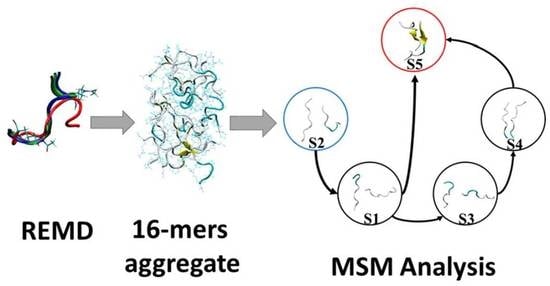
Deciphering the Effect of Lysine Acetylation on the Misfolding and Aggregation of Human Tau Fragment 171IPAKTPPAPK180 Using Molecular Dynamic Simulation and the Markov State Model
Abstract
:1. Introduction
2. Results and Discussion
2.1. Acetylation and Mutation of Lysine Residue (K174) Affects the Overall Structure of Tau Fragment 171IPAKTPPAPK180 Monomer
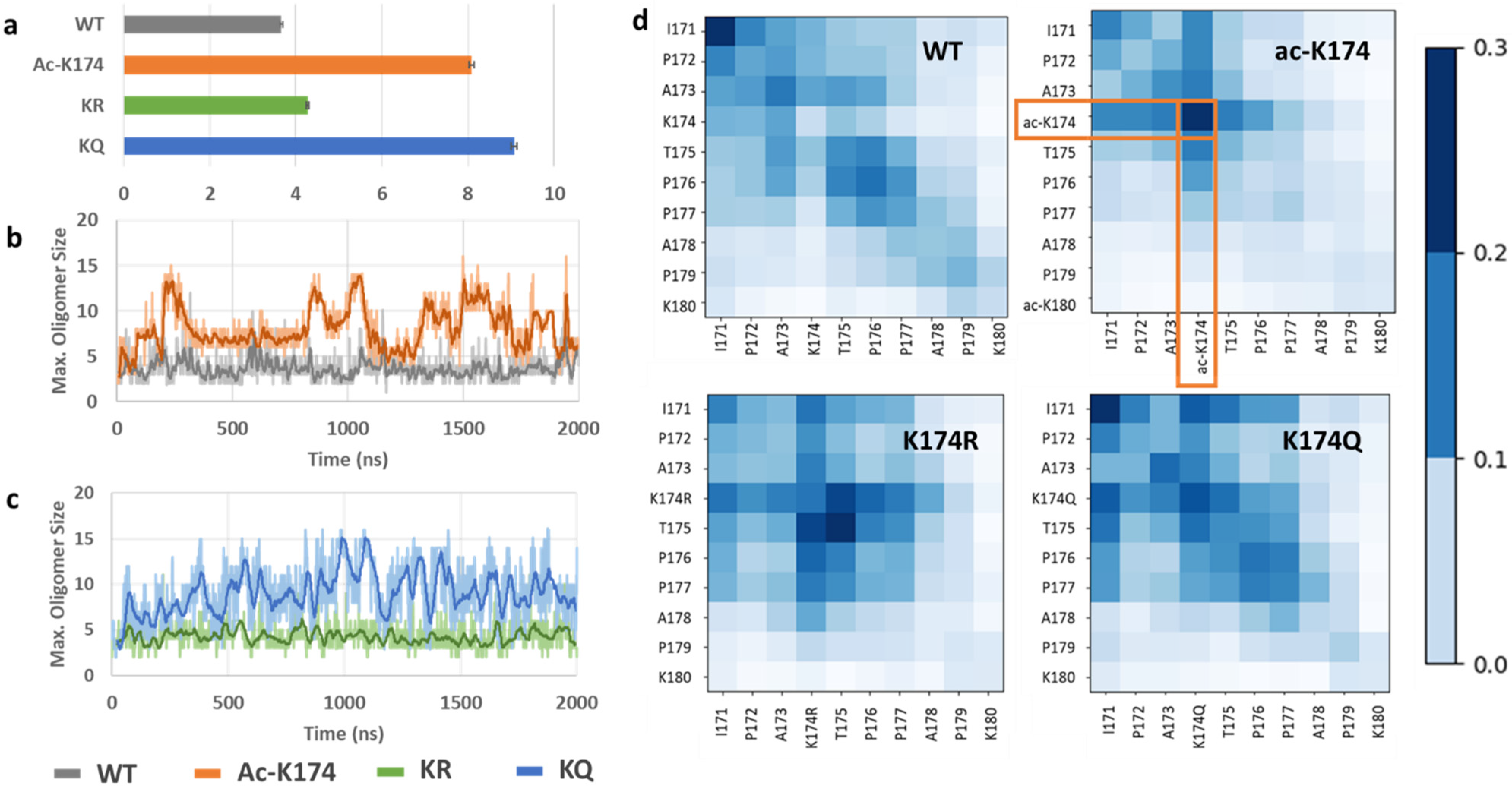
2.2. Spontaneous Aggregation of Acetylated (ac-K174) and Acetylation Mimic (ac-Mimic KQ) Containing Peptides
2.3. Dimerization Mechanism of the Tau 171IPAKTPPAPK180 Fragment under the Influence of Lysine Acetylation (ac-K174)—Insights from MSM Analysis
3. Materials and Methods
3.1. Simulation Details
3.2. Markov State Model (MSM) and Transition Pathway Analysis
3.3. Binding Free Energy and Per-Residue Energy Contribution in Dimer Formation and Stabilization
3.4. Residue Interaction Network Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bloom, G.S. Amyloid-β and tau: The trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014, 71, 505–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lasagna-Reeves, C.A.; Castillo-Carranza, D.L.; Sengupta, U.; Sarmiento, J.; Troncoso, J.; Jackson, G.R.; Kayed, R. Identification of oligomers at early stages of tau aggregation in Alzheimer’s disease. FASEB J. 2012, 26, 1946–1959. [Google Scholar] [CrossRef] [Green Version]
- Ren, Y.; Sahara, N. Characteristics of tau oligomers. Front. Neurol. 2013, 4, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeda, S.; Sahara, N.; Saito, Y.; Murayama, S.; Ikai, A.; Takashima, A. Increased levels of granular tau oligomers: An early sign of brain aging and Alzheimer’s disease. Neurosci. Res. 2006, 54, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Ballatore, C.; Lee, V.M.-Y.; Trojanowski, J.Q. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat. Rev. Neurosci. 2007, 8, 663–672. [Google Scholar] [CrossRef]
- Min, S.-W.; Cho, S.-H.; Zhou, Y.; Schroeder, S.; Haroutunian, V.; Seeley, W.W.; Huang, E.J.; Shen, Y.; Masliah, E.; Mukherjee, C. Acetylation of tau inhibits its degradation and contributes to tauopathy. Neuron 2010, 67, 953–966. [Google Scholar] [CrossRef] [Green Version]
- Cohen, T.J.; Guo, J.L.; Hurtado, D.E.; Kwong, L.K.; Mills, I.P.; Trojanowski, J.Q.; Lee, V.M. The acetylation of tau inhibits its function and promotes pathological tau aggregation. Nat. Commun. 2011, 2, 252. [Google Scholar] [CrossRef] [Green Version]
- Cook, C.; Carlomagno, Y.; Gendron, T.F.; Dunmore, J.; Scheffel, K.; Stetler, C.; Davis, M.; Dickson, D.; Jarpe, M.; DeTure, M. Acetylation of the KXGS motifs in tau is a critical determinant in modulation of tau aggregation and clearance. Hum. Mol. Genet. 2014, 23, 104–116. [Google Scholar] [CrossRef]
- Mandelkow, E.-M.; Mandelkow, E. Biochemistry and cell biology of tau protein in neurofibrillary degeneration. Cold Spring Harb. Perspect. Med. 2012, 2, a006247. [Google Scholar] [CrossRef]
- Min, S.-W.; Chen, X.; Tracy, T.E.; Li, Y.; Zhou, Y.; Wang, C.; Shirakawa, K.; Minami, S.S.; Defensor, E.; Mok, S.A. Critical role of acetylation in tau-mediated neurodegeneration and cognitive deficits. Nat. Med. 2015, 21, 1154–1162. [Google Scholar] [CrossRef] [Green Version]
- Chu, D.; Liu, F. Pathological changes of tau related to Alzheimer’s disease. ACS Chem. Neurosci. 2018, 10, 931–944. [Google Scholar] [CrossRef] [PubMed]
- Perilla, J.R.; Goh, B.C.; Cassidy, C.K.; Liu, B.; Bernardi, R.C.; Rudack, T.; Yu, H.; Wu, Z.; Schulten, K. Molecular dynamics simulations of large macromolecular complexes. Curr. Opin. Struct. Biol. 2015, 31, 64–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feig, M.; Nawrocki, G.; Yu, I.; Wang, P.-h.; Sugita, Y. Challenges and opportunities in connecting simulations with experiments via molecular dynamics of cellular environments. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2018; p. 012010. [Google Scholar]
- Chandrasekaran, P.; Kumar, C.S.; Rangachari, K.; Sekar, K. Disassociation of β1-α1-β2 from the α2-α3 domain of prion protein (PrP) is a prerequisite for the conformational conversion of PrPC into PrPSc: Driven by the free energy landscape. Int. J. Biol. Macromol. 2019, 136, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Narang, S.S.; Shuaib, S.; Goyal, B. Molecular insights into the inhibitory mechanism of rifamycin SV against β2–microglobulin aggregation: A molecular dynamics simulation study. Int. J. Biol. Macromol. 2017, 102, 1025–1034. [Google Scholar] [CrossRef]
- Khatua, P.; Mondal, S.; Bandyopadhyay, S. Effects of metal ions on Aβ42 peptide conformations from molecular simulation studies. J. Chem. Inf. Model. 2019, 59, 2879–2893. [Google Scholar] [CrossRef]
- Rayevsky, A.V.; Sharifi, M.; Samofalova, D.A.; Karpov, P.A.; Blume, Y.B. Structural and functional features of lysine acetylation of plant and animal tubulins. Cell Biol. Int. 2019, 43, 1040–1048. [Google Scholar] [CrossRef]
- Adhikari, R.; Yang, M.; Saikia, N.; Dutta, C.; Alharbi, W.F.; Shan, Z.; Pandey, R.; Tiwari, A. Acetylation of Aβ42 at Lysine 16 Disrupts Amyloid Formation. ACS Chem. Neurosci. 2020, 11, 1178–1191. [Google Scholar] [CrossRef]
- Chodera, J.D.; Noé, F. Markov state models of biomolecular conformational dynamics. Curr. Opin. Struct. Biol. 2014, 25, 135–144. [Google Scholar] [CrossRef] [Green Version]
- Case, D.A.; Ben-Shalom, I.Y.; Brozell, S.R.; Cerutti, D.S.; Cheatham, T.E.; Cruzeiro, V.W.D.; Darden, T.A.; Duke, R.E.; Ghoreishi, D.; Gilson, M.K.; et al. AMBER 18; University of California: San Francisco, CA, USA, 2018. [Google Scholar]
- Khoury, G.A.; Thompson, J.P.; Smadbeck, J.; Kieslich, C.A.; Floudas, C.A. Forcefield_PTM: Ab initio charge and AMBER forcefield parameters for frequently occurring post-translational modifications. J. Chem. Theory Comput. 2013, 9, 5653–5674. [Google Scholar] [CrossRef] [Green Version]
- Khoury, G.A.; Smadbeck, J.; Tamamis, P.; Vandris, A.C.; Kieslich, C.A.; Floudas, C.A. Forcefield_NCAA: Ab initio charge parameters to aid in the discovery and design of therapeutic proteins and peptides with unnatural amino acids and their application to complement inhibitors of the compstatin family. ACS Synth. Biol. 2014, 3, 855–869. [Google Scholar] [CrossRef] [Green Version]
- Qi, R.; Wei, G.; Ma, B.; Nussinov, R. Replica exchange molecular dynamics: A practical application protocol with solutions to common problems and a peptide aggregation and self-Assembly example. Methods Mol. Biol. 2018, 1777, 101. [Google Scholar] [PubMed]
- Kabsch, W.; Sander, C. Dictionary of protein secondary structure: Pattern recognition of hydrogen-bonded and geometrical features. Biopolym. Orig. Res. Biomol. 1983, 22, 2577–2637. [Google Scholar] [CrossRef] [PubMed]
- Samantray, S.; Schumann, W.; Illig, A.-M.; Carballo-Pacheco, M.; Paul, A.; Barz, B.; Strodel, B. Molecular dynamics simulations of protein aggregation: Protocols for simulation setup and analysis with Markov state models and transition networks. bioRxiv 2020, 1–34. [Google Scholar] [CrossRef]
- Prinz, J.-H.; Wu, H.; Sarich, M.; Keller, B.; Senne, M.; Held, M.; Chodera, J.D.; Schütte, C.; Noé, F. Markov models of molecular kinetics: Generation and validation. J. Chem. Phys. 2011, 134, 174105. [Google Scholar] [CrossRef] [PubMed]
- Deuflhard, P.; Weber, M. Robust Perron cluster analysis in conformation dynamics. Linear Algebra Its Appl. 2005, 398, 161–184. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Liu, H.; Sun, H.; Pan, P.; Li, Y.; Li, D.; Hou, T. Assessing the performance of the MM/PBSA and MM/GBSA methods. 6. Capability to predict protein–protein binding free energies and re-rank binding poses generated by protein–protein docking. Phys. Chem. Chem. Phys. 2016, 18, 22129–22139. [Google Scholar] [CrossRef]
- Sun, H.; Li, Y.; Tian, S.; Xu, L.; Hou, T. Assessing the performance of MM/PBSA and MM/GBSA methods. 4. Accuracies of MM/PBSA and MM/GBSA methodologies evaluated by various simulation protocols using PDBbind data set. Phys. Chem. Chem. Phys. 2014, 16, 16719–16729. [Google Scholar] [CrossRef]
- Qiao, Q.; Bowman, G.R.; Huang, X. Dynamics of an intrinsically disordered protein reveal metastable conformations that potentially seed aggregation. J. Am. Chem. Soc. 2013, 135, 16092–16101. [Google Scholar] [CrossRef]
- Sun, Y.; Qian, Z.; Guo, C.; Wei, G. Amphiphilic peptides A6K and V6K display distinct oligomeric structures and self-Assembly dynamics: A combined all-atom and coarse-grained simulation study. Biomacromolecules 2015, 16, 2940–2949. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, Q.; Wang, Y.; Yao, X.; Han, W.; Liu, H. The folding mechanism and key metastable state identification of the PrP127–147 monomer studied by molecular dynamics simulations and Markov state model analysis. Phys. Chem. Chem. Phys. 2017, 19, 11249–11259. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Essmann, U.; Perera, L.; Berkowitz, M.L.; Darden, T.; Lee, H.; Pedersen, L.G. A smooth particle mesh Ewald method. J. Chem. Phys. 1995, 103, 8577–8593. [Google Scholar] [CrossRef] [Green Version]
- Ryckaert, J.-P.; Ciccotti, G.; Berendsen, H.J. Numerical integration of the Cartesian equations of motion of a system with constraints: Molecular dynamics of n-alkanes. J. Comput. Phys. 1977, 23, 327–341. [Google Scholar] [CrossRef] [Green Version]
- Noé, F.; Schütte, C.; Vanden-Eijnden, E.; Reich, L.; Weikl, T.R. Constructing the equilibrium ensemble of folding pathways from short off-equilibrium simulations. Proc. Natl. Acad. Sci. USA 2009, 106, 19011–19016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da, L.-T.; Sheong, F.K.; Silva, D.-A.; Huang, X. Application of Markov State Models to simulate long timescale dynamics of biological macromolecules. In Protein Conformational Dynamics; Springer: Cham, Switzerland, 2014; pp. 29–66. [Google Scholar]
- Noé, F.; Fischer, S. Transition networks for modeling the kinetics of conformational change in macromolecules. Curr. Opin. Struct. Biol. 2008, 18, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhong, H.; Liu, X.; Zhou, S.; Tan, S.; Liu, H.; Yao, X. Disclosing the Mechanism of Spontaneous Aggregation and Template-Induced Misfolding of the Key Hexapeptide (PHF6) of Tau Protein Based on Molecular Dynamics Simulation. ACS Chem. Neurosci. 2019, 10, 4810–4823. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.; Wang, J.; Li, Y.; Wang, W. Assessing the performance of the MM/PBSA and MM/GBSA methods. 1. The accuracy of binding free energy calculations based on molecular dynamics simulations. J. Chem. Inf. Model. 2011, 51, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Dokholyan, N.V.; Li, L.; Ding, F.; Shakhnovich, E.I. Topological determinants of protein folding. Proc. Natl. Acad. Sci. USA 2002, 99, 8637–8641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, G.; Yan, W.; Zhou, J.; Shen, B. Residue interaction network analysis of Dronpa and a DNA clamp. J. Theor. Biol. 2014, 348, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Piovesan, D.; Minervini, G.; Tosatto, S.C. The RING 2.0 web server for high quality residue interaction networks. Nucleic Acids Res. 2016, 44, W367–W374. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]






| Acceptor | Donor | Occupied Percentage (%) |
|---|---|---|
| C3-acK174@O | C13-A173@N | 34.35 |
| C13-A173@O | C3-acK174@N | 34.35 |
| C13-A173@O | C3-A173@N | 31.05 |
| C3-I171@O | C13-T175@N | 23.10 |
| C13-T175@O | C3-I171@N | 11.20 |
| C3-I171@O | C13-T175@N | 9.75 |
| System | ΔEvdw | ΔEelc | ΔEGB | ΔEsurf | ΔGgas | ΔGsol | ΔGbind |
|---|---|---|---|---|---|---|---|
| C3–C13 | −31.1 | −8.6 | 14.2 | −3.7 | −39.7 | 10.5 | −29.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, S.J.A.; Zhong, H.; Zhang, Q.; Liu, H. Deciphering the Effect of Lysine Acetylation on the Misfolding and Aggregation of Human Tau Fragment 171IPAKTPPAPK180 Using Molecular Dynamic Simulation and the Markov State Model. Int. J. Mol. Sci. 2022, 23, 2399. https://doi.org/10.3390/ijms23052399
Shah SJA, Zhong H, Zhang Q, Liu H. Deciphering the Effect of Lysine Acetylation on the Misfolding and Aggregation of Human Tau Fragment 171IPAKTPPAPK180 Using Molecular Dynamic Simulation and the Markov State Model. International Journal of Molecular Sciences. 2022; 23(5):2399. https://doi.org/10.3390/ijms23052399
Chicago/Turabian StyleShah, Syed Jawad Ali, Haiyang Zhong, Qianqian Zhang, and Huanxiang Liu. 2022. "Deciphering the Effect of Lysine Acetylation on the Misfolding and Aggregation of Human Tau Fragment 171IPAKTPPAPK180 Using Molecular Dynamic Simulation and the Markov State Model" International Journal of Molecular Sciences 23, no. 5: 2399. https://doi.org/10.3390/ijms23052399
APA StyleShah, S. J. A., Zhong, H., Zhang, Q., & Liu, H. (2022). Deciphering the Effect of Lysine Acetylation on the Misfolding and Aggregation of Human Tau Fragment 171IPAKTPPAPK180 Using Molecular Dynamic Simulation and the Markov State Model. International Journal of Molecular Sciences, 23(5), 2399. https://doi.org/10.3390/ijms23052399