Abstract
The development of adsorption materials which can efficiently isolate and enrich uranium is of great scientific significance to sustainable development and environmental protection. In this work, a novel phosphonic acid-functionalized magnetic microsphere adsorbent Fe3O4/P (GMA-MBA)-PO4 was developed by functionalized Fe3O4/P (GMA-MBA) prepared by distill-precipitation polymerization with O-phosphoethanolamine. The adsorption process was endothermic, spontaneous and kinetically followed the pseudo second-order model. The maximum uranium adsorption capacity obtained from the Langmuir model was 333.33 mg g−1 at 298 K. In addition, the adsorbent also had good acid resistance and superparamagnetic properties, which could be quickly separated by a magnetic field. XPS analysis showed that the adsorption of adsorbent mainly depended on the complexation of phosphonic acid group with uranium. This work offers a promising candidate for the application of magnetic adsorbents in the field of uranium separation and enrichment.
1. Introduction
With the intensification of the energy crisis, nuclear energy as a kind of clean energy has been widely studied [1,2,3]. The demand for uranium as an important nuclear fuel is increasing. However, uranium is characterized by radioactivity and heavy metal toxicity. If it accumulates in large quantities in the environment, it will do harm to the environment. Therefore, it is important to develop the separation technology for the efficient separation and enrichment of uranium for the exploitation of uranium resources and environmental protection [4,5,6]. At present, many methods including ion exchange, solvent extraction, chemical precipitation, membrane separation, photocatalysis and adsorption have been used to recover uranium from an aqueous solution [7,8,9,10,11,12]. Among these methods, adsorption has proved to be a promising separation technique due to its wide source of materials, low cost, high adsorption selectivity and high volumetric value [13,14,15]. The key to the adsorption method is the choice of adsorption material. At present, many materials such as carbon materials, silicon materials, polymer materials and metal–organic frames have been used in the field of uranium adsorption. However, these materials often suffer from complicated separation steps such as filtration and centrifugation, which hinder their application in the field of uranium adsorption to a great extent. Therefore, the development of a uranium adsorption medium with a high adsorption efficiency and easy separation is one of the current research key points in the field of environmental radiochemistry [16,17].
Recently, magnetic polymer nanomaterials have received much attention [18,19,20,21,22]. On the one hand, the nano size can make this material have a larger specific surface area and more surface atoms than ordinary materials, thus showing strong adsorption and performance. On the other hand, magnetic polymer nanomaterials can be separated quickly by magnetic force, which solves the problem of the separation difficulty of traditional adsorption materials [23,24,25,26,27,28,29,30,31]. In addition, the surface of the material can also be rich in organic functional groups by means of copolymerization or post-modification, which can improve the adsorption capacity for uranium. Therefore, magnetic polymer nanomaterials have obvious advantages in the separation and enrichment of uranium, which cannot be replaced by conventional materials in many aspects. Based on these advantages, the development of magnetic polymer nanomaterials has important scientific significance and practical value for the healthy and rapid development of the nuclear industry.
Up to now, as an efficient ligand for uranium, the phosphonic acid group has been widely used for the separation and enrichment of uranium due to its strong complexing ability with uranyl ion. For example, in our preliminary work, many phosphonic acid-functionalized adsorbents were prepared by solvothermal polymerization, distillation precipitation polymerization and other methods, and all of them had good adsorption properties for uranium [1,16]. In addition, Broda et al. [32] reported a nanocomposite hydroxyapatite/white clay with excellent adsorption properties for uranium. Therefore, considering these factors, this work hoped to develop a phosphonic acid-functionalized magnetic polymer microsphere adsorbent for the efficient separation and enrichment of uranium from aqueous solutions [33,34].
In this work, a novel phosphonic acid-functionalized magnetic polymer microsphere adsorbent Fe3O4/P (GMA-MBA)-PO4 was developed by functionalizing Fe3O4/P (GMA-MBA) prepared by distill-precipitation polymerization with O-phosphoethanolamine. Surprisingly, the Fe3O4/P (GMA-MBA)-PO4 showed a good adsorption capacity, with the theoretical maximum adsorption capacity of uranium reaching 333.33 mg g−1 at pH 4.5. In addition, the adsorbent also had good structural stability and superparamagnetic character, resulting in the quick separation and recovery by magnetic force of the adsorbent. XPS analysis showed that the adsorption of the adsorbent mainly depended on the complexation of the phosphonic acid group with uranium. This work offers a promising candidate for the application of magnetic adsorbents in the field of uranium separation and enrichment.
2. Results and Discussion
2.1. Structural Analyses
To verify that Fe3O4/P (GMA-MBA)-PO4 was successfully synthesized by Scheme 1, the physical and chemical properties of the adsorbent were characterized by TEM, FT-IR, XRD, VSM and TGA, respectively.

Scheme 1.
Proposed synthesis of the magnetic adsorbent Fe3O4/P (GMA-MBA)-PO4.
The morphology of Fe3O4, activated Fe3O4, Fe3O4/P(GMA-MBA) and the magnetic adsorbent Fe3O4/P (GMA-MBA)-PO4 were investigated using TEM. As shown in Figure 1A, the prepared Fe3O4 had a uniform particle size with an average diameter of about 200 nm. The magnetic sphere surface had a fluffy structure [35]. The mean particle size and morphology of Fe3O4 did not change significantly after the modification of γ-MPS (Figure 1B), suggesting that the activation process did not have much effect on the morphology of Fe3O4. Figure 1C shows that there was a thick polymer shell layer around the Fe3O4, and the thickness of the polymer shell was around 35 nm, indicating that the copolymerization of GMA and MBA had occurred smoothly on the surface of Fe3O4 to obtain the matrix material Fe3O4/P (GMA-MBA). Figure 1D lists the TEM image of Fe3O4/P (GMA-MBA)-PO4. From Figure 1D, the magnetic adsorbent still maintained the core–shell structure, indicating the matrix material Fe3O4/P (GMA-MBA) had an excellent medium resistance.
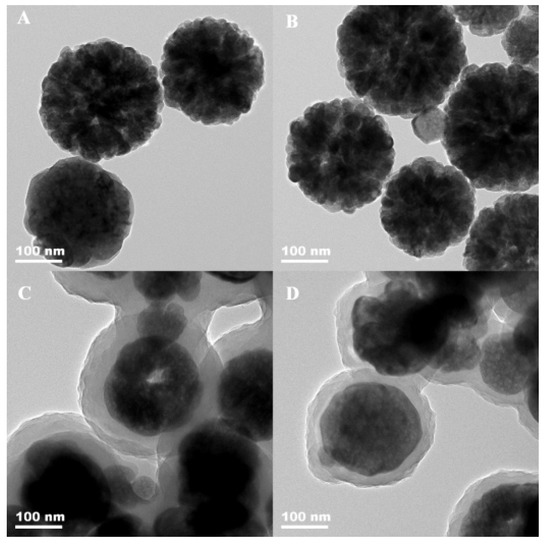
Figure 1.
The TEM images for (A) Fe3O4, (B) activated Fe3O4, (C) Fe3O4/P (GMA-MBA) and (D) magnetic adsorbent Fe3O4/P (GMA-MBA)-PO4.
Figure 2 shows the FTIR spectra of Fe3O4, Fe3O4 activated by KH570, Fe3O4/P(GMA-MBA) and Fe3O4/P (GMA-MBA)-PO4. As shown in Figure 2a, the characteristic peaks located around 585 cm−1 belonged to the characteristic absorption of Fe-O in Fe3O4. Compared to Fe3O4, a series of new peaks ascribed to γ-MPS can be observed in Figure 2b. For example, tow new peaks located at approximately, 1636 cm−1 and 1720 cm−1 were attributed to the -C=C and -C=O groups of γ-MPS, respectively, indicating that Fe3O4 had been functionalized by KH570 successfully [36]. In the spectrum of Fe3O4/P (GMA-MBA) (Figure 2c), many new characteristic absorption bands ascribed to P (GMA-MBA) could be observed. For instance, the two obvious peaks located at 1528 cm−1 and 1650 cm−1 belonged to the stretching vibration of N-H and C=O from MBA. In addition, the three obvious peaks located at 842 cm−1, 906 cm−1 and 1721 cm−1 belonged to the stretching vibration of epoxy group and C=O from GMA. Thus, all these results indicated that the co-polymerization of GMA and MBA had occurred on the surface of activated Fe3O4. Figure 2d shows the infrared spectrum of Fe3O4/P(GMA-MBA)-PO4. The two obvious peaks located at 940 cm−1 and 1232 cm−1 belonged to the stretching vibration of P-OH and P=O [37]. Besides, the epoxy group characteristic absorption peak (842 cm−1 and 906 cm−1) appearing in Figure 2c almost disappeared. Thus, all these results suggest that Fe3O4/P(GMA-MBA) was successfully modified with O-phosphoethanolamine.

Figure 2.
Infrared spectra of (a) Fe3O4; (b) activated Fe3O4; (c) Fe3O4/P (GMA-MBA) and (d) Fe3O4/P (GMA-MBA)-PO4.
The X-ray powder diffraction (XRD) pattern of Fe3O4 and Fe3O4/P(GMA-MBA)-PO4 was shown in Figure 3A. The XRD pattern of Fe3O4/P(GMA-MBA)-PO4 showed many characteristic peaks of Fe3O4. Moreover, a peak of dispersion at a 2θ of about 12.5° representing amorphous P(GMA-MBA) was also seen in addition to the characteristic peaks of Fe3O4. The VSM pattern of Fe3O4/P (GMA-MBA)-PO4 is shown in Figure 3B. As shown in Figure 3B, the hysteresis loop passed through the origin with a coercivity force of zero, confirming the magnetic polymer microsphere adsorbent was superparamagnetic with a specific saturation magnetization of about 10.0 emu g−1. Thus, Fe3O4/P (GMA-MBA)-PO4 could be quickly separated and recovered from the aqueous solution by applying an external magnetic field within 20 s (Figure 3B inset). The thermal stability of the material was tested, and the results are shown in Figure 3C. From Figure 3C, the lost weight of the sample was mainly caused by the volatilization of the small molecule including water, solvent and monomers remaining on the surface of Fe3O4/P (GMA-MBA)-PO4 within 50–293 °C. However, when the temperature was above 293 °C, the mass of the magnetic adsorbent dropped sharply, which might have been due to the oxidative degradation of the polymer chain. Thus, according to Figure 3C, the prepared magnetic adsorbent had a high thermal stability. As shown in Figure 3D, the elemental composition of Fe3O4/P (GMA-MBA)-PO4 was also analyzed by XPS, and the magnetic adsorbent contained C, N, O and P. Combined with the results of infrared spectroscopy analysis, it is clear that the phosphonic acid group was successfully introduced to the surface of the magnetic sphere. Based on these results, the phosphonic acid functionalized core–shell magnetic sorbent Fe3O4/P(GMA-MBA)-PO4 was successfully prepared.
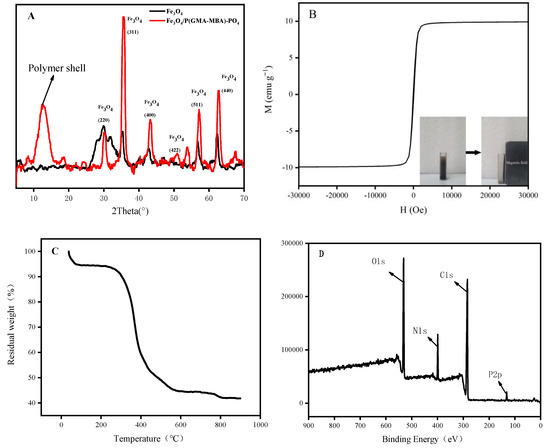
Figure 3.
(A) XRD pattern of Fe3O4, and Fe3O4/P(GMA-MBA)-PO4; (B) VSM curve; (C) TGA curve and (D) XPS spectrum of Fe3O4/P(GMA-MBA)-PO4.
2.2. Effect of pH
In general, the adsorption capacity of uranium is often significantly affected by pH, due to which the speciation of uranium and the surface charge of the adsorbent are affected to a large extent. Hence, the uranium adsorption capacity on Fe3O4/P (GMA-MBA) and Fe3O4/P (GMA-MBA)-PO4 was investigated with an initial pH ranging from 1.0 to 4.5, and the experimental results are shown in Figure 4A. From Figure 4A, the uranium adsorption capacity of the two magnetic adsorbents increased with the value of pH. In aqueous solutions of pH 4.5, the adsorption capacity of Fe3O4/P (GMA-MBA) and Fe3O4/P (GMA-MBA)-PO4 could reach up to 52.21 and 98.73 mg g−1, respectively. From Figure 4A, the uranium adsorption capacity of Fe3O4/P (GMA-MBA)-PO4 was much higher than that of Fe3O4/P (GMA-MBA). This result confirms that the prepared magnetic polymer microsphere adsorbent had the ability to efficiently separate the enriched uranyl ions in the aqueous solution, which was mainly due to the complexation of phosphoric acid functional groups on the surface of the adsorbent with uranyl ions.
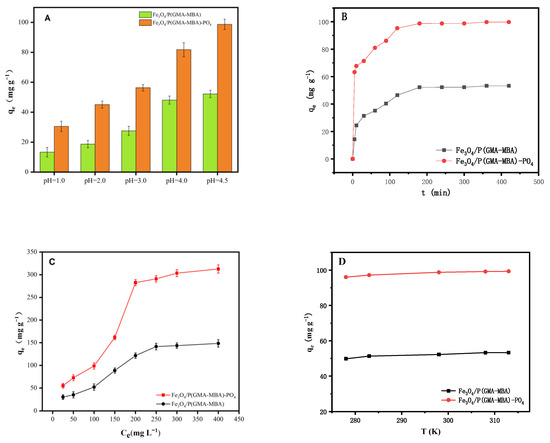
Figure 4.
(A) Adsorption performance of Fe3O4/P (GMA-MBA) and Fe3O4/P (GMA-MBA)-PO4 on uranium under different pH values; (B) influence of contact time on uranium adsorption capacity (C0 = 100 mg L−1, T = 298 K, pH = 4.5, m/V = 0.4 g L−1); (C) effect of uranium concentration on adsorption capacity of Fe3O4/P (GMA-MBA) and Fe3O4/P (GMA-MBA)-PO4 (T = 180 min, pH = 4.5, T = 298 K, m/V = 0.4 g L−1); (D) thermodynamic image of U (VI) adsorbed by Fe3O4/P (GMA-MBA) and Fe3O4/P (GMA-MBA)-PO4 (C0 = 100 mg L−1, pH = 4.5, m/V = 0.4 g L−1).
As shown in Figure 4A, under highly acidic conditions (at pH < 2), the U (VI) adsorption capacity of Fe3O4/P(GMA-MBA)-PO4 was very low. This was mainly because the material surface group was protonated when the solution pH value was very low, and the adsorbent surface was positively charged. Since uranium (VI) mainly exists in the form of UO22+ when the pH value was less than 4.5 (Figure S1), electrostatic repulsion between the adsorbed material and UO22+ reduced the adsorption performance of uranium. When the acidity of the system decreased, the protonation degree of the adsorbent surface reduced. In such a case, the electrostatic repulsion between the adsorption material and UO22+ was constantly reduced, and the complexation ability of the functional group of the adsorbent surface with uranyl ion was enhanced, resulting in the increase in the uranium adsorption capacity of the adsorbent. In addition, when the value of pH was greater than 4.5, uranium became unstable and easy to precipitate. Therefore, pH of 4.5 was applied as the best operation condition for further experiments.
2.3. Sorption Static Kinetics, Isotherms and Thermodynamic Analysis
To acquire sorption kinetics, isotherms and thermodynamic data, the effect of time, uranium concentration and temperature on the adsorption capacity of the adsorbent for uranium was evaluated, and the results are listed in Figure 4B–D.
The uranium adsorption capacity of Fe3O4/P(GMA-MBA) and Fe3O4/P(GMA-MBA)-PO4 at different adsorption times is shown in Figure 4B. From Figure 4B, the adsorption of uranium at pH 4.5 could be roughly divided into three stages. In the first 120 min, the adsorption rate was very fast and the adsorption capacity increased rapidly. At 120–180 min, the adsorption rate was reduced. The adsorption was gradually balanced after 180 min. Since the Fe3O4/P(GMA-MBA)-PO4 was rich in the phosphate acid group, the saturation adsorption capacity of Fe3O4/P(GMA-MBA)-PO4 was greater than that of Fe3O4/P(GMA-MBA).
Table S1 lists the kinetic parameters obtained by pseudo-first-order, pseudo-second-order and intra-particle diffusion models [38,39,40]. According to Table S1 and Figures S2–S4, the adsorption process was better correlated with the pseudo-second-order kinetic models. Calculated from the linear equation of the pseudo-second-order, the theoretical adsorption volume qe values of Fe3O4/P(GMA-MBA) and Fe3O4/P(GMA-MBA)-PO4 at pH 4.5 were 52.91 mg g−1 and 101.21 mg g−1, very close to the actual experimental results (52.21 mg g−1 and 98.73 mg g−1). This showed that the pseudo-second-order kinetic model was better suited for describing the adsorption process of uranium by Fe3O4/P(GMA-MBA)-PO4, since the pseudo-second-order model is based on the assumption that the adsorption rate is controlled by chemical adsorption. Thus, the adsorption mechanism of uranium by Fe3O4/P (GMA-MBA)-PO4 and Fe3O4/P(GMA-MBA) in pH 4.5 solution was mainly dominated by chemical adsorption.
The effect of the initial concentration of uranyl ion on the uranium adsorption capacity of Fe3O4/P(GMA-MBA) and Fe3O4/P(GMA-MBA)-PO4 in aqueous solutions at pH 4.5 was investigated, and the results are shown in Figure 4C. From Figure 4C, the adsorption capacity of the two magnetic adsorbents increased as the concentration of the uranium solution increased from 25 mg L−1 to 200 mg L−1. When the concentration of the solution reached above 250 mg L−1, the adsorption capacity of the two magnetic adsorbents increased. When the concentration was 300 mg L−1, the equilibrium adsorption capacity of Fe3O4/P (GMA-MBA) reached 148.59 mg g−1. When the adsorption capacity of Fe3O4/P (GMA-MBA)-PO4 increased to 303.59 mg g−1, the adsorption capacity remained unchanged with the continued increase in concentration. The main reason for this was that when the concentration of uranium ions in solution increased, the adsorption sites on the surface of the adsorbent were occupied by uranyl ions. When the concentration increased to a certain extent, the adsorption sites were occupied completely. In such case, there were no more phosphate groups to coordinate with the uranyl ion, meaning that the adsorption balance was reached. Moreover, the equilibrium adsorption capacity of Fe3O4/P (GMA-MBA)-PO4 was much higher than that of Fe3O4/P (GMA-MBA), due to the fact that Fe3O4/P (GMA-MBA)-PO4 had a high concentration of phosphonic acid groups.
The uranium adsorption performance of Fe3O4/P (GMA-MBA) and Fe3O4/P (GMA-MBA)-PO4 at different temperatures is shown in Figure 4D. With the increase in contact temperature, the uranium adsorption capacity of the two adsorbents to uranium gradually increased, indicating that the uranium adsorption process was endothermic, and the increase in temperature was beneficial to the adsorption. To further investigate the effect of temperature on uranium adsorption performance, the three parameters including enthalpy changes ΔH° (KJ mol−1), ΔS° (J mol−1 K−1) and Gibbs free energy changes ΔG° (KJ mol−1) were studied. ΔH° and ΔS° were calculated using the thermodynamic formulas Equations (S1) and (S2). The ΔH° and ΔS° were calculated from the linear plots of lnKd and 1/T (Figures S7 and S8). The ΔG° of Fe3O4/P (GMA-MBA) and Fe3O4/P (GMA-MBA)-PO4 were calculated, respectively, by using the Van’t Hoff equation. According to Table S3, since ΔH° was positive, the adsorption of Fe3O4/P (GMA-MBA)-PO4 to uranium was endothermic, suggesting the increase in the temperature was beneficial to the adsorption. The value of ΔS° was positive, indicating that the surface confusion and randomness of Fe3O4/P (GMA-MBA)-PO4 increased during the adsorption process. The value of ΔG° was negative, indicating that the uranium adsorption process was spontaneous.
Table S2 lists the sorption isotherm parameters of the Fe3O4/P (GMA-MBA) and Fe3O4/P (GMA-MBA)-PO4 calculated from the Langmuir and Freundlich models [41,42]. As shown in Figures S5 and S6 and Figures S5 and S6, the correlation coefficients R2 of Fe3O4/P (GMA-MBA) and Fe3O4/P (GMA-MBA)-PO4 were 0.995 and 0.9982 according to the Langmuir Model, which was much higher than that of Freundlich Model. The maximum adsorption capacity of Fe3O4/P (GMA-MBA)-PO4 for U (VI) (333.33 mg g−1) calculated by the Langmuir adsorption equation was closer to the experimental data (303.59 mg g−1). Compared with the Freundlich model, the Langmuir model was more compatible with the experimental data. Thus, it could be argued that the adsorption of Fe3O4/P (GMA-MBA)-PO4 for uranium was dominated by monolayer adsorption.
2.4. Structural Stability Analysis
To investigate the acid resistance of Fe3O4/P (GMA-MBA)-PO4, the adsorbent was soaked at a pH of 4.5 of HNO3 for 24 h, and then separated by an external magnet and freeze-dried. As shown in Figure 5, the soaked magnetic absorbent was characterized by FTIR, VSM and TEM. Figure 5A confirms that its structure did not significantly change after immersion and the surface functional group remained unchanged. The VSM test results are shown in Figure 5B, where the adsorbent magnetic response decreased slightly, but it still showed superparamagnetic character. The TEM images of the adsorbent before and after soaked are shown in Figure 5C,D. From Figure 5C,D, the material remained intact with a spherical structure after 24 h of soaking with no significant changes. The above results show that Fe3O4/P (GMA-MBA)-PO4 had a good structural stability at a pH of 4.5 of HNO3 for 24 h.
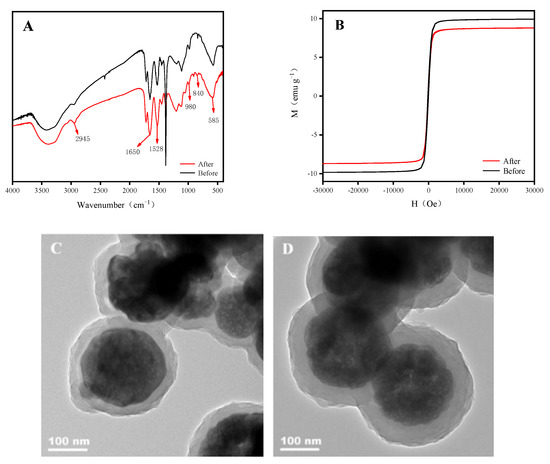
Figure 5.
Fe3O4/P(GMA-MBA)-PO4 was soaked in a HNO3 medium with pH 4.5 for 24 h before and after: (A) Infrared spectra; (B) VSM spectrum; (C) TEM images taken before immersion; (D) TEM images after immersion.
2.5. Comparison of the U(VI) Sorption Capacity of Fe3O4/P (GMA-MBA)-PO4 with That of Other Adsorbents in Aqueous Solutions
As shown in Table 1, the Fe3O4/P (GMA-MBA)-PO4 of adsorption properties were compared with other adsorbents. From Table 1, the qmax of Fe3O4/P (GMA-MBA)-PO4 was up to 333.33 mg g−1, which was better than that of the other magnetic adsorbents listed in Table. For example, unmodified Fe3O4 had a uranium adsorption capacity of less than 50 mg g−1 at pH 7.0. After modifying the Fe3O4 (such as Fe3O4/GO, Fe3O4@C-KO, Fe3O4@TiO2, MCFN, et al.) uranium adsorption capacity increased, but its saturation adsorption capacity was still lower than that of Fe3O4/P (GMA-MBA)-PO4. Most magnetic adsorbents show a good adsorption capacity at pH 5.5–6.0, but the adsorption capacity decreased with the increase in acidity. Thus, Fe3O4/P (GMA-MBA)-PO4 could be regarded as a promising candidate for the separation and preconcentration of uranium.

Table 1.
Comparison of uranium adsorption performance between Fe3O4/P(GMA-MBA)-PO4 and other adsorbents.
2.6. Analysis of the Interactions between Uranium and Fe3O4/P(GMA-MBA)-PO4
To study the interaction between magnetic adsorbent and uranium ions, XPS analysis of Fe3O4/P (GMA-MBA)-PO4 before and after uranium adsorption is shown in Figure 6A–D. As shown in Figure 6A, the two peaks located at 133.9 eV and 133.0 eV were the characteristic peaks of P 2p1/2 and P 2p3/2. Compared with Figure 6A, the binding energy strength and position of Fe3O4/P (GMA-MBA)-PO4 of P 2p1/2 changed significantly (Figure 6B), indicating a new complexation between uranyl with P. As shown in Figure 6C, the O 1s spectra could be decomposed into three peaks, and the characteristic peaks at 530.3 eV, 531.2 eV and 532.5 eV belonged to P=O, C=O, and P-OH. After adsorption of Fe3O4/P (GMA-MBA)-PO4 for uranium, the position of the binding energy and strength of O 1s of P=O and P-OH bond changed significantly, while the position of O 1s of C=O bond remained almost unchanged (Figure 6D). Thus, it could be concluded that the adsorption process mainly depended on the interaction between the phosphonic acid group with uranium.
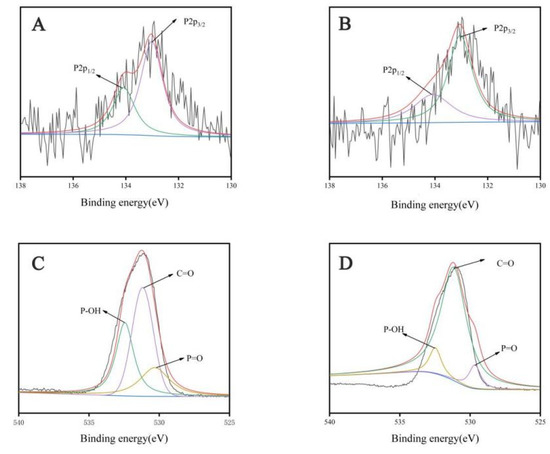
Figure 6.
(A) P 2p map of Fe3O4/P (GMA-MBA)-PO4; (B) P 2p map of Fe3O4/P (GMA-MBA)-PO4-U; (C) O 1s map of Fe3O4/P (GMA-MBA)-PO4; (D) O 1s map of Fe3O4/P (GMA-MBA)-PO4-U.
3. Materials and Methods
3.1. Materials
Bisacrylamide (MBA), O-phospethanolamine, γ-(Methacryloxypropyl) trimethoxy silane (KH570), glycidyl methacrylate (GMA) and azobisisobutyronitrile (AIBN) were bought from Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China) Acetonitrile, nitrate acid (HNO3), sodium hyfroxide (NaOH) and FeCl3·6H2O were supplied from Xilong Chemical Co., Ltd. (Shantou, China) O-phospethanolamine was purchased from Shanghai Perlingway Chemical Technology Co., Ltd. (Shanghai, China) UO2 (NO3)2·6H2O (ACS grade) was purchased from Shanghai Reagents (Shanghai, China). All other chemicals used in the experiments were of analytical grade. Deionized water used for all experiments was supplied from a Milli-Q (Milli-pore Corporation, Burlington, MA, USA) water purification system.
3.2. Preparation of Fe3O4
Fe3O4 microspheres were prepared by the solvothermal method. The synthetic procedure was as follows: 2.5 g of FeCl3·6H2O was dissolved in 80 g of ethylene glycol (EG), and then 7.2 g of sodium acetate (NaAc) and 2.0 g of polyethylene glycol acid (PEG) were added to the solution. The mixture was stirred at 50 °C for 1 h and transferred to a 100 mL autoclave lined with polytetrafluoroethylene. The autoclave was heated to 200 °C for 6 h, and then left to cool naturally to room temperature. The Fe3O4 microspheres were washed with ethanol several times to remove the impurities under ultrasonic condition. Finally, Fe3O4 microspheres were kept at room temperature.
3.3. Preparation of Activated Fe3O4
In order to improve the coating rate of Fe3O4 in the polymer shell, the Fe3O4 hollow pellets were activated by γ-(Methacryloxypropyl) trimethoxy silane (KH570) [55]. The synthetic procedure was as follows: 0.3 g of Fe3O4, 128.0 mL of anhydrous ethanol, 4.0 mL γ-(Methacryloxypropyl) trimethoxy silane (KH570), 4.0 mL NH3·H2O and 36.0 mL of distilled water were added to a three-neck flask with a capacity of 250 mL. The mixture was stirred for 12 h at 40 °C. After the reaction, the product was washed several times to remove impurities. Finally, activated Fe3O4 microspheres by KH570 were kept at room temperature.
3.4. Preparation of Fe3O4/P (GMA-MBA)
Fe3O4/P(GMA-MBA) was prepared by the distillation and precipitation method. In a typical run, 0.10 g of surface-activated Fe3O4 microsphere and 80.0 mL acetonitrile were moved into 250 mL three-neck round bottom flask under ultrasonic oscillations. Then, 0.08 g of AIBN, 0.30 g of GMA and 0.30 g of MBA were added to the mixture. The mixture was heated to 90 °C for the reaction for 2 h. At the end of the reaction, the obtained magnetic microspheres were separated by an external magnetic field, washed several times and dried by lyophilization.
3.5. Preparation of Fe3O4/P (GMA-MBA)-PO4
In a typical run, Fe3O4/P (GMA-MBA) microspheres (0.1 g), O-phospethanolamine (1 g), DMF (50 mL) and H2O (50 mL) were added to a round-bottom flask. The mixture was stirred at 80 °C for 8 h. Then, the obtained Fe3O4/P (GMA-MBA)-PO4 was separated by an external magnetic field, washed several times and dried by lyophilization. The synthesis process is described in Scheme 1.
3.6. Characterization
The XRD pattern was measured with a D8 ADVANCE Da Vinci. FT-IR spectroscopy analysis were acquired on a Nicolet 6700 (Thermo Fisher, Waltham, MA, USA). Thermogravimetric analysis (TGA) was measured with an STA 449 F3 (Nichi, Bavaria, Germany). TEM images were performed on a TALOS F200X (Thermo Fisher, Waltham, MA, USA). X-ray photoelectron spectra (XPS) were acquired on an AXIS UItraDLD (Shimadzu, Kyoto, Japan). Magnetic properties (VSM) were measured on a MPMS3 (Quantum Design, San Diego, CA, USA).
3.7. Adsorption Tests
The adsorption experiments were carried out using the batch method to measure the adsorption property of uranium of the magnetic adsorbent. In a typical run, 10 mg of Fe3O4/P (GMA-MBA)-PO4 was placed into 25 mL of uranium solution at different pH values that could be adjusted by adding negligible volumes of diluted HNO3 or NaOH. After stirring for an appropriate time at a given temperature, the Fe3O4/P (GMA-MBA)-PO4 was collected by magnetic separation. The concentrations of UO22+ in the aqueous solution, before and after adsorption, were determined by UV-vis spectrophotometer with arsenazo (III) as the complex agent.
The adsorption capacity qe (mg g−1) and the distribution coefficient, Kd (mL g−1), were calculated by Equations (1) and (2):
where C0 and Ce are the initial concentration and equilibrium concentration of metal cations (mg L−1), Kd is the distribution coefficient (mL g−1), V is the volume of testing solution (L) and M is the amount of adsorbent (g).
3.8. Stability Tests
To investigate the structural stability of the magnetic adsorbent in an acidic solution, the prepared adsorbent was immersed at a pH of 4.5 of HNO3 for 24 h, and then was magnetically separated and freeze-dried with an external magnet. The adsorbent was subsequently characterized using TEM, FTIR, XRD and VSM to determine whether the structure had changed.
4. Conclusions
In this work, a novel phosphonic acid-functionalized core–shell magnetic polymer microsphere adsorbent Fe3O4/P (GMA-MBA)-PO4 was prepared by functionalized Fe3O4/P (GMA-MBA) with O-phosphoethanolamine. The physical and chemical properties and microstructure of the material were characterized by TEM, FT-IR, TG, XRD, XP and, VSM. The effect of pH, time, concentration and temperature on the adsorption behavior of the adsorbent was studied. The results showed that the adsorbent had a good adsorption performance for uranium, and its theoretical saturated adsorption capacity was up to 333.3 mg g−1. The study of adsorption kinetics and thermodynamics showed that the adsorption process was a fast, spontaneous and endothermic process, which accorded with the pseudo-second-order model and the Langmuir model. In addition, the adsorbent also had a good acid resistance and superparamagnetic character, which could be quickly separated by an external magnet. The XPS analysis showed that the adsorption process was mainly dependent on the interaction between the phosphonic acid group with uranium. Based on these, Fe3O4/P(GMA-MBA)-PO4 could be a promising candidate for the separation and preconcentration of uranium in actual water treatment.
Supplementary Materials
The supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms232416227/s1.
Author Contributions
J.Z., conceptualization, investigation, data curation, and writing—original draft preparation; P.L., investigation, resources, methodology, and editing; T.H., methodology; J.H., S.Z., Y.L., Y.W. and C.M., methodology and validation; D.Y., conceptualization, supervision, project administration, funding acquisition, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grants No.22266002, 22066002, 21966005, 22166001), the Opening Project of Jiangxi Province Key Laboratory of Polymer Micro/Nano Manufacturing and Devices (PMND202102), the Natural Science Foundation of Jiangxi Province (20202BABL214058) and the Graduate Innovation Fund of East China University of Technology (DHYC-202111).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yuan, D.; Zhang, S.; Tan, J.; Dai, Y.; Wang, Y.; He, Y.; Liu, Y.; Zhao, X.; Zhang, M.; Zhang, Q. Highly efficacious entrapment of Th (IV) and U (VI) from rare earth elements in concentrated nitric acid solution using a phosphonic acid functionalized porous organic polymer adsorbent. Sep. Purif. Technol. 2020, 237, 116379. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, S.; Zhao, J.; Wei, P.; Wang, C.; Liu, P.; Zhao, X.; Zeng, K.; Wu, F.; Liu, Z. Unexpected ultrafast and highly efficient removal of uranium from aqueous solutions by a phosphonic acid and amine functionalized polymer adsorbent. New J. Chem. 2021, 45, 10777–10787. [Google Scholar] [CrossRef]
- Zhang, Q.; Zeng, K.; Wang, C.; Wei, P.; Zhao, X.; Wu, F.; Liu, Z. An imidazole functionalized porous organic polymer for the highly efficient extraction of uranium from aqueous solutions. New J. Chem. 2022, 46, 9238–9249. [Google Scholar] [CrossRef]
- Mori, T.; Takao, K.; Sasaki, K.; Suzuki, T.; Arai, T.; Ikeda, Y. Homogeneous liquid–liquid extraction of U (VI) from HNO3 aqueous solution to betainium bis(trifluoromethylsulfonyl)imide ionic liquid and recoveryof extracted U (VI). Sep. Purif. Technol. 2015, 155, 133–138. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, X.; Li, B.; Bai, C.; Li, Y.; Wang, L.; Wen, R.; Zhang, M.; Ma, L.; Li, S. “Stereoscopic” 2D super-microporous phosphazene-based covalent organic framework: Design, synthesis and selective sorption towards uranium at high acidic condition. J. Hazard. Mater. 2016, 314, 95–104. [Google Scholar] [CrossRef]
- Venkatesan, K.A.; Shyamala, K.V.; Antony, M.P.; Srinivasan, T.G.; Rao, P.R.V. Batch and dynamic extraction of uranium (VI) from nitric acid medium by commercial phosphinic acid resin, Tulsion CH-96. J. Radioanal. Nucl. Chem. Artic. 2008, 275, 563–570. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, X. Application of AnMBR Ion Exchange Technology in Water Treatment. IOP Conf. Series Earth Environ. Sci. 2021, 791, 012180. [Google Scholar] [CrossRef]
- Shimojo, K. Solvent Extraction in Analytical Separation Techniques. Anal. Sci. 2018, 34, 1345–1346. [Google Scholar] [CrossRef]
- Osmanlioglu, A.E. Decontamination of radioactive wastewater by two-staged chemical precipitation. Nucl. Eng. Technol. 2018, 50, 886–889. [Google Scholar] [CrossRef]
- Hao, S.; Jia, Z.; Wen, J.; Li, S.; Peng, W.; Huang, R.; Xu, X. Progress in adsorptive membranes for separation—A review. Sep. Purif. Technol. 2020, 255, 117772. [Google Scholar] [CrossRef]
- Li, H.; Qing, Q.; Zheng, L.; Xie, L.; Gan, Z.; Huang, L.; Liu, S.; Wang, Z.; Lu, Y.; Chen, J. Carbon dots and carbon nitride composite for photocatalytic removal of uranium under air atmosphere. Chin. Chem. Lett. 2022, 33, 3573–3576. [Google Scholar] [CrossRef]
- Liu, H.; Mao, Y. Graphene Oxide-based Nanomaterials for Uranium Adsorptive Uptake. ES Mater. Manuf. 2021, 13, 3–22. [Google Scholar] [CrossRef]
- Gaillard, C.; Boltoeva, M.; Billard, I.; Georg, S.; Mazan, V.; Ouadi, A. Ionic liquid-based uranium (VI) extraction with malonamide extractant: Cation exchange vs. neutral extraction. RSC Adv. 2016, 6, 70141–70151. [Google Scholar] [CrossRef][Green Version]
- Yuan, Y.; Zhao, S.; Wen, J.; Wang, D.; Guo, X.; Xu, L.; Wang, X.; Wang, N. Rational Design of Porous Nanofiber Adsorbent by Blow-Spinning with Ultrahigh Uranium Recovery Capacity from Seawater. Adv. Funct. Mater. 2019, 29, 1805380. [Google Scholar] [CrossRef]
- Luo, W.; Xiao, G.; Tian, F.; Richardson, J.J.; Wang, Y.; Zhou, J.; Guo, J.; Liao, X.; Shi, B. Engineering robust metal–phenolic network membranes for uranium extraction from seawater. Energy Environ. Sci. 2019, 12, 607–614. [Google Scholar] [CrossRef]
- Zhang, S.; Yuan, D.; Zhao, J.; Ren, G.; Zhao, X.; Liu, Y.; Wang, Y.; He, Y.; Ma, M.; Zhang, Q. Highly efficient extraction of uranium from strong HNO3 media achieved on phosphine oxide functionalized superparamagnetic composite polymer microspheres. J. Mater. Chem. A 2021, 9, 18393–18405. [Google Scholar] [CrossRef]
- Zhang, S.; Yuan, D.; Zhang, Q.; Wang, Y.; Liu, Y.; Zhao, J.; Chen, B. Highly efficient removal of uranium from highly acidic media achieved using a phosphine oxide and amino functionalized superparamagnetic composite polymer adsorbent. J. Mater. Chem. A 2020, 8, 10925–10934. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Xu, Z.; Rao, C.; Pi, L.; Fu, Y.; Dong, Y.; Shen, C.; Yao, L.; Xiong, C. Synthesis and application of recyclable core-shell structure microspheres MCTS-g-AT in detection of Hg (II) in aquatic products. J. Chin. Chem. Soc. 2021, 68, 1739–1747. [Google Scholar] [CrossRef]
- Zhang, Y.; Du, B.; Wu, Y.; Liu, Z.; Wang, J.; Xu, J.; Tong, Z.; Mu, X.; Liu, B. Fe3O4@PDA@PEI Core-Shell Microspheres as a Novel Magnetic Sorbent for the Rapid and Broad-Spectrum Separation of Bacteria in Liquid Phase. Materials 2022, 15, 2039. [Google Scholar] [CrossRef]
- Zuo, B.; Li, W.; Wu, X.; Wang, S.; Deng, Q.; Huang, M. Recent Advances in the Synthesis, Surface Modifications and Applications of Core-Shell Magnetic Mesoporous Silica Nanospheres. Chem. Asian J. 2020, 15, 1248–1265. [Google Scholar] [CrossRef]
- Shi, Z.; Xu, C.; Lu, P.; Fan, L.; Liu, Y.; Wang, Y.; Liu, L.; Li, L. Preparation and the adsorption ability of thiolated magnetic core-shell Fe3O4@SiO2@C-SH for removing Hg2+ in water solution. Mater. Lett. 2018, 225, 130–133. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, G.; Yang, F. The elaboration of multifunctional hollow core-shell Fe3O4@PDA@TiO2 architecture with dual magnetic and photo-responsive performance. New J. Chem. 2020, 44, 3487–3492. [Google Scholar] [CrossRef]
- Xu, M.; Han, X.; Wang, T.; Li, S.; Hua, D. Conjugated microporous polymers bearing phosphonate ligands as an efficient sorbent for potential uranium extraction from high-level liquid wastes. J. Mater. Chem. A 2018, 6, 13894–13900. [Google Scholar] [CrossRef]
- Xu, M.Y.; Hai, X.L.; Hua, D.B. Polyoxime-functionalized magnetic nanoparticles for uranium adsorption with high selectivity over vanadium. J. Mater. Chem. A 2017, 5, 12278–12284. [Google Scholar] [CrossRef]
- Calì, E.; Qi, J.; Preedy, O.; Chen, S.; Boldrin, D.; Branford, W.R.; Vandeperre, L.; Ryan, M.P. Functionalised magnetic nanoparticles for uranium adsorption with ultra-high capacity and selectivity. J. Mater. Chem. A 2018, 6, 3063–3073. [Google Scholar] [CrossRef]
- Husnain, S.M.; Um, W.Y.; Chang, W.L.; Chang, Y.S. Magnetite-based adsorbents for sequestration of radionuclides: A review. RSC Adv. 2018, 8, 2521–2540. [Google Scholar] [CrossRef]
- Dai, S.; Wang, N.; Qi, C.; Wang, X.; Ma, Y.; Yang, L.; Liu, X.; Huang, Q.; Nie, C.; Hu, B.; et al. Preparation of core-shell structure Fe3O4@C@MnO2 nanoparticles for efficient elimination of U (VI) and EU (III) ions. Sci. Total Environ. 2019, 685, 986–996. [Google Scholar] [CrossRef]
- Song, X.M.; Tan, L.C.; Ma, H.Y.; Guo, Y.; Zhu, L.; Yi, X.Q.; Gao, J.Y.; Yang, R.J.; Dong, Q. Facile preparation of S-doped magnetite hollow spheres for highly efficient sorption of uranium (VI). Dalton. Trans. 2017, 46, 3347–3352. [Google Scholar] [CrossRef]
- Yuan, D.; Xiong, X.; Chen, L.; Lv, Y.; Wang, Y.; Yuan, L.; Liao, S.; Zhang, Q. Removal of uranium from aqueous solution by phosphate functionalized superparamagnetic polymer microspheres Fe3O4/P(GMA-AA-MMA). J. Radioanal. Nucl. Chem. 2016, 309, 729–741. [Google Scholar] [CrossRef]
- Yuan, D.; Chen, L.; Xiong, X.; Zhang, Q.; Liao, S.; Yuan, L.; Wang, Y. Synthesis of PAMAM dendron functionalized superparamagnetic polymer microspheres for highly efficient sorption of uranium (VI). J. Radioanal. Nucl. Chem. 2016, 309, 1227–1240. [Google Scholar] [CrossRef]
- Nogami, M.; Sugiyama, Y.; Ikeda, Y. Adsorptivity of silica-supported adsorbents impregnated with polyphosphine polyoxides to U(VI) and some other metal ions in nitric acid media. J. Radioanal. Nucl. Chem. Artic. 2010, 284, 195–199. [Google Scholar] [CrossRef]
- Broda, E.; Gładysz-Płaska, A.; Skwarek, E.; Payentko, V.V. Structural properties and adsorption of uranyl ions on the nanocomposite hydroxyapatite/white clay. Appl. Nanosci. 2022, 12, 1101–1111. [Google Scholar] [CrossRef]
- Biedrzycka, A.; Skwarek, E.; Osypiuk, D.; Cristóvao, B. Synthesis of Hydroxyapatite/Iron Oxide Composite and Comparison of Selected Structural, Surface, and Electrochemical Properties. Materials 2022, 15, 1139. [Google Scholar] [CrossRef]
- Biedrzycka, A.; Skwarek, E.; Hanna, U.M. Hydroxyapatite with magnetic core: Synthesis methods, properties, adsorption and medical applications. Adv. Colloid Interface Sci. 2021, 291, 102401. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Guo, Z.; Zhang, S.; Wang, X. Enrich and seal radionuclides in magnetic agarose microspheres. Chem. Eng. J. 2011, 172, 892–897. [Google Scholar] [CrossRef]
- Yuan, D.; Zhang, S.; Xiang, Z.; He, Y.; Wang, Y.; Liu, Y.; Zhao, X.; Zhou, X.; Zhang, Q. Highly Efficient Removal of Thorium in Strong HNO3 Media Using a Novel Polymer Adsorbent Bearing a Phosphonic Acid Ligand: A Combined Experimental and Density Functional Theory Study. ACS Appl. Mater. Interfaces 2019, 11, 24512–24522. [Google Scholar] [CrossRef]
- Jonsson, M.; Nyström, D.; Nordin, O.; Malmström, E. Surface modification of thermally expandable microspheres by grafting poly(glycidyl methacrylate) using ARGET ATRP. Eur. Polym. J. 2009, 45, 2374–2382. [Google Scholar] [CrossRef]
- Liu, X.; Sun, J.; Xu, X.; Alsaedi, A.; Hayat, T.; Li, J. Adsorption and desorption of U(VI) on different-size graphene oxide. Chem. Eng. J. 2019, 360, 941–950. [Google Scholar] [CrossRef]
- Zhang, N.; Yuan, L.Y.; Guo, W.L.; Luo, S.Z.; Chai, Z.F.; Shi, W.Q. Extending the Use of Highly Porous and Functionalized MOFs to Th (IV) Capture. ACS Appl. Mater. Interfaces 2017, 9, 25216–25224. [Google Scholar] [CrossRef]
- Han, X.; Wang, Y.; Cao, X.; Dai, Y.; Liu, Y.; Dong, Z.; Zhang, Z.; Liu, Y. Adsorptive performance of ship-type nano-cage polyoxometalates for U (VI) in aqueous solution. Appl. Surf. Sci. 2019, 484, 1035–1040. [Google Scholar] [CrossRef]
- Wang, D.; Xu, Y.; Xiao, D.; Qiao, Q.; Yin, P.; Yang, Z.; Li, J.; Winchester, W.; Wang, Z.; Hayat, T. Ultra-thin iron phosphate nanosheets for high efficient U(VI) adsorption. J. Hazard. Mater. 2019, 371, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ouyang, Y.; Huang, D.; Jiang, C.; Liu, X.; Wang, Y.; Dai, Y.; Yuan, D.; Chew, J.W. N, P and S co-doped carbon materials derived from polyphosphazene for enhanced selective U (VI) adsorption. Sci. Total Environ. 2020, 706, 136019. [Google Scholar] [CrossRef] [PubMed]
- Amesh, P.; Suneesh, A.S.; Selvan, B.R.; Venkatesan, K.A.; Chandra, M. Magnetic assisted separation of uranium (VI) from aqueous phase using diethylenetriamine modified high capacity iron oxide adsorbent. J. Environ. Chem. Eng. 2020, 8, 103661. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, J.; Zhao, L.; Zhang, S.; Huang, Y.; Wu, X.; Wang, X. Synthesis of amidoxime-functionalized Fe3O4@SiO2 core–shell magnetic microspheres for highly efficient sorption of U(VI). Chem. Eng. J. 2014, 235, 275–283. [Google Scholar] [CrossRef]
- Zong, P.; Wang, S.; Zhao, Y.; Wang, H.; Pan, H.; He, C. Synthesis and application of magnetic graphene/iron oxides composite for the removal of U(VI) from aqueous solutions. Chem. Eng. J. 2013, 220, 45–52. [Google Scholar] [CrossRef]
- Liu, Q.; Li, W.; Zhao, W.; Tan, L.; Jing, X.; Liu, J.; Song, D.; Zhang, H.; Li, R.; Liu, L.; et al. Synthesis of ketoxime-functionalized Fe3O4@C core–shell magnetic microspheres for enhanced uranium(vi) removal. RSC Adv. 2016, 6, 22179–22186. [Google Scholar] [CrossRef]
- Sadeghi, S.; Azhdari, H.; Arabi, H.; Moghaddam, A.Z. Surface modified magnetic Fe3O4 nanoparticles as a selective sorbent for solid phase extraction of uranyl ions from water samples. J. Hazard. Mater. 2012, 215–216, 208–216. [Google Scholar] [CrossRef]
- Tan, L.; Zhang, X.; Liu, Q.; Jing, X.; Liu, J.; Song, D.; Hu, S.; Liu, L.; Wang, J. Synthesis of Fe3O4@TiO2 core–shell magnetic composites for highly efficient sorption of uranium (VI). Colloids Surfaces A Physicochem. Eng. Asp. 2015, 469, 279–286. [Google Scholar] [CrossRef]
- Song, W.; Liu, M.; Hu, R.; Tan, X.; Li, J. Water-soluble polyacrylamide coated-Fe3O4 magnetic composites for high-efficient enrichment of U(VI) from radioactive wastewater. Chem. Eng. J. 2014, 246, 268–276. [Google Scholar] [CrossRef]
- Fan, F.L.; Qin, Z.; Bai, J.; Rong, W.D.; Fan, F.Y.; Tian, W.; Wu, X.L.; Wang, Y.; Zhao, L. Rapid removal of uranium from aqueous solutions using magnetic Fe3O4@ SiO2 composite particles. J. Environ. Radioact. 2012, 106, 40–46. [Google Scholar] [CrossRef]
- Singhal, P.; Vats, B.G.; Yadav, A.; Pulhani, V. Efficient extraction of uranium from environmental samples using phosphoramide functionalized magnetic nanoparticles: Understanding adsorption and binding mechanisms. J. Hazard. Mater. 2020, 384, 121353. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Sureshkumar, M.; Koley, S.; Mithal, N.; Pillai, C. Sorption of uranium on magnetite nanoparticles. J. Radioanal. Nucl. Chem. 2010, 285, 447–454. [Google Scholar] [CrossRef]
- Li, L.; Huang, F.; Yuan, Y.; Hu, J.; Tang, Q.; Tang, S. Preparation and sorption performance of magnetic 18-crown-6/Fe3O4 nanocomposite for uranium (VI) in solution. J. Radioanal. Nucl. Chem. 2013, 298, 227–235. [Google Scholar] [CrossRef]
- El-Maghrabi, H.H.; Younes, A.A.; Salem, A.R.; Rabie, K.; El-Shereafy, E.S. Magnetically modified hydroxyapatite nanoparticles for the removal of uranium (VI): Preparation, characterization and adsorption optimization. J. Hazard. Mater. 2019, 378, 120703. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Y.; Ma, W.; Guo, J.; Lin, Y.; Wang, C. Uniform Magnetic Core/Shell Microspheres Functionalized with Ni2+–Iminodiacetic Acid for One Step Purification and Immobilization of His-Tagged Enzymes. ACS Appl. Mater. Interfaces 2013, 5, 2626–2633. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).