Distinct Cold Acclimation of Productivity Traits in Arabidopsis thaliana Ecotypes †
Abstract
:1. Introduction
1.1. Climate Resilience
1.2. Comparative Ecophysiology and the Study of Phenotypic Plasticity: Historical Perspective and Choice of Study System
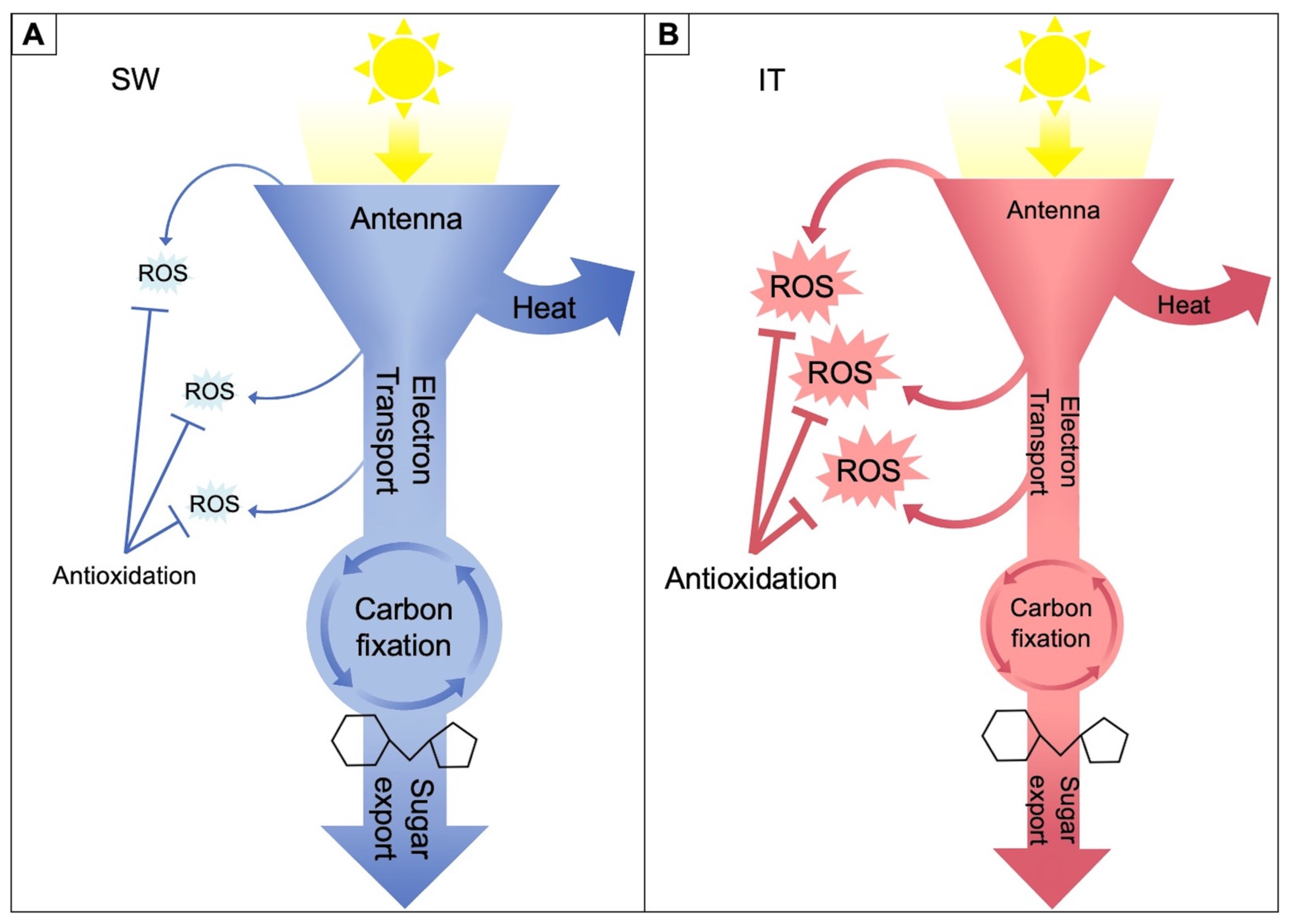
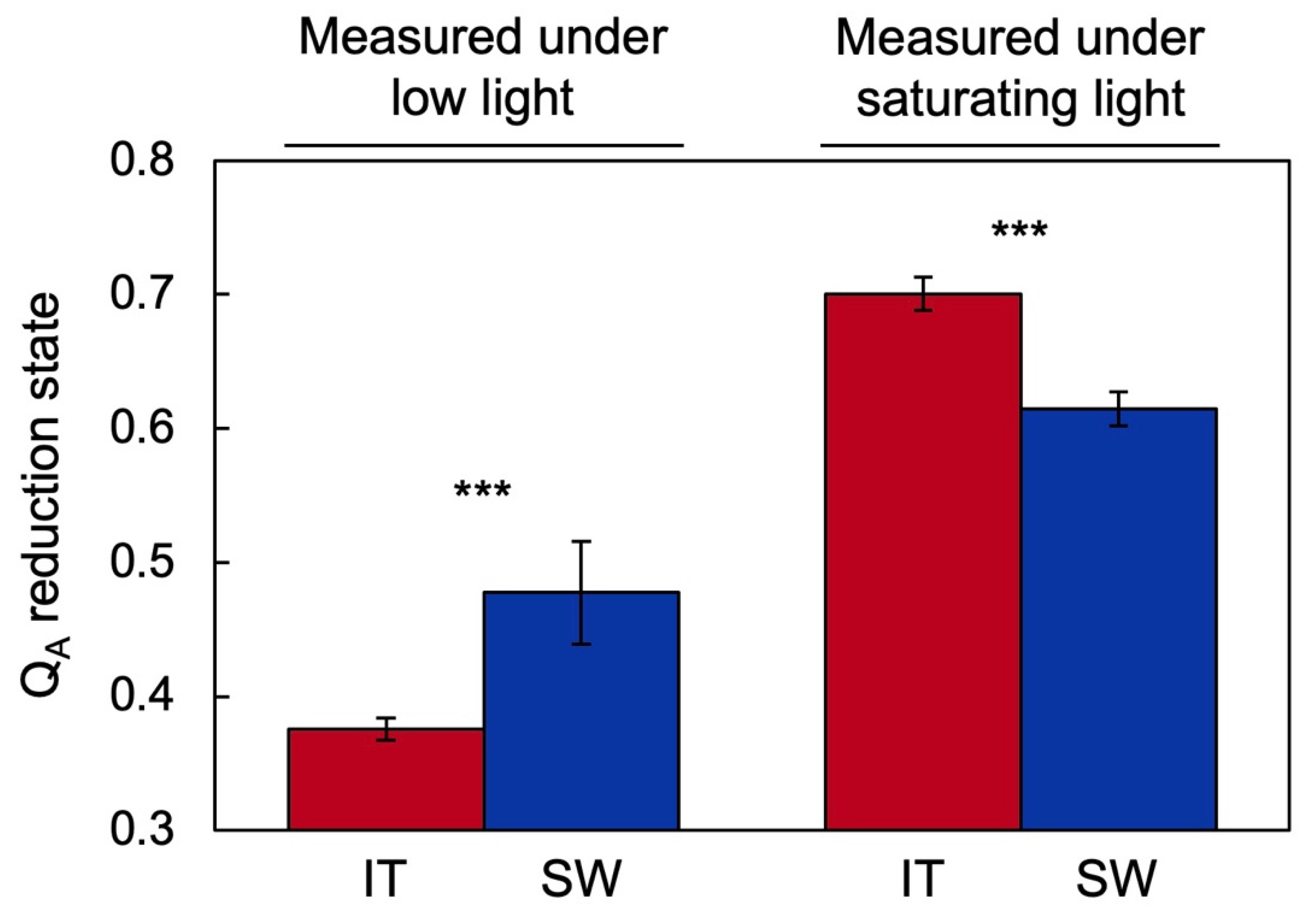
2. Differential Upregulation of Photosynthesis and Photoprotection, Sugar-Export Infrastructure, and Expression of Relevant Genes
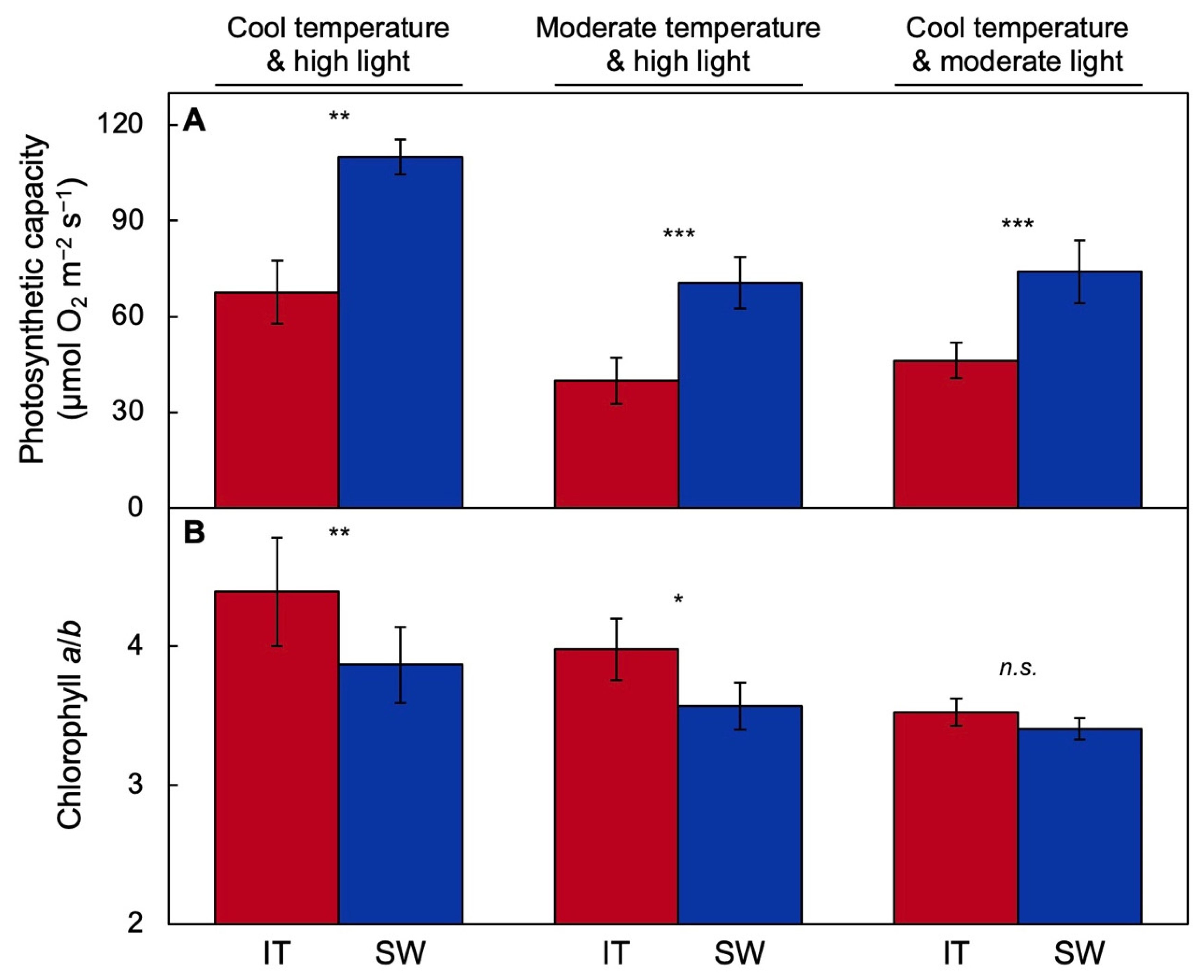
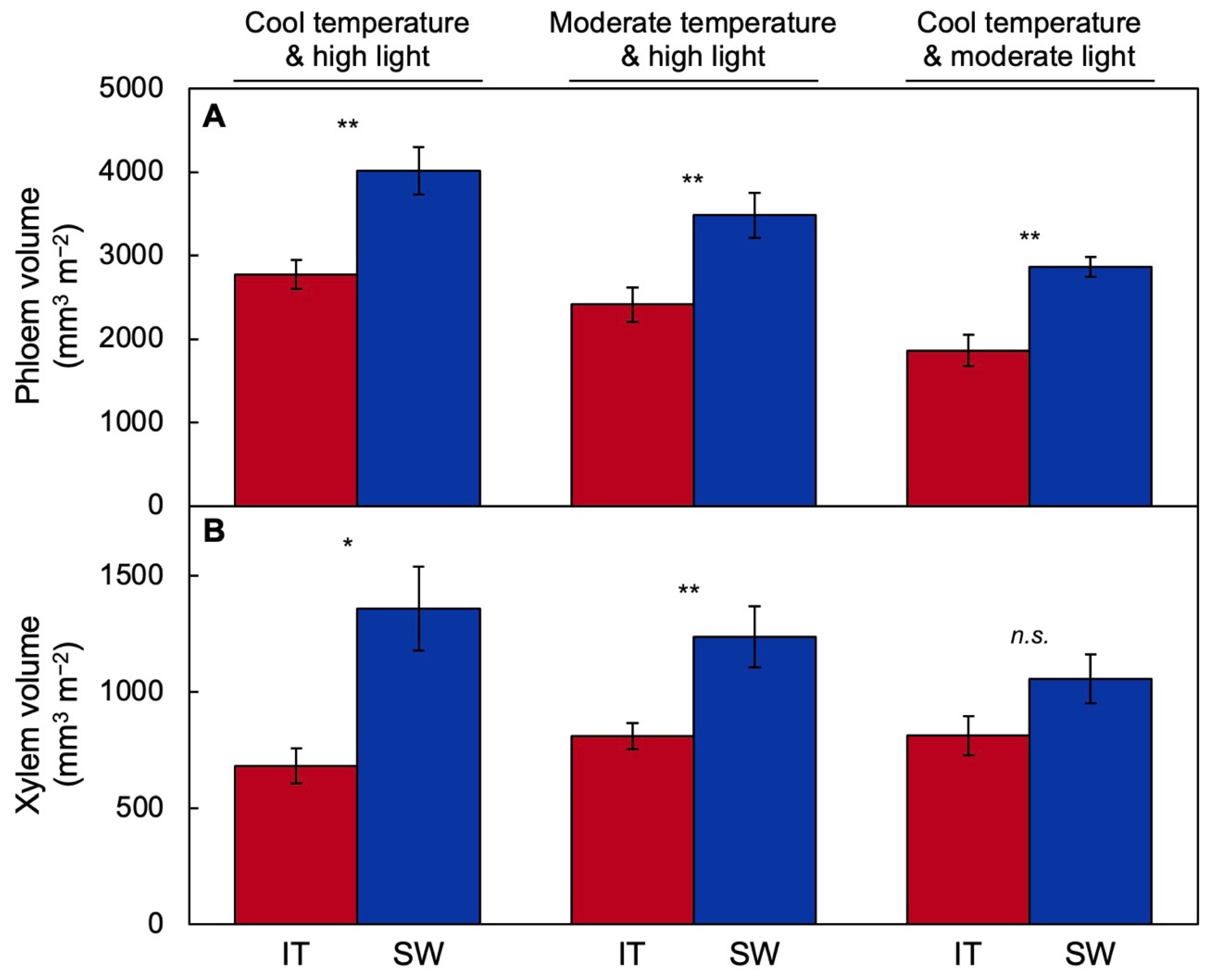
2.1. Functional and Anatomical Traits
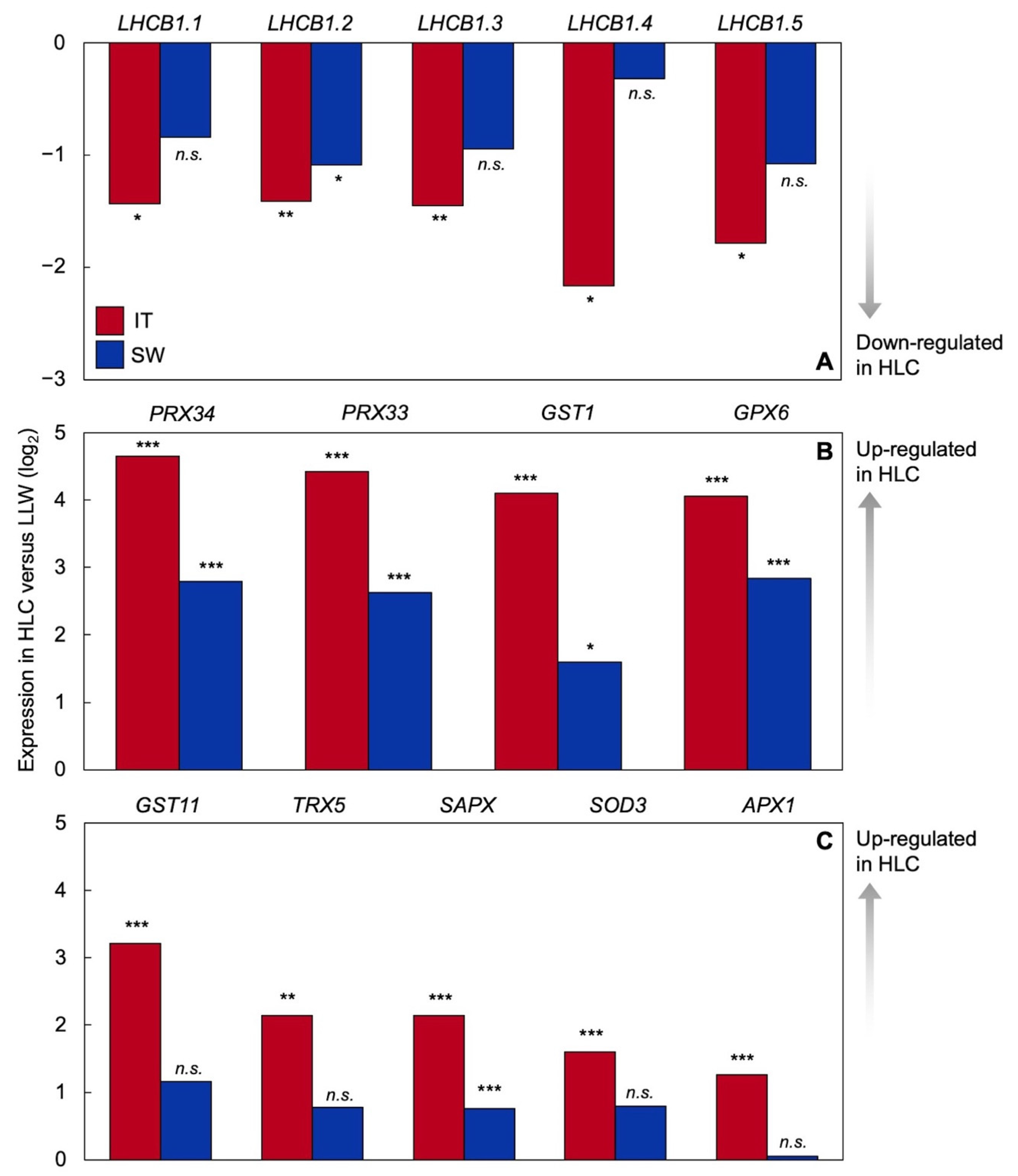
2.2. Differential Gene Expression
3. Growth Patterns as Affected by Growth Environment and Genotype
4. Summary and Conclusions in the Context of Climate Resilience and Dynamic Linkages
- The movement of agriculture to a cooler time of the year or a higher latitude, as an increasingly discussed agricultural approach [5,6,7,8], should be accompanied by crop development for each specific scenario. The design of climate-resilient crops should take into consideration the specific target latitude and associated degree of environmental variability.
- The development of improved crops for cultivation at high latitudes (with continuously low temperatures) should probably follow the acclimation pattern of SW, whereas improved crops for mid-latitudes (where cold spells are only intermittent) should probably follow the acclimation pattern of IT.
- Whereas this review focused on the comparison of two ecotypes of a winter annual, a comparison of either of these with summer annuals may be informative. It should be assessed whether IT, but not SW, combines traits of a winter annual (upregulation of freezing tolerance and photosynthetic and photosynthate-export capacity under cool growth temperatures) with those of a summer annual. IT’s higher growth rate in warm temperatures supports such a notion.
- More generally, environments with continuous exposure to cool temperatures that dramatically lower productivity in unacclimated plants may be best met with acclimatory adjustments of plant form and function supporting the maintenance of photosynthetic productivity and sugar export from source leaves, thereby pre-emptively minimizing shifts in cellular redox homeostasis. In contrast, environments with extended periods of warmer temperatures and the possibility of only brief interspersed cold spells may be adequately met with a relatively greater emphasis on upregulation of genes with roles in the maintenance of cellular redox homeostasis during intermittent periods of stress. Pronounced growth acceleration when temperatures rise in the spring may play a role in evasion of summer heat.
- Moreover, the trajectory from initial plant exposure to cold temperatures all the way to full acclimation (with leaves newly grown and fully developed under these conditions) likely involves a more pronounced transient upregulation of the antioxidation processes even in genotypes that eventually fully re-establish photosynthetic productivity. Leaves of winter annuals may thus rely to a relatively lesser degree on management rather than avoidance of oxidative stress in fully acclimated new leaves that have developed under cool growth temperatures.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dhankher, O.P.; Foyer, C.H. Climate resilient crops for improving global food security and safety. Plant. Cell Environ. 2018, 41, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Intergovernmental Panel on Climate Change. Available online: http://www.ipcc.ch (accessed on 23 December 2021).
- Rivero, R.M.; Mittler, R.; Blumwald, E.; Zandalinas, S.I. Developing climate-resilient crops: Improving plant tolerance to stress combination. Plant J. 2022, 109, 373–389. [Google Scholar] [CrossRef] [PubMed]
- Soriano, M.A.; Orgaz, F.; Villalobos, F.J.; Fereres, E. Efficiency of water use of early plantings of sunflower. Eur. J. Agron. 2004, 21, 465–476. [Google Scholar] [CrossRef]
- Vitt, P.; Havens, K.; Kramer, A.T.; Sollenberger, D.; Yates, E. Assisted migration of plants: Changes in latitudes, changes in attitudes. Biol. Conserv. 2010, 143, 18–27. [Google Scholar] [CrossRef]
- Hu, Y.; Fan, L.; Liu, Z.; Yu, Q.; Liang, S.; Chen, S.; You, L.; Wu, W.; Yang, P. Rice production and climate change in Northeast China: Evidence of adaptation through land use shifts. Environ. Res. Lett. 2019, 14, 024014. [Google Scholar] [CrossRef]
- Carlson-Nilsson, U.; Aloisi, K.; Vågen, I.M.; Rajala, A.; Mølmann, J.B.; Rasmussen, S.K.; Niemi, M.; Wojciechowska, E.; Pärssinen, P.; Poulsen, G.; et al. Trait expression and environmental eesponses of pea (Pisum sativum L.) genetic resources targeting cultivation in the Arctic. Front. Plant Sci. 2021, 12, 1476. [Google Scholar] [CrossRef]
- Mølmann, J.B.; Dalmannsdottir, S.; Hykkerud, A.L.; Hytönen, T.; Samkumar, A.; Jaakola, L. Influence of Arctic light conditions on crop production and quality. Physiol. Plant 2021, 172, 1931–1940. [Google Scholar] [CrossRef]
- Thomashow, M.F. Plant cold acclimation: Freezing tolerance genes and regulatory mechanisms. Annu. Rev. Plant Biol. 1999, 50, 571–599. [Google Scholar] [CrossRef] [Green Version]
- Zhen, Y.; Ungerer, M.C. Clinal variation in freezing tolerance among natural accessions of Arabidopsis thaliana. New Phytol. 2008, 177, 419–427. [Google Scholar] [CrossRef]
- Kang, J.; Zhang, H.; Sun, T.; Shi, Y.; Wang, J.; Zhang, B.; Wang, Z.; Zhou, Y.; Gu, H. Natural variation of C-repeat-binding factor (CBFs) genes is a major cause of divergence in freezing tolerance among a group of Arabidopsis thaliana populations along the Yangtze River in China. New Phytol. 2013, 199, 1069–1080. [Google Scholar] [CrossRef]
- Berry, J.; Bjorkman, O. Photosynthetic response and adaptation to temperature in higher plants. Annu. Rev. Plant. Physiol. 1980, 31, 491–543. [Google Scholar] [CrossRef]
- Hüner, N.P.A.; Öquist, G.; Sarhan, F. Energy balance and acclimation to light and cold. Trends Plant. Sci. 1998, 3, 224–230. [Google Scholar] [CrossRef]
- Savitch, L.V.; Leonardos, E.D.; Krol, M.; Jansson, S.; Grodzinski, B.; Hüner, N.P.A.; Öquist, G. Two different strategies for light utilization in photosynthesis in relation to growth and cold acclimation. Plant. Cell Environ. 2002, 25, 761–771. [Google Scholar] [CrossRef]
- Adams, W.W., III; Stewart, J.J.; Demmig-Adams, B. Photosynthetic modulation in response to plant activity and environment. In The Leaf: A Platform for Performing Photosynthesis, Advances in Photosynthesis and Respiration; Adams, W.W., III, Terashima, I., Eds.; Springer: Cham, Switzerland, 2018; Volume 44, pp. 493–563. [Google Scholar] [CrossRef]
- Cohu, C.M.; Muller, O.; Adams, W.W., III; Demmig-Adams, B. Leaf anatomical and photosynthetic acclimation to cool temperature and high light in two winter versus two summer annuals. Physiol. Plant. 2014, 152, 164–173. [Google Scholar] [CrossRef]
- Stewart, J.J.; Polutchko, S.K.; Adams, W.W., III; Cohu, C.M.; Wenzl, C.A.; Demmig-Adams, B. Light, temperature and tocopherol status influence foliar vascular anatomy and leaf function in Arabidopsis thaliana. Physiol. Plant. 2017, 160, 98–110. [Google Scholar] [CrossRef]
- Gorsuch, P.A.; Pandey, S.; Atkin, O.K. Temporal heterogeneity of cold acclimation phenotypes in Arabidopsis leaves. Plant. Cell Environ. 2010, 33, 244–258. [Google Scholar] [CrossRef]
- Boese, S.R.; Hüner, N.P.A. Effect of growth temperature and temperature shifts on spinach leaf morphology and photosynthesis. Plant. Physiol. 1990, 94, 1830–1836. [Google Scholar] [CrossRef] [Green Version]
- Adams, W.W., III; Hoehn, A.; Demmig-Adams, B. Chilling temperatures and the xanthophyll cycle. A comparison of warm-grown and overwintering spinach. Funct. Plant. Biol. 1995, 22, 75–85. [Google Scholar] [CrossRef]
- Dumlao, M.R.; Darehshouri, A.; Cohu, C.M.; Muller, O.; Mathias, J.; Adams, W.W., III; Demmig-Adams, B. Low temperature acclimation of photosynthetic capacity and leaf morphology in the context of phloem loading type. Photosynth. Res. 2012, 113, 181–189. [Google Scholar] [CrossRef]
- Muller, O.; Stewart, J.J.; Cohu, C.M.; Polutchko, S.K.; Demmig-Adams, B.; Adams, W.W., III. Leaf architectural, vascular and photosynthetic acclimation to temperature in two biennials. Physiol. Plant. 2014, 152, 763–772. [Google Scholar] [CrossRef]
- Adams, W.W., III; Cohu, C.M.; Muller, O.; Demmig-Adams, B. Foliar phloem infrastructure in support of photosynthesis. Front. Plant. Sci. 2013, 4, 194. [Google Scholar] [CrossRef] [Green Version]
- Cohu, C.M.; Muller, O.; Demmig-Adams, B.; Adams, W.W., III. Minor loading vein acclimation for three Arabidopsis thaliana ecotypes in response to growth under different temperature and light regimes. Front. Plant. Sci. 2013, 4, 240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muller, O.; Cohu, C.M.; Stewart, J.J.; Protheroe, J.A.; Demmig-Adams, B.; Adams, W.W., III. Association between photosynthesis and contrasting features of minor veins in leaves of summer annuals loading phloem via symplastic versus apoplastic routes. Physiol. Plant. 2014, 152, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Cohu, C.M.; Muller, O.; Stewart, J.J.; Demmig-Adams, B.; Adams, W.W., III. Association between minor loading vein architecture and light- and CO2-saturated rates of photosynthetic oxygen evolution among Arabidopsis thaliana ecotypes from different latitudes. Front. Plant. Sci. 2013, 4, 264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clausen, J.; Keck, D.D.; Hiesey, W.M. Experimental Studies on the Nature of Species. I. Effect of Varied Environments on Western North. American Plants; Carnegie Institution of Washington: Washington, DC, USA, 1940. [Google Scholar]
- Clausen, J.; Keck, D.D.; Hiesey, W.M. Regional differentiation in plant species. Am. Nat. 1941, 75, 231–250. [Google Scholar] [CrossRef]
- Clausen, J.; Keck, D.D.; Hiesey, W.M. Heredity of geographically and ecologically isolated races. Am. Nat. 1947, 81, 114–133. [Google Scholar] [CrossRef]
- Turesson, G. The species and variety as ecological units. Hereditas 1922, 3, 100–113. [Google Scholar] [CrossRef]
- Turesson, G. The genotypical response of the plant species to the habitat. Hereditas 1922, 3, 211–350. [Google Scholar] [CrossRef]
- Turesson, G. The plant species in relation to habitat and climate. Hereditas 1925, 6, 147–236. [Google Scholar] [CrossRef]
- Hiesey, W.M.; Nobs, M.A.; Björkman, O. Experimental Studies on the Nature of Species. Volume V: Biosystematics, Genetics, and Physiological Ecology of the Erythranthe Section of Mimulus; Carnegie Institution of Washington: Washington, DC, USA, 1971. [Google Scholar]
- Björkman, O. Comparative studies on photosynthesis in higher plants. In Photophysiology, Current Topics in Photobiology and Photochemistry; Giese, A.C., Ed.; Academic Press: New York, NY, USA, 1973; Volume VIII, pp. 1–63. [Google Scholar] [CrossRef]
- Osmond, C.B.; Björkman, O.; Anderson, D.J. Physiological Processes in Plant. Ecology: Toward a Synthesis with Atriplex; Springer: Berlin, Heidelberg, 1980. [Google Scholar] [CrossRef]
- Adams, W.W., III; Demmig-Adams, B. Lessons from nature: A personal perspective. In Non-Photochemical Quenching and Energy Dissipation in Plants, Algae and Cyanobacteria, Advances in Photosynthesis and Respiration; Demmig-Adams, B., Garab, G., Adams, W.W., III, Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2014; Volume 40, pp. 45–72. [Google Scholar] [CrossRef]
- Ågren, J.; Schemske, D.W. Reciprocal transplants demonstrate strong adaptive differentiation of the model organism Arabidopsis thaliana in its native range. New Phytol. 2012, 194, 1112–1122. [Google Scholar] [CrossRef]
- Lowry, D.B. Ecotypes and the controversy over stages in the formation of new species. Biol. J. Linn. Soc. 2012, 106, 241–257. [Google Scholar] [CrossRef] [Green Version]
- Ågren, J.; Oakley, C.G.; McKay, J.K.; Lovell, J.T.; Schemske, D.W. Genetic mapping of adaptation reveals fitness tradeoffs in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2013, 110, 21077–21082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ågren, J.; Oakley, C.G.; Lundemo, S.; Schemske, D.W. Adaptive divergence in flowering time among natural populations of Arabidopsis thaliana: Estimates of selection and QTL mapping. Evolution 2017, 71, 550–564. [Google Scholar] [CrossRef] [PubMed]
- Grillo, M.A.; Li, C.; Hammond, M.; Wang, L.; Schemske, D.W. Genetic architecture of flowering time differentiation between locally adapted populations of Arabidopsis thaliana. New Phytol. 2013, 197, 1321–1331. [Google Scholar] [CrossRef]
- Postma, F.M.; Ågren, J. Maternal environment affects the genetic basis of seed dormancy in Arabidopsis thaliana. Mol. Ecol. 2015, 24, 785–797. [Google Scholar] [CrossRef]
- Postma, F.M.; Ågren, J. Early life stages contribute strongly to local adaptation in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2016, 113, 7590–7595. [Google Scholar] [CrossRef] [Green Version]
- Postma, F.M.; Ågren, J. Among-year variation in selection during early life stages and the genetic basis of fitness in Arabidopsis thaliana. Mol. Ecol. 2018, 27, 2498–2511. [Google Scholar] [CrossRef]
- Postma, F.M.; Lundemo, S.; Ågren, J. Seed dormancy cycling and mortality differ between two locally adapted populations of Arabidopsis thaliana. Ann. Bot. 2016, 117, 249–256. [Google Scholar] [CrossRef] [Green Version]
- Ellis, T.J.; Postma, F.M.; Oakley, C.G.; Ågren, J. Life-history trade-offs and the genetic basis of fitness in Arabidopsis thaliana. Mol. Ecol. 2021, 30, 2846–2858. [Google Scholar] [CrossRef]
- Oakley, C.G.; Ågren, J.; Atchison, R.A.; Schemske, D.W. QTL mapping of freezing tolerance: Links to fitness and adaptive trade-offs. Mol. Ecol. 2014, 23, 4304–4315. [Google Scholar] [CrossRef]
- Gehan, M.A.; Park, S.; Gilmour, S.J.; An, C.; Lee, C.; Thomashow, M.F. Natural variation in the C-repeat binding factor cold response pathway correlates with local adaptation of Arabidopsis ecotypes. Plant. J. 2015, 84, 682–693. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Gilmour, S.J.; Grumet, R.; Thomashow, M.F. CBF-dependent and CBF-independent regulatory pathways contribute to the differences in freezing tolerance and cold-regulated gene expression of two Arabidopsis ecotypes locally adapted to sites in Sweden and Italy. PLoS ONE 2018, 13, e0207723. [Google Scholar] [CrossRef] [Green Version]
- Oakley, C.G.; Savage, L.; Lotz, S.; Larson, G.R.; Thomashow, M.F.; Kramer, D.M.; Schemske, D.W. Genetic basis of photosynthetic responses to cold in two locally adapted populations of Arabidopsis thaliana. J. Exp. Bot. 2018, 69, 699–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanderson, B.J.; Park, S.; Jameel, M.I.; Kraft, J.C.; Thomashow, M.F.; Schemske, D.W.; Oakley, C.G. Genetic and physiological mechanisms of freezing tolerance in locally adapted populations of a winter annual. Am. J. Bot. 2020, 107, 250–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, C.R.; Stewart, J.J.; Amstutz, C.L.; Ching, L.G.; Johnson, J.D.; Niyogi, K.K.; Adams, W.W., III; Demmig-Adams, B. Genotype-dependent contribution of CBF transcription factors to long-term acclimation to high light and cool temperature. Plant. Cell Environ. 2022, 45, 392–411. [Google Scholar] [CrossRef] [PubMed]
- Savitch, L.V.; Allard, G.; Seki, M.; Robert, L.S.; Tinker, N.A.; Hüner, N.P.A.; Shinozaki, K.; Singh, J. The effect of overexpression of two Brassica CBF/DREB1-like transcription factors on photosynthetic capacity and freezing tolerance in Brassica napus. Plant. Cell Physiol. 2005, 46, 1525–1539. [Google Scholar] [CrossRef]
- Hüner, N.P.A.; Dahal, K.; Bode, R.; Kurepin, L.V.; Ivanov, A.G. Photosynthetic acclimation, vernalization, crop productivity and ‘the grand design of photosynthesis’. J. Plant. Physiol. 2016, 203, 29–43. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Stewart, J.J.; Adams, W.W. III Environmental regulation of intrinsic photosynthetic capacity: An integrated view. Curr. Opin. Plant. Biol. 2017, 37, 34–41. [Google Scholar] [CrossRef]
- Stewart, J.J.; Adams, W.W., III; Cohu, C.M.; Polutchko, S.K.; Lombardi, E.M.; Demmig-Adams, B. Differences in light-harvesting, acclimation to growth-light environment, and leaf structural development between Swedish and Italian ecotypes of Arabidopsis thaliana. Planta 2015, 242, 1277–1290. [Google Scholar] [CrossRef]
- Adams, W.W., III; Stewart, J.J.; Cohu, C.M.; Muller, O.; Demmig-Adams, B. Habitat temperature and precipitation of Arabidopsis thaliana ecotypes determine the response of foliar vasculature, photosynthesis, and transpiration to growth temperature. Front. Plant. Sci. 2016, 7, 1026. [Google Scholar] [CrossRef] [Green Version]
- Stewart, J.J.; Demmig-Adams, B.; Cohu, C.M.; Wenzl, C.A.; Muller, O.; Adams, W.W., III. Growth temperature impact on leaf form and function in Arabidopsis thaliana ecotypes from northern and southern Europe. Plant. Cell Environ. 2016, 39, 1549–1558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, J.J.; Polutchko, S.K.; Adams, W.W., III; Demmig-Adams, B. Acclimation of Swedish and Italian ecotypes of Arabidopsis thaliana to light intensity. Photosynth. Res. 2017, 134, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Adams, W.W., III; Stewart, J.J.; Polutchko, S.K.; Demmig-Adams, B. Leaf vasculature and the upper limit of photosynthesis. In The Leaf: A Platform for Performing Photosynthesis, Advances in Photosynthesis and Respiration; Adams, W.W., III, Terashima, I., Eds.; Springer: Cham, Switzerland, 2018; Volume 44, pp. 27–54. [Google Scholar] [CrossRef]
- Mason, C.M.; LaScaleia, M.C.; De La Pascua, D.R.; Monroe, J.G.; Goolsby, E.W. Learning from dynamic traits: Seasonal shifts yield insights into ecophysiological tradeoffs across scales from macroevolutionary to intra-individual. Int. J. Plant. Sci. 2020, 181, 88–102. [Google Scholar] [CrossRef]
- Westerband, A.C.; Funk, J.L.; Barton, K.E. Intraspecific trait variation in plants: A renewed focus on its role in ecological processes. Ann. Bot. 2021, 127, 397–410. [Google Scholar] [CrossRef]
- Salguero-Gómez, R.; Jones, O.R.; Jongejans, E.; Blomberg, S.P.; Hodgson, D.J.; Mbeau-Ache, C.; Zuidema, P.A.; de Kroon, H.; Buckley, Y.M. Fast–slow continuum and reproductive strategies structure plant life-history variation worldwide. Proc. Natl. Acad. Sci. USA 2016, 113, 230–235. [Google Scholar] [CrossRef] [Green Version]
- Sulis, E.; Bacchetta, G.; Cogoni, D.; Fenu, G. From global to local scale: Where is the best for conservation purpose? Biodivers. Conserv. 2021, 30, 183–200. [Google Scholar] [CrossRef]
- Stearns, S.C. Trade-offs in life-history evolution. Funct. Ecol. 1989, 3, 259. [Google Scholar] [CrossRef] [Green Version]
- Kleyer, M.; Minden, V. Why functional ecology should consider all plant organs: An allocation-based perspective. Basic Appl. Ecol. 2015, 16, 1–9. [Google Scholar] [CrossRef]
- Sack, L.; Buckley, T.N. Trait multi-functionality in plant stress response. Integr. Comp. Biol. 2020, 60, 98–112. [Google Scholar] [CrossRef]
- Reich, P.B.; Wright, I.J.; Cavender-Bares, J.; Craine, J.M.; Oleksyn, J.; Westoby, M.; Walters, M.B. The evolution of plant functional variation: Traits, spectra, and strategies. Int. J. Plant. Sci. 2003, 164, S143–S164. [Google Scholar] [CrossRef]
- Van Nuland, M.E.; Vincent, J.B.; Ware, I.M.; Mueller, L.O.; Bayliss, S.L.; Beals, K.K.; Schweitzer, J.A.; Bailey, J.K. Intraspecific trait variation across elevation predicts a widespread tree species’ climate niche and range limits. Ecol. Evol. 2020, 10, 3856–3867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saltz, J.B.; Bell, A.M.; Flint, J.; Gomulkiewicz, R.; Hughes, K.A.; Keagy, J. Why does the magnitude of genotype-by-environment interaction vary? Ecol. Evol. 2018, 8, 6342–6353. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Blanco, C.; Gomez-Mena, C.; Llorente, F.; Koornneef, M.; Salinas, J.; Martínez-Zapater, J.M. Genetic and molecular analyses of natural variation indicate CBF2 as a candidate gene for underlying a freezing tolerance quantitative trait locus in Arabidopsis. Plant. Physiol. 2005, 139, 1304–1312. [Google Scholar] [CrossRef] [Green Version]
- Monroe, J.G.; McGovern, C.; Lasky, J.R.; Grogan, K.; Beck, J.; McKay, J.K. Adaptation to warmer climates by parallel functional evolution of CBF genes in Arabidopsis thaliana. Mol. Ecol. 2016, 25, 3632–3644. [Google Scholar] [CrossRef] [PubMed]
- Krapp, A.; Stitt, M. An evaluation of direct and indirect mechanisms for the “sink-regulation” of photosynthesis in spinach: Changes in gas exchange, carbohydrates, metabolites, enzyme activities and steady-state transcript levels after cold-girdling source leaves. Planta 1995, 195, 313–323. [Google Scholar] [CrossRef]
- Polutchko, S.K.; Stewart, J.J.; Demmig-Adams, B.; Adams, W.W. III. Evaluating the link between photosynthetic capacity and leaf vascular organization with principal component analysis. Photosynthetica 2018, 56, 392–403. [Google Scholar] [CrossRef]
- Adams, W.W., III; Stewart, J.J.; Polutchko, S.K.; Demmig-Adams, B. Foliar sieve elements: Nexus of the leaf. J. Plant. Physiol. 2022, 269, 153601. [Google Scholar] [CrossRef]
- Logan, B.A.; Demmig-Adams, B.; Adams, W.W., III; Bilger, W. Context, quantification, and measurement guide for non-photochemical quenching of chlorophyll fluorescence. In Non-Photochemical Quenching and Energy Dissipation in Plants, Algae and Cyanobacteria, Advances in Photosynthesis and Respiration; Demmig-Adams, B., Garab, G., Adams, W.W., III, Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2014; Volume 40, pp. 187–201. [Google Scholar] [CrossRef]
- Kramer, D.M.; Johnson, G.; Kiirats, O.; Edwards, G.E. New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Photosynth. Res. 2004, 79, 209–218. [Google Scholar] [CrossRef]
- Eremina, M.; Rozhon, W.; Poppenberger, B. Hormonal control of cold stress responses in plants. Cell. Mol. Life Sci. 2016, 73, 797–810. [Google Scholar] [CrossRef]
- Patten, D.T. Productivity and production efficiency of an upper Sonoran Desert ephemeral community. Am. J. Bot. 1978, 65, 891–895. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demmig-Adams, B.; Polutchko, S.K.; Baker, C.R.; Stewart, J.J.; Adams III, W.W. Distinct Cold Acclimation of Productivity Traits in Arabidopsis thaliana Ecotypes. Int. J. Mol. Sci. 2022, 23, 2129. https://doi.org/10.3390/ijms23042129
Demmig-Adams B, Polutchko SK, Baker CR, Stewart JJ, Adams III WW. Distinct Cold Acclimation of Productivity Traits in Arabidopsis thaliana Ecotypes. International Journal of Molecular Sciences. 2022; 23(4):2129. https://doi.org/10.3390/ijms23042129
Chicago/Turabian StyleDemmig-Adams, Barbara, Stephanie K. Polutchko, Christopher R. Baker, Jared J. Stewart, and William W. Adams III. 2022. "Distinct Cold Acclimation of Productivity Traits in Arabidopsis thaliana Ecotypes" International Journal of Molecular Sciences 23, no. 4: 2129. https://doi.org/10.3390/ijms23042129
APA StyleDemmig-Adams, B., Polutchko, S. K., Baker, C. R., Stewart, J. J., & Adams III, W. W. (2022). Distinct Cold Acclimation of Productivity Traits in Arabidopsis thaliana Ecotypes. International Journal of Molecular Sciences, 23(4), 2129. https://doi.org/10.3390/ijms23042129








