IQM-PC332, a Novel DREAM Ligand with Antinociceptive Effect on Peripheral Nerve Injury-Induced Pain
Abstract
1. Introduction
2. Results
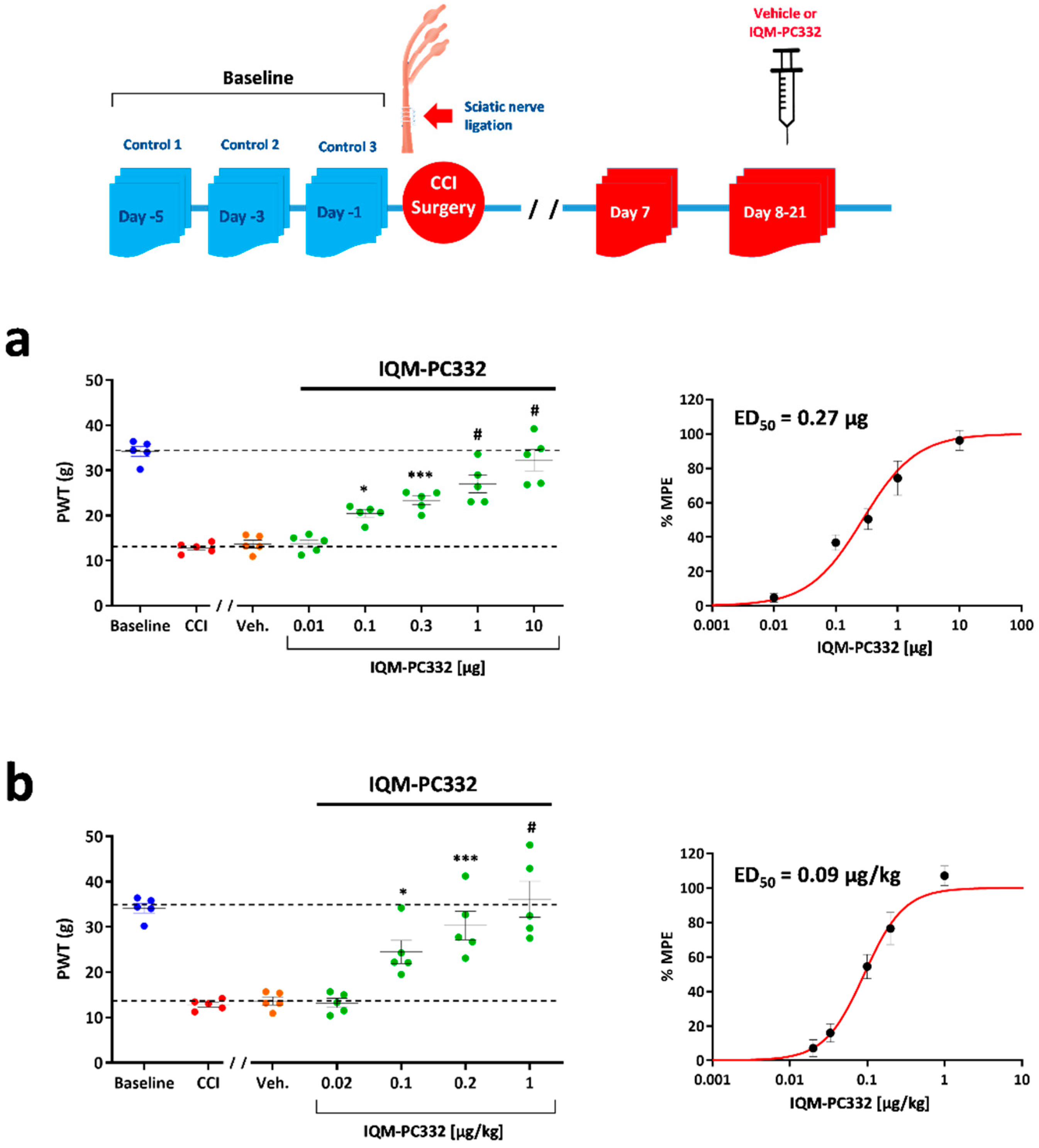
2.1. Effect of IQM-PC332 on Mechanical Sensitivity in CCI Animals
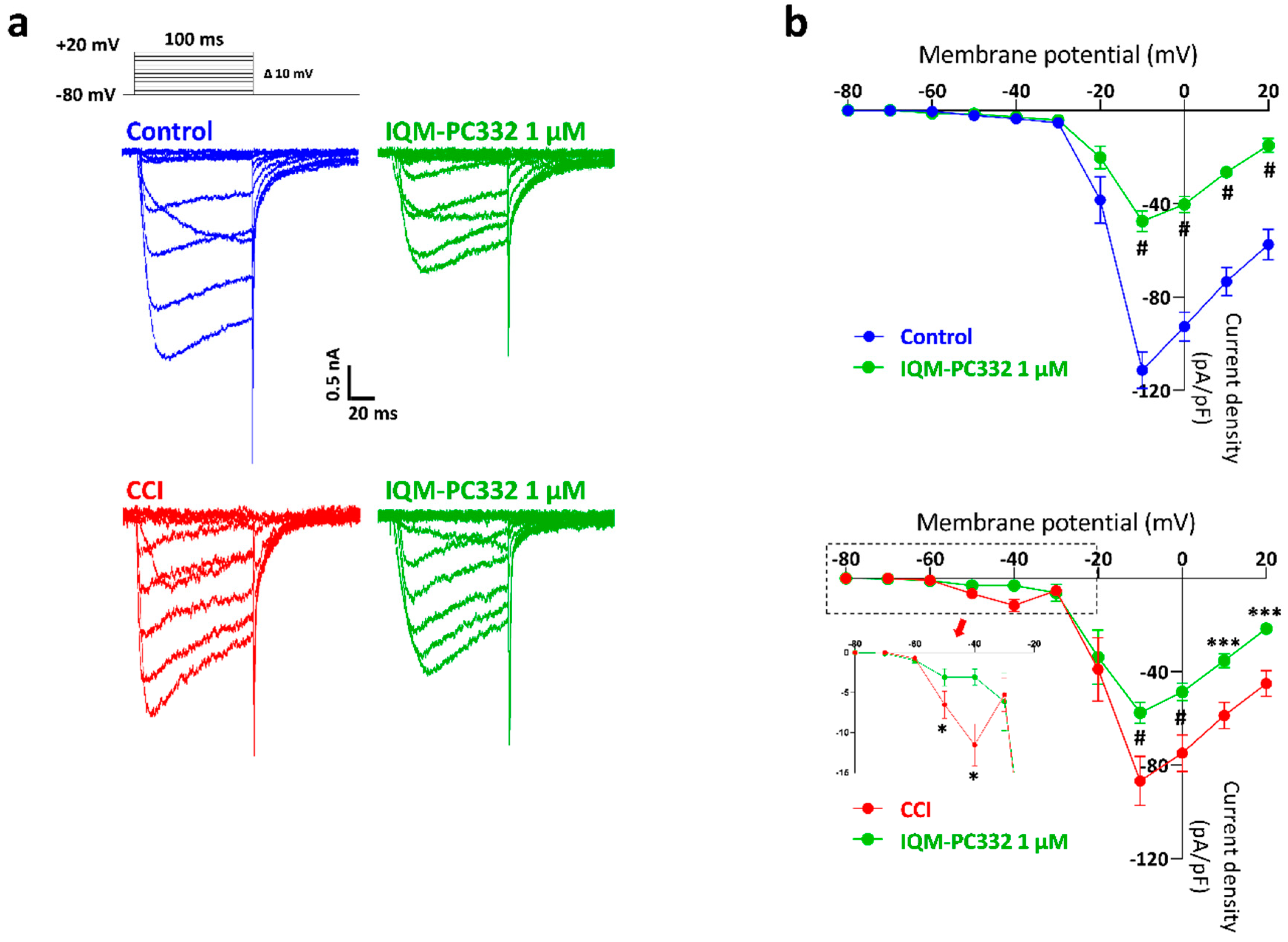
2.2. Effect of IQM-PC332 on IA in DRG Neurons
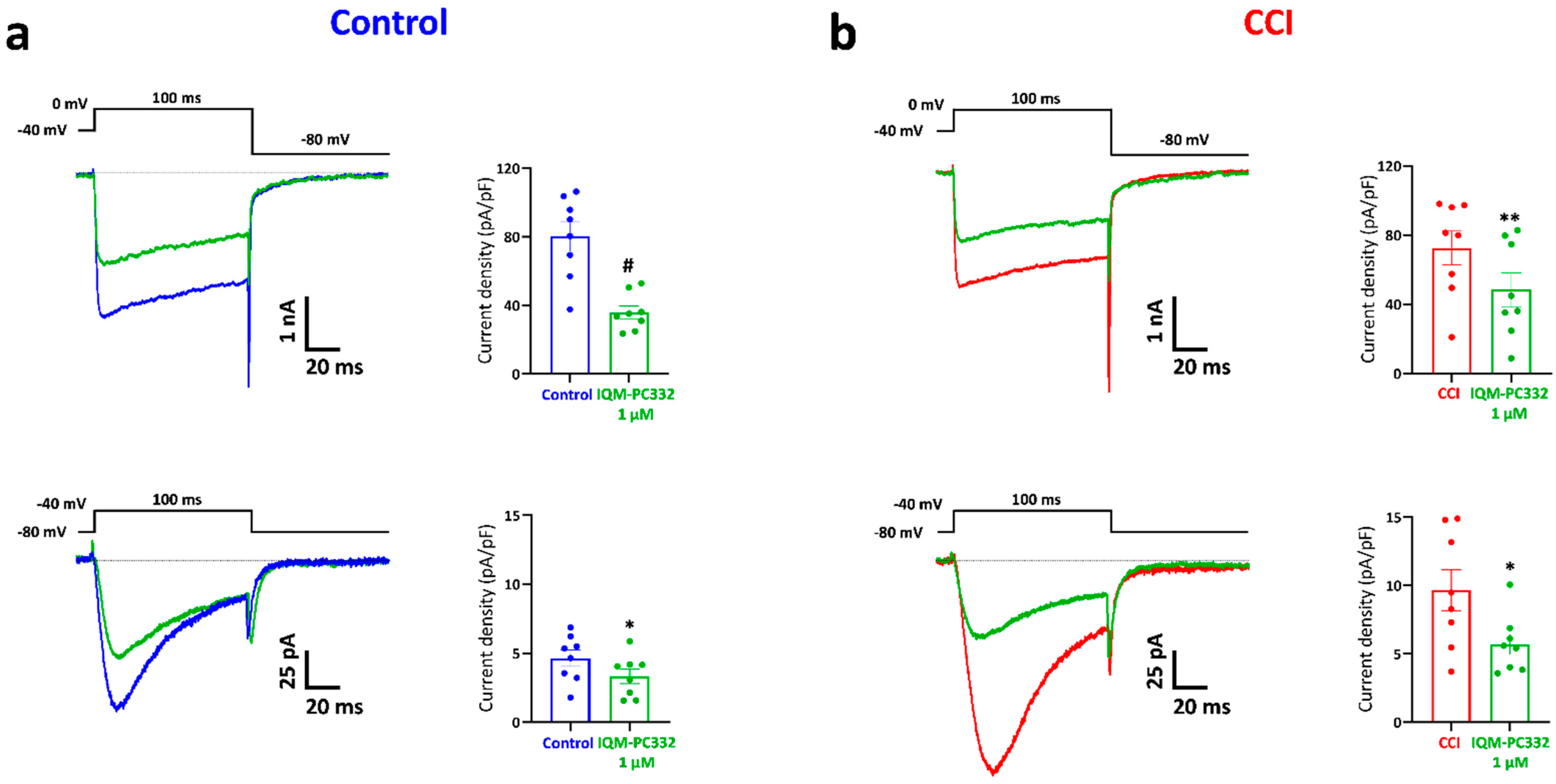
2.3. Effect of IQM-PC332 on T-Type and HVA Ca2+ Currents in DRG Neurons
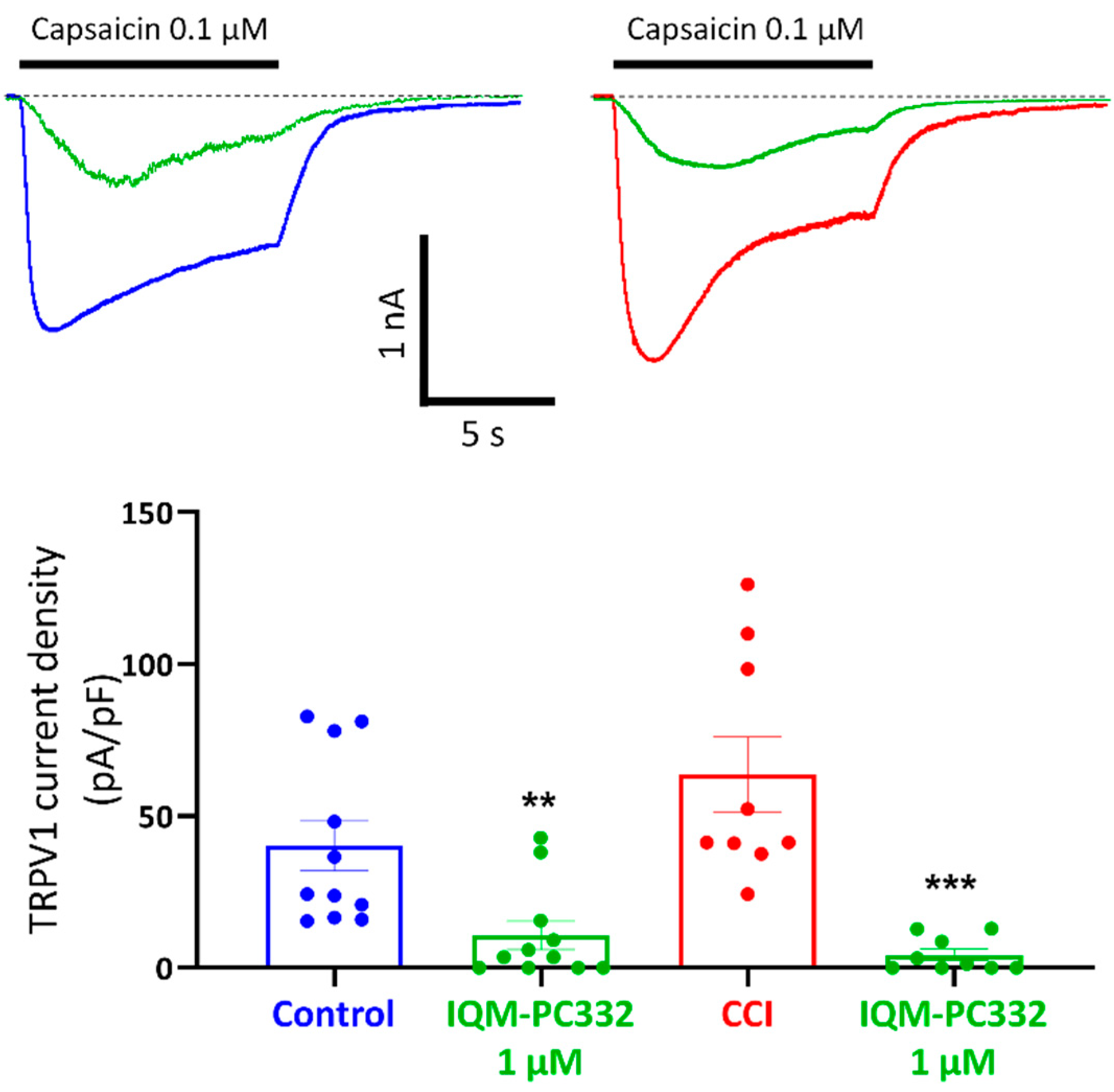
2.4. Effect of IQM-PC332 on TRPV1 Channels in DRG Neurons
2.5. Effect of IQM-PC332 on Electrical Activity of DRG Neurons
3. Discussion
4. Materials and Methods
4.1. Chronic Constriction Injury of The Sciatic Nerve
4.2. Behavioural Testing
4.2.1. Mechanical Sensitivity
4.2.2. Motor Coordination
4.3. Isolation of DRG Neurons
4.4. Electrophysiological Recordings
4.4.1. Solutions and Drug Application
4.4.2. Voltage/Current Protocols and Data Analysis
4.5. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BDNF | Brain derived neurotrophic factor |
| Cav | Voltage-activated calcium channel/current |
| CCI | Chronic constriction injury |
| DMEM | Dulbecco’s modified Eagle’s Medium-low glucose |
| DREAM | Downstream regulatory element antagonist modulator |
| DRG | Dorsal root ganglia |
| ED50 | Effective dose 50% |
| HVA | High voltage-activated |
| IC50 | Inhibitory concentration 50% |
| i.p. | Intraperitoneal |
| i.pl. | Intraplantar |
| IQM-PC332 | (2-[2-(3,4-Dichlorophenyl)acetylamino]-4-(4′-n-butylphenyl)benzoic acid) |
| KChIP3 | Potassium channel interacting protein 3 |
| KD | Dissociation constant |
| Kv | Voltage-activated potassium channel/current |
| MPE | Maximum possible effect |
| Nav | Voltage-activated sodium channel/current |
| PWT | Paw withdrawal threshold |
| TRPV1 | Transient receptor potential vanilloid-1 |
| Vh | Holding voltage |
| Vcomm | Voltage command |
| Vr | Resting membrane potential |
References
- Di Stefano, G.; Di Lionardo, A.; Di Pietro, G.; Cruccu, G.; Truini, A. Pharmacotherapeutic Options for Managing Neuropathic Pain: A Systematic Review and Meta-Analysis. Pain Res. Manag. 2021, 2021, 6656863. [Google Scholar] [CrossRef]
- St John Smith, E. Advances in Understanding Nociception and Neuropathic Pain. J. Neurol. 2018, 265, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Waxman, S.G.; Zamponi, G.W. Regulating Excitability of Peripheral Afferents: Emerging Ion Channel Targets. Nat. Neurosci. 2014, 17, 153–163. [Google Scholar] [CrossRef]
- Burgoyne, R.D.; Haynes, L.P. Understanding the Physiological Roles of the Neuronal Calcium Sensor Proteins. Mol. Brain 2012, 5, 2. [Google Scholar] [CrossRef] [PubMed]
- Buxbaum, J.D.; Choi, E.K.; Luo, Y.; Lilliehook, C.; Crowley, A.C.; Merriam, D.E.; Wasco, W. Calsenilin: A Calcium-Binding Protein That Interacts with the Presenilins and Regulates the Levels of a Presenilin Fragment. Nat. Med. 1998, 4, 1177–1181. [Google Scholar] [CrossRef] [PubMed]
- Carrión, A.M.; Link, W.A.; Ledo, F.; Mellström, B.; Naranjo, J.R. DREAM Is a Ca2+-Regulated Transcriptional Repressor. Nature 1999, 398, 80–84. [Google Scholar] [CrossRef]
- An, W.F.; Bowlby, M.R.; Betty, M.; Cao, J.; Ling, H.P.; Mendoza, G.; Hinson, J.W.; Mattsson, K.I.; Strassle, B.W.; Trimmer, J.S.; et al. Modulation of A-Type Potassium Channels by a Family of Calcium Sensors. Nature 2000, 403, 553–556. [Google Scholar] [CrossRef]
- Mellstrom, B.; Torres, B.; Link, W.A.; Naranjo, J.R. The BDNF Gene: Exemplifying Complexity in Ca2+ -Dependent Gene Expression. Crit. Rev. Neurobiol. 2004, 16, 43–49. [Google Scholar] [CrossRef]
- Rivera-Arconada, I.; Benedet, T.; Roza, C.; Torres, B.; Barrio, J.; Krzyzanowska, A.; Avendaño, C.; Mellström, B.; Lopez-Garcia, J.A.; Naranjo, J.R. DREAM Regulates BDNF-De.ependent Spinal Sensitization. Mol. Pain 2010, 6, 95. [Google Scholar] [CrossRef]
- Cheng, H.Y.; Pitcher, G.M.; Laviolette, S.R.; Whishaw, I.Q.; Tong, K.I.; Kockeritz, L.K.; Wada, T.; Joza, N.A.; Crackower, M.; Goncalves, J.; et al. DREAM Is a Critical Transcriptional Repressor for Pain Modulation. Cell 2002, 108, 31–43. [Google Scholar] [CrossRef]
- Benedet, T.; Gonzalez, P.; Oliveros, J.C.; Dopazo, J.M.; Ghimire, K.; Palczewska, M.; Mellstrom, B.; Naranjo, J.R. Transcriptional Repressor DREAM Regulates Trigeminal Noxious Perception. J. Neurochem. 2017, 141, 544–552. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Savignac, M.; Pintado, B.; Gutierrez-Adan, A.; Palczewska, M.; Mellström, B.; Naranjo, J.R. Transcriptional Repressor DREAM Regulates T-Lymphocyte Proliferation and Cytokine Gene Expression. EMBO J. 2005, 24, 3555–3564. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Su, P.; Liang, P.; Liu, T.; Liu, X.; Liu, X.Y.; Zhang, B.; Han, T.; Zhu, Y.B.; Yin, D.M.; et al. The DREAM Protein Negatively Regulates the NMDA Receptor through Interaction with the NR1 Subunit. J. Neurosci. 2010, 30, 7575–7586. [Google Scholar] [CrossRef] [PubMed]
- Tian, N.X.; Xu, Y.; Yang, J.Y.; Li, L.; Sun, X.H.; Wang, Y.; Zhang, Y. KChIP3 N-Terminal 31-50 Fragment Mediates Its Association with TRPV1 and Alleviates Inflammatory Hyperalgesia in Rats. J. Neurosci. 2018, 38, 1756–1773. [Google Scholar] [CrossRef]
- Jerng, H.H.; Pfaffinger, P.J.; Covarrubias, M. Molecular Physiology and Modulation of Somatodendritic A-Type Potassium Channels. Mol. Cell Neurosci. 2004, 27, 343–369. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.; Mehaffey, W.H.; Iftinca, M.; Rehak, R.; Engbers, J.D.; Hameed, S.; Zamponi, G.W.; Turner, R.W. Regulation of Neuronal Activity by Cav3-Kv4 Channel Signaling Complexes. Nat. Neurosci. 2010, 13, 333–337. [Google Scholar] [CrossRef]
- Thomsen, M.B.; Wang, C.; Ozgen, N.; Wang, H.G.; Rosen, M.R.; Pitt, G.S. Accessory Subunit KChIP2 Modulates the Cardiac L-Type Calcium Current. Circ. Res. 2009, 104, 1382–1389. [Google Scholar] [CrossRef]
- Matsuyoshi, H.; Takimoto, K.; Yunoki, T.; Erickson, V.L.; Tyagi, P.; Hirao, Y.; Wanaka, A.; Yoshimura, N. Distinct Cellular Distributions of Kv4 Pore-Forming and Auxiliary Subunits in Rat Dorsal Root Ganglion Neurons. Life Sci. 2012, 91, 258–263. [Google Scholar] [CrossRef]
- Cheng, C.F.; Wang, W.C.; Huang, C.Y.; Du, P.H.; Yang, J.H.; Tsaur, M.L. Coexpression of Auxiliary Subunits KChIP and DPPL in Potassium Channel Kv4-Positive Nociceptors and Pain-Modulating Spinal Interneurons. J. Comp. Neurol. 2016, 524, 846–873. [Google Scholar] [CrossRef]
- Lopez-Hurtado, A.; Peraza, D.A.; Cercos, P.; Lagartera, L.; Gonzalez, P.; Dopazo, X.M.; Herranz, R.; Gonzalez, T.; Martin-Martinez, M.; Mellström, B.; et al. Targeting the Neuronal Calcium Sensor DREAM with Small-Molecules for Huntington’s Disease Treatment. Sci. Rep. 2019, 9, 7260. [Google Scholar] [CrossRef]
- Cercós, P.; Peraza, D.A.; Benito-Bueno, A.; Socuéllamos, P.G.; Aziz-Nignan, A.; Arrechaga-Estévez, D.; Beato, E.; Peña-Acevedo, E.; Albert, A.; González-Vera, J.A.; et al. Pharmacological Approaches for the Modulation of the Potassium Channel KV4.x and KChIPs. Int. J. Mol. Sci. 2021, 22, 1419. [Google Scholar] [CrossRef] [PubMed]
- Phuket, T.R.; Covarrubias, M. Kv4 Channels Underlie the Subthreshold-Operating A-Type K+-Current in Nociceptive Dorsal Root Ganglion Neurons. Front. Mol. Neurosci. 2009, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Tsantoulas, C.; McMahon, S.B. Opening Paths to Novel Analgesics: The Role of Potassium Channels in Chronic Pain. Trends Neurosci. 2014, 37, 146–158. [Google Scholar] [CrossRef]
- Kim, D.S.; Choi, J.O.; Rim, H.D.; Cho, H.J. Downregulation of Voltage-Gated Potassium Channel Alpha Gene Expression in Dorsal Root Ganglia Following Chronic Constriction Injury of the Rat Sciatic Nerve. Brain Res. Mol. Brain Res. 2002, 105, 146–152. [Google Scholar] [CrossRef]
- Xiao, Y.; Wu, Y.; Zhao, B.; Xia, Z. Decreased Voltage-Gated Potassium Currents in Rat Dorsal Root Ganglion Neurons after Chronic Constriction Injury. Neuroreport 2016, 27, 104–109. [Google Scholar] [CrossRef] [PubMed]
- White, G.; Lovinger, D.M.; Weight, F.F. Transient Low-Threshold Ca2+ Current Triggers Burst Firing through an Afterdepolarizing Potential in an Adult Mammalian Neuron. Proc. Natl. Acad. Sci. USA 1989, 86, 6802–6806. [Google Scholar] [CrossRef] [PubMed]
- Bourinet, E.; Alloui, A.; Monteil, A.; Barrère, C.; Couette, B.; Poirot, O.; Pages, A.; McRory, J.; Snutch, T.P.; Eschalier, A.; et al. Silencing of the Cav3.2 T-Type Calcium Channel Gene in Sensory Neurons Demonstrates Its Major Role in Nociception. EMBO J. 2005, 24, 315–324. [Google Scholar] [CrossRef]
- Chi, X.X.; Schmutzler, B.S.; Brittain, J.M.; Wang, Y.; Hingtgen, C.M.; Nicol, G.D.; Khanna, R. Regulation of N-Type Voltage-Gated Calcium Channels (Cav2.2) and Transmitter Release by Collapsin Response Mediator Protein-2 (CRMP-2) in Sensory Neurons. J. Cell Sci. 2009, 122, 4351–4362. [Google Scholar] [CrossRef]
- Chaplan, S.R.; Pogrel, J.W.; Yaksh, T.L. Role of Voltage-Dependent Calcium Channel Subtypes in Experimental Tactile Allodynia. J. Pharmacol. Exp. Ther. 1994, 269, 1117–1123. [Google Scholar]
- Hatakeyama, S.; Wakamori, M.; Ino, M.; Miyamoto, N.; Takahashi, E.; Yoshinaga, T.; Sawada, K.; Imoto, K.; Tanaka, I.; Yoshizawa, T.; et al. Differential Nociceptive Responses in Mice Lacking the Alpha(1B) Subunit of N-Type Ca(2+) Channels. Neuroreport 2001, 12, 2423–2427. [Google Scholar] [CrossRef]
- François, A.; Laffray, S.; Pizzoccaro, A.; Eschalier, A.; Bourinet, E. T-Type Calcium Channels in Chronic Pain: Mouse Models and Specific Blockers. Pflugers Arch. 2014, 466, 707–717. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Montagut-Bordas, C.; Dickenson, A.H. Calcium Channel Modulation as a Target in Chronic Pain Control. Br. J. Pharmacol. 2018, 175, 2173–2184. [Google Scholar] [CrossRef] [PubMed]
- Carbone, E.; Lux, H.D. Kinetics and Selectivity of a Low-Voltage-Activated Calcium Current in Chick and Rat Sensory Neurones. J. Physiol. 1987, 386, 547–570. [Google Scholar] [CrossRef] [PubMed]
- Jagodic, M.M.; Pathirathna, S.; Joksovic, P.M.; Lee, W.; Nelson, M.T.; Naik, A.K.; Su, P.; Jevtovic-Todorovic, V.; Todorovic, S.M. Upregulation of the T-Type Calcium Current in Small Rat Sensory Neurons after Chronic Constrictive Injury of the Sciatic Nerve. J. Neurophysiol. 2008, 99, 3151–3156. [Google Scholar] [CrossRef] [PubMed]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The Capsaicin Receptor: A Heat-Activated Ion Channel in the Pain Pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Arribas-Blázquez, M.; Olivos-Oré, L.A.; Barahona, M.V.; Sánchez de la Muela, M.; Solar, V.; Jiménez, E.; Gualix, J.; McIntosh, J.M.; Ferrer-Montiel, A.; Miras-Portugal, M.T.; et al. Overexpression of P2X3 and P2X7 Receptors and TRPV1 Channels in Adrenomedullary Chromaffin Cells in a Rat Model of Neuropathic Pain. Int. J. Mol. Sci. 2019, 20, 155. [Google Scholar] [CrossRef]
- Cobianchi, S.; de Cruz, J.; Navarro, X. Assessment of Sensory Thresholds and Nociceptive Fiber Growth after Sciatic Nerve Injury Reveals the Differential Contribution of Collateral Reinnervation and Nerve Regeneration to Neuropathic Pain. Exp. Neurol. 2014, 255, 1–11. [Google Scholar] [CrossRef]
- Kambiz, S.; Baas, M.; Duraku, L.S.; Kerver, A.L.; Koning, A.H.J.; Walbeehm, E.T.; Ruigrok, T.J.H. Innervation Mapping of the Hind Paw of the Rat Using Evans Blue Extravasation, Optical Surface Mapping and CASAM. J. Neurosci. Methods 2014, 229, 15–27. [Google Scholar] [CrossRef]
- Attal, N.; Filliatreau, G.; Perrot, S.; Jazat, F.; Di Giamberardino, L.; Guilbaud, G. Behavioural Pain-Related Disorders and Contribution of the Saphenous Nerve in Crush and Chronic Constriction Injury of the Rat Sciatic Nerve. Pain 1994, 59, 301–312. [Google Scholar] [CrossRef]
- Julius, D. TRP Channels and Pain. Annu. Rev. Cell Dev. Biol. 2013, 29, 355–384. [Google Scholar] [CrossRef]
- Cardoso, F.C.; Lewis, R.J. Sodium Channels and Pain: From Toxins to Therapies. Br. J. Pharmacol. 2018, 175, 2138–2157. [Google Scholar] [CrossRef] [PubMed]
- Scroggs, R.S.; Fox, A.P. Multiple Ca2+ Currents Elicited by Action Potential Waveforms in Acutely Isolated Adult Rat Dorsal Root Ganglion Neurons. J. Neurosci. 1992, 12, 1789–1801. [Google Scholar] [CrossRef] [PubMed]
- Westenbroek, R.E.; Hoskins, L.; Catterall, W.A. Localization of Ca2+ Channel Subtypes on Rat Spinal Motor Neurons, Interneurons, and Nerve Terminals. J. Neurosci. 1998, 18, 6319–6330. [Google Scholar] [CrossRef]
- Rose, K.E.; Lunardi, N.; Boscolo, A.; Dong, X.; Erisir, A.; Jevtovic-Todorovic, V.; Todorovic, S.M. Immunohistological Demonstration of CaV3.2 T-Type Voltage-Gated Calcium Channel Expression in Soma of Dorsal Root Ganglion Neurons and Peripheral Axons of Rat and Mouse. Neuroscience 2013, 250, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Peraza, D.M. Efectos Electrofisiológicos de PC332 Y PC342 Sobre Los Canales Kv4.3 y Kv4.3+KChIP3. Master’s Thesis, Universidad Autónoma de Madrid, Madrid, Spain, 2015. [Google Scholar]
- Santicioli, P.; Del Bianco, E.; Tramontana, M.; Geppetti, P.; Maggi, C.A. Release of Calcitonin Gene-Related Peptide like-Immunoreactivity Induced by Electrical Field Stimulation from Rat Spinal Afferents Is Mediated by Conotoxin-Sensitive Calcium Channels. Neurosci. Lett. 1992, 136, 161–164. [Google Scholar] [CrossRef]
- Terashima, T.; Xu, Q.; Yamaguchi, S.; Yaksh, T.L. Intrathecal P/Q- and R-Type Calcium Channel Blockade of Spinal Substance P Release and c-Fos Expression. Neuropharmacology 2013, 75, 1–8. [Google Scholar] [CrossRef]
- Jacus, M.O.; Uebele, V.N.; Renger, J.J.; Todorovic, S.M. Presynaptic Cav3.2 Channels Regulate Excitatory Neurotransmission in Nociceptive Dorsal Horn Neurons. J. Neurosci. 2012, 32, 9374–9382. [Google Scholar] [CrossRef] [PubMed]
- Hogan, Q.H. Role of Decreased Sensory Neuron Membrane Calcium Currents in the Genesis of Neuropathic Pain. Croat. Med. J. 2007, 48, 9–21. [Google Scholar]
- Vydyanathan, A.; Wu, Z.Z.; Chen, S.R.; Pan, H.L. A-Type Voltage-Gated K+ Currents Influence Firing Properties of Isolectin B4-Positive but Not Isolectin B4-Negative Primary Sensory Neurons. J. Neurophysiol. 2005, 93, 3401–3409. [Google Scholar] [CrossRef]
- Wang, Z.; Ling, D.; Wu, C.; Han, J.; Zhao, Y. Baicalin Prevents the Up-Regulation of TRPV1 in Dorsal Root Ganglion and Attenuates Chronic Neuropathic Pain. Vet. Med. Sci. 2020, 6, 1034–1040. [Google Scholar] [CrossRef]
- Jerng, H.H.; Pfaffinger, P.J. Modulatory Mechanisms and Multiple Functions of Somatodendritic A-Type K (+) Channel Auxiliary Subunits. Front. Cell Neurosci. 2014, 8, 82. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.P.; Zhi, Y.R.; Liu, T.T.; Wang, Y.; Zhang, Y. Global Gene Knockout of Kcnip3 Enhances Pain Sensitivity and Exacerbates Negative Emotions in Rats. Front. Mol. Neurosci. 2019, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- Bennett, G.J.; Xie, Y.K. A Peripheral Mononeuropathy in Rat That Produces Disorders of Pain Sensation like Those Seen in Man. Pain 1988, 33, 87–107. [Google Scholar] [CrossRef]
- Arribas-Blázquez, M.; Olivos-Oré, L.A.; Barahona, M.V.; Wojnicz, A.; De Pascual, R.; Sánchez de la Muela, M.; García, A.G.; Artalejo, A.R. The Adrenal Medulla Modulates Mechanical Allodynia in a Rat Model of Neuropathic Pain. Int. J. Mol. Sci. 2020, 21, 8325. [Google Scholar] [CrossRef]
- Carabelli, V.; Giancippoli, A.; Baldelli, P.; Carbone, E.; Artalejo, A.R. Distinct Potentiation of L-Type Currents and Secretion by CAMP in Rat Chromaffin Cells. Biophys. J. 2003, 85, 1326–1337. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Socuéllamos, P.G.; Olivos-Oré, L.A.; Barahona, M.V.; Cercós, P.; Pérez Pascual, M.; Arribas-Blázquez, M.; Naranjo, J.R.; Valenzuela, C.; Gutiérrez-Rodríguez, M.; Artalejo, A.R. IQM-PC332, a Novel DREAM Ligand with Antinociceptive Effect on Peripheral Nerve Injury-Induced Pain. Int. J. Mol. Sci. 2022, 23, 2142. https://doi.org/10.3390/ijms23042142
Socuéllamos PG, Olivos-Oré LA, Barahona MV, Cercós P, Pérez Pascual M, Arribas-Blázquez M, Naranjo JR, Valenzuela C, Gutiérrez-Rodríguez M, Artalejo AR. IQM-PC332, a Novel DREAM Ligand with Antinociceptive Effect on Peripheral Nerve Injury-Induced Pain. International Journal of Molecular Sciences. 2022; 23(4):2142. https://doi.org/10.3390/ijms23042142
Chicago/Turabian StyleSocuéllamos, Paula G., Luis A. Olivos-Oré, María Victoria Barahona, Pilar Cercós, Marta Pérez Pascual, Marina Arribas-Blázquez, José Ramón Naranjo, Carmen Valenzuela, Marta Gutiérrez-Rodríguez, and Antonio R. Artalejo. 2022. "IQM-PC332, a Novel DREAM Ligand with Antinociceptive Effect on Peripheral Nerve Injury-Induced Pain" International Journal of Molecular Sciences 23, no. 4: 2142. https://doi.org/10.3390/ijms23042142
APA StyleSocuéllamos, P. G., Olivos-Oré, L. A., Barahona, M. V., Cercós, P., Pérez Pascual, M., Arribas-Blázquez, M., Naranjo, J. R., Valenzuela, C., Gutiérrez-Rodríguez, M., & Artalejo, A. R. (2022). IQM-PC332, a Novel DREAM Ligand with Antinociceptive Effect on Peripheral Nerve Injury-Induced Pain. International Journal of Molecular Sciences, 23(4), 2142. https://doi.org/10.3390/ijms23042142






