Bio-Efficacy of Chrysoeriol7, a Natural Chemical and Repellent, against Brown Planthopper in Rice
Abstract
:1. Introduction
2. Results
2.1. Analysis of Phenotypes in Rice after Infection with BPH
2.2. Analysis of Concentration of C7 after BPH Infection
2.3. Assessment of C7 Efficacy against BPH
2.4. Flavonoid and Plant Resistant Gene Expression Levels Analysis in BPH-Infected Rice
3. Discussion
4. Materials and Methods
4.1. Plant Material and Field Design
4.2. BPH Rearing
4.3. Evaluation of BPH Resistance in SNDH
4.4. Isolation of C7 in Rice
4.5. Evaluation of C7 Efficacy against BPH
4.6. Comparison of the Expression Levels of Plant Resistance-Related Genes
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taub, D.A.; Wei, J.T. The economics of benign prostatic hyperplasia and lower urinary tract symptoms in the United States. Curr. Urol. Rep. 2006, 7, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Zhou, X.; Zhang, C.X.; Yu, L.L.; Fan, H.W.; Wang, Z.; Xu, H.J.; Xi, Y.; Zhu, Z.R.; Zhou, W.W.; et al. Genomes of the rice pest brown planthopper and its endosymbionts reveal complex complementary contributions for host adaptation. Genome Biol. 2014, 15, 521. [Google Scholar] [CrossRef] [Green Version]
- Ling, Y.; Weilin, Z. Genetic and biochemical mechanisms of rice resistance to planthopper. Plant Cell Rep. 2016, 35, 1559–1572. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Shi, J.H.; Liu, H.; Yin, L.T.; Abdelnabby, H.; Wang, M.Q. Phytopathogenic infection alters rice–pest–parasitoid tri-trophic interactions. Pest Manag. Sci. 2021, 77, 4530–4538. [Google Scholar] [CrossRef] [PubMed]
- Jing, S.; Zhao, Y.; Du, B.; Chen, R.; Zhu, L.; He, G. Genomics of interaction between the brown planthopper and rice. Curr. Opin. Insect Sci. 2017, 19, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Chakraborty, G. Bio-efficacy of novel chemicals and tribal pesticide-based integrated modules against brown planthopper in rice. Int. J. Trop. Insect Sci. 2021, 1–11. [Google Scholar] [CrossRef]
- Zhang, F.; Zhu, L.; He, G. Differential gene expression in response to brown planthopper feeding in rice. J. Plant Physiol. 2004, 161, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.F.; Zeng, B.; Zheng, C.; Mu, X.C.; Zhang, Y.; Hu, J.; Zhang, S.; Gao, C.F.; Shen, J.L. The evolution of insecticide resistance in the brown planthopper (Nilaparvata lugens Stål) of China in the period 2012–2016. Sci. Rep. 2018, 8, 4586. [Google Scholar] [CrossRef]
- Tschoeke, P.H.; Oliveira, E.E.; Dalcin, M.S.; Silveira-Tschoeke, M.C.A.C.; Sarmento, R.A.; Santos, G.R. Botanical and synthetic pesticides alter the flower visitation rates of pollinator bees in Neotropical melon fields. Environ. Pollut. 2019, 251, 591–599. [Google Scholar] [CrossRef]
- Mesnage, R.; Séralini, G.-E. Editorial: Toxicity of Pesticides on Health and Environment. Front. Public Health 2018, 6, 1–2. [Google Scholar] [CrossRef]
- Dubey, N.K.; Shukla, R.; Kumar, A.; Singh, P.; Prakash, B. Prospects of botanical pesticides in sustainable agriculture. Curr. Sci. 2010, 98, 479–480. [Google Scholar]
- Amoabeng, B.W.; Johnson, A.C.; Gurr, G.M. Natural enemy enhancement and botanical insecticide source: A review of dual use companion plants. Appl. Entomol. Zool. 2019, 54, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Kedia, A.; Prakash, B.; Mishra, P.K.; Singh, P.; Dubey, N.K. Botanicals as eco friendly biorational alternatives of synthetic pesticides against Callosobruchus spp. (Coleoptera: Bruchidae)—A review. J. Food Sci. Technol. 2015, 52, 1239–1257. [Google Scholar] [CrossRef] [PubMed]
- Mfarrej, M.F.B.; Rara, F.M. Competitive, Sustainable Natural Pesticides. Acta Ecol. Sin. 2019, 39, 145–151. [Google Scholar] [CrossRef]
- Tomas, F.; Abbott, J.M.; Steinberg, C.; Balk, M.; Williams, S.L.; Stachowicz, J.J. Plant genotype and nitrogen loading influence seagrass productivity, biochemistry, and plant-herbivore interactions. Ecology 2011, 92, 1807–1817. [Google Scholar] [CrossRef]
- Bezemer, T.M.; Wagenaar, R.; Van Dam, N.M.; Wäckers, F.L. Interactions between above- and belowground insect herbivores as mediated by the plant defense system. Oikos 2003, 101, 555–562. [Google Scholar] [CrossRef]
- Rampe, H.L.; Tulung, M.; Pelealu, J.; Runtunuwu, S.D. The Antibiotic and Antixenotic Resistance of Some Peanut (Arachis hypogea L.) Varieties after the Organic Fertilizer Application. Int. J. Res. Eng. Sci. 2015, 3, 40–44. [Google Scholar]
- Aznar-Fernández, T.; Rubiales, D. Identification and characterisation of antixenosis and antibiosis to pea aphid (Acyrthosiphon pisum) in Pisum spp. germplasm. Ann. Appl. Biol. 2018, 172, 268–281. [Google Scholar] [CrossRef]
- Carr, J.P.; Murphy, A.M.; Tungadi, T.; Yoon, J.Y. Plant defense signals: Players and pawns in plant-virus-vector interactions. Plant Sci. 2019, 279, 87–95. [Google Scholar] [CrossRef]
- Bueno, A.F.; Panizzi, A.R.; Hunt, T.E.; Dourado, P.M.; Pitta, R.M.; Gonçalves, J. Challenges for Adoption of Integrated Pest Management (IPM): The Soybean Example. Neotrop. Entomol. 2021, 50, 5–20. [Google Scholar] [CrossRef]
- Lin, D.; Xu, Y.; Wu, H.; Liu, X.; Zhang, L.; Wang, J.; Rao, Q. Plant defense responses induced by two herbivores and consequences for whitefly Bemisia tabaci. Front. Physiol. 2019, 10, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F.; Xie, Y. Heavy metal stress and some mechanisms of plant defense response. Sci. World J. 2015, 25, 27–54. [Google Scholar] [CrossRef] [PubMed]
- Dalin, P.; Ågren, J.; Björkman, C.; Huttunen, P.; Kärkkäinen, K. Leaf trichome formation and plant resistance to herbivory. In Induced Plant Resistance to Herbivory; Springer: Dordrecht, The Netherlands, 2008; pp. 89–105. [Google Scholar]
- Wang, Y.; Cao, L.; Zhang, Y.; Cao, C.; Liu, F.; Huang, F.; Qiu, Y.; Li, R.; Lou, X. Map-based cloning and characterization of BPH29, a B3 domain-containing recessive gene conferring brown planthopper resistance in rice. J. Exp. Bot. 2015, 66, 6035–6045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saxena, S.C.; Salvi, P.; Kamble, N.U.; Joshi, P.K.; Majee, M.; Arora, S. Ectopic overexpression of cytosolic ascorbate peroxidase gene (Apx1) improves salinity stress tolerance in Brassica juncea by strengthening antioxidative defense mechanism. Acta Physiol. Plant. 2020, 42, 45. [Google Scholar] [CrossRef]
- Xing, Z.; Liu, Y.; Cai, W.; Huang, X.; Wu, S.; Lei, Z. Efficiency of trichome-based plant defense in phaseolus vulgaris depends on insect behavior, plant ontogeny, and structure. Front. Plant Sci. 2017, 8, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darvill, A.G.; Albersheim, P. Phytoalexins and their Elicitors-A Defense against Microbial Infection in Plants. Annu. Rev. Plant Physiol. 1984, 35, 243–275. [Google Scholar] [CrossRef]
- Saddique, M.; Kamran, M.; Shahbaz, M. Differential Responses of Plants to Biotic Stress and the Role of Metabolites; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128126905. [Google Scholar]
- Zhang, X.; Abrahan, C.; Colquhoun, T.A.; Liu, C.J. A proteolytic regulator controlling chalcone synthase stability and flavonoid biosynthesis in Arabidopsis. Plant Cell 2017, 29, 1157–1174. [Google Scholar] [CrossRef] [Green Version]
- Onkokesung, N.; Reichelt, M.; Van Doorn, A.; Schuurink, R.C.; Van Loon, J.J.A.; Dicke, M. Modulation of flavonoid metabolites in Arabidopsis thaliana through overexpression of the MYB75 transcription factor: Role of kaempferol-3,7- dirhamnoside in resistance to the specialist insect herbivore Pieris brassicae. J. Exp. Bot. 2014, 65, 2203–2217. [Google Scholar] [CrossRef] [Green Version]
- Simmonds, M.S.J. Flavonoid-insect interactions: Recent advances in our knowledge. Phytochemistry 2003, 64, 21–30. [Google Scholar] [CrossRef]
- Simmonds, M.S.J. Importance of flavonoids in insect-plant interactions: Feeding and oviposition. Phytochemistry 2001, 56, 245–252. [Google Scholar] [CrossRef]
- Aboshi, T.; Ishiguri, S.; Shiono, Y.; Murayama, T. Flavonoid glycosides in Malabar spinach Basella alba inhibit the growth of Spodoptera litura larvae. Biosci. Biotechnol. Biochem. 2018, 82, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Du, S.S.; Zhang, H.M.; Bai, C.Q.; Wang, C.F.; Liu, Q.Z.; Liu, Z.L.; Wang, Y.Y.; Deng, Z.W. Nematocidal flavone-C-glycosides against the root-knot nematode (Meloidogyne incognita) from Arisaema erubescens tubers. Molecules 2011, 16, 5079–5086. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Cui, B.; Yan, S.; Li, Y.; Xiao, H.; Li, Y.; Zhang, Y. Evaluation of tricin, a stylet probing stimulant of brown planthopper, in infested and non-infested rice plants. J. Appl. Entomol. 2017, 141, 393–401. [Google Scholar] [CrossRef]
- Kong, C.H.; Xu, X.H.; Zhang, M.; Zhang, S.Z. Allelochemical tricin in rice hull and its aurone isomer against rice seedling rot disease. Pest Manag. Sci. 2010, 66, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- Bing, L.; Hongxia, D.; Maoxin, Z.; Di, X.; Jingshu, W. Potential resistance of tricin in rice against brown planthopper Nilaparvata lugens (Stål). Acta Ecol. Sin. 2007, 27, 1300–1306. [Google Scholar] [CrossRef]
- Piasecka, A.; Jedrzejczak-Rey, N.; Bednarek, P. Secondary metabolites in plant innate immunity: Conserved function of divergent chemicals. New Phytol. 2015, 206, 948–964. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Wang, Q.; Erb, M.; Turlings, T.C.J.; Ge, L.; Hu, L.; Li, J.; Han, X.; Zhang, T.; Lu, J.; et al. Specific herbivore-induced volatiles defend plants and determine insect community composition in the field. Ecol. Lett. 2012, 15, 1130–1139. [Google Scholar] [CrossRef]
- Jang, Y.; Park, J.; Kim, K. Antimicrobial Activity of Chrysoeriol 7 and Chochlioquinone 9, White-Backed Planthopper-Resistant Compounds, Against Rice Pathogenic Strains. Biology 2020, 9, 382. [Google Scholar] [CrossRef]
- Wang, H.; Ye, S.; Mou, T. Molecular Breeding of Rice Restorer Lines and Hybrids for Brown Planthopper (BPH) Resistance Using the Bph14 and Bph15 Genes. Rice 2016, 9, 53. [Google Scholar] [CrossRef] [Green Version]
- Fan, F.; Li, N.; Chen, Y.; Liu, X.; Sun, H.; Wang, J.; He, G.; Zhu, Y.; Li, S. Development of elite BPH-resistant wide-spectrum restorer lines for three and two line hybrid rice. Front. Plant Sci. 2017, 8, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.; Chen, M.; Zhang, Y.; Gao, Q.; Noman, A.; Wang, Q.; Li, H.; Chen, L.; Zhou, P.; Lu, J.; et al. Osmkk3, a stress-responsive protein kinase, positively regulates rice resistance to nilaparvata lugens via phytohormone dynamics. Int. J. Mol. Sci. 2019, 20, 3023. [Google Scholar] [CrossRef] [Green Version]
- Verma, N.; Shukla, S. Impact of various factors responsible for fluctuation in plant secondary metabolites. J. Appl. Res. Med. Aromat. Plants 2015, 2, 105–113. [Google Scholar] [CrossRef]
- Mishra, B.; Priyadarsini, K.I.; Kumar, M.S.; Unnikrishnan, M.K.; Mohan, H. Effect of O-glycosilation on the antioxidant activity and free radical reactions of a plant flavonoid, chrysoeriol. Bioorg. Med. Chem. 2003, 11, 2677–2685. [Google Scholar] [CrossRef]
- Plazonić, A.; Bucar, F.; Maleŝ, Ẑeljan; Mornar, A.; Nigović, B.; Kujundẑić, N. Identification and quantification of flavonoids and phenolic acids in burr parsley (Caucalis platycarpos L.), using high-performance liquid chromatography with diode array detection and electrospray ionization mass spectrometry. Molecules 2009, 14, 2466–2490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelebek, H.; Canbas, A.; Jourdes, M.; Teissedre, P.L. Characterization of colored and colorless phenolic compounds in Öküzgözü wines from Denizli and Elazig regions using HPLC-DAD-MS. Ind. Crops Prod. 2010, 31, 499–508. [Google Scholar] [CrossRef]
- Gross, B.L.; Skare, K.J.; Olsen, K.M. Novel Phr1 mutations and the evolution of phenol reaction variation in US weedy rice (Oryza sativa). New Phytol. 2009, 184, 842–850. [Google Scholar] [CrossRef] [Green Version]
- Lou, Y.G.; Ma, B.; Cheng, J.A. Attraction of the parasitoid Anagrus nilaparvatae to rice volatiles induced by the rice brown planthopper Nilaparvata lugens. J. Chem. Ecol. 2005, 31, 2357–2372. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, U.; Kanu, A.S.; Mo, Z.; Hussain, S.; Anjum, S.A.; Khan, I.; Abbas, R.N.; Tang, X. Lead toxicity in rice: Effects, mechanisms, and mitigation strategies—A mini review. Environ. Sci. Pollut. Res. 2015, 22, 18318–18332. [Google Scholar] [CrossRef]
- Xu, T.; Zhou, Q.; Xia, Q.; Zhang, W.; Zhang, G.; Gu, D. Effects of herbivore-induced rice volatiles on the host selection behavior of brown planthopper, Nilaparvata lugens. Chin. Sci. Bull. 2002, 47, 1355–1360. [Google Scholar] [CrossRef]
- Shah, A.; Smith, D.L. Flavonoids in agriculture: Chemistry and roles in, biotic and abiotic stress responses, and microbial associations. Agronomy 2020, 10, 1209. [Google Scholar] [CrossRef]
- Jan, R.; Khan, M.A.; Asaf, S.; Lee, I.J.; Kim, K.M. Overexpression of OsF3H modulates WBPH stress by alteration of phenylpropanoid pathway at a transcriptomic and metabolomic level in Oryza sativa. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Park, J.R.; Yun, S.; Jan, R.; Kim, K.M. Screening and identification of brown planthopper resistance genes OsCM9 in rice. Agronomy 2020, 10, 1865. [Google Scholar] [CrossRef]
- Shimono, M.; Koga, H.; Akagi, A.; Hayashi, N.; Goto, S.; Sawada, M.; Kurihara, T.; Matsushita, A.; Sugano, S.; Jiang, C.J.; et al. Rice WRKY45 plays important roles in fungal and bacterial disease resistance. Mol. Plant Pathol. 2012, 13, 83–94. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhong, S.; Li, Q.; Zhu, Z.; Lou, Y.; Wang, L.; Wang, J.; Wang, M.; Li, Q.; Yang, D.; et al. Functional analysis of rice NPR1-like genes reveals that OsNPR1/NH1 is the rice orthologue conferring disease resistance with enhanced herbivore susceptibility. Plant Biotechnol. J. 2007, 5, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.; Mathesius, U. The role of flavonoids in root-rhizosphere signalling: Opportunities and challenges for improving plant-microbe interactions. J. Exp. Bot. 2012, 63, 3429–3444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IBPGR-IRRI Rice Advisory Committee. International Board for Plant Genetic Resources; Oryza, S., Ed.; International Rice Research Institute: Manilla, Philippines, 1980. [Google Scholar]
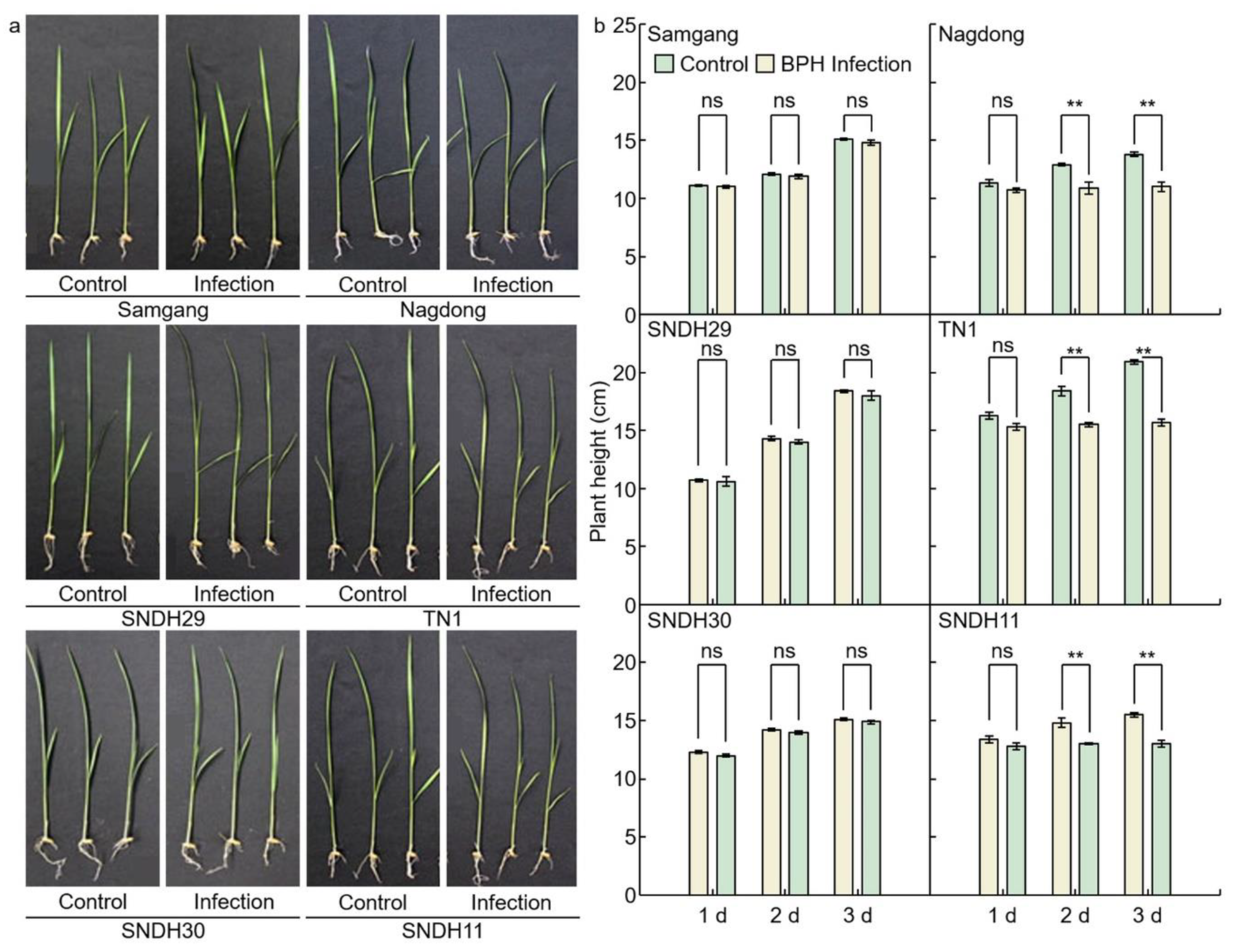
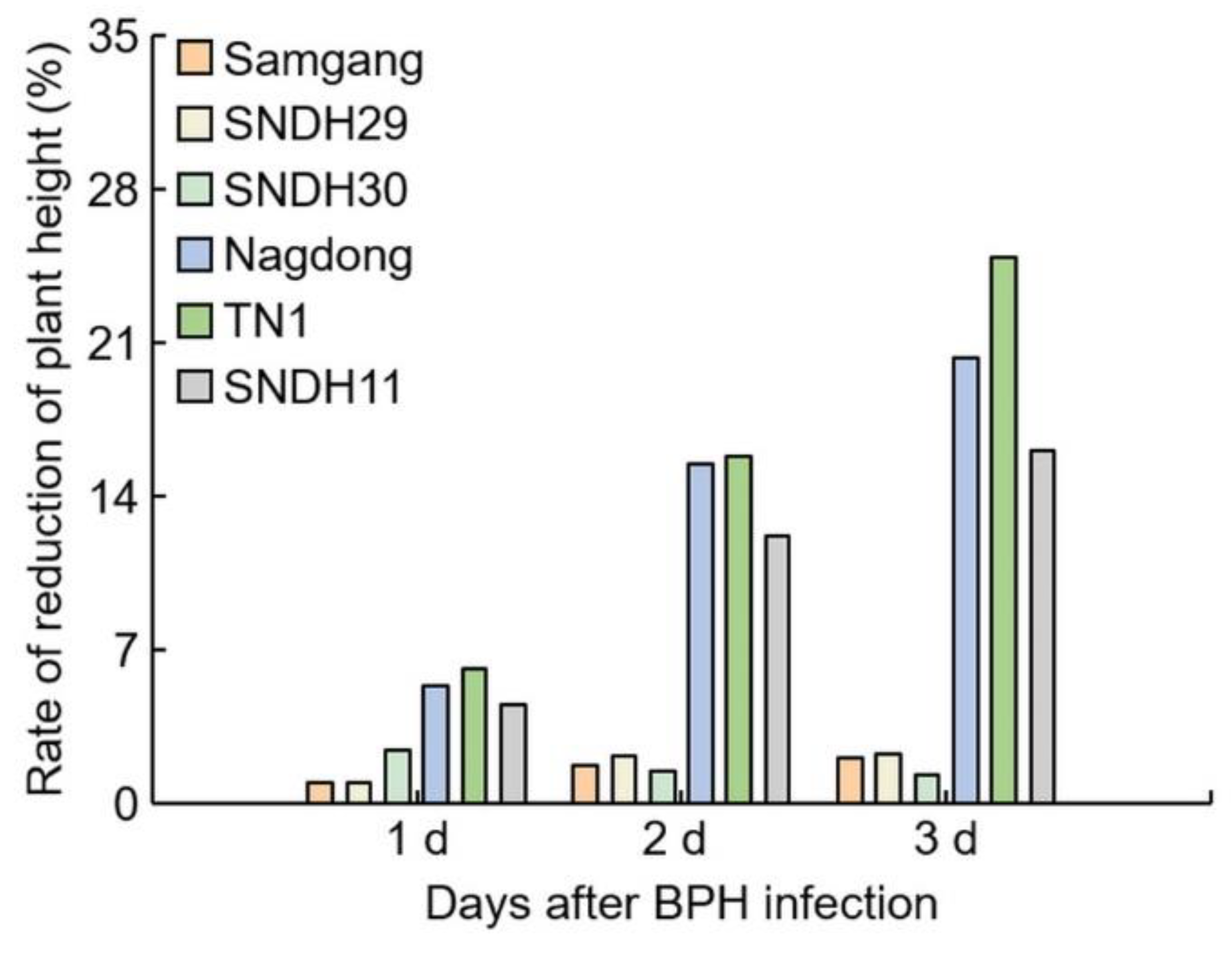
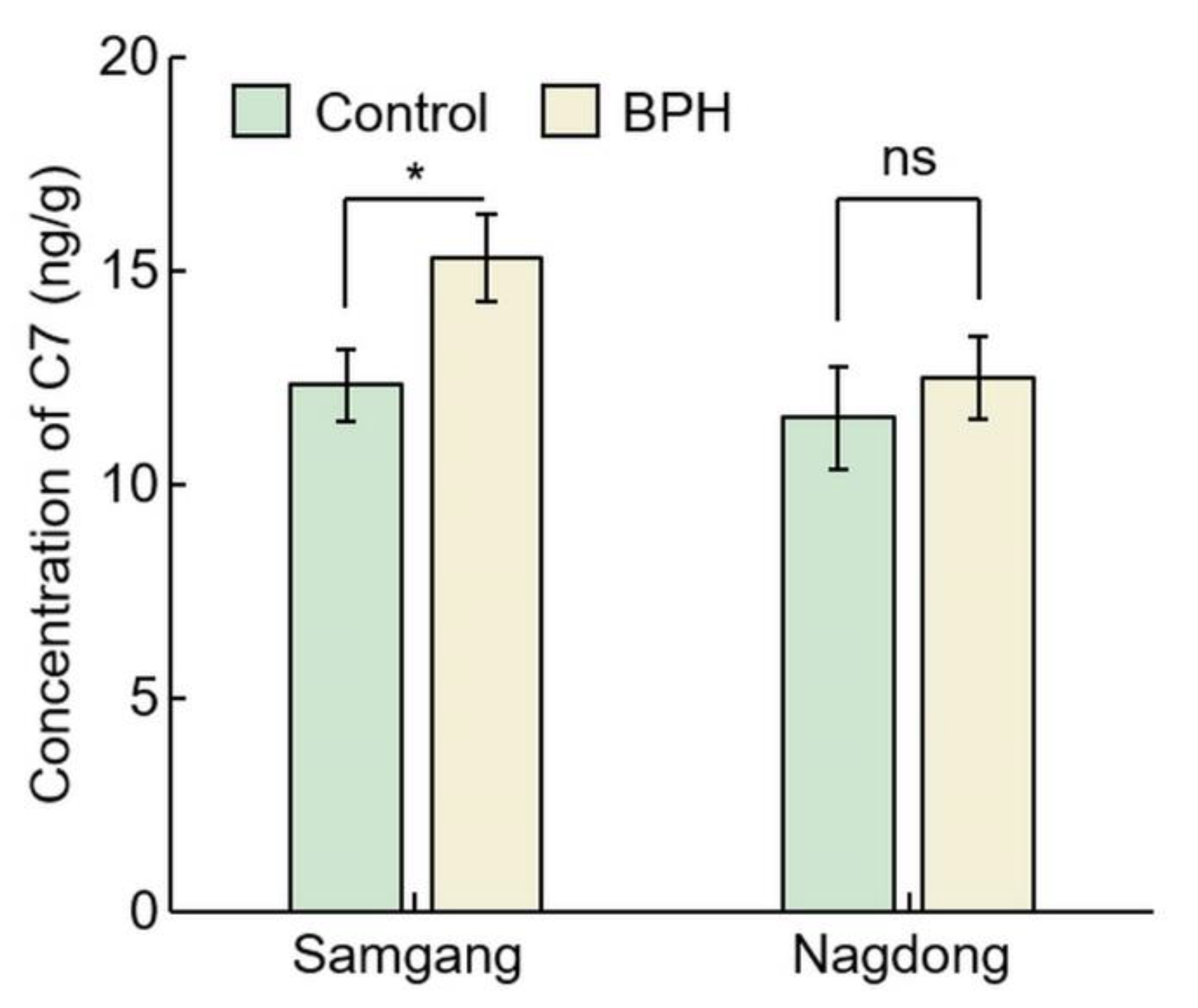
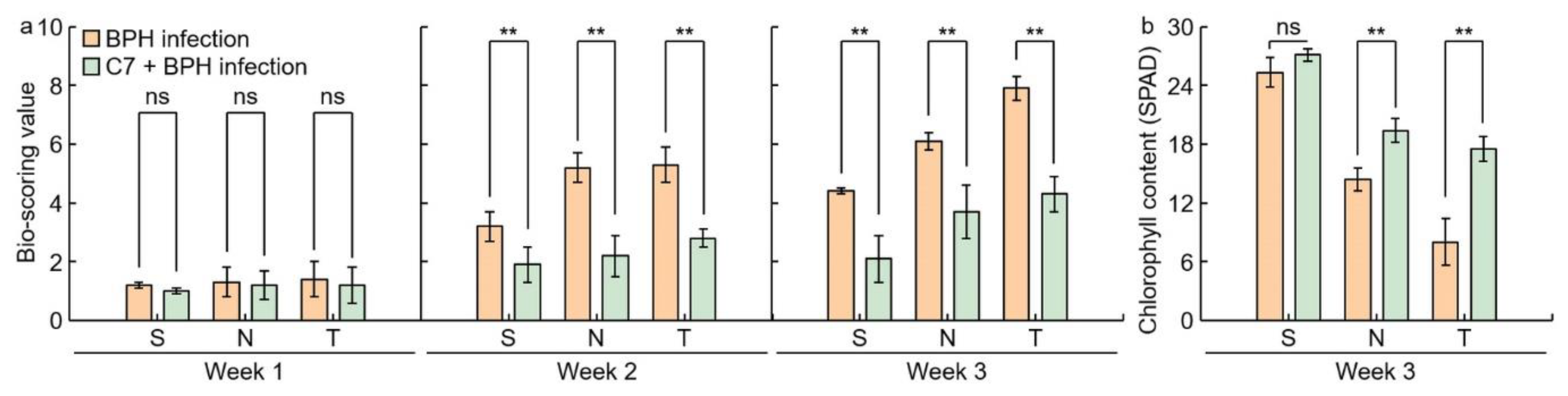

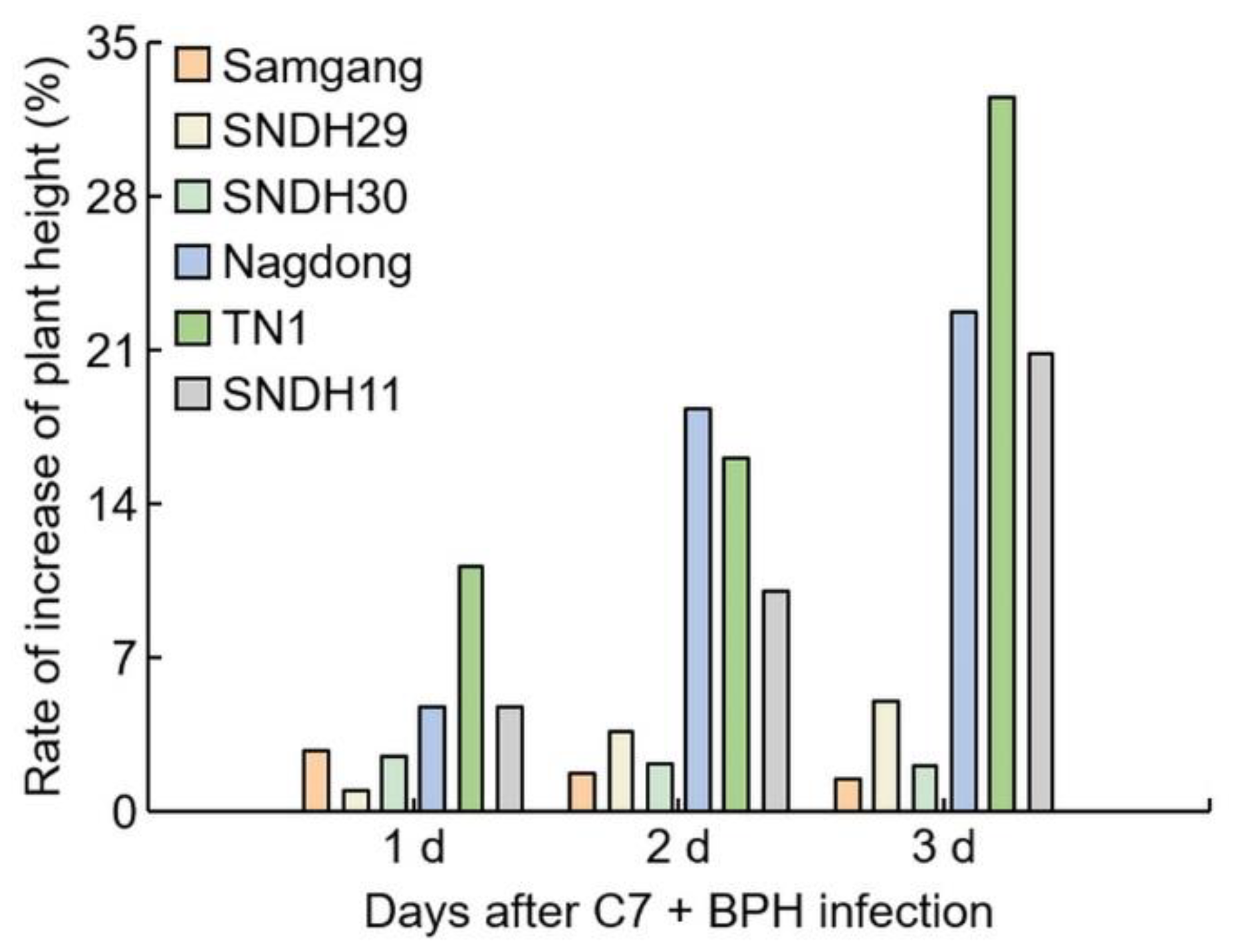
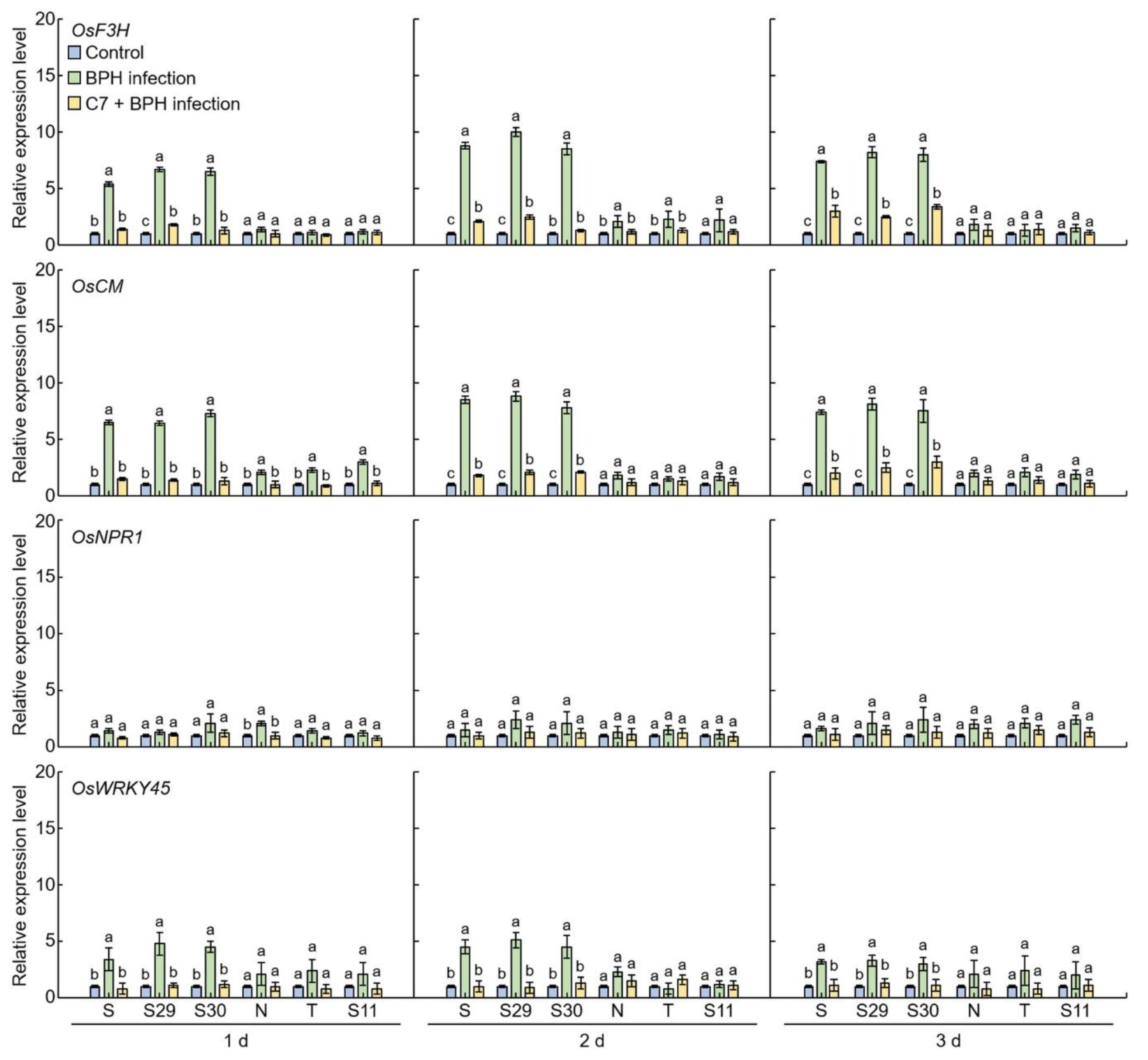
| Cultivar | Concentration of C7 (ng/g) | p Value | Rate of Increase (%) | |
|---|---|---|---|---|
| Control | BPH | |||
| Samgang | 12.32 ± 0.85 | 15.30 ± 1.01 | 0.0173 * | 24.24 ± 1.79 |
| Nagdong | 11.54 ± 1.21 | 12.48 ± 0.96 | 0.3434 ns | 08.41 ± 6.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, E.-G.; Yun, S.; Park, J.-R.; Jang, Y.-H.; Farooq, M.; Yun, B.-J.; Kim, K.-M. Bio-Efficacy of Chrysoeriol7, a Natural Chemical and Repellent, against Brown Planthopper in Rice. Int. J. Mol. Sci. 2022, 23, 1540. https://doi.org/10.3390/ijms23031540
Kim E-G, Yun S, Park J-R, Jang Y-H, Farooq M, Yun B-J, Kim K-M. Bio-Efficacy of Chrysoeriol7, a Natural Chemical and Repellent, against Brown Planthopper in Rice. International Journal of Molecular Sciences. 2022; 23(3):1540. https://doi.org/10.3390/ijms23031540
Chicago/Turabian StyleKim, Eun-Gyeong, Sopheap Yun, Jae-Ryoung Park, Yoon-Hee Jang, Muhammad Farooq, Byoung-Ju Yun, and Kyung-Min Kim. 2022. "Bio-Efficacy of Chrysoeriol7, a Natural Chemical and Repellent, against Brown Planthopper in Rice" International Journal of Molecular Sciences 23, no. 3: 1540. https://doi.org/10.3390/ijms23031540
APA StyleKim, E.-G., Yun, S., Park, J.-R., Jang, Y.-H., Farooq, M., Yun, B.-J., & Kim, K.-M. (2022). Bio-Efficacy of Chrysoeriol7, a Natural Chemical and Repellent, against Brown Planthopper in Rice. International Journal of Molecular Sciences, 23(3), 1540. https://doi.org/10.3390/ijms23031540







