The Role of Natural Polymorphic Variants of DNA Polymerase β in DNA Repair
Abstract
:1. Introduction
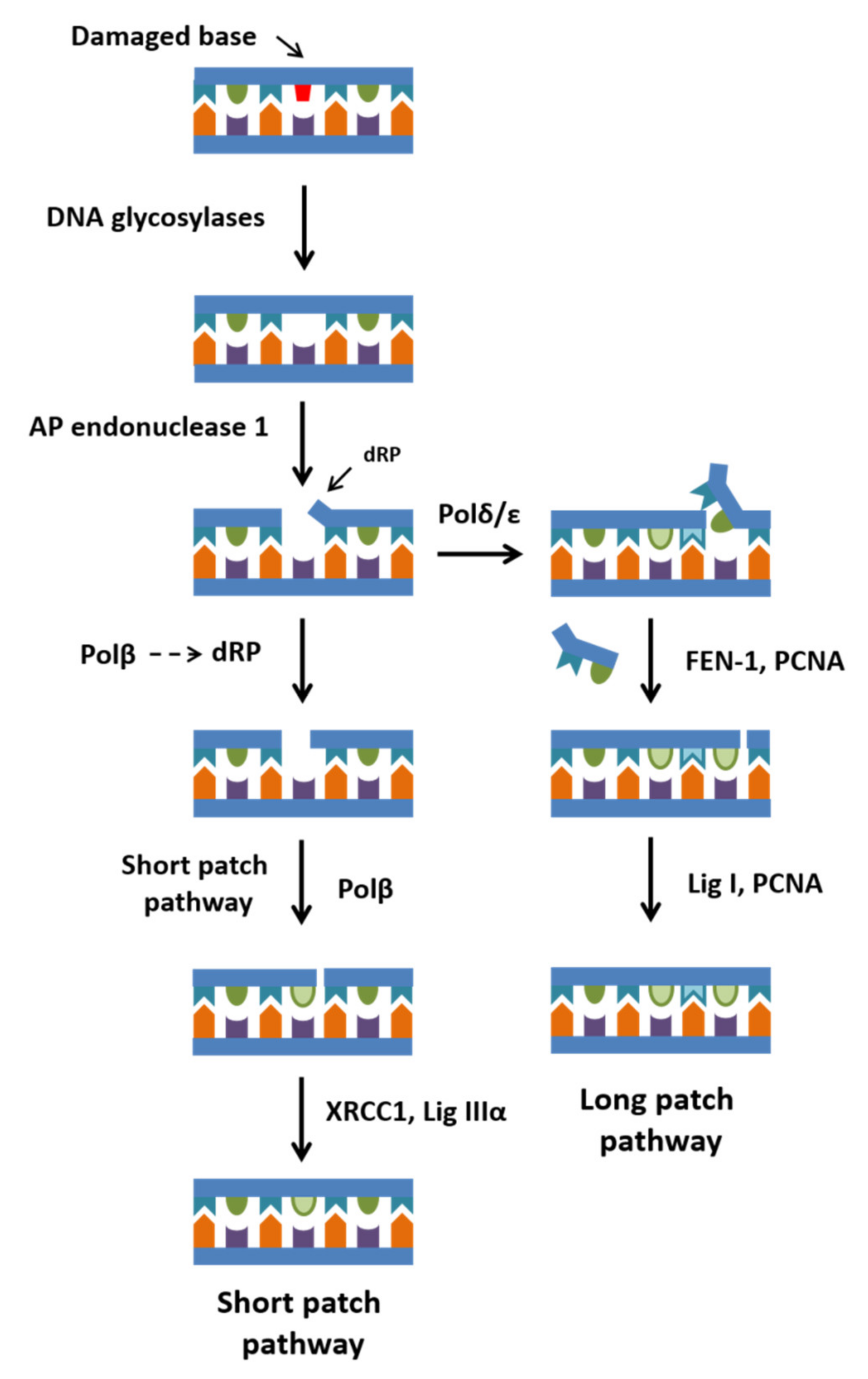
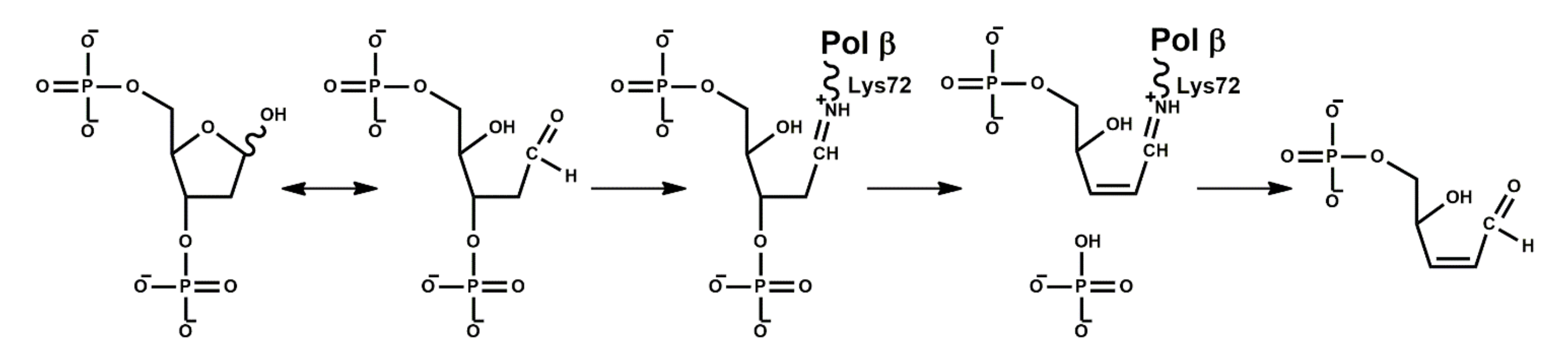
2. Functional Properties of Polβ
3. Effects of Single-Nucleotide Polymorphisms (SNPs) on Polβ Activity
3.1. In Silico Prediction of Effects of Polβ SNPs
3.2. Cancers Associated with SNPs of Polβ
3.3. Experimentally Tested Variants of Polβ
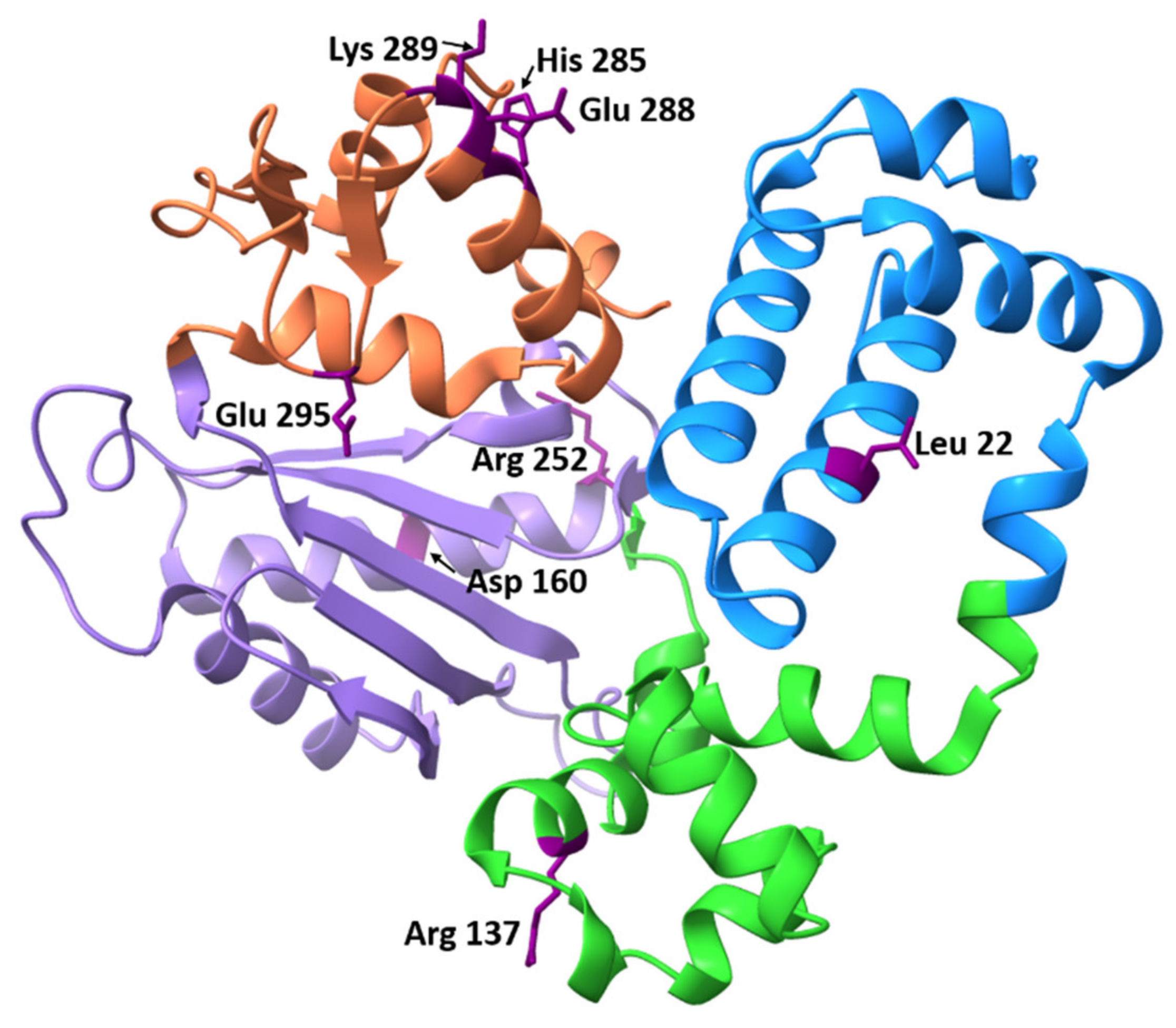
3.3.1. Glu295Lys
3.3.2. Leu22Pro
3.3.3. Glu288Lys
3.3.4. Arg152Cys
3.3.5. Arg137Gln
3.3.6. Asp160Gly
3.3.7. Lys289Met
3.3.8. His285Asp
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frederico, L.A.; Kunkel, T.A.; Shaw, B.R. A sensitive genetic assay for the detection of cytosine deamination: Determination of rate constants and the activation energy. Biochemistry 1990, 29, 2532–2537. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, J.; Walker, V.E.; Upton, P.B.; Chiang, S.Y.; Kow, Y.W.; Swenberg, J.A. Highly sensitive apurinic/apyrimidinic site assay can detect spontaneous and chemically induced depurination under physiological conditions. Cancer Res. 1998, 58, 222–225. [Google Scholar] [PubMed]
- Burcham, P.C. Internal hazards: Baseline DNA damage by endogenous products of normal metabolism. Mutat. Res. 1999, 443, 11–36. [Google Scholar] [CrossRef]
- Wallace, S.S. Biological consequences of free radical-damaged DNA bases. Free Radic. Biol. Med. 2002, 33, 1–14. [Google Scholar] [CrossRef]
- Boiteux, S.; Guillet, M. Abasic sites in DNA: Repair and biological consequences in Saccharomyces cerevisiae. DNA Repair 2004, 3, 1–12. [Google Scholar] [CrossRef]
- Evans, M.D.; Dizdaroglu, M.; Cooke, M.S. Oxidative DNA damage and disease: Induction, repair and significance. Mutat. Res. 2004, 567, 1–61. [Google Scholar] [CrossRef]
- Coppede, F.; Migliore, L. DNA damage in neurodegenerative diseases. Mutat. Res. Mol. Mech. Mutagen. 2015, 776, 84–97. [Google Scholar] [CrossRef]
- Leandro, G.S.; Sykora, P.; Bohr, V.A. The impact of base excision DNA repair in age-related neurodegenerative diseases. Mutat. Res. Mol. Mech. Mutagen. 2015, 776, 31–39. [Google Scholar] [CrossRef] [Green Version]
- Pascucci, B.; Stucki, M.; Jónsson, Z.O.; Dogliotti, E.; Hübscher, U. Long patch base excision repair with purified human proteins. DNA ligase I as patch size mediator for DNA polymerases delta and epsilon. J. Biol. Chem. 1999, 274, 33696–33702. [Google Scholar] [CrossRef] [Green Version]
- Frosina, G.; Fortini, P.; Rossi, O.; Carrozzino, F.; Raspaglio, G.; Cox, L.S.; Lane, D.P.; Abbondandolo, A.; Dogliotti, E. Two pathways for base excision repair in mammalian cells. J. Biol. Chem. 1996, 271, 9573–9578. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, D.K.; Vande Berg, B.J.; Prasad, R.; Molina, J.T.; Beard, W.A.; Tomkinson, A.E.; Wilson, S.H. Mammalian abasic site base excision repair: Identification of the reaction sequence and rate-determining steps. J. Biol. Chem. 1998, 273, 21203–21209. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, Y.; Kim, K. Excision of deoxyribose phosphate residues by DNA polymerase beta during DNA repair. Science 1995, 269, 699–702. [Google Scholar] [CrossRef]
- Klungland, A.; Lindahl, T. Second pathway for completion of human DNA base excision-repair: Reconstitution with purified proteins and requirement for DNase IV (FEN1). EMBO J. 1997, 16, 3341–3348. [Google Scholar] [CrossRef] [Green Version]
- Sobol, R.W.; Horton, J.K.; Kühn, R.; Gu, H.; Singhal, R.K.; Prasad, R.; Rajewsky, K.; Wilson, S.H. Requirement of mammalian DNA polymerase-β in base-excision repair. Nature 1996, 379, 183–186. [Google Scholar] [CrossRef]
- Schärer, O.D.; Nash, H.M.; Jiricny, J.; Laval, J.; Verdine, G.L. Specific Binding of a Designed Pyrrolidine Abasic Site Analog to Multiple DNA Glycosylases. J. Biol. Chem. 1998, 273, 8592–8597. [Google Scholar] [CrossRef] [Green Version]
- Hill, J.W.; Hazra, T.K.; Izumi, T.; Mitra, S. Stimulation of human 8-oxoguanine-DNA glycosylase by AP-endonuclease: Potential coordination of the initial steps in base excision repair. Nucleic Acids Res. 2001, 29, 430–438. [Google Scholar] [CrossRef]
- Petronzelli, F.; Riccio, A.; Markham, G.D.; Seeholzer, S.H.; Stoerker, J.; Genuardi, M.; Yeung, A.T.; Matsumoto, Y.; Bellacosa, A. Biphasic Kinetics of the Human DNA Repair Protein MED1 (MBD4), a Mismatch-specific DNA N-Glycosylase. J. Biol. Chem. 2000, 275, 32422–32429. [Google Scholar] [CrossRef] [Green Version]
- Waters, T.R.; Swann, P.F. Kinetics of the Action of Thymine DNA Glycosylase. J. Biol. Chem. 1998, 273, 20007–20014. [Google Scholar] [CrossRef] [Green Version]
- Kladova, O.A.O.A.; Bazlekowa-Karaban, M.; Baconnais, S.; Piétrement, O.; Ishchenko, A.A.A.A.; Matkarimov, B.T.B.T.; Iakovlev, D.A.D.A.; Vasenko, A.; Fedorova, O.S.O.S.; Le Cam, E.; et al. The role of the N-terminal domain of human apurinic/apyrimidinic endonuclease 1, APE1, in DNA glycosylase stimulation. DNA Repair 2018, 64, 10–25. [Google Scholar] [CrossRef]
- Sidorenko, V.S.; Nevinsky, G.A.; Zharkov, D.O. Mechanism of interaction between human 8-oxoguanine-DNA glycosylase and AP endonuclease. DNA Repair 2007, 6, 317–328. [Google Scholar] [CrossRef]
- Xia, L.; Zheng, L.; Lee, H.W.; Bates, S.E.; Federico, L.; Shen, B.; O’Connor, T.R. Human 3-Methyladenine-DNA Glycosylase: Effect of Sequence Context on Excision, Association with PCNA, and Stimulation by AP Endonuclease. J. Mol. Biol. 2005, 346, 1259–1274. [Google Scholar] [CrossRef]
- Waters, T.R.; Gallinari, P.; Jiricnyl, J.; Swann, P.F. Human Thymine DNA Glycosylase Binds to Apurinic Sites in DNA but Is Displaced by Human Apurinic Endonuclease 1. J. Biol. Chem. 1999, 274, 67–74. [Google Scholar] [CrossRef] [Green Version]
- Esadze, A.; Rodriguez, G.; Cravens, S.L.; Stivers, J.T. AP-Endonuclease 1 Accelerates Turnover of Human 8-Oxoguanine DNA Glycosylase by Preventing Retrograde Binding to the Abasic-Site Product. Biochemistry 2017, 56, 1974–1986. [Google Scholar] [CrossRef]
- Wiederhold, L.; Leppard, J.B.; Kedar, P.; Karimi-Busheri, F.; Rasouli-Nia, A.; Weinfeld, M.; Tomkinson, A.E.; Izumi, T.; Prasad, R.; Wilson, S.H.; et al. AP endonuclease-independent DNA base excision repair in human cells. Mol. Cell 2004, 15, 209–220. [Google Scholar] [CrossRef]
- Kubota, Y.; Nash, R.A.; Klungland, A.; Schär, P.; Barnes, D.E.; Lindahl, T. Reconstitution of DNA base excision-repair with purified human proteins: Interaction between DNA polymerase β and the XRCC1 protein. EMBO J. 1996, 15, 6662–6670. [Google Scholar] [CrossRef]
- Dianova, I.I.; Sleeth, K.M.; Allinson, S.L.; Parsons, J.L.; Breslin, C.; Caldecott, K.W.; Dianov, G.L. XRCC1-DNA polymerase β interaction is required for efficient base excision repair. Nucleic Acids Res. 2004, 32, 2550–2555. [Google Scholar] [CrossRef] [Green Version]
- Marintchev, A.; Robertson, A.; Dimitriadis, E.K.; Prasad, R.; Wilson, S.H.; Mullen, G.P. Domain specific interaction in the XRCC1-DNA polymerase β complex. Nucleic Acids Res. 2000, 28, 2049–2059. [Google Scholar] [CrossRef] [Green Version]
- Gryk, M.R.; Marintchev, A.; Maciejewski, M.W.; Robertson, A.; Wilson, S.H.; Mullen, G.P. Mapping of the interaction interface of DNA polymerase β with XRCC1. Structure 2002, 10, 1709–1720. [Google Scholar] [CrossRef] [Green Version]
- Kedar, P.S.; Kim, S.J.; Robertson, A.; Hou, E.; Prasad, R.; Horton, J.K.; Wilson, S.H. Direct interaction between mammalian DNA polymerase β and proliferating cell nuclear antigen. J. Biol. Chem. 2002, 277, 31115–31123. [Google Scholar] [CrossRef] [Green Version]
- Nash, R.A.; Caldecott, K.W.; Barnes, D.E.; Lindahl, T. XRCC1 Protein Interacts with One of Two Distinct Forms of DNA Ligase III. Biochemistry 1997, 36, 5207–5211. [Google Scholar] [CrossRef]
- Masson, M.; Niedergang, C.; Schreiber, V.; Muller, S.; Menissier-de Murcia, J.; de Murcia, G. XRCC1 is specifically associated with poly(ADP-ribose) polymerase and negatively regulates its activity following DNA damage. Mol. Cell. Biol. 1998, 18, 3563–3571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, A.; Wiederhold, L.; Leppard, J.B.; Kedar, P.; Prasad, R.; Wang, H.; Boldogh, I.; Karimi-Busheri, F.; Weinfeld, M.; Tomkinson, A.E.; et al. NEIL2-initiated, APE-independent repair of oxidized bases in DNA: Evidence for a repair complex in human cells. DNA Repair 2006, 5, 1439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campalans, A.; Marsin, S.; Nakabeppu, Y.; O’Connor, T.R.; Boiteux, S.; Radicella, J.P. XRCC1 interactions with multiple DNA glycosylases: A model for its recruitment to base excision repair. DNA Repair 2005, 4, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Akbari, M.; Solvang-Garten, K.; Hanssen-Bauer, A.; Lieske, N.V.; Pettersen, H.S.; Pettersen, G.K.; Wilson, D.M.; Krokan, H.E.; Otterlei, M. Direct interaction between XRCC1 and UNG2 facilitates rapid repair of uracil in DNA by XRCC1 complexes. DNA Repair 2010, 9, 785–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennett, R.A.O.; Wilson, D.M.; Wong, D.; Demple, B. Interaction of human apurinic endonuclease and DNA polymerase beta in the base excision repair pathway. Proc. Natl. Acad. Sci. USA 1997, 94, 7166–7169. [Google Scholar] [CrossRef] [Green Version]
- Moor, N.A.; Vasil’eva, I.A.; Anarbaev, R.O.; Antson, A.A.; Lavrik, O.I. Quantitative characterization of protein-protein complexes involved in base excision DNA repair. Nucleic Acids Res. 2015, 43, 6009–6022. [Google Scholar] [CrossRef] [Green Version]
- Lavrik, O.I.; Prasad, R.; Sobol, R.W.; Horton, J.K.; Ackerman, E.J.; Wilson, S.H. Photoaffinity labeling of mouse fibroblast enzymes by a base excision repair intermediate: Evidence for the role of poly(ADP-ribose) polymerase-1 in DNA repair. J. Biol. Chem. 2001, 276, 25541–25548. [Google Scholar] [CrossRef] [Green Version]
- Zhou, T.; Pan, F.; Cao, Y.; Han, Y.; Zhao, J.; Sun, H.; Zhou, X.; Wu, X.; He, L.; Hu, Z.; et al. R152C DNA Pol β mutation impairs base excision repair and induces cellular transformation. Oncotarget 2016, 7, 6902–6915. [Google Scholar] [CrossRef] [Green Version]
- Pan, F.; Zhao, J.; Zhou, T.; Kuang, Z.; Dai, H.; Wu, H.; Sun, H.; Zhou, X.; Wu, X.; Hu, Z.; et al. Mutation of DNA Polymerase β R137Q Results in Retarded Embryo Development Due to Impaired DNA Base Excision Repair in Mice. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Nemec, A.A.; Abriola, L.; Merkel, J.S.; De Stanchina, E.; DeVeaux, M.; Zelterman, D.; Glazer, P.M.; Sweasy, J.B. DNA polymerase beta germline variant confers cellular response to cisplatin therapy. Mol. Cancer Res. 2017, 15, 269–280. [Google Scholar] [CrossRef] [Green Version]
- Almeida, K.H.; Sobol, R.W. A unified view of base excision repair: Lesion-dependent protein complexes regulated by post-translational modification. DNA Repair 2007, 6, 695–711. [Google Scholar] [CrossRef] [Green Version]
- Fotiadou, P.; Henegariu, O.; Sweasy, J.B. DNA polymerase β interacts with TRF2 and induces telomere dysfunction in a murine mammary cell line. Cancer Res. 2004, 64, 3830–3837. [Google Scholar] [CrossRef] [Green Version]
- Muftuoglu, M.; Wong, H.K.; Imam, S.Z.; Wilson, D.M.; Bohr, V.A.; Opresko, P.L. Telomere repeat binding factor 2 interacts with base excision repair proteins and stimulates DNA synthesis by DNA polymerase β. Cancer Res. 2006, 66, 113–124. [Google Scholar] [CrossRef] [Green Version]
- Kidane, D.; Jonason, A.S.; Gorton, T.S.; Mihaylov, I.; Pan, J.; Keeney, S.; De Rooij, D.G.; Ashley, T.; Keh, A.; Liu, Y.; et al. DNA polymerase Β is critical for mouse meiotic synapsis. EMBO J. 2010, 29, 410–423. [Google Scholar] [CrossRef] [Green Version]
- Horton, J.K.; Srivastava, D.K.; Zmudzka, B.Z.; Wilson, S.H. Strategic down-regulation of DNA polymerase β by antisense RNA sensitizes mammalian cells to specific DNA damaging agents. Nucleic Acids Res. 1995, 23, 3810–3815. [Google Scholar] [CrossRef] [Green Version]
- Ray, S.; Breuer, G.; DeVeaux, M.; Zelterman, D.; Bindra, R.; Sweasy, J.B. DNA polymerase beta participates in DNA end-joining. Nucleic Acids Res. 2018, 46, 242–255. [Google Scholar] [CrossRef]
- Burgers, P.M.J.; Koonin, E.V.; Bruford, E.; Blanco, L.; Burtis, K.C.; Christman, M.F.; Copeland, W.C.; Friedberg, E.C.; Hanaoka, F.; Hinkle, D.C.; et al. Eukaryotic DNA Polymerases: Proposal for a Revised Nomenclature. J. Biol. Chem. 2001, 276, 43487–43490. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.S.F.; Korn, D. Specificity of the Catalytic Interaction of Human DNA Polymerase β with Nucleic Acid Substrates. Biochemistry 1982, 21, 1597–1608. [Google Scholar] [CrossRef]
- Prasad, R.; Beard, W.A.; Wilson, S.H. Studies of gapped DNA substrate binding by mammalian DNA polymerase β. Dependence on 5′-phosphate group. J. Biol. Chem. 1994, 269, 18096–18101. [Google Scholar] [CrossRef]
- Shu-Fong Wang, T.; Korn, D. Reactivity of KB Cell Deoxyribonucleic Acid Polymerases α and β with Nicked and Gapped Deoxyribonucleic Acid. Biochemistry 1980, 19, 1782–1790. [Google Scholar] [CrossRef]
- Freemont, P.S.; Ollis, D.L.; Steitz, T.A.; Joyce, C.M. A domain of the klenow fragment of Escherichia coli DNA polymerase I has polymerase but no exonuclease activity. Proteins Struct. Funct. Bioinform. 1986, 1, 66–73. [Google Scholar] [CrossRef]
- Steitz, T.A. DNA- and RNA-dependent DNA polymerases. Curr. Opin. Struct. Biol. 1993, 3, 31–38. [Google Scholar] [CrossRef]
- Pelletier, H.; Sawaya, M.R.; Kumar, A.; Wilson, S.H.; Kraut, J. Structures of Ternary Complexes of Rat DNA Polymerase, a DNA Template-Primer, and ddCTP. Science 1994, 264, 1891–1903. [Google Scholar] [CrossRef]
- Arndt, J.W.; Gong, W.; Zhong, X.; Showalter, A.K.; Liu, J.; Dunlap, C.A.; Lin, Z.; Paxson, C.; Tsai, M.-D.; Chan, M.K. Insight into the Catalytic Mechanism of DNA Polymerase : Structures of Intermediate Complexes †,‡. The coordinates have been deposited in the Protein Data Bank. PDB ID: Pol-DNA-Cr(III)‚dTMPPCP, 1huo; Pol-DNA-Cr(III). Biochemistry 2001, 40, 5368–5375. [Google Scholar] [CrossRef]
- Pelletier, H.; Sawaya, M.R.; Wolfle, W.; Wilson, S.H.; Kraut, J. Crystal structures of human DNA polymerase β complexed with DNA: Implications for catalytic mechanism, processivity, and fidelity. Biochemistry 1996, 35, 12742–12761. [Google Scholar] [CrossRef]
- Bose-Basu, B.; DeRose, E.F.; Kirby, T.W.; Mueller, G.A.; Beard, W.A.; Wilson, S.H.; London, R.E. Dynamic characterization of a DNA repair enzyme: NMR studies of [methyl-13C]methionine-labeled DNA polymerase β. Biochemistry 2004, 43, 8911–8922. [Google Scholar] [CrossRef]
- Joyce, C.M.; Steitz, T.A. MINIREVIEW Polymerase Structures and Function: Variations on a Theme? J. Bacteriol. 1995, 177, 6321–6329. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, T.; Zhao, Y.; Yamagata, Y.; Hua, Y.J.; Yang, W. Watching DNA polymerase η make a phosphodiester bond. Nature 2012, 487, 196–201. [Google Scholar] [CrossRef] [Green Version]
- Whitaker, A.M.; Smith, M.R.; Schaich, M.A.; Freudenthal, B.D. Capturing a mammalian DNA polymerase extending from an oxidized nucleotide. Nucleic Acids Res. 2017, 45, 6934. [Google Scholar] [CrossRef] [Green Version]
- Reed, A.J.; Suo, Z. Time-Dependent Extension from an 8-Oxoguanine Lesion by Human DNA Polymerase Beta. J. Am. Chem. Soc. 2017, 139, 9684–9690. [Google Scholar] [CrossRef]
- Reed, A.J.; Vyas, R.; Raper, A.T.; Suo, Z. Structural insights into the post-chemistry steps of nucleotide incorporation catalyzed by a DNA polymerase. J. Am. Chem. Soc. 2017, 139, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Vyas, R.; Reed, A.J.; Tokarsky, E.J.; Suo, Z. Viewing Human DNA Polymerase β Faithfully and Unfaithfully Bypass an Oxidative Lesion by Time-Dependent Crystallography. J. Am. Chem. Soc. 2015, 137, 5225–5230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freudenthal, B.D.; Beard, W.A.; Perera, L.; Shock, D.D.; Kim, T.; Schlick, T.; Wilson, S.H. Uncovering the polymerase-induced cytotoxicity of an oxidized nucleotide. Nature 2015, 517, 635–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freudenthal, B.D.; Beard, W.A.; Shock, D.D.; Wilson, S.H. Observing a DNA polymerase choose right from wrong. Cell 2013, 154, 157. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Yang, W. Capture of A Third Mg2+ is Essential for Catalyzing DNA Synthesis. Science 2016, 352, 1334. [Google Scholar] [CrossRef] [Green Version]
- Raper, A.T.; Reed, A.J.; Suo, Z. Kinetic Mechanism of DNA Polymerases: Contributions of Conformational Dynamics and a Third Divalent Metal Ion. Chem. Rev. 2018, 118, 6000–6025. [Google Scholar] [CrossRef]
- Yang, W.; Weng, P.J.; Gao, Y. A new paradigm of DNA synthesis: Three-metal-ion catalysis. Cell Biosci. 2016, 6, 51. [Google Scholar] [CrossRef] [Green Version]
- Jamsen, J.A.; Beard, W.A.; Pedersen, L.C.; Shock, D.D.; Moon, A.F.; Krahn, J.M.; Bebenek, K.; Kunkel, T.A.; Wilson, S.H. Time-lapse crystallography snapshots of a double-strand break repair polymerase in action. Nat. Commun. 2017, 8, 253. [Google Scholar] [CrossRef]
- Singhal, R.K.; Wilson, S.H. Short gap-filling synthesis by DNA polymerase β is processive. J. Biol. Chem. 1993, 268, 15906–15911. [Google Scholar] [CrossRef]
- Nowak, R.; Kulik, J.; Siedlecki, J.A. The ability of DNA polymerase beta to synthesize DNA beyond the gap with displacement of the non-replicated strand. Acta. Biochim. Pol. 1987, 34, 205–215. [Google Scholar]
- Prasad, R.; Dianov, G.L.; Bohr, V.A.; Wilson, S.H. FEN1 stimulation of DNA polymerase β mediates an excision step in mammalian long patch base excision repair. J. Biol. Chem. 2000, 275, 4460–4466. [Google Scholar] [CrossRef] [Green Version]
- Prasad, R.; Lavrik, O.I.; Kim, S.J.; Kedar, P.; Yang, X.P.; Vande Berg, B.J.; Wilson, S.H. DNA polymerase β-mediated long patch base excision repair: Poly(ADP-ribose) polymerase-1 stimulates strand displacement DNA synthesis. J. Biol. Chem. 2001, 276, 32411–32414. [Google Scholar] [CrossRef] [Green Version]
- Sukhanova, M.V.; Khodyreva, S.N.; Lebedeva, N.A.; Prasad, R.; Wilson, S.H.; Lavrik, O.I. Human base excision repair enzymes apurinic/apyrimidinic endonuclease1 (APE1), DNA polymerase β and poly(ADP-ribose) polymerase 1: Interplay between strand-displacement DNA synthesis and proofreading exonuclease activity. Nucleic Acids Res. 2005, 33, 1222–1229. [Google Scholar] [CrossRef] [Green Version]
- Prasad, R.; Beard, W.A.; Strauss, P.R.; Wilson, S.H. Human DNA polymerase β deoxyribose phosphate lyase: Substrate specificity and catalytic mechanism. J. Biol. Chem. 1998, 273, 15263–15270. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, Y.; Kim, K.; Katz, D.S.; Feng, J.A. Catalytic center of DNA polymerase β for excision of deoxyribose phosphate groups. Biochemistry 1998, 37, 6456–6464. [Google Scholar] [CrossRef]
- Xu, G.; Herzig, M.; Rotrekl, V.; Walter, C.A. Base excision repair, aging and health span. Mech. Ageing Dev. 2008, 129, 366–382. [Google Scholar] [CrossRef] [Green Version]
- Starcevic, D.; Dalal, S.; Sweasy, J.B. Is there a link between DNA polymerase β and cancer? Cell Cycle 2004, 3, 998–1001. [Google Scholar] [CrossRef]
- Copani, A.; Hoozemans, J.J.M.; Caraci, F.; Calafiore, M.; Van Haastert, E.S.; Veerhuis, R.; Rozemuller, A.J.M.; Aronica, E.; Sortino, M.A.; Nicoletti, F. DNA polymerase-β is expressed early in neurons of Alzheimer’s disease brain and is loaded into DNA replication forks in neurons challenged with β-amyloid. J. Neurosci. 2006, 26, 10949–10957. [Google Scholar] [CrossRef] [Green Version]
- Copani, A.; Caraci, F.; Hoozemans, J.J.M.; Calafiore, M.; Angela Sortino, M.; Nicoletti, F. The nature of the cell cycle in neurons: Focus on a “non-canonical” pathway of DNA replication causally related to death. Biochim. Biophys. Acta. Mol. Basis Dis. 2007, 1772, 409–412. [Google Scholar] [CrossRef] [Green Version]
- Loeb, L.A.; Springgate, C.F.; Battula, N. Errors in DNA Replication as a Basis of Malignant Changes. Cancer Res. 1974, 34, 2311–2321. [Google Scholar]
- Loeb, L.A. Human cancers express a mutator phenotype: Hypothesis, origin, and consequences. Cancer Res. 2016, 76, 2057–2059. [Google Scholar] [CrossRef] [Green Version]
- Bronner, C.E.; Baker, S.M.; Morrison, P.T.; Warren, G.; Smith, L.G.; Lescoe, M.K.; Kane, M.; Earabino, C.; Lipford, J.; Lindblom, A.; et al. Mutation in the DNA mismatch repair gene homologue hMLH 1 is associated with hereditary non-polyposis colon cancer. Nature 1994, 368, 258–261. [Google Scholar] [CrossRef]
- Kothandapani, A.; Sawant, A.; Dangeti, V.S.M.N.; Sobol, R.W.; Patrick, S.M. Epistatic role of base excision repair and mismatch repair pathways in mediating cisplatin cytotoxicity. Nucleic Acids Res. 2013, 41, 7332–7343. [Google Scholar] [CrossRef] [Green Version]
- Lang, T.; Dalal, S.; Chikova, A.; DiMaio, D.; Sweasy, J.B. The E295K DNA Polymerase Beta Gastric Cancer-Associated Variant Interferes with Base Excision Repair and Induces Cellular Transformation. Mol. Cell. Biol. 2007, 27, 5587–5596. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharyya, N.; Chen, H.-C.; Comhair, S.; Erzurum, S.C.; Banerjee, S. Variant Forms of DNA Polymerase beta in Primary Lung Carcinomas. DNA Cell Biol. 1999, 18, 549–554. [Google Scholar] [CrossRef]
- Opresko, P.L.; Sweasy, J.B.; Eckert, K.A. The mutator form of polymerase β with amino acid substitution at tyrosine 265 in the hinge region displays an increase in both base substitution and frame shift errors. Biochemistry 1998, 37, 2111–2119. [Google Scholar] [CrossRef]
- Starcevic, D.; Dalal, S.; Sweasy, J. Hinge residue Ile260 of DNA polymerase β is important for enzyme activity and fidelity. Biochemistry 2005, 44, 3775–3784. [Google Scholar] [CrossRef]
- Collins, F.; Brooks, L.; Chakravarti, A. A DNA polymorphism discovery resource for research on human genetic variation. Genome Res. 1998, 8, 1229–1231. [Google Scholar] [CrossRef] [Green Version]
- Donigan, K.A.; Hile, S.E.; Eckert, K.A.; Sweasy, J.B. The human gastric cancer-associated DNA polymerase β variant D160N is a mutator that induces cellular transformation. DNA Repair 2012, 11, 381–390. [Google Scholar] [CrossRef] [Green Version]
- Hall, J.; Marcel, V.; Bolin, C.; Fernet, M.; Tartier, L.; Vaslin, L.; Hainaut, P. The associations of sequence variants in DNA-repair and cell-cycle genes with cancer risk: Genotype-phenotype correlations. Biochem. Soc. Trans. 2009, 37, 527–533. [Google Scholar] [CrossRef]
- Nemec, A.A.; Donigan, K.A.; Murphy, D.L.; Jaegers, J.; Sweasy, J.B. Colon cancer-associated DNA polymerase β variant induces genomic instability and cellular transformation. J. Biol. Chem. 2012, 287, 23840–23849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nemec, A.A.; Murphy, D.L.; Donigan, K.A.; Sweasy, J.B. The S229L colon tumor-associated variant of DNA polymerase β induces cellular transformation as a result of decreased polymerization efficiency. J. Biol. Chem. 2014, 289, 13708–13716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, P.C.; Henikoff, S. Accounting for Human Polymorphisms Predicted to Affect Protein Function. Genome Res. 2002, 12, 436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, P.C.; Henikoff, S. Predicting Deleterious Amino Acid Substitutions. Genome Res. 2001, 11, 863. [Google Scholar] [CrossRef] [Green Version]
- Adzhubei, I.; Schmidt, S.; Peshkin, L.; Ramensky, V.; Gerasimova, A.; Bork, P.; Kondrashov, A.; Sunyaev, S. A method and server for predicting damaging missense mutations. Nat. Methods 2010, 7, 248–249. [Google Scholar] [CrossRef] [Green Version]
- Martin, K.; Daniela, M.W.; Preti, J.; Brain, J.O.; Gregory, M.C.; Jay, S. A general framework for estimating the relative pathogenicity of human genetic variants. Nat. Genet. 2014, 46, 310–315. [Google Scholar]
- Ioannidis, N.M.; Rothstein, J.H.; Pejaver, V.; Middha, S.; McDonnell, S.K.; Baheti, S.; Musolf, A.; Li, Q.; Holzinger, E.; Karyadi, D.; et al. REVEL: An Ensemble Method for Predicting the Pathogenicity of Rare Missense Variants. Am. J. Hum. Genet. 2016, 99, 877. [Google Scholar] [CrossRef] [Green Version]
- Dong, C.; Wei, P.; Jian, X.; Gibbs, R.; Boerwinkle, E.; Wang, K.; Liu, X. Comparison and integration of deleteriousness prediction methods for nonsynonymous SNVs in whole exome sequencing studies. Hum. Mol. Genet. 2015, 24, 2125–2137. [Google Scholar] [CrossRef] [Green Version]
- Sato, Y.; Yoshizato, T.; Shiraishi, Y.; Maekawa, S.; Okuno, Y.; Kamura, T.; Shimamura, T.; Sato-Otsubo, A.; Nagae, G.; Suzuki, H.; et al. Integrated molecular analysis of clear-cell renal cell carcinoma. Nat. Genet. 2013, 45, 860–867. [Google Scholar] [CrossRef]
- Kirby, T.W.; Derose, E.F.; Beard, W.A.; Shock, D.D.; Wilson, S.H.; London, R.E. Substrate rescue of DNA polymerase β containing a catastrophic L22P mutation. Biochemistry 2014, 53, 2413–2422. [Google Scholar] [CrossRef]
- Rozacky, J.; Nemec, A.A.; Sweasy, J.B.; Kidane, D. Gastric cancer associated variant of DNA polymerase beta (Leu22Pro) promotes DNA replication associated double strand breaks. Oncotarget 2015, 6, 24474–24487. [Google Scholar] [CrossRef] [Green Version]
- Dalal, S.; Chikova, A.; Jaeger, J.; Sweasy, J.B. The Leu22Pro tumor-associated variant of DNA polymerase beta is dRP lyase deficient. Nucleic Acids Res. 2008, 36, 411–422. [Google Scholar] [CrossRef] [Green Version]
- El-Andaloussi, N.; Valovka, T.; Toueille, M.; Steinacher, R.; Focke, F.; Gehrig, P.; Covic, M.; Hassa, P.O.; Schär, P.; Hübscher, U.; et al. Arginine Methylation Regulates DNA Polymerase β. Mol. Cell 2006, 22, 51–62. [Google Scholar] [CrossRef]
- Gu, H.; Marth, J.D.; Orban, P.C.; Mossmann, H.; Rajewsky, K. Deletion of a DNA Polymerase β Gene Segment in T Cells Using Cell Type-Specific Gene Targeting. Science 1994, 265, 103–106. [Google Scholar] [CrossRef] [Green Version]
- Poltoratsky, V.; Horton, J.K.; Prasad, R.; Wilson, S.H. REV1 mediated mutagenesis in base excision repair deficient mouse fibroblast. DNA Repair 2005, 4, 1182–1188. [Google Scholar] [CrossRef]
- Albertella, M.R.; Lau, A.; O’Connor, M.J. The overexpression of specialized DNA polymerases in cancer. DNA Repair 2005, 4, 583–593. [Google Scholar] [CrossRef]
- Yang, J.; Parsons, J.; Nicolay, N.H.; Caporali, S.; Harrington, C.F.; Singh, R.; Finch, D.; Datri, S.; Farmer, P.B.; Johnston, P.G.; et al. Cells deficient in the base excision repair protein, DNA polymerase beta, are hypersensitive to oxaliplatin chemotherapy. Oncogene 2009, 29, 463–468. [Google Scholar] [CrossRef]
- Canitrot, Y.; Cazaux, C.; Fréchet, M.; Bouayadi, K.; Lesca, C.; Salles, B.; Hoffmann, J.S. Overexpression of DNA polymerase β in cell results in a mutator phenotype and a decreased sensitivity to anticancer drugs. Proc. Natl. Acad. Sci. USA 1998, 95, 12586–12590. [Google Scholar] [CrossRef] [Green Version]
- Nicolay, N.H.; Helleday, T.; Sharma, R.A. Biological Relevance of DNA Polymerase Beta and Translesion Synthesis Polymerases to Cancer and its Treatment. Curr. Mol. Pharmacol. 2011, 5, 54–67. [Google Scholar] [CrossRef]
- Yamtich, J.; Starcevic, D.; Lauper, J.; Smith, E.; Shi, I.; Rangarajan, S.; Jaeger, J.; Sweasy, J.B. Hinge residue I174 is critical for proper dNTP selection by DNA polymerase beta. Biochemistry 2010, 49, 2326–2334. [Google Scholar] [CrossRef] [Green Version]
- Yamtich, J.; Nemec, A.A.; Keh, A.; Sweasy, J.B. A Germline Polymorphism of DNA Polymerase Beta Induces Genomic Instability and Cellular Transformation. PLoS Genet. 2012, 8, e1003052. [Google Scholar] [CrossRef]
- Iwanaga, A.; Ouchida, M.; Miyazaki, K.; Hori, K.; Mukai, T. Functional mutation of DNA polymerase β found in human gastric cancer-Inability of the base excision repair in vitro. Mutat. Res. DNA Repair 1999, 435, 121–128. [Google Scholar] [CrossRef]
- Sawaya, M.R.; Pelletier, H.; Kumar, A.; Wilson, S.H.; Kraut, J. Crystal structure of rat DNA polymerase β: Evidence for a common polymerase mechanism. Science 1994, 264, 1930–1935. [Google Scholar] [CrossRef]
- Prasad, R.; Batra, V.K.; Yang, X.P.; Krahn, J.M.; Pedersen, L.C.; Beard, W.A.; Wilson, S.H. Structural insight into the DNA polymerase β deoxyribose phosphate lyase mechanism. DNA Repair 2005, 4, 1347–1357. [Google Scholar] [CrossRef]
- Lyu, P.C.; Sherman, J.C.; Chen, A.; Kallenbach, N.R. α-Helix stabilization by natural and unnatural amino acids with alkyl side chains. Proc. Natl. Acad. Sci. USA 1991, 88, 5317–5320. [Google Scholar] [CrossRef] [Green Version]
- Murphy, D.L.; Donigan, K.A.; Jaeger, J.; Sweasy, J.B. The E288K colon tumor variant of DNA polymerase β is a sequence specific mutator. Biochemistry 2012, 51, 5269–5275. [Google Scholar] [CrossRef] [Green Version]
- Chan, K.; Houlbrook, S.; Zhang, Q.M.; Harrison, M.; Hickson, I.D.; Dianov, G.L. Overexpression of DNA polymerase β results in an increased rate of frameshift mutations during base excision repair. Mutagenesis 2007, 22, 183–188. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.; Zheng, L.; Dai, H.; Zhou, M.; Xu, H.; Shen, B. Human DNA polymerase β polymorphism, Arg137Gln, impairs its polymerase activity and interaction with PCNA and the cellular base excision repair capacity. Nucleic Acids Res. 2009, 37, 3431–3441. [Google Scholar] [CrossRef]
- Wang, M.; Li, E.; Lin, L.; Kumar, A.K.; Pan, F.; He, L.; Zhang, J.; Hu, Z.; Guo, Z. Enhanced activity of variant DNA polymerase b (D160G) contributes to cisplatin therapy by impeding the efficiency of NER. Mol. Cancer Res. 2019, 17, 2077–2088. [Google Scholar] [CrossRef] [Green Version]
- Alnajjar, K.S.; Garcia-Barboza, B.; Negahbani, A.; Nakhjiri, M.; Kashemirov, B.; McKenna, C.; Goodman, M.F.; Sweasy, J.B. A change in the rate-determining step of polymerization by the K289M DNA polymerase β cancer-associated variant. Biochemistry 2017, 56, 2096–2105. [Google Scholar] [CrossRef] [Green Version]
- Lang, T.; Maitra, M.; Starcevic, D.; Li, S.-X.; Sweasy, J.B. A DNA polymerase mutant from colon cancer cells induces mutations. Proc. Natl. Acad. Sci. USA 2004, 101, 6074–6079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, D.L.; Kosa, J.; Jaeger, J.; Sweasy, J.B. The Asp285 variant of DNA polymerase beta extends mispaired primer termini via increased nucleotide binding. Biochemistry 2008, 47, 8048–8057. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| SNP | SIFT | PolyPhen | CADD | REVEL | MetaLR | Provean | Refs. for Experimental Confirmation | |
|---|---|---|---|---|---|---|---|---|
| 1 | L19P | Deleterious | Probably damaging | Likely deleterious | Likely disease causing | Damaging | Deleterious | |
| 2 | L22P | Deleterious | Probably damaging | Likely deleterious | Likely disease causing | Tolerated | Deleterious | [100,101,102] |
| 3 | K35E | Deleterious | Probably damaging | Likely deleterious | Likely disease causing | Tolerated | Deleterious | [75] |
| 4 | A42T | Deleterious | Probably damaging | Likely benign | Likely disease causing | Damaging | Deleterious | |
| 5 | G66R | Deleterious | Probably damaging | Likely benign | Likely disease causing | Damaging | Deleterious | |
| 6 | G118V | Deleterious | Probably damaging | Likely deleterious | Likely disease causing | Damaging | Deleterious | |
| 7 | L122R | Deleterious | Probably damaging | Likely benign | Likely disease causing | Damaging | Deleterious | |
| 8 | R149I | Deleterious | Possibly damaging | Likely deleterious | Likely disease causing | Tolerated | Deleterious | |
| 9 | P151L | Deleterious | Possibly damaging | Likely benign | Likely disease causing | Damaging | Deleterious | |
| 10 | R152L | Deleterious | Probably damaging | Likely deleterious | Likely benign | Damaging | Deleterious | [103] |
| 11 | E154A | Deleterious | Probably damaging | Likely deleterious | Likely disease causing | Damaging | Deleterious | |
| 12 | G189D | Deleterious | Probably damaging | Likely benign | Likely disease causing | Damaging | Deleterious | |
| 13 | D192G | Deleterious | Probably damaging | Likely deleterious | Likely disease causing | Damaging | Deleterious | |
| 14 | M236T | Deleterious | Probably damaging | Likely deleterious | Likely disease causing | Tolerated | Deleterious | |
| 15 | G237V | Deleterious | Probably damaging | Likely deleterious | Likely disease causing | Tolerated | Deleterious | |
| 16 | R254I | Deleterious | Probably damaging | Likely deleterious | Likely disease causing | Tolerated | Deleterious | |
| 17 | G274R | Deleterious | Probably damaging | Likely benign | Likely disease causing | Damaging | Deleterious | |
| 18 | G290C | Deleterious | Probably damaging | Likely deleterious | Likely disease causing | Damaging | Deleterious | |
| 19 | E316K | Deleterious | Probably damaging | Likely benign | Likely disease causing | Damaging | Deleterious | |
| 20 | P330L | Deleterious | Probably damaging | Likely benign | Likely disease causing | Damaging | Deleterious | |
| 21 | R333W | Deleterious | Probably damaging | Likely benign | Likely disease causing | Damaging | Deleterious | |
| 22 | R333Q | Deleterious | Possibly damaging | Likely benign | Likely disease causing | Damaging | Deleterious |
| SNP | Cbioportal https://www.cbioportal.org/ (accessed on 21 February 2021) | HiveBiochemistry https://hive.biochemistry.gwu.edu/biomuta (accessed on 21 February 2021) | COSMIC https://cancer.sanger.ac.uk/cosmic (accessed on 21 February 2021) | |
|---|---|---|---|---|
| 1 | L22P | Carcinoma: L22F | ||
| 2 | R152L | Rectal adenocarcinoma: R152C | Lung cancer: R152P, malignant glioma: R152H | Lung cancer: R152P |
| 3 | G189D | Liver cancer: G189V | ||
| 4 | D192G | Colon adenocarcinoma: D192H | Melanoma, colorectal cancer: D192H | |
| 5 | M236T | Liver cancer: M236I | ||
| 6 | R254I | Uterine endometrioid carcinoma | Uterine cancer | |
| 7 | G274R | Melanoma: G274V | Malignant melanoma: G274V | |
| 8 | G290C | Uterine cancer: G290D | ||
| 9 | R333W | Prostate cancer | Prostate adenocarcinoma | |
| 10 | R333Q | Colon adenocarcinoma | Colorectal cancer |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kladova, O.A.; Fedorova, O.S.; Kuznetsov, N.A. The Role of Natural Polymorphic Variants of DNA Polymerase β in DNA Repair. Int. J. Mol. Sci. 2022, 23, 2390. https://doi.org/10.3390/ijms23042390
Kladova OA, Fedorova OS, Kuznetsov NA. The Role of Natural Polymorphic Variants of DNA Polymerase β in DNA Repair. International Journal of Molecular Sciences. 2022; 23(4):2390. https://doi.org/10.3390/ijms23042390
Chicago/Turabian StyleKladova, Olga A., Olga S. Fedorova, and Nikita A. Kuznetsov. 2022. "The Role of Natural Polymorphic Variants of DNA Polymerase β in DNA Repair" International Journal of Molecular Sciences 23, no. 4: 2390. https://doi.org/10.3390/ijms23042390
APA StyleKladova, O. A., Fedorova, O. S., & Kuznetsov, N. A. (2022). The Role of Natural Polymorphic Variants of DNA Polymerase β in DNA Repair. International Journal of Molecular Sciences, 23(4), 2390. https://doi.org/10.3390/ijms23042390







