Thiolated Hydroxypropyl-β-cyclodextrin: A Potential Multifunctional Excipient for Ocular Drug Delivery
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of Thiolated Hydroxypropyl-β-cyclodextrin
2.2. HP-β-CD-SH Mucoadhesivity Determination
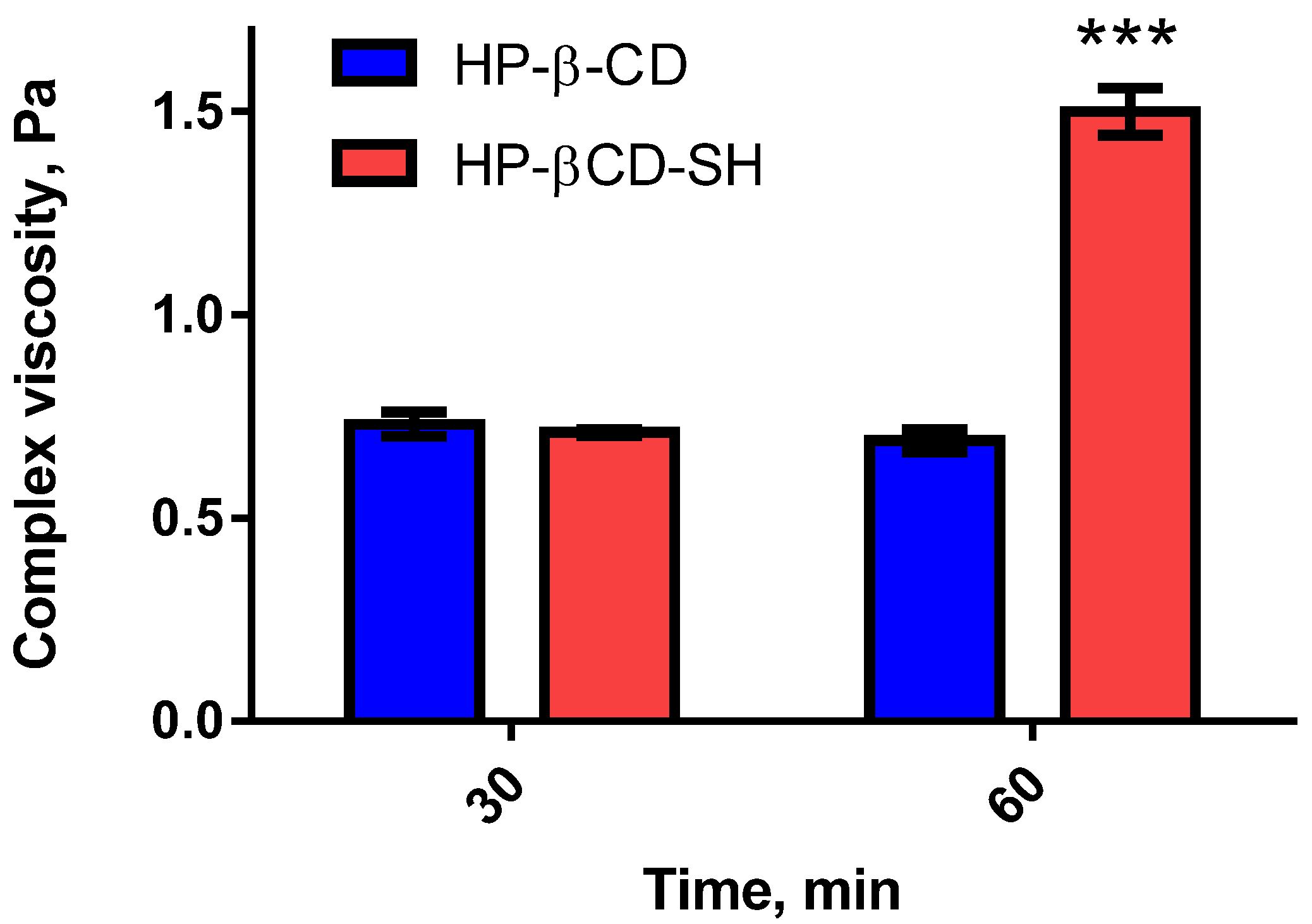
2.2.1. Viscosity Tests
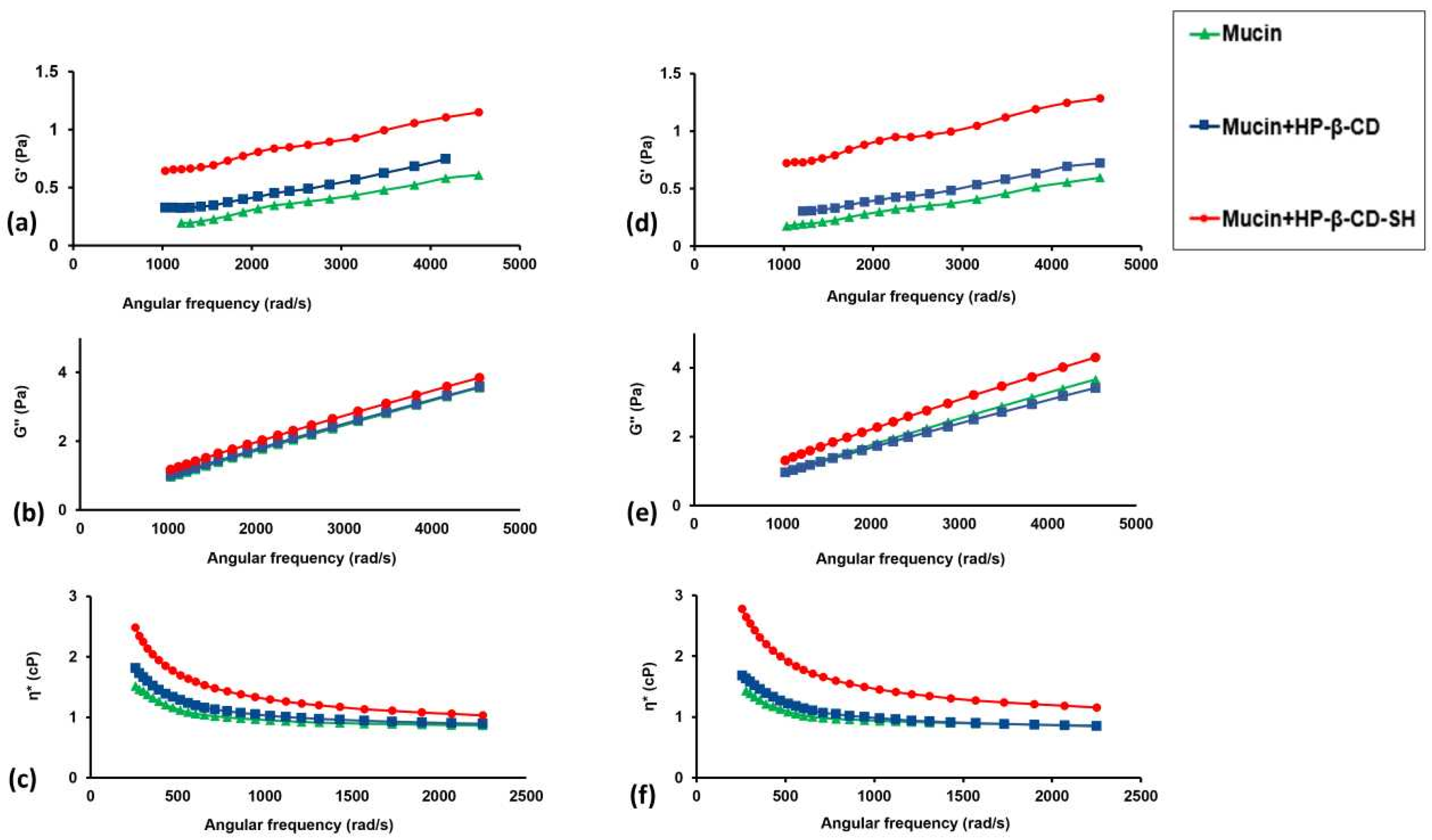
2.2.2. Microrheological Tests
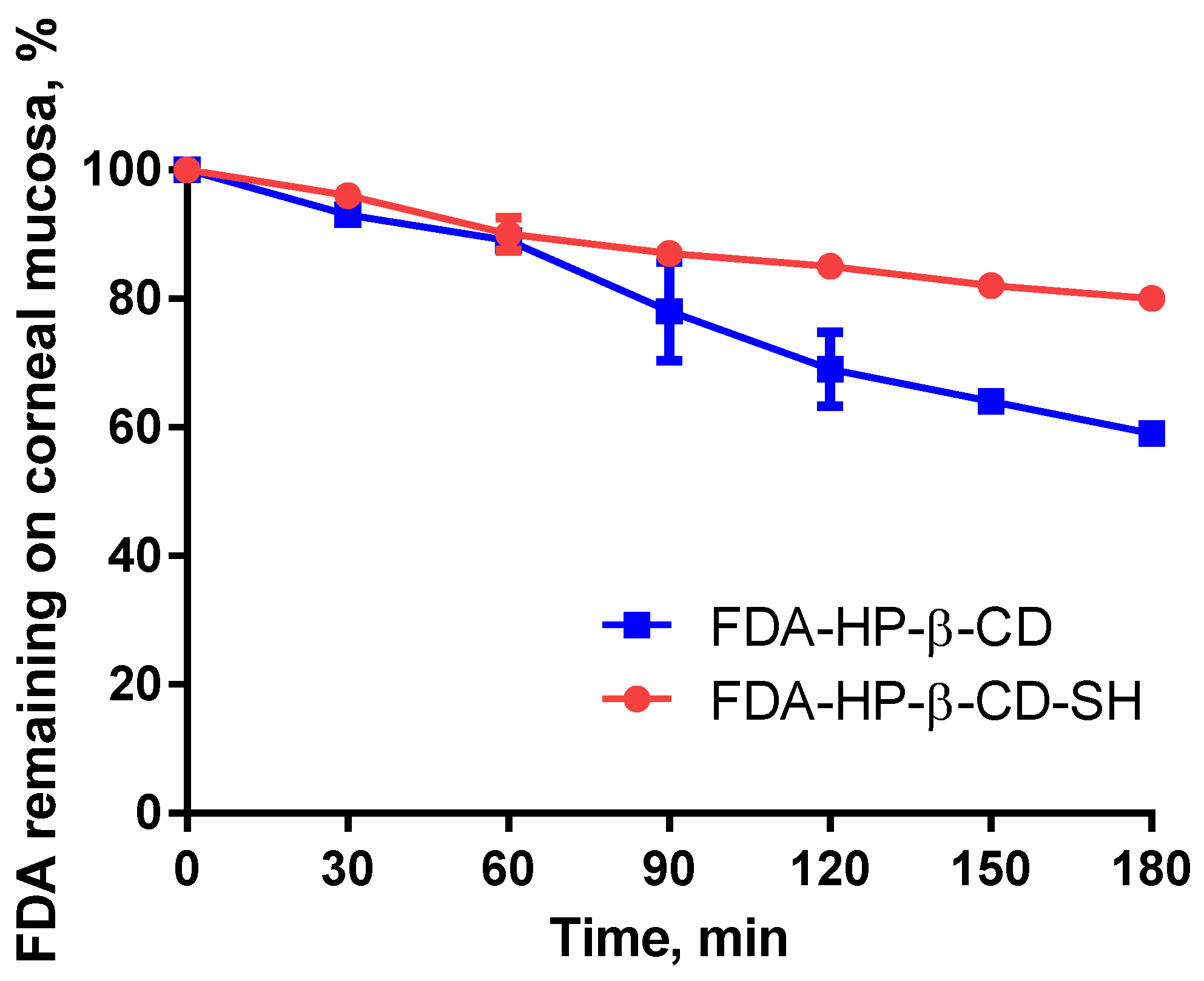
2.2.3. Ex-Vivo Tests
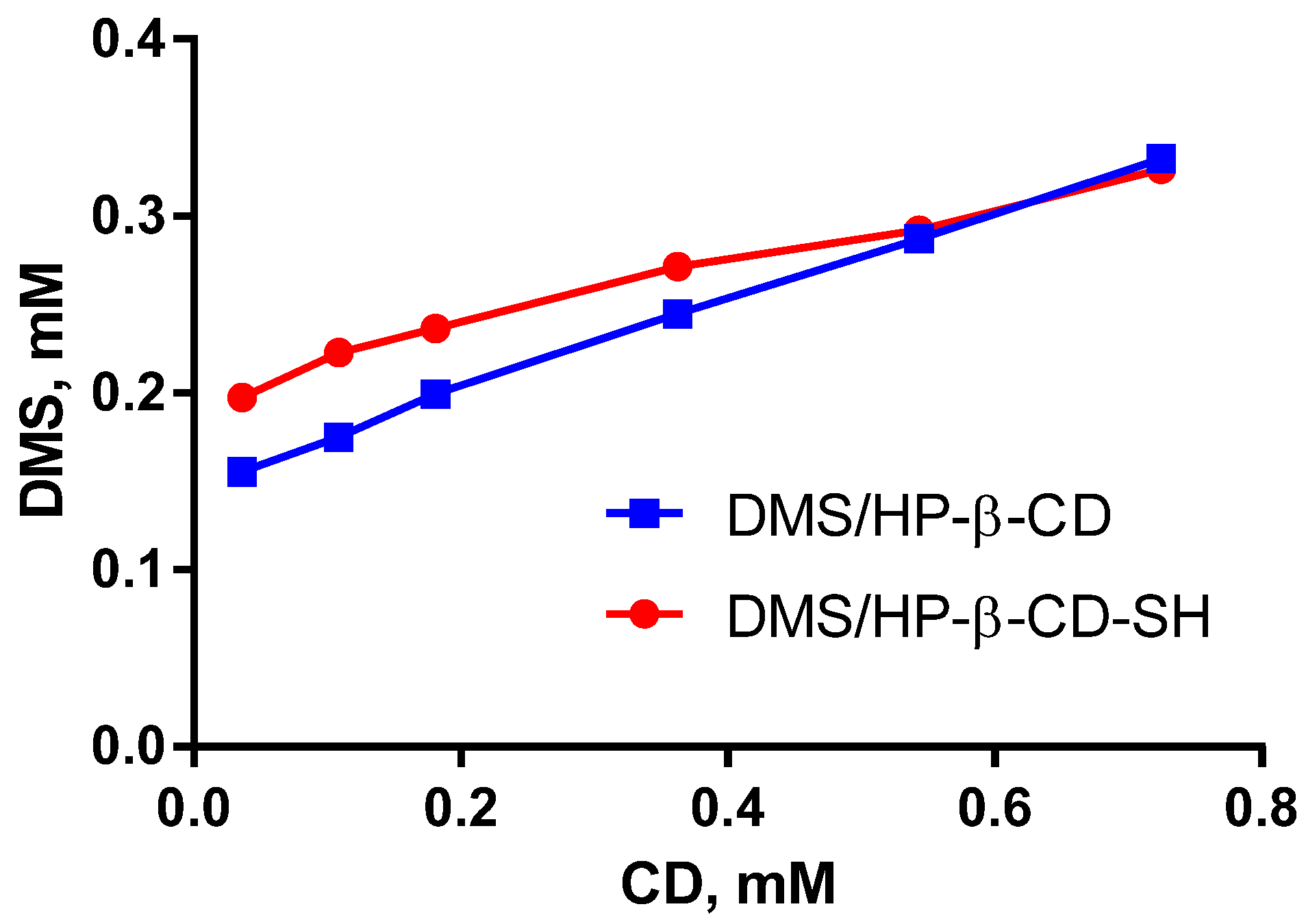
2.3. Determination of the Dexamethasone/CD Association Constant
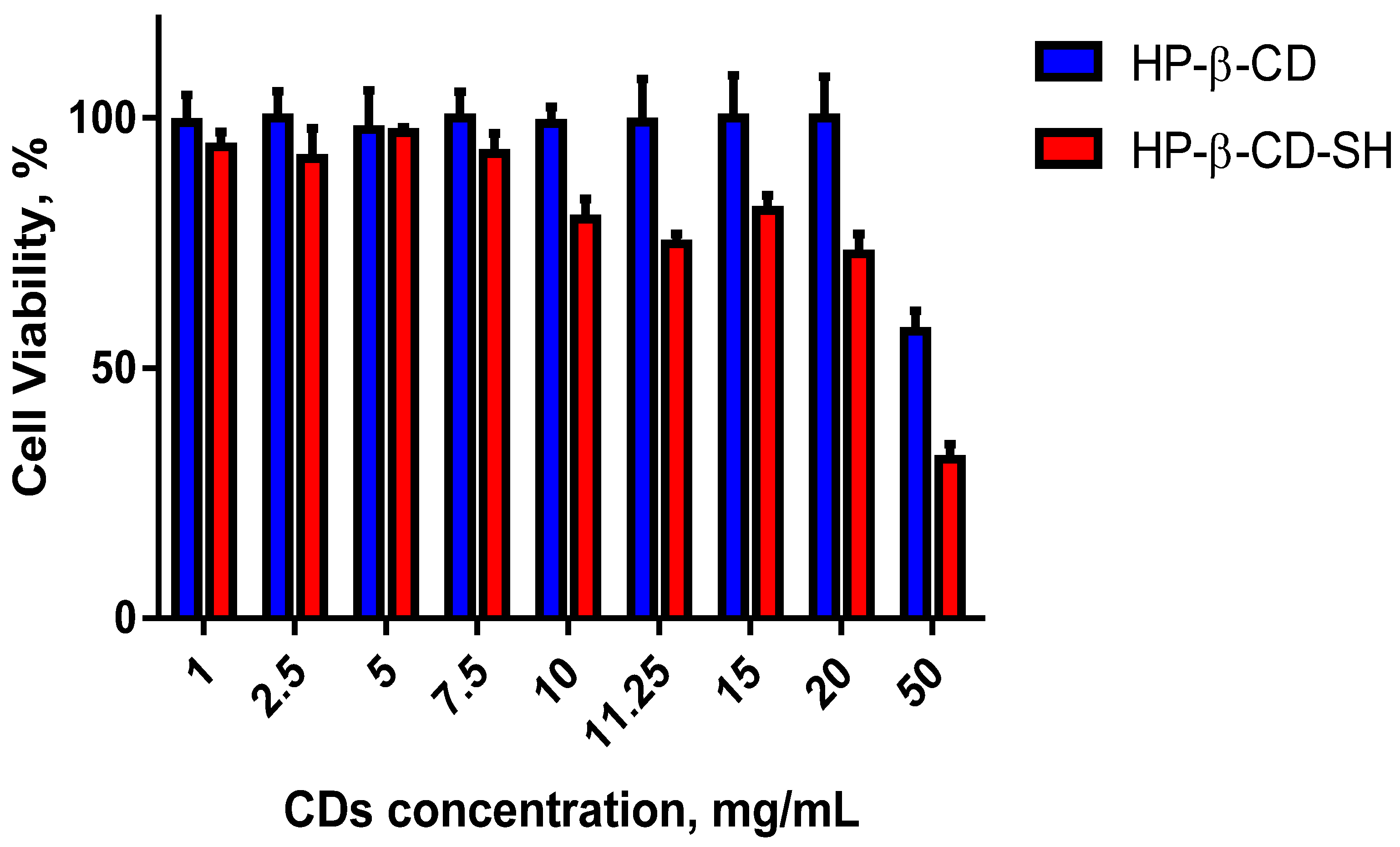
2.4. Cell Viability Assay
2.5. In Vivo Studies
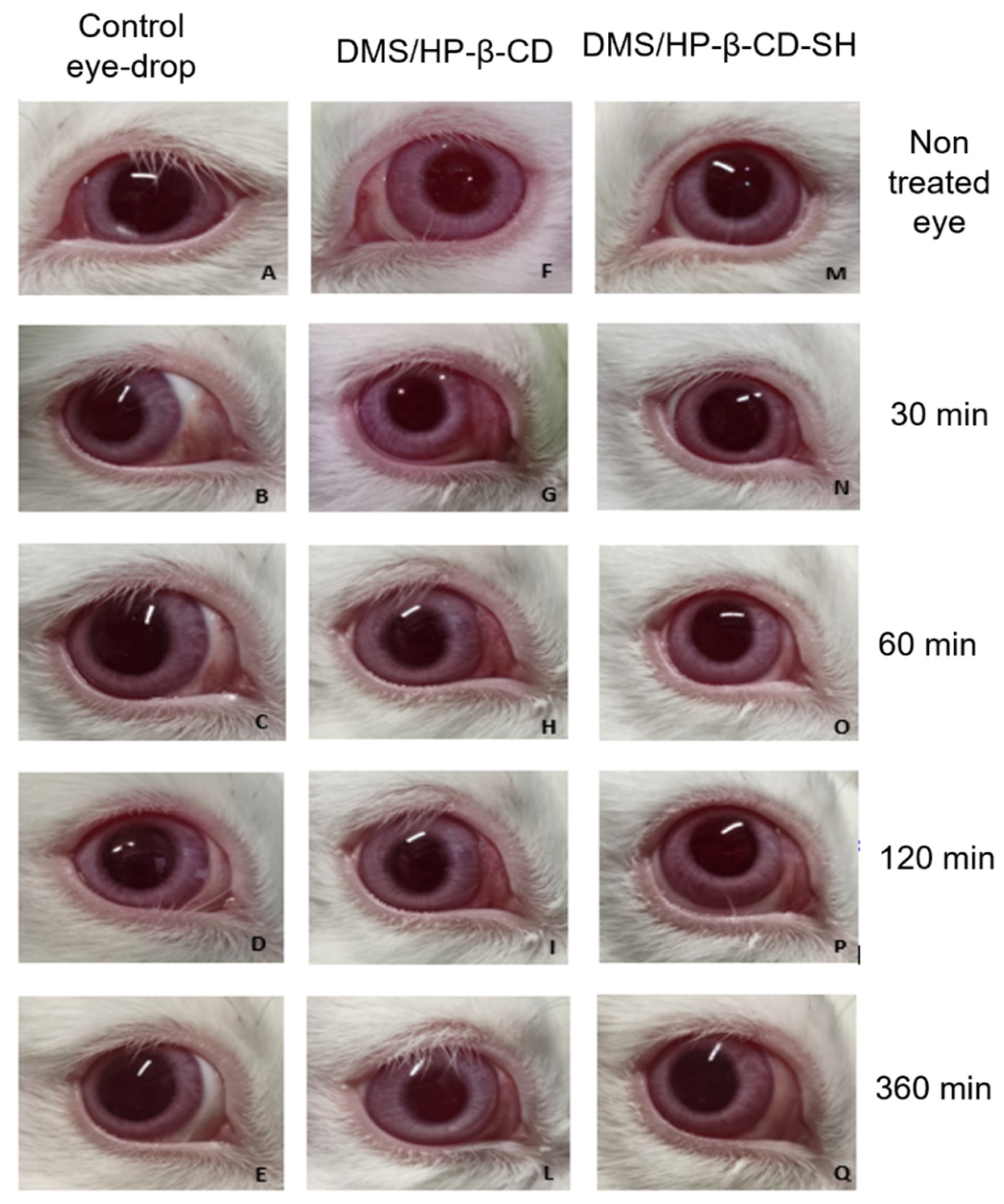
2.5.1. Draize Test
2.5.2. Kinetics of DMS Elimination from Tear Fluid
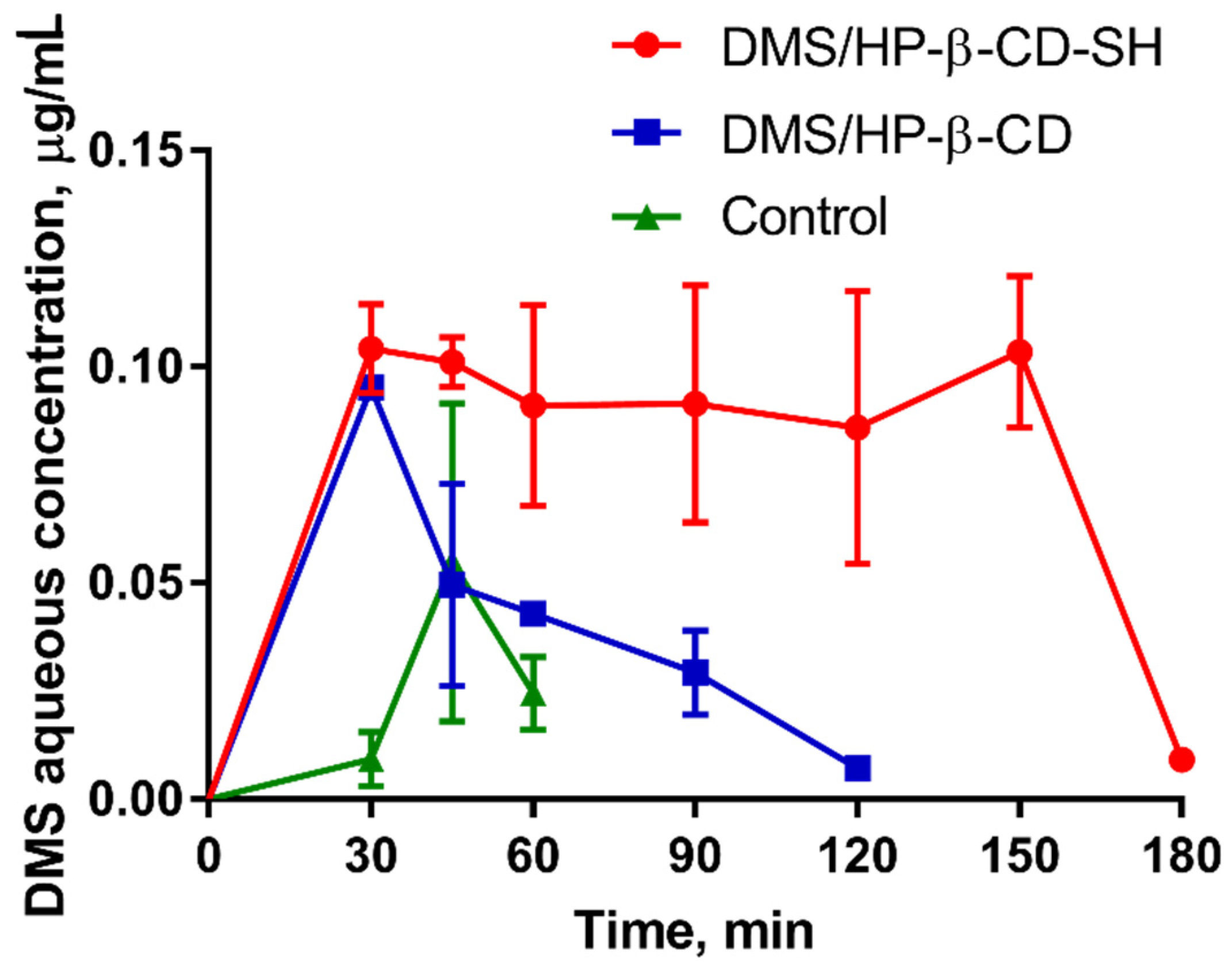
2.5.3. Measurement of DMS Intraocular Penetration
3. Materials and Methods
3.1. Materials
3.2. Thiolation of HP-β-CD via Microwave Irradiation
3.3. Purification of HP-β-CD-SH
3.4. NMR Characterization
3.5. Determination of Thiol Content in HP-β-CD-SH
3.6. Mucoadhesivity Determination of HP-β-CD-SH
3.6.1. Viscosity Tests
3.6.2. Microrheological Tests
3.6.3. Ex Vivo Tests
3.7. Determination of the DMS/Cyclodextrins Association Constant
3.8. Cell Viability Assay
3.9. In Vivo Studies
- 0.3% (w/v) DMS (control);
- 0.3% (w/v) DMS containing 12.5% (w/v) of HP-β-CD (DMS/HP-β-CD);
- 0.3% (w/v) DMS containing 12.5% (w/v) of HP-β-CD-SH (DMS/HP-β-CD-SH).
3.9.1. Draize Test
3.9.2. Determination of DMS Elimination Kinetics from the Tear Fluid
3.9.3. DMS Pharmacokinetics in the Aqueous Humour
3.10. Statistical Data Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Grassiri, B.; Zambito, Y.; Bernkop-Schnürch, A. Strategies to prolong the residence time of drug delivery systems on ocular surface. Adv. Colloid Interface Sci. 2021, 288, 102342. [Google Scholar] [CrossRef]
- Ijaz, M.; Bernkop-Schnürch, A. Preactivated thiomers: Their role in drug delivery. Expert Opin. Drug Deliv. 2015, 12, 1269–1281. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, D.H.; Engelke, A.; Wenz, G. Solubilizing steroidal drugs by β-cyclodextrin derivatives. Int. J. Pharm. 2017, 531, 559–567. [Google Scholar] [CrossRef]
- Loftsson, T.; Stefánsson, E. Cyclodextrins in eye drop formulations: Enhanced topical delivery of corticosteroids to the eye. Acta Ophthalmol. Scand. 2002, 80, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E.; Brewster, M.E. Cyclodextrin-based pharmaceutics: Past, present and future. Nat. Rev. Drug Discov. 2004, 3, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Asim, M.H.; Ijaz, M.; Roesch, A.C.; Bernkop-Schnürch, A. Thiolated cyclodextrins: New perspectives for old excipients. Coord. Chem. Rev. 2020, 420, 213433. [Google Scholar] [CrossRef]
- Asim, M.H.; Nazir, I.; Jalil, A.; Matuszczak, B.; Bernkop-Schnürch, A. Tetradeca-thiolated cyclodextrins: Highly mucoadhesive and in-situ gelling oligomers with prolonged mucosal adhesion. Int. J. Pharm. 2020, 577, 119040. [Google Scholar] [CrossRef] [PubMed]
- Racaniello, G.F.; Laquintana, V.; Summonte, S.; Lopedota, A.; Cutrignelli, A.; Lopalco, A.; Franco, M.; Bernkop-Schnürch, A.; Denora, N. Spray-dried mucoadhesive microparticles based on S-protected thiolated hydroxypropyl-β-cyclodextrin for budesonide nasal delivery. Int. J. Pharm. 2021, 603, 120728. [Google Scholar] [CrossRef]
- Laquintana, V.; Asim, M.H.; Lopedota, A.; Cutrignelli, A.; Lopalco, A.; Franco, M.; Bernkop-Schnürch, A.; Denora, N. Thiolated hydroxypropyl-β-cyclodextrin as mucoadhesive excipient for oral delivery of budesonide in liquid paediatric formulation. Int. J. Pharm. 2019, 572, 118820. [Google Scholar] [CrossRef]
- Mortazavi, S.A.; Smart, J.D. Factors influencing gel-strengthening at the mucoadhesive-mucus interface. J. Pharm. Pharmacol. 1994, 46, 86–90. [Google Scholar] [CrossRef]
- Asim, M.H.; Nazir, I.; Jalil, A.; Laffleur, F.; Matuszczak, B.; Bernkop-Schnürch, A. Per-6-Thiolated Cyclodextrins: A Novel Type of Permeation Enhancing Excipients for BCS Class IV Drugs. ACS Appl. Mater. Interfaces 2020, 12, 7942–7950. [Google Scholar] [CrossRef] [Green Version]
- Asim, M.H.; Moghadam, A.; Ijaz, M.; Mahmood, A.; Götz, R.X.; Matuszczak, B.; Bernkop-Schnürch, A. S-protected thiolated cyclodextrins as mucoadhesive oligomers for drug delivery. J. Colloid Interface Sci. 2018, 531, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Leitner, M.V.; Marschütz, M.K.; Bernkop-Schnürch, A. Mucoadhesive and cohesive properties of poly(acrylic acid)-cysteine conjugates with regard to their molecular mass. Eur. J. Pharm. Sci. 2003, 18, 89–96. [Google Scholar] [CrossRef]
- Lupo, N.; Fodor, B.; Muhammad, I.; Yaqoob, M.; Matuszczak, B.; Bernkop-Schnürch, A. Entirely S-protected chitosan: A promising mucoadhesive excipient for metronidazole vaginal tablets. Acta Biomater. 2017, 64, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Fabiano, A.; Piras, A.M.; Guazzelli, L.; Storti, B.; Bizzarri, R.; Zambito, Y. Impact of Different Mucoadhesive Polymeric Nanoparticles Loaded in Thermosensitive Hydrogels on Transcorneal Administration of 5-Fluorouracil. Pharmaceutics 2019, 11, 623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bansil, R.; Turner, B.S. Mucin structure, aggregation, physiological functions and biomedical applications. Int. J. Pharm. 2006, 22, 164–170. [Google Scholar] [CrossRef]
- Bernkop-Schnürch, A. Thiolated polymers: Bioinspired polymers utilizing one of the most important bridging structure in nature. Adv. Drug Deliv. Rev. 2019, 151–152, 17–24. [Google Scholar]
- Higuchi, T.; Connors, K.A. Phase Solubility Techniques. Adv. Anal. Chem. Instrum. 1965, 4, 117–212. [Google Scholar]
- Worakul, N.; Robinson, J.R. Ocular pharmacokinetics/pharmacodynamics. Eur. J. Pharm. Biopharm. 1997, 44, 71–83. [Google Scholar] [CrossRef]
- Stella, V.J.; He, Q. Cyclodextrins. Toxicol. Pathol. 2008, 36, 30–42. [Google Scholar] [CrossRef]
- EMA. Cyclodextrins Used as Excipients, Report Published in Support of the ‘Questions and Answers on Cyclodextrins Used as Excipients in Medicinal Products for Human Use’ ; EMA/CHMP/495747/2013; EMA: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Gibaldi, M.; Perrier, D. Pharmacokinetics, 2nd ed.; Marcell Dekker: New York, NY, USA, 1982. [Google Scholar]
- Di Colo, G.; Zambito, Y.; Zaino, C.; Sansò, M. Selected polysaccharides at comparison for their mucoadhesiveness and effect on precorneal residence of different drugs in the rabbit model. Drug Dev. Ind. Pharm. 2009, 35, 9–941. [Google Scholar] [CrossRef] [PubMed]
- Usayapant, A.; Karara, A.H.; Narurkar, M.M. Effect of 2-hydroxypropyl-beta-cyclodextrin on the ocular absorption of dexamethasone and dexamethasone acetate. Pharm. Res. 1991, 8, 1495–1499. [Google Scholar] [CrossRef]
- Morrison, P.W.; Connon, C.J.; Khutoryanskiy, V.V. Cyclodextrin-mediated enhancement of riboflavin solubility and corneal permeability. Mol. Pharm. 2013, 10, 756–762. [Google Scholar] [CrossRef]
- Asim, M.H.; Ijaz, M.; Mahmood, A.; Knoll, P.; Jalil, A.; Arshad, S.; Bernkop-Schnürch, A. Thiolated cyclodextrins: Mucoadhesive and permeation enhancing excipients for ocular drug delivery. Int. J. Pharm. 2021, 599, 120451. [Google Scholar] [CrossRef] [PubMed]
- Fabiano, A.; Zambito, Y.; Bernkop-Schnürch, A. About the impact of water movement on the permeation behaviour of nanoparticles in mucus. Int. J. Pharm. 2017, 517, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Dodero, A.; Williams, R.; Gagliardi, S.; Vicini, S.; Alloisio, M.; Castellano, M. A micro-rheological and rheological study of biopolymers solutions: Hyaluronic acid. Carbohydr. Polym. 2019, 203, 349–355. [Google Scholar] [CrossRef]
- Ceulemans, J.; Vermeire, A.; Adriaens, E.; Remon, J.; Ludwig, A. Evaluation of a mucoadhesive tablet for ocular use. J. Control. Release 2011, 77, 333–344. [Google Scholar] [CrossRef]
- Elbahwy, I.A.; Lupo, N.; Ibrahim, H.M.; Ismael, H.R.; Kasem, A.A.; Caliskan, C.; Matuszczak, B.; Bernkop-Schnürch, A. Mucoadhesive self-emulsifying delivery systems for ocular administration of econazole. Int. J. Pharm. 2018, 541, 72–80. [Google Scholar] [CrossRef]
- Hornof, M.; Weyenberg, W.; Ludwig, A.; Bernkop-Schnürch, A. Mucoadhesive ocular insert based on thiolated poly(acrylic acid): Development and in vivo evaluation in humans. J. Control. Release 2003, 89, 419–428. [Google Scholar] [CrossRef]
- Lallemand, F.; Furrer, P.; Felt-Baeyens, O.; Gex-Fabry, M.; Dumont, J.-M.; Besseghir, K.; Gurny, R. A novel water-soluble cyclosporine A prodrug: Ocular tolerance and in vivo kinetics. Int. J. Pharm. 2005, 295, 7–14. [Google Scholar] [CrossRef]
- Baeyens, V.; Felt-Baeyens, O.; Rougier, S.; Pheulpin, S.; Boisrame, B.; Gurny, R. Clinical evaluation of bioadhesive ophthalmic drug inserts (BODI) for the treatment of external ocular infections in dogs. J. Control. Release 2002, 85, 163–168. [Google Scholar] [CrossRef]
- Zambito, Y.; Zaino, C.; di Colo, G. Effects of N-trimethylchitosan on transcellular and paracellular transcorneal drug transport. Eur. J. Pharm. Biopharm. 2006, 64, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Zambito, Y.; Di Colo, G. Thiolated quaternary ammonium-chitosan conjugates for enhanced precorneal retention, transcorneal permeation and intraocular absorption of dexamethasone. Eur. J. Pharm. Biopharm. 2010, 75, 94–199. [Google Scholar] [CrossRef] [PubMed]
- Schoenwald, R.D.; Harris, R.G.; Turner, D.; Knowles, W.; Chien, D.-S. Ophthalmic bioequivalence of steroid/antibiotic combination formulations. Biopharm. Drug Dispos. 1987, 8, 527–548. [Google Scholar] [CrossRef] [PubMed]
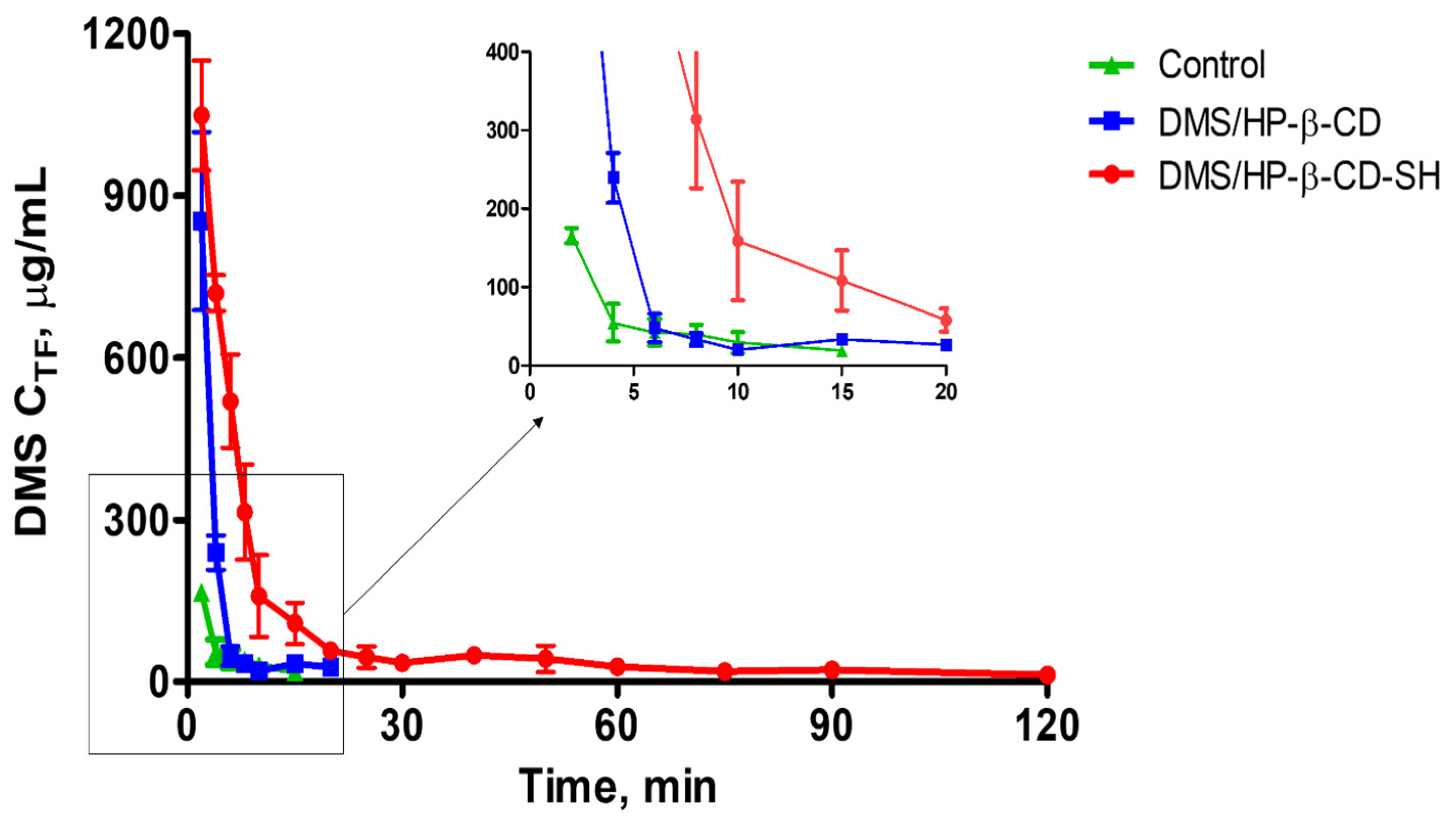
| Formulation | MRT ± SD (min) | RTmax (min) |
|---|---|---|
| Control | 6.2 ± 0.6 | 15 |
| DMS/HP-β-CD | 5.1 ± 0.5 ** | 20 |
| DMS/HP-β-CD-SH | 24.4 ± 1.4 *,** | 120 |
| Formulation | Cmax±SD, μg mL−1 | Tmax, min | AUC±SD, μg mL−1 min | AUCrel |
|---|---|---|---|---|
| Control | 0.055 ± 0.036 | 45 | 1.1 ± 0.4 | - |
| DMS/HP-β-CD | 0.095 ± 0.014 * | 30 | 3.4 ± 0.3 *,** | 3.1 |
| DMS/HP-β-CD-SH | - | - | 12.9 ± 1.0 *,** | 11.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grassiri, B.; Knoll, P.; Fabiano, A.; Piras, A.M.; Zambito, Y.; Bernkop-Schnürch, A. Thiolated Hydroxypropyl-β-cyclodextrin: A Potential Multifunctional Excipient for Ocular Drug Delivery. Int. J. Mol. Sci. 2022, 23, 2612. https://doi.org/10.3390/ijms23052612
Grassiri B, Knoll P, Fabiano A, Piras AM, Zambito Y, Bernkop-Schnürch A. Thiolated Hydroxypropyl-β-cyclodextrin: A Potential Multifunctional Excipient for Ocular Drug Delivery. International Journal of Molecular Sciences. 2022; 23(5):2612. https://doi.org/10.3390/ijms23052612
Chicago/Turabian StyleGrassiri, Brunella, Patrick Knoll, Angela Fabiano, Anna Maria Piras, Ylenia Zambito, and Andreas Bernkop-Schnürch. 2022. "Thiolated Hydroxypropyl-β-cyclodextrin: A Potential Multifunctional Excipient for Ocular Drug Delivery" International Journal of Molecular Sciences 23, no. 5: 2612. https://doi.org/10.3390/ijms23052612
APA StyleGrassiri, B., Knoll, P., Fabiano, A., Piras, A. M., Zambito, Y., & Bernkop-Schnürch, A. (2022). Thiolated Hydroxypropyl-β-cyclodextrin: A Potential Multifunctional Excipient for Ocular Drug Delivery. International Journal of Molecular Sciences, 23(5), 2612. https://doi.org/10.3390/ijms23052612








