The Enzymatic and Non-Enzymatic Antioxidant System Response of the Seagrass Cymodocea nodosa to Bisphenol-A Toxicity
Abstract
:1. Introduction
2. Results
2.1. Effects of BPA on Oxidative Stress Parameters
2.1.1. Hydrogen Peroxide Production
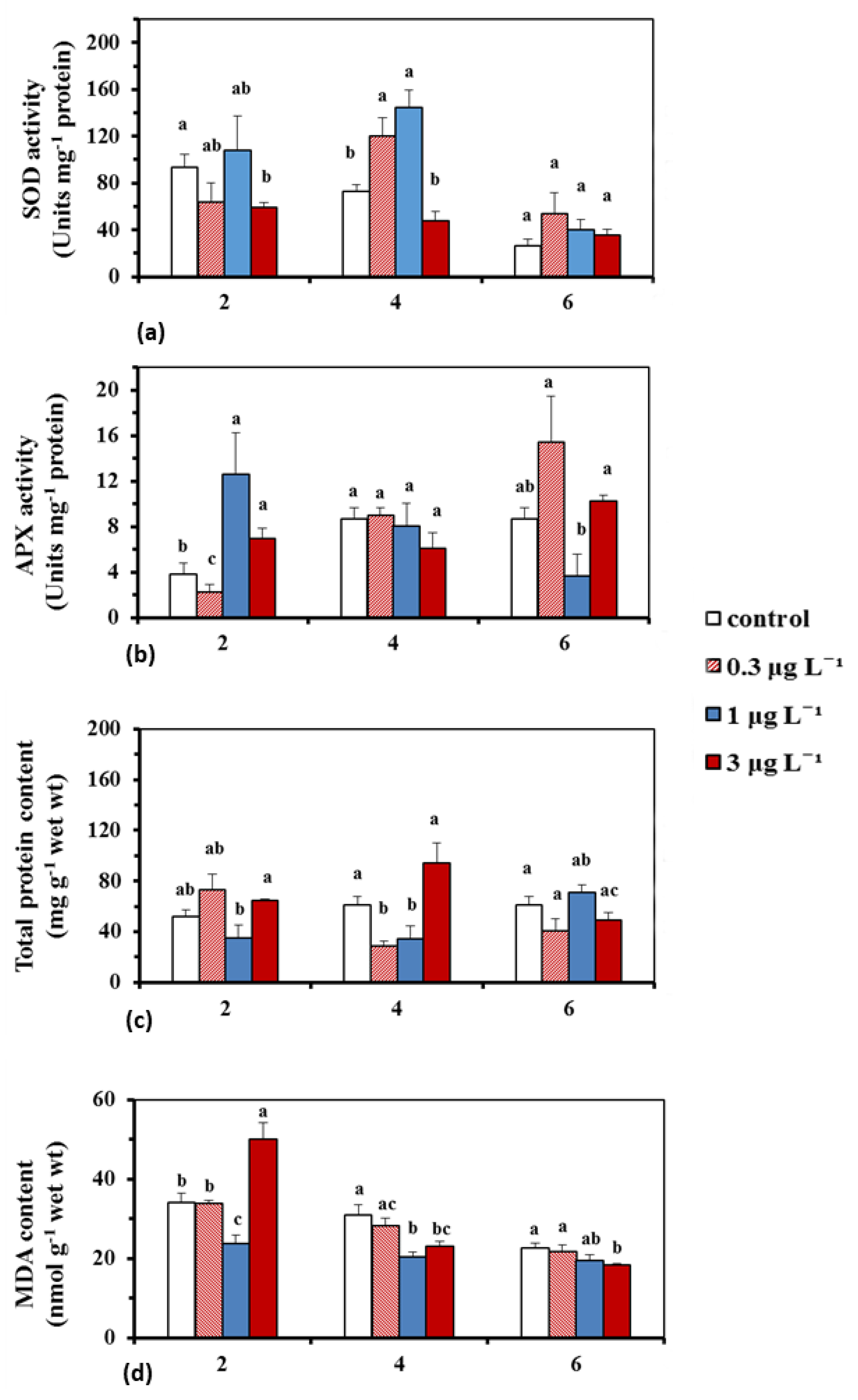
2.1.2. BPA Effects on Antioxidant Enzyme Activity, Total Protein Content and Lipid Peroxidation
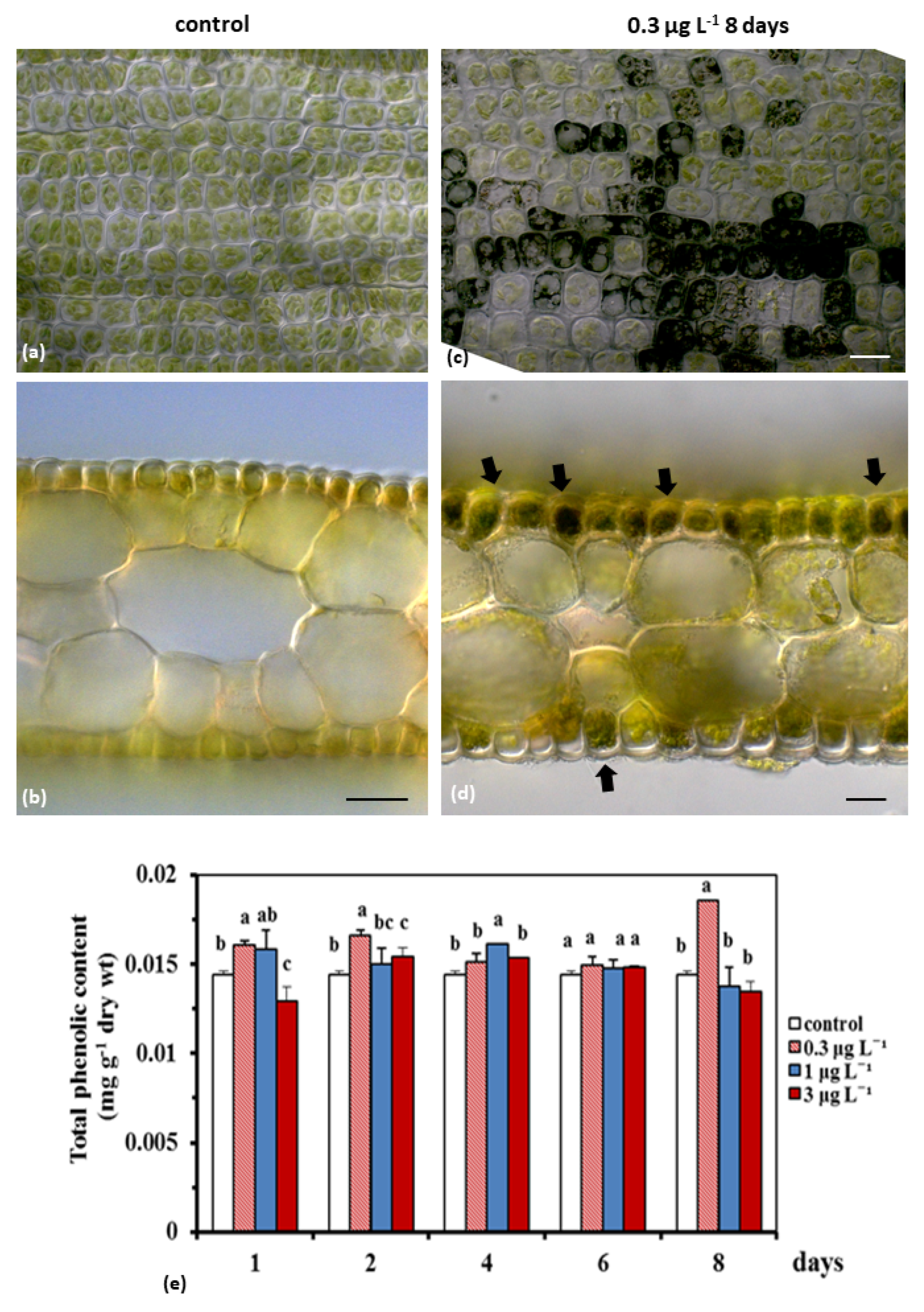
2.1.3. Total Phenolic Content
3. Discussion
4. Materials and Methods
4.1. Plant Collection and Experimental Conditions
4.2. Hydrogen Peroxide Production
4.3. Estimation of Antioxidant Enzyme Activities, Total Protein and MDA Content
4.4. Total Phenolic Compound Content
4.5. Microscopy
4.6. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Zhou, J. Endocrine disrupting chemicals in aquatic environments: A potential reason for organism extinction? Aquat. Ecosyst. Health Manag. 2013, 16, 88–93. [Google Scholar] [CrossRef]
- Windsor, F.M.; Ormerod, S.J.; Tyler, C.R. Endocrine disruption in aquatic systems: Up-scaling research to address ecological consequences. Biol. Rev. 2018, 93, 626–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, N.C.; Seebacher, F. Effect of the plastic pollutant bisphenol A on the biology of aquatic organisms: A meta-analysis. Glob. Chang. Biol. 2020, 26, 3821–3833. [Google Scholar] [CrossRef] [PubMed]
- Staples, C.; van der Hoeven, N.; Clark, K.; Mihaich, E.; Woelz, J.; Hentges, S. Distributions of concentrations of bisphenol A in North American and European surface waters and sediments determined from 19 years of monitoring data. Chemosphere 2018, 201, 448–458. [Google Scholar] [CrossRef]
- Xu, S.Y.; Zhang, H.; He, P.J.; Shao, L.M. Leaching behaviour of bisphenol A from municipal solid waste under landfill environment. Environ. Technol. 2011, 32, 1269–1277. [Google Scholar] [CrossRef]
- Rotimi, O.A.; Olawole, T.D.; De Campos, O.C.; Adelani, I.B.; Rotimi, S.O. Bisphenol A in Africa: A review of environmental and biological levels. Sci. Total Environ. 2021, 764, 142854. [Google Scholar] [CrossRef]
- Careghini, A.; Mastorgio, A.F.; Saponaro, S.; Sezenna, E. Bisphenol A, nonylphenols, benzophenones, and benzotriazoles in soils, groundwater, surface water, sediments, and food: A review. Environ. Sci. Pollut. Res. 2015, 22, 5711–5741. [Google Scholar] [CrossRef] [Green Version]
- Flint, S.; Markle, T.; Thompson, S.; Wallace, E. Bisphenol A exposure, effects, and policy: A wildlife perspective. J. Environ. Manag. 2012, 104, 19–34. [Google Scholar] [CrossRef]
- Mihaich, E.; Staples, C.; Ortego, L.; Klečka, G.; Woelz, J.; Dimond, S.; Hentges, S. Life-cycle studies with 2 marine species and bisphenol A: The mysid shrimp (Americamysis bahia) and sheepshead minnow (Cyprinodon variegatus). Environ. Toxicol. Chem. 2018, 37, 398–410. [Google Scholar] [CrossRef] [Green Version]
- Falcão, V.G.O.; de Carvalho Carneiro, D.; Pereira, S.A.; da Silva, M.R.D.; Candé, A.A.; da Cunha Lima, S.T. Analyzing the toxicity of bisphenol-A to microalgae for ecotoxicological applications. Environ. Monit. Assess. 2020, 192, 8. [Google Scholar] [CrossRef]
- Rykowska, I.; Wasiak, W. Properties, threats, and methods of analysis of bisphenol A and its derivatives. Acta Chromatogr. 2006, 16, 7–27. [Google Scholar]
- Šutiaková, I.; Kovalkovičová, N.; Tulenková, M.; Šutiak, V. Bisphenol a and Its Potential Toxic Effects on Living Organisms. J. Microbiol. Biotechnol. Food Sci. 2021, 2021, 526–535. [Google Scholar]
- Ali, I.; Jan, M.; Wakeel, A.; Azizullah, A.; Liu, B.; Islam, F.; Ali, A.; Daud, M.K.; Liu, Y.; Gan, Y. Biochemical responses and ultrastructural changes in ethylene insensitive mutants of Arabidopsis thialiana subjected to bisphenol A exposure. Ecotoxicol. Environ. Saf. 2017, 144, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Feng, Y.; Liu, Y.W.; Chang, H.Q.; Li, Z.J.; Xue, J.M. Uptake and translocation of organic pollutants in plants: A review. J. Integr. Agric. 2017, 16, 1659–1668. [Google Scholar] [CrossRef] [Green Version]
- Pop, C.E.; Draga, S.; Măciucă, R.; Niță, R.; Crăciun, N.; Wolff, R. Bisphenol A effects in aqueous environment on Lemna minor. Processes 2021, 9, 1512. [Google Scholar] [CrossRef]
- Bourgeade, P.; Aleya, E.; Alaoui-Sosse, L.; Herlem, G.; Alaoui-Sosse, B.; Bourioug, M. Growth, pigment changes, and photosystem II activity in the aquatic macrophyte Lemna minor exposed to bisphenol A. Environ. Sci. Pollut. Res. 2021, 28, 68671–68678. [Google Scholar] [CrossRef]
- Xiao, C.; Wang, L.; Zhou, Q.; Huang, X. Hazards of bisphenol A (BPA) exposure: A systematic review of plant toxicology studies. J. Hazard. Mater. 2020, 384, 121488. [Google Scholar] [CrossRef]
- Adamakis, I.D.S.; Panteris, E.; Cherianidou, A.; Eleftheriou, E.P. Effects of bisphenol A on the microtubule arrays in root meristematic cells of Pisum sativum L. Mutat. Res.-Genet. Toxicol. Environ. Mutagen. 2013, 750, 111–120. [Google Scholar] [CrossRef]
- Adamakis, I.D.S.; Panteris, E.; Eleftheriou, E.P. Bisphenol A disrupts microtubules and induces multipolar spindles in dividing root tip cells of the gymnosperm Abies cephalonica. Chemosphere 2016, 149, 202–210. [Google Scholar] [CrossRef]
- Adamakis, I.-D.S.; Malea, P.; Panteris, E. The effects of Bisphenol A on the seagrass Cymodocea nodosa: Leaf elongation impairment and cytoskeleton disturbance. Ecotoxicol. Environ. Saf. 2018, 157, 431–440. [Google Scholar] [CrossRef]
- Adamakis, I.-D.S.; Panteris, E.; Eleftheriou, E.P. Tubulin acetylation mediates bisphenol a effects on the microtubule arrays of Allium cepa and Triticum turgidum. Biomolecules 2019, 9, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamakis, I.D.S.; Sperdouli, I.; Eleftheriou, E.P.; Moustakas, M. Hydrogen Peroxide Production by the Spot-Like Mode Action of Bisphenol A. Front. Plant Sci. 2020, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Adamakis, I.-D.S.; Malea, P.; Sperdouli, I.; Panteris, E.; Kokkinidi, D.; Moustakas, M. Evaluation of the spatiotemporal effects of bisphenol A on the leaves of the seagrass Cymodocea nodosa. J. Hazard. Mater. 2021, 404, 124001. [Google Scholar] [CrossRef] [PubMed]
- Stavropoulou, K.; Adamakis, I.D.S.; Panteris, E.; Arseni, E.M.; Eleftheriou, E.P. Disruption of actin filaments in Zea mays by bisphenol A depends on their crosstalk with microtubules. Chemosphere 2018, 195, 653–665. [Google Scholar] [CrossRef] [PubMed]
- Malea, P.; Kokkinidi, D.; Kevrekidou, A.; Adamakis, I.-D.S. Environmentally relevant bisphenol A concentrations effects on the seagrass Cymodocea nodosa different parts elongation: Perceptive assessors of toxicity. Environ. Sci. Pollut. Res. 2020, 27, 7267–7279. [Google Scholar] [CrossRef]
- Ali, I.; Liu, B.; Farooq, M.A.; Islam, F.; Azizullah, A.; Yu, C.; Su, W.; Gan, Y. Toxicological effects of bisphenol A on growth and antioxidant defense system in Oryza sativa as revealed by ultrastructure analysis. Ecotoxicol. Environ. Saf. 2016, 124, 277–284. [Google Scholar] [CrossRef]
- Beniah Obinna, I.; Ebere, E.C. A review: Water pollution by heavy metal and organic pollutants: Brief review of sources, effects and progress on remediation with aquatic plants. Anal. Methods Environ. Chem. J. 2019, 2, 5–38. [Google Scholar] [CrossRef] [Green Version]
- Effrosynidis, D.; Arampatzis, A.; Sylaios, G. Seagrass detection in the mediterranean: A supervised learning approach. Ecol. Inform. 2018, 48, 158–170. [Google Scholar] [CrossRef]
- Nugegoda, D.; Kibria, G. Encyclopedia of Aquatic Ecotoxicology; Springer: Dordrecht, The Netherlands, 2013. [Google Scholar]
- Tsioli, S.; Koutalianou, M.; Gkafas, G.A.; Exadactylos, A.; Papathanasiou, V.; Katsaros, C.I.; Orfanidis, S.; Küpper, F.C. Responses of the Mediterranean seagrass Cymodocea nodosa to combined temperature and salinity stress at the ionomic, transcriptomic, ultrastructural and photosynthetic levels. Mar. Environ. Res. 2021, 105512. [Google Scholar] [CrossRef]
- Malea, P.; Adamakis, I.-D.S.; Kevrekidis, T. Kinetics of cadmium accumulation and its effects on microtubule integrity and cell viability in the seagrass Cymodocea nodosa. Aquat. Toxicol. 2013, 144–145, 257–264. [Google Scholar] [CrossRef]
- Malea, P.; Adamakis, I.D.S.; Kevrekidis, T. Microtubule integrity and cell viability under metal (Cu, Ni and Cr) stress in the seagrass Cymodocea nodosa. Chemosphere 2013, 93, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Malea, P.; Adamakis, I.D.S.; Kevrekidis, T. Effects of lead uptake on microtubule cytoskeleton organization and cell viability in the seagrass Cymodocea nodosa. Ecotoxicol. Environ. Saf. 2014, 104, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, M.; Malea, P.; Haritonidou, K.; Sperdouli, I. Copper bioaccumulation, photosystem II functioning, and oxidative stress in the seagrass Cymodocea nodosa exposed to copper oxide nanoparticles. Environ. Sci. Pollut. Res. 2017, 24, 16007–16018. [Google Scholar] [CrossRef] [PubMed]
- Malea, P.; Charitonidou, K.; Sperdouli, I.; Mylona, Z.; Moustakas, M. Zinc uptake, photosynthetic efficiency and oxidative stress in the seagrass Cymodocea nodosa exposed to ZnO nanoparticles. Materials 2019, 12, 2101. [Google Scholar] [CrossRef] [Green Version]
- Orlando-Bonaca, M.; Francé, J.; Mavrič, B.; Grego, M.; Lipej, L.; Flander-Putrle, V.; Šiško, M.; Falace, A. A new index (MediSkew) for the assessment of the Cymodocea nodosa (Ucria) Ascherson meadow’s status. Mar. Environ. Res. 2015, 110, 132–141. [Google Scholar] [CrossRef]
- Orfanidis, S.; Papathanasiou, V.; Mittas, N.; Theodosiou, T.; Ramfos, A.; Tsioli, S.; Kosmidou, M.; Kafas, A.; Mystikou, A.; Papadimitriou, A. Further improvement, validation, and application of CymoSkew biotic index for the ecological status assessment of the Greek coastal and transitional waters. Ecol. Indic. 2020, 118, 106727. [Google Scholar] [CrossRef]
- Mihaich, E.M.; Friederich, U.; Caspers, N.; Hall, A.T.; Klecka, G.M.; Dimond, S.S.; Staples, C.A.; Ortego, L.S.; Hentges, S.G. Acute and chronic toxicity testing of bisphenol A with aquatic invertebrates and plants. Ecotoxicol. Environ. Saf. 2009, 72, 1392–1399. [Google Scholar] [CrossRef] [PubMed]
- Doğan, M.; Sahin Yigit, S.; Dogancay, G.; Koçum, D.; Sevindik, M. Effects of single and binary applications of bisphenol A and NaCl on Ceratopyllum demersum. Turk. J. Agric.-Food Sci. Technol. 2020, 8, 2164–2169. [Google Scholar] [CrossRef]
- Kim, D.; Kwak, J.I.; An, Y.J. Effects of bisphenol A in soil on growth, photosynthesis activity, and genistein levels in crop plants (Vigna radiata). Chemosphere 2018, 209, 875–882. [Google Scholar] [CrossRef]
- Li, Y.T.; Liang, Y.; Li, Y.N.; Che, X.K.; Zhao, S.J.; Zhang, Z.S.; Gao, H.Y. Mechanisms by which Bisphenol A affect the photosynthetic apparatus in cucumber (Cucumis sativus L.) leaves. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Qiu, Z.; Wang, L.; Zhou, Q. Effects of bisphenol A on growth, photosynthesis and chlorophyll fluorescence in above-ground organs of soybean seedlings. Chemosphere 2013, 90, 1274–1280. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, X.; Zhou, L.; Wang, L.; Zhou, Q.; Huang, X. Analysis of effects of a new environmental pollutant, bisphenol A, on antioxidant systems in soybean roots at different growth stages. Sci. Rep. 2016, 6, 23782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrat, L.; Pergent-Martini, C.; Roméo, M. Assessment of the use of biomarkers in aquatic plants for the evaluation of environmental quality: Application to seagrasses. Aquat. Toxicol. 2003, 65, 187–204. [Google Scholar] [CrossRef]
- Xiang, R.; Shi, J.; Yu, Y.; Zhang, H.; Dong, C.; Yang, Y.; Wu, Z. The Effect of Bisphenol A on Growth, Morphology, Lipid Peroxidation, Antioxidant Enzyme Activity, and PS II in Cylindrospermopsis raciborskii and Scenedesmus quadricauda. Arch. Environ. Contam. Toxicol. 2018, 74, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Ben Ouada, S.; Ben Ali, R.; Leboulanger, C.; Ben Ouada, H.; Sayadi, S. Effect of Bisphenol A on the extremophilic microalgal strain Picocystis sp. (Chlorophyta) and its high BPA removal ability. Ecotoxicol. Environ. Saf. 2018, 158, 1–8. [Google Scholar] [CrossRef]
- M’Rabet, C.; Yahia, O.K.-D.; Couet, D.; Gueroun, S.K.M.; Pringault, O. Consequences of a contaminant mixture of bisphenol A (BPA) and di-(2-ethylhexyl) phthalate (DEHP), two plastic-derived chemicals, on the diversity of coastal phytoplankton. Mar. Pollut. Bull. 2019, 138, 385–396. [Google Scholar] [CrossRef]
- Aljahdali, M.O.; Alhassan, A.B. Heavy metal accumulation and anti-oxidative feedback as a biomarker in Seagrass Cymodocea serrulata. Sustainability 2020, 12, 2841. [Google Scholar] [CrossRef] [Green Version]
- Mylona, Z.; Panteris, E.; Kevrekidis, T.; Malea, P. Silver nanoparticle toxicity effect on the seagrass Halophila stipulacea. Ecotoxicol. Environ. Saf. 2020, 189, 109925. [Google Scholar] [CrossRef]
- Černý, M.; Habánová, H.; Berka, M.; Luklová, M.; Brzobohatý, B. Hydrogen peroxide: Its role in plant biology and crosstalk with signalling networks. Int. J. Mol. Sci. 2018, 19, 2812. [Google Scholar] [CrossRef] [Green Version]
- Palma, J.M.; Gupta, D.K.; Corpas, F.J. Hydrogen Peroxide and Nitric Oxide Generation in Plant Cells: Overview and Queries. In Nitric Oxide and Hydrogen Peroxide Signaling in Higher Plants; Gupta, D.K., Palma, J.M., Corpas, F.J., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–16. ISBN 978-3-030-11129-8. [Google Scholar]
- Orabi, S.; Abouhussein, S. Antioxidant defense mechanisms enhance oxidative stress tolerance in plants. A review. Curr. Sci. Int. 2019, 8, 565–576. [Google Scholar]
- Stephenie, S.; Chang, Y.P.; Gnanasekaran, A.; Esa, N.M.; Gnanaraj, C. An insight on superoxide dismutase (SOD) from plants for mammalian health enhancement. J. Funct. Foods 2020, 68, 103917. [Google Scholar] [CrossRef]
- Nadarajah, K.K. Ros homeostasis in abiotic stress tolerance in plants. Int. J. Mol. Sci. 2020, 21, 5208. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Wakeel, A.; Upreti, S.; Liu, D.; Azizullah, A.; Jan, M.; Ullah, W.; Liu, B.; Ali, A.; Daud, M.K.; et al. Effect of bisphenol a-induced oxidative stress on the ultra structure and antioxidant defence system of Arabidopsis thialiana leaves. Pol. J. Environ. Stud. 2018, 27, 967–978. [Google Scholar] [CrossRef]
- Morales, M.; Munné-Bosch, S. Malondialdehyde: Facts and artifacts. Plant Physiol. 2019, 180, 1246–1250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, C.; Wang, L.; Hu, D.; Zhou, Q.; Huang, X. Effects of exogenous bisphenol A on the function of mitochondria in root cells of soybean (Glycine max L.) seedlings. Chemosphere 2019, 222, 619–627. [Google Scholar] [CrossRef]
- Liu, Y.; Guan, Y.; Gao, Q.; Tam, N.F.Y.; Zhu, W. Cellular responses, biodegradation and bioaccumulation of endocrine disrupting chemicals in marine diatom Navicula incerta. Chemosphere 2010, 80, 592–599. [Google Scholar] [CrossRef]
- Rapała, M.; Pluciński, B.; Jedynak, P. The effect of bisphenol A on growth, pigment composition and photosystem II activity of Arabidopsis thaliana. Acta Biochim. Pol. 2017, 64, 407–413. [Google Scholar] [CrossRef]
- Sun, H.; Wang, L.H.; Zhou, Q.; Huang, X.H. Effects of bisphenol A on ammonium assimilation in soybean roots. Environ. Sci. Pollut. Res. 2013, 20, 8484–8490. [Google Scholar] [CrossRef]
- Šamec, D.; Karalija, E.; Šola, I.; Vujčić Bok, V.; Salopek-Sondi, B. The role of polyphenols in abiotic stress response: The influence of molecular structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef]
- Migliore, L.; Rotini, A.; Randazzo, D.; Albanese, N.N.; Giallongo, A. Phenols content and 2-D electrophoresis protein pattern: A promising tool to monitor Posidonia meadows health state. BMC Ecol. 2007, 7, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Rotini, A.; Anello, L.; Di Bernardo, M.; Giallongo, A.; Valiante, L.; Migliore, L. Comparative analysis of bed density, total phenol content and protein expression pattern in Posidonia oceanica (L.) Delile. Open J. Ecol. 2013, 3, 438–444. [Google Scholar] [CrossRef] [Green Version]
- Rotini, A.; Belmonte, A.; Barrote, I.; Micheli, C.; Peirano, A.; Santos, R.O.; Silva, J.; Migliore, L. Effectiveness and consistency of a suite of descriptors for assessing the ecological status of seagrass meadows (Posidonia oceanica L. Delile). Estuar. Coast. Shelf Sci. 2013, 130, 252–259. [Google Scholar] [CrossRef] [Green Version]
- Piro, A.; Bernardo, L.; Serra, I.A.; Barrote, I.; Olivé, I.; Costa, M.M.; Lucini, L.; Santos, R.; Mazzuca, S.; Silva, J. Leaf proteome modulation and cytological features of seagrass Cymodocea nodosa in response to long-term high CO2 exposure in volcanic vents. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Khaled Abdel-Halim, Y. Biomarkers in Ecotoxicological Research Trails. J. Forensic Sci. Toxicol. 2018, 1, 1005. [Google Scholar]
- Andelić, I.; Roje-Busatto, R.; Ujević, I.; Vuletić, N.; Matijević, S. Distribution of bisphenol a in sediment and suspended matter and its possible impact on marine life in Kastela Bay, Adriatic Sea, Croatia. J. Mar. Sci. Eng. 2020, 8, 480. [Google Scholar] [CrossRef]
- Pedrol, N.; Ramos, P. Protein Content Quantification By Bradford Method. Handb. Plant Ecophysiol. Tech. 2001, 283–295. [Google Scholar]
- Elavarthi, S.; Martin, B. Spectrophotometric Assays for Antioxidant Enzymes in Plants. In Plant Stress Tolerance: Methods and Protocols; Sunkar, R., Ed.; Humana Press: Totowa, NJ, USA, 2010; pp. 273–280. ISBN 978-1-60761-702-0. [Google Scholar]
- Davey, M.W.; Stals, E.; Panis, B.; Keulemans, J.; Swennen, R.L. High-throughput determination of malondialdehyde in plant tissues. Anal. Biochem. 2005, 347, 201–207. [Google Scholar] [CrossRef]
- Boumaza, S.; Boudefoua, N.; Semroud, R. Effects of urban effluents on spatial structure, morphology and total phenols of Posidonia oceanica: Comparison with a reference site. J. Exp. Mar. Biol. Ecol. 2014, 457, 113–119. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Kesraoui, O.; Marzouki, M.N.; Maugard, T.; Limam, F. In vitro evaluation of antioxidant activitities of free and bound phenolic compounds from Posidonia oceanica (I.) Delile leaves. Afr. J. Biotechnol. 2011, 10, 3176–3185. [Google Scholar]
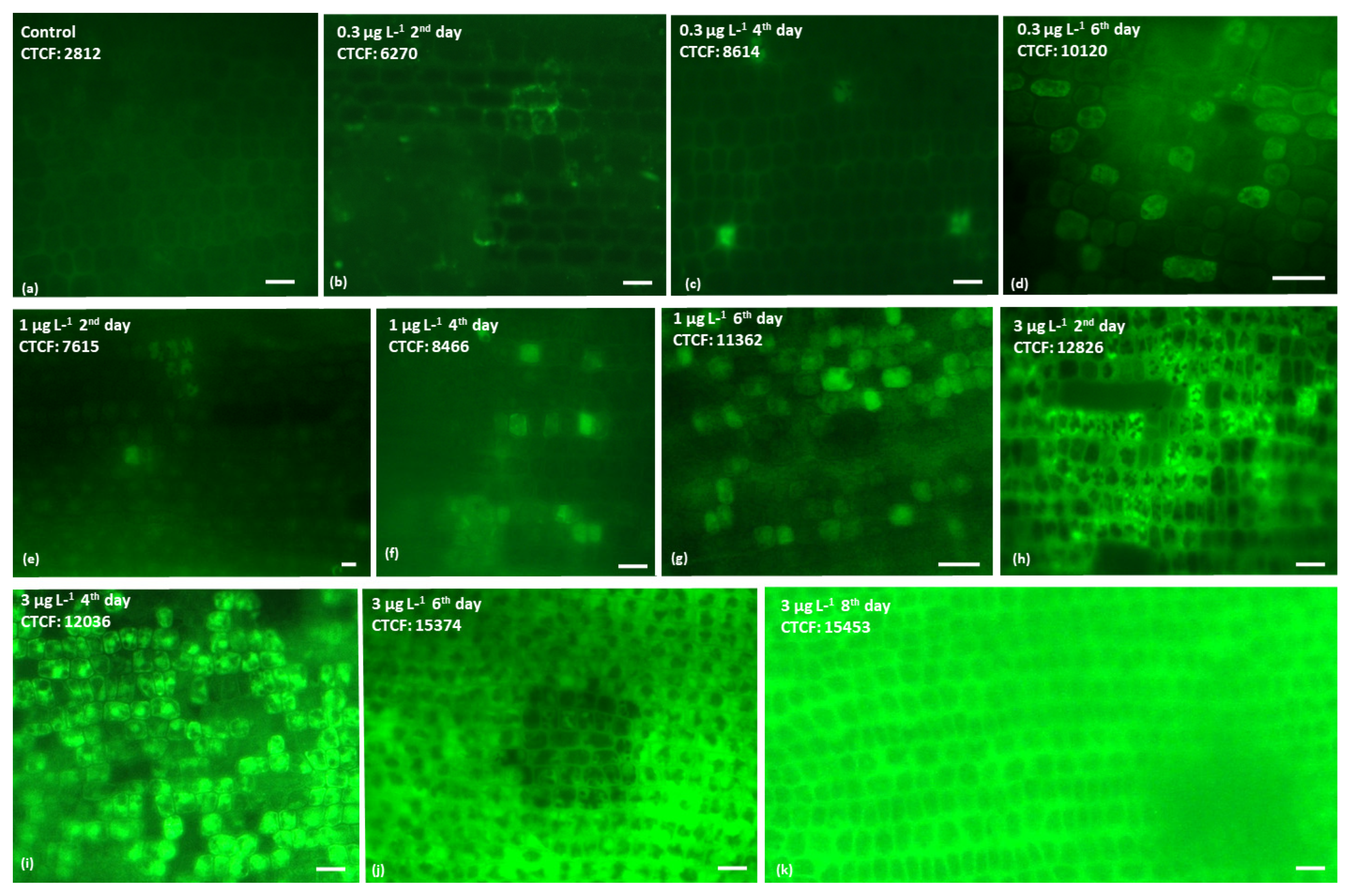

| BPA Concentrations (μg L−1) | Comparison of CTCF Values | ||||||
|---|---|---|---|---|---|---|---|
| Days | Control | 0.3 | 1 | 3 | C-0.3 | C-1 | C-3 |
| 2 | 3571.2 ± 78.7 | 6808.3 ± 103.5 | 7795.3 ± 261.3 | 11,638.8 ± 152.7 | *** | *** | *** |
| 4 | 3583.5 ± 76.7 | 9153.5 ± 104.5 | 9140.3 ± 261.3 | 10,404.8 ± 150.7 | *** | *** | *** |
| 6 | 3505.5 ± 75.7 | 10,388.3 ± 102.5 | 9712.1 ± 121.8 | 10,402.1 ± 155.7 | *** | *** | *** |
| 8 | 3437.6 ± 78.7 | 10,445.2 ± 104.5 | 9767.0 ± 121.8 | 10,481.1 ± 151.6 | *** | *** | *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malea, P.; Kokkinidi, D.; Kevrekidou, A.; Adamakis, I.-D.S. The Enzymatic and Non-Enzymatic Antioxidant System Response of the Seagrass Cymodocea nodosa to Bisphenol-A Toxicity. Int. J. Mol. Sci. 2022, 23, 1348. https://doi.org/10.3390/ijms23031348
Malea P, Kokkinidi D, Kevrekidou A, Adamakis I-DS. The Enzymatic and Non-Enzymatic Antioxidant System Response of the Seagrass Cymodocea nodosa to Bisphenol-A Toxicity. International Journal of Molecular Sciences. 2022; 23(3):1348. https://doi.org/10.3390/ijms23031348
Chicago/Turabian StyleMalea, Paraskevi, Danae Kokkinidi, Alkistis Kevrekidou, and Ioannis-Dimosthenis S. Adamakis. 2022. "The Enzymatic and Non-Enzymatic Antioxidant System Response of the Seagrass Cymodocea nodosa to Bisphenol-A Toxicity" International Journal of Molecular Sciences 23, no. 3: 1348. https://doi.org/10.3390/ijms23031348
APA StyleMalea, P., Kokkinidi, D., Kevrekidou, A., & Adamakis, I.-D. S. (2022). The Enzymatic and Non-Enzymatic Antioxidant System Response of the Seagrass Cymodocea nodosa to Bisphenol-A Toxicity. International Journal of Molecular Sciences, 23(3), 1348. https://doi.org/10.3390/ijms23031348







