Oncogenic Mutation BRAF V600E Changes Phenotypic Behavior of THLE-2 Liver Cells through Alteration of Gene Expression
Abstract
1. Introduction
2. Results
2.1. Selected BRAF Mutations Are Located in the Kinase Domain of BRAF
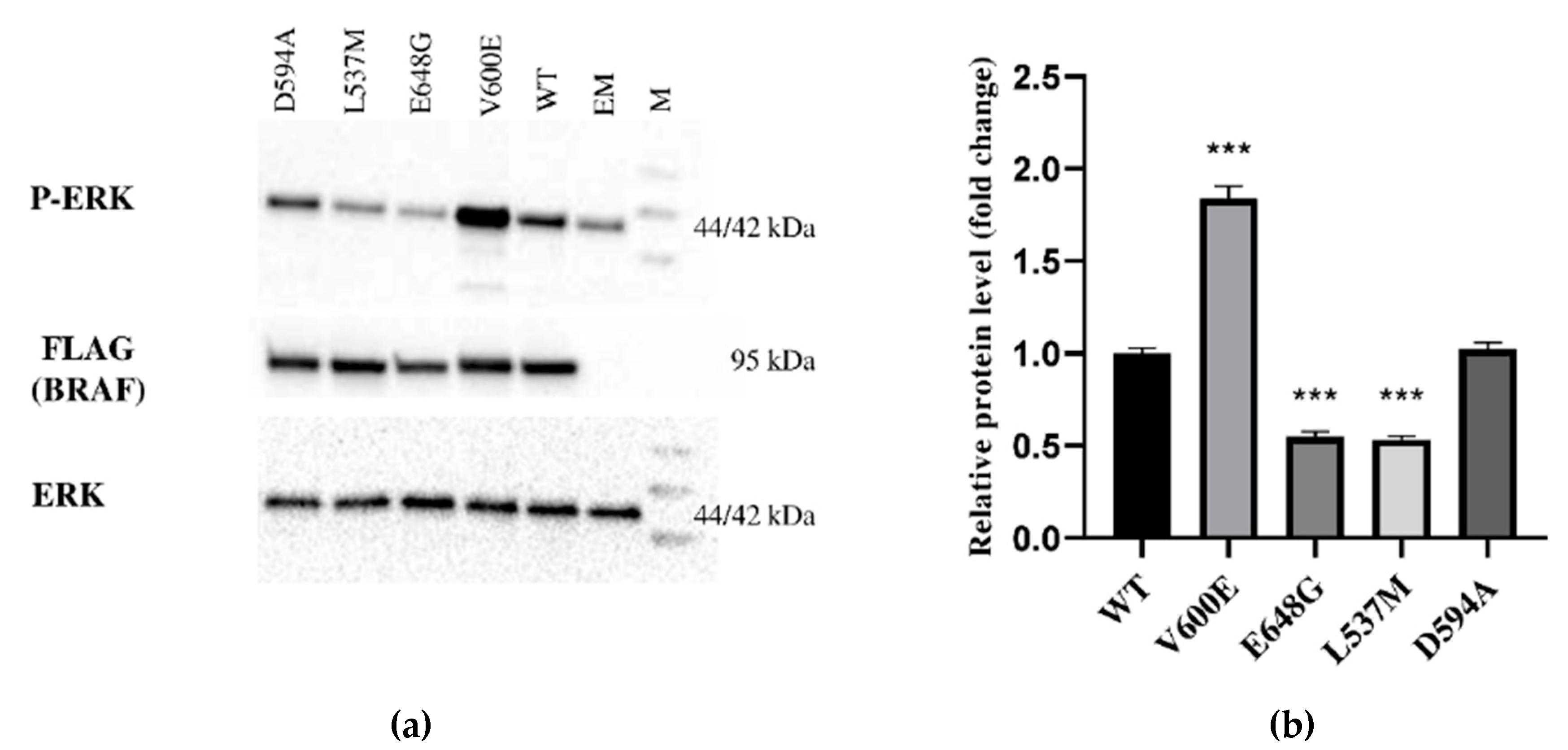
2.2. Effect of BRAF Mutations on Its Kinase Activity
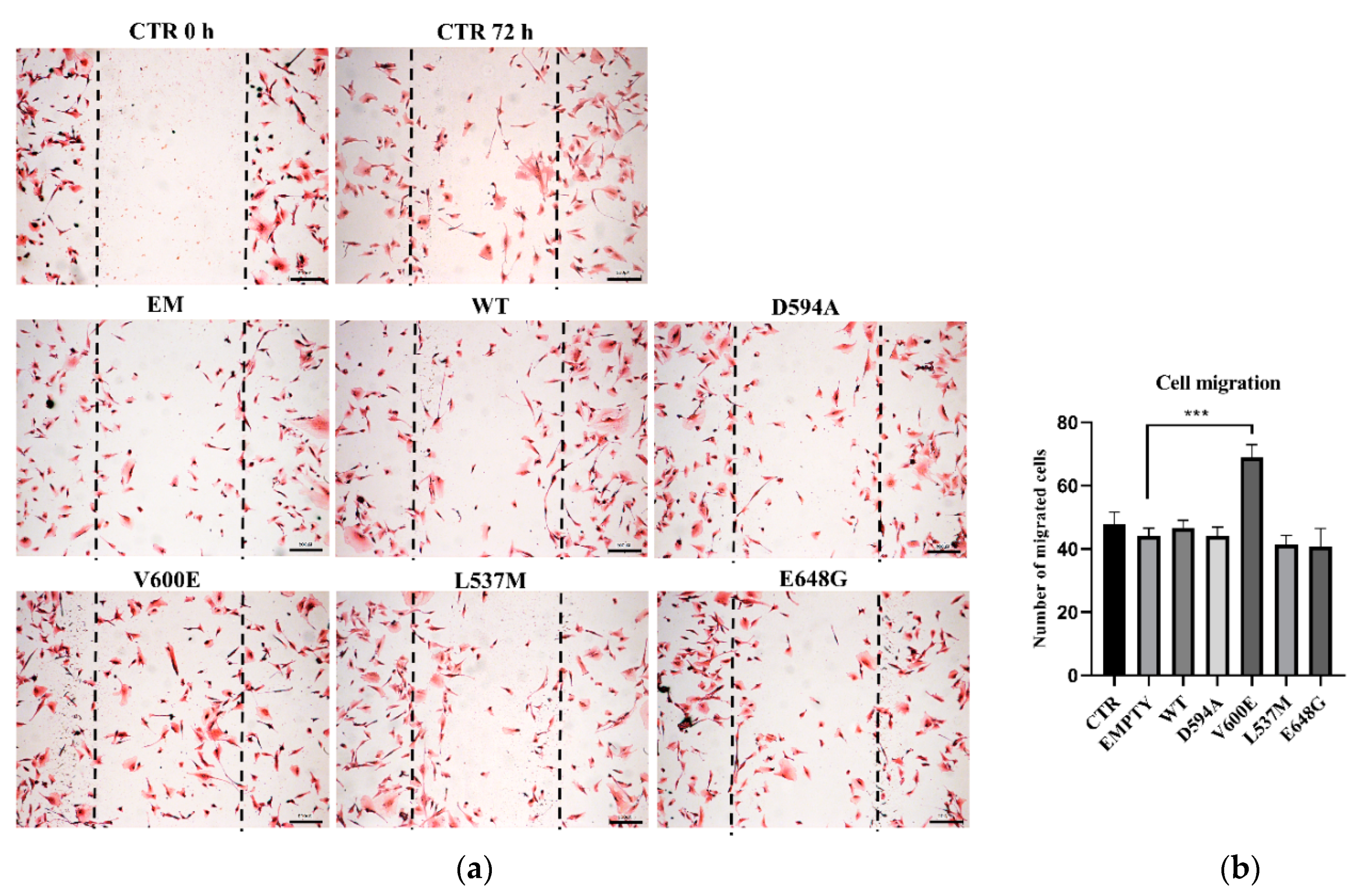
2.3. Motility of THLE-2 Cells with BRAF Mutations
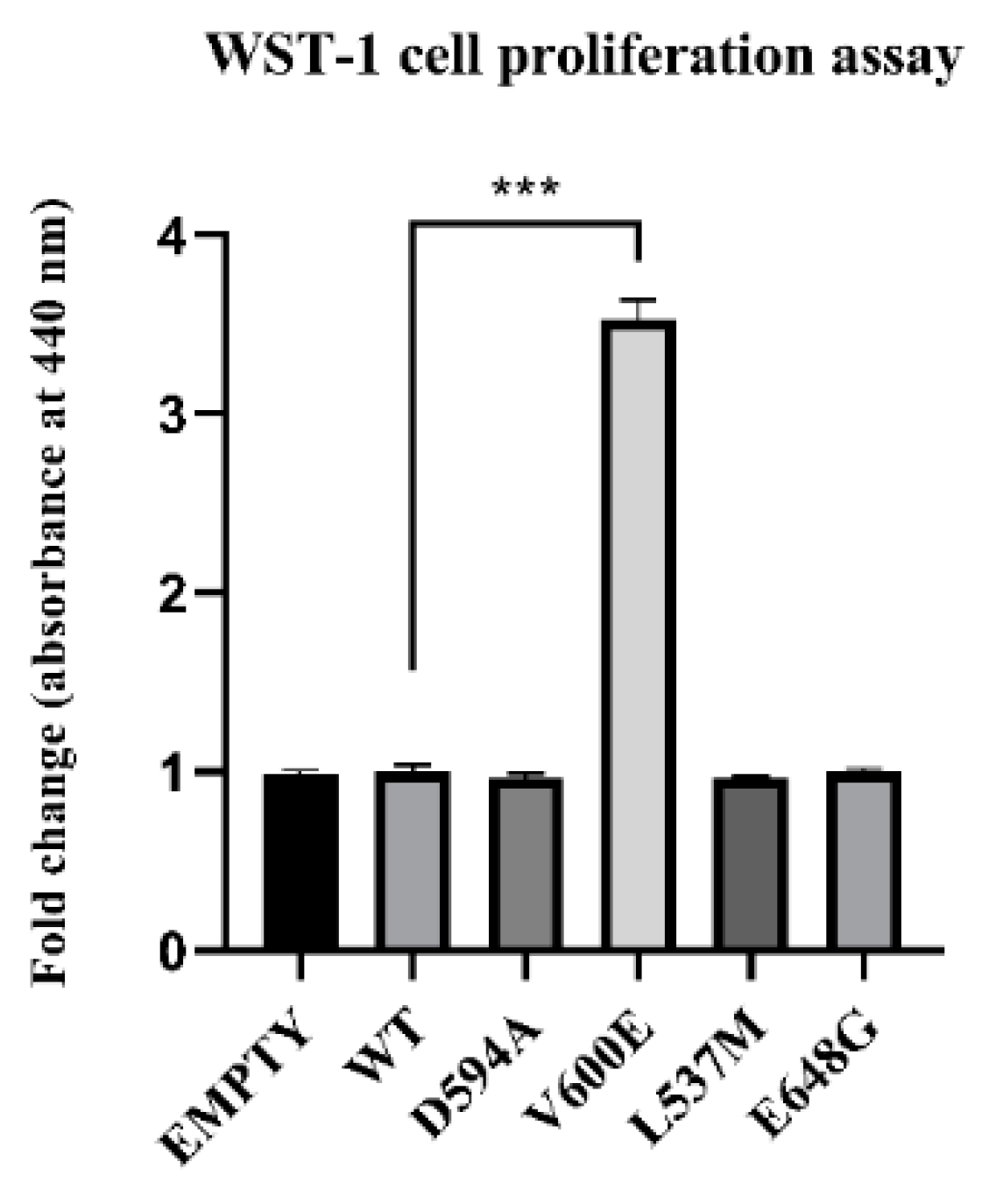
2.4. Effect of BRAF Mutations on THLE-2 Cells Proliferation
2.5. BRAF V600E Mutation Influences on Cell Invasion
2.6. Analysis of Differentially Expressed Genes (DEGs) and Involved Pathways
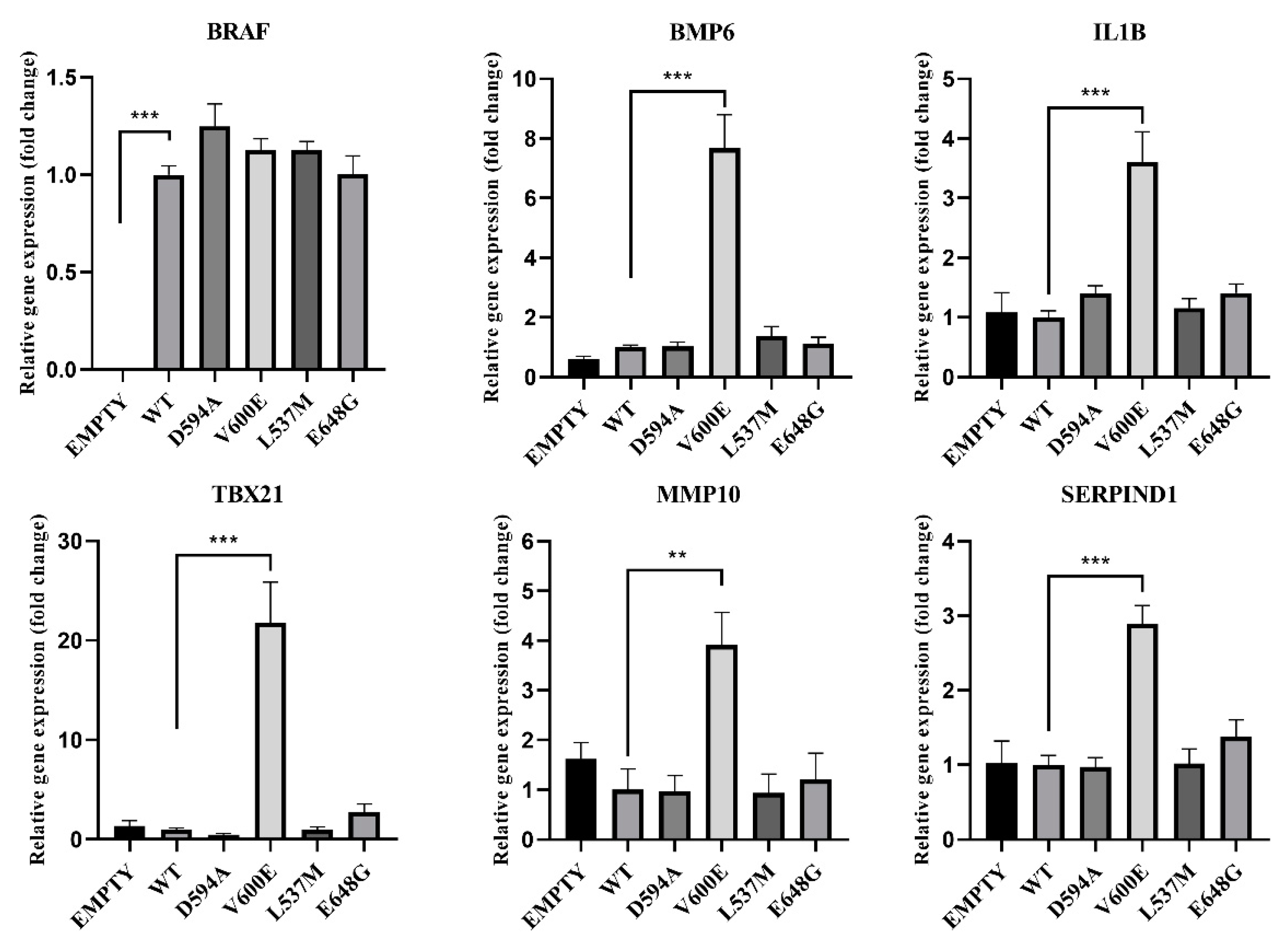
2.7. Validation of RNA-seq Data by qPCR
2.8. Canonical Pathway Analysis
2.9. Affected Pathway Analysis
2.10. Gene Expression Analysis in BRAF Mutant-Transfected THLE-2 Cells
3. Discussion
4. Materials and Methods
4.1. Sequence Alignment
4.2. Cell Culture
4.3. Plasmid Construction
4.4. Cell Transfection
4.5. Western Blotting
4.6. Wound Healing Assay
4.7. Cell Proliferation Test (WST-1 Test)
4.8. Cell Invasion Assay
4.9. BGI RNA-Seq (Transcriptome) Sequencing
4.10. Network Analysis
4.11. cDNA Synthesis
4.12. Real-Time PCR (qPCR)
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.V.; Asch, A.S.; Yamada, H.Y. Frequently mutated genes/pathways and genomic instability as prevention targets in liver cancer. Carcinogenesis 2017, 38, 2–11. [Google Scholar] [CrossRef] [PubMed]
- McGlynn, K.A.; Petrick, J.L.; London, W.T. Global epidemiology of hepatocellular carcinoma: An emphasis on demographic and regional variability. Clin. Liver Dis. 2015, 19, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Bosch, F.X.; Ribes, J.; Díaz, M.; Cléries, R. Primary liver cancer: Worldwide incidence and trends. Gastroenterology 2004, 127, S5–S16. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, H.; Takaki, A. Alcohol and hepatocellular carcinoma. BMJ Open Gastroenterol. 2019, 6, e000260. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, A.I.; Khan, S.A.; Toledano, M.B.; Waked, I.; Taylor-Robinson, S.D. Hepatocellular carcinoma: Epidemiology, risk factors and pathogenesis. World J. Gastroenterol. 2008, 14, 4300–4308. [Google Scholar] [CrossRef]
- Henry, S.H.; Bosch, F.X.; Bowers, J.C. Aflatoxin, hepatitis and worldwide liver cancer risks. Adv. Exp. Med. Biol. 2002, 504, 229–233. [Google Scholar] [CrossRef]
- Marengo, A.; Rosso, C.; Bugianesi, E. Liver Cancer: Connections with Obesity, Fatty Liver, and Cirrhosis. Annu. Rev. Med. 2016, 67, 103–117. [Google Scholar] [CrossRef]
- McGlynn, K.A.; Petrick, J.L. Epidemiology of Hepatocellular Carcinoma. Liver Biol. Pathobiol 2021, 73, 4–13. [Google Scholar] [CrossRef]
- Müller, M.; Bird, T.G.; Nault, J.C. The landscape of gene mutations in cirrhosis and hepatocellular carcinoma. J. Hepatol. 2020, 72, 990–1002. [Google Scholar] [CrossRef]
- Shiraha, H.; Yamamoto, K.; Namba, M. Human hepatocyte carcinogenesis (review). Int. J. Oncol. 2013, 42, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Cajuso, T.; Hänninen, U.A.; Kondelin, J.; Gylfe, A.E.; Tanskanen, T.; Katainen, R.; Pitkänen, E.; Ristolainen, H.; Kaasinen, E.; Taipale, M.; et al. Exome sequencing reveals frequent inactivating mutations in ARID1A, ARID1B, ARID2 and ARID4A in microsatellite unstable colorectal cancer. Int. J. Cancer 2014, 135, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Devereux, T.R.; Stern, M.C.; Flake, G.P.; Yu, M.C.; Zhang, Z.Q.; London, S.J.; Taylor, J.A. CTNNB1 mutations and beta-catenin protein accumulation in human hepatocellular carcinomas associated with high exposure to aflatoxin B1. Mol. Carcinog. 2001, 31, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Guichard, C.; Amaddeo, G.; Imbeaud, S.; Ladeiro, Y.; Pelletier, L.; Maad, I.B.; Calderaro, J.; Bioulac-Sage, P.; Letexier, M.; Degos, F.; et al. Integrated analysis of somatic mutations and focal copy-number changes identifies key genes and pathways in hepatocellular carcinoma. Nat. Genet. 2012, 44, 694–698. [Google Scholar] [CrossRef] [PubMed]
- Nigro, J.M.; Baker, S.J.; Preisinger, A.C.; Jessup, J.M.; Hostetter, R.; Cleary, K.; Bigner, S.H.; Davidson, N.; Baylin, S.; Devilee, P.; et al. Mutations in the p53 gene occur in diverse human tumour types. Nature 1989, 342, 705–708. [Google Scholar] [CrossRef] [PubMed]
- Hollstein, M.; Sidransky, D.; Vogelstein, B.; Harris, C.C. p53 mutations in human cancers. Science 1991, 253, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Hudson, T.J.; Anderson, W.; Artez, A.; Barker, A.D.; Bell, C.; Bernabé, R.R.; Bhan, M.K.; Calvo, F.; Eerola, I.; Gerhard, D.S.; et al. International network of cancer genome projects. Nature 2010, 464, 993–998. [Google Scholar] [CrossRef] [PubMed]
- Hindley, A.; Kolch, W. Extracellular signal regulated kinase (ERK)/mitogen activated protein kinase (MAPK)-independent functions of Raf kinases. J. Cell Sci. 2002, 115, 1575–1581. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/gene/673 (accessed on 6 January 2021).
- Guo, Y.J.; Pan, W.W.; Liu, S.B.; Shen, Z.F.; Xu, Y.; Hu, L.L. ERK/MAPK signalling pathway and tumorigenesis. Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef]
- Garnett, M.J.; Marais, R. Guilty as charged: B-RAF is a human oncogene. Cancer Cell 2004, 6, 313–319. [Google Scholar] [CrossRef]
- Cantwell-Dorris, E.R.; O’Leary, J.J.; Sheils, O.M. BRAFV600E: Implications for carcinogenesis and molecular therapy. Mol. Cancer Ther. 2011, 10, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Śmiech, M.; Leszczyński, P. Emerging BRAF Mutations in Cancer Progression and Their Possible Effects on Transcriptional Networks. Genes 2020, 11, 1342. [Google Scholar] [CrossRef] [PubMed]
- Loo, E.; Khalili, P.; Beuhler, K.; Siddiqi, I.; Vasef, M.A. BRAF V600E Mutation Across Multiple Tumor Types: Correlation Between DNA-based Sequencing and Mutation-specific Immunohistochemistry. Appl. Immunohistochem. Mol. Morphol. 2018, 26, 709–713. [Google Scholar] [CrossRef] [PubMed]
- International Cancer Genome Consortium Data Portal. Available online: https://dcc.icgc.org/ (accessed on 18 February 2021).
- Fujimoto, A.; Furuta, M.; Totoki, Y.; Tsunoda, T.; Kato, M.; Shiraishi, Y.; Tanaka, H.; Taniguchi, H.; Kawakami, Y.; Ueno, M.; et al. Whole-genome mutational landscape and characterization of noncoding and structural mutations in liver cancer. Nat. Genet. 2016, 48, 500–509. [Google Scholar] [CrossRef]
- Van Lint, P.; Libert, C. Chemokine and cytokine processing by matrix metalloproteinases and its effect on leukocyte migration and inflammation. J. Leukoc. Biol. 2007, 82, 1375–1381. [Google Scholar] [CrossRef]
- Chen, K.; Liu, J.; Cao, X. Regulation of type I interferon signaling in immunity and inflammation: A comprehensive review. J. Autoimmun. 2017, 83, 1–11. [Google Scholar] [CrossRef]
- Xing, L.; Cheng, Q.; Zha, G. Transcriptional Profiling at High Temporal Resolution Reveals Robust Immune/Inflammatory Responses during Rat Sciatic Nerve Recovery. Mediat. Inflamm. 2017, 2017, 3827841. [Google Scholar] [CrossRef]
- Pon, J.R.; Marra, M.A. Driver and passenger mutations in cancer. Annu. Rev. Pathol. 2015, 10, 25–50. [Google Scholar] [CrossRef]
- Caronia, L.M.; Phay, J.E.; Shah, M.H. Role of BRAF in thyroid oncogenesis. Clin. Cancer Res. 2011, 17, 7511–7517. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Kirkwood, J.M.; Grob, J.J.; Simeone, E.; Grimaldi, A.M.; Maio, M.; Palmieri, G.; Testori, A.; Marincola, F.M.; Mozzillo, N. The role of BRAF V600 mutation in melanoma. J. Transl. Med. 2012, 10, 85. [Google Scholar] [CrossRef]
- Pelizzo, M.R.; Dobrinja, C.; Casal Ide, E.; Zane, M.; Lora, O.; Toniato, A.; Mian, C.; Barollo, S.; Izuzquiza, M.; Guerrini, J.; et al. The role of BRAF(V600E) mutation as poor prognostic factor for the outcome of patients with intrathyroid papillary thyroid carcinoma. Biomed. Pharmacother. 2014, 68, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Eblen, S.T. Extracellular-Regulated Kinases: Signaling From Ras to ERK Substrates to Control Biological Outcomes. Adv. Cancer Res. 2018, 138, 99–142. [Google Scholar] [CrossRef]
- Schulze, A.; Lehmann, K.; Jefferies, H.B.; McMahon, M.; Downward, J. Analysis of the transcriptional program induced by Raf in epithelial cells. Genes Dev. 2001, 15, 981–994. [Google Scholar] [CrossRef] [PubMed]
- Rubinfeld, H.; Seger, R. The ERK cascade: A prototype of MAPK signaling. Mol. Biotechnol. 2005, 31, 151–174. [Google Scholar] [CrossRef]
- Maegdefrau, U.; Bosserhoff, A.K. BMP activated Smad signaling strongly promotes migration and invasion of hepatocellular carcinoma cells. Exp. Mol. Pathol. 2012, 92, 74–81. [Google Scholar] [CrossRef]
- Chang, C.F.; D’Souza, W.N.; Ch’en, I.L.; Pages, G.; Pouyssegur, J.; Hedrick, S.M. Polar opposites: Erk direction of CD4 T cell subsets. J. Immunol. 2012, 189, 721–731. [Google Scholar] [CrossRef]
- Koukouli, E.; Wang, D. A regularized functional regression model enabling transcriptome-wide dosage-dependent association study of cancer drug response. PLoS Comput. Biol. 2021, 17, e1008066. [Google Scholar] [CrossRef]
- Guo, Q.; Zhu, L.; Wang, C.; Wang, S.; Nie, X.; Liu, J.; Liu, Q.; Hao, Y.; Li, X.; Lin, B. SERPIND1 Affects the Malignant Biological Behavior of Epithelial Ovarian Cancer via the PI3K/AKT Pathway: A Mechanistic Study. Front. Oncol. 2019, 9, 954. [Google Scholar] [CrossRef]
- Cavalcante Mde, S.; Torres-Romero, J.C.; Lobo, M.D.; Moreno, F.B.; Bezerra, L.P.; Lima, D.S.; Matos, J.C.; Moreira Rde, A.; Monteiro-Moreira, A.C. A panel of glycoproteins as candidate biomarkers for early diagnosis and treatment evaluation of B-cell acute lymphoblastic leukemia. Biomark. Res. 2016, 4, 1. [Google Scholar] [CrossRef]
- The Human Protein Atlas. Available online: https://www.proteinatlas.org/ (accessed on 28 February 2021).
- Liao, W.Y.; Ho, C.C.; Hou, H.H.; Hsu, T.H.; Tsai, M.F.; Chen, K.Y.; Chen, H.Y.; Lee, Y.C.; Yu, C.J.; Lee, C.H.; et al. Heparin co-factor II enhances cell motility and promotes metastasis in non-small cell lung cancer. J. Pathol. 2015, 235, 50–64. [Google Scholar] [CrossRef]
- Abdolvahab, M.H.; Darvishi, B. Interferons: Role in cancer therapy. Immunotherapy 2020, 12, 833–855. [Google Scholar] [CrossRef] [PubMed]
- Tas, F.; Erturk, K. BRAF mutation status might contribute an effect on both disease-free and overall survival in stage III cutaneous melanomas treated with intermediate dose interferon-alpha. Cancer Chemother. Pharmacol. 2019, 84, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Davar, D.; Fuchs, S.Y.; Kirkwood, J.M. BRAF Inhibitors and IFNα: Plus, Minus, or Indeterminate? J. Natl. Cancer Inst. 2016, 108. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sabbatino, F.; Wang, Y.; Scognamiglio, G.; Favoino, E.; Feldman, S.A.; Villani, V.; Flaherty, K.T.; Nota, S.; Giannarelli, D.; Simeone, E.; et al. Antitumor Activity of BRAF Inhibitor and IFNα Combination in BRAF-Mutant Melanoma. J. Natl. Cancer Inst. 2016, 108. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, S.; Yano, H.; Momosaki, S.; Akiba, J.; Nishida, N.; Kojiro, S.; Moriya, F.; Ishizaki, H.; Kuratomi, K.; Kojiro, M. Growth inhibitory effects of IFN-beta on human liver cancer cells in vitro and in vivo. J. Interferon. Cytokine Res. 2007, 27, 507–516. [Google Scholar] [CrossRef]
- Sharma, A.; Huard, C.; Vernochet, C.; Ziemek, D.; Knowlton, K.M.; Tyminski, E.; Paradis, T.; Zhang, Y.; Jones, J.E.; von Schack, D.; et al. Brown fat determination and development from muscle precursor cells by novel action of bone morphogenetic protein 6. PLoS ONE 2014, 9, e92608. [Google Scholar] [CrossRef]
- Camaschella, C. BMP6 orchestrates iron metabolism. Nat. Genet. 2009, 41, 386–388. [Google Scholar] [CrossRef]
- Yang, S.; Du, J.; Wang, Z.; Yuan, W.; Qiao, Y.; Zhang, M.; Zhang, J.; Gao, S.; Yin, J.; Sun, B.; et al. BMP-6 promotes E-cadherin expression through repressing deltaEF1 in breast cancer cells. BMC Cancer 2007, 7, 211. [Google Scholar] [CrossRef]
- Darby, S.; Cross, S.S.; Brown, N.J.; Hamdy, F.C.; Robson, C.N. BMP-6 over-expression in prostate cancer is associated with increased Id-1 protein and a more invasive phenotype. J. Pathol. 2008, 214, 394–404. [Google Scholar] [CrossRef]
- Stieglitz, D.; Lamm, S.; Braig, S.; Feuerer, L.; Kuphal, S.; Dietrich, P.; Arndt, S.; Echtenacher, B.; Hellerbrand, C.; Karrer, S.; et al. BMP6-induced modulation of the tumor micro-milieu. Oncogene 2019, 38, 609–621. [Google Scholar] [CrossRef]
- Maurer, G.; Tarkowski, B.; Baccarini, M. Raf kinases in cancer-roles and therapeutic opportunities. Oncogene 2011, 30, 3477–3488. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lv, B.; Yi, C.; Cui, X.; Sui, S.; Li, X.; Qi, M.; Hao, C.; Han, B.; Liu, Z. Genistein inhibits human papillary thyroid cancer cell detachment, invasion and metastasis. J. Cancer 2019, 10, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, S.J.; Xu, Z.; Ha, S.Y.; Byeon, J.H.; Kang, E.J.; Shin, S.H.; Yoo, S.K.; Jee, H.G.; Yoon, S.G.; et al. BRAF(V600E) Transduction of an SV40-Immortalized Normal Human Thyroid Cell Line Induces Dedifferentiated Thyroid Carcinogenesis in a Mouse Xenograft Model. Thyroid 2020, 30, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Barajon, I.; Garlanda, C. IL-1 and IL-1 regulatory pathways in cancer progression and therapy. Immunol. Rev. 2018, 281, 57–61. [Google Scholar] [CrossRef]
- Matanić, D.; Beg-Zec, Z.; Stojanović, D.; Matakorić, N.; Flego, V.; Milevoj-Ribić, F. Cytokines in patients with lung cancer. Scand. J. Immunol. 2003, 57, 173–178. [Google Scholar] [CrossRef]
- Jin, L.; Yuan, R.Q.; Fuchs, A.; Yao, Y.; Joseph, A.; Schwall, R.; Schnitt, S.J.; Guida, A.; Hastings, H.M.; Andres, J.; et al. Expression of interleukin-1beta in human breast carcinoma. Cancer 1997, 80, 421–434. [Google Scholar] [CrossRef]
- Voronov, E.; Apte, R.N. IL-1 in Colon Inflammation, Colon Carcinogenesis and Invasiveness of Colon Cancer. Cancer Microenviron. 2015, 8, 187–200. [Google Scholar] [CrossRef]
- Rébé, C.; Ghiringhelli, F. Interleukin-1β and Cancer. Cancers 2020, 12, 1791. [Google Scholar] [CrossRef]
- Khalili, J.S.; Liu, S.; Rodríguez-Cruz, T.G.; Whittington, M.; Wardell, S.; Liu, C.; Zhang, M.; Cooper, Z.A.; Frederick, D.T.; Li, Y.; et al. Oncogenic BRAF(V600E) promotes stromal cell-mediated immunosuppression via induction of interleukin-1 in melanoma. Clin. Cancer Res. 2012, 18, 5329–5340. [Google Scholar] [CrossRef]
- Kerkelä, E.; Ala-aho, R.; Lohi, J.; Grénman, R.; M-Kähäri, V.; Saarialho-Kere, U. Differential patterns of stromelysin-2 (MMP-10) and MT1-MMP (MMP-14) expression in epithelial skin cancers. Br. J. Cancer 2001, 84, 659–669. [Google Scholar] [CrossRef]
- Klupp, F.; Neumann, L.; Kahlert, C.; Diers, J.; Halama, N.; Franz, C.; Schmidt, T.; Koch, M.; Weitz, J.; Schneider, M.; et al. Serum MMP7, MMP10 and MMP12 level as negative prognostic markers in colon cancer patients. BMC Cancer 2016, 16, 494. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Miyake, M.; Lawton, A.; Goodison, S.; Rosser, C.J. Matrix metalloproteinase-10 promotes tumor progression through regulation of angiogenic and apoptotic pathways in cervical tumors. BMC Cancer 2014, 14, 310. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhu, S.; Luo, G.; Zheng, L.; Wei, J.; Zhu, J.; Mu, Q.; Xu, N. Expression of MMP-10 in lung cancer. Anticancer Res. 2007, 27, 2791–2795. [Google Scholar] [PubMed]
- García-Irigoyen, O.; Latasa, M.U.; Carotti, S.; Uriarte, I.; Elizalde, M.; Urtasun, R.; Vespasiani-Gentilucci, U.; Morini, S.; Benito, P.; Ladero, J.M.; et al. Matrix metalloproteinase 10 contributes to hepatocarcinogenesis in a novel crosstalk with the stromal derived factor 1/C-X-C chemokine receptor 4 axis. Hepatology 2015, 62, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Shen, W.; Yu, J.; Wang, L. TBX21 predicts prognosis of patients and drives cancer stem cell maintenance via the TBX21-IL-4 pathway in lung adenocarcinoma. Stem Cell Res. Ther. 2018, 9, 89. [Google Scholar] [CrossRef]
- Yu, H.; Yang, J.; Jiao, S.; Li, Y.; Zhang, W.; Wang, J. T-box transcription factor 21 expression in breast cancer and its relationship with prognosis. Int. J. Clin. Exp. Pathol. 2014, 7, 6906–6913. [Google Scholar]
- Barretina, J.; Caponigro, G.; Stransky, N.; Venkatesan, K.; Margolin, A.A.; Kim, S.; Wilson, C.J.; Lehár, J.; Kryukov, G.V.; Sonkin, D.; et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature 2012, 483, 603–607. [Google Scholar] [CrossRef]
- CCLE Cell Line Gene Mutation Profiles. Available online: https://maayanlab.cloud/Harmonizome/gene_set/HUH7/CCLE+Cell+Line+Gene+Mutation+Profiles (accessed on 6 September 2021).
- Kasai, F.; Hirayama, N.; Ozawa, M.; Satoh, M.; Kohara, A. HuH-7 reference genome profile: Complex karyotype composed of massive loss of heterozygosity. Human Cell 2018, 31, 261–267. [Google Scholar] [CrossRef]
- Wan, P.T.; Garnett, M.J.; Roe, S.M.; Lee, S.; Niculescu-Duvaz, D.; Good, V.M.; Jones, C.M.; Marshall, C.J.; Springer, C.J.; Barford, D.; et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell 2004, 116, 855–867. [Google Scholar] [CrossRef]
- Heidorn, S.J.; Milagre, C.; Whittaker, S.; Nourry, A.; Niculescu-Duvas, I.; Dhomen, N.; Hussain, J.; Reis-Filho, J.S.; Springer, C.J.; Pritchard, C.; et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell 2010, 140, 209–221. [Google Scholar] [CrossRef]
- Cremolini, C.; Di Bartolomeo, M.; Amatu, A.; Antoniotti, C.; Moretto, R.; Berenato, R.; Perrone, F.; Tamborini, E.; Aprile, G.; Lonardi, S.; et al. BRAF codons 594 and 596 mutations identify a new molecular subtype of metastatic colorectal cancer at favorable prognosis. Ann. Oncol. 2015, 26, 2092–2097. [Google Scholar] [CrossRef] [PubMed]
- PRALINE Multiple Sequence Alignment. Available online: https://www.ibi.vu.nl/programs/pralinewww/ (accessed on 3 February 2021).
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Smyth, G.K.; Shi, W. FeatureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Systems, I. The Ingenuity Pathways Analysis. Available online: www.ingenuity.com/ (accessed on 18 May 2020).
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
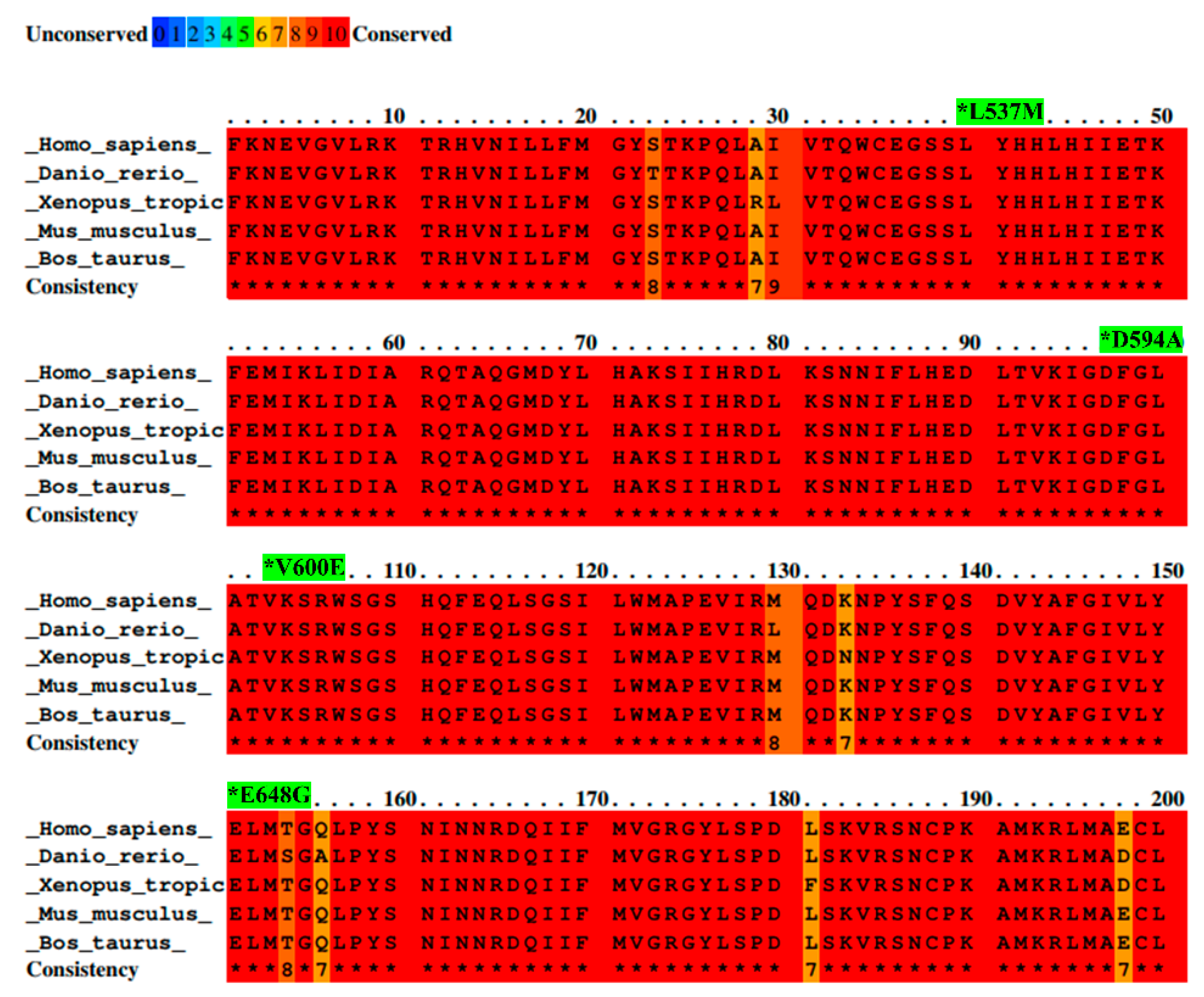

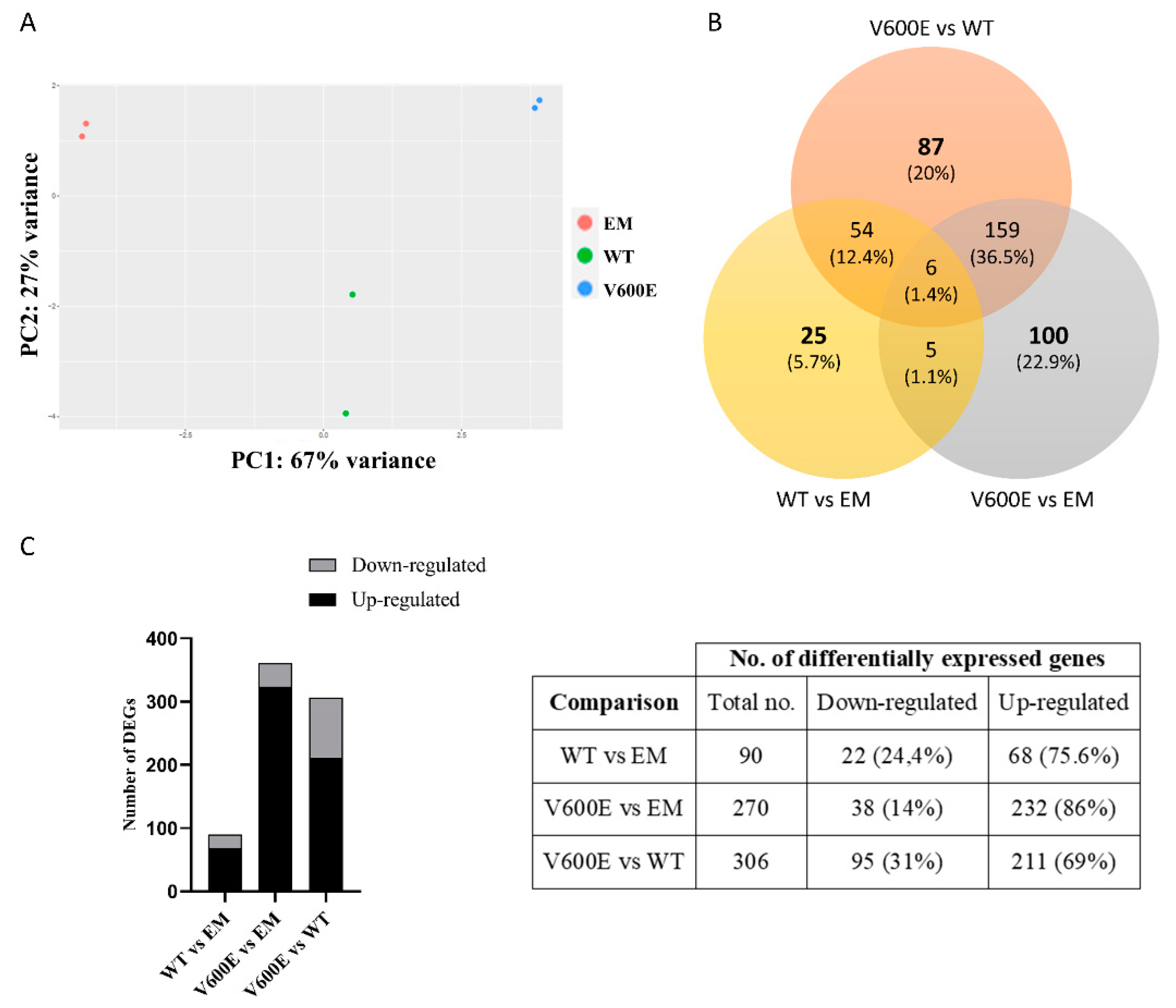
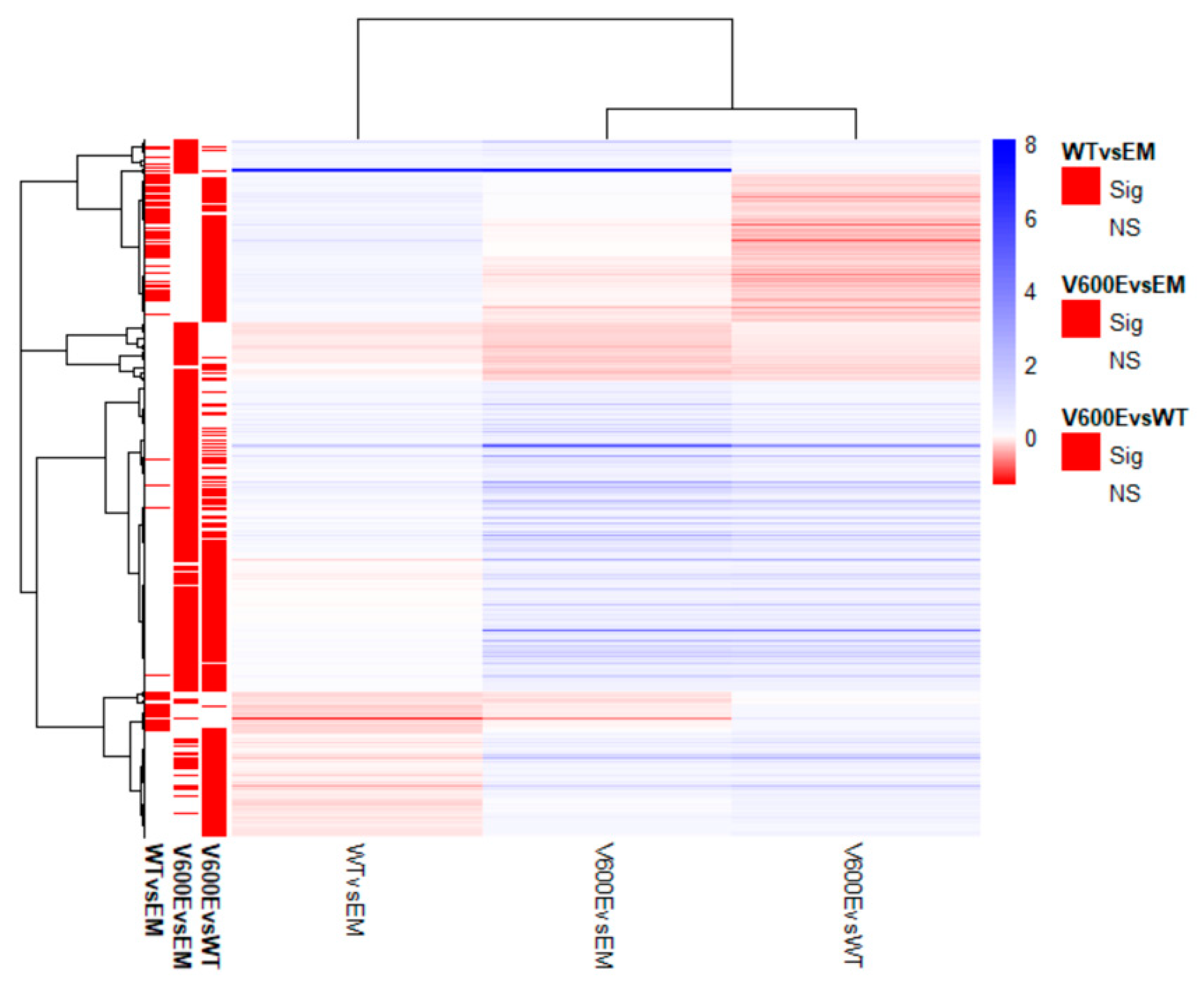
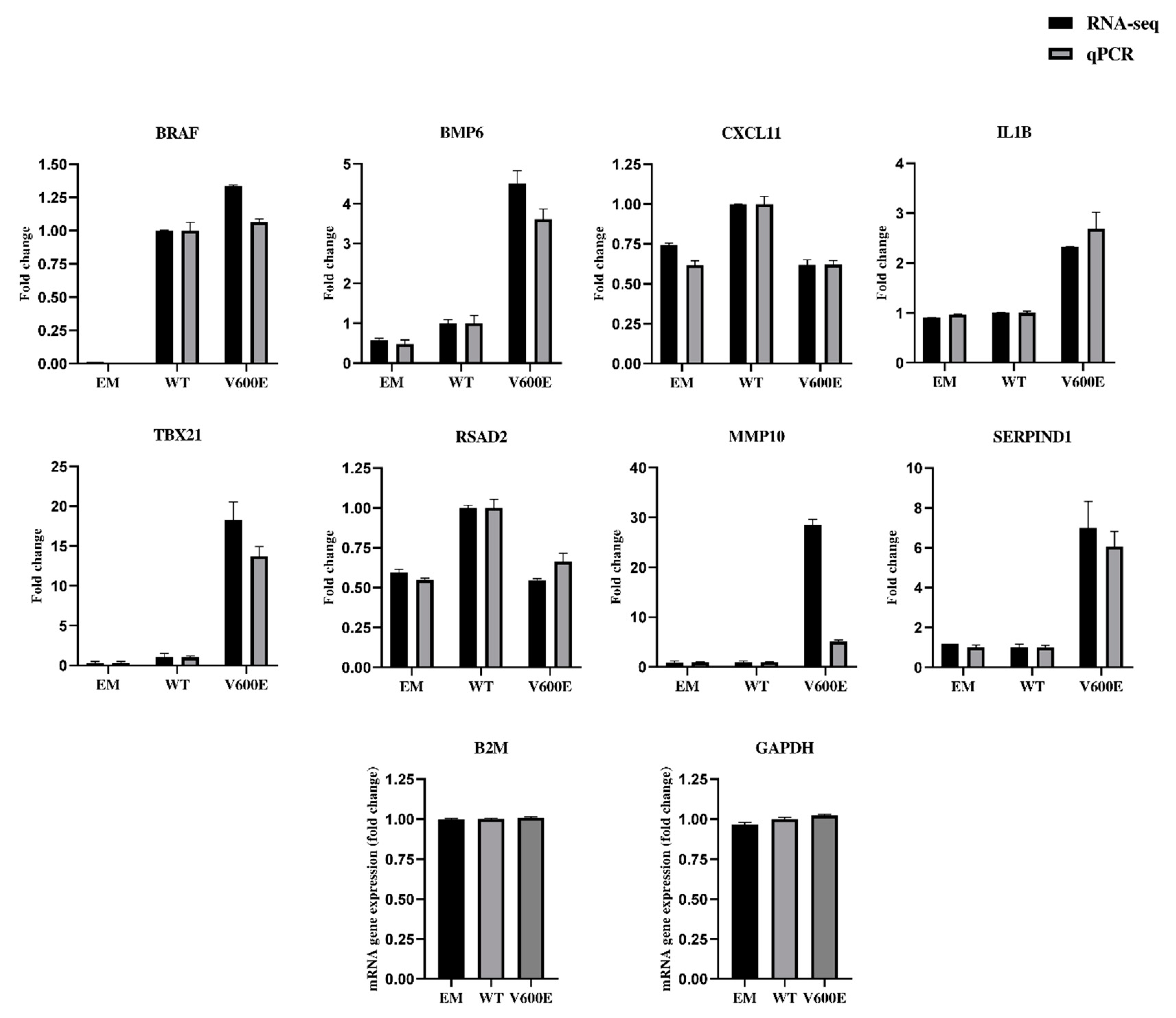
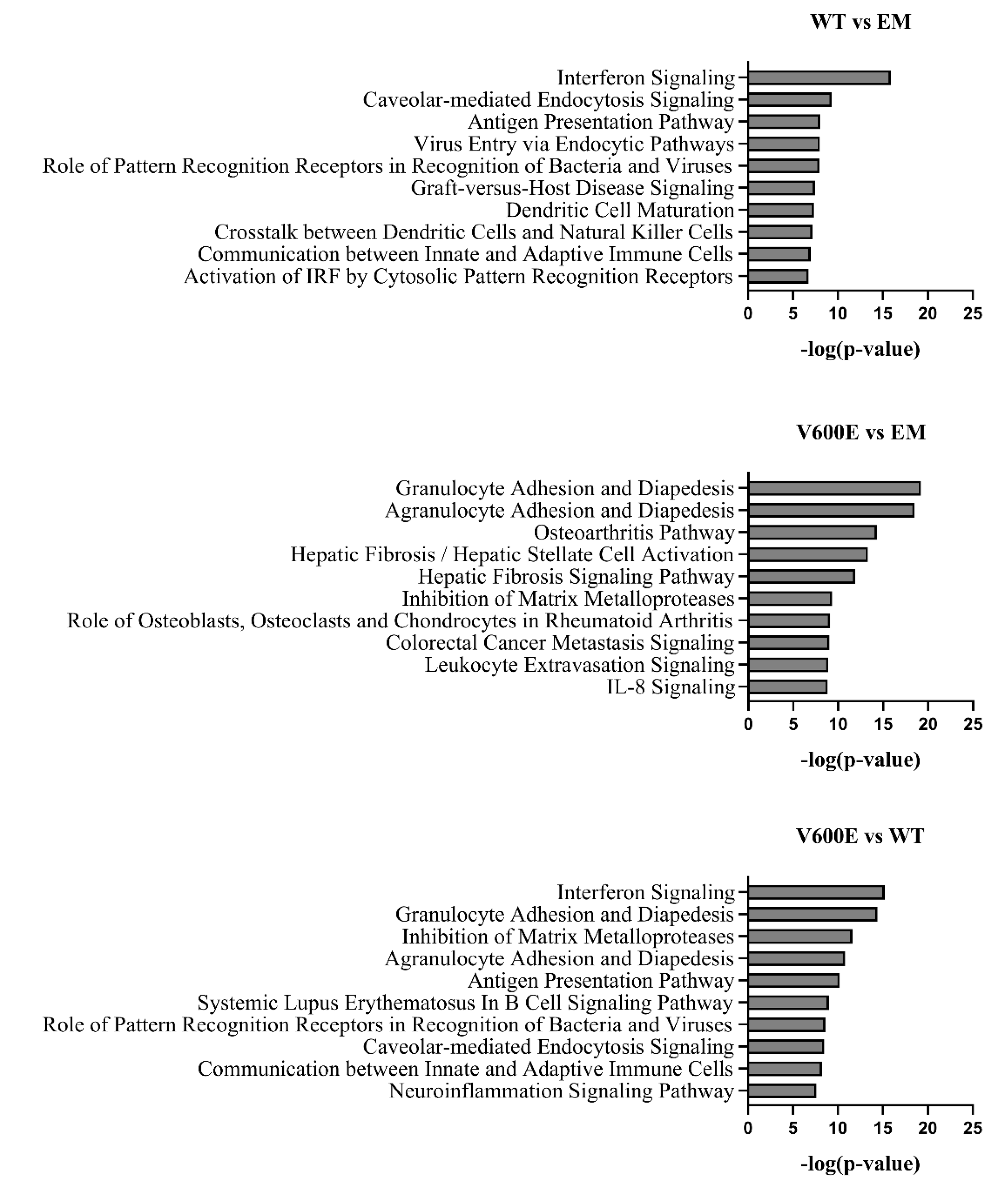

| Mutation | Mutation ID | Genomic DNA Change | Type | Projects Mutation Observed | Country |
|---|---|---|---|---|---|
| L537M | MU3683890 | chr7:g.140476797 A > T | Single base substitution leading to a missense mutation | LIRI-JP:1/258 | Japan |
| D594A | MU30632423 | chr7:g.140453154 T > G | LIRI-JP:1/258 | Japan | |
| V600E | MU62030 | chr7:g.140453136 A > T | LICA-FR:1/240 | France | |
| E648G | MU1115756 | chr7:g.140449136 T > C | LIRI-JP:1/258 | Japan |
| Up-Regulated | Down-Regulated | ||
|---|---|---|---|
| BRAF V600E vs. BRAF WT | |||
| Gene | Fold Change (Log Ratio) | Gene | Fold Change (Log Ratio) |
| MMP10 | 4.847 | CXCL10 | −0.948 |
| TBX21 | 4.205 | RSAD2 | −0.855 |
| SERPIND1 | 2.823 | CXCL11 | −0.675 |
| COL5A3 | 2.600 | CMPK2 | −0.619 |
| ADAMTS18 | 2.338 | CCL5 | −0.557 |
| IL33 | 2.325 | HSH2D | −0.500 |
| BMP6 | 2.185 | NT5C3A | −0.488 |
| NTSR1 | 2.173 | IFIT2 | −0.449 |
| LRRC15 | 1.903 | BATF2 | −0.435 |
| TAGLN3 | 1.859 | RTP4 | −0.435 |
| ESM1 | 1.834 | IFIT1 | −0.429 |
| MMP9 | 1.809 | IFIT3 | −0.413 |
| SIGLEC15 | 1.741 | USP18 | −0.405 |
| ITGAX | 1.716 | GBP4 | −0.400 |
| TRPV3 | 1.681 | GMPR | −0.400 |
| DIRAS3 | 1.625 | SLC15A3 | −0.391 |
| MMP1 | 1.569 | C4orf33 | −0.381 |
| IL13RA2 | 1.545 | LGALS9 | −0.377 |
| MMP3 | 1.493 | TNFSF10 | −0.376 |
| RCSD1 | 1.464 | OASL | −0.373 |
| TNFSF15 | 1.447 | BST2 | −0.371 |
| NOX5 | 1.445 | GBP1 | −0.362 |
| CHRNA9 | 1.287 | MX2 | −0.358 |
| EGR3 | 1.250 | PSMB9 | −0.352 |
| IL1B | 1.233 | HERC5 | −0.342 |
| Primer Name | Primer Sequence | Amplicon Size |
|---|---|---|
| HindIII_18 | Forward: GGAAGCTTGCGGCGCTGAGCGGTGGC | 2298 bp |
| XbaI_20 | Reverse:GGTCTAGATCAGTGGACAGGAAACGCAC |
| Mutation | Position | Primer Sequences | Length (nt.) | |
|---|---|---|---|---|
| D594A | a1781c | F R | 5′-TCACTGTAGCTAGACCAAAAGCACCTATTTTTACTGTGAGG-3′ 5′-CCTCACAGTAAAAATAGGTGCTTTTGGTCTAGCTACAGTGA-3′ | 41 |
| L537M | t1609a | F R | 5′-GGAGATGGTGATACATGCTGGAGCCCTCACA-3′ 5′-TGTGAGGGCTCCAGCATGTATCACCATCTCC-3′ | 31 |
| V600E | t1799a | F R | 5′-CCACTCCATCGAGATTTCTCTGTAGCTAGACCAAAAT-3′ 5′-ATTTTGGTCTAGCTACAGAGAAATCTCGATGGAGTGG-3′ | 37 |
| E648G | a1943g | F R | 5′-GGTAACTGTCCAGTCATCAATCCATACAGAACAATTCCAAATG-3′ 5′-CATTTGGAATTGTTCTGTATGGATTGATGACTGGACAGTTACC-3′ | 43 |
| Primary Antibody | Secondary Antibody | |||||
|---|---|---|---|---|---|---|
| Antibody | Dilution | Host | Molecular Weight (kDa) | Supplier | Antibody | Dilution |
| Anti-Flag M2 | 1:2000 | mouse, monoclonal | 94 | Sigma-Aldrich | Anti-Mouse IgG (whole molecule)–Peroxidase antibody produced in goat | 1:5000 |
| ERK1/2 (C-9) | 1:1000 | mouse, monoclonal | 44/42 | Santa Cruz Biotechnology | Anti-Mouse IgG (whole molecule)–Peroxidase antibody produced in goat | 1:5000 |
| Phospho-p44/42 MAPK (Erk1/2) | 1:1000 | rabbit, monoclonal | 44/42 | Cell Signaling Technology | Anti-Rabbit IgG (whole molecule)–Peroxidase antibody produced in goat | 1:5000 |
| β-Actin (13E5) | 1:1000 | rabbit, monoclonal | 45 | Cell Signaling Technology | Anti-Rabbit IgG (whole molecule)–Peroxidase antibody produced in goat | 1:5000 |
| Gene | GenBank Acc. No. | Primer Sequence | Length | Exon Boundary |
|---|---|---|---|---|
| BRAF | NM_004333 | F: CCCCAAGTCACCACAAAAACC R: CGGACTGTAACTCCACACCTT | 90 | 3–4 |
| BMP6 | NM_001718 | F: AAGAAGGCTGGCTGGAATTT R: GAAGGGCTGCTTGTCGTAAG | 170 | 3–4 |
| CXCL11 | NM_005409 | F: TCGAAGCAAGCAAGGCTTAT R: GTCCTTTCACCCACCTTTCA | 221 | 2–3 |
| IL1B | NM_000576 | F: TCCAGGGACAGGATATGGAG R: TCTTTCAACACGCAGGACAG | 133 | 5–6 |
| TBX21 | NM_013351 | F: CCGTGACTGCCTACCAGAAT R: ATCTCCCCCAAGGAATTGAC | 158 | 4–6 |
| RSAD2 | NM_080657 | F: CTCGCCAGTGCAACTACAAA R: CACCAACTTGCCCAGGTATT | 182 | 1–2 |
| MMP10 | NM_002425 | F: GTGGAGTTCCTGACGTTGGT R: AGCCTGGAGAATGTGAGTGG | 181 | 2–3 |
| SERPIND1 | NM_000185 | F: GAAGTTGATGGGGATCAGGA R: GTCGACAGTGAAGCGGACTT | 190 | 4–5 |
| B2M | NM_004048 | F: GAGGCTATCCAGCGTACTCCA R: CGGCAGGCATACTCATCTTTT | 248 | 1–2 |
| GAPDH | NM_001256799 | F: GGAGCGAGATCCCTCCAAAAT R: GGCTGTTGTCATACTTCTCATGG | 197 | 4–6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Śmiech, M.; Leszczyński, P.; Wardell, C.; Poznański, P.; Pierzchała, M.; Taniguchi, H. Oncogenic Mutation BRAF V600E Changes Phenotypic Behavior of THLE-2 Liver Cells through Alteration of Gene Expression. Int. J. Mol. Sci. 2022, 23, 1548. https://doi.org/10.3390/ijms23031548
Śmiech M, Leszczyński P, Wardell C, Poznański P, Pierzchała M, Taniguchi H. Oncogenic Mutation BRAF V600E Changes Phenotypic Behavior of THLE-2 Liver Cells through Alteration of Gene Expression. International Journal of Molecular Sciences. 2022; 23(3):1548. https://doi.org/10.3390/ijms23031548
Chicago/Turabian StyleŚmiech, Magdalena, Paweł Leszczyński, Christopher Wardell, Piotr Poznański, Mariusz Pierzchała, and Hiroaki Taniguchi. 2022. "Oncogenic Mutation BRAF V600E Changes Phenotypic Behavior of THLE-2 Liver Cells through Alteration of Gene Expression" International Journal of Molecular Sciences 23, no. 3: 1548. https://doi.org/10.3390/ijms23031548
APA StyleŚmiech, M., Leszczyński, P., Wardell, C., Poznański, P., Pierzchała, M., & Taniguchi, H. (2022). Oncogenic Mutation BRAF V600E Changes Phenotypic Behavior of THLE-2 Liver Cells through Alteration of Gene Expression. International Journal of Molecular Sciences, 23(3), 1548. https://doi.org/10.3390/ijms23031548







