Electroretinography as a Biomarker to Monitor the Progression of Stargardt Disease
Abstract
1. Introduction
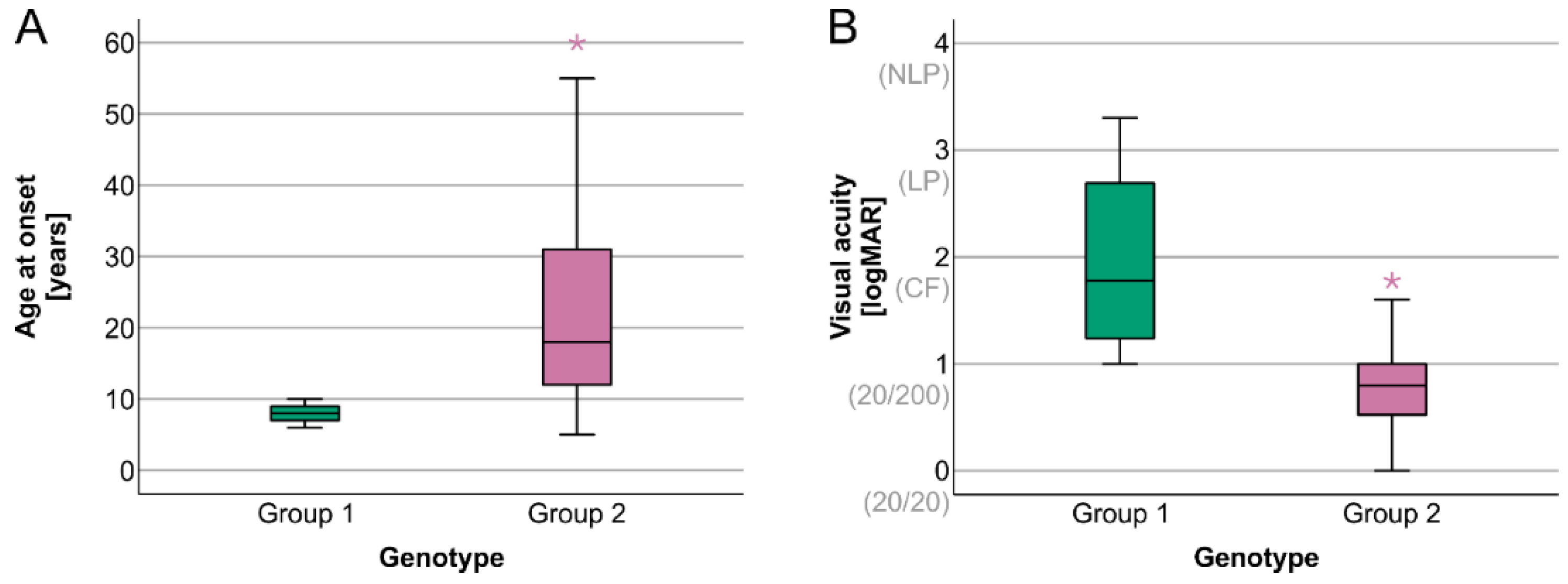
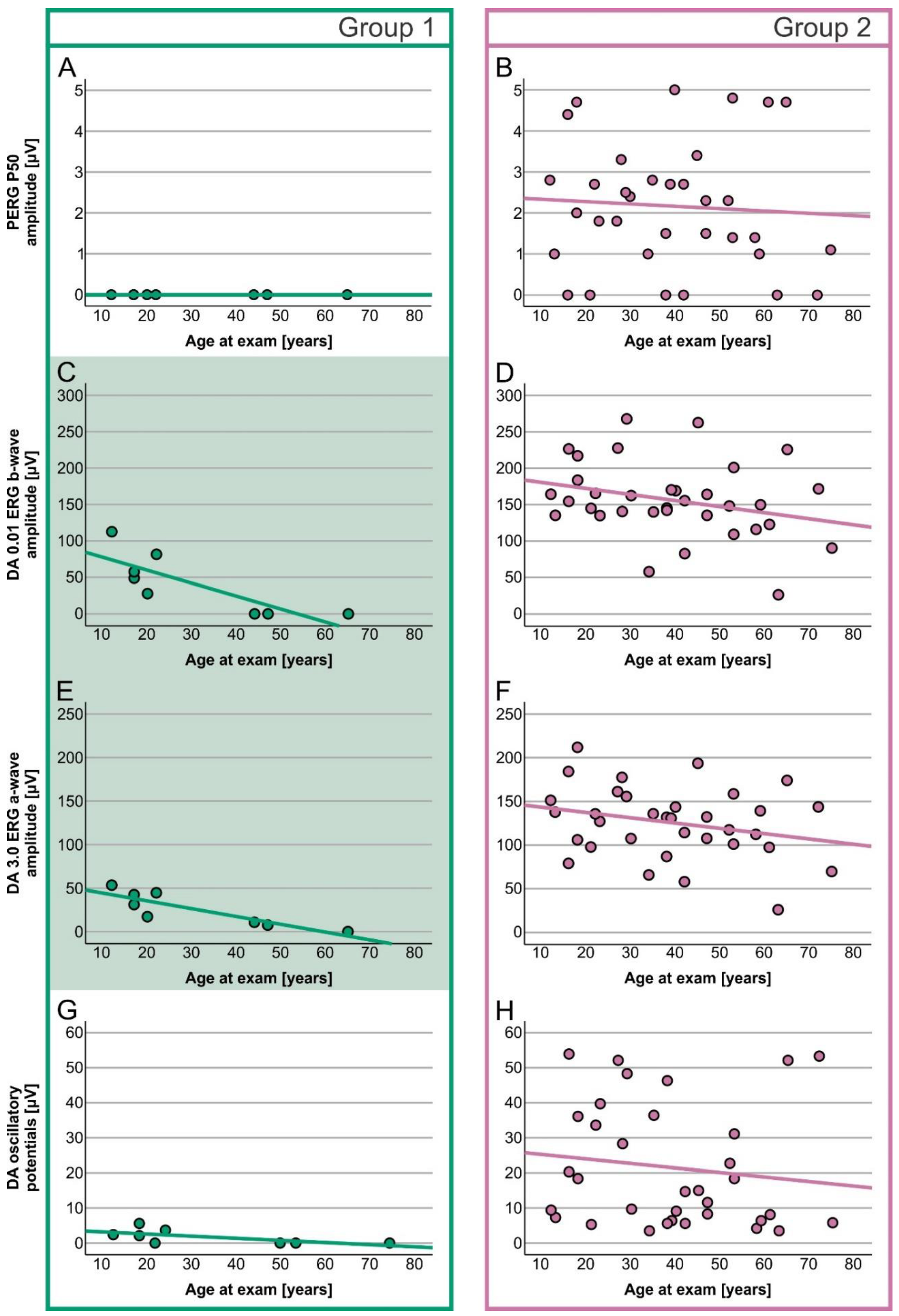
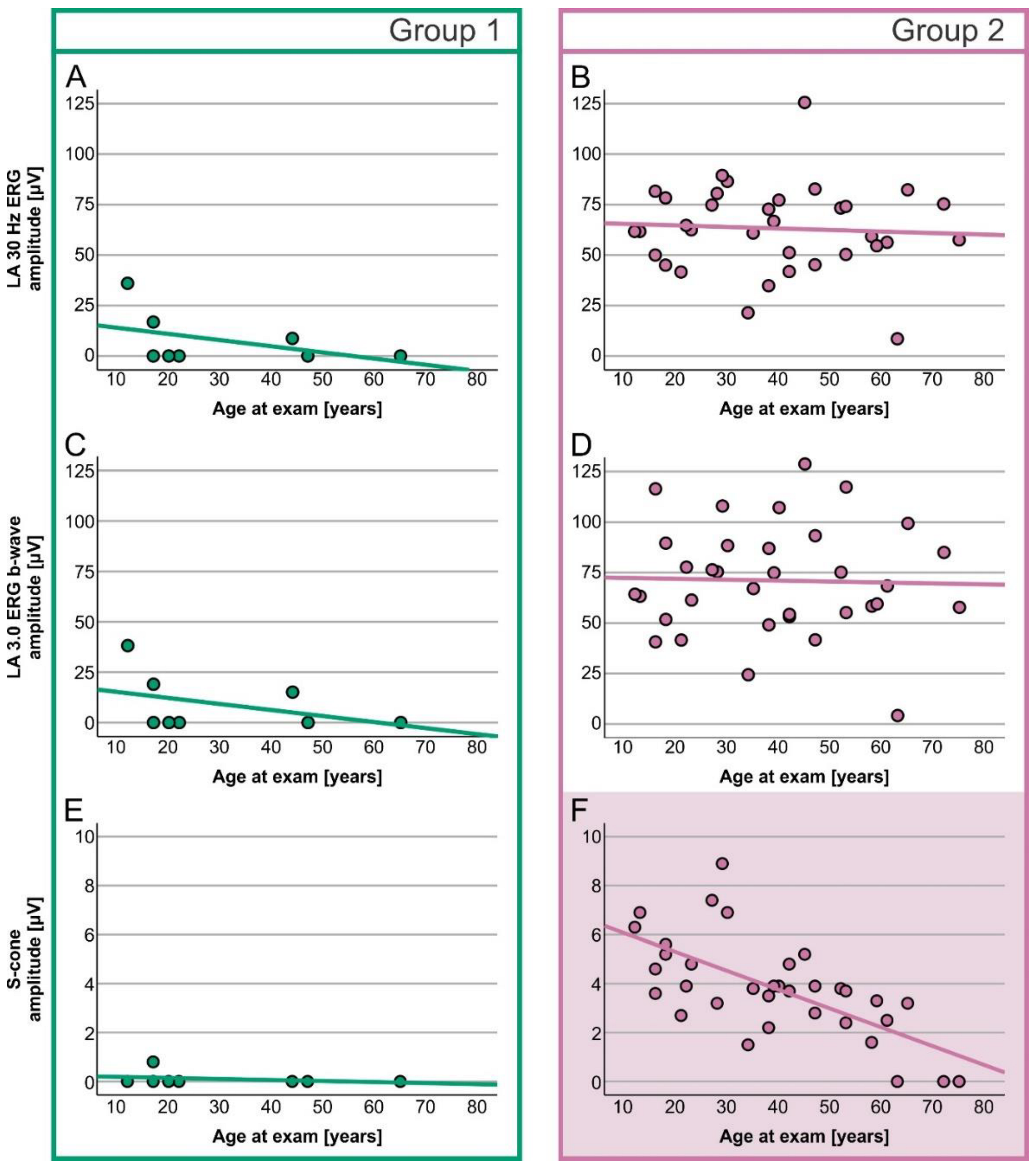
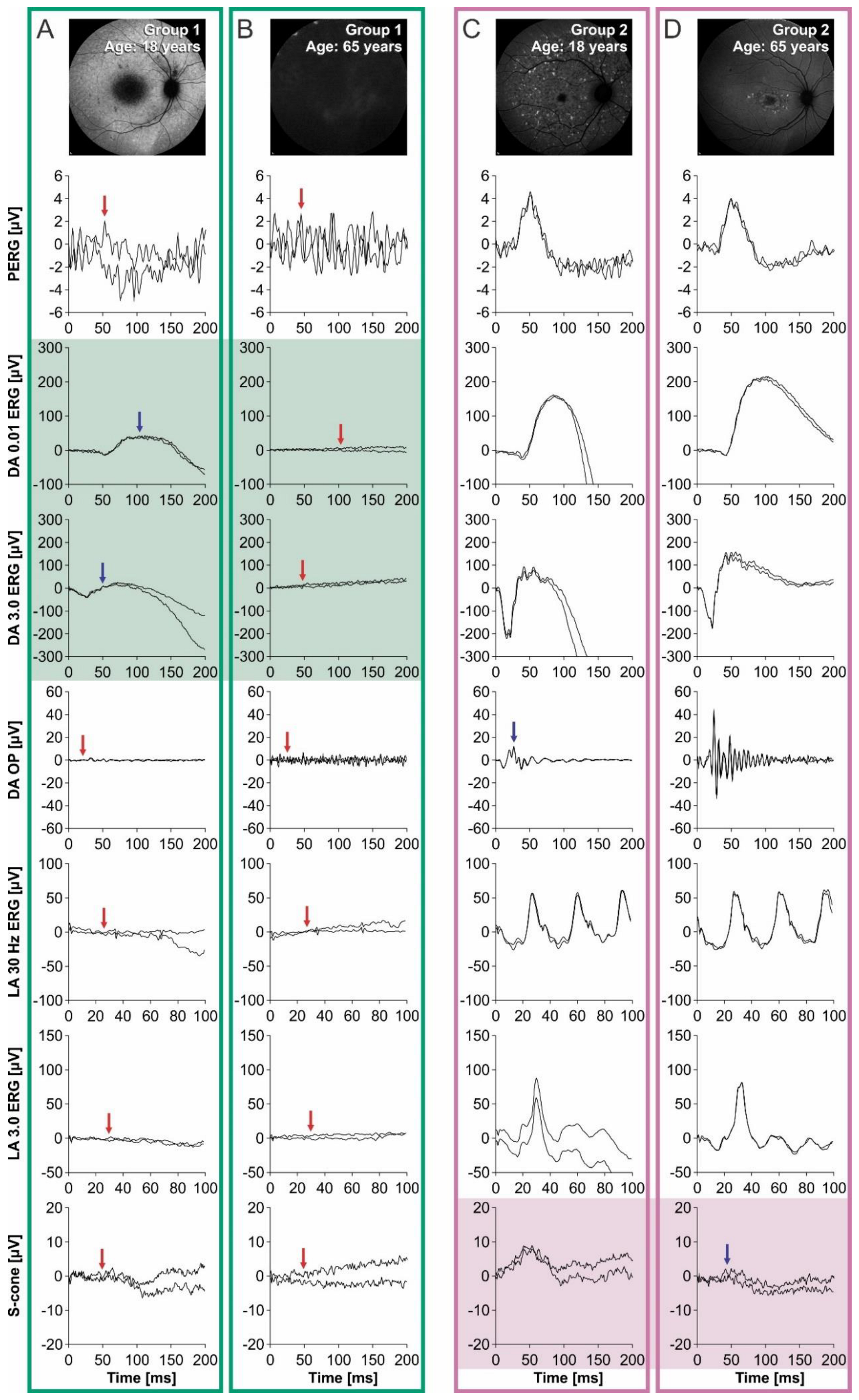
2. Results
Electrophysiology
3. Discussion
Study Strength and Limitations
4. Materials and Methods
4.1. Patients
4.2. Clinical Evaluation
4.3. Electrophysiology
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| STGD1 | Stargardt disease |
| RPE | retinal pigment epithelium |
| ERG | electroretinography |
| BCVA | best-corrected visual acuity |
| DA | dark-adapted |
| LA | light-adapted |
| L-cone | long-wave-sensitive cone |
| M-cone | middle-wave-sensitive cone |
| S-cone | short-wavelength-sensitive cone |
| PERG | pattern electroretinography |
| logMAR | logarithm of the minimum angle of resolution |
| ffERG | full-field ERG |
| ISCEV | International Society of Clinical Electrophysiology of Vision |
References
- Cremers, F.P.M.; Lee, W.; Collin, R.W.J.; Allikmets, R. Clinical spectrum, genetic complexity and therapeutic approaches for retinal disease caused by ABCA4 mutations. Prog. Retin. Eye Res. 2020, 79, 100861. [Google Scholar] [CrossRef] [PubMed]
- Tanna, P.; Strauss, R.W.; Fujinami, K.; Michaelides, M. Stargardt disease: Clinical features, molecular genetics, animal models and therapeutic options. Br. J. Ophthalmol. 2017, 101, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Fishman, G.A. Fundus flavimaculatus. A clinical classification. Arch. Ophthalmol. 1976, 94, 2061–2067. [Google Scholar] [CrossRef] [PubMed]
- Molday, L.L.; Rabin, A.R.; Molday, R.S. ABCR expression in foveal cone photoreceptors and its role in Stargardt macular dystrophy. Nat. Genet. 2000, 25, 257–258. [Google Scholar] [CrossRef] [PubMed]
- Lenis, T.L.; Hu, J.; Ng, S.Y.; Jiang, Z.; Sarfare, S.; Lloyd, M.B.; Esposito, N.J.; Samuel, W.; Jaworski, C.; Bok, D.; et al. Expression of ABCA4 in the retinal pigment epithelium and its implications for Stargardt macular degeneration. Proc. Natl. Acad. Sci. USA 2018, 115, e11120–e11127. [Google Scholar] [CrossRef]
- Illing, M.; Molday, L.L.; Molday, R.S. The 220-kDa rim protein of retinal rod outer segments is a member of the ABC transporter superfamily. J. Biol. Chem. 1997, 272, 10303–10310. [Google Scholar] [CrossRef]
- Fakin, A.; Robson, A.G.; Fujinami, K.; Moore, A.T.; Michaelides, M.; Holder, G.E.; Webster, A.R. Phenotype and Progression of Retinal Degeneration Associated with Nullizigosity of ABCA4. Investig. Ophthalmol. Vis. Sci. 2016, 57, 4668–4678. [Google Scholar] [CrossRef]
- Conley, S.M.; Cai, X.; Makkia, R.; Wu, Y.; Sparrow, J.R.; Naash, M.I. Increased cone sensitivity to ABCA4 deficiency provides insight into macular vision loss in Stargardt’s dystrophy. Biochim. Biophys. Acta 2012, 1822, 1169–1179. [Google Scholar] [CrossRef]
- Molday, R.S.; Zhong, M.; Quazi, F. The role of the photoreceptor ABC transporter ABCA4 in lipid transport and Stargardt macular degeneration. Biochim. Biophys. Acta 2009, 1791, 573–583. [Google Scholar] [CrossRef]
- Papermaster, D.S.; Reilly, P.; Schneider, B.G. Cone lamellae and red and green rod outer segment disks contain a large intrinsic membrane protein on their margins: An ultrastructural immunocytochemical study of frog retinas. Vis. Res. 1982, 22, 1417–1428. [Google Scholar] [CrossRef]
- Papermaster, D.S.; Schneider, B.G.; Zorn, M.A.; Kraehenbuhl, J.P. Immunocytochemical localization of a large intrinsic membrane protein to the incisures and margins of frog rod outer segment disks. J. Cell Biol. 1978, 78, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Molday, R.S. Insights into the Molecular Properties of ABCA4 and Its Role in the Visual Cycle and Stargardt Disease. Prog. Mol. Biol. Transl. Sci. 2015, 134, 415–431. [Google Scholar] [CrossRef] [PubMed]
- Fakin, A.; Robson, A.G.; Chiang, J.P.; Fujinami, K.; Moore, A.T.; Michaelides, M.; Holder, G.E.; Webster, A.R. The Effect on Retinal Structure and Function of 15 Specific ABCA4 Mutations: A Detailed Examination of 82 Hemizygous Patients. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5963–5973. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, M.; Kane, T.; Tanna, P.; Bouzia, Z.; Singh, N.; Kalitzeos, A.; Strauss, R.W.; Fujinami, K.; Michaelides, M. Prospective Cohort Study of Childhood-Onset Stargardt Disease: Fundus Autofluorescence Imaging, Progression, Comparison with Adult-Onset Disease, and Disease Symmetry. Am. J. Ophthalmol. 2020, 211, 159–175. [Google Scholar] [CrossRef]
- Westeneng-van Haaften, S.C.; Boon, C.J.; Cremers, F.P.; Hoefsloot, L.H.; den Hollander, A.I.; Hoyng, C.B. Clinical and genetic characteristics of late-onset Stargardt’s disease. Ophthalmology 2012, 119, 1199–1210. [Google Scholar] [CrossRef] [PubMed]
- Burke, T.R.; Fishman, G.A.; Zernant, J.; Schubert, C.; Tsang, S.H.; Smith, R.T.; Ayyagari, R.; Koenekoop, R.K.; Umfress, A.; Ciccarelli, M.L.; et al. Retinal phenotypes in patients homozygous for the G1961E mutation in the ABCA4 gene. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4458–4467. [Google Scholar] [CrossRef]
- Lee, W.; Nõupuu, K.; Oll, M.; Duncker, T.; Burke, T.; Zernant, J.; Bearelly, S.; Tsang, S.H.; Sparrow, J.R.; Allikmets, R. The external limiting membrane in early-onset Stargardt disease. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6139–6149. [Google Scholar] [CrossRef]
- Arepalli, S.; Traboulsi, E.I.; Ehlers, J.P. Ellipsoid zone mapping and outer retinal assessment in stargardt disease. Retina 2018, 38, 1427–1431. [Google Scholar] [CrossRef]
- Burke, T.R.; Duncker, T.; Woods, R.L.; Greenberg, J.P.; Zernant, J.; Tsang, S.H.; Smith, R.T.; Allikmets, R.; Sparrow, J.R.; Delori, F.C. Quantitative fundus autofluorescence in recessive Stargardt disease. Investig. Ophthalmol. Vis. Sci. 2014, 55, 2841–2852. [Google Scholar] [CrossRef]
- Cukras, C.A.; Wong, W.T.; Caruso, R.; Cunningham, D.; Zein, W.; Sieving, P.A. Centrifugal Expansion of Fundus Autofluorescence Patterns in Stargardt Disease Over Time. Arch. Ophthalmol. 2012, 130, 171–179. [Google Scholar] [CrossRef]
- Burke, T.R.; Rhee, D.W.; Smith, R.T.; Tsang, S.H.; Allikmets, R.; Chang, S.; Lazow, M.A.; Hood, D.C.; Greenstein, V.C. Quantification of peripapillary sparing and macular involvement in Stargardt disease (STGD1). Investig. Ophthalmol. Vis. Sci. 2011, 52, 8006–8015. [Google Scholar] [CrossRef]
- Lois, N.; Holder, G.E.; Bunce, C.; Fitzke, F.W.; Bird, A.C. Phenotypic subtypes of Stargardt macular dystrophy-fundus flavimaculatus. Arch. Ophthalmol. 2001, 119, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Fujinami, K.; Lois, N.; Davidson, A.E.; Mackay, D.S.; Hogg, C.R.; Stone, E.M.; Tsunoda, K.; Tsubota, K.; Bunce, C.; Robson, A.G.; et al. A longitudinal study of stargardt disease: Clinical and electrophysiologic assessment, progression, and genotype correlations. Am. J. Ophthalmol. 2013, 155, 1075–1088.e1013. [Google Scholar] [CrossRef] [PubMed]
- Fujinami, K.; Sergouniotis, P.I.; Davidson, A.E.; Wright, G.; Chana, R.K.; Tsunoda, K.; Tsubota, K.; Egan, C.A.; Robson, A.G.; Moore, A.T.; et al. Clinical and molecular analysis of Stargardt disease with preserved foveal structure and function. Am. J. Ophthalmol. 2013, 156, 487–501.e481. [Google Scholar] [CrossRef]
- Lambertus, S.; van Huet, R.A.; Bax, N.M.; Hoefsloot, L.H.; Cremers, F.P.; Boon, C.J.; Klevering, B.J.; Hoyng, C.B. Early-onset stargardt disease: Phenotypic and genotypic characteristics. Ophthalmology 2015, 122, 335–344. [Google Scholar] [CrossRef]
- Bonilha, V.L.; Rayborn, M.E.; Bell, B.A.; Marino, M.J.; Fishman, G.A.; Hollyfield, J.G. Retinal Histopathology in Eyes from a Patient with Stargardt disease caused by Compound Heterozygous ABCA4 Mutations. Ophthalmic Genet. 2016, 37, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Werner, J.S. The Verriest Lecture: Short-wave-sensitive cone pathways across the life span. J. Opt. Soc. Am. Opt. Image Sci. Vis. 2016, 33, A104–A122. [Google Scholar] [CrossRef]
- Curcio, C.A.; Allen, K.A.; Sloan, K.R.; Lerea, C.L.; Hurley, J.B.; Klock, I.B.; Milam, A.H. Distribution and morphology of human cone photoreceptors stained with anti-blue opsin. J. Comp. Neurol. 1991, 312, 610–624. [Google Scholar] [CrossRef] [PubMed]
- Cornish, E.E.; Hendrickson, A.E.; Provis, J.M. Distribution of short-wavelength-sensitive cones in human fetal and postnatal retina: Early development of spatial order and density profiles. Vis. Res. 2004, 44, 2019–2026. [Google Scholar] [CrossRef]
- Perlman, I.; Kondo, M.; Chelva, E.; Robson, A.G.; Holder, G.E. ISCEV extended protocol for the S-cone ERG. Doc. Ophthalmol. 2020, 140, 95–101. [Google Scholar] [CrossRef]
- Marmor, M.F.; Jain, A.; Moshfeghi, D. Total rod ERG suppression with high dose compassionate Fenretinide usage. Doc. Ophthalmol. 2008, 117, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kiser, P.D.; Badiee, M.; Palczewska, G.; Dong, Z.; Golczak, M.; Tochtrop, G.P.; Palczewski, K. Molecular pharmacodynamics of emixustat in protection against retinal degeneration. J. Clin. Investig. 2015, 125, 2781–2794. [Google Scholar] [CrossRef] [PubMed]
- Dobri, N.; Qin, Q.; Kong, J.; Yamamoto, K.; Liu, Z.; Moiseyev, G.; Ma, J.X.; Allikmets, R.; Sparrow, J.R.; Petrukhin, K. A1120, a nonretinoid RBP4 antagonist, inhibits formation of cytotoxic bisretinoids in the animal model of enhanced retinal lipofuscinogenesis. Investig. Ophthalmol. Vis. Sci. 2013, 54, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Mata, N.L.; Lichter, J.B.; Vogel, R.; Han, Y.; Bui, T.V.; Singerman, L.J. Investigation of oral fenretinide for treatment of geographic atrophy in age-related macular degeneration. Retina 2013, 33, 498–507. [Google Scholar] [CrossRef]
- Charbel Issa, P.; Barnard, A.R.; Herrmann, P.; Washington, I.; MacLaren, R.E. Rescue of the Stargardt phenotype in ABCA4 knockout mice through inhibition of vitamin A dimerization. Proc. Natl. Acad. Sci. USA 2015, 112, 8415–8420. [Google Scholar] [CrossRef]
- Lenassi, E.; Jarc-Vidmar, M.; Glavac, D.; Hawlina, M. Pattern electroretinography of larger stimulus field size and spectral-domain optical coherence tomography in patients with Stargardt disease. Br. J. Ophthalmol. 2009, 93, 1600–1605. [Google Scholar] [CrossRef]
- Robson, A.G.; Frishman, L.J.; Grigg, J.; Hamilton, R.; Jeffrey, B.G.; Kondo, M.; Li, S.; McCulloch, D.L. ISCEV Standard for full-field clinical electroretinography (2022 update). Doc. Ophthalmol. 2022, 144, 165–177. [Google Scholar] [CrossRef]
- Bach, M.; Brigell, M.G.; Hawlina, M.; Holder, G.E.; Johnson, M.A.; McCulloch, D.L.; Meigen, T.; Viswanathan, S. ISCEV standard for clinical pattern electroretinography (PERG): 2012 update. Doc. Ophthalmol. 2013, 126, 1–7. [Google Scholar] [CrossRef]
- Hawlina, M.; Konec, B. New noncorneal HK-loop electrode for clinical electroretinography. Doc. Ophthalmol. 1992, 81, 253–259. [Google Scholar] [CrossRef]

| Parameter | Group 1 (Median, Range) | Group 2 (Median, Range) | Mann–Whitney Test (U, z, p Values) |
|---|---|---|---|
| Age at exam | 21 years (12–65 years) | 39 years (12–75 years) | U = 174.500, z = 1.234, p = 0.222 |
| Age at onset | 8 years (6–10 years) | 18.5 years (5–60 years) | U = 260.000, z = 3.976, p < 0.001 |
| Disease duration | 12.5 years (4–55 years) | 12 years (0–51 years) | U = 104.500, z = −1.010, p = 0.320 |
| Visual acuity | 1.8 (1.0–3.3) | 0.8 (0.0–1.8) | U = 28.500, z = −3.463, p < 0.001 |
| PERG P50 amplitude | 0.0 µV (0.0–0.0) | 2.2 µV (0.0–5.0) | U = 248.000, z = 3.658, p < 0.001 |
| S-cone ERG amplitude | 0.0 µV (0.0–0.8) | 3.8 µV (0.0–8.9) | U = 258.500, z = 3.953, p < 0.001 |
| DA 0.01 ERG b-wave amplitude | 38.3 µV (0.0–122.5) | 152.2 (26.5–267.8) | U = 262.000, z = 4.037, p < 0.001 |
| DA 3.0 ERG a-wave amplitude | 24.2 µV (0.0–53.4) | 131.4 µV (26.0–211.9) | U = 268.000, z = 4.228, p < 0.001 |
| Oscillatory potentials | 1.1 µV (0.0–5.6) | 14.9 µV (3.5–53.9) | U = 265.000, z = 4.135, p < 0.001 |
| LA 30 Hz ERG amplitude | 0.0 µV (0.0–36.0) | 62.1 µV (8.5–125.6) | U = 267.000, z = 4.200, p < 0.001 |
| LA 3.0 ERG b-wave amplitude | 0.0 µV (0.0–38.2) | 67.8 µV (4.2–128.7) | U = 268.000, z = 4.232, p < 0.001 |
| ERG Group | Patient Group 1 (Two Null Variants) | Patient Group 2 (All Other Genotypes) |
|---|---|---|
| Normal ERG | 23.5% (8/34) | |
| ERG Group 1 (normal ffERG) | / | 47.1% (16/34) |
| ERG Group 2 (cone dystrophy) | / | 17.6% (6/34) |
| ERG Group 3 (cone-rod dystrophy) | 100% (8/8) | 11.8% (4/34) |
| ERG Response | Group 1 | Group 2 | ||
|---|---|---|---|---|
| Simple Linear Regression | Simple Linear Regression | |||
| ANOVA Results | B and β Coefficients | ANOVA Results | B and β Coefficients | |
| PERG P50 amplitude | Variable is constant | (F(1, 32) = 0.135, p = 0.715, R2 = 0.004, R2adjusted = −0.027 | B = −0.006, 95% CI [−0.037, 0.026]), β = −0.065 | |
| DA 0.01 ERG b-wave amplitude | F(1, 6) = 11.134, p = 0.016, R2 = 0.650, R2adjusted = 0.591 | B = −1.776, 95% CI [−3.079, −0.377]), β = −0.806 | F(1, 32) = 2.782, p = 0.105, R2 = 0.080, R2adjusted = 0.041 | B = −0.606, 95% CI [−1.402, 0.189]), β = −0.283 |
| DA 3.0 ERG a-wave amplitude | F(1, 6) = 17.839, p = 0.006, R2 = 0.748, R2adjusted = 0.706 | B = −0.897, 95% CI [−1.417, −0.474]), β = −0.865 | F(1, 32) = 2.410, p = 0.130, R2 = 0.070, R2adjusted = 0.051 | B = −0.833, 95% CI [−1.849, 0.184]), β = −0.265 |
| Oscillatory potentials | F(1, 6) = 4.103, p = 0.089, R2 = 0.406, R2adjusted = 0.307 | B = −0.071, 95% CI [−0.157, 0.015]), β = −0.637 | F(1, 32) = 0.558, p = 0.461, R2 = 0.017, R2adjusted = −0.014 | B = −0.128, 95% CI [−0.479, −0.222]), β = −0.131 |
| LA 30 Hz ERG amplitude | F(1, 6) = 1.508, p = 0.265, R2 = 0.201, R2adjusted = 0.068 | B = −0.306, 95% CI [−0.916, 0.304]), β = −0.448 | F(1, 32) = 0.124, p = 0.727, R2 = 0.004, R2adjusted = −0.027 | B = −0.076, 95% CI [−0.514, 0.326]), β = −0.062 |
| LA 3.0 ERG b-wave amplitude | F(1, 6) = 1.170, p = 0.321, R2 = 0.163, R2adjusted = 0.024 | B = −0.300, 95% CI [−0.978, −0.378]), β = −0.404 | F(1, 32) = 0.537, p = 0.868, R2 = 0.001, R2adjusted = −0.030 | B = −0.046, 95% CI [−0.599, 0.508]), β = −0.030 |
| S-cone ERG amplitude | F(1, 6) = 0.537, p = 0.491, R2 = 0.082, R2adjusted = −0.071 | B = −0.004, 95% CI [−0.018, −0.010]), β = −0.287 | F(1, 32) = 25.810, p < 0.001, R2 = 0.446, R2adjusted = 0.429 | B = −0.077, 95% CI [−0.108, −0.046]), β = −0.065 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sajovic, J.; Meglič, A.; Hawlina, M.; Fakin, A. Electroretinography as a Biomarker to Monitor the Progression of Stargardt Disease. Int. J. Mol. Sci. 2022, 23, 16161. https://doi.org/10.3390/ijms232416161
Sajovic J, Meglič A, Hawlina M, Fakin A. Electroretinography as a Biomarker to Monitor the Progression of Stargardt Disease. International Journal of Molecular Sciences. 2022; 23(24):16161. https://doi.org/10.3390/ijms232416161
Chicago/Turabian StyleSajovic, Jana, Andrej Meglič, Marko Hawlina, and Ana Fakin. 2022. "Electroretinography as a Biomarker to Monitor the Progression of Stargardt Disease" International Journal of Molecular Sciences 23, no. 24: 16161. https://doi.org/10.3390/ijms232416161
APA StyleSajovic, J., Meglič, A., Hawlina, M., & Fakin, A. (2022). Electroretinography as a Biomarker to Monitor the Progression of Stargardt Disease. International Journal of Molecular Sciences, 23(24), 16161. https://doi.org/10.3390/ijms232416161






