Early Intervention of Cold-Water Swimming on Functional Recovery and Spinal Pain Modulation Following Brachial Plexus Avulsion in Rats
Abstract
1. Introduction
2. Results
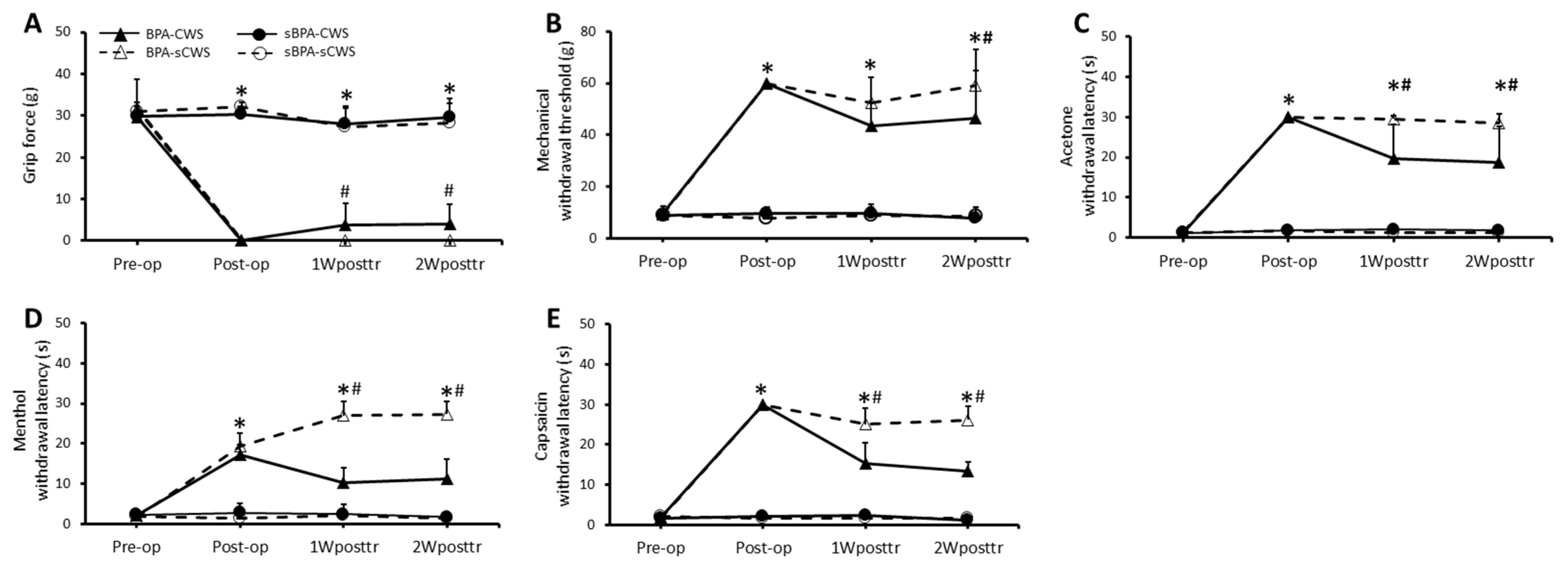
2.1. Effects of CWS on Motor Function and Sensory Behavioral Assessments in BPA Rats
2.1.1. Motor Function
2.1.2. Mechanical Sensitivity
2.1.3. Cold Sensitivity
2.1.4. Thermal Sensitivity
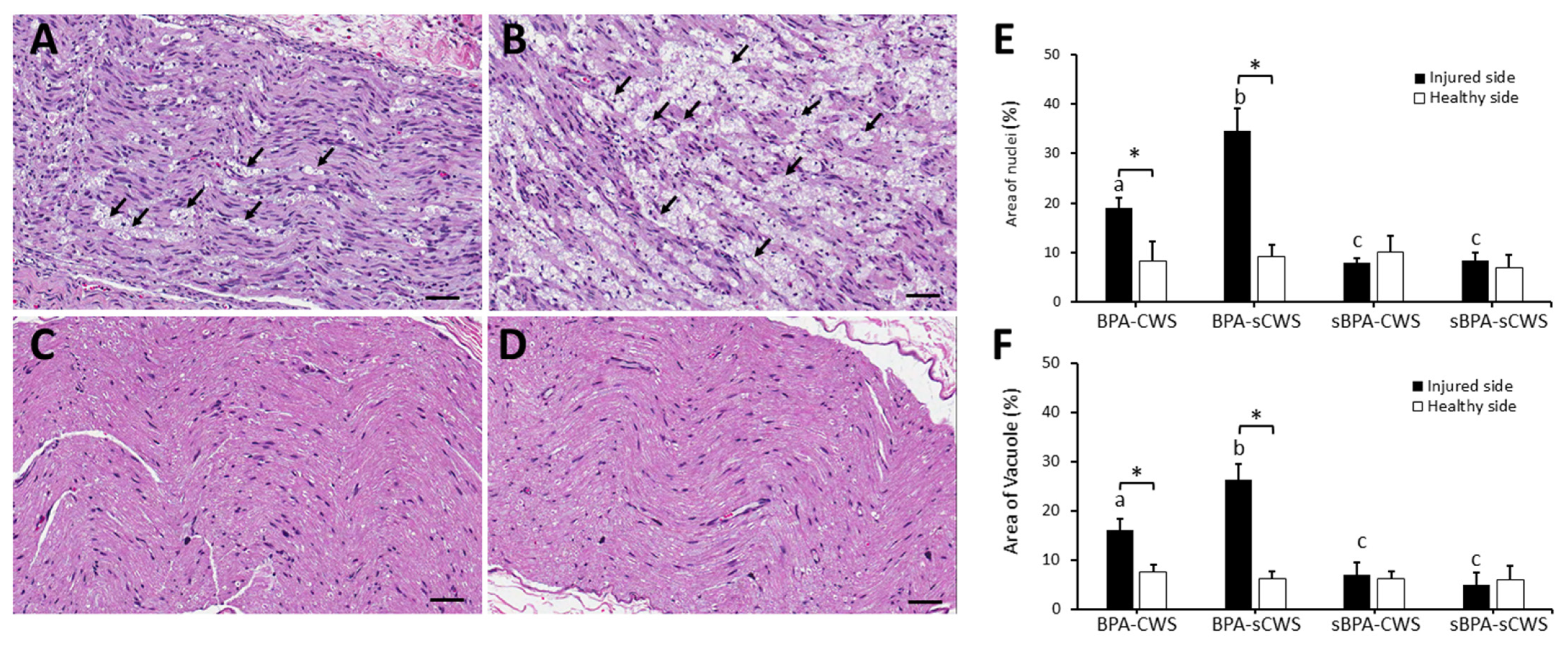
2.2. Effects of CWS on Morphological Studies in Brachial Plexus in BPA Rats
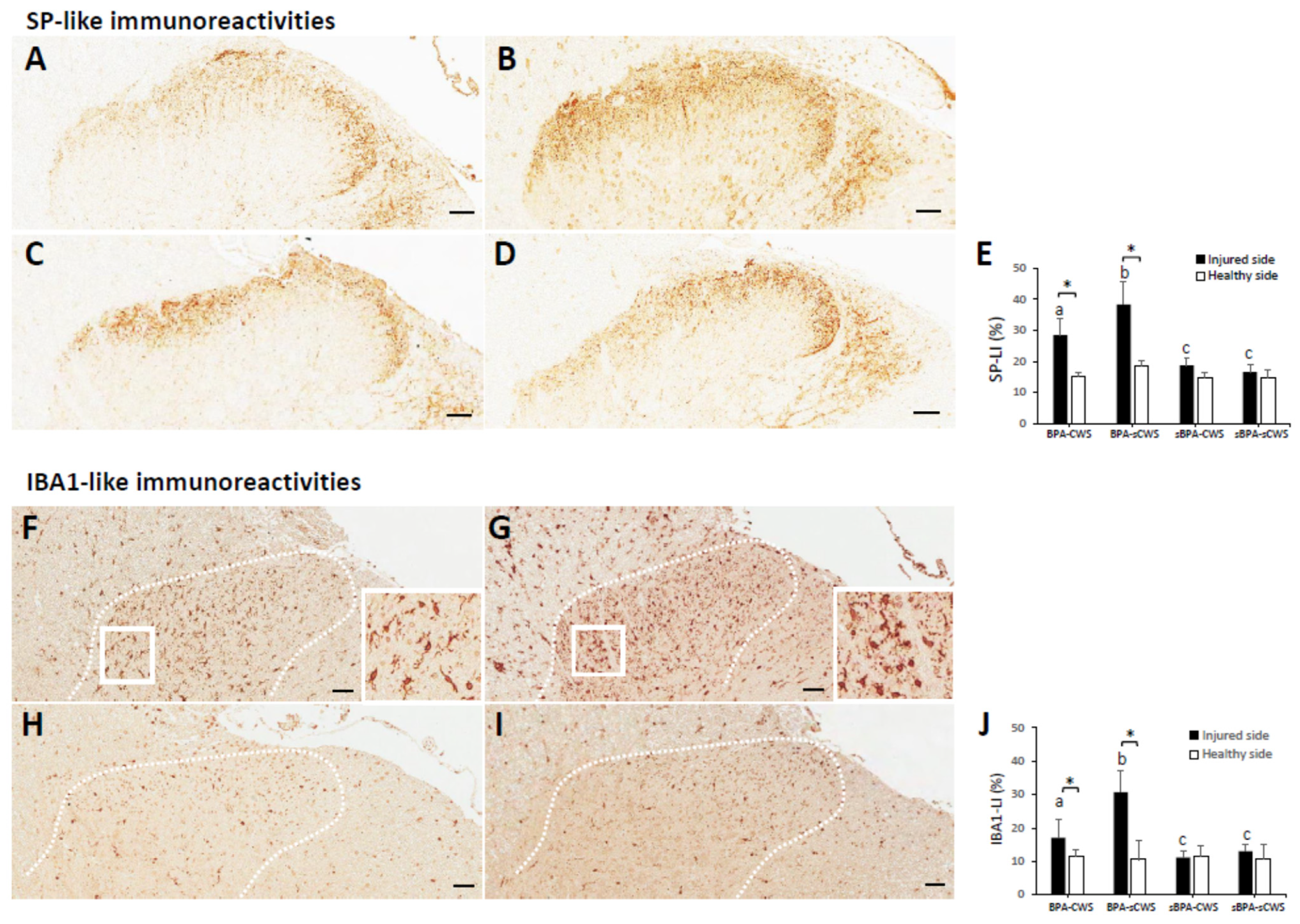
2.3. Effects of CWS on Protein Levels of SP and IBA1 in Superficial Dorsal Horns in BPA Rats
3. Discussion
4. Materials and Methods
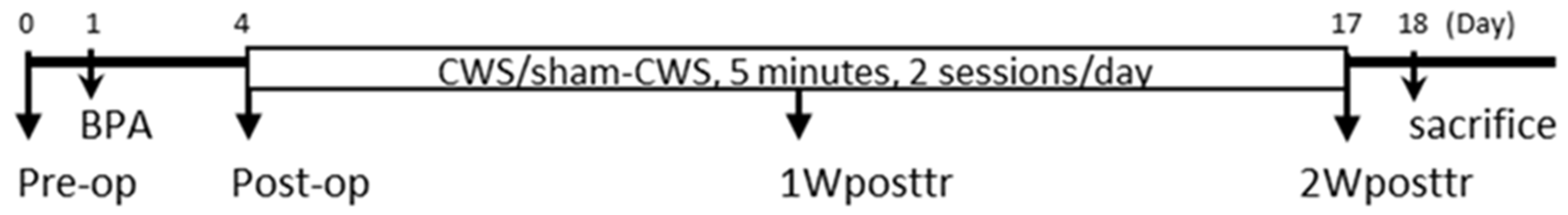
4.1. Study Design
4.2. Animal Care and Preparation
4.3. Surgical Procedures for Brachial Plexus Injury
4.4. Swimming Intervention
4.5. Functional Assessments
4.5.1. Motor Function Test
4.5.2. Sensory Behavioral Examinations
Mechanical Sensitivity
Cold Sensitivity
Thermal Sensitivity
4.6. Morphology and Immunoassays
4.6.1. Tissue Preparation and Morphological Examination
4.6.2. Immunohistochemical Staining and Quantitative Analyses
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xian, H.; Xie, R.; Luo, C.; Cong, R. Comparison of different in vivo animal models of brachial plexus avulsion and its application in pain study. Neural Plast. 2020, 2020, 8875915. [Google Scholar] [CrossRef] [PubMed]
- Hou, A.L.; Xu, W.D. A model of neuropathic pain in brachial plexus avulsion injury and associated spinal glial cell activation. J. Pain Res. 2018, 11, 3171–3179. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.J.; da Paz, M.G.; Bina, M.T.; Santos, S.N.; Raicher, I.; Galhardoni, R.; Fernandes, D.T.; Yeng, L.T.; Baptista, A.F.; de Andrade, D.C. Neuropathic pain after brachial plexus avulsion—Central and peripheral mechanisms. BMC Neurol. 2015, 15, 73. [Google Scholar] [CrossRef] [PubMed]
- Khasabov, S.G.; Rogers, S.D.; Ghilardi, J.R.; Peters, C.M.; Mantyh, P.W.; Simone, D.A. Spinal neurons that possess the substance P receptor are required for the development of central sensitization. J. Neurosci. Off. J. Soc. Neurosci. 2002, 22, 9086–9098. [Google Scholar] [CrossRef]
- Popiolek-Barczyk, K.; Mika, J. Targeting the Microglial Signaling Pathways: New Insights in the Modulation of Neuropathic Pain. Curr. Med. Chem. 2016, 23, 2908–2928. [Google Scholar] [CrossRef]
- Matsuura, Y.; Iwakura, N.; Ohtori, S.; Suzuki, T.; Kuniyoshi, K.; Murakami, K.; Hiwatari, R.; Hashimoto, K.; Okamoto, S.; Shibayama, M.; et al. The effect of Anti-NGF receptor (p75 Neurotrophin Receptor) antibodies on nociceptive behavior and activation of spinal microglia in the rat brachial plexus avulsion model. Spine (Phila Pa 1976) 2013, 38, E332–E338. [Google Scholar] [CrossRef]
- Van Meeteren, N.L.; Brakkee, J.H.; Hamers, F.P.; Helders, P.J.; Gispen, W.H. Exercise training improves functional recovery and motor nerve conduction velocity after sciatic nerve crush lesion in the rat. Arch. Phys. Med. Rehabil. 1997, 78, 70–77. [Google Scholar] [CrossRef]
- Udina, E.; Puigdemasa, A.; Navarro, X. Passive and active exercise improve regeneration and muscle reinnervation after peripheral nerve injury in the rat. Muscle Nerve 2011, 43, 500–509. [Google Scholar] [CrossRef]
- Yang, C.C.; Wang, J.; Chen, S.C.; Jan, Y.M.; Hsieh, Y.L. Enhanced functional recovery from sciatic nerve crush injury through a combined treatment of cold-water swimming and mesenchymal stem cell transplantation. Neurol. Res. 2015, 37, 816–826. [Google Scholar] [CrossRef]
- Kafka, J.; Lukacova, N.; Sulla, I.; Maloveska, M.; Vikartovska, Z.; Cizkova, D. Hypothermia in the course of acute traumatic spinal cord injury. Acta Neurobiol. Exp. (Wars) 2020, 80, 172–178. [Google Scholar] [CrossRef]
- Drury, P.P.; Gunn, E.R.; Bennet, L.; Gunn, A.J. Mechanisms of hypothermic neuroprotection. Clin. Perinatol. 2014, 41, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, W.D.; Atkins, C.M.; Bramlett, H.M. Protection in animal models of brain and spinal cord injury with mild to moderate hypothermia. J. Neurotrauma 2009, 26, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Pei, B.; Li, Z.; Ou, X.L.; Sun, T.; Zhu, Z. PLGA-PEG-PLGA hydrogel with NEP1-40 promotes the functional recovery of brachial plexus root avulsion in adult rats. PeerJ 2021, 9, e12269. [Google Scholar] [CrossRef] [PubMed]
- González Rodríguez, A.; González Porto, S.A.; Comellas Melero, N.; Arufe, M.C. Acellular nerve graft enriched with mesenchymal stem cells in the transfer of the phrenic nerve to the musculocutaneous nerve in a C5-C6 brachial plexus avulsion in a rat model. Microsurgery 2022, 42, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Ding, L.; Shui, H.; Liang, Y.; Zhang, X.; Wang, T.; Li, L.; Liu, S.; Wu, H. MiR-615 agomir encapsulated in pluronic f-127 alleviates neuron damage and facilitates function recovery after brachial plexus avulsion. J. Mol. Neurosci. MN 2021, 72, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Debastiani, J.C.; Santana, A.J.; Ribeiro, L.F.C.; Brancalhão, R.M.C.; Bertolini, G.R.F. Sericin silk protein in peripheral nervous repair associated with the physical exercise of swimming in Wistar rats. Neurol. Res. 2019, 41, 326–334. [Google Scholar] [CrossRef]
- Farzad, B.; Rajabi, H.; Gharakhanlou, R.; Allison, D.J.; Hayat, P.; Jameie, S.B. Swimming training attenuates allodynia and hyperalgesia induced by peripheral nerve injury in an adult male rat neuropathic model: Effects on irisin and GAD65. Pain Med. 2018, 19, 2236–2245. [Google Scholar] [CrossRef]
- Lovaglio, A.C.; Socolovsky, M.; Di Masi, G.; Bonilla, G. Treatment of neuropathic pain after peripheral nerve and brachial plexus traumatic injury. Neurol. India 2019, 67, S32–S37. [Google Scholar] [CrossRef]
- Boeltz, T.; Ireland, M.; Mathis, K.; Nicolini, J.; Poplavski, K.; Rose, S.J.; Wilson, E.; English, A.W. Effects of treadmill training on functional recovery following peripheral nerve injury in rats. J. Neurophysiol. 2013, 109, 2645–2657. [Google Scholar] [CrossRef]
- Maugeri, G.; D’Agata, V.; Trovato, B.; Roggio, F.; Castorina, A.; Vecchio, M.; Di Rosa, M.; Musumeci, G. The role of exercise on peripheral nerve regeneration: From animal model to clinical application. Heliyon 2021, 7, e08281. [Google Scholar] [CrossRef]
- Wang, J.; Yang, C.C.; Chen, S.C.; Hsieh, Y.L. No synergistic effect of mesenchymal stem cells and exercise on functional recovery following sciatic nerve transection. Funct. Neurol. 2010, 25, 33–43. [Google Scholar] [PubMed]
- Cobianchi, S.; Marinelli, S.; Florenzano, F.; Pavone, F.; Luvisetto, S. Short- but not long-lasting treadmill running reduces allodynia and improves functional recovery after peripheral nerve injury. Neuroscience 2010, 168, 273–287. [Google Scholar] [CrossRef] [PubMed]
- McGregor, C.E.; Irwin, A.M.; English, A.W. The Val66Met BDNF polymorphism and peripheral nerve injury: Enhanced regeneration in mouse met-carriers is not further improved with activity-dependent treatment. Neurorehabil. Neural Repair 2019, 33, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Abel, E.L.; Bilitzke, P.J. A possible alarm substance in the forced swimming test. Physiol. Behav. 1990, 48, 233–239. [Google Scholar] [CrossRef]
- Oliveira, L.S.; Sobral, L.L.; Takeda, S.Y.; Betini, J.; Guirro, R.R.; Somazz, M.C.; Teodori, R.M. Electrical stimulation and swimming in the acute phase of axonotmesis: Their influence on nerve regeneration and functional recovery. Rev. Neurol. 2008, 47, 11–15. [Google Scholar]
- De Moraes, A.A.; de Almeida, C.A.S.; Lucas, G.; Thomazini, J.A.; DeMaman, A.S. Effect of swimming training on nerve morphological recovery after compressive injury. Neurol. Res. 2018, 40, 955–962. [Google Scholar] [CrossRef]
- Teodori, R.M.; Betini, J.; de Oliveira, L.S.; Sobral, L.L.; Takeda, S.Y.; de Lima Montebelo, M.I. Swimming exercise in the acute or late phase after sciatic nerve crush accelerates nerve regeneration. Neural Plast. 2011, 2011, 783901. [Google Scholar] [CrossRef]
- Herbison, G.J.; Jaweed, M.M.; Ditunno, J.F. Effect of swimming on reinnervation of rat skeletal muscle. J. Neurol. Neurosurg. Psychiatry 1974, 37, 1247–1251. [Google Scholar] [CrossRef][Green Version]
- Andrade, I.R.S.; Nakachima, L.R.; Fernandes, M.; Fernandes, C.H.; Santos, J.; Valente, S.G. Assessment of the effects of swimming as a postoperative rehabilitation on nerve regeneration of wistar rats submitted to grafting of autologous nerves after injury to the sciatic nerve. Rev. Bras. Ortop. 2020, 55, 323–328. [Google Scholar]
- Guo, J.B.; Chen, B.L.; Wang, Y.; Zhu, Y.; Song, G.; Yang, Z.; Zheng, Y.L.; Wang, X.Q.; Chen, P.J. Meta-Analysis of the Effect of Exercise on Neuropathic Pain Induced by Peripheral Nerve Injury in Rat Models. Front. Neurol. 2019, 10, 636. [Google Scholar] [CrossRef]
- Lapo, I.B.; Konarzewski, M.; Sadowski, B. Effect of cold acclimation and repeated swimming on opioid and nonopioid swim stress-induced analgesia in selectively bred mice. Physiol. Behav. 2003, 78, 345–350. [Google Scholar] [CrossRef]
- Mogil, J.S.; Sternberg, W.F.; Balian, H.; Liebeskind, J.C.; Sadowski, B. Opioid and nonopioid swim stress-induced analgesia: A parametric analysis in mice. Physiol. Behav. 1996, 59, 123–132. [Google Scholar] [CrossRef]
- Chen, Y.W.; Li, Y.T.; Chen, Y.C.; Li, Z.Y.; Hung, C.H. Exercise training attenuates neuropathic pain and cytokine expression after chronic constriction injury of rat sciatic nerve. Anesth. Analg. 2012, 114, 1330–1337. [Google Scholar] [CrossRef] [PubMed]
- Calvo, M.; Bennett, D.L. The mechanisms of microgliosis and pain following peripheral nerve injury. Exp. Neurol. 2012, 234, 271–282. [Google Scholar] [CrossRef]
- Eriksson, N.P.; Persson, J.K.; Svensson, M.; Arvidsson, J.; Molander, C.; Aldskogius, H. A quantitative analysis of the microglial cell reaction in central primary sensory projection territories following peripheral nerve injury in the adult rat. Exp. Brain Res. 1993, 96, 19–27. [Google Scholar] [CrossRef]
- Ohsawa, K.; Imai, Y.; Sasaki, Y.; Kohsaka, S. Microglia/macrophage-specific protein Iba1 binds to fimbrin and enhances its actin-bundling activity. J. Neurochem. 2004, 88, 844–856. [Google Scholar] [CrossRef]
- Hayati, A.A.; Zalina, I.; Myo, T.; Badariah, A.A.; Azhar, A.; Idris, L. Modulation of formalin-induced fos-like immunoreactivity in the spinal cord by swim stress-induced analgesia, morphine and ketamine. Ger. Med. Sci 2008, 6, Doc05. [Google Scholar]
- Bruijnzeel, A.W.; Stam, R.; Compaan, J.C.; Croiset, G.; Akkermans, L.M.; Olivier, B.; Wiegant, V.M. Long-term sensitization of Fos-responsivity in the rat central nervous system after a single stressful experience. Brain Res. 1999, 819, 15–22. [Google Scholar] [CrossRef]
- De Lange, R.P.; Geerse, G.J.; Dahlhaus, M.; van Laar, T.J.; Wiegant, V.M.; Stam, R. Altered brain stem responsivity to duodenal pain after a single stressful experience. Neurosci. Lett. 2005, 381, 144–148. [Google Scholar] [CrossRef]
- Xiong, M.; Cheng, G.Q.; Ma, S.M.; Yang, Y.; Shao, X.M.; Zhou, W.H. Post-ischemic hypothermia promotes generation of neural cells and reduces apoptosis by Bcl-2 in the striatum of neonatal rat brain. Neurochem. Int. 2011, 58, 625–633. [Google Scholar] [CrossRef]
- Terman, G.W.; Morgan, M.J.; Liebeskind, J.C. Opioid and non-opioid stress analgesia from cold water swim: Importance of stress severity. Brain Res. 1986, 372, 167–171. [Google Scholar] [CrossRef]
- Killian, P.; Holmes, B.B.; Takemori, A.E.; Portoghese, P.S.; Fujimoto, J.M. Cold water swim stress- and delta-2 opioid-induced analgesia are modulated by spinal gamma-aminobutyric acidA receptors. J. Pharm. Exp. 1995, 274, 730–734. [Google Scholar]
- Łapo, I.B.; Konarzewski, M.; Sadowski, B. Analgesia induced by swim stress: Interaction between analgesic and thermoregulatory mechanisms. Pflug. Arch. Eur. J. Physiol. 2003, 446, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, R.J.; Glusman, M.; Brutus, M.; Spiaggia, A.; Kelly, D.D. Analgesia induced by cold-water stress: Attenuation following hypophysectomy. Physiol. Behav. 1979, 23, 53–62. [Google Scholar] [CrossRef]
- Bodnar, R.J.; Kelly, D.D.; Spiaggia, A.; Glusman, M. Stress-induced analgesia: Adaptation following chronic cold water swims. Bull. Psychon. Soc. 1978, 11, 337–340. [Google Scholar] [CrossRef]
- Marqueste, T.; Alliez, J.R.; Alluin, O.; Jammes, Y.; Decherchi, P. Neuromuscular rehabilitation by treadmill running or electrical stimulation after peripheral nerve injury and repair. J. Appl. Physiol. (1985) 2004, 96, 1988–1995. [Google Scholar] [CrossRef]
- López-Álvarez, V.M.; Modol, L.; Navarro, X.; Cobianchi, S. Early increasing-intensity treadmill exercise reduces neuropathic pain by preventing nociceptor collateral sprouting and disruption of chloride cotransporters homeostasis after peripheral nerve injury. Pain 2015, 156, 1812–1825. [Google Scholar] [CrossRef]
- Kumar, S.; Behera, S.; Basu, A.; Dey, S.; Ghosh-Roy, A. Swimming exercise promotes post-injury axon regeneration and functional restoration through AMPK. eNeuro 2021, 8, ENEURO.0414-20.2021. [Google Scholar] [CrossRef]
- Kawamura, N.; Schmelzer, J.D.; Wang, Y.; Schmeichel, A.M.; Low, P.A. The therapeutic window of hypothermic neuroprotection in experimental ischemic neuropathy: Protection in ischemic phase and potential deterioration in later reperfusion phase. Exp. Neurol. 2005, 195, 305–312. [Google Scholar] [CrossRef]
- Zimmermann, M. Ethical considerations in relation to pain in animal experimentation. Acta Physiol. Scand. 1986, 128 (Suppl. 554), 221–233. [Google Scholar]
- Rodrigues-Filho, R.; Santos, A.R.; Bertelli, J.A.; Calixto, J.B. Avulsion injury of the rat brachial plexus triggers hyperalgesia and allodynia in the hindpaws: A new model for the study of neuropathic pain. Brain Res. 2003, 982, 186–194. [Google Scholar] [CrossRef]
- Hsieh, Y.L.; Chou, L.W.; Chang, P.L.; Yang, C.C.; Kao, M.J.; Hong, C.Z. Low-level laser therapy alleviates neuropathic pain and promotes function recovery in rats with chronic constriction injury: Possible involvements in hypoxia-inducible factor 1alpha (HIF-1alpha). J. Comp. Neurol. 2012, 520, 2903–2916. [Google Scholar] [CrossRef] [PubMed]
- Pingle, S.C.; Matta, J.A.; Ahern, G.P. Capsaicin receptor: TRPV1 a promiscuous TRP channel. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2007; pp. 155–171. [Google Scholar]
- McKemy, D.D. TRPM8: The cold and menthol receptor. In TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades; Liedtke, W.B., Heller, S., Eds.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh, Y.-L.; Yang, N.-P.; Chen, S.-F.; Lu, Y.-L.; Yang, C.-C. Early Intervention of Cold-Water Swimming on Functional Recovery and Spinal Pain Modulation Following Brachial Plexus Avulsion in Rats. Int. J. Mol. Sci. 2022, 23, 1178. https://doi.org/10.3390/ijms23031178
Hsieh Y-L, Yang N-P, Chen S-F, Lu Y-L, Yang C-C. Early Intervention of Cold-Water Swimming on Functional Recovery and Spinal Pain Modulation Following Brachial Plexus Avulsion in Rats. International Journal of Molecular Sciences. 2022; 23(3):1178. https://doi.org/10.3390/ijms23031178
Chicago/Turabian StyleHsieh, Yueh-Ling, Nian-Pu Yang, Shih-Fong Chen, Yu-Lin Lu, and Chen-Chia Yang. 2022. "Early Intervention of Cold-Water Swimming on Functional Recovery and Spinal Pain Modulation Following Brachial Plexus Avulsion in Rats" International Journal of Molecular Sciences 23, no. 3: 1178. https://doi.org/10.3390/ijms23031178
APA StyleHsieh, Y.-L., Yang, N.-P., Chen, S.-F., Lu, Y.-L., & Yang, C.-C. (2022). Early Intervention of Cold-Water Swimming on Functional Recovery and Spinal Pain Modulation Following Brachial Plexus Avulsion in Rats. International Journal of Molecular Sciences, 23(3), 1178. https://doi.org/10.3390/ijms23031178






