Human Chorionic Gonadotropin and Early Embryogenesis: Review
Abstract
1. Introduction
2. hCG Isoforms and Secretion
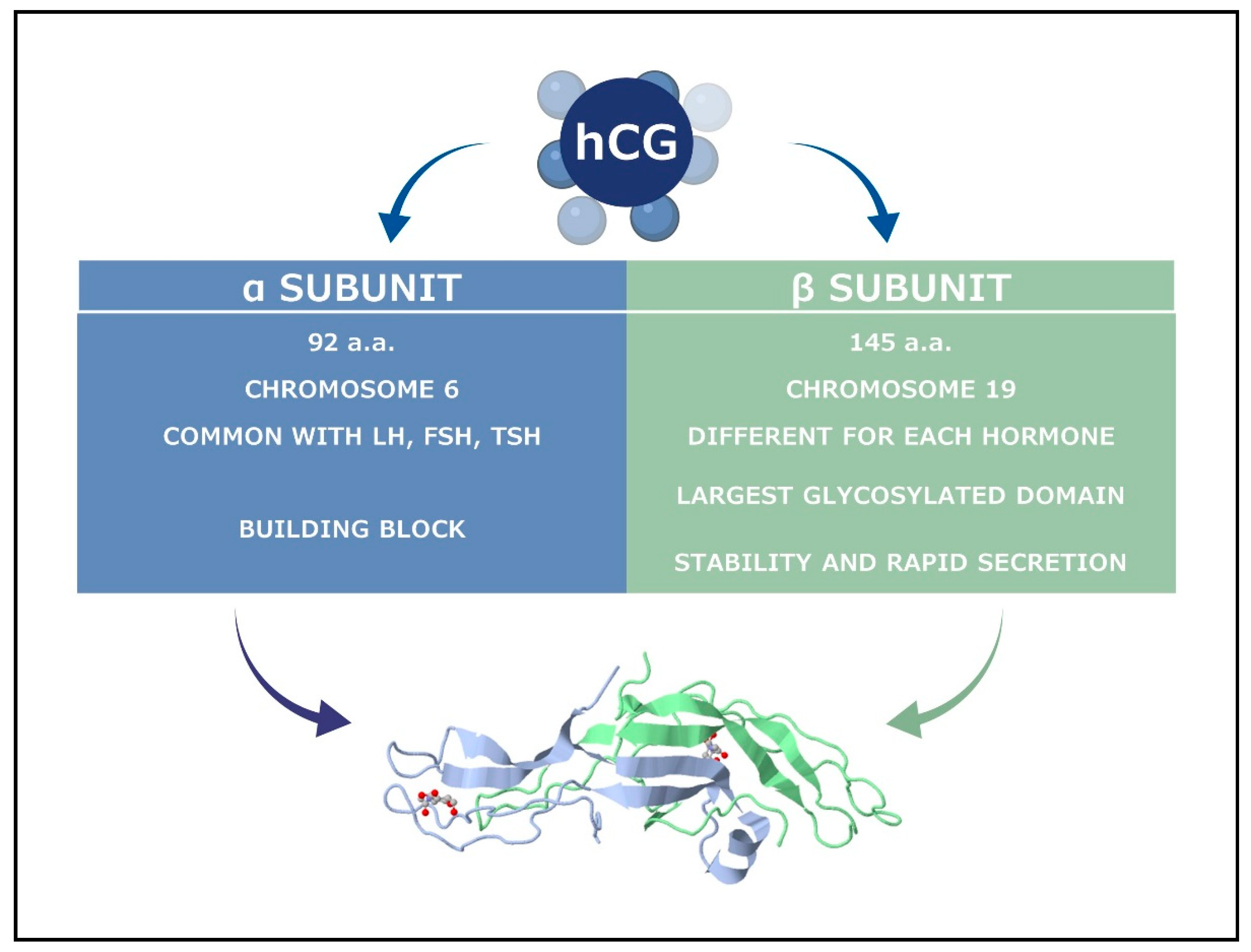
2.1. The Classical hCG
2.2. Hyperglycosylated hCG
2.3. The Free β Subunit
2.4. The Sulphated hCG
3. hCG Levels and Pregnancy
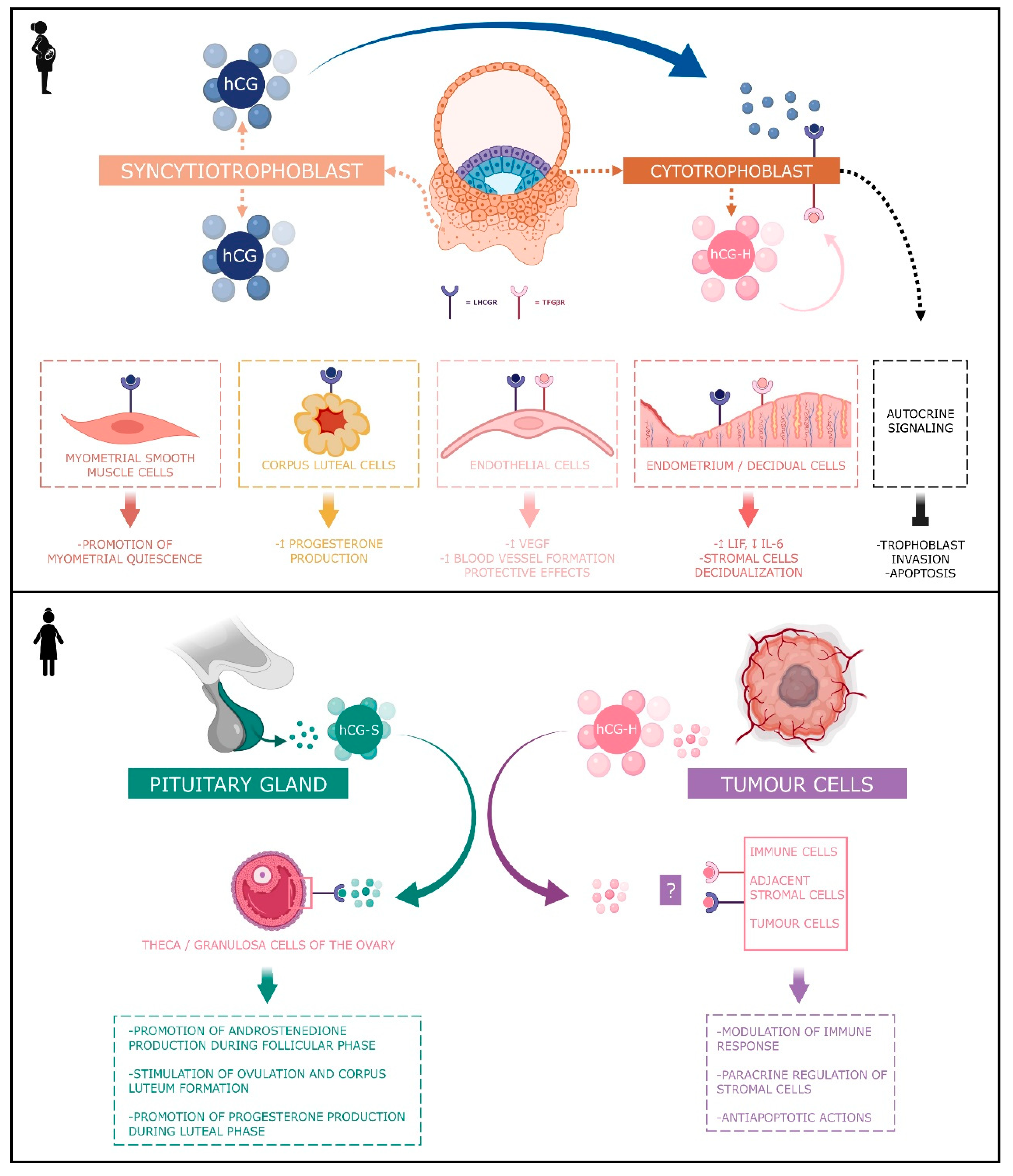
4. Angiogenic Actions of hCG
5. Immunological Actions of hCG
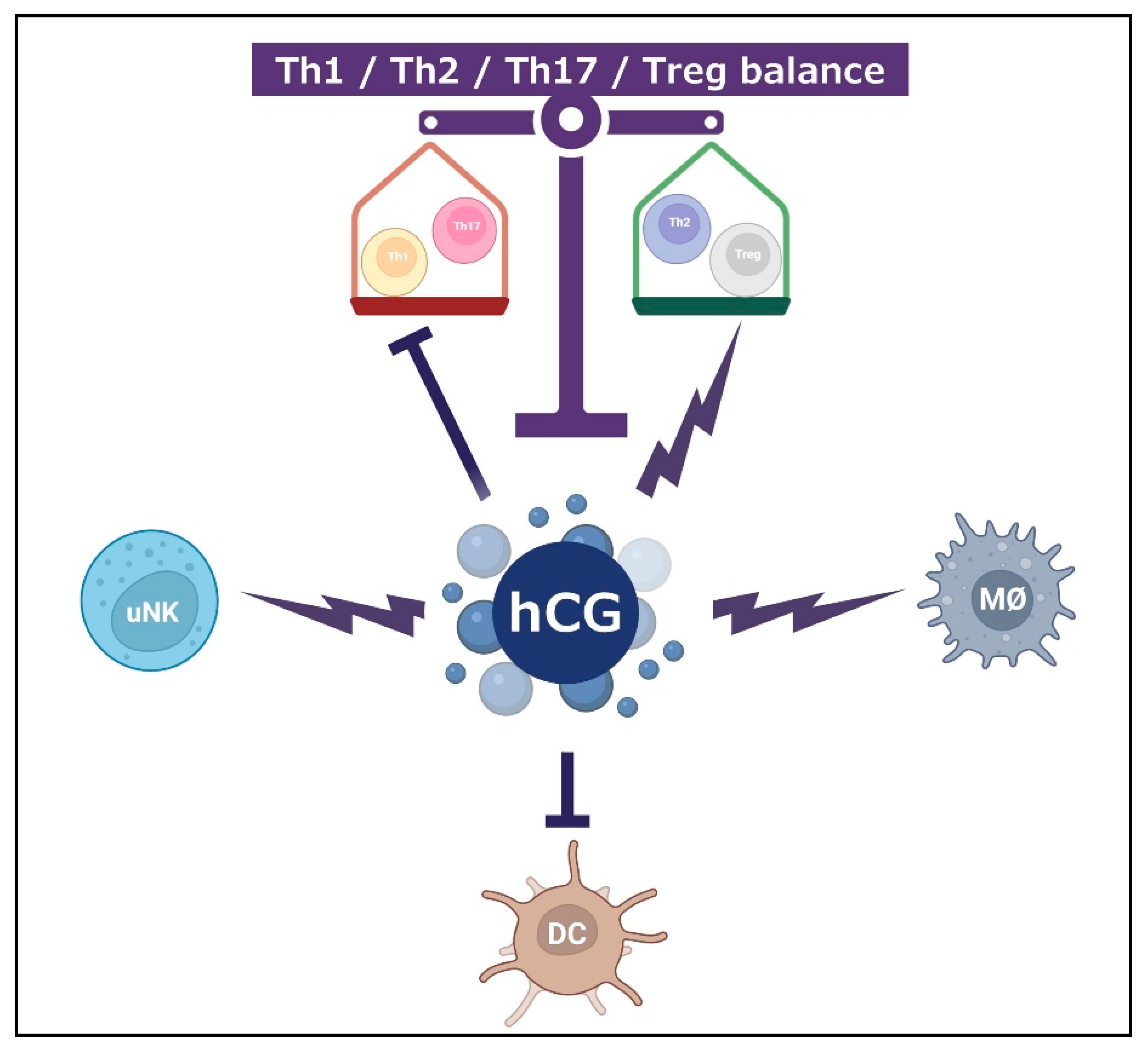
5.1. Th1/Th2/Th17/Treg Paradigm
5.2. T-Cell
5.3. Uterine Natural Killer Cells
5.4. Bone Marrow-Derived Dendritic Cells
5.5. Monocytes and Macrophages
5.6. Other Immunological Molecules
6. Infusion of hCG during Embryo Transfer
7. hCG and Miscarriages
8. hCG and Thyroid Fonction
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lunenfeld, B. Historical perspectives in gonadotrophin therapy. Hum. Reprod. Updat. 2004, 10, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Strott, C.A.; Yoshimi, T.; Ross, G.T.; Lipsett, M.B. Ovarian Physiology: Relationship Between Plasma LH and Steroidogenesis by the Follicle and Corpus Luteum; Effect of HCG1. J. Clin. Endocrinol. Metab. 1969, 29, 1157–1167. [Google Scholar] [CrossRef] [PubMed]
- Cole, L. Hyperglycosylated hCG, a review. Placenta 2010, 31, 653–664. [Google Scholar] [CrossRef]
- Montagnana, M.; Trenti, T.; Aloe, R.; Cervellin, G.; Lippi, G. Human chorionic gonadotropin in pregnancy diagnostics. Clin. Chim. Acta 2011, 412, 1515–1520. [Google Scholar] [CrossRef] [PubMed]
- De Medeiros, S.; Norman, R. Human choriogonadotrophin protein core and sugar branches heterogeneity: Basic and clinical insights. Hum. Reprod. Updat. 2009, 15, 69–95. [Google Scholar] [CrossRef][Green Version]
- Rivero-Müller, A.; Huhtaniemi, I. Genetic variants of gonadotrophins and their receptors: Impact on the diagnosis and management of the infertile patient. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 101596. [Google Scholar] [CrossRef]
- Sjunnesson, Y. In vitro fertilisation in domestic mammals—A brief overview. Upsala J. Med. Sci. 2020, 125, 68–76. [Google Scholar] [CrossRef]
- Stenman, U.-H.; Tiitinen, A.; Alfthan, H.; Valmu, L. The classification, functions and clinical use of different isoforms of HCG. Hum. Reprod. Updat. 2006, 12, 769–784. [Google Scholar] [CrossRef]
- Cole, L.A. New discoveries on the biology and detection of human chorionic gonadotropin. Reprod. Biol. Endocrinol. 2009, 7, 8. [Google Scholar] [CrossRef]
- Jurisicova, A.; Antenos, M.; Kapasi, K.; Meriano, J.; Casper, R.F. Variability in the expression of trophectodermal markers β-human chorionic gonadotrophin, human leukocyte antigen-G and pregnancy specific β-1 glycoprotein by the human blastocyst. Hum. Reprod. 1999, 14, 1852–1858. [Google Scholar] [CrossRef]
- Bonduelle, M.-L.; Dodd, R.; Liebaers, I.; Van Steirteghem, A.; Williamson, R.; Akhurst, R. Chorionic gonadotrophin-β mRNA, a trophoblast marker, is expressed in human 8-cell embryos derived from tripronucleate zygotes. Hum. Reprod. 1988, 3, 909–914. [Google Scholar] [CrossRef]
- Lopata, A.; Hay, D.L. The potential of early human embryos to form blastocysts, hatch from their zona and secrete HCG in culture. Hum. Reprod. 1989, 4, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Betz, D.; Fane, K. Human Chorionic Gonadotropin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Braunstein, G.D.; Rasor, J.; Danzer, H.; Adler, D.; Wade, M.E. Serum human chorionic gonadotropin levels throughout normal pregnancy. Am. J. Obstet. Gynecol. 1976, 126, 678–681. [Google Scholar] [CrossRef]
- Ohlsson, R.; Larsson, E.; Nilsson, O.; Wahlström, T.; Sundström, P. Blastocyst implantation precedes induction of insulin-like growth factor II gene expression in human trophoblasts. Development 1989, 106, 555–559. [Google Scholar] [CrossRef] [PubMed]
- D’Hauterive, S.P.; Charlet-Renard, C.; Berndt, S.; Dubois, M.; Munaut, C.; Goffin, F.; Hagelstein, M.-T.; Noel, A.; Hazout, A.; Foidart, J.-M.; et al. Human chorionic gonadotropin and growth factors at the embryonic–endometrial interface control leukemia inhibitory factor (LIF) and interleukin 6 (IL-6) secretion by human endometrial epithelium. Hum. Reprod. 2004, 19, 2633–2643. [Google Scholar] [CrossRef]
- Srisuparp, S.; Strakova, Z.; Fazleabas, A.T. The Role of Chorionic Gonadotropin (CG) in Blastocyst Implantation. Arch. Med. Res. 2001, 32, 627–634. [Google Scholar] [CrossRef]
- Lobo, S.C.; Srisuparp, S.; Peng, X.; Fazleabas, A.T. Uterine Receptivity in the Baboon: Modulation by Chorionic Gonadotropin. Semin. Reprod. Med. 2001, 19, 069–074. [Google Scholar] [CrossRef]
- Shi, Q.J.; Lei, Z.M.; Rao, C.V.; Lin, J. Novel role of human chorionic gonadotropin in differentiation of human cytotrophoblasts. Endocrinology 1993, 132, 1387–1395. [Google Scholar] [CrossRef]
- North, R.A.; Whitehead, R.; Larkins, R.G. Stimulation by Human Chorionic Gonadotropin of Prostaglandin Synthesis by Early Human Placental Tissue. J. Clin. Endocrinol. Metab. 1991, 73, 60–70. [Google Scholar] [CrossRef]
- Weedon-Fekjær, M.; Taskén, K. Review: Spatiotemporal dynamics of hCG/cAMP signaling and regulation of placental function. Placenta 2012, 33, S87–S91. [Google Scholar] [CrossRef]
- Prast, J.; Saleh, L.; Husslein, H.; E Sonderegger, S.; Helmer, H.; Knöfler, M. Human Chorionic Gonadotropin Stimulates Trophoblast Invasion through Extracellularly Regulated Kinase and AKT Signaling. Endocrinology 2007, 149, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Palaniappan, M.; Menon, K. Human Chorionic Gonadotropin Stimulates Theca-Interstitial Cell Proliferation and Cell Cycle Regulatory Proteins by a cAMP-Dependent Activation of AKT/mTORC1 Signaling Pathway. Mol. Endocrinol. 2010, 24, 1782–1793. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, C.-L.; Chiu, C.N.; Hautala, L.; Salo, T.; Yeung, S.B.W.; Stenman, U.-H.; Koistinen, H. Human chorionic gonadotropin and its free β-subunit stimulate trophoblast invasion independent of LH/hCG receptor. Mol. Cell. Endocrinol. 2013, 375, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Cole, L.A.; Butler, S.A. Hyperglycosylated human chorionic gonadotropin and human chorionic gonadotropin free beta-subunit: Tumor markers and tumor promoters. J. Reprod. Med. 2008, 53, 499–512. [Google Scholar]
- Guibourdenche, J.; Handschuh, K.; Tsatsaris, V.; Gerbaud, P.; Leguy, M.C.; Müller, F.; Brion, D.E.; Fournier, T. Hyperglycosylated hCG Is a Marker of Early Human Trophoblast Invasion. J. Clin. Endocrinol. Metab. 2010, 95, E240–E244. [Google Scholar] [CrossRef]
- Salas, A.; Gastón, B.; Barrenetxea, J.; Sendino, T.; Jurado, M.; Alcázar, J.L. Predictive value of hyperglycosylated human chorionic gonadotropin for pregnancy outcomes in threatened abortion in first-trimester viable pregnancies. An. Sist. Sanit. Navar. 2021, 44, 23–31. [Google Scholar] [CrossRef]
- Hamada, A.L.; Nakabayashi, K.; Sato, A.; Kiyoshi, K.; Takamatsu, Y.; Laoag-Fernandez, J.B.; Ohara, N.; Maruo, T. Transfection of Antisense Chorionic Gonadotropin β Gene into Choriocarcinoma Cells Suppresses the Cell Proliferation and Induces Apoptosis. J. Clin. Endocrinol. Metab. 2005, 90, 4873–4879. [Google Scholar] [CrossRef]
- Sasaki, Y.; Ladner, D.G.; Cole, L.A. Hyperglycosylated human chorionic gonadotropin and the source of pregnancy failures. Fertil. Steril. 2008, 89, 1781–1786. [Google Scholar] [CrossRef]
- Cole, L. Hyperglycosylated hCG. Placenta 2007, 28, 977–986. [Google Scholar] [CrossRef]
- Cole, L.A.; Dai, D.; Butler, S.A.; Leslie, K.K.; Kohorn, E.I. Gestational trophoblastic diseases: 1. Pathophysiology of hyperglycosylated hCG. Gynecol. Oncol. 2006, 102, 145–150. [Google Scholar] [CrossRef]
- Kovalevskaya, G.; Kakuma, T.; Schlatterer, J.; O’Connor, J.F. Hyperglycosylated HCG expression in pregnancy: Cellular origin and clinical applications. Mol. Cell. Endocrinol. 2007, 260–262, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Bersinger, N.A.; Wunder, D.M.; Nicolas, M.; Birkhäuser, M.H.; Porquet, D.; Guibourdenche, J. Serum Hyperglycosylated Human Chorionic Gonadotropin to Predict the Gestational Outcome in in vitro Fertilization/Intracytoplasmic Sperm Injection Pregnancies. Fetal Diagn. Ther. 2008, 24, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Chen, L.; Wang, X.; Liu, Y. Association between serum beta-human chorionic gonadotropin and inflammation, oxidative stress in pregnancy-induced hypertension. Microvasc. Res. 2021, 135, 104130. [Google Scholar] [CrossRef] [PubMed]
- Ballantyne, A.; Rashid, L.; Pattenden, R. Stability of maternal serum free β-hCG following whole blood sample transit: First trimester Down’s syndrome screening in Scotland. Ann. Clin. Biochem. 2022, 59, 87–91. [Google Scholar] [CrossRef]
- Cole, L.A.; Butler, S. Hyperglycosylated hCG, hCGβ and Hyperglycosylated hCGβ: Interchangeable cancer promoters. Mol. Cell. Endocrinol. 2012, 349, 232–238. [Google Scholar] [CrossRef]
- Butler, S.A.; Ikram, M.S.; Mathieu, S.; Iles, R.K. The increase in bladder carcinoma cell population induced by the free beta subunit of human chorionic gonadotrophin is a result of an anti-apoptosis effect and not cell proliferation. Br. J. Cancer 2000, 82, 1553–1556. [Google Scholar] [CrossRef]
- Sirikunalai, P.; Wanapirak, C.; Sirichotiyakul, S.; Tongprasert, F.; Srisupundit, K.; Luewan, S.; Traisrisilp, K.; Tongsong, T. Associations between maternal serum free beta human chorionic gonadotropin (β-hCG) levels and adverse pregnancy outcomes. J. Obstet. Gynaecol. 2016, 36, 178–182. [Google Scholar] [CrossRef]
- Cole, L.A.; Gutierrez, J.M. Production of human chorionic gonadotropin during the normal menstrual cycle. J. Reprod. Med. 2009, 54, 245–250. [Google Scholar]
- Cole, L.A. “Background” Human Chorionic Gonadotropin in Healthy, Nonpregnant Women. Clin. Chem. 2005, 51, 1765–1766. [Google Scholar] [CrossRef][Green Version]
- Cole, L.A.; Laidler, L.L.; Muller, C.Y. USA hCG reference service, 10-year report. Clin. Biochem. 2010, 43, 1013–1022. [Google Scholar] [CrossRef]
- Birken, S.; Maydelman, Y.; Gawinowicz, M.A.; Pound, A.; Liu, Y.; Hartree, A.S. Isolation and characterization of human pituitary chorionic gonadotropin. Endocrinology 1996, 137, 1402–1411. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.Y.; Haddow, J.E.; Palomaki, G.E.; Roberson, M. Major fetal abnormalities associated with positive screening tests for Smith-Lemli-Opitz syndrome (SLOS). Prenat. Diagn. 2007, 27, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Norris, W.; Nevers, T.; Sharma, S.; Kalkunte, S. Review: hCG, preeclampsia and regulatory T cells. Placenta 2011, 32, S182–S185. [Google Scholar] [CrossRef] [PubMed]
- Barjaktarovic, M.; Korevaar, T.I.M.; Jaddoe, V.W.V.; de Rijke, Y.B.; Peeters, R.P.; Steegers, E.A.P. Human chorionic gonadotropin and risk of pre-eclampsia: Prospective population-based cohort study. Ultrasound Obstet. Gynecol. 2019, 54, 477–483. [Google Scholar] [CrossRef]
- Barjaktarovic, M.; Korevaar, T.I.; Jaddoe, V.W.; de Rijke, Y.B.; Visser, T.J.; Peeters, R.P.; Steegers, E.A. Human chorionic gonadotropin (hCG) concentrations during the late first trimester are associated with fetal growth in a fetal sex-specific manner. Eur. J. Epidemiol. 2017, 32, 135–144. [Google Scholar] [CrossRef]
- Stevens, F.T.; Katzorke, N.; Tempfer, C.; Kreimer, U.; Bizjak, G.I.; Fleisch, M.C.; Fehm, T.N. Gestational Trophoblastic Disorders: An Update in 2015. Geburtshilfe Und Frauenheilkd. 2015, 75, 1043–1050. [Google Scholar] [CrossRef]
- De Backer, B.; Goffin, F.; Nisolle, M.; Minon, J.M. Élévation faible d’hCG en dehors d’un contexte gravidique: À propos de deux cas et revue de la littérature [Persistent low hCG levels beyond pregnancy: Report of two cases and review of the literature]. Ann. Biol. Clin. 2013, 71, 496–502. [Google Scholar] [CrossRef]
- Katabuchi, H.; Ohba, T. Human chorionic villous macrophages as a fetal biological shield from maternal chorionic gonadotropin. Dev. Growth Differ. 2008, 50, 299–306. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Ohba, T.; Tashiro, H.; Yamada, G.; Katabuchi, H. Human Chorionic Gonadotropin Induces Human Macrophages to Form Intracytoplasmic Vacuoles Mimicking Hofbauer Cells in Human Chorionic Villi. Cells Tissues Organs 2013, 197, 127–135. [Google Scholar] [CrossRef]
- Paulesu, L.; Rao, C.; Ietta, F.; Pietropolli, A.; Ticconi, C. hCG and Its Disruption by Environmental Contaminants during Human Pregnancy. Int. J. Mol. Sci. 2018, 19, 914. [Google Scholar] [CrossRef]
- Tsampalas, M.; Gridelet, V.; Berndt, S.; Foidart, J.-M.; Geenen, V.; D’Hauterive, S.P. Human chorionic gonadotropin: A hormone with immunological and angiogenic properties. J. Reprod. Immunol. 2010, 85, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Polese, B.; Gridelet, V.; Araklioti, E.; Martens, H.; Perrier d’Hauterive, S.; Geenen, V. The Endocrine Milieu and CD4 T-Lymphocyte Polarization during Pregnancy. Front. Endocrinol. 2014, 5, 106. [Google Scholar] [CrossRef] [PubMed]
- Ogino, M.H.; Tadi, P. Physiology, Chorionic Gonadotropin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Berndt, S.; D’Hauterive, S.P.; Blacher, S.; Pequeux, C.; Lorquet, S.; Munaut, C.; Applanat, M.; Hervé, M.A.; Lamandé, N.; Corvol, P.; et al. Angiogenic activity of human chorionic gonadotropin through LH receptor activation on endothelial and epithelial cells of the endometrium. FASEB J. 2006, 20, 2630–2632. [Google Scholar] [CrossRef] [PubMed]
- Berndt, S.; Blacher, S.; D’Hauterive, S.P.; Thiry, M.; Tsampalas, M.; Cruz, A.; Péqueux, C.; Lorquet, S.; Munaut, C.; Noël, A.; et al. Chorionic Gonadotropin Stimulation of Angiogenesis and Pericyte Recruitment. J. Clin. Endocrinol. Metab. 2009, 94, 4567–4574. [Google Scholar] [CrossRef][Green Version]
- Herr, F.; Baal, N.; Reisinger, K.; Lorenz, A.; McKinnon, T.; Preissner, K.; Zygmunt, M. hCG in the Regulation of Placental Angiogenesis. Results of an In Vitro Study. Placenta 2007, 28, S85–S93. [Google Scholar] [CrossRef]
- Bourdiec, A.; Bédard, D.; Rao, C.V.; Akoum, A. Human Chorionic Gonadotropin Regulates Endothelial Cell Responsiveness to Interleukin 1 and Amplifies the Cytokine-Mediated Effect on Cell Proliferation, Migration and the Release of Angiogenic Factors. Am. J. Reprod. Immunol. 2013, 70, 127–138. [Google Scholar] [CrossRef]
- Reisinger, K.; Baal, N.; McKinnon, T.; Münstedt, K.; Zygmunt, M. The gonadotropins: Tissue-specific angiogenic factors? Mol. Cell. Endocrinol. 2007, 269, 65–80. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, Y.; Zhang, J.; Liu, Z.; Lin, Q.; Wang, Z. Activation of NF-κB signaling pathway during HCG-induced VEGF expression in luteal cells. Cell Biol. Int. 2019, 43, 344–349. [Google Scholar] [CrossRef]
- Surico, D.; Farruggio, S.; Marotta, P.; Raina, G.; Mary, D.; Surico, N.; Vacca, G.; Grossini, E. Human Chorionic Gonadotropin Protects Vascular Endothelial Cells from Oxidative Stress by Apoptosis Inhibition, Cell Survival Signalling Activation and Mitochondrial Function Protection. Cell. Physiol. Biochem. 2015, 36, 2108–2120. [Google Scholar] [CrossRef]
- Jing, G.; Yao, J.; Dang, Y.; Liang, W.; Xie, L.; Chen, J.; Li, Z. The role of β-HCG and VEGF-MEK/ERK signaling pathway in villi angiogenesis in patients with missed abortion. Placenta 2021, 103, 16–23. [Google Scholar] [CrossRef]
- Fournier, T.; Guibourdenche, J.; Evain-Brion, D. Review: hCGs: Different sources of production, different glycoforms and functions. Placenta 2015, 36, S60–S65. [Google Scholar] [CrossRef] [PubMed]
- Berndt, S.; Blacher, S.; Munaut, C.; Detilleux, J.; D’Hauterive, S.P.; Huhtaniemi, I.; Evain-Brion, D.; Noël, A.; Fournier, T.; Foidart, J. Hyperglycosylated human chorionic gonadotropin stimulates angiogenesis through TGF-β receptor activation. FASEB J. 2013, 27, 1309–1321. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, V.; González, M.; Toledo, F.; Sobrevia, L. Role of heme oxygenase 1 and human chorionic gonadotropin in pregnancy associated diseases. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2020, 1866, 165522. [Google Scholar] [CrossRef] [PubMed]
- Raghupathy, R. Th 1-type immunity is incompatible with successful pregnancy. Immunol. Today 1997, 18, 478–482. [Google Scholar] [CrossRef]
- Schumacher, A.; Heinze, K.; Witte, J.; Poloski, E.; Linzke, N.; Woidacki, K.; Zenclussen, A.C. Human Chorionic Gonadotropin as a Central Regulator of Pregnancy Immune Tolerance. J. Immunol. 2013, 190, 2650–2658. [Google Scholar] [CrossRef]
- Lea, R.G.; Sandra, O. Immunoendocrine aspects of endometrial function and implantation. Reproduction 2007, 134, 389–404. [Google Scholar] [CrossRef]
- Fujiwara, H. Do circulating blood cells contribute to maternal tissue remodeling and embryo-maternal cross-talk around the implantation period? Mol. Hum. Reprod. 2009, 15, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Raghupathy, R.; Makhseed, M.; Azizieh, F.; Hassan, N.; Al-Azemi, M.; Al-Shamali, E. Maternal Th1- and Th2-type reactivity to placental antigens in normal human pregnancy and unexplained recurrent spontaneous abortions. Cell Immunol. 1999, 196, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-J.; Hao, C.-F.; Lin, Y.; Yin, G.-J.; Bao, S.-H.; Qiu, L.-H.; Lin, Q.-D. Increased prevalence of T helper 17 (Th17) cells in peripheral blood and decidua in unexplained recurrent spontaneous abortion patients. J. Reprod. Immunol. 2010, 84, 164–170. [Google Scholar] [CrossRef]
- Sereshki, N.; Gharagozloo, M.; Ostadi, V.; Ghahiri, A.; Roghaei, M.A.; Mehrabian, F.; Andalib, A.A.; Hassanzadeh, A.; Hosseini, H.; Rezaei, A. Variations in T-helper 17 and Regulatory T Cells during The Menstrual Cycle in Peripheral Blood of Women with Recurrent Spontaneous Abortion. Int. J. Fertil. Steril. 2014, 8, 59–66. [Google Scholar]
- Schumacher, A. Human Chorionic Gonadotropin as a Pivotal Endocrine Immune Regulator Initiating and Preserving Fetal Tolerance. Int. J. Mol. Sci. 2017, 18, 2166. [Google Scholar] [CrossRef] [PubMed]
- Ricketts, R.M.; Jones, D.B. Differential effect of human chorionic gonadotrophin on lymphocyte proliferation induced by mitogens. J. Reprod. Immunol. 1985, 7, 225–232. [Google Scholar] [CrossRef]
- Yagel, S.; Parhar, R.S.; Lala, P.K. Trophic effects of first-trimester human trophoblasts and human chorionic gonadotropin on lymphocyte proliferation. Am. J. Obstet. Gynecol. 1989, 160, 946–953. [Google Scholar] [CrossRef]
- Carbone, F.; Procaccini, C.; De Rosa, V.; Alviggi, C.; DE Placido, G.; Kramer, D.; Longobardi, S.; Matarese, G. Divergent immunomodulatory effects of recombinant and urinary-derived FSH, LH, and hCG on human CD4+ T cells. J. Reprod. Immunol. 2010, 85, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Khil, L.-Y.; Jun, H.-S.; Kwon, H.J.; Yoo, J.K.; Kim, S.; Notkins, A.L.; Yoon, J.-W. Human chorionic gonadotropin is an immune modulator and can prevent autoimmune diabetes in NOD mice. Diabetologia 2007, 50, 2147–2155. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, A.; Brachwitz, N.; Sohr, S.; Engeland, K.; Langwisch, S.; Dolaptchieva, M.; Alexander, T.; Taran, A.; Malfertheiner, S.F.; Costa, S.-D.; et al. Human Chorionic Gonadotropin Attracts Regulatory T Cells into the Fetal-Maternal Interface during Early Human Pregnancy. J. Immunol. 2009, 182, 5488–5497. [Google Scholar] [CrossRef] [PubMed]
- Freis, A.; Schlegel, J.; Daniel, V.; Jauckus, J.; Strowitzki, T.; Germeyer, A. Cytokines in relation to hCG are significantly altered in asymptomatic women with miscarriage—a pilot study. Reprod. Biol. Endocrinol. 2018, 16, 93. [Google Scholar] [CrossRef]
- Liu, X.; Ma, D.; Wang, W.; Qu, Q.; Zhang, N.; Wang, X.; Fang, J.; Ma, Z.; Hao, C. Intrauterine administration of human chorionic gonadotropin improves the live birth rates of patients with repeated implantation failure in frozen-thawed blastocyst transfer cycles by increasing the percentage of peripheral regulatory T cells. Arch. Gynecol. Obstet. 2019, 299, 1165–1172. [Google Scholar] [CrossRef]
- Laokirkkiat, P.; Thanaboonyawat, I.; Boonsuk, S.; Petyim, S.; Prechapanich, J.; Choavaratana, R. Increased implantation rate after intrauterine infusion of a small volume of human chorionic gonadotropin at the time of embryo transfer: A randomized, double-blind controlled study. Arch. Gynecol Obstet. 2019, 299, 267–275. [Google Scholar] [CrossRef]
- Zamorina, S.A.; Litvinova, L.S.; Yurova, K.A.; Khaziakhmatova, O.G.; Timganova, V.P.; Bochkova, M.S.; Khramtsov, P.V.; Rayev, M.B. The role of human chorionic gonadotropin in regulation of naïve and memory T cells activity in vitro. Int. Immunopharmacol. 2018, 54, 33–38. [Google Scholar] [CrossRef]
- Lash, G.E.; Robson, S.C.; Bulmer, J.N. Review: Functional role of uterine natural killer (uNK) cells in human early pregnancy decidua. Placenta 2010, 31, S87–S92. [Google Scholar] [CrossRef] [PubMed]
- Yagel, S. The developmental role of natural killer cells at the fetal-maternal interface. Am. J. Obstet. Gynecol. 2009, 201, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Moffett, A.; Colucci, F. Uterine NK cells: Active regulators at the maternal-fetal interface. J. Clin. Investig. 2014, 124, 1872–1879. [Google Scholar] [CrossRef]
- Sauerbrun-Cutler, M.; Huber, W.J.; Krueger, P.M.; Sung, C.J.; Has, P.; Sharma, S. Do endometrial natural killer and regulatory T cells differ in infertile and clinical pregnancy patients? An analysis in patients undergoing frozen embryo transfer cycles. Am. J. Reprod. Immunol. 2021, 85, e13393. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.S.; Bora, S.A.; Saso, S.; Smith, J.R.; Johnson, M.R.; Thum, M.Y. Mechanism of human chorionic gonadotrophin-mediated immunomodulation in pregnancy. Expert Rev. Clin. Immunol. 2012, 8, 747–753. [Google Scholar] [CrossRef]
- Kane, N.; Kelly, R.; Saunders, P.T.; Critchley, H.O. Proliferation of Uterine Natural Killer Cells Is Induced by Human Chorionic Gonadotropin and Mediated via the Mannose Receptor. Endocrinology 2009, 150, 2882–2888. [Google Scholar] [CrossRef]
- Ashkar, A.A.; Croy, B.A. Functions of uterine natural killer cells are mediated by interferon gamma production during murine pregnancy. Semin. Immunol. 2001, 13, 235–241. [Google Scholar] [CrossRef]
- Hanna, J.; Goldman-Wohl, D.; Hamani, Y.; Avraham, I.; Greenfield, C.; Natanson-Yaron, S.; Prus, D.; Cohen-Daniel, L.; I Arnon, T.; Manaster, I.; et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat. Med. 2006, 12, 1065–1074. [Google Scholar] [CrossRef]
- Wan, H.; Versnel, M.A.; Leijten, L.M.; van Helden-Meeuwsen, C.G.; Fekkes, D.; Leenen, P.J.; Khan, N.A.; Benner, R.; Kiekens, R.C. Chorionic gonadotropin induces dendritic cells to express a tolerogenic phenotype. J. Leukoc. Biol. 2008, 83, 894–901. [Google Scholar] [CrossRef]
- Dauven, D.; Ehrentraut, S.; Langwisch, S.; Zenclussen, A.C.; Schumacher, A. Immune Modulatory Effects of Human Chorionic Gonadotropin on Dendritic Cells Supporting Fetal Survival in Murine Pregnancy. Front. Endocrinol. 2016, 7, 146. [Google Scholar] [CrossRef]
- Kosaka, K.; Fujiwara, H.; Tatsumi, K.; Yoshioka, S.; Sato, Y.; Egawa, H.; Higuchi, T.; Nakayama, T.; Ueda, M.; Maeda, M.; et al. Human Chorionic Gonadotropin (HCG) Activates Monocytes to Produce Interleukin-8 via a Different Pathway from Luteinizing Hormone/HCG Receptor System. J. Clin. Endocrinol. Metab. 2002, 87, 5199–5208. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Versnel, M.A.; Cheung, W.Y.; Leenen, P.J.; Khan, N.A.; Benner, R.; Kiekens, R.C. Chorionic gonadotropin can enhance innate immunity by stimulating macrophage function. J. Leukoc. Biol. 2007, 82, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, T.; Fujiwara, H.; Maeda, M.; Inoue, T.; Yoshioka, S.; Mori, T.; Fujii, S. Human peripheral blood mononuclear cells (PBMC) in early pregnancy promote embryo invasion in vitro: HCG enhances the effects of PBMC. Hum. Reprod. 2002, 17, 207–212. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sauss, K.; Ehrentraut, S.; Zenclussen, A.C.; Schumacher, A. The pregnancy hormone human chorionic gonadotropin differentially regulates plasmacytoid and myeloid blood dendritic cell subsets. Am. J. Reprod. Immunol. 2018, 79, e12837. [Google Scholar] [CrossRef]
- Licht, P.; von Wolff, M.; Berkholz, A.; Wildt, L. Evidence for cycle-dependent expression of full-length human chorionic gonadotropin/luteinizing hormone receptor mRNA in human endometrium and decidua. Fertil. Steril. 2003, 79, 718–723. [Google Scholar] [CrossRef]
- Schjenken, J.E.; Glynn, D.J.; Sharkey, D.J.; Robertson, S.A. TLR4 Signaling Is a Major Mediator of the Female Tract Response to Seminal Fluid in Mice1. Biol. Reprod. 2015, 93, 68. [Google Scholar] [CrossRef]
- Bourdiec, A.; Calvo, E.; Rao, C.V.; Akoum, A. Transcriptome Analysis Reveals New Insights into the Modulation of Endometrial Stromal Cell Receptive Phenotype by Embryo-Derived Signals Interleukin-1 and Human Chorionic Gonadotropin: Possible Involvement in Early Embryo Implantation. PLoS ONE 2013, 8, e64829. [Google Scholar] [CrossRef]
- Srivastava, A.; Sengupta, J.; Kriplani, A.; Roy, K.K.; Ghosh, D. Profiles of cytokines secreted by isolated human endometrial cells under the influence of chorionic gonadotropin during the window of embryo implantation. Reprod. Biol. Endocrinol. 2013, 11, 116. [Google Scholar] [CrossRef]
- Wang, H.; Liu, F.; Liu, X.; Zhu, X.-W. The effect of hCG bind immunoglobulin G on maternalinterface’s Th1/Th2 type cytokines and pregnancy’s outcome of abortion model. Chin. J. Birth Health Hered 2013, 21, 53–55. [Google Scholar]
- Bai, H.; Pan, J.; Jia, X.; Huang, H. The inhibition effect of human chorionic gonadotropin (hCG) on mRNA expression of cytokines initiated inflammatory reaction. Chin. J. Immunol. 2003, 19, 193–203. [Google Scholar]
- Palomino, W.A.; Argandoña, F.; Azúa, R.; Kohen, P.; Devoto, L. Complement C3 and Decay-Accelerating Factor Expression Levels Are Modulated by Human Chorionic Gonadotropin in Endometrial Compartments during the Implantation Window. Reprod. Sci. 2013, 20, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Simon, A.; Laufer, N. Repeated implantation failure: Clinical approach. Fertil. Steril. 2012, 97, 1039–1043. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; Chang, H.; Zhou, S.; Zhang, S.; Yuan, D.; Yu, L.-L.; Qu, T. Intrauterine administration of peripheral blood mononuclear cells activated by human chorionic gonadotropin in patients with repeated implantation failure: A meta-analysis. J. Reprod. Immunol. 2021, 145, 103323. [Google Scholar] [CrossRef] [PubMed]
- Pourmoghadam, Z.; Soltani-Zangbar, M.S.; Sheikhansari, G.; Azizi, R.; Eghbal-Fard, S.; Mohammadi, H.; Siahmansouri, H.; Aghebati-Maleki, L.; Danaii, S.; Mehdizadeh, A.; et al. Intrauterine administration of autologous hCG- activated peripheral blood mononuclear cells improves pregnancy outcomes in patients with recurrent implantation failure; A double-blind, randomized control trial study. J. Reprod. Immunol. 2020, 142, 103182. [Google Scholar] [CrossRef]
- Tesarik, J.; Hazout, A.; Mendoza, C. Luteinizing hormone affects uterine receptivity independently of ovarian function. Reprod. Biomed. Online 2003, 7, 59–64. [Google Scholar] [CrossRef]
- Nwabuobi, C.; Arlier, S.; Schatz, F.; Guzeloglu-Kayisli, O.; Lockwood, C.J.; Kayisli, U.A. hCG: Biological Functions and Clinical Applications. Int. J. Mol. Sci. 2017, 18, 2037. [Google Scholar] [CrossRef]
- Craciunas, L.; Tsampras, N.; Raine-Fenning, N.; Coomarasamy, A. Intrauterine administration of human chorionic gonadotropin (hCG) for subfertile women undergoing assisted reproduction. Cochrane Database Syst. Rev. 2018, 10, CD011537. [Google Scholar] [CrossRef]
- Hong, K.H.; Forman, E.J.; Werner, M.D.; Upham, K.M.; Gumeny, C.L.; Winslow, A.D.; Kim, T.J.; Scott, R.T. Endometrial infusion of human chorionic gonadotropin at the time of blastocyst embryo transfer does not impact clinical outcomes: A randomized, double-blind, placebo-controlled trial. Fertil. Steril. 2014, 102, 1591–1595.e2. [Google Scholar] [CrossRef][Green Version]
- Diao, L.H.; Li, G.G.; Zhu, Y.C.; Tu, W.W.; Huang, C.Y.; Lian, R.C.; Chen, X.; Li, Y.Y.; Zhang, T.; Huang, Y.; et al. Human chorionic gonadotropin potentially affects pregnancy outcome in women with recurrent implantation failure by regulating the homing preference of regulatory T cells. Am. J. Reprod. Immunol. 2017, 77, e12618. [Google Scholar] [CrossRef]
- Santibañez, A.; García, J.; Pashkova, O.; Colín, O.; Castellanos, G.; Sánchez, A.P.; De la Jara, J.F. Effect of intrauterine injection of human chorionic gonadotropin before embryo transfer on clinical pregnancy rates from in vitro fertilisation cycles: A prospective study. Reprod. Biol. Endocrinol. 2014, 12, 9. [Google Scholar] [CrossRef]
- Hafezi, M.; Madani, T.; Arabipoor, A.; Zolfaghari, Z.; Sadeghi, M.; Ramezanali, F. The effect of intrauterine human chorionic gonadotropin flushing on live birth rate after vitrified-warmed embryo transfer in programmed cycles: A randomized clinical trial. Arch. Gynecol. Obstet. 2018, 297, 1571–1576. [Google Scholar] [CrossRef] [PubMed]
- Wirleitner, B.; Schuff, M.; Vanderzwalmen, P.; Stecher, A.; Okhowat, J.; Hradecký, L.; Kohoutek, T.; Králícková, M.; Spitzer, D.; Zech, N.H. Intrauterine administration of human chorionic gonadotropin does not improve pregnancy and life birth rates independently of blastocyst quality: A randomised prospective study. Reprod. Biol. Endocrinol. 2015, 13, 70. [Google Scholar] [CrossRef] [PubMed]
- Bielfeld, A.P.; Pour, S.J.; Poschmann, G.; Stühler, K.; Krüssel, J.-S.; Baston-Büst, D.M. A Proteome Approach Reveals Differences between Fertile Women and Patients with Repeated Implantation Failure on Endometrial Level–Does hCG Render the Endometrium of RIF Patients? Int. J. Mol. Sci. 2019, 20, 425. [Google Scholar] [CrossRef] [PubMed]
- Fazleabas, A.T.; Donnelly, K.M.; Srinivasan, S.; Fortman, J.D.; Miller, J.B. Modulation of the baboon (Papio anubis) uterine endometrium by chorionic gonadotrophin during the period of uterine receptivity. Proc. Natl. Acad. Sci. USA 1999, 96, 2543–2548. [Google Scholar] [CrossRef]
- Uchida, H.; Maruyama, T.; Nishikawa-Uchida, S.; Miyazaki, K.; Masuda, H.; Yoshimura, Y. Glycodelin in reproduction. Reprod. Med. Biol. 2013, 12, 79–84. [Google Scholar] [CrossRef]
- Hosseinisadat, R.; Saeed, L.; Ashourzadeh, S.; Heidari, S.S.; Habibzadeh, V. Effects of human chorionic gonadotropin intrauterine injection on oocyte retrieval day on assisted reproductive techniques outcomes: An RCT. Int. J. Reprod. Biomed. 2021, 773–780. [Google Scholar] [CrossRef]
- Puget, C.; Joueidi, Y.; Bauville, E.; Laviolle, B.; Bendavid, C.; Lavoué, V.; Le Lous, M. Serial hCG and progesterone levels to predict early pregnancy outcomes in pregnancies of uncertain viability: A prospective study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 220, 100–105. [Google Scholar] [CrossRef]
- Kim, Y.J.; Shin, J.H.; Hur, J.Y.; Kim, H.; Ku, S.-Y.; Suh, C.S. Predictive value of serum progesterone level on β-hCG check day in women with previous repeated miscarriages after in vitro fertilization. PLoS ONE 2017, 12, e0181229. [Google Scholar] [CrossRef]
- Xiong, F.; Li, G.; Sun, Q.; Chen, P.; Wang, Z.; Wan, C.; Yao, Z.; Zhong, H.; Zeng, Y. Obstetric and perinatal outcomes of pregnancies according to initial maternal serum HCG concentrations after vitrified–warmed single blastocyst transfer. Reprod. Biomed. Online 2019, 38, 455–464. [Google Scholar] [CrossRef]
- Brady, P.C.; Farland, L.V.; Racowsky, C.; Ginsburg, E.S. Hyperglycosylated human chorionic gonadotropin as a predictor of ongoing pregnancy. Am. J. Obstet. Gynecol. 2020, 222, 68.e1–68.e12. [Google Scholar] [CrossRef]
- Pillai, R.N.; Konje, J.C.; Tincello, D.G.; Potdar, N. Role of serum biomarkers in the prediction of outcome in women with threatened miscarriage: A systematic review and diagnostic accuracy meta-analysis. Hum. Reprod. Updat. 2016, 22, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Morley, L.C.; Simpson, N.; Tang, T. Human chorionic gonadotrophin (hCG) for preventing miscarriage. Cochrane Database Syst. Rev. 2013. [Google Scholar] [CrossRef] [PubMed]
- Jayasena, C.N.; Abbara, A.; Izzi-Engbeaya, C.; Comninos, A.; Harvey, R.A.; Maffe, J.G.; Sarang, Z.; Ganiyu-Dada, Z.; Padilha, A.; Dhanjal, M.; et al. Reduced Levels of Plasma Kisspeptin During the Antenatal Booking Visit Are Associated With Increased Risk of Miscarriage. J. Clin. Endocrinol. Metab. 2014, 99, E2652–E2660. [Google Scholar] [CrossRef] [PubMed]
- Sha, J.; Liu, F.; Zhai, J.; Liu, X.; Zhang, Q.; Zhang, B. Alteration of Th17 and Foxp3+ regulatory T cells in patients with unexplained recurrent spontaneous abortion before and after the therapy of hCG combined with immunoglobulin. Exp. Ther. Med. 2017, 14, 1114–1118. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, C.; Yang, X.; Yang, S.; Meng, Y.; Liu, Z.; Peeters, R.P.; Huang, H.F.; Korevaar, T.I.M.; Fan, J. Association of Maternal Thyroid Function and Thyroidal Response to Human Chorionic Gonadotropin with Early Fetal Growth. Thyroid 2019, 29, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Liu, A.; Li, J.; Wang, H.; Yang, Y.; Li, Y.; Fan, C.; Zhang, H.; Wang, H.; Ding, S.; et al. Different Thyroidal Responses to Human Chorionic Gonadotropin Under Different Thyroid Peroxidase Antibody and/or Thyroglobulin Antibody Positivity Conditions During the First Half of Pregnancy. Thyroid 2019, 29, 577–585. [Google Scholar] [CrossRef]
- Korevaar, T.I.M.; Steegers, E.A.P.; Chaker, L.; Medici, M.; Jaddoe, V.W.V.; Visser, T.J.; De Rijke, Y.B.; Peeters, R.P. Thyroid Function and Premature Delivery in TPO Antibody−Negative Women: The Added Value of hCG. J. Clin. Endocrinol. Metab. 2017, 102, 3360–3367. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, F.; Maraka, S.; Zhang, Y.; Zhang, C.; Korevaar, T.I.M.; Fan, J. Associations between Human Chorionic Gonadotropin, Maternal Free Thyroxine, and Gestational Diabetes Mellitus. Thyroid 2021, 31, 1282–1288. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
d’Hauterive, S.P.; Close, R.; Gridelet, V.; Mawet, M.; Nisolle, M.; Geenen, V. Human Chorionic Gonadotropin and Early Embryogenesis: Review. Int. J. Mol. Sci. 2022, 23, 1380. https://doi.org/10.3390/ijms23031380
d’Hauterive SP, Close R, Gridelet V, Mawet M, Nisolle M, Geenen V. Human Chorionic Gonadotropin and Early Embryogenesis: Review. International Journal of Molecular Sciences. 2022; 23(3):1380. https://doi.org/10.3390/ijms23031380
Chicago/Turabian Styled’Hauterive, Sophie Perrier, Romann Close, Virginie Gridelet, Marie Mawet, Michelle Nisolle, and Vincent Geenen. 2022. "Human Chorionic Gonadotropin and Early Embryogenesis: Review" International Journal of Molecular Sciences 23, no. 3: 1380. https://doi.org/10.3390/ijms23031380
APA Styled’Hauterive, S. P., Close, R., Gridelet, V., Mawet, M., Nisolle, M., & Geenen, V. (2022). Human Chorionic Gonadotropin and Early Embryogenesis: Review. International Journal of Molecular Sciences, 23(3), 1380. https://doi.org/10.3390/ijms23031380





