5-Methoxyindole, a Chemical Homolog of Melatonin, Adversely Affects the Phytopathogenic Fungus Fusarium graminearum
Abstract
:1. Introduction
2. Results and Discussion
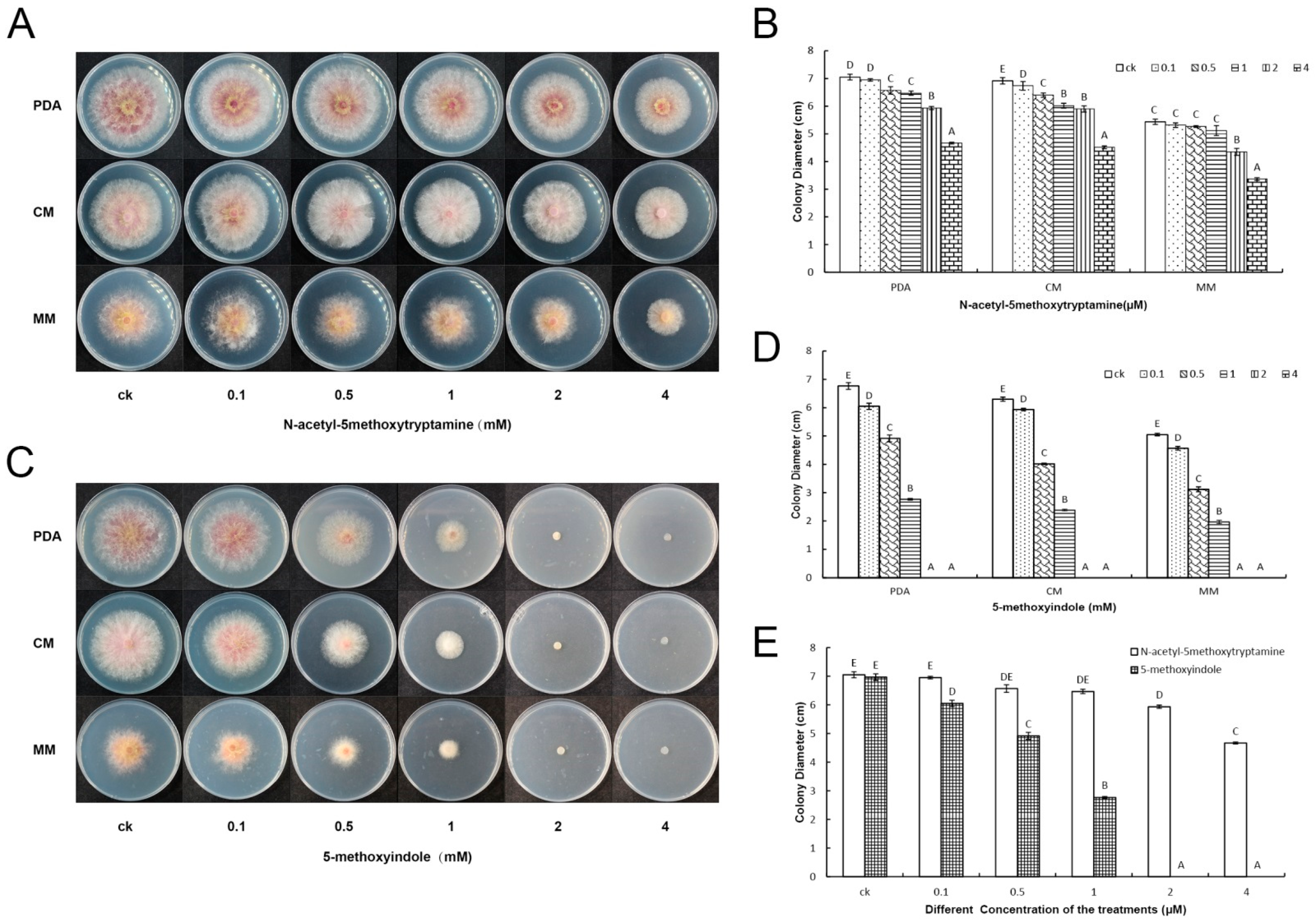
2.1. 5-Methoxyindole Displayed Strong Antagonistic Activity against F. graminearum PH-1 Similar to Melatonin
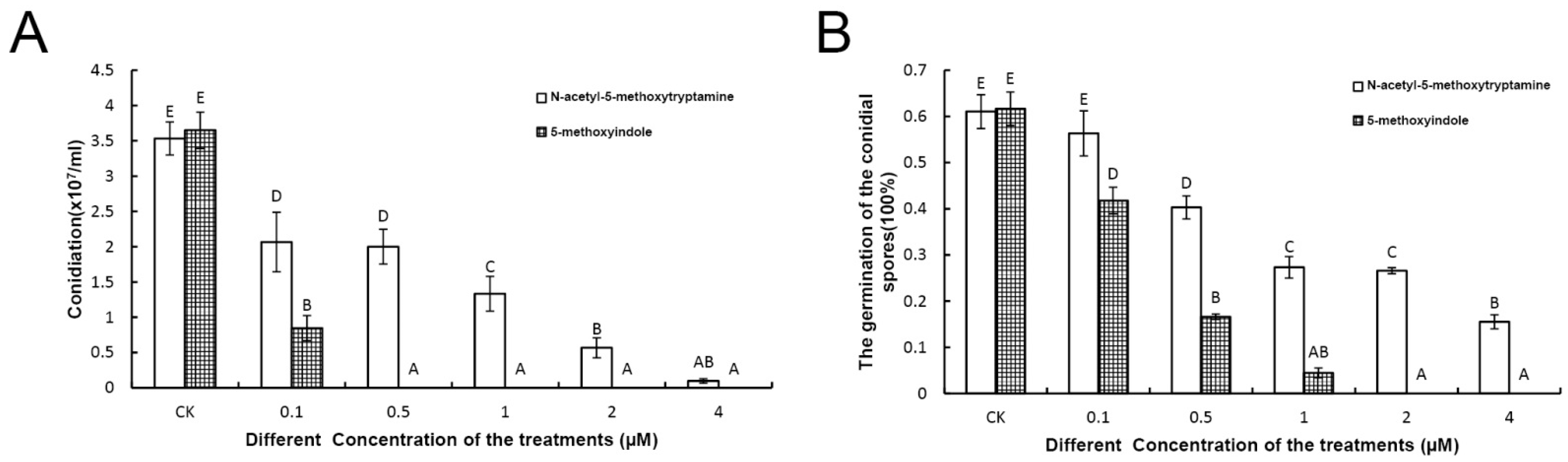
2.2. Melatonin and 5-Methoxyindole Exhibited Strong Inhibitory Properties against the Conidiation and Germination of F. graminearum
2.3. Melatonin and 5-Methoxyindole Released the Inhibitory Function of H2O2 against F. graminearum
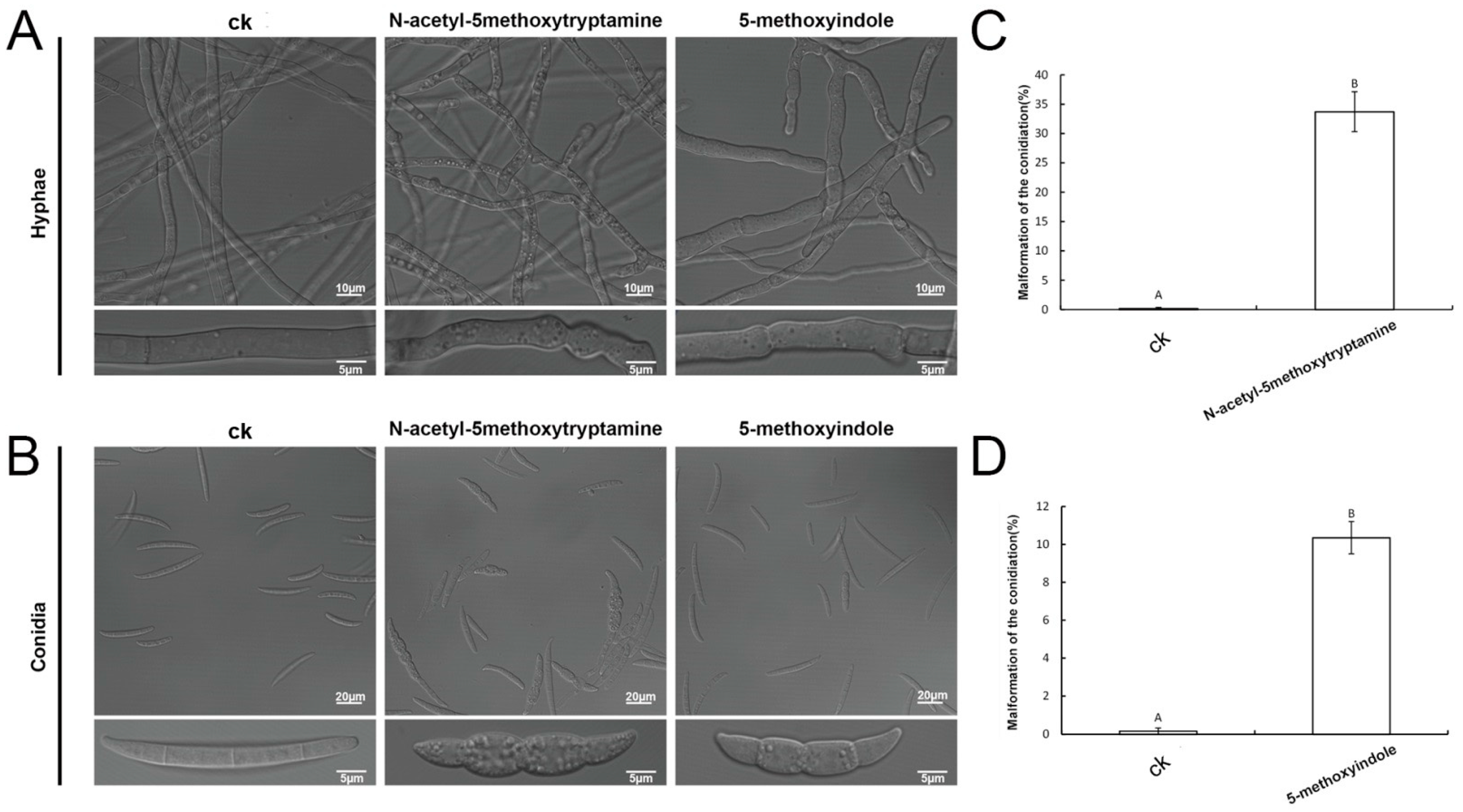
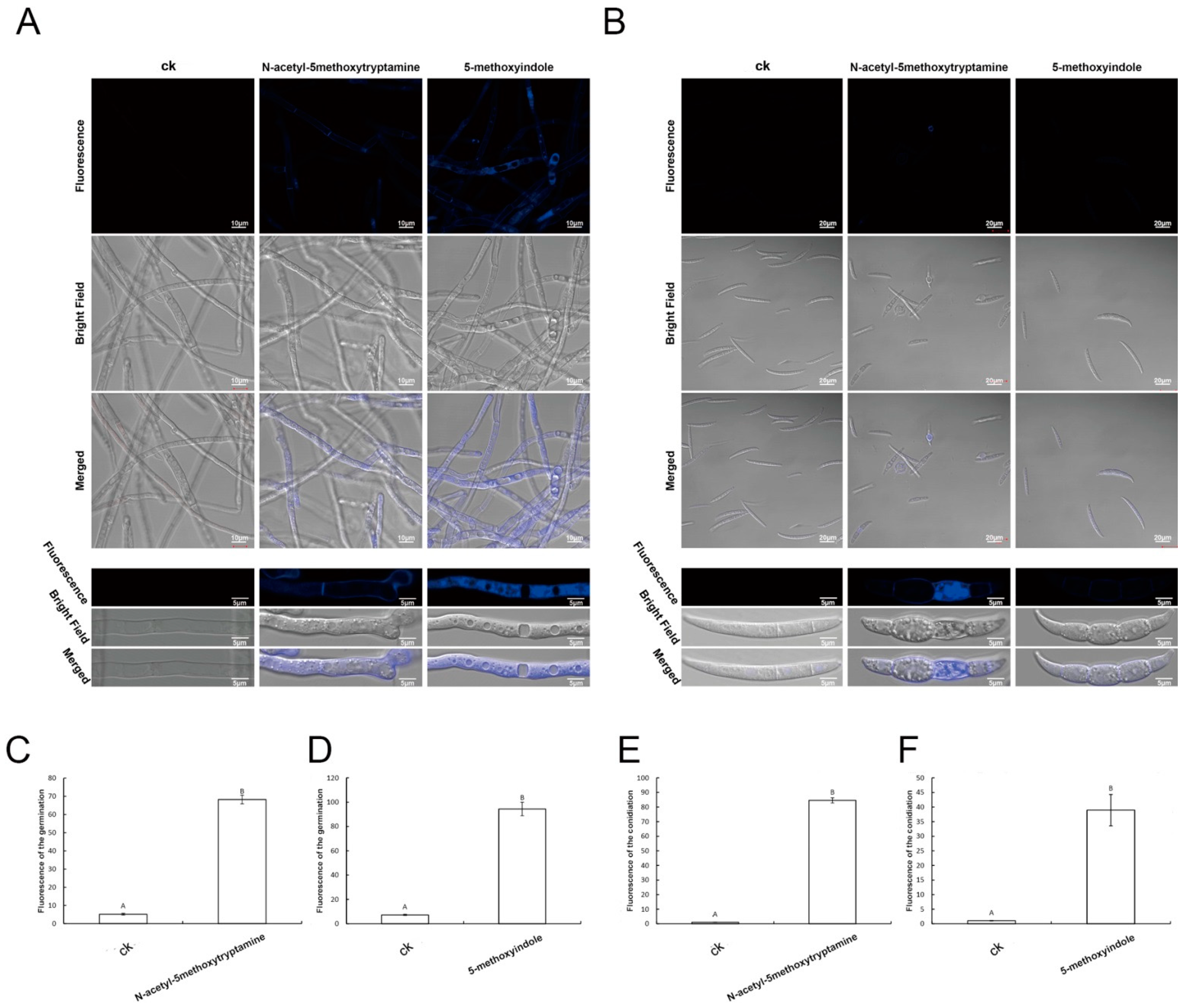
2.4. Melatonin and 5-Methoxyindole Led to Morphologic Changes in F. graminearum Hyphae and Conidia
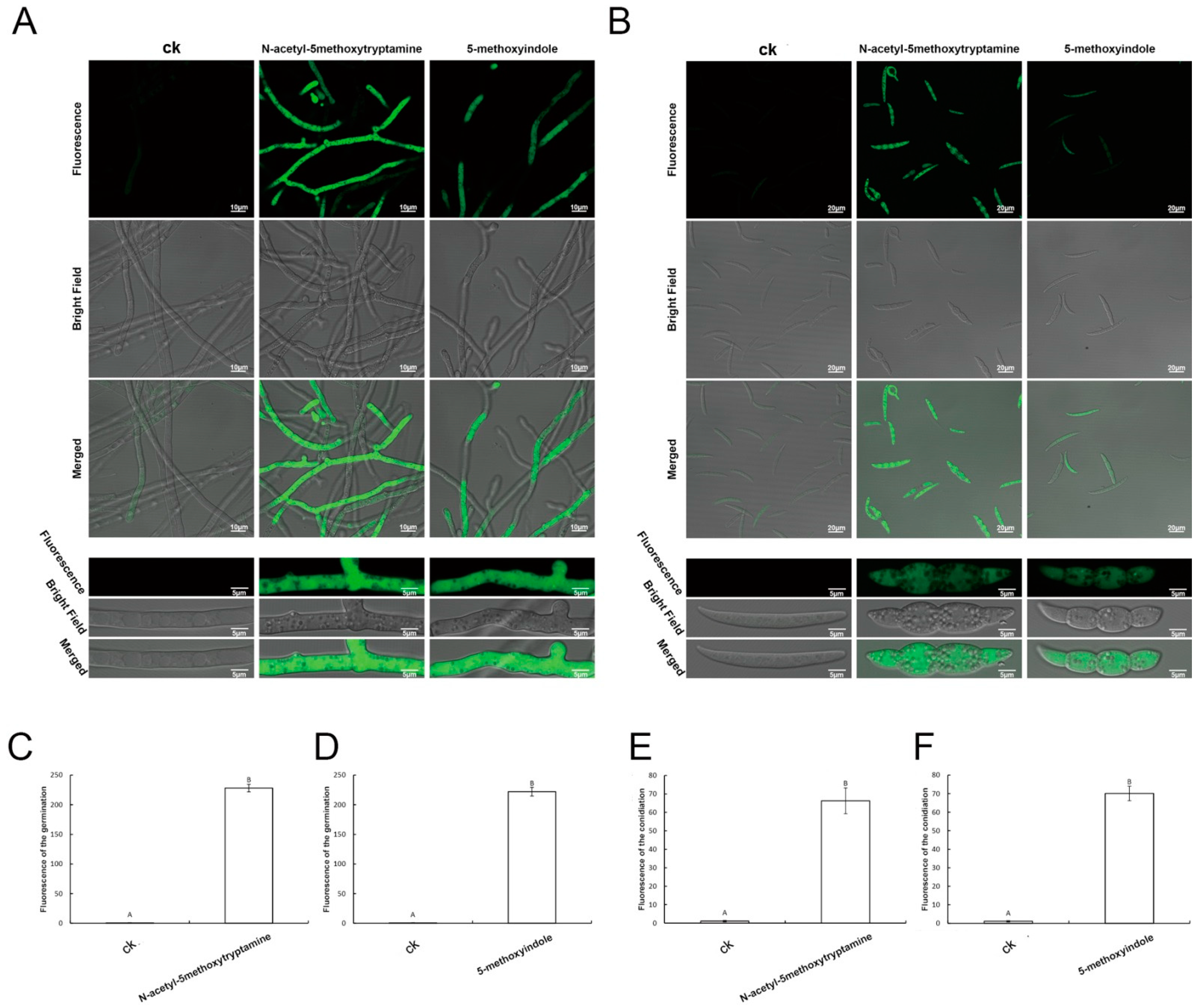
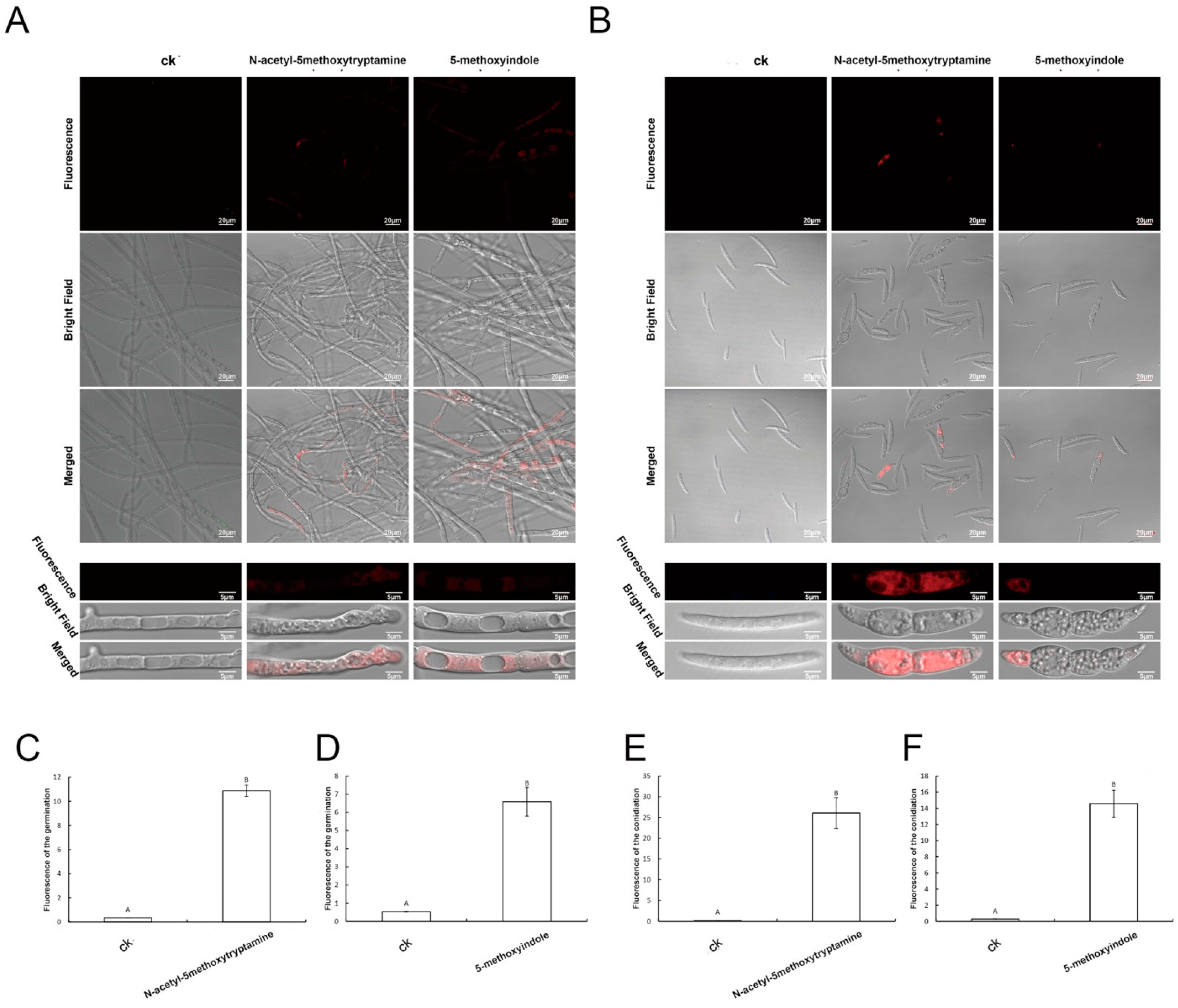
2.5. Accumulation of Reactive Oxygen Species by F. graminearum Hyphae and Conidia Treated with Melatonin and 5-Methoxyindole
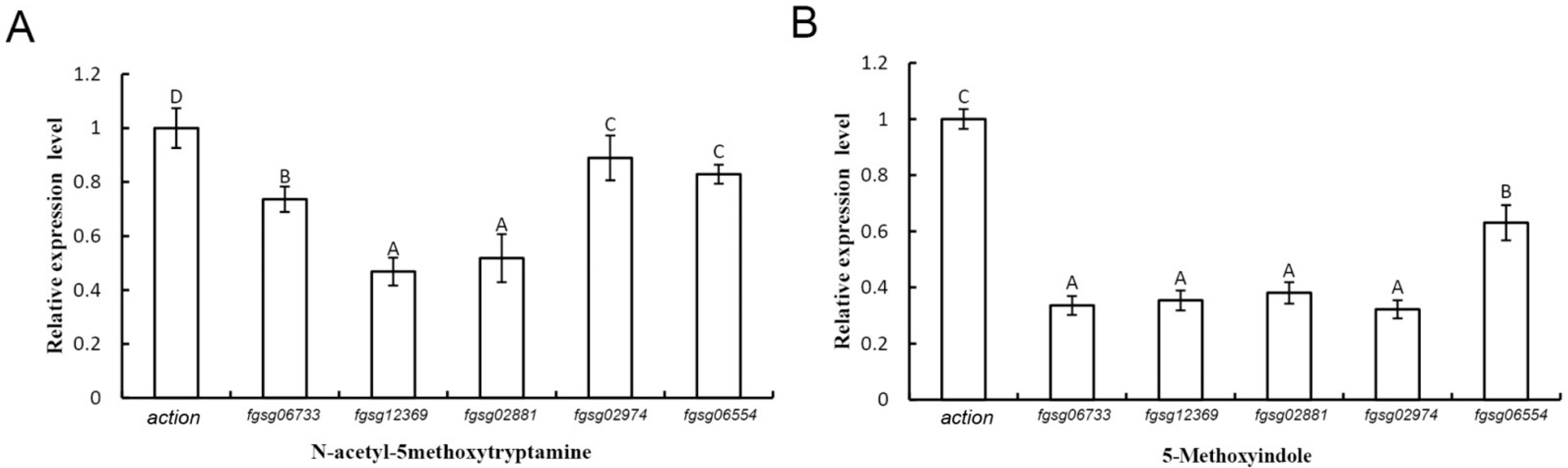
2.6. Extracellular ROS-Scavenging Enzyme Genes and Peroxidase Genes of F. graminearum Were Downregulated in the Presence of Melatonin and 5-Methoxyindole
2.7. Apoptosis of F. graminearum Hyphae and Conidia Was Caused by Melatonin and 5-Methoxyindole
2.8. Melatonin and 5-Methoxyindole Induced the Cell Death of F. graminearum Hyphae and Conidia
3. Materials and Methods
3.1. Fungal Strains and Growth Conditions
3.2. Antifungal Activity Assay and EC50 Determination
3.3. Fungus Inhibitor Compound Assays with Melatonin and 5-Methoxyindole against F. graminearum
3.4. Laser Scanning Confocal Microscopic Observation of Hyphal and Conidial Morphologies
3.5. Reactive Oxygen Species Detection
3.6. Formation and Germination of Conidia
3.7. Live/Dead Fungus Viability Staining
3.8. RNA Isolation and Reverse Transcription-Quantitative PCR (RT-qPCR)
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goswami, R.S.; Kistler, H.C. Heading for disaster: Fusarium graminearum on cereal crops. Mol. Plant Pathol. 2004, 5, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Stack, R.W. Return of an old problem: Fusarium head blight of small grains. Plant health Prog. 2000, 1, 19. [Google Scholar] [CrossRef]
- Nganje, W.E.; Bangsund, D.A.; Leistritz, F.L.; Wilson, W.W.; Tiapo, N.M. Estimating the economic impact of a crop disease: The case of Fusarium head blight in U.S. wheat and barley. In 2002 National Fusarium Head Blight Forum Proceedings; Michigan State University: East Lansing, MI, USA, 2002; pp. 275–281. [Google Scholar]
- McMullen, M.; Jones, R.; Gallenberg, D. Scab of wheat and barley: A re-emerging disease of devastating impact. Plant Dis. 1997, 81, 1340–1348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pestka, J.J.; Smolinski, A.T. Deoxynivalenol: Toxicology and potential effects on humans. J. Toxicol. Environ. Health Part B 2005, 8, 39–69. [Google Scholar] [CrossRef] [PubMed]
- Mesterhazy, A. Types and components of resistance to Fusarium head blight of wheat. Plant Breed. 1995, 114, 377–386. [Google Scholar] [CrossRef]
- Anand, A.; Zhou, T.; Trick, H.N.; Gill, B.S.; Bockus, W.W.; Muthukrishnan, S. Greenhouse and field testing of transgenic wheat plants stably expressing genes for thaumatin-like protein, chitinase and glucanase against Fusarium graminearum. J. Exp. Bot. 2003, 54, 1101–1111. [Google Scholar] [CrossRef] [Green Version]
- Liu, N.; Fan, F.; Qiu, D.; Jiang, L. The transcription cofactor FgSwi6 plays a role in growth and development, carbendazim sensitivity, cellulose utilization, lithium tolerance, deoxynivalenol production and virulence in the filamentous fungus Fusarium graminearum. Fungal Genet. Biol. 2013, 58, 42–52. [Google Scholar] [CrossRef]
- Sun, H.Y.; Zhu, Y.F.; Liu, Y.Y.; Deng, Y.Y.; Li, W.; Zhang, A.X.; Chen, H.G. Evaluation of tebuconazole for the management of Fusarium head blight in China. Australas. Plant Pathol. 2014, 43, 631–638. [Google Scholar] [CrossRef]
- Duan, Y.; Zhang, X.; Ge, C.; Wang, Y.; Cao, J.; Jia, X.; Wang, J.; Zhou, M. Development and application of loop-mediated isothermal amplification for detection of the F167Y mutation of carbendazim-resistant isolates in Fusarium graminearum. Sci. Rep. 2014, 4, 7094. [Google Scholar] [CrossRef] [Green Version]
- Hou, Y.; Luo, Q.; Chen, C.; Zhou, M. Application of tetra primer ARMS-PCR approach for detection of Fusarium graminearum genotypes with resistance to carbendazim. Australas. Plant Pathol. 2013, 42, 73–78. [Google Scholar] [CrossRef]
- Wang, P.; Yin, L.H.; Liang, D.; Li, C.; Ma, F.W.; Yue, Z.Y. Delayed senescence of apple leaves by exogenous melatonin treatment: Toward regulating the ascorbate-glutathione cycle. J. Pineal Res. 2012, 53, 11–20. [Google Scholar] [CrossRef]
- Byeon, Y.; Back, K. An increase in melatonin in transgenic rice causes pleiotropic phenotypes, including enhanced seedling growth, delayed flowering, and low grain yield. J. Pineal Res. 2014, 56, 408–414. [Google Scholar] [CrossRef]
- Byeon, Y.; Park, S.; Lee, H.; Kim, Y.; Back, K. Elevated production of melatonin in transgenic rice seeds expressing rice tryptophan decarboxylase. J. Pineal Res. 2014, 56, 275–282. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, H.J.; Zhao, B.; Sun, Q.Q.; Cao, Y.Y.; Li, R.; Wu, X.X.; Weeda, S.; Li, L.; Ren, S.X.; et al. The RNA-seq approach to discriminate gene expression profiles in response to melatonin on cucumber lateral root formation. J. Pineal Res. 2014, 56, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Zhang, N.; Wang, J.F.; Zhang, H.J.; Li, D.B.; Shi, J.; Li, R.; Weeda, S.; Zhao, B.; Ren, S.; et al. Melatonin promotes ripening and improves quality of tomato fruit during postharvest life. J. Exp. Bot. 2015, 66, 657–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, H.; Jiang, C.; Ye, T.; Tan, D.X.; Reiter, R.J.; Zhang, H.; Liu, R.; Chan, Z. Comparative physiological, metabolomic, and transcriptomic analyses reveal mechanisms of improved abiotic stress resistance in bermudagrass [Cynodon dactylon (L). Pers.] by exogenous melatonin. J. Exp. Bot. 2015, 66, 681–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, W.; Li, Q.T.; Chu, Y.N.; Reiter, R.J.; Yu, X.M.; Zhu, D.H.; Zhang, W.K.; Ma, B.A.; Lin, Q.; Zhang, J.S.; et al. Melatonin enhances plant growth and abiotic stress tolerance in soybean plants. J. Exp. Bot. 2015, 66, 695–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.Q.; Hasan, M.K.; Li, C.X.; Ahammed, G.J.; Xia, X.J.; Shi, K.; Zhou, Y.H.; Reiter, R.J.; Yu, J.Q.; Xu, M.X.; et al. Melatonin mediates selenium induced tolerance to cadmium stress in tomato plants. J. Pineal Res. 2016, 61, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Wang, G.; Wang, M.; Zhang, S. Exogenous melatonin improves tolerance to water deficit by promoting cuticle formation in tomato plants. Molecules 2018, 23, 1605. [Google Scholar] [CrossRef] [Green Version]
- Yin, L.H.; Wang, P.; Li, M.J.; Ke, X.W.; Li, C.Y.; Liang, D.; Wu, S.; Ma, X.L.; Li, C.; Zou, Y.; et al. Exogenous melatonin improves Malus resistance to Marssonina apple blotch. J. Pineal Res. 2013, 54, 426–434. [Google Scholar] [CrossRef]
- Lee, H.; Byeon, Y.; Tan, D.X.; Reiter, R.J.; Back, K. Arabidopsis serotonin N-acetyltransferase knockout mutant plants exhibit decreased melatonin and salicylic acid levels resulting in susceptibility to an avirulent pathogen. J. Pineal Res. 2015, 58, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Qian, Y.; Tan, D.X.; Reiter, R.J.; He, C. Melatonin induces the transcripts of CBFDREB1s and their involvement in both abiotic and biotic stresses in Arabidopsis. J. Pineal Res. 2015, 59, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Tan, D.X.; Reiter, R.J.; Ye, T.; Yang, F.; Chan, Z. Melatonin induces class A1 heat-shock factors (HSFA1s) and their possible involvement of thermotolerance in Arabidopsis. J. Pineal Res. 2015, 58, 335–342. [Google Scholar] [CrossRef]
- Mandal, M.K.; Suren, H.; Ward, B.; Boroujerdi, A.; Kousik, C. Differential roles of melatonin in plant-host resistance and pathogen suppression in cucurbits. J. Pineal Res. 2018, 65, 12505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, J.; Li, D.X.; Zhang, J.R.; Shan, C. Phytomelatonin Receptor PMTR1-Mediated Signaling Regulates Stomatal Closure in Arabidopsis thaliana. J. Pineal Res. 2018, 65, e12500. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.F.; Liu, Z.L.; Shao, Y.D.; Sun, F.; Zhang, Y.L.; Cui, J.; Zhou, Y.J.; Shen, W.B.; Zhou, T. Melatonin is responsible for rice resistance to rice stripe virus infection through a nitric oxide-dependent pathway. Virol. J. 2019, 16, 141. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, A.; Roychoudhury, A. Melatonin application reduces fluoride uptake and toxicity in rice seedlings by altering abscisic acid, gibberellin, auxin and antioxidant homeostasis. Plant Physiol. Biochem. 2019, 145, 164–173. [Google Scholar] [CrossRef]
- Kong, M.; Sheng, T.; Liang, J.; Ali, Q.; Gu, Q.; Wu, H.; Chen, J.; Liu, J.; Gao, X. Melatonin and its homologs induce immune responses via receptors trP47363-trP13076 in Nicotiana benthamiana. Front. Plant Sci. 2021, 12, 691835. [Google Scholar] [CrossRef]
- Walters, D.R. Why Do Plants Need Defenses? In Plant Defense; Wiley-Blackwell: Edinburgh, UK, 2010; Volume 1, p. 14. [Google Scholar]
- Vielma, J.R.; Bonilla, E.; Chacin-Bonilla, L.; Mora, M.; Medina-Leendertz, S.; Bravo, Y. Effects of melatonin on oxidative stress, and resistance to bacterial, parasitic, and viral infections: A review. Acta Trop. 2014, 137, 31–38. [Google Scholar] [CrossRef]
- Nabavi, S.M.; Nabavi, S.F.; Sureda, A.; Xiao, J.B.; Dehpour, A.R.; Shirooie, S.; Silva, A.S.; Baldi, A.; Khan, H.; Daglia, M. Anti-inflammatory effects of Melatonin: A mechanistic review. Crit. Rev. Food Sci. Nutr. 2019, 59, 4–16. [Google Scholar] [CrossRef]
- Regodon, S.; Martín-Palomino, P.; Fernandez-Montesinos, R.; Herrera, J.L.; Carrascosa-Salmoral, M.P.; Píriz, S.; Vadillo, S.; Guerrero, J.M.; Pozo, D. The use of melatonin as a vaccine agent. Vaccine 2005, 23, 5321–5327. [Google Scholar] [CrossRef]
- Tekbas, Ö.F.; Ogur, R.; Korkmaz, A.; Kilic, A.; Reiter, R.J. Melatonin as an antibiotic: New insights into the actions of this ubiquitous molecule. J. Pineal Res. 2008, 44, 222–226. [Google Scholar] [CrossRef]
- Liu, C.X.; Chen, L.L.; Zhao, R.R.; Li, R.; Zhang, S.J.; Yu, W.Q.; Sheng, J.P.; Shen, L. Melatonin Induces Disease Resistance to Botrytis cinerea in Tomato Fruit by Activating Jasmonic Acid Signaling Pathway. J. Agric. Food Chem. 2019, 67, 6116–6124. [Google Scholar] [CrossRef]
- Zhang, S.; Zheng, X. Melatonin Attenuates Potato Late Blight by Disrupting Cell Growth, Stress Tolerance, Fungicide Susceptibility and Homeostasis of Gene Expression in Phytophthora infestans. Front. Plant Sci. 2017, 8, 1993. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.M.; Liu, S.; Zhang, J.K.; Reiter, R.J.; Wang, Y.; Qiu, D.; Luo, X.M.; Khalid, A.R.; Wang, H.; Feng, L.; et al. Synergistic anti-oomycete effect of melatonin with a biofungicide against oomycetic black shank disease. J. Pineal Res. 2018, 65, 12492. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhao, Q.; Gao, T.T.; Wang, H.Y.; Zhang, Z.J.; Liang, B.W.; Wei, Z.W.; Liu, C.H.; Ma, F.W. The mitigation effects of exogenous melatonin on replant disease in apple. J. Pineal Res. 2018, 65, 12523. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Functions of melatonin in plants: A review. J. Pineal Res. 2015, 59, 133–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lerner, A.; Case, J.; Takahashi, Y.; Lee, T.H. Isolation of melatonin, the pineal gland factor that lightens melanocytes. J. Am. Chem. Soc. 1958, 80, 2587. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernándezruiz, J. The physiological function of melatonin in plants. Plant Signal. Behav. 2006, 1, 89–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reiter, R.; Tan, D.; Fuentesbroto, L. Melatonin: A multitasking molecule. Prog. Brain. Res. 2010, 181, 127–151. [Google Scholar] [PubMed]
- Liang, C.; Zheng, G.; Li, W.; Wang, Y.; Hu, B.; Wang, H.; Wu, H.; Qian, Y.; Zhu, X.; Tan, D.; et al. Melatonin delays leaf senescence and enhances salt stress tolerance in Rice. J. Pineal Res. 2015, 59, 91–101. [Google Scholar] [CrossRef]
- Zhang, N.; Sun, Q.; Zhang, H.; Zhao, C.; Li, L.; Chen, M. Roles of melatonin in abiotic stress resistance in plants. J. Exp. Bot. 2015, 66, 647–656. [Google Scholar] [CrossRef] [Green Version]
- Bonilla, E.; Valero, N.; Chacín-Bonilla, L.; Medina-Leendertz, S.J. Melatonin and viral infections. J Pineal Res. 2004, 36, 73–79. [Google Scholar] [CrossRef]
- Dollins, A.B.; Zhdanova, I.V.; Wurtman, R.J.; Lynch, H.J.; Deng, M.H. Effect of inducing nocturnal serum melatonin concentrations in daytime on sleep, mood, body temperature, and performance. Proc. Natl. Acad. Sci. USA 1994, 91, 1824–1828. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, C.; Mayo, J.C.; Sainz, R.M.; Antolin, I.; Herrera, F.; Martin, V.; Reiter, R.J. Regulation of antioxidant enzymes: A significant role for melatonin. J. Pineal Res. 2004, 36, 1–9. [Google Scholar] [CrossRef]
- Brainard, G.C.; Hanifin, J.P.; Greeson, J.M.; Byrne, B.; Glickman, G.; Gerner, E.; Rollag, M.D. Action spectrum for melatonin regulation in humans: Evidence for a novel circadian photoreceptor. J. Neurosci. 2011, 21, 6405–6412. [Google Scholar] [CrossRef] [Green Version]
- Carrillo-Vico, A.; Lardone, P.J.; Alvarez-Sanchez, N.; Rodriguez-Rodriguez, A.; Guerrero, J.M. Melatonin: Buffering the immune system. Int. J. Mol. 2013, 14, 8638–8683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.; Byeon, Y.; Back, K. Melatonin as a signal molecule triggering defense responses against pathogen attack in Arabidopsis and tobacco. J. Pineal Res. 2014, 57, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Murch, S.; Saxena, P. Melatonin: A potential regulator of plant growth and development? Vitr. Cell. Dev. Biol.-Plant 2002, 38, 531–536. [Google Scholar] [CrossRef]
- Reppert, S.M.; Weaver, D.R.; Ebisawa, T. Cloning and characterization of a mammalian melatonin receptor that mediates reproductive and circadian responses. Neuron 1994, 13, 1177–1185. [Google Scholar] [CrossRef]
- Reppert, S.M.; Godson, C.; Mahle, C.D.; Weaver, D.R.; Slaugenhaupt, S.A.; Gusella, J.F. Molecular characterization of a second melatonin receptor expressed in human retina and brain: The Mel1b melatonin receptor. Proc. Natl. Acad. Sci. USA. 1995, 92, 8734–8738. [Google Scholar] [CrossRef] [Green Version]
- Levoye, A.; Dam, J.; Ayoub, M.A.; Guillaume, J.L.; Couturier, C.; Delagrange, P.; Jockers, R. The orphan GPR50 receptor specifically inhibits MT1 melatonin receptor function through heterodimerization. EMBO J. 2006, 25, 3012–3023. [Google Scholar] [CrossRef] [PubMed]
- Nosjean, O.; Ferro, M.; Coge, F.; Beauverger, P.; Henlin, J.M.; Lefoulon, F.; Fauchere, J.L.; Delagrange, P.; Canet, E.; Boutin, J.A. Identification of the melatonin-binding site MT3 as the quinone reductase 2. J. Biol. Chem. 2000, 275, 31311–31317. [Google Scholar] [CrossRef] [Green Version]
- Mittler, R.; Vanderauwera, S.; Suzuki, N.; Miller, G.; Tognetti, V.B.; Vandepoele, K.; Gollery, M.; Shulaev, V.; Van Breusegem, F. ROS signaling: The new wave? Trends Plant Sci. 2011, 16, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Cadenas, E.; Davies, K.J. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic. Biol. Med. 2000, 29, 222–230. [Google Scholar] [CrossRef]
- Cuomo, C.A.; Gueldener, U.; Xu, J.R.; Trail, F.; Turgeon, B.G.; Di Pietro, A.; Walton, J.D.; Ma, L.J.; Baker, S.E.; Rep, M.; et al. The Fusarium graminearum genome reveals a link between localized polymorphism and pathogen specialization. Science 2007, 317, 1400–1402. [Google Scholar] [CrossRef] [Green Version]
- Han, Q.; Wu, F.; Wang, X.; Qi, H.; Shi, L.; Ren, A.; Liu, Q.; Zhao, M.; Tang, C. The bacterial lipopeptide iturins induce Verticillium dahliae cell death by affecting fungal signalling pathways and mediate plant defence responses involved in pathogen-associated molecular pattern-triggered immunity. Environ. Microbiol. 2015, 17, 1166–1188. [Google Scholar] [CrossRef]
- Gu, Q.; Yang, Y.; Yuan, Q.; Shi, G.; Wu, L.; Lou, Z.; Huo, R.; Wu, H.; Borriss, R.; Gao, X. Bacillomycin D Produced by Bacillus amyloliquefaciens is involved in the antagonistic interaction with the Plant-Pathogenic Fungus Fusarium graminearum. Appl. Environ. Microbiol. 2017, 83, e01075-17. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Luo, L.; Guo, J.; Liu, H.; Wang, B.; Deng, B.; Long, C.A.; Cheng, Y. Farnesol induces apoptosis and oxidative stress in the fungal pathogen Penicillium expansum. Mycologia 2010, 102, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Gong, A.D.; Li, H.P.; Yuan, Q.S.; Song, X.S.; Yao, W.; He, W.J.; Zhang, J.B.; Liao, Y.C. Antagonistic mechanism of iturin A and plipastatin A form Bacillus amyloliquefaciens S76-3 from wheat spikes against Fusarium graminearum. PLoS ONE 2015, 10, e0116871. [Google Scholar]
- Ayaz, M.; Ali, Q.; Farzand, A.; Khan, A.R.; Ling, H.; Gao, X. Nematicidal Volatiles from Bacillus atrophaeus GBSC56 Promote Growth and Stimulate Induced Systemic Resistance in Tomato against Meloidogyne incognita. Int. J. Mol. Sci. 2021, 22, 5049. [Google Scholar] [CrossRef] [PubMed]
- Saddique, M.A.B.; Ali, Z.; Sher, M.A.; Farid, B.; Ikram, R.M.; Ahmad, M.S. Proline, Total Antioxidant Capacity, and OsP5CS Gene Activity in Radical and Plumule of Rice are Efficient Drought Tolerance Indicator Traits. Int. J. Agron. 2020, 2020. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, M.; Liang, J.; Ali, Q.; Wen, W.; Wu, H.; Gao, X.; Gu, Q. 5-Methoxyindole, a Chemical Homolog of Melatonin, Adversely Affects the Phytopathogenic Fungus Fusarium graminearum. Int. J. Mol. Sci. 2021, 22, 10991. https://doi.org/10.3390/ijms222010991
Kong M, Liang J, Ali Q, Wen W, Wu H, Gao X, Gu Q. 5-Methoxyindole, a Chemical Homolog of Melatonin, Adversely Affects the Phytopathogenic Fungus Fusarium graminearum. International Journal of Molecular Sciences. 2021; 22(20):10991. https://doi.org/10.3390/ijms222010991
Chicago/Turabian StyleKong, Mengmeng, Jing Liang, Qurban Ali, Wen Wen, Huijun Wu, Xuewen Gao, and Qin Gu. 2021. "5-Methoxyindole, a Chemical Homolog of Melatonin, Adversely Affects the Phytopathogenic Fungus Fusarium graminearum" International Journal of Molecular Sciences 22, no. 20: 10991. https://doi.org/10.3390/ijms222010991
APA StyleKong, M., Liang, J., Ali, Q., Wen, W., Wu, H., Gao, X., & Gu, Q. (2021). 5-Methoxyindole, a Chemical Homolog of Melatonin, Adversely Affects the Phytopathogenic Fungus Fusarium graminearum. International Journal of Molecular Sciences, 22(20), 10991. https://doi.org/10.3390/ijms222010991




