Hypertriglyceridemia and Atherosclerotic Carotid Artery Stenosis
Abstract
1. Introduction
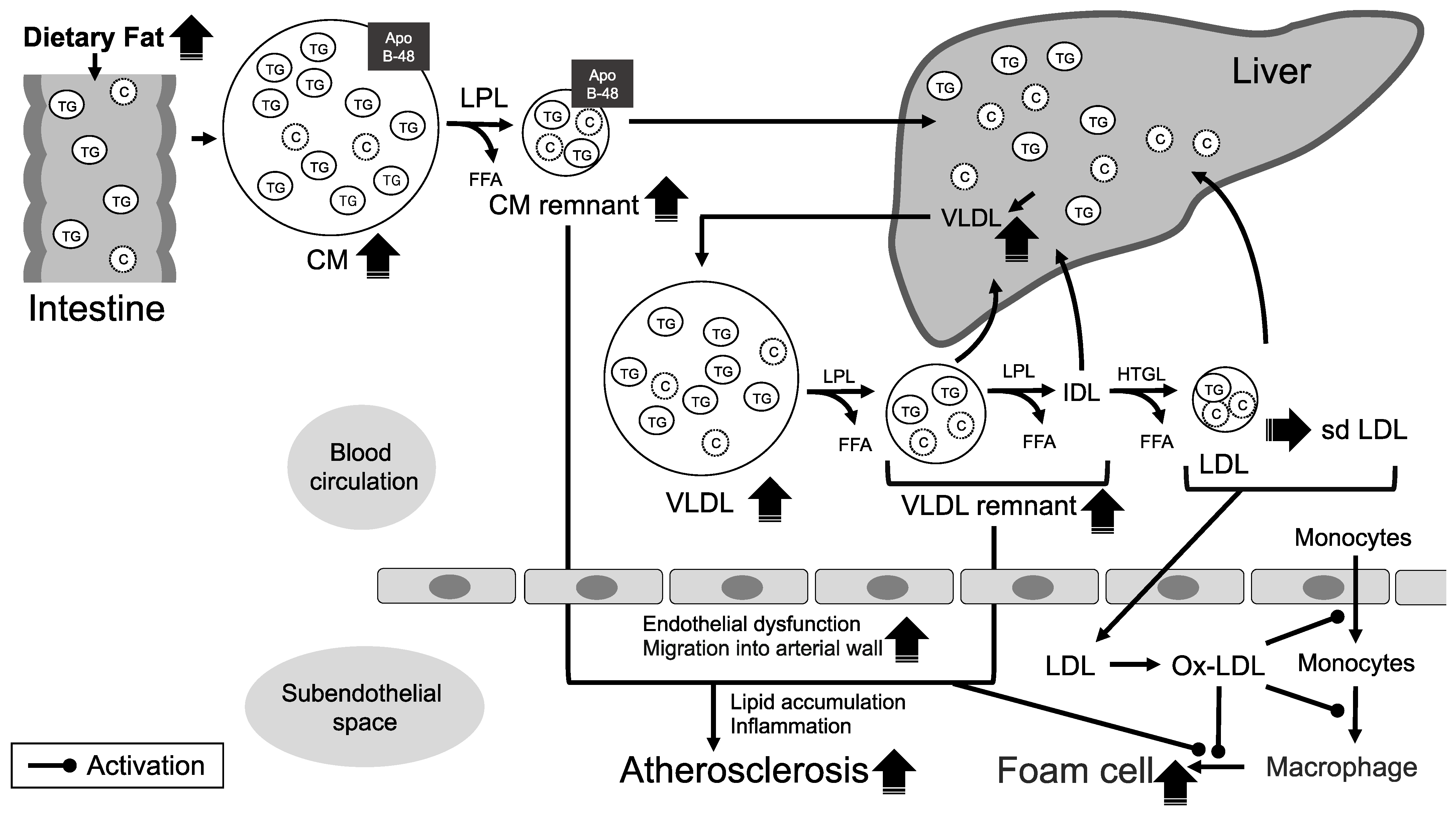
2. The Mechanisms of Atherosclerosis Formation Associated with TGs
3. Impact of Elevated Levels of Non-Fasting Serum TG on Atherosclerosis
4. The Role of Lipid-Lowering Drugs in Patients with Atherosclerotic CAS
5. Previous Reports Regarding the Association of Hypertriglyceridemia with Atherosclerotic CAS
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AHA | American Heart Association |
| Apo | Apolipoprotein |
| BMT | best medical treatment |
| CA | carotid artery |
| CAS | CA stenosis |
| CEA | carotid endarterectomy |
| CM | Chylomicron |
| cIMT | carotid intima-media thickness |
| CLZ | Cilostazol |
| DM | diabetes mellitus |
| EPA | eicosapentaenoic acid |
| HDL-C | high-density lipoprotein cholesterol |
| LDL-C | low-density lipoprotein cholesterol |
| LPL | lipoprotein lipase |
| MI | myocardial infarction |
| PPAR | peroxisome proliferator-activated receptor |
| sd LDL | small dense LDL |
| TC | total cholesterol |
| TG | Triglyceride |
| TRL | TG-rich lipoprotein |
| VLDL | very LDL |
References
- Miller, M.; Stone, N.J.; Ballantyne, C.; Bittner, V.; Criqui, M.H.; Ginsberg, H.N.; Goldberg, A.C.; Howard, W.J.; Jacobson, M.S.; Kris-Etherton, P.M.; et al. Triglycerides and cardiovascular disease: A scientific statement from the American Heart Association. Circulation 2011, 123, 2292–2333. [Google Scholar] [CrossRef]
- Nordestgaard, B.G.; Langsted, A.; Mora, S.; Kolovou, G.; Baum, H.; Bruckert, E.; Watts, G.F.; Sypniewska, G.; Wiklund, O.; Borén, J.; et al. Fasting is not routinely required for determination of a lipid profile: Clinical and laboratory implications including flagging at desirable concentration cut-points-a joint consensus statement from the European Atherosclerosis Society and European Federation of Clinical Chemistry and Laboratory Medicine. Eur. Heart J. 2016, 37, 1944–1958. [Google Scholar] [CrossRef]
- Japan Atherosclerosis Society (JAS) Guideline for Prevention of Atherosclerotic Cardiovascular Diseases 2022. Available online: https://www.j-athero.org/jp/wp-content/uploads/publications/pdf/GL2022_s/jas_gl2022_2_220926.pdf (accessed on 28 November 2022).
- Savory, W.S. Case of a young woman in whom the main arteries of both upper extremities and of the left side of the neck were throughout completely obliterated. Med. Chir. Trans. 1856, 39, 205–219. [Google Scholar] [CrossRef]
- Fine-Edelstein, J.S.; Wolf, P.A.; O’Leary, D.H.; Poehlman, H.; Belanger, A.J.; Kase, C.S.; D’Agostino, R.B. Precursors of extracranial carotid atherosclerosis in the Framingham Study. Neurology 1994, 44, 1046–1050. [Google Scholar] [CrossRef]
- Rockman, C.B.; Hoang, H.; Guo, Y.; Maldonado, T.S.; Jacobowitz, G.R.; Talishinskiy, T.; Riles, T.S.; Berger, J.S. The prevalence of carotid artery stenosis varies significantly by race. J. Vasc. Surg. 2013, 57, 327–337. [Google Scholar] [CrossRef]
- Petty, G.; Brown, R.D., Jr.; Whisnant, J.P.; Sicks, J.D.; O’Fallon, W.M.; Wiebers, D.O. Ischemic stroke subtypes: A population-based study of incidence and risk factors. Stroke 1999, 30, 2513–2516. [Google Scholar] [CrossRef]
- Markl, M.; Wegent, F.; Zech, T.; Bauer, S.; Strecker, C.; Schumacher, M.; Weiller, C.; Hennig, J.; Harloff, A. In vivo wall shear stress distribution in the carotid artery: Effect of bifurcation geometry, internal carotid artery stenosis, and recanalization therapy. Circ. Cardiovasc. Imaging 2010, 3, 647–655. [Google Scholar] [CrossRef]
- Fukuda, S.; Shimogonya, Y.; Yonemoto, N.; Fukuda, M.; Watanabe, A.; Fujiwara, K.; Enomoto, R.; Hasegawa, K.; Yasoda, A.; Tsukahara, T.; et al. Hemodynamic risk factors for the development of carotid stenosis in patients with unilateral carotid stenosis. World Neurosurg. 2022, 160, e353–e371. [Google Scholar] [CrossRef]
- Prasad, K. Pathophysiology and medical treatment of carotid artery stenosis. Int. J. Angiol. 2015, 24, 158–172. [Google Scholar] [CrossRef]
- Miura, Y.; Suzuki, H. Dyslipidemia and atherosclerotic carotid artery stenosis. Vessel Plus 2019, 3, 1. [Google Scholar] [CrossRef]
- Jebari-Benslaiman, S.; Galicia-García, U.; Larrea-Sebal, A.; Olaetxea, J.R.; Alloza, I.; Vandenbroeck, K.; Benito-Vicente, A.; Martín, C. Pathophysiology of Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3346. [Google Scholar] [CrossRef]
- Jorgensen, A.B.; Frikke-Schmidt, R.; West, A.S.; Grande, P.; Nordesrgaard, B.G.; Tybjærg-Hansen, A. Genetically elevated non-fasting triglycerides and calculated remnant cholesterol as causal risk factors for myocardial infarction. Eur. Heart J. 2013, 34, 1826–1833. [Google Scholar] [CrossRef]
- Holmes, M.V.; Asselbergs, F.W.; Palmer, T.M.; Drenos, F.; Lanktree, M.B.; Nelson, C.P.; Dale, C.E.; Padmanabhan, S.; Finan, C.; Swerdlow, D.I.; et al. Mendelian randomization of blood lipids for coronary heart disease. Eur. Heart J. 2015, 36, 539–550. [Google Scholar] [CrossRef]
- Labreuche, J.; Deplanque, D.; Touboul, P.J.; Bruckert, E.; Amarenco, P. Association between change in plasma triglyceride levels and risk of stroke and carotid atherosclerosis systematic review and meta-regression analysis. Atherosclerosis 2010, 212, 9–15. [Google Scholar] [CrossRef]
- Teno, S.; Uto, Y.; Nagashima, H.; Endoh, Y.; Iwamoto, Y.; Omori, Y.; Takizawa, T. Association of postprandial hypertriglyceridemia and carotid intima-media thickness in patient with type 2 diabetes. Diabetes Care 2000, 23, 1401–1406. [Google Scholar] [CrossRef]
- Vouillarmet, J.; Helfre, M.; Maucort-Boulch, D.; Riche, B.; Thivolet, C.; Grange, C. Carotid atherosclerosis progression and cerebrovascular events in patients with diabetes. J. Diabetes Complicat. 2016, 30, 638–643. [Google Scholar] [CrossRef]
- Kitagami, M.; Yasuda, R.; Toma, N.; Shiba, M.; Nampei, M.; Yamamoto, Y.; Nakatsuka, Y.; Sakaida, H.; Suzuki, H. Impact of hypertriglyceridemia on carotid stenosis progression under normal low-density lipoprotein cholesterol levels. J. Stroke Cerebrovasc. Dis. 2017, 26, 1793–1800. [Google Scholar] [CrossRef]
- Mori, Y.; Itoh, Y.; Komiya, H.; Tajima, N.; Kon, M.; Horiuchi, Y.; Ueno, T.; Miyake, K.; Satoh, N.; Yoshii, H.; et al. Association between postprandial remnant-like particle triglyceride (RLP-TG) levels and carotid intima-media thickness (IMT) in Japanese patients with type 2 diabetes: Assessment by meal tolerance tests (MTT). Endocrine 2005, 28, 157–163. [Google Scholar] [CrossRef]
- Idei, M.; Hirayama, S.; Miyake, N.; Kon, M.; Horiuchi, Y.; Ueno, T.; Miyake, K.; Satoh, N.; Yoshii, H.; Yamashiro, K.; et al. Mean postprandial triglyceride concentration is an independent risk factor for carotid atherosclerosis in patients with type 2 diabetes. Clin. Chim. Acta 2014, 430, 134–139. [Google Scholar] [CrossRef]
- Miura, Y.; Suzuki, Y.; Kanamaru, H.; Shiba, M.; Yasuda, R.; Toma, N.; Suzuki, H. Higher non-fasting serum triglyceride preceding the carotid stenosis progression. Neurol. Med. Chir. 2021, 15, 422–432. [Google Scholar] [CrossRef]
- Miura, Y.; Yasuda, R.; Toma, N.; Suzuki, H. Non-fasting hypertriglyceridemia burden as a residual risk of the progression of carotid artery stenosis. Int. J. Mol. Sci. 2022, 23, 9197. [Google Scholar] [CrossRef]
- Karpe, K. Postprandial lipoprotein metabolism and atherosclerosis. J. Intern. Med. 1999, 246, 341–355. [Google Scholar] [CrossRef]
- Nordestgaard, B.G.; Benn, M.; Schnohr, P.; Tybjaerg-Hansen, A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA 2007, 298, 299–308. [Google Scholar] [CrossRef]
- Fruchart, J.C.; Santos, R.D.; Aguilar-Salinas, C.; Aikawa, M.; Al Rasadi, K.; Amarenco, P.; Barter, P.J.; Ceska, R.; Corsini, A.; Després, J.P.; et al. The selective peroxisome proliferator-activated receptor alpha modulator (SPPARMα) paradigm: Conceptual framework and therapeutic potential: A consensus statement from the International Atherosclerosis Society (IAS) and the Residual Risk Reduction Initiative (R3i) Foundation. Cardiovasc. Diabetol. 2019, 18, 71. [Google Scholar] [CrossRef]
- Fujioka, Y.; Cooper, A.D.; Fong, L.G. Multiple processes are involved in the uptake of chylomicron remnants by mouse peritoneal macrophages. J. Lipid Res. 1998, 39, 2339–2349. [Google Scholar] [CrossRef]
- Masuda, D.; Yamashita, S. Postprandial hyperlipidemia and remnant lipoproteins. J. Atheroscler. Thromb. 2017, 24, 95–109. [Google Scholar] [CrossRef]
- Fogelstrand, P.; Boren, J. Retention of atherogenic lipoproteins in the artery wall and its role in atherogenesis. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 1–7. [Google Scholar] [CrossRef]
- Boren, J.; Taskinen, M.R.; Olofsson, S.O.; Levin, M. Ectopic lipid storage and insulin resistance: A harmful relationship. J. Intern. Med. 2013, 274, 25–40. [Google Scholar] [CrossRef]
- Zhang, X.; Sessa, W.C.; Fernández-Hernando, C. Endothelial transcytosis of lipoproteins in atherosclerosis. Front. Cardiovasc. Med. 2018, 5, 130. [Google Scholar] [CrossRef]
- Thuenauer, R.; Muller, S.K.; Romer, W. Pathways of protein and lipid receptormediated transcytosis in drug delivery. Expert Opin. Drug Deliv. 2017, 14, 341–351. [Google Scholar] [CrossRef]
- Zaima, N.; Sasaki, T.; Tanaka, H.; Cheng, X.W.; Onoue, K.; Hayasaka, T.; Goto-Inoue, N.; Enomoto, H.; Unno, N.; Kuzuya, M.; et al. Imaging mass spectrometry-based histopathologic examination of atherosclerotic lesions. Atherosclerosis 2011, 217, 427–432. [Google Scholar] [CrossRef]
- Chung, B.H.; Segrest, J.P.; Smith, K.; Griffin, F.M.; Brouillette, C.G. Lipolytic surface remnants of triglyceride-rich lipoproteins are cytotoxic to macrophages but not in the presence of high density lipoprotein: A possible mechanism of atherogenesis? J. Clin. Investig. 1989, 83, 1363–1374. [Google Scholar] [CrossRef]
- Tomono, S.; Kawazu, S.; Kato, N.; Ono, T.; Ishii, C.; Ito, Y.; Shimizu, M.; Shimoyama, M.; Nakano, T.; Nakajima, K. Uptake of remnant like particles (RLP) in diabetic patients from mouse peritoneal macrophages. J. Atheroscler. Thromb. 1994, 1, 98–102. [Google Scholar] [CrossRef]
- Ginsberg, H.N.; Packard, C.J.; Chapman, M.J.; Borén, J.; Aguilar-Salinas, C.A.; Averna, M.; Ference, B.A.; Gaudet, D.; Hegele, R.A.; Kersten, S.; et al. Triglyceride-rich lipoproteins and their remnants: Metabolic insights, role in atherosclerotic cardiovascular disease, and emerging therapeutic strategies-a consensus statement from the European Atherosclerosis Society. Eur. Heart J. 2021, 42, 4791–4806. [Google Scholar] [CrossRef]
- Rosenson, R.S.; Davidson, M.H.; Hirsh, B.J.; Kathiresan, S.; Gaudet, D. Genetics and causality of triglyceride-rich lipoproteins in atherosclerotic cardiovascular disease. J. Am. Coll. Cardiol. 2014, 64, 2525–2540. [Google Scholar] [CrossRef]
- Wang, L.; Gill, R.; Pedersen, T.L.; Higgins, L.J.; Newman, J.W.; Rutledge, J.C. Triglyceride-rich lipoprotein lipolysis releases neutral and oxidized FFAs that induce endothelial cell inflammation. J. Lipid Res. 2009, 50, 204–213. [Google Scholar] [CrossRef]
- Eiselein, L.; Wilson, D.W.; Lamé, M.W.; Rutledge, J.C. Lipolysis products from triglyceride-rich lipoproteins increase endothelial permeability, perturb zonula occludens-1 and F-actin, and induce apoptosis. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H2745–H2753. [Google Scholar] [CrossRef]
- Norata, G.D.; Tsimikas, S.; Pirillo, A.; Catapano, A.L. Apolipoprotein CIII: From pathophysiology to pharmacology. Trends Pharmacol. Sci. 2015, 36, 675–687. [Google Scholar] [CrossRef]
- Welty, F.K. How do elevated triglycerides and low HDL-cholesterol affect inflammation and atherothrombosis? Curr. Cardiol. Rep. 2013, 15, 400. [Google Scholar] [CrossRef]
- Patel, S.; Puranik, R.; Nakhla, S.; Lundman, P.; Stocker, R.; Wang, X.S.; Lambert, G.; Rye, K.A.; Barter, P.J.; Nicholls, S.J.; et al. Acute hypertriglyceridaemia in humans increases the triglyceride content and decreases the anti-inflammatory capacity of high density lipoproteins. Atherosclerosis 2009, 204, 424–428. [Google Scholar] [CrossRef]
- Linsel-Nitschke, P.; Jansen, H.; Aherrarhou, Z.; Belz, S.; Mayer, B.; Lieb, W.; Huber, F.; Kremer, W.; Kalbitzer, H.R.; Erdmann, J.; et al. Macrophage cholesterol efflux correlates with lipoprotein subclass distribution and risk of obstructive coronary artery disease in patients undergoing coronary angiography. Lipids Health Dis. 2009, 8, 14. [Google Scholar] [CrossRef]
- Skartlien, A.H.; Lyberg-Beckmann, S.; Holme, I.; Hjermann, I.; Prydz, H. Effect of alteration in triglyceride levels on factor VII phospholipid complexes in plasma. Arteriosclerosis 1989, 9, 798–801. [Google Scholar] [CrossRef]
- Simpson, H.C.R.; Mann, J.I.; Meade, T.W.; Chakrabarti, R.; Stirling, Y.; Woolf, L. Hypertriglyceridaemia and hypercoagulability. Lancet 1983, 1, 786–790. [Google Scholar] [CrossRef]
- Hamsten, A.; Wiman, B.; de Faire, U.; Blombäck, M. Increased plasma levels of a rapid inhibitor of tissue plasminogen activator in young survivors of myocardial infarction. N. Engl. J. Med. 1985, 313, 1557–1563. [Google Scholar] [CrossRef]
- Vanschoonbeek, K.; Feijge, M.A.; Saris, W.H.; de Maat, M.P.; Heemskerk, J.W. Plasma triacylglycerol and coagulation factor concentrations predict the anticoagulant effect of dietary fish oil in overweight subjects. J. Nutr. 2007, 137, 7–13. [Google Scholar] [CrossRef]
- Bjorkegren, J.; Karpe, F.; Milne, R.W.; Hamsten, A. Differences in apolipoprotein and lipid composition between human chylomicron remnants and very low density lipoproteins isolated from fasting and postprandial plasma. J. Lipid Res. 1998, 39, 1412–1420. [Google Scholar] [CrossRef]
- Iso, H.; Naito, Y.; Sato, S.; Kitamura, A.; Okamura, T.; Sankai, T.; Shimamoto, T.; Iida, M.; Komachi, Y. Serum triglycerides and risk of coronary heart disease among Japanese men and women. Am. J. Epidemiol. 2001, 153, 490–499. [Google Scholar] [CrossRef]
- Kamstrup, P.R.; Tybjaerg-Hansen, A.; Nordestgaard, B.G. Elevated lipoprotein(a) and risk of aortic valve stenosis in the general population. J. Am. Coll. Cardiol. 2014, 63, 470–477. [Google Scholar] [CrossRef]
- Hirata, A.; Okamur, T.; Hirata, T.; Sugiyama, D.; Ohkubo, T.; Okuda, N.; Kita, Y.; Hayakawa, T.; Kadota, A.; Kondo, K.; et al. Relationship between non-fasting triglycerides and cardiovascular disease mortality in a 20-year follow-up study of a Japanese general population: NIPPON DATA90. J. Epidemiol. 2021, 16, 303–313. [Google Scholar] [CrossRef]
- Ginsberg, H.N. Lipoprotein physiology. Endocrinol. Metab. Clin. N. Am. 1998, 27, 503–519. [Google Scholar] [CrossRef]
- Masuda, D.; Sakai, N.; Sugimoto, T.; Kitazume-Taneike, R.; Yamashita, T.; Kawase, R.; Nakaoka, H.; Inagaki, M.; Nakatani, K.; Yuasa-Kawase, M.; et al. Fasting serum apolipoprotein B-48 can be a marker of postprandial hyperlipidemia. J. Atheroscler. Thromb. 2011, 18, 1062–1070. [Google Scholar] [CrossRef]
- Cohn, J.S.; Johnson, E.J.; Millar, J.S.; Cohn, S.D.; Milne, R.W.; Marcel, Y.L.; Russell, R.M.; Schaefer, E.J. Contribution of apoB-48 and apoB-100 triglyceride-rich lipoproteins (TRL) to postprandial increases in the plasma concentration of TRL triglycerides and retinyl esters. J. Lipid Res. 1993, 34, 2033–2040. [Google Scholar] [CrossRef]
- Karpe, F.; Bell, M.; Bjorkegren, J.; Hamsten, A. Quantification of postprandial triglyceride-rich lipoproteins in healthy men by retinyl ester labeling and simultaneous measurement of apolipoproteins B-48 and B-100. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Schneeman, B.O.; Kotite, L.; Todd, K.M.; Havel, R.J. Relationships between the responses of triglyceride-rich lipoproteins in blood plasma containing apolipoproteins B-48 and B-100 to a fat-containing meal in normolipidemic humans. Proc. Natl. Acad. Sci. USA 1993, 90, 2069–2073. [Google Scholar] [CrossRef] [PubMed]
- Chait, A.; Ginsberg, H.N.; Vaisar, T.; Heinecke, J.W.; Goldberg, I.J.; Bornfeldt, K.E. Remnants of the triglyceride-rich lipoproteins, diabetes, and cardiovascular disease. Diabetes 2020, 69, 508–516. [Google Scholar] [CrossRef]
- Norata, G.D.; Grigore, L.; Raselli, S.; Redaelli, L.; Hamsten, A.; Maggi, F.; Eriksson, P.; Catapano, A.L. Post-prandial endothelial dysfunction in hypertriglyceridemic subjects: Molecular mechanisms and gene expression studies. Atherosclerosis 2007, 193, 321–327. [Google Scholar] [CrossRef]
- Shin, H.K.; Kim, Y.K.; Kim, K.Y.; Lee, J.H.; Hong, K.W. Remnant lipoprotein particles induce apoptosis in endothelial cells by NAD(P)H oxidasemediated production of superoxide and cytokines via lectin-like oxidized low-density lipoprotein receptor-1 activation: Prevention by cilostazol. Circulation 2004, 109, 1022–1028. [Google Scholar] [CrossRef]
- Ting, H.J.; Stice, J.P.; Schaff, U.Y.; Hui, D.Y.; Rutledge, J.C.; Knowlton, A.A.; Passerini, A.G.; Simon, S.I. Triglyceride-rich lipoproteins prime aortic endothelium for an enhanced inflammatory response to tumor necrosis factor-alpha. Circ. Res. 2007, 100, 381–390. [Google Scholar] [CrossRef]
- Taskinen, M.R. Diabetic dyslipidaemia: From basic research to clinical practice. Diabetologia 2003, 46, 733–749. [Google Scholar] [CrossRef]
- Ginsberg, H.N.; Illingworth, D.R. Postprandial dyslipidemia: An atherogenic disorder common in patients with diabetes mellitus. Am. J. Cardiol. 2001, 88, 9H–15H. [Google Scholar] [CrossRef]
- Naylor, A.R.; Ricco, J.B.; de Borst, G.J.; Debus, S.; de Haro, J.; Halliday, A.; Halliday, A.; Hamilton, G.; Kakisis, J.; Kakkos, S.; et al. Editor’s choice–management of atherosclerotic carotid and vertebral artery disease: 2017 clinical practice guidelines of the European Society for Vascular Surgery (ESVS). Eur. J. Vasc. Endovasc. Surg. 2018, 55, 3–81. [Google Scholar] [CrossRef] [PubMed]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.C.; Watts, G.F.; Barrett, P.H.; Martins, I.J.; James, A.P.; Mamo, J.C.L.; Mori, T.A.; Redgrave, T.G. Effect of atorvastatin on chylomicron remnant metabolism in visceral obesity: A study employing a new stable isotope breath test. J. Lipid Res. 2002, 43, 706–712. [Google Scholar] [CrossRef]
- Hogue, J.C.; Lamarche, B.; Deshaies, Y.; Tremblay, A.J.; Bergeron, J.; Gagné, C.; Couture, P. Differential effect of fenofibrate and atorvastatin on in vivo kinetics of apolipoproteins B-100 and B-48 in subjects with type 2 diabetes mellitus with marked hypertriglyceridemia. Metabolism 2008, 57, 246–254. [Google Scholar] [CrossRef]
- Fisch, U.; von Felten, S.; Wiencierz, A.; Jansen, O.; Howard, G.; Hendrikse, J.; Halliday, A.; Fraedrich, G.; Eckstein, H.H.; Calvet, D.; et al. Risk of stroke before revascularisation in patients with symptomatic carotid stenosis: A pooled analysis of randomised controlled trials. Eur. J. Vasc. Endovasc. Surg. 2021, 61, 881–887. [Google Scholar] [CrossRef]
- Reiff, T.; Eckstein, H.H.; Mansmann, U.; Jansen, O.; Fraedrich, G.; Mudra, H.; Hacke, W.; Ringleb, P.A.; SPACE-2 study group. Successful implementation of best medical treatment for patients with asymptomatic carotid artery stenosis within a randomized controlled trial (SPACE-2). Neurol. Res. Pract. 2021, 3, 62. [Google Scholar] [CrossRef]
- Merwick, Á.; Albers, G.W.; Arsava, E.M.; Ay, H.; Calvet, D.; Coutts, S.B.; Cucchiara, B.L.; Demchuk, A.M.; Giles, M.F.; Mas, J.L.; et al. Reduction in early stroke risk in carotid stenosis with transient ischemic attack associated with statin treatment. Stroke 2013, 44, 2814–2820. [Google Scholar] [CrossRef]
- McGirt, M.J.; Perler, B.A.; Brooke, B.S.; Woodworth, G.F.; Coon, A.; Jain, S.; Buck, D.; Roseborough, G.S.; Tamargo, R.J.; Heller, J.; et al. 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors reduce the risk of perioperative stroke and mortality after carotid endarterectomy. J. Vasc. Surg. 2005, 42, 829–836. [Google Scholar] [CrossRef]
- Crisby, M.; Nordin-Fredriksson, G.; Shah, P.K.; Yano, J.; Zhu, J.; Nilsson, J. Pravastatin treatment increases collagen content and decreases lipid content, inflammation, metalloproteinases, and cell death in human carotid plaques: Implications for plaque stabilization. Circulation 2001, 103, 926–933. [Google Scholar] [CrossRef]
- Satny, M.; Hubacek, J.A.; Vrablik, M. Statins and Inflammation. Curr. Atheroscler. Rep. 2021, 23, 80. [Google Scholar] [CrossRef]
- Calvo, M.J.; Martínez, M.S.; Torres, W.; Chávez-Castillo, M.; Luzardo, E.; Villasmil, N.; Salazar, J.; Velasco, M.; Bermúdez, V. Omega-3 polyunsaturated fatty acids and cardiovascular health: A molecular view into structure and function. Vessel Plus 2017, 1, 116–128. [Google Scholar] [CrossRef][Green Version]
- Slivkoff-Clark, K.M.; James, A.P.; Mamo, J.C. The chronic effects of fish oil with exercise on postprandial lipaemia and chylomicron homeostasis in insulin resistant viscerally obese men. Nutr. Metab. 2012, 9, 9. [Google Scholar] [CrossRef]
- Park, Y.; Harris, W.S. Omega-3 fatty acid supplementation accelerates chylomicron triglyceride clearance. J. Lipid Res. 2003, 44, 455–463. [Google Scholar] [CrossRef]
- Tinker, L.F.; Parks, E.J.; Behr, S.R.; Schneeman, O.; Davis, P.A. (n-3) fatty acid supplementation in moderately hypertriglyceridemic adults changes postprandial lipid and apolipoprotein B responses to a standardized test meal. J. Nutr. 1999, 129, 1126–1134. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle, R.T., Jr.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N. Engl. J. Med. 2019, 380, 11–22. [Google Scholar] [CrossRef]
- Saito, Y.; Yokoyama, M.; Origasa, H.; Matsuzaki, M.; Matsuzawa, Y.; Ishikawa, Y.; Oikawa, S.; Sasaki, J.; Hishida, H.; Itakura, H.; et al. Effects of EPA on coronary artery disease in hypercholesterolemic patients with multiple risk factors: Sub-analysis of primary prevention cases from the Japan EPA Lipid Intervention Study (JELIS). Atherosclerosis 2008, 200, 135–140. [Google Scholar] [CrossRef]
- Kolovou, G.D.; Kostakou, P.M.; Anagnostopoulou, K.K.; Cokkinos, D.V. Therapeutic effects of fibrates in postprandial lipemia. Am. J. Cardiovasc. Drugs 2008, 8, 243–255. [Google Scholar] [CrossRef]
- Birjmohun, R.S.; Hutten, B.A.; Kastelein, J.J.; Stroes, E.S. Efficacy and safety of high-density lipoprotein cholesterol-increasing compounds: A meta-analysis of randomized controlled trials. J. Am. Coll. Cardiol. 2005, 45, 185–197. [Google Scholar] [CrossRef]
- Rubins, H.B.; Robins, S.J.; Collins, D.; Fye, C.L.; Anderson, J.W.; Elam, M.B.; Faas, F.H.; Linares, E.; Schaefer, E.J.; Schectman, G.; et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. N. Engl. J. Med. 1999, 341, 410–418. [Google Scholar] [CrossRef]
- Jun, M.; Foote, C.; Lv, J.; Neal, B.; Patel, A.; Nicholls, S.J.; Grobbee, D.E.; Cass, A.; Chalmers, J.; Perkovic, V. Effects of fibrates on cardiovascular outcomes: A systematic review and meta-analysis. Lancet 2010, 375, 1875–1884. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Ye, X.F.; Yu, F.F.; Zhang, X.; Qin, Y.Y.; Lu, J.; He, J. Lipid management in the prevention of stroke: A meta-analysis of fibrates for stroke prevention. BMC Neurol. 2013, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, T.; Ishizuka, K.; Toi, S.; Seki, M.; Kitagawa, K. Effects of pemafibrate in patients with stroke and hypertriglyceridemia: Baseline cerebral artery diseases and 3-month laboratory outcomes. J. Atheroscler. Thromb. 2022, 29, 1020–1030. [Google Scholar] [CrossRef] [PubMed]
- Das Pradhan, A.; Glynn, R.J.; Fruchart, J.C.; MacFadyen, J.G.; Zaharris, E.S.; Everett, B.M.; Campbell, S.E.; Oshima, R.; Amarenco, P.; Blom, D.J.; et al. Triglyceride lowering with pemafibrate to reduce cardiovascular risk. N. Engl. J. Med. 2022, 387, 1923–1934. [Google Scholar] [CrossRef] [PubMed]
- Bozzetto, L.; Annuzzi, G.; Corte, G.D.; Patti, L.; Cipriano, P.; Mangione, A.; Riccardi, G.; Rivellese, A.A. Ezetimibe beneficially influences fasting and postprandial triglyceride-rich lipoproteins in type 2 diabetes. Atherosclerosis 2011, 217, 142–148. [Google Scholar] [CrossRef]
- Hajer, G.R.; Dallinga-Thie, G.M.; van Vark-van der Zee, L.C.; Visseren, F.L.J. The effect of statin alone or in combination with ezetimibe on postprandial lipoprotein composition in obese metabolic syndrome patients. Atherosclerosis 2009, 202, 216–224. [Google Scholar] [CrossRef]
- Kastelein, J.J.; Akdim, F.; Stroes, E.S.; Zwinderman, A.H.; Bots, M.L.; Stalenhoef, A.F.; Visseren, F.L.; Sijbrands, E.J.; Trip, M.D.; Stein, E.A.; et al. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N. Engl. J. Med. 2008, 358, 1431–1443. [Google Scholar] [CrossRef]
- Fleg, J.L.; Mete, M.; Howard, B.V.; Umans, J.G.; Roman, M.J.; Ratner, R.E.; Silverman, A.; Galloway, J.M.; Henderson, J.A.; Weir, M.R.; et al. Effect of statins alone versus statins plus ezetimibe on carotid atherosclerosis in type 2 diabetes: The SANDS (Stop Atherosclerosis in Native Diabetics Study) trial. J. Am. Coll. Cardiol. 2008, 52, 2198–2205. [Google Scholar] [CrossRef]
- Meaney, A.; Ceballos, G.; Asbun, J.; Solache, G.; Mendoza, E.; Vela, A.; Meaney, A. The VYtorin on Carotid intima-media thickness and overall arterial rigidity (VYCTOR) study. J. Clin. Pharmacol. 2009, 49, 838–847. [Google Scholar] [CrossRef]
- O’Keefe, J.H., Jr.; Harris, W.S.; Nelson, J.; Windsor, S.L. Effects of pravastatin with niacin or magnesium on lipid levels and postprandial lipemia. Am. J. Cardiol. 1995, 76, 480–484. [Google Scholar] [CrossRef]
- Sorrentino, S.A.; Besler, C.; Rohrer, L.; Meyer, M.; Heinrich, K.; Bahlmann, F.H.; Mueller, M.; Horváth, T.; Doerries, C.; Heinemann, M.; et al. Endothelial-vasoprotective effects of high-density lipoprotein are impaired in patients with type 2 diabetes mellitus but are improved after extended-release niacin therapy. Circulation 2010, 121, 110–122. [Google Scholar] [CrossRef]
- Villines, T.C.; Stanek, E.J.; Devine, P.J.; Turco, M.; Miller, M.; Weissman, N.J.; Griffen, L.; Taylor, A.J. The ARBITER 6-HALTS trial (arterial biology for the investigation of the treatment effects of reducing cholesterol 6-HDL and LDL treatment strategies in atherosclerosis): Final results and the impact of medication adherence, dose, and treatment duration. J. Am. Coll. Cardiol. 2010, 55, 2721–2726. [Google Scholar] [CrossRef] [PubMed]
- Lavigne, P.M.; Karas, R.H. The current state of niacin in cardiovascular disease prevention: A systematic review and meta-regression. J. Am. Coll. Cardiol. 2013, 61, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Geng, D.F.; Deng, J.; Jin, D.M.; Wu, W.; Wang, J.F. Effect of cilostazol on the progression of carotid intima-media thickness: A meta-analysis of randomized controlled trials. Atherosclerosis 2012, 220, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Uehara, K.; Matsumoto, Y.; Hashimoto, A.; Nagano, C.; Niimi, M.; Miyakoda, G.; Nagano, K. Cilostazol inhibits accumulation of triglyceride in aorta and platelet aggregation in cholesterol-fed rabbits. PLoS ONE 2012, 7, e39374. [Google Scholar] [CrossRef]
- Ishizaka, N.; Taguchi, J.; Kimura, Y.; Ikari, Y.; Aizawa, T.; Togo, M.; Miki, K.; Kurokawa, K.; Ohno, M. Effects of a single local administration of cilostazol on neointimal formation in balloon-injured rat carotid artery. Atherosclerosis 1999, 142, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Zaima, N.; Ito, H.; Hattori, K.; Yamamoto, N.; Konno, H.; Setou, M.; Unno, N. Cilostazol inhibits accumulation of triglycerides in a rat model of carotid artery ligation. J. Vasc. Surg. 2013, 58, 1366–1374. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lorenz, M.W.; Markus, H.S.; Bots, M.L.; Rosvall, M.; Sitzer, M. Prediction of clinical cardiovascular events with carotid intima-media thickness: A systematic review and meta-analysis. Circulation 2007, 115, 459–467. [Google Scholar] [CrossRef]
- Irie, Y.; Katakami, N.; Kaneto, H.; Kasami, R.; Sumitsuji, S.; Yamasaki, K.; Tachibana, K.; Kuroda, T.; Sakamoto, K.; Umayahara, Y.; et al. Maximum carotid intima-media thickness improves the prediction ability of coronary artery stenosis in type 2 diabetic patients without history of coronary artery disease. Atherosclerosis 2012, 221, 438–444. [Google Scholar] [CrossRef]
- Den Ruijter, H.M.; Peters, S.A.; Anderson, T.J.; Britton, A.R.; Dekker, J.M.; Eijkemans, M.J.; Engström, G.; Evans, G.W.; de Graaf, J.; Grobbee, D.E.; et al. Common carotid intima-media thickness measurements in cardiovascular risk prediction: A meta-analysis. JAMA 2012, 308, 796–803. [Google Scholar] [CrossRef]
- Lorenz, M.W.; Polak, J.F.; Kavousi, M.; Mathiesen, E.B.; Völzke, H.; Tuomainen, T.P.; Sander, D.; Plichart, M.; Catapano, A.L.; Robertson, C.M.; et al. Carotid intima-media thickness progression to predict cardiovascular events in the general population (the PROG-IMT collaborative project): A meta-analysis of individual participant data. Lancet 2012, 379, 2053–2062. [Google Scholar] [CrossRef]
- Wang, A.; Li, H.; Yuan, J.; Zuo, Y.; Zhang, Y.; Chen, S.; Wu, S.; Wang, Y. Visit-to-visit variability of lipids measurements and the risk of stroke and stroke types: A prospective cohort study. J. Stroke 2020, 22, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Kivioja, R.; Pietilä, A.; Martinez-Majander, N.; Gordin, D.; Havulinna, A.S.; Salomaa, V.; Aarnio, K.; Curtze, S.; Leiviskä, J.; Rodríguez-Pardo, J.; et al. Risk factors for early-onset ischemic stroke: A case-control study. J. Am. Heart Assoc. 2018, 7, e009774. [Google Scholar] [CrossRef] [PubMed]
- Hindy, G.; Engström, G.; Larsson, S.C.; Traylor, M.; Markus, H.S.; Melander, O.; Stroke Genetics Network (SiGN). Role of blood lipids in the development of ischemic stroke and its subtypes: A Mendelian randomization study. Stroke 2018, 49, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Chang, P.Y.; Zhang, Y.; Kizer, J.R.; Best, L.G.; Howard, B.V. Triglyceride and HDL-C dyslipidemia and risks of coronary heart disease and ischemic stroke by glycemic dysregulation status: The strong heart study. Diabetes Care 2017, 40, 529–537. [Google Scholar] [CrossRef]
| Study | Design | No. of Cases | Variables Associated with Carotid Atherosclerosis | OR or HR (95% CI) | p Value |
|---|---|---|---|---|---|
| Teno et al. [16] | Cross-sectional | 61 patients with T2DM | Fasting TG ≥ 150 mg/dL | N/A | 0.02 |
| Non-fasting TG ≥ 200 mg/dL | N/A | Tendency | |||
| Mori et al. [19] | Cross-sectional | 68 patients with T2DM | Fasting TG ≥ 150 mg/dL | N/A | <0.05 |
| Non-fasting TG ≥ 200 mg/dL | N/A | <0.05 | |||
| Idei et al. [20] | Retrospective | 115 patients with T2DM | Mean non-fasting TG during follow-up | OR, 1.20 (1.05–1.37) | 0.009 |
| Vouillarmet et al. [17] | Retrospective | 342 patients with T2DM | Fasting TG ≥ 150 mg/dL | N/A | Tendency |
| Kitagami et al. [18] | Retrospective | 71 patients with CAS with LDL-C < 140 mg/dL | Fasting TG ≥ 150 mg/dL | HR, 6.228 (1.533−25.309) | 0.011 |
| Miura et al. [21] | Retrospective | 121 carotid arteries in 96 patients with CAS | Non-fasting TG ≥ 175 mg/dL | OR, 4.703 (1.511−14.638) | 0.008 |
| Miura et al. [22] | Retrospective | 111 carotid arteries in 88 patients with CAS | Area [TG ≥ 175] (cumulative non-fasting TG ≥ 175 mg/dL during follow-up) ≥ 6.35 year-mg/dL | OR, 4.21 | 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miura, Y.; Suzuki, H. Hypertriglyceridemia and Atherosclerotic Carotid Artery Stenosis. Int. J. Mol. Sci. 2022, 23, 16224. https://doi.org/10.3390/ijms232416224
Miura Y, Suzuki H. Hypertriglyceridemia and Atherosclerotic Carotid Artery Stenosis. International Journal of Molecular Sciences. 2022; 23(24):16224. https://doi.org/10.3390/ijms232416224
Chicago/Turabian StyleMiura, Yoichi, and Hidenori Suzuki. 2022. "Hypertriglyceridemia and Atherosclerotic Carotid Artery Stenosis" International Journal of Molecular Sciences 23, no. 24: 16224. https://doi.org/10.3390/ijms232416224
APA StyleMiura, Y., & Suzuki, H. (2022). Hypertriglyceridemia and Atherosclerotic Carotid Artery Stenosis. International Journal of Molecular Sciences, 23(24), 16224. https://doi.org/10.3390/ijms232416224






