Fatty Liver Disease-Alcoholic and Non-Alcoholic: Similar but Different
Abstract
1. Introduction
2. Epidemiology and Clinical Features of NAFLD and ALD
3. The Importance of Fatty Liver Disease
4. Pathogenesis of ALD
5. The Impact of Different Types of Alcohol on Alcohol Consumption
6. Pathogenesis of NAFLD
6.1. Insulin Resistance
6.2. Hepatocellular Injury
6.3. Iron Overload
7. Role of the Intestinal Microbiota
7.1. Alcoholic Liver Disease (ALD)
7.2. Non-Alcoholic Fatty Liver Disease (NAFLD)
8. Endotoxins, Oxidative Status in NAFLD and ALD
9. Clinical Manifestations and Diagnosis of Fatty Liver Disease
9.1. Alcoholic Liver Disease
9.2. Non Alcoholic Fatty Liver Disease
10. Liver Histology in ALD and NAFLD
10.1. Alcoholic Liver Disease
10.2. Non-Alcoholic Fatty Liver Disease
10.3. Revised Nomenclature for Fatty Liver Disease
10.4. Treatment of MAFLD
10.5. Treatment of ALD
11. NAFLD, T2D, CVD
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACoAC | acetyl CoA carboxylase |
| ACS | acute coronary syndrome |
| ADH | alcohol dehydrogenase |
| ALDH | Acetaldehyde dehydrogenase. |
| ALT | alanine aminotransferase |
| ALD | Alcoholic liver disease |
| ALP | Alkaline phosphatase |
| AST | aspartate aminotransferase |
| AST/ALT | Ratio AST/ALT |
| AUD | alcohol use disorder |
| BAC | Blood alcohol concentration |
| BMI | Body mass index |
| CAD | Coronary artery disease |
| CDT | Carbohydrate deficiency transferrin |
| CRP | C reactive protein |
| CYP | Cytochrome |
| DILI | Drug induced liver injury |
| DM | diabetes mellitus |
| FAS | fatty acid synthetase |
| FFA | free fatty acid |
| GGT | Gamma gluthamyl transferase |
| GSH | Glutathione |
| HCC | Hepatocellular carcinoma |
| HEF | Hepcidin gene mutation |
| IL | Interleukin |
| LPS | lipopolysaccharides |
| MAFLD | Metabolic Associated Fatty Liver Disease |
| MCV | Mean corpuscular erythrocyte volume |
| MEOS | Microsomal ethanol-oxidizing system |
| MiRNA | Micro-Ribonucleic acid |
| MIF | Migration inflammatory factor |
| mi-RNA | Micro-RNA |
| MS | Metabolic syndrome |
| NAFLD | Nonalcoholic fatty liver disease |
| NAC | N-acetylcysteine |
| NHANES | National Health and Nutrition Examination Survey |
| NAS | NASH fibrosis score |
| NK | Natural killer |
| NOX-4 | NADH oxidase 4 |
| OSBP | Oxysterol-binding protein |
| PAMP | pathogen-associated molecular pattern. |
| Peth | phosphatidylethanol |
| PLP | patatin-like phospholipases |
| PPARg-C1a | peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| ROS | Reactive oxygen species |
| SCoAD | stearoyl-CoA desaturase |
| SGLT2 | sodium/glucose transport protein 2 |
| SRBEP | Sterol regulatory element binding proteins |
| SSB | sweet sugary beverages |
| T2D | Type 2 Diabetes |
| TLR | toll like receptor |
| ULN | upper limit of normal |
References
- Lieber, C.S. Role of oxidative stress and antioxidant therapy in alcoholic and nonalcoholic liver diseases. Adv. Pharmacol. 1997, 38, 601–628. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, J.; Viggiano, T.R.; McGill, D.B.; Oh, B.J. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin. Proc. 1980, 55, 434–438. [Google Scholar] [PubMed]
- Kumarendran, B.; O’Reilly, M.W.; Manolopoulos, K.N.; Toulis, K.A.; Gokhale, K.M.; Sitch, A.J.; Wijeyaratne, C.N.; Coomarasamy, A.; Arlt, W.; Nirantharakumar, K. Polycystic ovary syndrome, androgen excess, and the risk of nonalcoholic fatty liver disease in women: A longitudinal study based on a United Kingdom primary care database. PLoS Med. 2018, 15, e1002542. [Google Scholar] [CrossRef] [PubMed]
- Thapa, K.; Grewal, A.S.; Kanojia, N.; Rani, L.; Sharma, N.; Singh, S. Alcoholic and Non-Alcoholic Liver Diseases: Promising Molecular Drug Targets and their Clinical Development. Curr. Drug Discov. Technol. 2021, 18, 333–353. [Google Scholar] [CrossRef]
- Lazo, M.; Hernaez, R.; Eberhardt, M.S.; Bonekamp, S.; Kamel, I.; Guallar, E.; Koteish, A.; Brancati, F.L.; Clark, J.M. Prevalence of nonalcoholic fatty liver disease in the United States: The Third National Health and Nutrition Examination Survey, 1988–1994. Am. J. Epidemiol. 2013, 178, 38–45. [Google Scholar] [CrossRef]
- Lee, R.G. Nonalcoholic steatohepatitis: A study of 49 patients. Hum. Pathol. 1989, 20, 594–598. [Google Scholar] [CrossRef]
- Powell, E.E.; Cooksley, W.G.; Hanson, R.; Searle, J.; Halliday, J.W.; Powell, L.W. The natural history of nonalcoholic steatohepatitis: A follow-up study of forty-two patients for up to 21 years. Hepatology 1990, 11, 74–80. [Google Scholar] [CrossRef]
- Diehl, A.M.; Goodman, Z.; Ishak, K.G. Alcohol like liver disease in nonalcoholics. A clinical and histologic comparison with alcohol-induced liver injury. Gastroenterology 1988, 95, 1056–1062. [Google Scholar] [CrossRef]
- Angulo, P.; Keach, J.C.; Batts, K.P.; Lindor, K.D. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology 1999, 30, 1356–1362. [Google Scholar] [CrossRef]
- Matteoni, C.A.; Younossi, Z.M.; Gramlich, T.; Boparai, N.; Liu, Y.C.; McCullough, A.J. Nonalcoholic fatty liver disease: A spectrum of clinical and pathological severity. Gastroenterology 1999, 116, 1413–1419. [Google Scholar] [CrossRef]
- Cortez-Pinto, H.; Camilo, M.E.; Baptista, A.; De Oliveira, A.G.; De Moura, M.C. Non-alcoholic fatty liver: Another feature of the metabolic syndrome? Clin. Nutr. 1999, 18, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.D.; Stengel, J.; Asike, M.I.; Torres, D.M.; Shaw, J.; Contreras, M.; Landt, C.L.; Harrison, S.A. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: A prospective study. Gastroenterology 2011, 140, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Browning, J.D.; Szczepaniak, L.S.; Dobbins, R.; Nuremberg, P.; Horton, J.D.; Cohen, J.C.; Grundy, S.M.; Hobbs, H.H. Prevalence of hepatic steatosis in an urban population in the United States: Impact of ethnicity. Hepatology 2004, 40, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Bacon, B.R.; Farahvash, M.J.; Janney, C.G.; Neuschwander-Tetri, B.A. Nonalcoholic steatohepatitis: An expanded clinical entity. Gastroenterology 1994, 107, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Arun, J.; Clements, R.H.; Lazenby, A.J.; Leeth, R.R.; Abrams, G.A. The prevalence of nonalcoholic steatohepatitis is greater in morbidly obese men compared to women. Obes. Surg. 2006, 16, 1351–1358. [Google Scholar] [CrossRef]
- Marchesini, G.; Bugianesi, E.; Forlani, G.; Cerrelli, F.; Lenzi, M.; Manini, R.; Natale, S.; Vanni, E.; Villanova, N.; Melchionda, N.; et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology 2003, 37, 917–923. [Google Scholar] [CrossRef]
- Chitturi, S.; Abeygunasekera, S.; Farrell, G.C.; Holmes-Walker, J.; Hui, J.M.; Fung, C.; Karim, R.; Lin, R.; Samarasinghe, D.; Liddle, C.; et al. NASH and insulin resistance: Insulin hypersecretion and specific association with the insulin resistance syndrome. Hepatology 2002, 35, 373–379. [Google Scholar] [CrossRef]
- Younossi, Z.M. Non-alcoholic fatty liver disease—A global public health perspective. J. Hepatol. 2019, 70, 531–544. [Google Scholar] [CrossRef]
- Wong, V.W.S.; Chu, W.C.W.; Wong, G.L.H.; Chan, R.; Chim, A.M.-L.; Ong, A.; Yeung, D.K.-W.; Yiu, K.K.-L.; Chu, S.H.-T.; Woo, J.; et al. Prevalence of non-alcoholic fatty liver disease and advanced fibrosis in Hong Kong Chinese: A population study using proton-magnetic resonance spectroscopy and transient elastography. Gut 2011, 61, 409–415. [Google Scholar] [CrossRef]
- Wong, T.; Dang, K.; Ladhani, S.; Singal, A.K.; Wong, R.J. Prevalence of Alcoholic Fatty Liver Disease Among Adults in the United States, 2001–2016. JAMA 2019, 321, 1723–1725. [Google Scholar] [CrossRef]
- Peery, A.F.; Crockett, S.D.; Murphy, C.C.; Lund, J.L.; Dellon, E.S.; Williams, J.L.; Jensen, E.T.; Shaheen, N.J.; Barritt, A.S.; Lieber, S.R.; et al. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2018. Gastroenterology 2019, 156, 254–272.e11. [Google Scholar] [CrossRef]
- Askgaard, G.; Grønbæk, M.; Kjær, M.S.; Tjønneland, A.; Tolstrup, J.S. Alcohol drinking pattern and risk of alcoholic liver cirrhosis: A prospective cohort study. J. Hepatol. 2015, 62, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Traversy, G.; Chaput, J.P. Alcohol Consumption and Obesity: An Update. Curr. Obes. Rep. 2015, 4, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Crabb, D.W.; Im, G.Y.; Szabo, G.; Mellinger, J.L.; Lucey, M.R. Diagnosis and Treatment of Alcohol-Associated Liver Diseases: 2019 Practice Guidance From the American Association for the Study of Liver Diseases. Hepatology 2020, 71, 306–333. [Google Scholar] [CrossRef] [PubMed]
- Dam, M.K.; Flensborg-Madsen, T.; Eliasen, M.; Becker, U.; Tolstrup, J.S. Smoking and risk of liver cirrhosis: A population-based cohort study. Scand. J. Gastroenterol. 2013, 48, 585–591. [Google Scholar] [CrossRef]
- Lelbach, W.K. Cirrhosis in the alcoholic and its relation to the volume of alcohol abuse. Ann. N. Y. Acad. Sci. 1975, 252, 85–105. [Google Scholar] [CrossRef]
- Kaplowitz, N.; Than, T.A.; Shinohara, M.; Ji, C. Endoplasmic reticulum stress and liver injury. Semin. Liver Dis. 2007, 27, 367–377. [Google Scholar] [CrossRef]
- Shiba, S.; Nakamoto, N.; Chu, P.S.; Ojiro, K.; Taniki, N.; Yamaguchi, A.; Morikawa, R.; Katayama, T.; Yoshida, A.; Aoki, R.; et al. Acetaldehyde exposure underlies functional defects in monocytes induced by excessive alcohol consumption. Sci. Rep. 2021, 11, 13690. [Google Scholar] [CrossRef]
- Frezza, M.; di Padova, C.; Pozzato, G.; Terpin, M.; Baraona, E.; Lieber, C.S. High blood alcohol levels in women. The role of decreased gastric alcohol dehydrogenase activity and first-pass metabolism. N. Engl. J. Med. 1990, 322, 95–99. [Google Scholar] [CrossRef]
- Thuluvath, P.; Wojno, K.J.; Yardley, J.H.; Mezey, E. Effects of Helicobacter pylori infection and gastritis on gastric alcohol dehydrogenase activity. Alcohol. Clin. Exp. Res. 1994, 18, 795–798. [Google Scholar] [CrossRef]
- Walker, J.E.C.; Gordon, E.R. Biochemical aspects associated with an ethanol-induced fatty liver. Biochem. J. 1970, 119, 511–516. [Google Scholar] [CrossRef] [PubMed]
- You, M.; Fischer, M.; Deeg, M.A.; Crabb, D.W. Ethanol induces fatty acid synthesis pathways by activation of sterol regulatory element-binding protein (SREBP). J. Biol. Chem. 2002, 277, 29342–29347. [Google Scholar] [CrossRef] [PubMed]
- Lazo, M.; Bilal, U.; Mitchell, M.C.; Potter, J.; Hernaez, R.; Clark, J.M. Interaction Between Alcohol Consumption and PNPLA3 Variant in the Prevalence of Hepatic Steatosis in the US Population. Clin. Gastroenterol. Hepatol. 2021, 19, 2606–2614.e4. [Google Scholar] [CrossRef]
- Li, S.; Tan, H.Y.; Wang, N.; Feng, Y.; Wang, X.; Feng, Y. Recent Insights Into the Role of Immune Cells in Alcoholic Liver Disease. Front. Immunol. 2019, 10, 1328. [Google Scholar] [CrossRef] [PubMed]
- Sheron, N.; Bird, G.; Koskinas, J.; Portmann, B.; Ceska, M.; Lindley, I.; William, R. Circulating and tissue levels of the neutrophil chemotaxin interleukin-8 are elevated in severe acute alcoholic hepatitis, and tissue levels correlate with neutrophil infiltration. Hepatology 1993, 18, 41–46. [Google Scholar] [PubMed]
- Artru, F.; Bou Saleh, M.; Maggiotto, F.; Lassailly, G.; Ningarhari, M.; Demaret, J.; Ntandja-Wandji, L.-C.; de Barros, J.-P.P.; Labreuche, J.; Drumez, E.; et al. IL-33/ST2 pathway regulates neutrophil migration and predicts outcome in patients with severe alcoholic hepatitis. J. Hepatol. 2020, 72, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, K.L.; Fan, X.; Kibler, C.D.; Huang, E.; Wu, X.; McMullen, M.R.; Leng, L.; Bucala, R.; Ventura-Cots, M.; Argemi, J.; et al. Role of MIF in coordinated expression of hepatic chemokines in patients with alcohol-associated hepatitis. JCI Insight 2021, 6, 141420. [Google Scholar] [CrossRef]
- Niemelä, O.; Parkkila, S.; Ylä-Herttuala, S.; Halsted, C.; Witztum, J.L.; Lanca, A.; Israel, Y. Covalent protein adducts in the liver as a result of ethanol metabolism and lipid peroxidation. Lab. Investig. 1994, 70, 537–546. [Google Scholar] [PubMed]
- Colmenero, J.; Bataller, R.; Sancho-Bru, P.; Bellot, P.; Miquel, R.; Moreno, M.; Jares, P.; Bosch, J.; Arroyo, V.; Caballería, J.; et al. Hepatic expression of candidate genes in patients with alcoholic hepatitis: Correlation with disease severity. Gastroenterology 2007, 132, 687–697. [Google Scholar] [CrossRef]
- McClain, C.; Barve, S.; Joshi-Barve, S.; Song, Z.; Deaciuc, I.; Chen, T.; Hill, D. Dysregulated cytokine metabolism, altered hepatic methionine metabolism and proteasome dysfunction in alcoholic liver disease. Alcohol. Clin. Exp. Res. 2005, 29 (Suppl. 11), 180S–188S. [Google Scholar] [CrossRef]
- Ma, H.Y.; Yamamoto, G.; Xu, J.; Liu, X.; Karin, D.; Kim, J.Y.; Alexandrov, L.B.; Koyama, Y.; Nishio, T.; Benner, C.; et al. IL-17 signaling in steatotic hepatocytes and macrophages promotes hepatocellular carcinoma in alcohol-related liver disease. J. Hepatol. 2020, 72, 946–959. [Google Scholar] [CrossRef] [PubMed]
- Nanji, A.A.; Zakim, D.; Rahemtulla, A.; Daly, T.; Miao, L.; Zhao, S.; Khwaja, S.; Tahan, S.R.; Dannenberg, A.J. Dietary saturated fatty acids down-regulate cyclooxygenase-2 and tumor necrosis factor alfa and reverse fibrosis in alcohol-induced liver disease in the rat. Hepatology 1997, 26, 1538–1545. [Google Scholar] [CrossRef] [PubMed]
- Parola, M.; Robino, G. Oxidative stress-related molecules and liver fibrosis. J. Hepatol. 2001, 35, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Sampey, B.P.; Stewart, B.J.; Petersen, D.R. Ethanol-induced modulation of hepatocellular extracellular signal-regulated kinase-1/2 activity via 4-hydroxynonenal. J. Biol. Chem. 2007, 282, 1925–1937. [Google Scholar] [CrossRef]
- Sastre, J.; Serviddio, G.; Pereda, J.; Minana, J.B.; Arduini, A.; Vendemiale, G.; Poli, G.; Pallardo, F.V.; Vina, J. Mitochondrial function in liver disease. Front. Biosci. 2007, 12, 1200–1209. [Google Scholar] [CrossRef]
- Blaya, D.; Coll, M.; Rodrigo-Torres, D.; Vila-Casadesús, M.; Altamirano, J.; Llopis, M.; Graupera, I.; Perea, L.; Aguilar-Bravo, B.; Díaz, A.; et al. Integrative microRNA profiling in alcoholic hepatitis reveals a role for microRNA-182 in liver injury and inflammation. Gut 2016, 65, 1535–1545. [Google Scholar] [CrossRef]
- Wang, X.; He, Y.; Mackowiak, B.; Gao, B. MicroRNAs as regulators, biomarkers and therapeutic targets in liver diseases. Gut 2021, 70, 784–795. [Google Scholar] [CrossRef]
- Torres, J.L.; Novo-Veleiro, I.; Manzanedo, L.; Alvela-Suárez, L.; Macías, R.; Laso, F.J.; Marcos, M. Role of microRNAs in alcohol-induced liver disorders and non-alcoholic fatty liver disease. World J. Gastroenterol. 2018, 24, 4104–4118. [Google Scholar] [CrossRef]
- Zuo, Z.; Li, Y.; Zeng, C.; Xi, Y.; Tao, H.; Guo, Y. Integrated Analyses Identify Key Molecules and Reveal the Potential Mechanism of miR-182-5p/FOXO1 Axis in Alcoholic Liver Disease. Front. Med. 2021, 8, 767584. [Google Scholar] [CrossRef]
- Straka, Ľ.; Janík, M.; Novomeský, F.; Komáreková, I.; Krajčovič, J.; Štuller, F. Alcohol congeners and their implications for medicolegal assessment of drunkeness. Soud. Lek. 2015, 60, 7–8. [Google Scholar]
- Roerecke, M.; Nanau, R.; Rehm, J.; Neuman, M. Ethnicity matters: A Systematic Review and Meta-Analysis of the Non-Linear Relationship Between Alcohol Consumption and Prevalence and Incidence of Hepatic Steatosis. EBioMedicine 2016, 8, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Willner, I.R.; Waters, B.; Patil, S.R.; Reuben, A.; Morelli, J.; Riely, C.A. Ninety patients with nonalcoholic steatohepatitis: Insulin resistance, familial tendency, and severity of disease. Am. J. Gastroenterol. 2001, 96, 2957–2961. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, H.J.; Lee, K.E.; Kim, D.J.; Kim, S.K.; Ahn, C.W.; Lim, S.-K.; Kim, K.R.; Lee, H.C.; Huh, K.B.; et al. Metabolic significance of nonalcoholic fatty liver disease in nonobese, nondiabetic adults. Arch. Intern. Med. 2004, 164, 2169–2175. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.F.; Dufour, S.; Hariri, A.; Nelson-Williams, C.; Foo, J.N.; Zhang, X.-M.; Dziura, J.; Lifton, R.P.; Shulman, G.I. Apolipoprotein C3 gene variants in nonalcoholic fatty liver disease. N. Engl. J. Med. 2010, 362, 1082–1089. [Google Scholar] [CrossRef]
- Carulli, L.; Canedi, I.; Rondinella, S.; Lombardini, S.; Ganazzi, D.; Fargion, S.; De Palma, M.; Lonardo, A.; Ricchi, M.; Bertolotti, M.; et al. Genetic polymorphisms in non-alcoholic fatty liver disease: Interleukin-6-174G/C polymorphism is associated with non-alcoholic steatohepatitis. Dig. Liver Dis. 2009, 41, 823–828. [Google Scholar] [CrossRef]
- Rotman, Y.; Koh, C.; Zmuda, J.M.; Kleiner, D.E.; Liang, T.J.; The NASH CRN. The association of genetic variability in patatin-like phospholipase domain-containing protein 3 (PNPLA3) with histological severity of nonalcoholic fatty liver disease. Hepatology 2010, 52, 894–903. [Google Scholar] [CrossRef]
- Sookoian, S.; Rosselli, M.S.; Gemma, C.; Burgueño, A.L.; Gianotti, T.F.; Castaño, G.O.; Pirola, C.J. Epigenetic regulation of insulin resistance in nonalcoholic fatty liver disease: Impact of liver methylation of the peroxisome proliferator-activated receptor γ coactivator 1α promoter. Hepatology 2010, 52, 1992–2000. [Google Scholar] [CrossRef]
- Domenici, F.A.; Brochado, M.J.F.; Martinelli, A.D.L.C.; Zucoloto, S.; da Cunha, S.F.D.C.; Vannucchi, H. Peroxisome proliferator-activated receptors alpha and gamma2 polymorphisms in nonalcoholic fatty liver disease: A study in Brazilian patients. Gene 2013, 529, 326–331. [Google Scholar] [CrossRef]
- Lin, Y.C.; Chang, P.F.; Hu, F.C.; Yang, W.S.; Chang, M.H.; Ni, Y.H. A common variant in the PNPLA3 gene is a risk factor for non-alcoholic fatty liver disease in obese Taiwanese children. J. Pediatr. 2011, 158, 740–744. [Google Scholar] [CrossRef]
- Gastaldelli, A.; Cusi, K.; Pettiti, M.; Hardies, J.; Miyazaki, Y.; Berria, R.; Buzzigoli, E.; Sironi, A.M.; Cersosimo, E.; Ferrannini, E.; et al. Relationship between hepatic/visceral fat and hepatic insulin resistance in nondiabetic and type 2 diabetic subjects. Gastroenterology 2007, 133, 496–506. [Google Scholar] [CrossRef]
- van der Poorten, D.; Milner, K.L.; Hui, J.; Hodge, A.; Trenell, M.I.; Kench, J.G.; London, R.; Peduto, T.; Chisholm, D.J.; George, J. Visceral fat: A key mediator of steatohepatitis in metabolic liver disease. Hepatology 2008, 48, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Wieckowska, A.; Papouchado, B.G.; Li, Z.; Lopez, R.; Zein, N.N.; Feldstein, A.E. Increased hepatic and circulating interleukin-6 levels in human nonalcoholic steatohepatitis. Am. J. Gastroenterol. 2008, 103, 1372–1379. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, L.; Vivanti, A.; Cavalera, M.; Marzano, V.; Ronci, M.; Fabrizi, M.; Menini, S.; Pugliese, G.; Menghini, R.; Khokha, R.; et al. Increased tumor necrosis factor alpha-converting enzyme activity induces insulin resistance and hepatosteatosis in mice. Hepatology 2010, 51, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Calvert, V.S.; Collantes, R.; Elariny, H.; Afendy, A.; Baranova, A.; Mendoza, M.; Goodman, Z.; Liotta, L.A.; Petricoin, E.F.; Younossi, Z.M. A systems biology approach to the pathogenesis of obesity-related nonalcoholic fatty liver disease using reverse phase protein microarrays for multiplexed cell signaling analysis. Hepatology 2007, 46, 166–172. [Google Scholar] [CrossRef]
- Kral, J.G.; Lundholm, K.; Björntorp, P.; Sjöström, L.; Scherstén, T. Hepatic lipid metabolism in severe human obesity. Metabolism 1977, 26, 1025–1031. [Google Scholar] [CrossRef]
- Ferrannini, E.; Barrett, E.J.; Bevilacqua, S.; DeFronzo, R.A. Effect of fatty acids on glucose production and utilization in man. J. Clin. Investig. 1983, 72, 1737–1747. [Google Scholar] [CrossRef]
- Angulo, P. Nonalcoholic fatty liver disease. N. Engl. J. Med. 2002, 346, 1221–1231. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Campbell-Sargent, C.; Mirshahi, F.; Rizzo, W.B.; Contos, M.J.; Sterling, R.K.; Luketic, V.A.; Shiffman, M.L.; Clore, J.N. Nonalcoholic steatohepatitis: Association of insulin resistance and mitochondrial abnormalities. Gastroenterology 2001, 120, 1183–1192. [Google Scholar] [CrossRef]
- Sreekumar, R.; Rosado, B.; Rasmussen, D.; Charlton, M. Hepatic gene expression in histologically progressive nonalcoholic steatohepatitis. Hepatology 2003, 38, 244–251. [Google Scholar] [CrossRef]
- Rensen, S.S.; Slaats, Y.; Driessen, A.; Peutz-Kootstra, C.J.; Nijhuis, J.; Steffensen, R.; Greve, J.W.; Buurman, W.A. Activation of the complement system in human nonalcoholic fatty liver disease. Hepatology 2009, 50, 1809–1817. [Google Scholar] [CrossRef]
- Rensen, S.S.; Slaats, Y.; Nijhuis, J.; Jans, A.; Bieghs, V.; Driessen, A.; Malle, E.; Greve, J.W.; Buurman, W.A. Increased hepatic myeloperoxidase activity in obese subjects with nonalcoholic steatohepatitis. Am. J. Pathol. 2009, 175, 1473–1482. [Google Scholar] [CrossRef] [PubMed]
- Tajiri, K.; Shimizu, Y.; Tsuneyama, K.; Sugiyama, T. Role of liver-infiltrating CD3+CD56+ natural killer T cells in the pathogenesis of nonalcoholic fatty liver disease. Eur. J. Gastroenterol. Hepatol. 2009, 21, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Guy, C.D.; Suzuki, A.; Zdanowicz, M.; Abdelmalek, M.F.; Burchette, J.; Unalp, A.; Diehl, A.M.; The NASH CRN. Hedgehog pathway activation parallels histologic severity of injury and fibrosis in human nonalcoholic fatty liver disease. Hepatology 2012, 55, 1711–1721. [Google Scholar] [CrossRef]
- Machado, M.V.; Michelotti, G.A.; Pereira, T.D.A.; Boursier, J.; Kruger, L.; Swiderska-Syn, M.; Karaca, G.; Xie, G.; Guy, C.D.; Bohinc, B.; et al. Reduced lipoapoptosis, hedgehog pathway activation and fibrosis in caspase-2 deficient mice with non-alcoholic steatohepatitis. Gut 2015, 64, 1148–1157. [Google Scholar] [CrossRef] [PubMed]
- Valenti, L.; Fracanzani, A.L.; Bugianesi, E.; Dongiovanni, P.; Galmozzi, E.; Vanni, E.; Canavesi, E.; Lattuada, E.; Roviaro, G.; Marchesini, G.; et al. HFE genotype, parenchymal iron accumulation, and liver fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology 2010, 138, 905–912. [Google Scholar] [CrossRef] [PubMed]
- George, D.K.; Goldwurm, S.; MacDonald, G.A.; Cowley, L.L.; Walker, N.I.; Ward, P.J.; Jazwinska, E.C.; Powell, L.W. Increased hepatic iron concentration in nonalcoholic steatohepatitis is associated with increased fibrosis. Gastroenterology 1998, 114, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, L.M.; Powell, L.W. Hemochromatosis and alcoholic liver disease. Alcohol 2003, 30, 131–136. [Google Scholar] [CrossRef]
- Nelson, J.E.; Wilson, L.; Brunt, E.M.; Yeh, M.M.; Kleiner, D.E.; Unalp-Arida, A.; Kowdley, K.V. Relationship between the pattern of hepatic iron deposition and histological severity in nonalcoholic fatty liver disease. Hepatology 2011, 53, 448–457. [Google Scholar] [CrossRef]
- Mendler, M.H.; Turlin, B.; Moirand, R.; Jouanolle, A.-M.; Sapey, T.; Guyader, D.; le Gall, J.-Y.; Brissot, P.; David, V.; Deugnier, Y. Insulin resistance-associated hepatic iron overload. Gastroenterology 1999, 117, 1155–1163. [Google Scholar] [CrossRef]
- Bonkovsky, H.L.; Jawaid, Q.; Tortorelli, K.; LeClair, P.; Cobb, J.; Lambrecht, R.W.; Banner, B.F. Non-alcoholic steatohepatitis and iron: Increased prevalence of mutations of the HFE gene in non-alcoholic steatohepatitis. J. Hepatol. 1999, 31, 421–429. [Google Scholar] [CrossRef]
- Chitturi, S.; Weltman, M.; Farrell, G.C.; McDonald, D.; Liddle, C.; Samarasinghe, D.; Lin, R.; Abeygunasekera, S.; George, J. HFE mutations, hepatic iron, and fibrosis: Ethnic-specific association of NASH with C282Y but not with fibrotic severity. Hepatology 2002, 36, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Gramlich, T.; Bacon, B.R.; Matteoni, C.A.; Boparai, N.; O’Neill, R.; McCullough, A.J. Hepatic iron and nonalcoholic fatty liver disease. Hepatology 1999, 30, 847–850. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.A.; Crawford, D.H.; Stuart, K.; House, M.J.; Pierre, T.G.S.; Webb, M.; Ching, H.L.; Kava, J.; Bynevelt, M.; MacQuillan, G.C.; et al. The impact of phlebotomy in nonalcoholic fatty liver disease: A prospective, randomized, controlled trial. Hepatology 2015, 61, 1555–1564. [Google Scholar] [CrossRef] [PubMed]
- Viganò, M.; Vergani, A.; Trombini, P.; Paleari, F.; Piperno, A. Insulin resistance influence iron metabolism and hepatic steatosis in type II diabetes. Gastroenterology 2000, 118, 986–987. [Google Scholar] [CrossRef] [PubMed]
- Nobili, V.; Manco, M.; Ciampalini, P.; Diciommo, V.; Devito, R.; Piemonte, F.; Comparcola, D.; Guidi, R.; Marcellini, M. Leptin, free leptin index, insulin resistance and liver fibrosis in children with non-alcoholic fatty liver disease. Eur. J. Endocrinol. 2006, 155, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Musso, G.; Gambino, R.; Durazzo, M.; Biroli, G.; Carello, M.; Fagà, E.; Pacini, G.; De Michieli, F.; Rabbione, L.; Premoli, A.; et al. Adipokines in NASH: Postprandial lipid metabolism as a link between adiponectin and liver disease. Hepatology 2005, 42, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Muse, E.D.; Obici, S.; Bhanot, S.; Monia, B.P.; McKay, R.A.; Rajala, M.W.; Scherer, P.E.; Rossetti, L. Role of resistin in diet-induced hepatic insulin resistance. J. Clin. Investig. 2004, 114, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.J.; Gaunt, P.; Aithal, G.P.; Barton, D.; Hull, D.; Parker, R.; Hazlehurst, J.M.; Guo, K.; Abouda, G.; Aldersley, M.A.; et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): A multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 2016, 387, 679–690. [Google Scholar] [CrossRef]
- The Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef]
- Bajaj, J.S. Alcohol, liver disease and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 235–246. [Google Scholar] [CrossRef]
- Abdul-Hai, A.; Abdallah, A.; Malnick, S.D. Influence of gut bacteria on development and progression of non-alcoholic fatty liver disease. World J. Hepatol. 2015, 7, 1679–1684. [Google Scholar] [CrossRef] [PubMed]
- Vancamelbeke, M.; Vermeire, S. The intestinal barrier: A fundamental role in health and disease. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Fukui, H.; Brauner, B.; Bode, J.C.; Bode, C. Plasma endotoxin concentrations in patients with alcoholic and non-alcoholic liver disease: Reevaluation with an improved chromogenic assay. J. Hepatol. 1991, 12, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Bode, C.; Kugler, V.; Bode, J.C. Endotoxemia in patients with alcoholic and non-alcoholic cirrhosis and in subjects with no evidence of chronic liver disease following acute alcohol excess. J. Hepatol. 1987, 4, 8–14. [Google Scholar] [CrossRef]
- Lin, R.S.; Lee, F.Y.; Lee, S.D.; Tsai, Y.-T.; Lin, H.C.; Rei-Hwa, L.; Wan-Ching, H.; Cheng-Chun, H.; Sun-Sang, W.; Kwang-Juei, L. Endotoxemia in patients with chronic liver diseases: Relationship to severity of liver diseases, presence of esophageal varices, and hyperdynamic circulation. J. Hepatol. 1995, 22, 165–172. [Google Scholar] [CrossRef]
- Purohit, V.; Bode, J.C.; Bode, C.; Brenner, D.; Choudhry, M.A.; Hamilton, F.; Kang, Y.J.; Keshavarzian, A.; Rao, R.; Sartor, R.B.; et al. Alcohol, intestinal bacterial growth, intestinal permeability to endotoxin, and medical consequences: Summary of a symposium. Alcohol 2008, 42, 349–361. [Google Scholar] [CrossRef]
- Ansaldo, E.; Farley, T.K.; Belkaid, Y. Control of Immunity by the Microbiota. Annu. Rev. Immunol. 2021, 39, 449–479. [Google Scholar] [CrossRef]
- Casafont Morencos, F.; de las Heras Castaño, G.; Martín Ramos, L.; López Arias, M.J.; Ledesma, F.; Pons Romero, F. Small bowel bacterial overgrowth in patients with alcoholic cirrhosis. Dig. Dis. Sci. 1996, 41, 552–556. [Google Scholar] [CrossRef]
- Llopis, M.; Cassard, A.M.; Wrzosek, L.; Boschat, L.; Bruneau, A.; Ferrere, G.; Puchois, V.; Martin, J.C.; Lepage, P.; Le Roy, T.; et al. Intestinal microbiota contributes to individual susceptibility to alcoholic liver disease. Gut 2016, 65, 830–839. [Google Scholar] [CrossRef]
- Leclercq, S.; Matamoros, S.; Cani, P.D.; Neyrinck, A.M.; Jamar, F.; Stärkel, P.; Windey, K.; Tremaroli, V.; Bäckhed, F.; Verbeke, K.; et al. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc. Natl. Acad. Sci. USA 2014, 111, E4485–E4493. [Google Scholar] [CrossRef]
- Chu, H.; Duan, Y.; Lang, S.; Jiang, L.; Wang, Y.; Llorente, C.; Liu, J.; Mogavero, S.; Bosques-Padilla, F.; Abraldes, J.G.; et al. The Candida albicans exotoxin candidalysin promotes alcohol-associated liver disease. J. Hepatol. 2020, 72, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.M.; Inamine, T.; Hochrath, K.; Chen, P.; Wang, L.; Llorente, C.; Bluemel, S.; Hartmann, P.; Xu, J.; Koyama, Y.; et al. Intestinal fungi contribute to development of alcoholic liver disease. J. Clin. Investig. 2017, 127, 2829–2841. [Google Scholar] [CrossRef] [PubMed]
- Puri, P.; Liangpunsakul, S.; Christensen, J.E.; Shah, V.H.; Kamath, P.S.; Gores, G.J.; Walker, S.; Comerford, M.; Katz, B.; Borst, A.; et al. The circulating microbiome signature and inferred functional metagenomics in alcoholic hepatitis. Hepatology 2018, 67, 1284–1302. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, E.A.; Gillevet, P.M.; Rangwala, H.; Sikaroodi, M.; Naqvi, A.; Engen, P.A.; Kwasny, M.; Lau, C.K.; Keshavarzian, A. Colonic microbiome is altered in alcoholism. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G966–G978. [Google Scholar] [CrossRef]
- Couch, R.D.; Dailey, A.; Zaidi, F.; Navarro, K.; Forsyth, C.B.; Mutlu, E.; Engen, P.A.; Keshavarzian, A. Alcohol induced alterations to the human fecal VOC metabolome. PLoS ONE 2015, 10, e0119362. [Google Scholar] [CrossRef]
- Dantzer, R.; Kelley, K.W. Twenty years of research on cytokine-induced sickness behavior. Brain Behav. Immun. 2007, 21, 153–160. [Google Scholar] [CrossRef]
- Henao-Mejia, J.; Elinav, E.; Jin, C.; Hao, L.; Mehal, W.Z.; Strowig, T.; Thaiss, C.A.; Kau, A.L.; Eisenbarth, S.C.; Jurczak, M.J.; et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 2012, 482, 179–185. [Google Scholar] [CrossRef]
- Le Roy, T.; Llopis, M.; Lepage, P.; Bruneau, A.; Rabot, S.; Bevilacqua, C.; Martin, P.; Philippe, C.; Walker, F.; Bado, A.; et al. Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut 2013, 62, 1787–1794. [Google Scholar] [CrossRef]
- Farrell, G.; Schattenberg, J.M.; Leclercq, I.; Yeh, M.M.; Goldin, R.; Teoh, N.; Schuppan, D. Mouse Models of Nonalcoholic Steatohepatitis: Toward Optimization of Their Relevance to Human Nonalcoholic Steatohepatitis. Hepatology 2019, 69, 2241–2257. [Google Scholar] [CrossRef]
- Chiu, C.C.; Ching, Y.H.; Li, Y.P.; Liu, J.-Y.; Huang, Y.-T.; Huang, Y.-W.; Yang, S.-S.; Huang, W.-C.; Chuang, H.-L. Nonalcoholic Fatty Liver Disease Is Exacerbated in High-Fat Diet-Fed Gnotobiotic Mice by Colonization with the Gut Microbiota from Patients with Nonalcoholic Steatohepatitis. Nutrients 2017, 9, 1220. [Google Scholar] [CrossRef]
- Hoyles, L.; Fernández-Real, J.M.; Federici, M.; Serino, M.; Abbott, J.; Charpentier, J.; Heymes, C.; Luque, J.L.; Anthony, E.; Barton, R.H.; et al. Molecular phenomics and metagenomics of hepatic steatosis in non-diabetic obese women. Nat. Med. 2018, 24, 1070–1080. [Google Scholar] [CrossRef] [PubMed]
- Elshaghabee, F.M.; Bockelmann, W.; Meske, D.; de Vrese, M.; Walte, H.G.; Schrezenmeir, J.; Heller, K.J. Ethanol Production by Selected Intestinal Microorganisms and Lactic Acid Bacteria Growing under Different Nutritional Conditions. Front. Microbiol. 2016, 7, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Elshaghabee, F.M.F.; Ghadimi, D.; Habermann, D.; de Vrese, M.; Bockelmann, W.; Kaatsch, H.J.; Heller, K.J.; Schrezenmeir, J. Effect of Oral Administration of Weissella confusa on Fecal and Plasma Ethanol Concentrations, Lipids and Glucose Metabolism in Wistar Rats Fed High Fructose and Fat Diet. Hepatic Med. 2020, 12, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Mezey, E.; Imbembo, A.L.; Potter, J.J.; Rent, K.C.; Lombardo, R.; Holt, P.R. Endogenous ethanol production and hepatic disease following jejunoileal bypass for morbid obesity. Am. J. Clin. Nutr. 1975, 28, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Aron-Wisnewsky, J.; Vigliotti, C.; Witjes, J.; Le, P.; Holleboom, A.G.; Verheij, J.; Nieuwdorp, M.; Clément, K. Gut microbiota and human NAFLD: Disentangling microbial signatures from metabolic disorders. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 279–297. [Google Scholar] [CrossRef]
- Baraona, E.; Julkunen, R.; Tannenbaum, L.; Lieber, C.S. Role of intestinal bacterial overgrowth in ethanol production and metabolism in rats. Gastroenterology 1986, 90, 103–110. [Google Scholar] [CrossRef]
- Nair, S.; Cope, K.; Risby, T.H.; Diehl, A.M. Obesity and female gender increase breath ethanol concentration: Potential implications for the pathogenesis of nonalcoholic steatohepatitis. Am. J. Gastroenterol. 2001, 96, 1200–1204. [Google Scholar] [CrossRef]
- Sookoian, S.; Pirola, C.J. Systems Biology Elucidates Common Pathogenic Mechanisms between Nonalcoholic and Alcoholic-Fatty Liver Disease. PLoS ONE 2013, 8, e58895. [Google Scholar] [CrossRef]
- Gupta, A.; Osadchiy, V.; Mayer, E.A. Brain–gut–microbiome interactions in obesity and food addiction. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 655–672. [Google Scholar] [CrossRef]
- Mehacagel, W.Z.; Schwabe, R.F. A disease-promoting role of the intestinal mycobiome in non-alcoholic fatty liver disease. J. Hepatol. 2022, 76, 765–767. [Google Scholar] [CrossRef]
- Demir, M.; Lang, S.; Hartmann, P.; Duan, Y.; Martin, A.; Miyamoto, Y.; Bondareva, M.; Zhang, X.; Wang, Y.; Kasper, P.; et al. The fecal mycobiome in non-alcoholic fatty liver disease. J. Hepatol. 2022, 76, 788–799. [Google Scholar] [CrossRef] [PubMed]
- Malnick, S.D.H.; Fisher, D.; Somin, M.; Neuman, M.G. Treating the Metabolic Syndrome by Fecal Transplantation-Current Status. Biology 2021, 10, 447. [Google Scholar] [CrossRef] [PubMed]
- Kessoku, T.; Kobayashi, T.; Imajo, K.; Tanaka, K.; Yamamoto, A.; Takahashi, K.; Kasai, Y.; Ozaki, A.; Iwaki, M.; Nogami, A.; et al. Endotoxins and Non-Alcoholic Fatty Liver Disease. Front. Endocrinol. 2021, 12, 770986. [Google Scholar] [CrossRef] [PubMed]
- Nier, A.; Huber, Y.; Labenz, C.; Michel, M.; Bergheim, I.; Schattenberg, J.M. Adipokines and Endotoxemia Correlate with Hepatic Steatosis in Non-Alcoholic Fatty Liver Disease (NAFLD). Nutrients 2020, 12, 699. [Google Scholar] [CrossRef]
- Lee, K.H.; Bartsch, H.; Nair, J.; Yoo, D.-H.; Hong, Y.-C.; Cho, S.-H.; Kang, D. Effect of short-term fasting on urinary excretion of primary lipid peroxidation products and on markers of oxidative DNA damage in healthy women. Carcinogenesis 2006, 27, 1398–1403. [Google Scholar] [CrossRef]
- Carpino, G.; Pastori, D.; Baratta, F.; Overi, D.; Labbadia, G.; Polimeni, L.; Di Costanzo, A.; Pannitteri, G.; Carnevale, R.; Del Ben, M.; et al. PNPLA3 variant and portal/periportal histological pattern in patients with biopsy-proven non-alcoholic fatty liver disease: A possible role for oxidative stress. Sci. Rep. 2017, 7, 15756. [Google Scholar] [CrossRef]
- Masarone, M.; Rosato, V.; Dallio, M.; Gravina, A.G.; Aglitti, A.; Loguercio, C.; Federico, A.; Persico, M. Role of Oxidative Stress in Pathophysiology of Nonalcoholic Fatty Liver Disease. Oxidative Med. Cell. Longev. 2018, 2018, 9547613. [Google Scholar] [CrossRef]
- Gonzalez, A.; Huerta-Salgado, C.; Orozco-Aguilar, J.; Aguirre, F.; Tacchi, F.; Simon, F.; Cabello-Verrugio, C. Role of Oxidative Stress in Hepatic and Extrahepatic Dysfunctions during Nonalcoholic Fatty Liver Disease (NAFLD). Oxid. Med. Cell. Longev. 2020, 2020, 1617805. [Google Scholar] [CrossRef]
- Nascè, A.; Gariani, K.; Jornayvaz, F.R.; Szanto, I. NADPH Oxidases Connecting Fatty Liver Disease, Insulin Resistance and Type 2 Diabetes: Current Knowledge and Therapeutic Outlook. Antioxidants 2022, 11, 1131. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, W.; Zhong, W.; Sun, X.; Zhou, Z. Pharmacological inhibition of NOX4 ameliorates alcohol-induced liver injury in mice through improving oxidative stress and mitochondrial function. Biochim. Et Biophys. Acta Gen. Subj. 2017, 1861 Pt A, 2912–2921. [Google Scholar] [CrossRef]
- Heard, K.J. Acetylcysteine for acetaminophen poisoning. N. Engl. J. Med. 2008, 359, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Morley, K.C.; Baillie, A.; Van Den Brink, W.; Chitty, K.E.; Brady, K.; Back, S.E.; Seth, D.; Sutherland, G.; Leggio, L.; Haber, P.S. N-acetyl cysteine in the treatment of alcohol use disorder in patients with liver disease: Rationale for further research. Expert Opin. Investig. Drugs 2018, 27, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Ronis, M.J.J.; Butura, A.; Sampey, B.P.; Shankar, K.; Prior, R.L.; Korourian, S.; Albano, E.; Ingelman-Sundberg, M.; Petersen, D.R.; Badger, T.M. Effects of N-acetylcysteine on ethanol-induced hepatotoxicity in rats fed via total enteral nutrition. Free Radic. Biol. Med. 2005, 39, 619–630. [Google Scholar] [CrossRef]
- Yang, Y.M.; Cho, Y.E.; Hwang, S. Crosstalk between Oxidative Stress and Inflammatory Liver Injury in the Pathogenesis of Alcoholic Liver Disease. IJMS 2022, 23, 774. [Google Scholar] [CrossRef] [PubMed]
- Hock, B.; Schwarz, M.; Domke, I.; Grunert, V.P.; Wuertemberger, M.; Schiemann, U.; Horster, S.; Limmer, C.; Stecker, G.; Soyka, M. Validity of carbohydrate-deficient transferrin (%CDT), gamma-glutamyltransferase (gamma-GT) and mean corpuscular erythrocyte volume (MCV) as biomarkers for chronic alcohol abuse: A study in patients with alcohol dependence and liver disorders of non-alcoholic and alcoholic origin. Addiction 2005, 100, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Diehl, A.M. Liver disease in alcohol abusers: Clinical perspective. Alcohol 2002, 27, 7–11. [Google Scholar] [CrossRef]
- Amarapurka, D.N.; Amarapurkar, A.D.; Patel, N.D.; Agal, S.; Baigal, R.; Gupte, P.; Pramanik, S. Nonalcoholic steatohepatitis (NASH) with diabetes: Predictors of liver fibrosis. Ann. Hepatol. 2006, 5, 30–33. [Google Scholar] [CrossRef]
- Sorbi, D.; Boynton, J.; Lindor, K.D. The ratio of aspartate aminotransferase to alanine aminotransferase: Potential value in differentiating nonalcoholic steatohepatitis from alcoholic liver disease. Am. J. Gastroenterol. 1999, 94, 1018–1022. [Google Scholar] [CrossRef]
- Nyblom, H.; Berggren, U.; Balldin, J.; Olsson, R. High AST/ALT ratio may indicate advanced alcoholic liver disease rather than heavy drinking. Alcohol Alcohol. 2004, 39, 336–339. [Google Scholar] [CrossRef]
- Cohen, J.A.; Kaplan, M.M. The SGOT/SGPT ratio—An indicator of alcoholic liver disease. Dig. Dis. Sci. 1979, 24, 835–838. [Google Scholar] [CrossRef]
- Moussavian, S.N.; Becker, R.C.; Piepmeyer, J.L.; Mezey, E.; Bozian, R.C. Serum gamma-glutamyl transpeptidase and chronic alcoholism. Influence of alcohol ingestion and liver disease. Dig. Dis. Sci. 1985, 30, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Palmentieri, B.; de Sio, I.; La Mura, V.; Masarone, M.; Vecchione, R.; Bruno, S.; Torella, R.; Persico, M. The role of bright liver echo pattern on ultrasound B-mode examination in the diagnosis of liver steatosis. Dig. Liver Dis. 2006, 38, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Neuman, M.G.; Mueller, J.; Mueller, S. Non-invasive Biomarkers of Liver Inflammation and Cell Death in Response to Alcohol Detoxification. Front. Physiol. 2021, 12, 678118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.N.; Fowler, K.J.; Hamilton, G.; Cui, J.Y.; Sy, E.Z.; Balanay, M.; Hooker, J.C.; Szeverenyi, N.; Sirlin, C.B. Liver fat imaging a clinical overview of ultrasound, CT, and MR imaging. Br. J. Radiol. 2018, 91, 20170959. [Google Scholar] [CrossRef]
- Mofrad, P.; Contos, M.J.; Haque, M.; Sargeant, C.; Fisher, R.A.; Luketic, V.A.; Sterling, R.K.; Shiffman, M.L.; Stravitz, R.T.; Sanyal, A.J. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology 2003, 37, 1286–1292. [Google Scholar] [CrossRef]
- Kowdley, K.V.; Belt, P.; Wilson, L.A.; Yeh, M.M.; Neuschwander-Tetri, B.A.; Chalasani, N.; Sanyal, A.J.; Nelson, J.E.; The NASH Clinical Research Network. Serum ferritin is an independent predictor of histologic severity and advanced fibrosis in patients with nonalcoholic fatty liver disease. Hepatology 2012, 55, 77–85. [Google Scholar] [CrossRef]
- Mottin, C.C.; Moretto, M.; Padoin, A.V.; Swarowsky, A.M.; Toneto, M.G.; Glock, L.; Repetto, G. The role of ultrasound in the diagnosis of hepatic steatosis in morbidly obese patients. Obes. Surg. 2004, 14, 635–637. [Google Scholar] [CrossRef]
- Rofsky, N.M.; Fleishaker, H. CT and MRI of diffuse liver disease. Semin. Ultrasound CT MR 1995, 16, 16–33. [Google Scholar] [CrossRef]
- Springer, F.; Machann, J.; Claussen, C.D.; Schick, F.; Schwenzer, N.F. Liver fat content determined by magnetic resonance imaging and spectroscopy. World J. Gastroenterol. 2010, 16, 1560–1566. [Google Scholar] [CrossRef]
- Anstee, Q.M.; Targher, G.; Day, C.P. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 330–344. [Google Scholar] [CrossRef]
- Goland, S.; Shimoni, S.; Zornitzki, T.; Knobler, H.; Azoulai, O.; Lutaty, G.; Melzer, E.; Orr, A.; Caspi, A.; Malnick, S. Cardiac abnormalities as a new manifestation of nonalcoholic fatty liver disease: Echocardiographic and tissue Doppler imaging assessment. J. Clin. Gastroenterol. 2006, 40, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Minhas, A.M.K.; Bhopalwala, H.M.; Dewaswala, N.; Salah, H.M.; Khan, M.S.; Shahid, I.; Biegus, J.; Lopes, R.D.; Pandey, A.; Fudim, M. Association of Non-Alcoholic Fatty Liver Disease with In-Hospital Outcomes in Primary Heart Failure Hospitalizations with Reduced or Preserved Ejection Fraction. Curr. Probl. Cardiol. 2022, 101199. [Google Scholar] [CrossRef] [PubMed]
- Rubin, E.; Lieber, C.S. Alcohol-induced hepatic injury in nonalcoholic volunteers. N. Engl. J. Med. 1968, 278, 869–876. [Google Scholar] [CrossRef]
- Lefkowitch, J.H. Morphology of alcoholic liver disease. Clin. Liver Dis. 2005, 9, 37–53. [Google Scholar] [CrossRef]
- French, S.W.; Nash, J.; Shitabata, P.; Kachi, K.; Hara, C.; Chedid, A.; Mendenhall, C. Pathology of alcoholic liver disease. VA Cooperative Study Group 119. Semin. Liver Dis. 1993, 13, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Teli, M.R.; Day, C.P.; Burt, A.D.; Bennett, M.K.; James, O.F. Determinants of progression to cirrhosis or fibrosis in pure alcoholic fatty liver. Lancet 1995, 346, 987–990. [Google Scholar] [CrossRef] [PubMed]
- Cameron, R.G.; Neuman, M.G. Novel morphologic findings in alcoholic liver disease. Clin. Biochem. 1999, 32, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Beste, L.A.; Leipertz, S.L.; Green, P.K.; Dominitz, J.A.; Ross, D.; Ioannou, G.N. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001-2013. Gastroenterology. 2015, 149, 1471–1482.e5. [Google Scholar] [CrossRef]
- Turlin, B.; Mendler, M.H.; Moirand, R.; Guyader, D.; Guillygomarc’h, A.; Deugnier, Y. Histologic features of the liver in insulin resistance-associated iron overload. A study of 139 patients. Am. J. Clin. Pathol. 2001, 116, 263–270. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M. Nonalcoholic Fatty liver disease: Pathologic patterns and biopsy evaluation in clinical research. Semin. Liver Dis. 2012, 32, 3–13. [Google Scholar] [CrossRef]
- Brunt, E.M.; Tiniakos, D.G. Histopathology of nonalcoholic fatty liver disease. World J. Gastroenterol. 2010, 16, 5286–5296. [Google Scholar] [CrossRef] [PubMed]
- Brunt, E.M.; Ramrakhiani, S.; Cordes, B.G.; Neuschwander-Tetri, B.A.; Janney, C.G.; Bacon, B.R.; Di Bisceglie, A.M. Concurrence of Histologic Features of Steatohepatitis with Other Forms of Chronic Liver Disease. Mod. Pathol. 2003, 16, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Sanyal, A.J.; George, J. Toward More Accurate Nomenclature for Fatty Liver Diseases. Gastroenterology 2019, 157, 590–593. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Sanyal, A.J.; George, J.; Sanyal, A.; Neuschwander-Tetri, B.; Tiribelli, C.; Kleiner, D.E.; Brunt, E.; Bugianesi, E.; Yki-Järvinen, H.; et al. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020, 158, 1999–2014.e1. [Google Scholar] [CrossRef] [PubMed]
- Åberg, F.; Helenius-Hietala, J.; Puukka, P.; Färkkilä, M.; Jula, A. Interaction between alcohol consumption and metabolic syndrome in predicting severe liver disease in the general population. Hepatology 2018, 67, 2141–2149. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Stepanova, M.; Ong, J.; Yilmaz, Y.; Duseja, A.; Eguchi, Y.; El Kassas, M.; Fernández, M.I.C.; George, J.; Jacobson, I.M.; et al. Effects of Alcohol Consumption and Metabolic Syndrome on Mortality in Patients With Nonalcoholic and Alcohol-Related Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2019, 17, 1625–1633.e1. [Google Scholar] [CrossRef] [PubMed]
- Ajmera, V.; Belt, P.; Wilson, L.A.; Gill, R.M.; Loomba, R.; Kleiner, D.E.; Neuschwander-Tetri, B.A.; Terrault, N. Among Patients With Nonalcoholic Fatty Liver Disease, Modest Alcohol Use Is Associated With Less Improvement in Histologic Steatosis and Steatohepatitis. Clin. Gastroenterol. Hepatol. 2018, 16, 1511–1520.e5. [Google Scholar] [CrossRef]
- Chang, Y.; Cho, J.; Cho, Y.K.; Cho, A.; Hong, Y.S.; Zhao, D.; Ahn, J.; Sohn, C.I.; Shin, H.; Guallar, E.; et al. Alcoholic and Nonalcoholic Fatty Liver Disease and Incident Hospitalization for Liver and Cardiovascular Diseases. Clin. Gastroenterol. Hepatol. 2020, 18, 205–215.e7. [Google Scholar] [CrossRef]
- Chang, Y.; Cho, Y.K.; Cho, J.; Jung, H.-S.; Yun, K.E.; Ahn, J.; Sohn, C.I.; Shin, H.; Ryu, S. Alcoholic and Nonalcoholic Fatty Liver Disease and Liver-Related Mortality: A Cohort Study. Am. J. Gastroenterol. 2019, 114, 620–629. [Google Scholar] [CrossRef]
- Chang, Y.; Ryu, S.; Sung, K.C.; Cho, Y.K.; Sung, E.; Kim, H.-N.; Jung, H.-S.; Yun, K.E.; Ahn, J.; Shin, H.; et al. Alcoholic and non-alcoholic fatty liver disease and associations with coronary artery calcification: Evidence from the Kangbuk Samsung Health Study. Gut 2019, 68, 1667–1675. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, S.Y.; Jung, E.S.; Jung, S.W.; Koo, J.S.; Kim, J.H.; Yeon, J.E.; Kwon, S.Y.; Lee, S.W.; Byun, K.S.; et al. Carotid Intima-Media Thickness Is Increased Not Only in Non-Alcoholic Fatty Liver Disease Patients but Also in Alcoholic Fatty Liver Patients. Digestion 2011, 84, 149–155. [Google Scholar] [CrossRef]
- Maor, Y.; Ergaz, D.; Malnick, S.D.H.; Melzer, E.; Neuman, M.G. Liraglutide-Induced Hepatotoxicity. Biomedicines 2021, 9, 106. [Google Scholar] [CrossRef] [PubMed]
- Neuman, M.G.; Seitz, H.K.; Teschke, R.; Malnick, S.; Johnson-Davis, K.L.; Cohen, L.B.; German, A.; Hohmann, N.; Moreira, B.; Moussa, G.; et al. Cellular, molecular, vial and clnical featues of alcohol and non-alcohol induced liver disease. Curr. Issue Mol. Biol. 2022, 44, 1294–1315. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wong, V.W.-S.; Dufour, J.-F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Zelber-Sagi, S. Dietary Treatment for NAFLD: New Clinical and Epidemiological Evidence and Updated Recommendations. Semin. Liver Dis. 2021, 41, 248–262. [Google Scholar] [CrossRef]
- Głuszyńska, P.; Lemancewicz, D.; Dzięcioł, J.B.; Razak Hady, H. Non-Alcoholic Fatty Liver Disease (NAFLD) and Bariatric/Metabolic Surgery as Its Treatment Option: A Review. JCM 2021, 10, 5721. [Google Scholar] [CrossRef]
- Powell, E.E.; Wong, V.W.S.; Rinella, M. Non-alcoholic fatty liver disease. Lancet 2021, 397, 2212–2224. [Google Scholar] [CrossRef]
- Vilar-Gomez, E.; Martinez-Perez, Y.; Calzadilla-Bertot, L.; Torres-Gonzalez, A.; Gra-Oramas, B.; Gonzalez-Fabian, L.; Friedman, S.L.; Diago, M.; Romero-Gomez, M. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology 2015, 149, 367–378.e5. [Google Scholar] [CrossRef]
- von Loeffelholz, C.; Roth, J.; Coldewey, S.; Birkenfeld, A. The Role of Physical Activity in Nonalcoholic and Metabolic Dysfunction Associated Fatty Liver Disease. Biomedicines 2021, 9, 1853. [Google Scholar] [CrossRef]
- Sumida, Y.; Naito, Y.; Tanaka, S.; Sakai, K.; Inada, Y.; Taketani, H.; Kanemasa, K.; Yasui, K.; Itoh, Y.; Okanoue, T.; et al. Long-term (≥2 y) efficacy of vitamin E for non-alcoholic steatohepatitis. Hepatogastroenterology 2013, 60, 1445–1450. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Ratziu, V.; Loomba, R.; Rinella, M.; Anstee, Q.M.; Goodman, Z.; Bedossa, P.; Geier, A.; Beckebaum, S.; Newsome, P.N.; et al. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: Interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2019, 394, 2184–2196. [Google Scholar] [CrossRef] [PubMed]
- Stofan, M.; Guo, G.L. Bile Acids and FXR: Novel Targets for Liver Diseases. Front. Med. 2020, 7, 544. [Google Scholar] [CrossRef] [PubMed]
- Boeckmans, J.; Natale, A.; Rombaut, M.; Buyl, K.; Rogiers, V.; De Kock, J.; Vanhaecke, T.; Rodrigues, R.M. Anti-NASH Drug Development Hitches a Lift on PPAR Agonism. Cells 2019, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Diehl, A.M.; Brunt, E.M.; Cusi, K.; Charlton, M.; Sanyal, A.J. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012, 55, 2005–2023. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver; European Association for the Study of Diabetes; European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef]
- Westerouen Van Meeteren, M.J.; Drenth, J.P.H.; Tjwa, E.T. Elafibranor: A potential drug for the treatment of nonalcoholic steatohepatitis (NASH). Expert Opin. Investig. Drugs 2020, 29, 117–123. [Google Scholar] [CrossRef]
- Siddiqui, M.S.; Idowu, M.O.; Parmar, D.; Borg, B.B.; Denham, D.; Loo, N.M.; Lazas, D.; Younes, Z.; Sanyal, A.J. A Phase 2 Double Blinded, Randomized Controlled Trial of Saroglitazar in Patients With Nonalcoholic Steatohepatitis. Clin. Gastroenterol. Hepatol. 2021, 19, 2670–2672. [Google Scholar] [CrossRef]
- Sven, M.F.; Pierre, B.; Manal, F.A.; Quentin, M.A.; Elisabetta, B.; Vlad, R.; Philippe, H.-M.; Bruno, S.; Jean-Louis, J.; Pierre, B.; et al. A randomised, double-blind, placebo-controlled, multi-centre, dose-range, proof-of-concept, 24-week treatment study of lanifibranor in adult subjects with non-alcoholic steatohepatitis: Design of the NATIVE study. Contemp. Clin. Trials 2020, 98, 106170. [Google Scholar] [CrossRef]
- Li, Y.; Liu, L.; Wang, B.; Wang, J.; Chen, D. Metformin in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Biomed. Rep. 2013, 1, 57–64. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Houlihan, D.D.; Rowe, I.A. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N. Engl. J. Med. 2010, 363, 1185–1186. [Google Scholar] [CrossRef]
- Newsome, P.N.; Buchholtz, K.; Cusi, K.; Linder, M.; Okanoue, T.; Ratziu, V.; Sanyal, A.J.; Sejling, A.-S.; Harrison, S.A. A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. N. Engl. J. Med. 2021, 384, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Latva-Rasku, A.; Honka, M.J.; Kullberg, J.; Mononen, N.; Lehtimäki, T.; Saltevo, J.; Kirjavainen, A.K.; Saunavaara, V.; Iozzo, P.; Johansson, L.; et al. The SGLT2 Inhibitor Dapagliflozin Reduces Liver Fat but Does Not Affect Tissue Insulin Sensitivity: A Randomized, Double-Blind, Placebo-Controlled Study With 8-Week Treatment in Type 2 Diabetes Patients. Diabetes Care 2019, 42, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Filipovic, B.; Lukic, S.; Mijac, D.; Marjanovic-Haljilji, M.; Vojnovic, M.; Bogdanovic, J.; Glisic, T.; Filipovic, N.; Al Kiswani, J.; Djokovic, A.; et al. The New Therapeutic Approaches in the Treatment of Non-Alcoholic Fatty Liver Disease. IJMS 2021, 22, 13219. [Google Scholar] [CrossRef] [PubMed]
- Gulyás, G.; Sohn, M.; Kim, Y.J.; Várnai, P.; Balla, T. ORP3 phosphorylation regulates phosphatidylinositol 4-phosphate and Ca2+ dynamics at plasma membrane-ER contact sites. J. Cell Sci. 2020, 133, jcs237388. [Google Scholar] [CrossRef] [PubMed]
- Boroumand, P.; Klip, A. Bone marrow adipose cells—Cellular interactions and changes with obesity. J. Cell Sci. 2020, 133, jcs238394. [Google Scholar] [CrossRef]
- Harford, K.A.; Reynolds, C.M.; McGillicuddy, F.C.; Roche, H.M. Fats, inflammation and insulin resistance: Insights to the role of macrophage and T-cell accumulation in adipose tissue. Proc. Nutr. Soc. 2011, 70, 408–417. [Google Scholar] [CrossRef]
- Tarantino, G.; Citro, V.; Balsano, C.; Capone, D. Could SCGF-Beta Levels Be Associated with Inflammation Markers and Insulin Resistance in Male Patients Suffering from Obesity-Related NAFLD? Diagnostics 2020, 10, 395. [Google Scholar] [CrossRef]
- Muntaner, L.; Altamirano, J.T.; Augustin, S.; González, A.; Esteban, R.; Guardia, J.; Genesca, J. High doses of beta-blockers and alcohol abstinence improve long-term rebleeding and mortality in cirrhotic patients after an acute variceal bleeding. Liver Int. 2010, 30, 1123–1130. [Google Scholar] [CrossRef]
- Tang-Barton, P.; Vas, W.; Weissman, J.; Salimi, Z.; Patel, R.; Morris, L. Focal fatty liver lesions in alcoholic liver disease: A broadened spectrum of CT appearances. Gastrointest. Radiol. 1985, 10, 133–137. [Google Scholar] [CrossRef]
- Singal, A.K.; Bataller, R.; Ahn, J.; Kamath, P.S.; Shah, V.H. ACG Clinical Guideline: Alcoholic Liver Disease. Am. J. Gastroenterol. 2018, 113, 175–194. [Google Scholar] [CrossRef]
- Van Melkebeke, L.; Korf, H.; Tsochatzis, E.A.; van der Merwe, S.; Nevens, F.; Verbeek, J. Treatment of severe alcoholic hepatitis: A systematic review. Curr. Opin. Pharmacol. 2021, 60, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Ballestri, S.; Zona, S.; Targher, G.; Romagnoli, D.; Baldelli, E.; Nascimbeni, F.; Roverato, A.; Guaraldi, G.; Lonardo, A. Nonalcoholic fatty liver disease is associated with an almost twofold increased risk of incident type 2 diabetes and metabolic syndrome. Evidence from a systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2016, 31, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.; Qi, Q.; Kizer, J.R.; Williams-Nguyen, J.; Strickler, H.D.; Thyagarajan, B.; Daviglus, M.; Talavera, G.A.; Schneiderman, N.; Cotler, S.J.; et al. Association of liver enzymes with incident diabetes in US Hispanic/Latino adults. Diabet. Med. 2021, 38, e14522. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.X.; Trasolini, R.; Heer, M.; Cox, B.; Galts, C.; Marquez, V.; Yoshida, E.M. Non-alcoholic fatty liver disease (NAFLD) in Filipino North American patients: Results from a multi-ethnic cohort. Can. Liver J. 2022, 5, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Byrne, C.D.; Lonardo, A.; Zoppini, G.; Barbui, C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: A meta-analysis. J. Hepatol. 2016, 65, 589–600. [Google Scholar] [CrossRef]
- Chen, K.; Sng, W.K.; Quah, J.H.; Liu, J.; Chong, B.Y.; Lee, H.K.; Wang, X.F.; Tan, N.C.; Chang, P.E.; Tan, H.C.; et al. Clinical spectrum of non-alcoholic fatty liver disease in patients with diabetes mellitus. PLoS ONE 2020, 15, e0236977. [Google Scholar] [CrossRef]
- Araujo, A.R.; Rosso, N.; Bedogni, G.; Tiribelli, C.; Bellentani, S. Global epidemiology of non-alcoholic fatty liver disease/non-alcoholic steatohepatitis: What we need in the future. Liver Int. 2018, 38 (Suppl. 1), 47–51. [Google Scholar] [CrossRef]
- Wortmann, S.B.; Mayr, J.A. Choline-related-inherited metabolic diseases—A mini review. J. Inherit. Metab. Dis. 2019, 42, 237–242, Erratum in J. Inherit. Metab. Dis. 2020, 43, 156. [Google Scholar] [CrossRef]
- Pallayova, M.; Taheri, S. Non-alcoholic fatty liver disease in obese adults: Clinical aspects and current management strategies. Clin. Obes. 2014, 4, 243–253. [Google Scholar] [CrossRef]
- Leoni, S.; Tovoli, F.; Napoli, L.; Serio, I.; Ferri, S.; Bolondi, L. Current guidelines for the management of non-alcoholic fatty liver disease: A systematic review with comparative analysis. World J. Gastroenterol. 2018, 24, 3361–3373. [Google Scholar] [CrossRef]
- Méndez-Sánchez, N.; Bugianesi, E.; Gish, R.G.; Lammert, F.; Tilg, H.; Nguyen, M.H.; Sarin, S.K.; Fabrellas, N.; Zelber-Sagi, S.; Fan, J.-G.; et al. Global multi-stakeholder consensus on the redefinition of fatty liver disease. Global multi-stakeholder endorsement of the MAFLD definition. Lancet Gastroenterol. Hepatol. 2022, 7, 388–390. [Google Scholar] [CrossRef] [PubMed]
- Neuman, M.G.; Dumitrascu, D.L. Non-alcoholic fatty liver disease: An update on diagnosis. Clujul Med. 2018, 91, 147–150. [Google Scholar] [CrossRef]
- Neuman, M.G.; Seitz, H.K.; Tuma, P.L.; Osna, N.A.; Casey, C.A.; Kharbanda, K.K.; Cohen, L.B.; Malnick, S.D.H.; Adhikari, R.; Mitra, R.; et al. Alcohol: Basic and translational research 15th annual Charles Lieber &1st Samuel French satellite symposium. Exp. Mol. Pathol. 2022, 126, 104750. [Google Scholar] [CrossRef] [PubMed]
- Teschke, R.; Eickhoff, A.; Brown, A.C.; Neuman, M.G.; Schulze, J. Diagnostic Biomarkers in Liver Injury by Drugs, Herbs, and Alcohol: Tricky Dilemma after EMA Correctly and Officially Retracted Letter of Support. Int. J. Mol. Sci. 2019, 21, 212. [Google Scholar] [CrossRef]
- Westman, E.C.; Feinman, R.D.; Mavropoulos, J.C.; Vernon, M.C.; Volek, J.S.; Wortman, J.A.; Yancy, W.S.; Phinney, S.D. Low-carbohydrate nutrition and metabolism. Am. J. Clin. Nutr. 2007, 86, 276–284. [Google Scholar] [CrossRef]
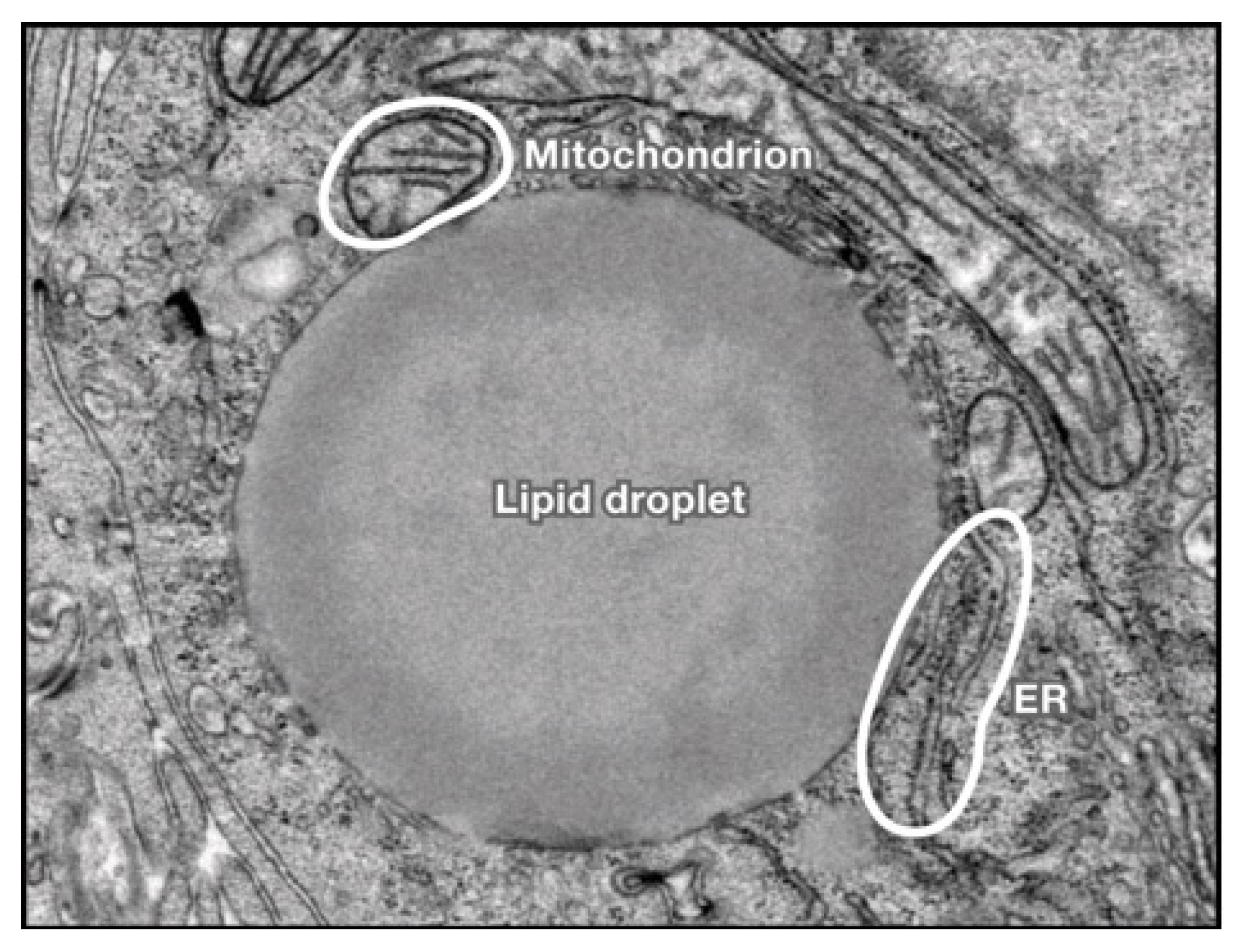
| NAFLD | ALD | |
|---|---|---|
| Risk factors | Metabolic syndrome, sedentary lifestyle, processed food, sweet beverages, genetics, intestinal microbiota, type 2 diabetes | Alcohol consumption, genetics, microbiome, endotoxemia |
| Clinical | Asymptomatic to cirrhosis | Asymptomatic to cirrhosis |
| Imaging | US/CT- normal to steatosis to cirrhosis MRI? | US/CT- normal to steatosis to cirrhosis; MRI? |
| Pathology | Steatohepatitis, fibrosis, Mallory–Denk Bodies | Steatohepatitis, fibrosis, Mallory–Denk Bodies |
| Microbiome | Increase in Bacteroidetes sp. and Proteobacteriae sp. Decrease in Firmicutes sp. | Increase in Proteobacteriae sp., decrease in Faecalibacterium prausnitzi. Addictive behavior. |
| Pathogenesis | Inflammatory cytokines, insulin resistance, iron/copper overload, bacterial translocation. carbohydrate metabolism impairment, obesity, sugar excess of dietary long-chain fatty acids | Continuous excess drinking, binge drinking, genetic predisposition, endotoxemia Drugs of misuse combination, malnutrition |
| Inflammatory cytokines and chemokines | TGF β, TNF α IL-6, IL-8; IL-17; IL-23 RANTES | TGF β, TNF α IL-6, IL-8; IL-17; IL-23 RANTES |
| Cell death | Apoptosis, necrosis | Apoptosis, necrosis, necroptosis |
| Treatment | Lifestyle changes, exercise, coffee, dietary changes | Abstinence |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malnick, S.D.H.; Alin, P.; Somin, M.; Neuman, M.G. Fatty Liver Disease-Alcoholic and Non-Alcoholic: Similar but Different. Int. J. Mol. Sci. 2022, 23, 16226. https://doi.org/10.3390/ijms232416226
Malnick SDH, Alin P, Somin M, Neuman MG. Fatty Liver Disease-Alcoholic and Non-Alcoholic: Similar but Different. International Journal of Molecular Sciences. 2022; 23(24):16226. https://doi.org/10.3390/ijms232416226
Chicago/Turabian StyleMalnick, Stephen D. H., Pavel Alin, Marina Somin, and Manuela G. Neuman. 2022. "Fatty Liver Disease-Alcoholic and Non-Alcoholic: Similar but Different" International Journal of Molecular Sciences 23, no. 24: 16226. https://doi.org/10.3390/ijms232416226
APA StyleMalnick, S. D. H., Alin, P., Somin, M., & Neuman, M. G. (2022). Fatty Liver Disease-Alcoholic and Non-Alcoholic: Similar but Different. International Journal of Molecular Sciences, 23(24), 16226. https://doi.org/10.3390/ijms232416226







