] [CrossRef] [PubMed]
- Naidu, S.; Wijayanti, N.; Santoso, S.; Kietzmann, T.; Immenschuh, S. An Atypical NF-ΚB-Regulated Pathway Mediates Phorbol Ester-Dependent Heme Oxygenase-1 Gene Activation in Monocytes. J. Immunol. 2008, 181, 4113–4123. [Google Scholar] [CrossRef]
- Vijayan, V.; Wagener, F.A.; Immenschuh, S. The Macrophage Heme-Heme Oxygenase-1 System and Its Role in Inflammation. Biochem. Pharmacol. 2018, 153, 159–167. [Google Scholar] [CrossRef]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef]
- Dulak, J.; Jozkowicz, A. Novel Faces of Heme Oxygenase-1: Mechanisms and Therapeutic Potentials. Antioxid. Redox Signal. 2014, 20, 1673–1676. [Google Scholar] [CrossRef]
- Wajant, H.; Siegmund, D. TNFR1 and TNFR2 in the Control of the Life and Death Balance of Macrophages. Front. Cell Dev. Biol. 2019, 7, 91. [Google Scholar] [CrossRef]
- Lopez, C.A.; Skaar, E.P. The Impact of Dietary Transition Metals on Host-Bacterial Interactions. Cell Host Microbe 2018, 23, 737–748. [Google Scholar] [CrossRef]
- ImageJ. Available online: https://imagej.nih.gov/ij/index.html (accessed on 23 September 1997).
- Riemer, J.; Hoepken, H.H.; Czerwinska, H.; Robinson, S.R.; Dringen, R. Colorimetric Ferrozine-Based Assay for the Quantitation of Iron in Cultured Cells. Anal. Biochem. 2004, 331, 370–375. [Google Scholar] [CrossRef] [PubMed]
| Primer | Sequence 5′ → 3′ |
|---|---|
| HAMP forward | CAGCTGGATGCCCATGTT |
| HAMP reverse | TGCAGCACATCCCACATC |
| β-actin forward | AGAAAATCTGGCACCACACC |
| β-actin reverse | GGGGTGTTGAAGGTGTCAAA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandur, E.; Tamási, K.; Pap, R.; Jánosa, G.; Sipos, K. Modulatory Effects of Fractalkine on Inflammatory Response and Iron Metabolism of Lipopolysaccharide and Lipoteichoic Acid-Activated THP-1 Macrophages. Int. J. Mol. Sci. 2022, 23, 2629. https://doi.org/10.3390/ijms23052629
Pandur E, Tamási K, Pap R, Jánosa G, Sipos K. Modulatory Effects of Fractalkine on Inflammatory Response and Iron Metabolism of Lipopolysaccharide and Lipoteichoic Acid-Activated THP-1 Macrophages. International Journal of Molecular Sciences. 2022; 23(5):2629. https://doi.org/10.3390/ijms23052629
Chicago/Turabian StylePandur, Edina, Kitti Tamási, Ramóna Pap, Gergely Jánosa, and Katalin Sipos. 2022. "Modulatory Effects of Fractalkine on Inflammatory Response and Iron Metabolism of Lipopolysaccharide and Lipoteichoic Acid-Activated THP-1 Macrophages" International Journal of Molecular Sciences 23, no. 5: 2629. https://doi.org/10.3390/ijms23052629
APA StylePandur, E., Tamási, K., Pap, R., Jánosa, G., & Sipos, K. (2022). Modulatory Effects of Fractalkine on Inflammatory Response and Iron Metabolism of Lipopolysaccharide and Lipoteichoic Acid-Activated THP-1 Macrophages. International Journal of Molecular Sciences, 23(5), 2629. https://doi.org/10.3390/ijms23052629







