1. Introduction
The usage of anesthetic ketamine in treating pain, depression, and mood swings has been practiced for years because of its versatile nature. The ecstasy-inducing nature of ketamine leads to extensive abuse of this drug, and remarkably, this has increased in recent years [
1]. The availability, accessibility, inexpensive nature, and rise in rapid-acting effects have enhanced the illicit usage of ketamine among teenagers for entertainment purposes. More importantly, ketamine has been labeled a schedule 3 controlled drug in Taiwan [
2]. The accumulation of ketamine and its metabolites mainly affects the pulmonary, cardiovascular system and induces gastrointestinal and urological pathology [
3]. According to recent reports, the prolonged usage of ketamine leads to histopathological alterations in the central nervous system, which reflects behavioral changes in the juvenile animal models [
4]. The abuse or recurrent use of ketamine will initially lead to minor disorientation, sedation, and blood pressure variations. Later, it will result in short-term memory bounces, orientation issues, acute urinary tract symptoms, and liver toxicity [
5]. Several studies have revealed that ketamine precipitously affects the urinary bladder, resulting in increased urination frequency, urgency, nocturia, and hematuria. It also causes painful, burning episodes of micturition that resemble bladder pain syndrome/interstitial cystitis (BPS/IS) [
6,
7].
Ketamine cystitis (KC) has become a perilous condition in recent years. It has been identified as an ulcerative cystitis condition due to chronic ketamine usage [
2], and the symptoms, as mentioned earlier, are classified as chronic KC conditions. Besides this, KC patients also experience other side effects such as decreased bladder volume, low bladder compliance, elevated bladder contractive pressure, and overactive bladder condition [
8]. The pathogenesis of KC is still elusive; additionally, the risk, severity, and symptoms are related to the drug usage frequency and are dosage-dependent [
9]. Several pathological approaches have been postulated, which elucidate the toxic effects of ketamine and its metabolites, such as norketamine, dehydronorketamine, hydroxyketamine, and hydroxynorketamine [
8], their interaction with the urothelial cells, and induction of inflammatory responses. The urothelium acts as a guardian by playing the first layer of defense to the bladder and protecting the bladder stroma. It also exhibits signaling properties such as signaling bladder voiding function and managing contractile activity [
2]. Ketamine and its metabolites directly rupture the urothelial layer by diminishing the expression of gap junction proteins; hence, the urinary irritant particles rapidly enter via the slackened and conceded bladder wall that ultimately elicits inflammatory responses [
9].
Currently, no specific treatments are available for KC, yet few cost-effective strategies are being practiced in managing the severity, such as cystoscopy, oral medications, intravesical medicine injection, and hyperbaric oxygen therapy or surgical interventions [
10]. The time course experiments have been designed to observe the cellular processes and responses to stimuli or treatment. In this research study, we have proposed a time course investigation as a tool for analysis of bladder dysfunctions followed by associated histological alterations in the bladder with response to ketamine treatment. Previous research studies have mainly focused on ketamine metabolites and their accumulation or interactive effects with receptors or transporters in organisms. Hitherto, no affirmative report has been found exclusively to exhibit the interaction and direct effects of ketamine on bladder function in a time-dependent sequel. Hence, we consider this to be a novel attempt, where the specific actions of ketamine over the bladder functions and the subsequent cascade of histological changes at fixed time course intervals have been effectively examined and precisely reported.
3. Discussions
As mentioned earlier, the pathophysiology of KC is still elusive. Herein, we have detected several mechanisms at several time course levels that might be involved in the pathophysiology of KC, such as bladder dysfunction, bladder wall inflammation and thickening, structural damages in the blood vessels, nerve, and disruption of the detrusor muscle and gap junction. In general, KC patients predominantly exhibited pain, increased frequency of urination, and a burning sensation at the time of urination and post-urination [
6].
The cystometric measurements of ketamine-injected rats demonstrated bladder dysfunction with impaired bladder contractions at all time course levels in the present study. For instance, all the ketamine-injected rats from three groups (weeks 2, 4, and 8) exhibited bladder dysfunction such as increased frequency of micturition and significantly reduced ICI and voided volumes. This scenario precisely portrayed the typical KC condition, and the complexity of the bladder dysfunction followed an increasing trend according to the dosage duration of the drug from 2 weeks to 8 weeks. This scenario indicated that the severity of voiding function was dependent on the usage of the drug on its time course level, which also proved the association between frequency of drug exposure and progress of bladder dysfunction. Besides this, the increased urinary frequency and reduced ICI and residual volume were mainly due to the small capacity and low bladder compliance, resulting from a contracted bladder condition due to the severity of inflammation [
7].
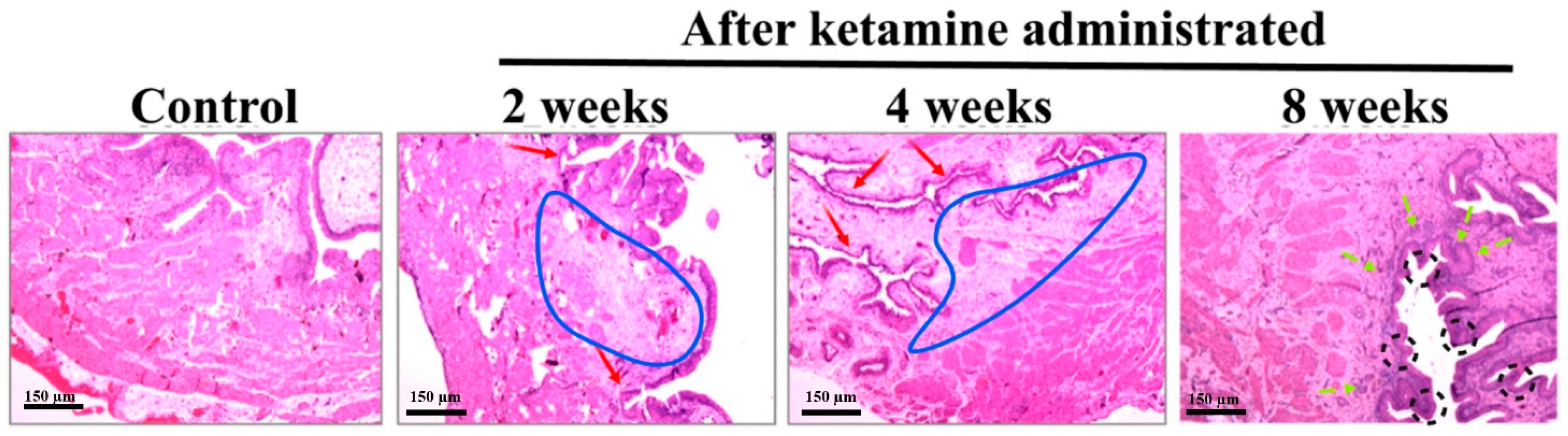
In the present study, lymphoid infiltrates were observed in the lamina propria of the rats belonging to the time course of week 2 and 4 groups. Recent pathological studies on ketamine-administered mice indicated mononuclear infiltration, a similar condition that mimics a clinical situation of interstitial cystitis [
11]. The bladder wall inflammations might initially lead to an ulcerative condition and, later on, to chronic KC. Here, the histological assessment of the bladder tissue with the aid of HE staining revealed an increased level of inflammation. Abnormal tissue morphology and submucosal edema were identified in ketamine-administered rats of 2 and 4 weeks. The rats of 8 weeks exhibited proliferation of urothelial cells with a progressive increase in fibrosis and significant bladder detrusor hypertrophy. These findings indicated that the time and exposure of the drug increased the severity of the disease and its symptoms. Research evidence also substantiated that prolonged ketamine usage caused bladder tumors and premalignant conditions such as squamous metaplasia and nephrogenic metaplasia. Moreover, ketamine-induced urothelial alterations that simulate carcinoma in situ have also been documented [
12]. Therefore, diffused bladder wall conditions were observed in 2- and 4-week ketamine-treated rats. Similar conditions and thickened bladder walls were also observed in 8-week ketamine-treated rats. Furthermore, no thickened bladder walls were observed in the remaining 2- and 4-week ketamine-treated rats. Rats with nonneoplastic conditions exhibited a diffused bladder wall thickening and reduced bladder capacity due to ketamine exposure [
13]. The thickening of bladder walls might be due to severe inflammatory conditions associated with the infiltration of mast cells in the mucosa and muscle layers of the bladder. However, sustained exposure to ketamine might induce persistent inflammations mediated by neurogenic, IgE, or NOS–COX, tailed by collagen accumulation and fibrosis in the urinary bladder, which leads to the thickened bladder wall conditions [
7]. Additionally, muscle hypertrophy, submucosal fibrosis, and alterations in the collagen muscle ratio are further common pathological attributes noted in KC patient bladders [
14], which were found in the present study observations at different time courses.
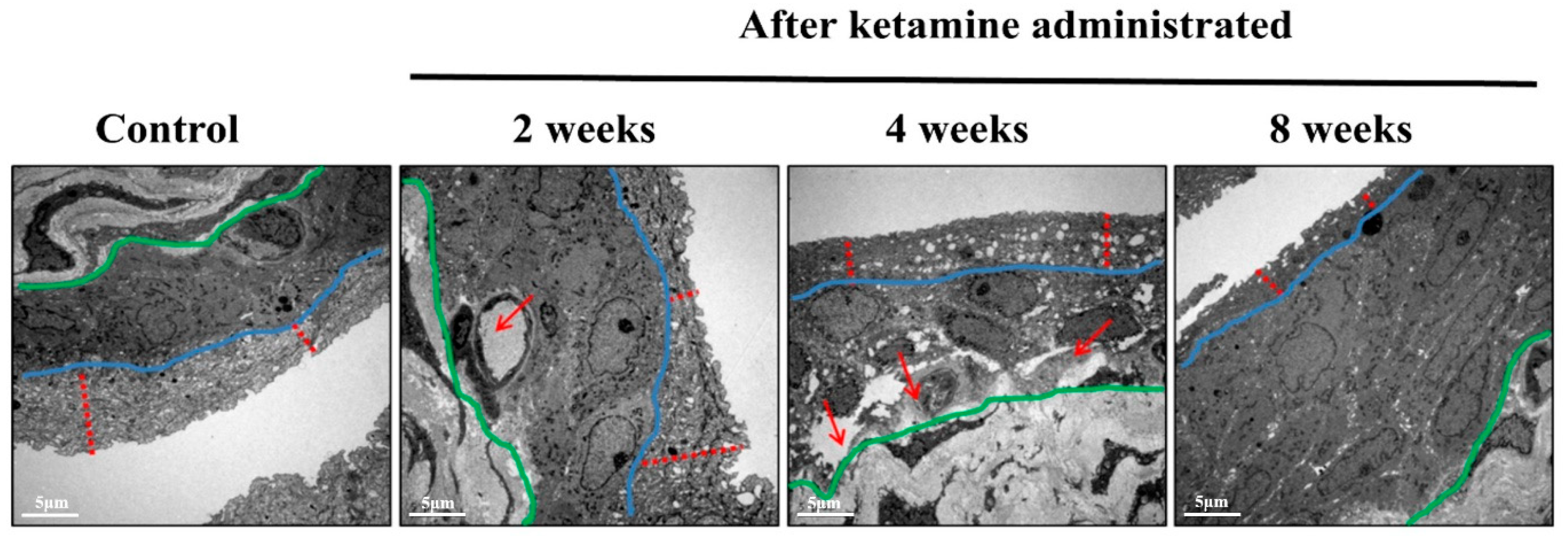
The ultrastructural changes in the nerve and blood vessels have been considered a substantial symptom of KC, and the TEM results of the current study reported significant vessel damages in the endothelium of the bladder in the 4- and 8-week ketamine-treated rats. Recent research has also supported the outcomes of the present study by conforming that ketamine can cause microvascular alterations in the bladder by inducing endothelial cell injury of microvessels and consequent compromised intrinsic microcirculation [
15]. Besides this, it has been reported that ketamine might potentially trigger various signaling pathways to initiate microvascular injury with increased apoptosis, fibrosis, and angiogenesis. Capillary fibrosis increases the tendency of fragility and capillary bleeding, and these alterations lead to a compromised intrinsic microcirculation, which might result in an ischemic condition of the bladder. It has also been documented that the ischemic condition contributes to bladder hyperactivity, underactivity, and low bladder compliance [
16]. Besides this, apoptosis in the endothelium increases microvascular permeability and leakage in the bladders of KC patients; eventually, the leakage triggers the inflammation and disease progression in the KC patients. Previous studies have also enumerated that the vessels in the bladders were the primary target sites for the disease progression in KC conditions [
15].
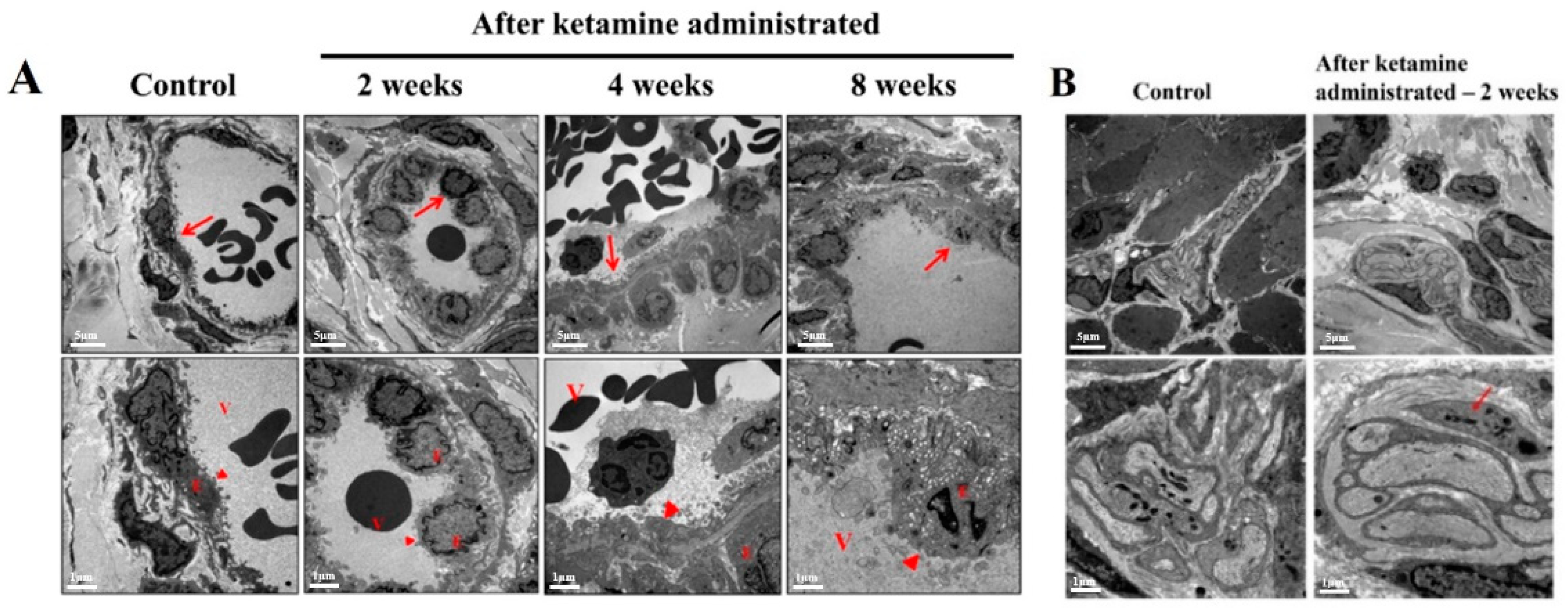
Interstitial inflammatory disease prognosis in the animal model following ketamine exposure has never been depicted before. In the present study, the TEM observations spotted a small nerve fiber in the submucosal layer of the week 2 ketamine-administered rats. The rats’ bladders exhibited a similar condition to human KC with inflammation in the submucosal area, and such alterations in the submucosal area of the rat urinary system might be due to muscular contractability in the urinary bladder or a possible decrease in nerve fiber due to pathophysiological changes induced by ketamine administration [
17]. However, additional investigations are required to support these findings. Elevated concentrations of ketamine induce a chronic state of the submucosal inflammatory response, which results in a state of detrusor muscle inflammation [
12]. Here, the ketamine-administered rats of the 2-, 4-, and 8-week periods exhibited certain levels of urodynamic dysfunction according to the dosage, exposure period, and severity of the disease condition. This scenario depicted the severity and inflamed status of the detrusor muscle and submucosal layer. The TEM images also conformed to the exact status of the detrusor muscle and submucosal layer. The foremost reason for this condition is ketamine-induced oxidative stress tailed with persistent inflammation, which insistently leads to tissue injuries significantly in the detrusor muscle and enhances KC’s pathogenesis [
16].
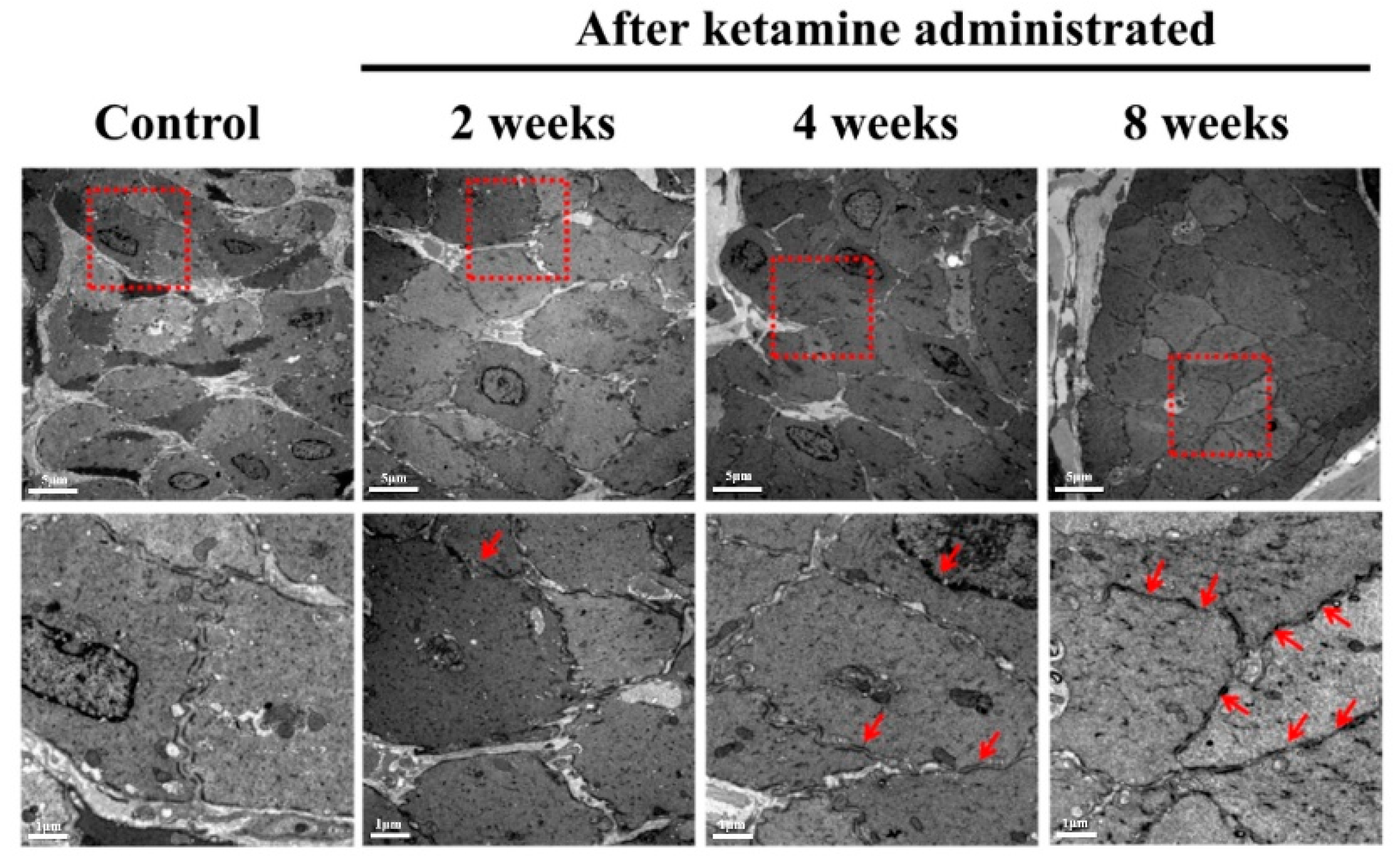
The integrity of the gap junctions plays a pivotal role in maintaining the fundamental epithelial function, and defects in this scenario lead to epithelial denaturation and barrier dysfunction. Ketamine induces enormous oxidative stress, and these ROS and RNS play a crucial role in disrupting the gap junction proteins and initiating urothelial barrier dysfunction through altering reduced glutathione/oxidized glutathione (GHS/GSSG) homeostasis [
16]. The TEM images of the urothelial layer spectacled several abnormal structures increased with loss of gap junctions and disrupted smooth muscle cells in ketamine administered 4- and 8-week groups of rats. To affirm these findings, recent research has stated that the ROS and RNS produced by the ketamine oxidizing the glutathione leads to the activation of protein tyrosine phosphatase and tyrosine kinases. The tyrosine kinase phosphorylates certain specific proteins such as ZO1, occluding, and E-cadherin and ultimately leads to epithelial barrier disruption. Furthermore, the actin cytoskeleton is eminent for gathering and maintaining tight and adherent junctions. The ROS and RNS initiate actin cytoskeleton reorganization in endothelial cells, resulting in the opening of gap junctions and gap development among endothelial cells [
18]. Eventually, the loss of integrity in the gap junctions would lead to epithelial denaturation and barrier dysfunction and reflects a poor bladder function.
4. Materials and Methods
4.1. Study Layout
For this study, 8-week-old adult Sprague Dawley (SD) rats were used. A sum of 40 rats were equally divided into 10 rats and labeled into 4 groups according to the time course schedule of 2, 4, and 8 weeks along with normal control (NC). The experimental rats (30 rats) received intraperitoneal injection of ketamine hydrochloride according to their time course schedule, and the NC group of rats (10 rats) received saline injection similar to the schedule of the experimental animals. The succeeding experiments such as cystometric measurements (CMG), histology, and transmission electron microscopic TEM analyses were also scheduled within the time course boundary.
4.2. Experimental Animals
Male SD rats (8 weeks old at the time of purchase) (BioLasco Taiwan Co., Ltd., Taipei, Taiwan) were used for this study. All the protocols and methods used in the study followed the guidelines of the declaration of Helsinki and were authorized by the Fu Jen Catholic University Institutional Animal Care and Use Committee (approval No.: A 10609 dated: 3 May 2017). All the rats were maintained in the animal house in a standard cage at 25 °C under a 12 h light/12 h dark cycle in an aseptic condition with food and water ad libitum.
4.3. Ketamine Administration Procedure
The intraperitoneal ketamine administration schedule was divided into specific time course intervals such as weeks 2, 4, and 8. Ketamine injections were executed according to the previously explained protocol [
19]. In brief, the experimental animals received a daily intraperitoneal injection of ketamine (100 mg/kg/day) (Imalgene 1000
®, Merial, Lyon, France) for 2, 4, and 8 weeks, respectively, according to the time course. The NC animal received an intraperitoneal saline injection for the duration, similar to the experimental animals. The bodyweight of the rats was monitored once a week to adjust the ketamine dosage.
4.4. Surgical Procedures and Cystometric Measurements for the Analysis of Bladder Functions
All the rats underwent bladder function analysis using CMG measurements. After exposing the rats to isoflurane anesthesia with the aid of a subcutaneous instillator, the PE90 micro-tubing was substituted for the instillator, and the free end of the PE90 tube was latched to a saline injector for saline filling into the bladder at a rate of 0.1 mL/min. The infusion rate was adapted from our trial experiment results of various infusion rates in CMG analysis. This infusion rate could only increase the frequency of urination without altering the normal bladder function. For obtaining the real-time voiding responses, the rats were kept awake throughout the experiment, and the voiding responses were recorded in the MP36 pressure transducer (Biopac Systems Inc., Santa Barbara, CA, USA) and computer-installed recording software, Biopac Student Lab 4.1 (Biopac Systems Inc., Santa Barbara, CA, USA).
4.5. Bladder Histology, Hematoxylin, and Eosin (H&E) Staining
Two rats were allotted from each group for the histological and following TEM analyses. The rats were euthanized by an IP injection overdose of pentobarbital sodium solution. Later, the bladders were collected, and 10% formaldehyde (
v/
v) was used to fix the tissues. The tissue samples remained in formaldehyde solution for 24 h. The samples were then dehydrated, postfixed, and carefully embedded into paraffin blocks for the microtomy process and were sliced into 5 µm-thin sections. Subsequently, the sections were deparaffinized for the staining process and subjected to hydrated in the graded concentration of ethanol (100%, 95%, 80%, 70%) and double-distilled H
2O. Later, the tissue samples were stained in Hematoxylin and Eosin, as described in our previous study [
8].
4.6. Transmission Electron Microscopy (TEM)
The sample preparation for TEM was followed as per our previous study [
20]. Overnight, the bladder tissues were sliced into small pieces and fixed in 2.5% phosphate-buffered glutaraldehyde (0.1 M, pH 7.2). Later, they were postfixed in 1% phosphate-buffered osmium tetroxide (0.1 M, pH 7.2). Subsequently, the samples were subjected to dehydration in the graded concentration ethanol and carefully embedded in Epon-812. Initially, 1µm semi-thin sections were stained with toluidine blue. Further, the ultra-thin sections from the chosen blocks were stained with uranyl acetate and lead citrate. All the sections were observed in JOEL JEM-1400 transmission electron microscope (JOEL, Tokyo, Japan).
4.7. Statistical Analysis
The results are expressed in means ± standard deviations. A one-way analysis of variance (ANOVA) followed by Scheffe post hoc analysis was used to compare the three different time course animal groups. All statistical analyses were performed using the SPSS v.18.0 (SPSS Inc., Chicago, IL, USA), and statistical significance was set at p < 0.05.
5. Conclusions
In this study, we have explained that the urological anomalies observed in KC extended to the damage of the urothelial layer of the bladder, and the risk, severity, and symptoms are dependent on frequency of usage and dosage. This time course study renders a clear note on the severity, disease onset, pathophysiological changes, stage-by-stage tissue damage according to the dosage, and recurrent exposure to ketamine. The only hope for fast recovery is abandoning ketamine usage along with a prescribed rehabilitation session. This time course analysis might provide a better understanding of the bladder function followed by changes in its histopathology at each time point after ketamine administration in a time course experiment. We strongly believe these novel findings will provide significant information in understanding the onset and progression of ketamine-induced bladder dysfunction and its pathophysiological conditions, which enlighten new ideas for developing more effective therapeutic strategies. Since KC is an agonizing pathological condition with prolonged complications, further research must be warranted in this field to battle against ketamine addiction.