Cellular and Molecular Nature of Fragmentation of Human Embryos
Abstract
1. Introduction
2. Results
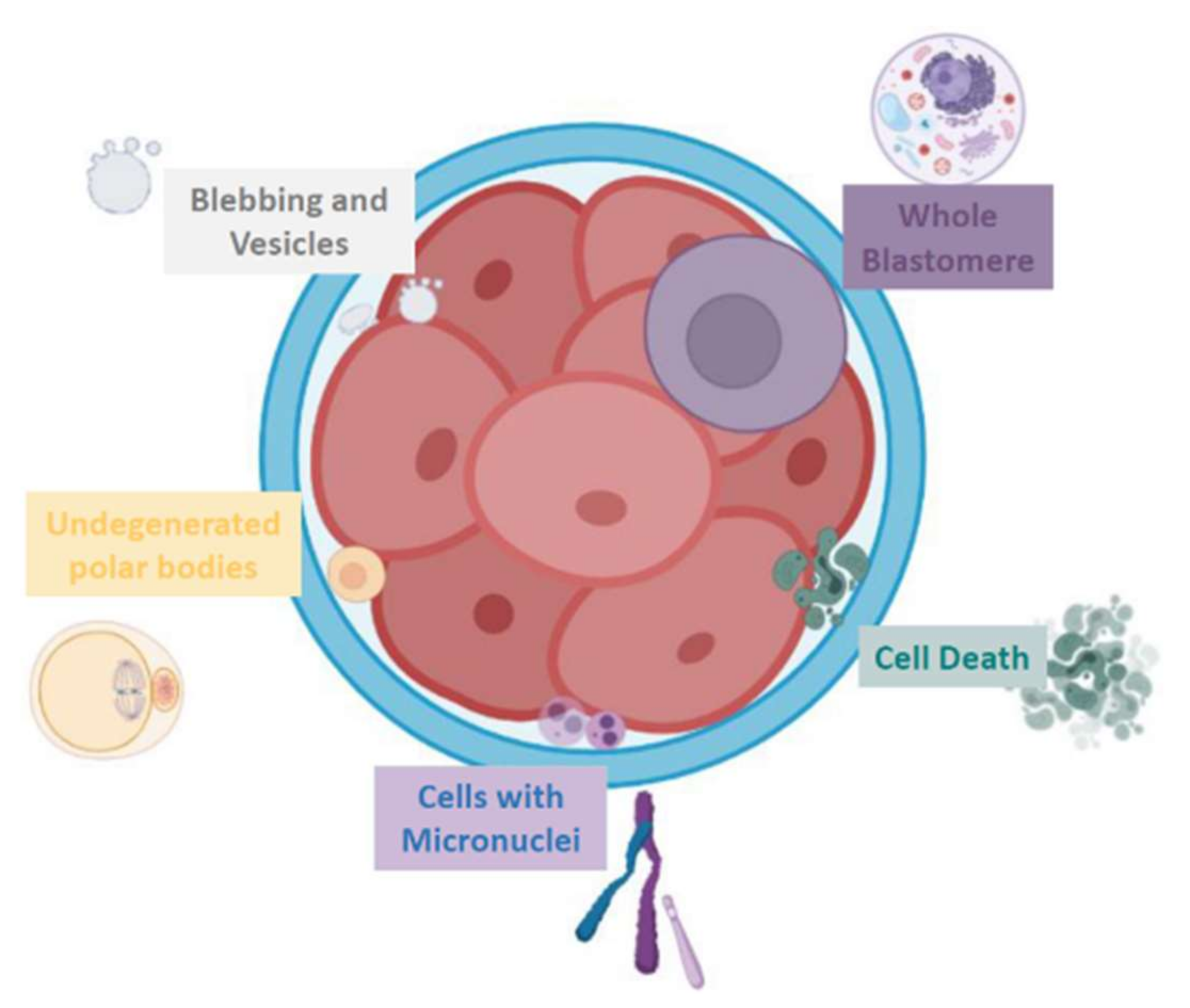
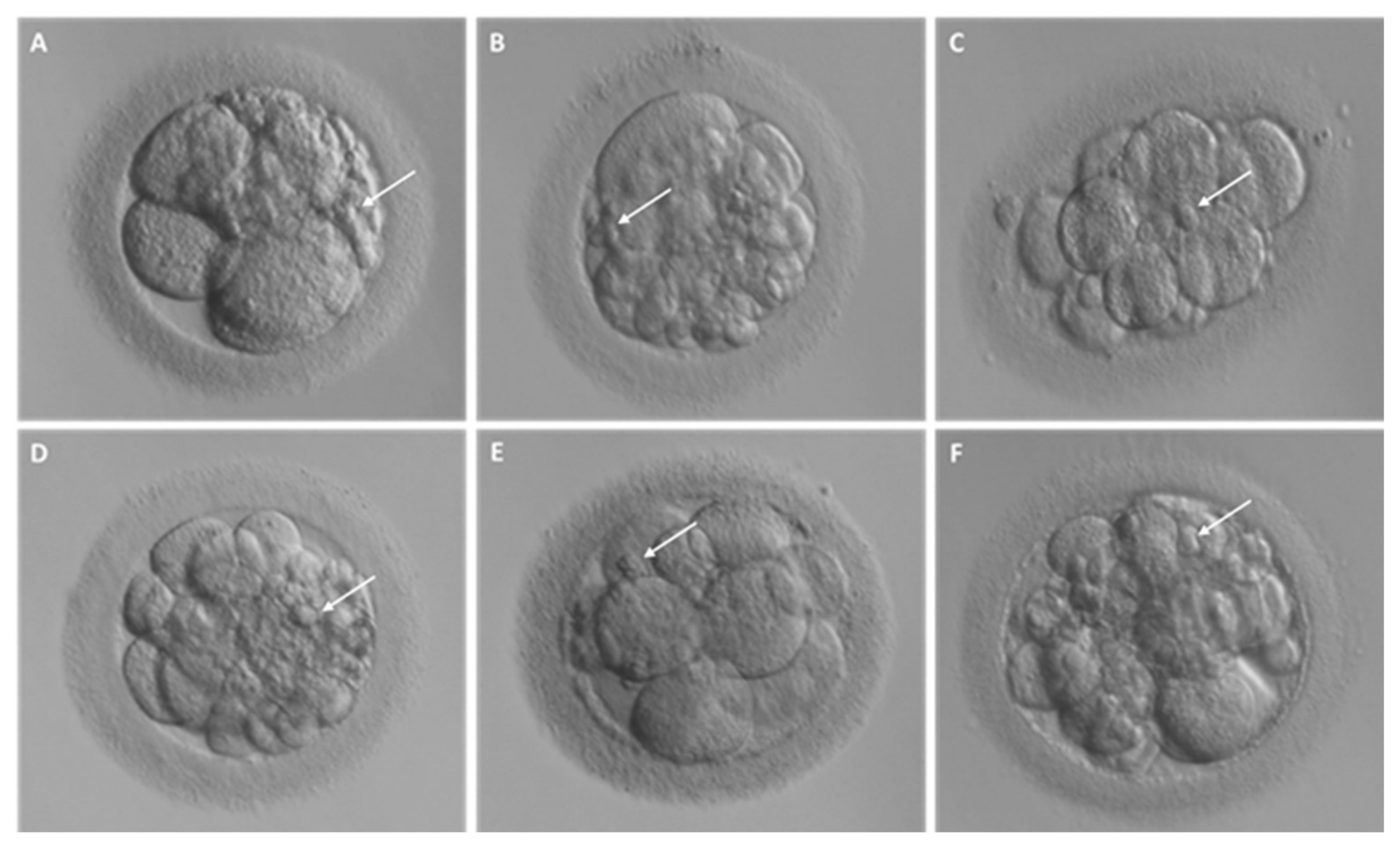
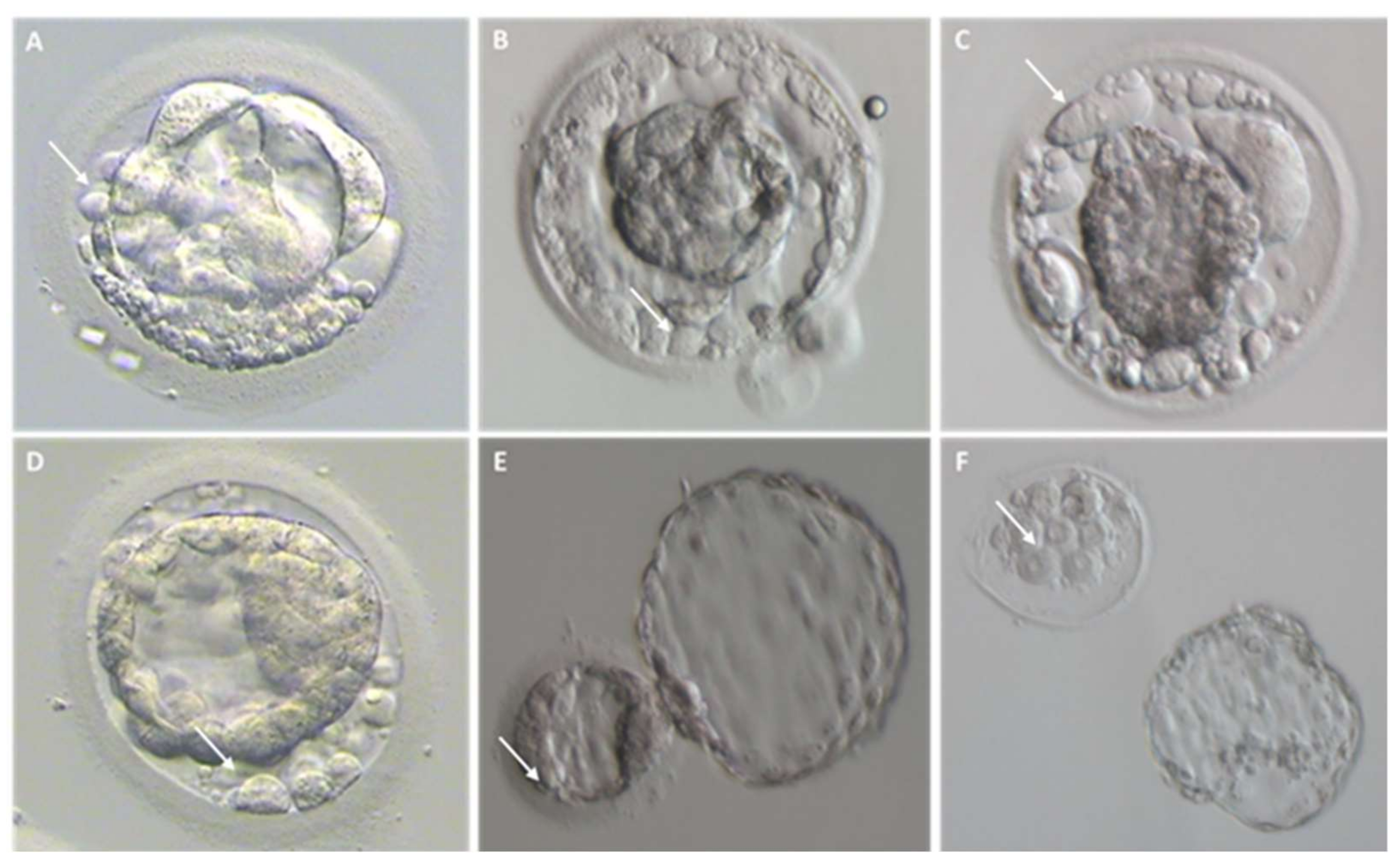
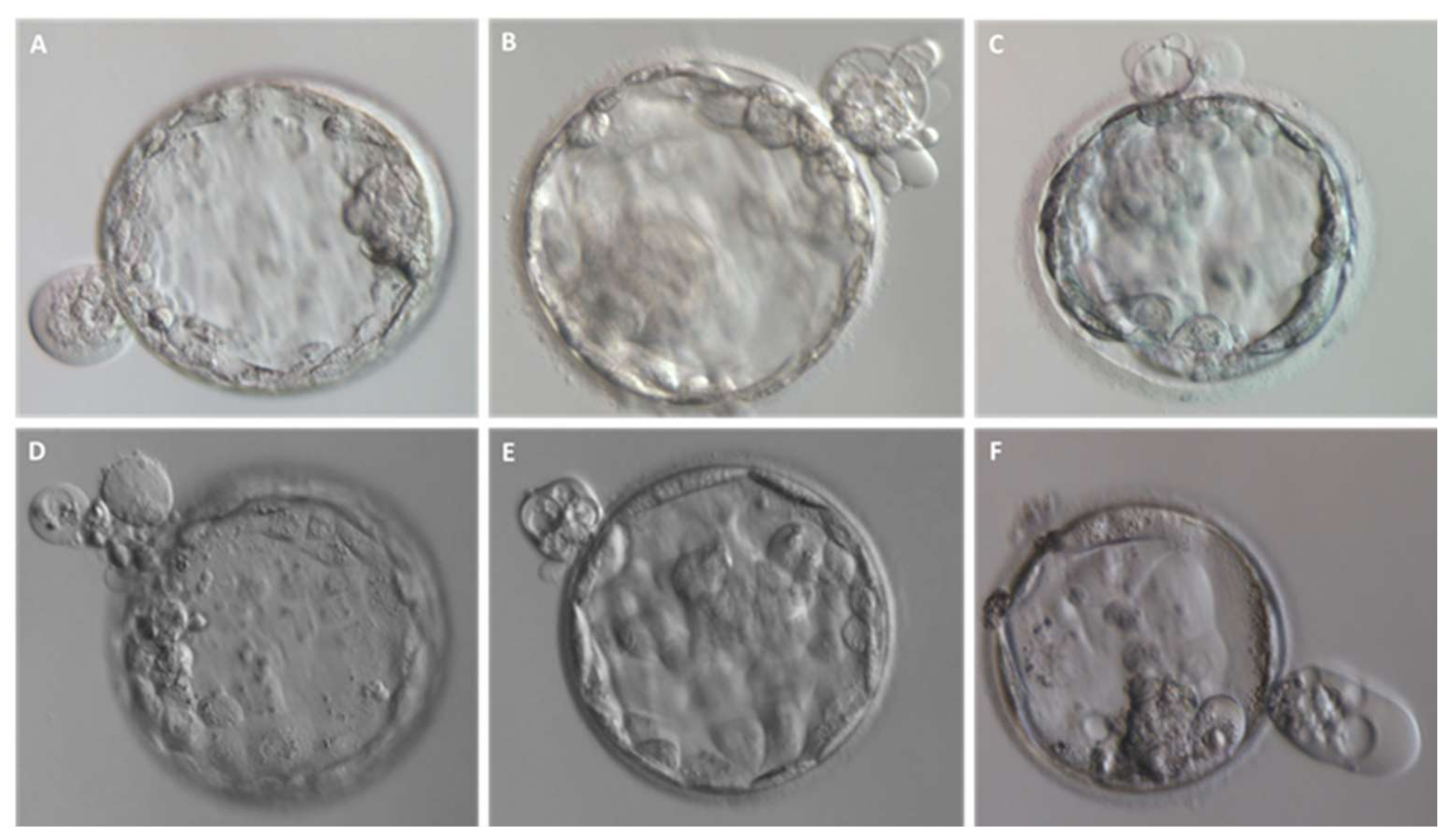
2.1. Different Fragment-like Cellular Structures: Characterization, Timing, and Cargo
- Fragment size
- Timing of cytoplasmic fragment formation
- Fragment cargo
The Different Origins Proposed for Embryo Fragment-like Entities
- Extruded blastomeres
- 2.
- Chromosome-containing micronuclei
- 3.
- Apoptotic bodies
- 4.
- Persisting polar bodies
- 5.
- Extracellular Vesicles
- 6.
- Others
- Mitochondria
- Perivitelline threads
2.2. Theories on the Origins over the Years
- Apoptotic cell death
- 2.
- Reactive oxygen species effect
- 3.
- Membrane compartmentalization of DNA
- 4.
- Abnormal cytokinesis and cytoskeletal disorder
- 5.
- Extracellular vesicle formation
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| ART | assisted reproduction technology |
| CENP-A | centromere protein-A |
| DAPI | 4′,6-diamidino-2-phenylindole |
| EV | extracellular vesicles |
| ICM | inner cell mass |
| ILV | intraluminal vesicle |
| MVB | multivesicular body |
| PB | polar body |
| PI | propidium iodide |
| PVT | perivitelline thread |
| ROS | reactive oxygen species |
| SCNT | somatic cell nuclear transfer |
| TEM | transmission electron microscopy |
| TLM | time-lapse microscopy |
| TUNEL | terminal deoxynucleotidyl transferase dUTP nick end labeling |
| ZP | zona pellucida |
References
- Trounson, A.; Sathananthan, A.H. The application of electron microscopy in the evaluation of two—to four-cell human embryos cultured in vitro for embryo transfer. J. Vitr. Fertil. Embryo Transf. 1984, 1, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Pereda, J.; Croxatto, H.B. Ultrastructure of a Seven-Cell Human Embryo. Biol. Reprod. 1978, 18, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Van Blerkom, J.; Davis, P.; Alexander, S. A microscopic and biochemical study of fragmentation phenotypes in stage-appropriate human embryos. Hum. Reprod. 2001, 16, 719–729. [Google Scholar] [CrossRef]
- Kim, S.-G.; Kim, Y.-Y.; Park, J.-Y.; Kwak, S.-J.; Yoo, C.-S.; Park, I.-H.; Sun, H.-G.; Kim, J.-W.; Lee, K.-H.; Park, H.-D.; et al. Early fragment removal on in vitro fertilization day 2 significantly improves the subsequent development and clinical outcomes of fragmented human embryos. Clin. Exp. Reprod. Med. 2018, 45, 122–128. [Google Scholar] [CrossRef]
- Hardarson, T.; Löfman, C.; Coull, G.; Sjögren, A.; Hamberger, L.; Edwards, R. Internalization of cellular fragments in a human embryo: Time-lapse recordings. Reprod. Biomed. Online 2002, 5, 36–38. [Google Scholar] [CrossRef]
- Johansson, M.; Hardarson, T.; Lundin, K. There Is a Cutoff Limit in Diameter Between a Blastomere and a Small Anucleate Fragment. J. Assist. Reprod. Genet. 2003, 20, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Barile, L.; Vassalli, G. Exosomes: Therapy delivery tools and biomarkers of diseases. Pharmacol. Ther. 2017, 174, 63–78. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Giacomini, E.; Vago, R.; Sanchez, A.M.; Podini, P.; Zarovni, N.; Murdica, V.; Rizzo, R.; Bortolotti, D.; Candiani, M.; Viganã, P. Secretome of in vitro cultured human embryos contains extracellular vesicles that are uptaken by the maternal side. Sci. Rep. 2017, 7, 5210. [Google Scholar] [CrossRef]
- Borges, F.T.; Reis, L.A.; Schor, N. Extracellular vesicles: Structure, function, and potential clinical uses in renal diseases. Braz. J. Med. Biol. Res. 2013, 46, 824–830. [Google Scholar] [CrossRef]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [PubMed]
- Yáñez-Mó, M.; Siljander, P.R.-M.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed]
- Alikani, M. Epithelial cadherin distribution in abnormal human pre-implantation embryos. Hum. Reprod. 2005, 20, 3369–3375. [Google Scholar] [CrossRef] [PubMed]
- Lagalla, C.; Coticchio, G.; Sciajno, R.; Tarozzi, N.; Zacà, C.; Borini, A. Alternative patterns of partial embryo compaction: Prevalence, morphokinetic history and possible implications. Reprod. Biomed. Online 2020, 40, 347–354. [Google Scholar] [CrossRef]
- Giacomini, E.; Alleva, E.; Fornelli, G.; Quartucci, A.; Privitera, L.; Vanni, V.S.; Viganò, P. Embryonic extracellular vesicles as informers to the immune cells at the maternal–fetal interface. Clin. Exp. Immunol. 2019, 198, 15–23. [Google Scholar] [CrossRef]
- Vyas, P.; Balakier, H.; Librach, C. Ultrastructural identification of CD9 positive extracellular vesicles released from human embryos and transported through the zona pellucida. Syst. Biol. Reprod. Med. 2019, 65, 273–280. [Google Scholar] [CrossRef]
- Chi, H.-J.; Koo, J.-J.; Choi, S.-Y.; Jeong, H.-J.; Roh, S.-I. Fragmentation of embryos is associated with both necrosis and apoptosis. Fertil. Steril. 2011, 96, 187–192. [Google Scholar] [CrossRef]
- Halvaei, I.; Khalili, M.A.; Esfandiari, N.; Safari, S.; Talebi, A.R.; Miglietta, S.; Nottola, S.A. Ultrastructure of cytoplasmic fragments in human cleavage stage embryos. J. Assist. Reprod. Genet. 2016, 33, 1677–1684. [Google Scholar] [CrossRef] [PubMed]
- Chavez, S.; Loewke, K.E.; Han, J.; Moussavi, F.; Colls, P.; Munne, S.; Behr, B.; Pera, R.A.R. Dynamic blastomere behaviour reflects human embryo ploidy by the four-cell stage. Nat. Commun. 2012, 3, 1251. [Google Scholar] [CrossRef]
- Daughtry, B.L.; Chavez, S.L. Time-Lapse Imaging for the Detection of Chromosomal Abnormalities in Primate Preimplantation Embryos. Methods Mol. Biol. 2018, 1769, 293–317. [Google Scholar]
- Calarco, P.G.; Pedersen, R.A. Ultrastructural observations of lethal yellow (Ay/Ay) mouse embryos. J. Embryol. Exp. Morphol. 1976, 35, 73–80. [Google Scholar] [CrossRef]
- Lindner, G.M.; Wright, R.W. Bovine embryo morphology and evaluation. Theriogenology 1983, 20, 407–416. [Google Scholar] [CrossRef]
- Alikani, M.; Cohen, J.; Tomkin, G.; Garrisi, G.J.; Mack, C.; Scott, R.T. Human embryo fragmentation in vitro and its implications for pregnancy and im-plantation. Fertil. Steril. 1999, 71, 836–842. [Google Scholar] [CrossRef]
- Alikani, M.; Willadsen, S.M. Human blastocysts from aggregated mononucleated cells of two or more non-viable zygote-derived embryos. Reprod. Biomed. Online 2002, 5, 56–58. [Google Scholar] [CrossRef]
- Hardy, K. Apoptosis in the human embryo. Rev. Reprod. 1999, 4, 125–134. [Google Scholar] [CrossRef]
- Van Soom, A.; Mateusen, B.; Leroy, J.; de Kruif, A. Assessment of mammalian embryo quality: What can we learn from embryo morphology? Reprod. Biomed. Online 2003, 7, 664–670. [Google Scholar] [CrossRef]
- Prados, F.J.; Debrock, S.; Lemmen, J.G.; Agerholm, I. The cleavage stage embryo. Hum. Reprod. 2012, 27, i50–i71. [Google Scholar] [CrossRef] [PubMed]
- Chavez, S.L.; McElroy, S.L.; Bossert, N.L.; De Jonge, C.J.; Rodriguez, M.V.; Leong, D.E.; Behr, B.; Westphal, L.M.; Pera, R.A.R. Comparison of epigenetic mediator expression and function in mouse and human embryonic blastomeres. Hum. Mol. Genet. 2014, 23, 4970–4984. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Watson, A.J. The cell biology of blastocyst development. Mol. Reprod. Dev. 1992, 33, 492–504. [Google Scholar] [CrossRef]
- Eftekhari-Yazdi, P.; Valojerdi, M.R.; Ashtiani, S.K.; Eslaminejad, M.B.; Karimian, L. Effect of fragment removal on blastocyst formation and quality of human embryos. Reprod. Biomed. Online 2006, 13, 823–832. [Google Scholar] [CrossRef]
- Keltz, M.D.; Skorupski, J.C.; Bradley, K.; Stein, D. Predictors of embryo fragmentation and outcome after fragment removal in in vitro fertilization. Fertil. Steril. 2006, 86, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Luzhna, L.; Kathiria, P.; Kovalchuk, O. Micronuclei in genotoxicity assessment: From genetics to epigenetics and beyond. Front. Genet. 2013, 4, 131. [Google Scholar] [CrossRef] [PubMed]
- Sedelnikova, O.A.; Nakamura, A.; Kovalchuk, O.; Koturbash, I.; Mitchell, S.A.; Marino, S.A.; Brenner, D.J.; Bonner, W.M. DNA Double-Strand Breaks Form in Bystander Cells after Microbeam Irradiation of Three-dimensional Human Tissue Models. Cancer Res. 2007, 67, 4295–4302. [Google Scholar] [CrossRef] [PubMed]
- Fenech, M.; Kirsch-Volders, M.; Natarajan, A.T.; Surralles, J.; Crott, J.W.; Parry, J.; Norppa, H.; Eastmond, D.A.; Tucker, J.D.; Thomas, P. Molecular mechanisms of micronucleus, nucleoplasmic bridge and nuclear bud formation in mammalian and human cells. Mutagenesis 2010, 26, 125–132. [Google Scholar] [CrossRef]
- Mohr, L.R.; Trounson, A.O. Comparative ultrastructure of hatched human, mouse and bovine blastocysts. J. Reprod. Fertil. 1982, 66, 499–504. [Google Scholar] [CrossRef]
- Hurst, P.R.; Jefferies, K.; Eckstein, P.; Wheeler, A.G. An ultrastructural study of preimplantation uterine embryos of the rhesus monkey. J. Anat. 1978, 126, 209–220. [Google Scholar]
- Blander, J.M. The many ways tissue phagocytes respond to dying cells. Immunol. Rev. 2017, 277, 158–173. [Google Scholar] [CrossRef]
- Li, Y.; Xu, J.; Zhou, C.-Q.; Zhang, C.-L.; Zhuang, G.-L. Nonprofessional phagocytosis in trophectoderm cells of human preimplantation blastocysts. Syst. Biol. Reprod. Med. 2016, 62, 243–248. [Google Scholar] [CrossRef][Green Version]
- Pisko, J.; Špirková, A.; Čikoš, Š.; Olexiková, L.; Kovaříková, V.; Šefčíková, Z.; Fabian, D. Apoptotic cells in mouse blastocysts are eliminated by neighbouring blastomeres. Sci. Rep. 2021, 11, 9228. [Google Scholar] [CrossRef]
- Longo, F.J. Fertilization; Chapman & Hall: New York, NY, USA, 1997. [Google Scholar]
- Ottolini, C.S.; Rogers, S.; Sage, K.; Summers, M.C.; Capalbo, A.; Griffin, D.K.; Sarasa, J.; Wells, D.; Handyside, A.H. Karyomapping identifies second polar body DNA persisting to the blastocyst stage: Implications for embryo biopsy. Reprod. Biomed. Online 2015, 31, 776–782. [Google Scholar] [CrossRef]
- Battaglia, R.; Palini, S.; Vento, M.E.; La Ferlita, A.; Faro, M.J.L.; Caroppo, E.; Borzì, P.; Falzone, L.; Barbagallo, D.; Ragusa, M.; et al. Identification of extracellular vesicles and characterization of miRNA expression profiles in human blastocoel fluid. Sci. Rep. 2019, 9, 84. [Google Scholar] [CrossRef] [PubMed]
- DesRochers, L.M.; Bordeleau, F.; Reinhart-King, C.A.; Cerione, R.A.; Antonyak, M.A. Microvesicles provide a mechanism for intercellular communication by embryonic stem cells during embryo implantation. Nat. Commun. 2016, 7, 11958. [Google Scholar] [CrossRef] [PubMed]
- Wilding, M.; Dale, B.; Marino, M.; Di Matteo, L.; Alviggi, C.; Pisaturo, M.L.; Lombardi, L.; De Placido, G. Mitochondrial aggregation patterns and activity in human oocytes and preimplantation embryos. Hum. Reprod. 2001, 16, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Van Blerkom, J.; Davis, P.W.; Lee, J. ATP content of human oocytes and developmental potential and outcome after in-vitro fertilization and embryo transfer. Hum. Reprod. 1995, 10, 415–424. [Google Scholar] [CrossRef]
- Gilula, N.B.; Epstein, M.L.; Beers, W.H. Cell-to-cell communication and ovulation. A study of the cumulus-oocyte complex. J. Cell Biol. 1978, 78, 58–75. [Google Scholar] [CrossRef]
- Li, R.; Albertini, D.F. The road to maturation: Somatic cell interaction and self-organization of the mammalian oocyte. Nat. Rev. Mol. Cell Biol. 2013, 14, 141–152. [Google Scholar] [CrossRef]
- Derrick, R.; Hickman, C.; Oliana, O.; Wilkinson, T.; Gwinnett, D.; Whyte, L.B.; Carby, A.; Lavery, S. Perivitelline threads associated with fragments in human cleavage stage embryos observed through time-lapse microscopy. Reprod. Biomed. Online 2017, 35, 640–645. [Google Scholar] [CrossRef]
- Fujimoto, V.Y.; Browne, R.W.; Bloom, M.S.; Sakkas, D.; Alikani, M. Pathogenesis, developmental consequences, and clinical correlations of human embryo fragmentation. Fertil. Steril. 2011, 95, 1197–1204. [Google Scholar] [CrossRef]
- Munné, S.; Grifo, J.; Cohen, J.; Weier, H.U. Chromosome abnormalities in human arrested preimplantation embryos: A multi-ple-probe FISH study. Am. J. Hum. Genet. 1994, 55, 150–159. [Google Scholar]
- Vanneste, E.; Voet, T.; Le Caignec, C.; Ampe, M.; Konings, P.; Melotte, C.; Debrock, S.; Amyere, M.; Vikkula, M.; Schuit, F.; et al. Chromosome instability is common in human cleavage-stage embryos. Nat. Med. 2009, 15, 577–583. [Google Scholar] [CrossRef]
- Johnson, D.S.; Cinnioglu, C.; Ross, R.; Filby, A.; Gemelos, G.; Hill, M.; Ryan, A.; Smotrich, D.; Rabinowitz, M.; Murray, M.J. Comprehensive analysis of karyotypic mosaicism between trophectoderm and inner cell mass. Mol. Hum. Reprod. 2010, 16, 944–949. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.F.; Yeung, W.S.; Lau, E.Y.; Lee, V.C.; Ng, E.H.; Ho, P.-C. Array comparative genomic hybridization analyses of all blastomeres of a cohort of embryos from young IVF patients revealed significant contribution of mitotic errors to embryo mosaicism at the cleavage stage. Reprod. Biol. Endocrinol. 2014, 12, 105. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yan, L.; Fan, W.; Zhao, N.; Zhang, Y.; Tang, F.; Xie, X.S.; Qiao, J. Validation of multiple annealing and looping-based amplification cycle sequencing for 24-chromosome aneuploidy screening of cleavage-stage embryos. Fertil. Steril. 2014, 102, 1685–1691. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.-C.; Ogilvie, C.M.; Handyside, A.H. Chromosomal Mosaicism in Cleavage-Stage Human Embryos and the Accuracy of Single-Cell Genetic Analysis. J. Assist. Reprod. Genet. 1998, 15, 276–280. [Google Scholar] [CrossRef]
- Baart, E.; Martini, E.; Berg, I.V.D.; Macklon, N.; Galjaard, R.-J.; Fauser, B.; Van Opsta, D. Preimplantation genetic screening reveals a high incidence of aneuploidy and mosaicism in embryos from young women undergoing IVF. Hum. Reprod. 2005, 21, 223–233. [Google Scholar] [CrossRef]
- Munné, S.; Velilla, E.; Colls, P.; Bermúdez, M.G.; Vemuri, M.C.; Steuerwald, N.; Garrisi, J.; Cohen, J. Self-correction of chromosomally abnormal embryos in culture and implications for stem cell production. Fertil. Steril. 2005, 84, 1328–1334. [Google Scholar] [CrossRef]
- Barbash-Hazan, S.; Frumkin, T.; Malcov, M.; Yaron, Y.; Cohen, T.; Azem, F.; Amit, A.; Ben-Yosef, D. Preimplantation aneuploid embryos undergo self-correction in correlation with their developmental potential. Fertil. Steril. 2009, 92, 890–896. [Google Scholar] [CrossRef]
- Pellestor, F. Chromothripsis: How does such a catastrophic event impact human reproduction? Hum. Reprod. 2014, 29, 388–393. [Google Scholar] [CrossRef]
- Pellestor, F.; Girardet, A.; Andréo, B.; Arnal, F.; Humeau, C. Relationship between morphology and chromosomal constitution in human preimplantation embryo. Mol. Reprod. Dev. 1994, 39, 141–146. [Google Scholar] [CrossRef]
- Munné, S.; Alikani, M.; Tomkin, G.; Grifo, J.; Cohen, J. Embryo morphology, developmental rates, and maternal age are correlated with chromosome abnormalities. Fertil. Steril. 1995, 64, 382–391. [Google Scholar] [CrossRef]
- Munne, S. Chromosome abnormalities in human embryos. Hum. Reprod. Updat. 1998, 4, 842–855. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Apoptosis: Mechanisms of life and death in the immune system. J. Allergy Clin. Immunol. 1999, 103, 548–554. [Google Scholar] [CrossRef]
- Maderna, P.; Godson, C. Phagocytosis of apoptotic cells and the resolution of inflammation. Biochim Biophys Acta. 2003, 1639, 141–151. [Google Scholar] [CrossRef]
- Martin, S.; Reutelingsperger, C.E.M.; McGahon, A.J.; Rader, J.; Van Schie, R.C.A.A.; LaFace, D.M.; Green, D. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: Inhibition by overexpression of Bcl-2 and Abl. J. Exp. Med. 1995, 182, 1545–1556. [Google Scholar] [CrossRef] [PubMed]
- Koopman, G.; Reutelingsperger, C.P.; Kuijten, G.A.; Keehnen, R.M.; Pals, S.T.; van Oers, M.H. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood 1994, 84, 1415–1420. [Google Scholar] [CrossRef] [PubMed]
- Gavrieli, Y.; Sherman, Y.; Ben-Sasson, S.A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J. Cell Biol. 1992, 119, 493–501. [Google Scholar] [CrossRef]
- Yang, H.W.; Hwang, K.J.; Kwon, H.C.; Kim, H.S.; Choi, K.W.; Oh, K.S. Detection of reactive oxygen species (ROS) and apoptosis in human fragmented embryos. Hum. Reprod. 1998, 13, 998–1002. [Google Scholar] [CrossRef]
- Antczak, M.; Van Blerkom, J. Temporal and spatial aspects of fragmentation in early human embryos: Possible effects on de-velopmental competence and association with the differential elimination of regulatory proteins from polarized domains. Hum. Reprod. 1999, 14, 429–447. [Google Scholar] [CrossRef]
- Levy, R.; Benchaib, M.; Cordonier, H.; Souchier, C.; Guerin, J.F. Annexin V labelling and terminal transferase-mediated DNA end labelling (TUNEL) assay in human arrested embryos. Mol. Hum. Reprod. 1998, 4, 775–783. [Google Scholar] [CrossRef]
- Jurisicova, A.; Varmuza, S.; Casper, R. Programmed cell death and human embryo fragmentation. Mol. Hum. Reprod. 1996, 2, 93–98. [Google Scholar] [CrossRef]
- Jurisicova, A.; Antenos, M.; Varmuza, S.; Tilly, J.L.; Casper, R.F. Expression of apoptosis-related genes during human preimplantation embryo development: Potential roles for the Harakiri gene product and Caspase-3 in blastomere fragmentation. Mol. Hum. Reprod. 2003, 9, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.; Rienzi, L.; Iacobelli, M.; Ubaldi, F.; Mendoza, C.; Greco, E.; Tesarik, J. Caspase activity in preimplantation human embryos is not associated with apoptosis. Hum. Reprod. 2002, 17, 1584–1590. [Google Scholar] [CrossRef] [PubMed]
- Hardy, K.; Spanos, S.; Becker, D.; Iannelli, P.; Winston, R.M.L.; Stark, J. From cell death to embryo arrest: Mathematical models of human preimplantation embryo development. Proc. Natl. Acad. Sci. USA 2001, 98, 1655–1660. [Google Scholar] [CrossRef] [PubMed]
- Dale, B.; Gualtieri, R.; Talevi, R.; Tosti, E.; Santella, L.; Elder, K. Intercellular communication in the early human embryo. Mol. Reprod. Dev. 1991, 29, 22–28. [Google Scholar] [CrossRef]
- Hardy, K.; Warner, A.; Winston, R.M.; Becker, D.L. Expression of intercellular junctions during preimplantation development of the human embryo. Mol. Hum. Reprod. 1996, 2, 621–632. [Google Scholar] [CrossRef]
- Mantikou, E.; Wong, K.M.; Repping, S.; Mastenbroek, S. Molecular origin of mitotic aneuploidies in preimplantation embryos. Biochim. Biophys. Acta 2012, 1822, 1921–1930. [Google Scholar] [CrossRef]
- Santos, M.A.; Teklenburg, G.; Macklon, N.S.; Van Opstal, D.; Schuring-Blom, G.H.; Krijtenburg, P.-J.; de Vreeden-Elbertse, J.; Fauser, B.C.; Baart, E.B. The fate of the mosaic embryo: Chromosomal constitution and development of Day 4, 5 and 8 human embryos. Hum. Reprod. 2010, 25, 1916–1926. [Google Scholar] [CrossRef]
- Athavale, D.M.; Barré, A.; Kranyak, A.C.; Lal, A.; Blalock, J.L.; Zimmerman, S.; Chang, T.A.; Robinson, R.D.; Wininger, J.D.; Roudebush, W.E.; et al. Pro-apoptotic gene expression in blastocoel fluid from euploid day-5 embryos is associated with negative pregnancy outcomes. Fertil. Steril. 2019, 112, e261. [Google Scholar] [CrossRef]
- Rule, K.; Chosed, R.; Chang, T.A.; Wininger, J.D.; Roudebush, W.E. Relationship between blastocoel cell-free DNA and day-5 blastocyst morphology. J. Assist. Reprod. Genet. 2018, 35, 1497–1501. [Google Scholar] [CrossRef]
- Goto, Y.; Noda, Y.; Mori, T.; Nakano, M. Increased generation of reactive oxygen species in embryos cultured in vitro. Free. Radic. Biol. Med. 1993, 15, 69–75. [Google Scholar] [CrossRef]
- Agarwal, A.; Saleh, R.; Bedaiwy, M. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil. Steril. 2003, 79, 829–843. [Google Scholar] [CrossRef]
- Guérin, P.; El Mouatassim, S.; Ménézo, Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum. Reprod. Update 2001, 7, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Bedaiwy, M.A.; Falcone, T.; Mohamed, M.S.; Aleem, A.A.N.; Sharma, R.K.; Worley, S.E.; Thornton, J.; Agarwal, A. Differential growth of human embryos in vitro: Role of reactive oxygen species. Fertil. Steril. 2004, 82, 593–600. [Google Scholar] [CrossRef]
- Bedaiwy, M.A.; Mahfouz, R.Z.; Goldberg, J.M.; Sharma, R.; Falcone, T.; Hafez, M.F.A.; Agarwal, A. Relationship of reactive oxygen species levels in day 3 culture media to the outcome of in vitro fertilization/intracytoplasmic sperm injection cycles. Fertil. Steril. 2010, 94, 2037–2042. [Google Scholar] [CrossRef]
- Lee, T.-H.; Lee, M.-S.; Liu, C.-H.; Tsao, H.-M.; Huang, C.-C.; Yang, Y.-S. The Association Between Microenvironmental Reactive Oxygen Species and Embryo Development in Assisted Reproduction Technology Cycles. Reprod. Sci. 2012, 19, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Lan, K.-C.; Lin, Y.-C.; Chang, Y.-C.; Lin, H.-J.; Tsai, Y.-R.; Kang, H.-Y. Limited relationships between reactive oxygen species levels in culture media and zygote and embryo development. J. Assist. Reprod. Genet. 2019, 36, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Daughtry, B.L.; Rosenkrantz, J.L.; Lazar, N.H.; Fei, S.S.; Redmayne, N.; Torkenczy, K.A.; Adey, A.; Yan, M.; Gao, L.; Park, B.; et al. Single-cell sequencing of primate preimplantation embryos reveals chromosome elimination via cellular fragmentation and blastomere exclusion. Genome Res. 2019, 29, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Lemmen, J.; Agerholm, I.; Ziebe, S. Kinetic markers of human embryo quality using time-lapse recordings of IVF/ICSI-fertilized oocytes. Reprod. Biomed. Online 2008, 17, 385–391. [Google Scholar] [CrossRef]
- Stensen, M.H.; Tanbo, T.G.; Storeng, R.; Åbyholm, T.; Fedorcsak, P. Fragmentation of human cleavage-stage embryos is related to the progression through meiotic and mitotic cell cycles. Fertil. Steril. 2015, 103, 374–381. [Google Scholar] [CrossRef]
- Hassold, T.; Hunt, P. To err (meiotically) is human: The genesis of human aneuploidy. Nat. Rev. Genet. 2001, 2, 280–291. [Google Scholar] [CrossRef]
- Magli, M.C.; Capoti, A.; Resta, S.; Stanghellini, I.; Ferraretti, A.P.; Gianaroli, L. Prolonged absence of meiotic spindles by birefringence imaging negatively affects normal fertilization and embryo development. Reprod. Biomed. Online 2011, 23, 747–754. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Magli, M.C.; Ferraretti, A.P.; Crippa, A.; Lappi, M.; Feliciani, E.; Gianaroli, L. First meiosis errors in immature oocytes generated by stimulated cycles. Fertil. Steril. 2006, 86, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Minciacchi, V.R.; Freeman, M.R.; Di Vizio, D. Extracellular Vesicles in Cancer: Exosomes, Microvesicles and the Emerging Role of Large Oncosomes. Semin. Cell Dev. Biol. 2015, 40, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Teng, F.; Fussenegger, M. Shedding Light on Extracellular Vesicle Biogenesis and Bioengineering. Adv. Sci. 2021, 8, 202003505. [Google Scholar] [CrossRef]
- Trajkovic, K.; Hsu, C.; Chiantia, S.; Rajendran, L.; Wenzel, D.; Wieland, F.; Schwille, P.; Brügger, B.; Simons, M. Ceramide Triggers Budding of Exosome Vesicles into Multivesicular Endosomes. Science 2008, 319, 1244–1247. [Google Scholar] [CrossRef]
- Murphy, D.E.; De Jong, O.G.; Brouwer, M.; Wood, M.J.; Lavieu, G.; Schiffelers, R.M.; Vader, P. Extracellular vesicle-based therapeutics: Natural versus engineered targeting and trafficking. Exp. Mol. Med. 2019, 51, 1–12. [Google Scholar] [CrossRef]
- Muralidharan-Chari, V.; Clancy, J.; Plou, C.; Romao, M.; Chavrier, P.; Raposo, G.; D’Souza-Schorey, C. ARF6-Regulated Shedding of Tumor Cell-Derived Plasma Membrane Microvesicles. Curr. Biol. 2009, 19, 1875–1885. [Google Scholar] [CrossRef]
- Giacomini, E.; Makieva, S.; Murdica, V.; Vago, R.; Viganó, P. Extracellular vesicles as a potential diagnostic tool in assisted reproduction. Curr. Opin. Obstet. Gynecol. 2020, 32, 179–184. [Google Scholar] [CrossRef]
- Aleksejeva, E.; Zarovni, N.; Dissanayake, K.; Godakumara, K.; Vigano, P.; Fazeli, A.; Jaakma, Ü.; Salumets, A. Extracellular vesicle research in reproductive science—Paving the way for clinical achievements. Biol. Reprod. 2022. [Google Scholar] [CrossRef]
- Giacomini, E.; Scotti, G.M.; Vanni, V.S.; Lazarevic, D.; Makieva, S.; Privitera, L.; Signorelli, S.; Cantone, L.; Bollati, V.; Murdica, V.; et al. Global transcriptomic changes occur in uterine fluid-derived extracellular vesicles during the endometrial window for embryo implantation. Hum. Reprod. 2021, 36, 2249–2274. [Google Scholar] [CrossRef]
- Abu-Halima, M.; Häusler, S.; Backes, C.; Fehlmann, T.; Staib, C.; Nestel, S.; Nazarenko, I.; Meese, E.; Keller, A. Micro-ribonucleic acids and extracellular vesicles repertoire in the spent culture media is altered in women undergoing In Vitro Fertilization. Sci. Rep. 2017, 7, 13525. [Google Scholar] [CrossRef]
- Kovács Árpád, F.; Fekete, N.; Turiák, L.; Ács, A.; Kőhidai, L.; Buzás, E.I.; Pállinger, É. Unravelling the Role of Trophoblastic-Derived Extracellular Vesicles in Regulatory T Cell Differentiation. Int. J. Mol. Sci. 2019, 20, 3457. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, K.; Nõmm, M.; Lättekivi, F.; Ressaissi, Y.; Godakumara, K.; Lavrits, A.; Midekessa, G.; Viil, J.; Bæk, R.; Jørgensen, M.M.; et al. Individually cultured bovine embryos produce extracellular vesicles that have the potential to be used as non-invasive embryo quality markers. Theriogenology 2020, 149, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Mellisho, E.; Briones, M.A.; Velasquez, A.E.; Cabezas, J.; Castro, F.O.; Rodriguez-Alvarez, L. Extracellular vesicles secreted during blastulation show viability of bovine embryos. Reproduction 2019, 158, 477–492. [Google Scholar] [CrossRef] [PubMed]
- Boiso, I.; Veiga, A.; Edwards, R.G. Fundamentals of human embryonic growth in vitro and the selection of high-quality embryos for transfer. Reprod. Biomed. Online 2002, 5, 328–350. [Google Scholar] [CrossRef]
- Borini, A.; Lagalla, C.; Cattoli, M.; Sereni, E.; Sciajno, R.; Flamigni, C.; Coticchio, G. Predictive factors for embryo implantation potential. Reprod. Biomed. Online 2005, 10, 653–668. [Google Scholar] [CrossRef]
- Giorgetti, C.; Terriou, P.; Auquier, P.; Hans, E.; Spach, J.-L.; Salzmann, J.; Roulier, R. Implantation: Embryo score to predict implantation after in-vitro fertilization: Based on 957 single embryo transfers. Hum. Reprod. 1995, 10, 2427–2431. [Google Scholar] [CrossRef]
- Staessen, C.; Camus, M.; Bollen, N.; Devroey, P.; Van Steirteghem, A.C. The relationship between embryo quality and the occurrence of multiple pregnancies. Fertil. Steril. 1992, 57, 626–630. [Google Scholar] [CrossRef]
- Urman, B.; Yakin, K.; Balaban, B. Recurrent implantation failure in assisted reproduction: How to counsel and manage. B. Treatment options that have not been proven to benefit the couple. Reprod. Biomed. Online 2005, 11, 382–391. [Google Scholar] [CrossRef]
- Sordia-Hernandez, L.H.; Morales-Martinez, F.A.; Frazer-Moreira, L.M.; Villarreal-Pineda, L.; Sordia-Piñeyro, M.O.; Valdez-Martinez, O.H. Clinical Pregnancy after Elimination of Embryo Fragments Before Fresh Cleavage-Stage Embryo Transfer. J. Fam. Reprod. Heal. 2020, 14, 198–204. [Google Scholar] [CrossRef]
- Viganò, P.; Alteri, A.; Busnelli, A.; Vanni, V.; Somigliana, E. Frozen IVF Cycles to Circumvent the Hormonal Storm on Endometrium. Trends Endocrinol. Metab. 2020, 31, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Halvaei, I.; Ghazali, S.; Nottola, S.A.; Khalili, M.A. Cleavage-stage embryo micromanipulation in the clinical setting. Syst. Biol. Reprod. Med. 2018, 64, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, W.; Zhang, L.; Ji, Y.; Qin, J.; Wang, L.; Wang, M.; Qi, L.; Xue, J.; Lv, B.; et al. Effect of Sperm Cryopreservation on miRNA Expression and Early Embryonic Development. Front. Cell Dev. Biol. 2021, 9, 749486. [Google Scholar] [CrossRef] [PubMed]
- Timofeeva, A.V.; Fedorov, I.S.; Shamina, M.A.; Chagovets, V.V.; Makarova, N.P.; Kalinina, E.A.; Nazarenko, T.A.; Sukhikh, G.T. Clinical Relevance of Secreted Small Noncoding RNAs in an Embryo Implantation Potential Prediction at Morula and Blastocyst Development Stages. Life 2021, 11, 1328. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Lin, W.; Peng, S.-Y.; Lee, J.-W.; Lin, Y.-H.; Yu, C.; Shen, P.-C. Effects of intracytoplasmic sperm injection timing and fertilization methods on the development of bovine spindle transferred embryos. Theriogenology 2021, 180, 63–71. [Google Scholar] [CrossRef]
- Samiec, M.; Skrzyszowska, M. The use of different methods of oocyte activation for generation of porcine fibroblast cell nu-clear-transferred embryos. Ann. Anim. Sci. 2010, 10, 399–411. [Google Scholar]
- Kamimura, S.; Inoue, K.; Mizutani, E.; Kim, J.-M.; Inoue, H.; Ogonuki, N.; Miyamoto, K.; Ihashi, S.; Itami, N.; Wakayama, T.; et al. Improved development of mouse somatic cell nuclear transfer embryos by chlamydocin analogues, class I and IIa histone deacetylase inhibitors†. Biol. Reprod. 2021, 105, 543–553. [Google Scholar] [CrossRef]
- Gorczyca, G.; Wartalski, K.; Wiater, J.; Samiec, M.; Tabarowski, Z.; Duda, M. Anabolic Steroids-Driven Regulation of Porcine Ovarian Putative Stem Cells Favors the Onset of Their Neoplastic Transformation. Int. J. Mol. Sci. 2021, 22, 11800. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cecchele, A.; Cermisoni, G.C.; Giacomini, E.; Pinna, M.; Vigano, P. Cellular and Molecular Nature of Fragmentation of Human Embryos. Int. J. Mol. Sci. 2022, 23, 1349. https://doi.org/10.3390/ijms23031349
Cecchele A, Cermisoni GC, Giacomini E, Pinna M, Vigano P. Cellular and Molecular Nature of Fragmentation of Human Embryos. International Journal of Molecular Sciences. 2022; 23(3):1349. https://doi.org/10.3390/ijms23031349
Chicago/Turabian StyleCecchele, Anna, Greta Chiara Cermisoni, Elisa Giacomini, Monica Pinna, and Paola Vigano. 2022. "Cellular and Molecular Nature of Fragmentation of Human Embryos" International Journal of Molecular Sciences 23, no. 3: 1349. https://doi.org/10.3390/ijms23031349
APA StyleCecchele, A., Cermisoni, G. C., Giacomini, E., Pinna, M., & Vigano, P. (2022). Cellular and Molecular Nature of Fragmentation of Human Embryos. International Journal of Molecular Sciences, 23(3), 1349. https://doi.org/10.3390/ijms23031349






