Targeting a Silent Disease: Vascular Calcification in Chronic Kidney Disease
Abstract
1. Introduction
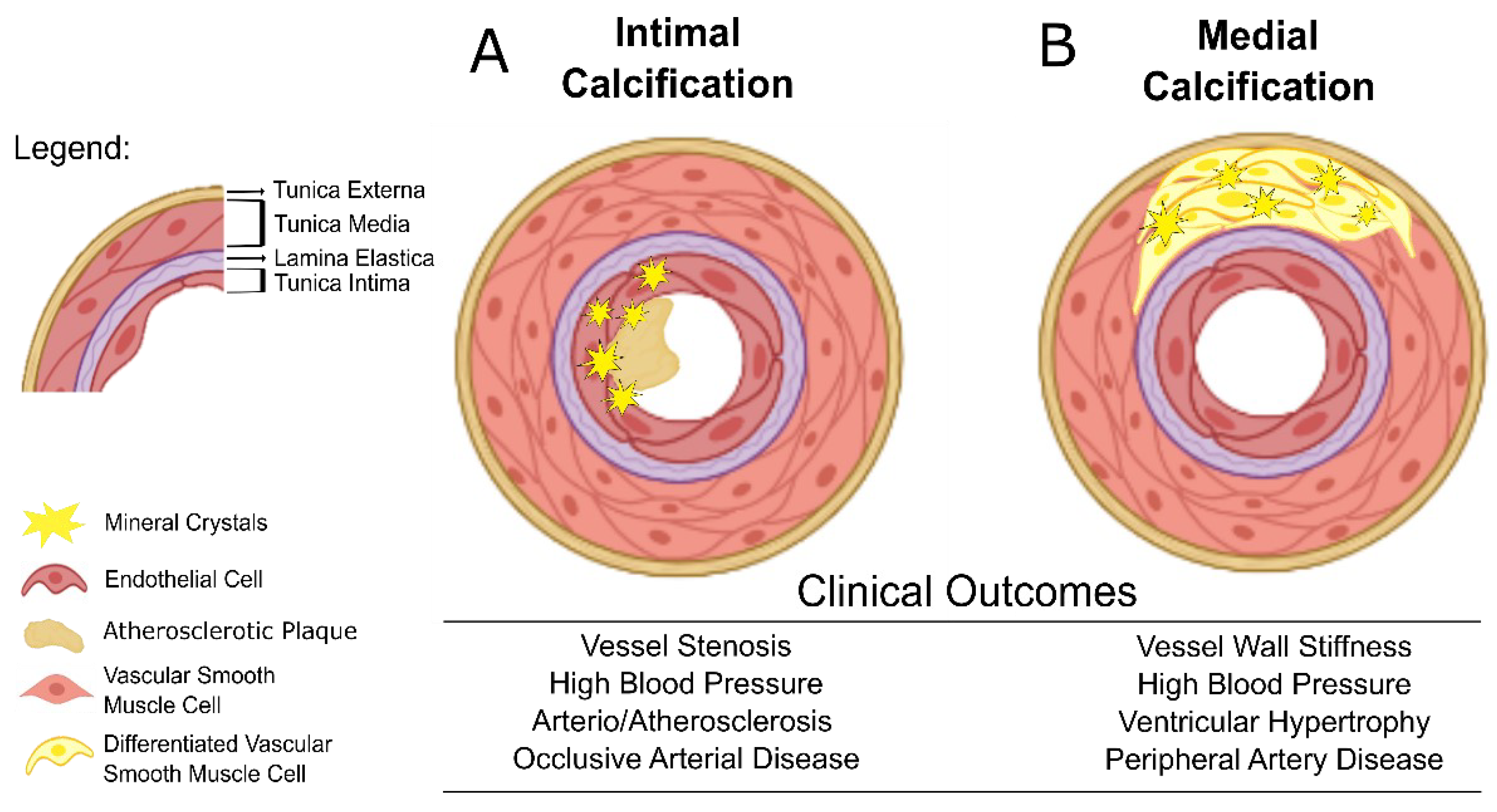
2. Vascular Calcification and Cardiovascular Clinical Outcomes
3. Imaging Cardiovascular Calcification in CKD Patients
3.1. Radiology Techniques
3.2. Ultrasonography Techniques
3.3. Molecular Imaging Techniques
4. Circulating Biomarkers of Vascular Calcification in CKD
4.1. Fetuin-A, Matrix Gla Protein and Gla Rich Protein in Vascular Calcification in CKD: Potential Biomarker Utility
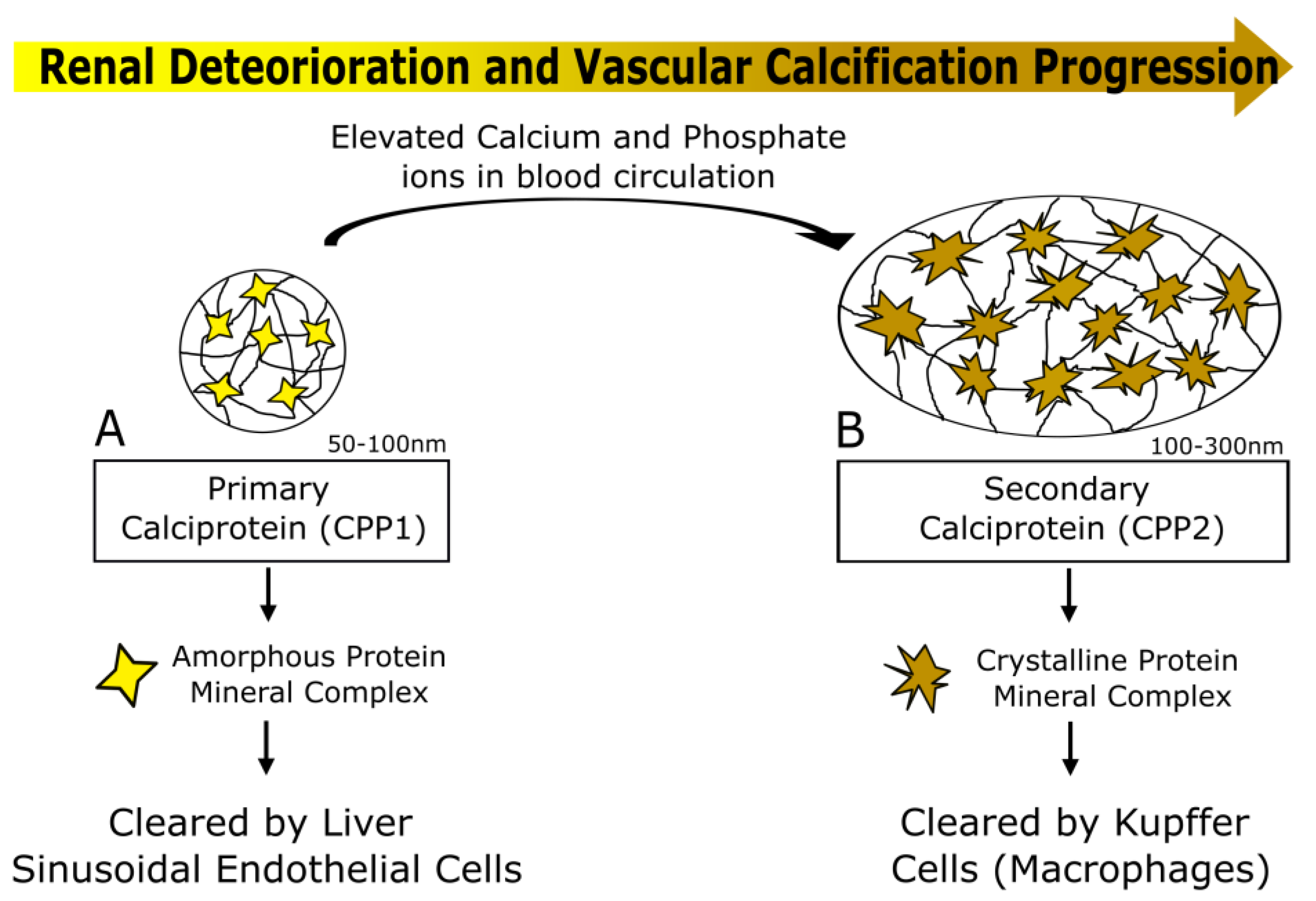
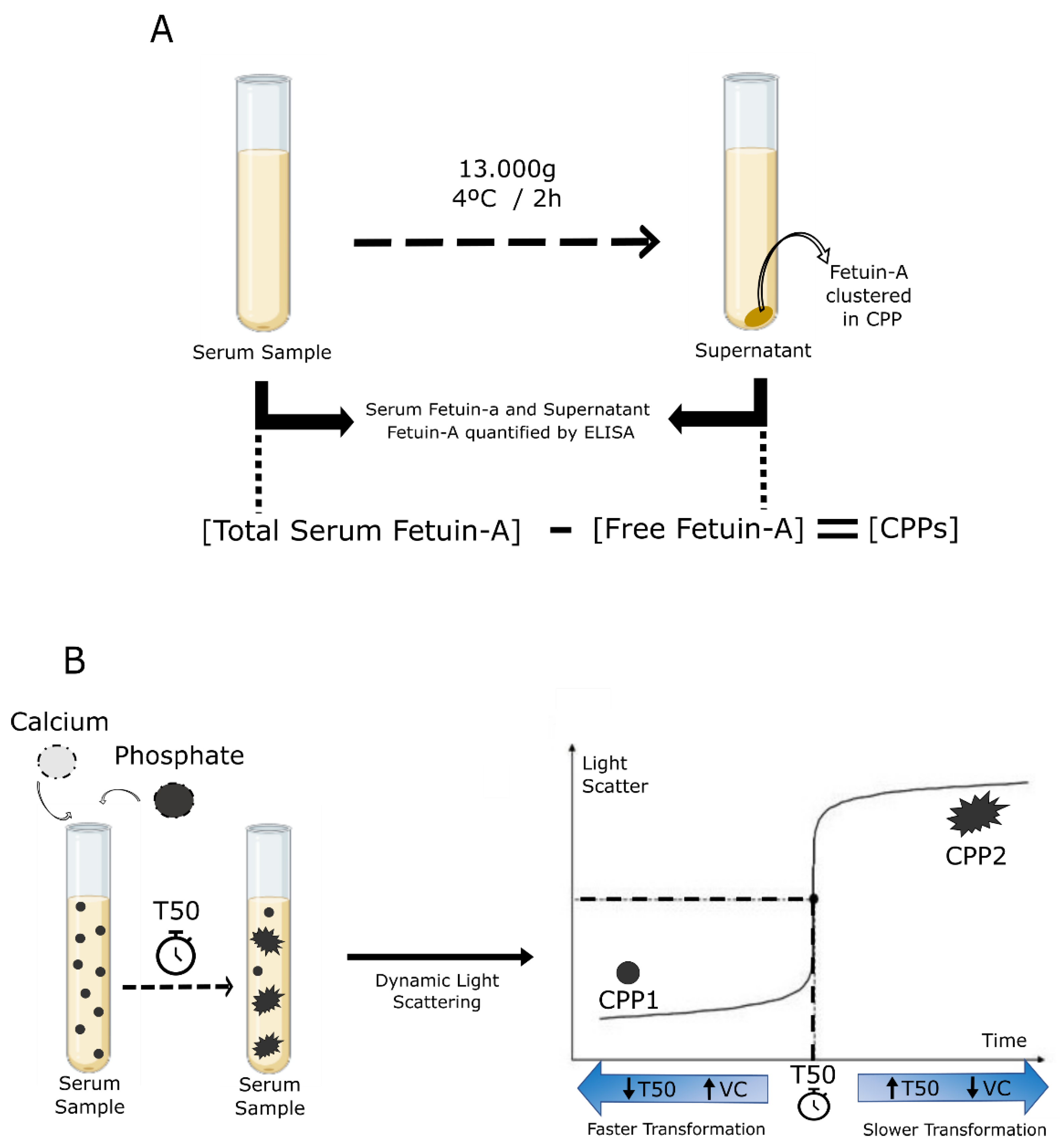
4.2. Calciprotein Particles (CPPs) as a Potential Biomarker of Vascular Calcification Progression in CKD
CPP-Fetuin-A and T50 Calcification and Tests
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kramer, A.; Boenink, R.; Stel, V.S.; Santiuste De Pablos, C.; Tomović, F.; Golan, E.; Kerschbaum, J.; Seyahi, N.; Ioanou, K.; Beltrán, P.; et al. The ERA-EDTA Registry Annual Report 2018: A summary. Clin. Kidney J. 2021, 14, 107–123. [Google Scholar] [CrossRef] [PubMed]
- Cancer. 2015. Available online: OurWorldInData.org (accessed on 24 August 2022).
- HIV Data and Statistics, Health Topics. 2020. Available online: WHO.int/health-topics (accessed on 24 August 2022).
- Herzog, C.A.; Asinger, R.W.; Berger, A.K.; Charytan, D.M.; Díez, J.; Hart, R.G.; Eckardt, K.U.; Kasiske, B.L.; McCullough, P.A.; Passman, R.S.; et al. Cardiovascular disease in chronic kidney disease. A clinical update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2011, 80, 572–586. [Google Scholar] [CrossRef]
- D’agostino, R.B.; Vasan, R.S.; Pencina, M.J.; Wolf, P.A.; Cobain, M.; Massaro, J.M.; Kannel, W.B. General Cardiovascular Risk Profile for Use in Primary Care The Framingham Heart Study. Circulation 2008, 117, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Cardiovascular, E.S.C. SCORE2 risk prediction algorithms: New models to estimate 10-year risk of cardiovascular disease in Europe. Eur. Heart J. 2021, 42, 2439–2454. [Google Scholar]
- De Vries, T.; Hageman, S.; Xu, Z.; Westerink, J. SCORE2-OP risk prediction algorithms: Estimating incident cardiovascular event risk in older persons in four geographical risk regions. Eur. Heart J. 2021, 42, 2455–2467. [Google Scholar]
- Schlendorf, K.H.; Nasir, K.; Blumenthal, R.S. Limitations of the Framingham risk score are now much clearer. Prev. Med. (Baltim) 2009, 48, 115–116. [Google Scholar] [CrossRef]
- Mortensen, M.B.; Falk, E. Limitations of the SCORE-guided European guidelines on cardiovascular disease prevention. Eur. Heart J. 2017, 38, 2259–2263. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, X.-C.; Lu, L.; Cao, Y.; Sun, R.-R.; Chen, S.; Zhang, P.-Y. Cardiovascular Disease and Its Relationship with Chronic Kidney Disease. Available online: https://www.europeanreview.org/article/7900 (accessed on 7 July 2022).
- Mizobuchi, M.; Towler, D.; Slatopolsky, E. Vascular Calcification: The Killer of Patients with Chronic Kidney Disease. J. Am. Soc. Nephrol. 2009, 20, 1453–1464. [Google Scholar] [CrossRef] [PubMed]
- Zwakenberg, S.R.; De Jong, P.A.; Hendriks, E.J.; Westerink, J.; Spiering, W.; De Borst, G.J.; Cramer, M.J.; Bartstra, J.W.; Doesburg, T.; Rutters, F.; et al. Intimal and medial calcification in relation to cardiovascular risk factors. PLoS ONE 2020, 15, e0235228. [Google Scholar] [CrossRef]
- Tomiyama, C.; Higa, A.; Dalboni, M.A.; Cendoroglo, M.; Draibe, S.A.; Cuppari, L.; Carvalho, A.B.; Neto, E.M.; Canziani, M.E.F. The impact of traditional and non-traditional risk factors on coronary calcification in pre-dialysis patients. Nephrol. Dial. Transplant. 2006, 21, 2464–2471. [Google Scholar] [CrossRef]
- Visseren, F.L.J.; MacH, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.J.; Raggi, P.; Wolf, M.; Gold, A.M.; Chertow, G.M.; Roe, M.T. Targeting Vascular Calcification in Chronic Kidney Disease. JACC Basic Transl. Sci. 2020, 5, 398–412. [Google Scholar] [CrossRef] [PubMed]
- Paloian, N.J.; Giachelli, C.M. A current understanding of vascular calcification in CKD. Am. J. Physiol.-Ren. Physiol. 2014, 307, F891–F900. [Google Scholar] [CrossRef] [PubMed]
- Giachelli, C.M. Vascular Calcification Mechanisms. J. Am. Soc. Nephrol. 2004, 15, 2959–2964. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Lee, I.K.; Jeon, J.H. Vascular calcification—New insights into its mechanism. Int. J. Mol. Sci. 2020, 21, 2685. [Google Scholar] [CrossRef]
- Wu, M.; Rementer, C.; Giachelli, C.M. Vascular Calcification: An Update on Mechanisms and Challenges in Treatment. Calcif. Tissue Int. 2013, 93, 365–373. [Google Scholar] [CrossRef]
- Vancheri, F.; Longo, G.; Vancheri, S.; Danial, J.S.H.; Henein, M.Y. Coronary Artery Microcalcification: Imaging and Clinical Implications. Diagnostics 2019, 9, 125. [Google Scholar] [CrossRef]
- Moccia, F.; Hutcheson, J.D.; Montecucco, F.; Ye, R.; Shi, X.; Gao, J.; Lv, Q.; Cai, H.; Wang, F.; Liu, X. Calcification in Atherosclerotic Plaque Vulnerability: Friend or Foe? Front. Physiol. 2020, 11, 56. [Google Scholar]
- Kawtharany, L.; Bessueille, L.; Issa, H.; Hamade, E.; Zibara, K.; Magne, D. Inflammation and Microcalcification: A Never-Ending Vicious Cycle in Atherosclerosis? Atherosclerosis: A Chronic Inflammatory Disease. J. Vasc. Res. 2022, 59, 137–150. [Google Scholar] [CrossRef]
- Baber, U.; Stone, G.W.; Weisz, G.; Moreno, P.; Dangas, G.; Maehara, A.; Mintz, G.S.; Cristea, E.; Fahy, M.; Xu, K.; et al. Coronary Plaque Composition, Morphology, and Outcomes in Patients With and Without Chronic Kidney Disease Presenting With Acute Coronary Syndromes. JACC Cardiovasc. Imaging 2012, 5, S53–S61. [Google Scholar] [CrossRef]
- Kono, K.; Fujii, H.; Nakai, K.; Goto, S.; Shite, J.; Hirata, K.I.; Fukagawa, M.; Nishi, S. Composition and plaque patterns of coronary culprit lesions and clinical characteristics of patients with chronic kidney disease. Kidney Int. 2012, 82, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, S.; Ahmed, S.; Ali, A.; Han, K.H.; Sechopoulos, I.; O’Neill, A.; Fei, B.; O’Neill, W.C. Progression of Medial Arterial Calcification in CKD. Kidney Int. Rep. 2018, 3, 1328–1335. [Google Scholar] [CrossRef] [PubMed]
- London, G.M.; Guérin, A.P.; Marchais, S.J.; Métivier, F.; Pannier, B.; Adda, H. Arterial media calcification in end-stage renal disease: Impact on all-cause and cardiovascular mortality. Nephrol. Dial. Transpl. 2003, 18, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Melamed, M.L. Vascular Calcification in Predialysis CKD: Common and Deadly. Clin. J. Am. Soc. Nephrol. 2015, 10, 551–553. [Google Scholar] [CrossRef]
- Adragao, T.; Pires, A.; Lucas, C.; Birne, R.; Gonçalves, M.; Pita Negrao, A. A simple vascular calcification score predicts cardiovascular risk in haemodialysis patients. Nephrol. Dial. Transpl. 2004, 19, 1480–1488. [Google Scholar] [CrossRef]
- Petrauskiene, V.; Vaiciuniene, R.; Bumblyte, I.A.; Kuzminskis, V.; Ziginskiene, E.; Grazulis, S.; Jonaitiene, E. Association between vascular calcification assessed by simple radiography and non-fatal cardiovascular events in hemodialysis patients. Nephrol. Ther. 2016, 12, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Kauppila, L.I.; Polak, J.F.; Cupples, L.A.; Hannan, M.T.; Kiel, D.P.; Wilson, P.W.F. New indices to classify location, severity and progression of calcific lesions in the abdominal aorta: A 25-year follow-up study. Atherosclerosis 1997, 132, 245–250. [Google Scholar] [CrossRef]
- Toussaint, N.D.; Lau, K.K.; Strauss, B.J.; Polkinghorne, K.R.; Kerr, P.G. Determination and Validation of Aortic Calcification Measurement from Lateral Bone Densitometry in Dialysis Patients. Clin. J. Am. Soc. Nephrol. 2009, 4, 119–127. [Google Scholar] [CrossRef]
- Taylor, C.; Zielinski, L.P.; Chowdhury, M.M.; Coughlin, P.A. Defining the Role of Duplex Ultrasound Assessment to Determine Severity of Arterial Calcification: An Analysis of the Superficial Femoral Artery. J. Vasc. Ultrasound 2020, 44, 74–78. [Google Scholar] [CrossRef]
- Utsunomiya, H.; Yamamoto, H.; Urabe, Y.; Tsushima, H.; Kunita, E.; Kitagawa, T.; Hidaka, T.; Kihara, Y. Association between heart calcification assessed by echocardiography and future cardiovascular disease mortality and morbidity. Int. J. Cardiol. Heart Vessel. 2013, 2, 15. [Google Scholar] [CrossRef]
- Hirschberg, K.; Reinhart, M.; Konstandin, M.; Uhlmann, L.; Katus, H.A.; Mereles, D. Diagnostic and prognostic value of a novel cardiac calcification score for coronary artery disease by transthoracic echocardiography. Int. J. Cardiol. 2015, 190, 332–334. [Google Scholar] [CrossRef] [PubMed]
- Flore, R.; Zocco, M.A.; Ainora, M.E.; Fonnesu, C.; Nesci, A.; Gasbarrini, A.; Ponziani, F.R. A novel ultrasound-based vascular calcification score (CALCS) to detect subclinical atherosclerosis. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 736–742. [Google Scholar] [PubMed]
- Liu, S.; Neleman, T.; Hartman, E.M.J.; Ligthart, J.M.R.; Witberg, K.T.; van der Steen, A.F.W.; Wentzel, J.J.; Daemen, J.; van Soest, G. Automated Quantitative Assessment of Coronary Calcification Using Intravascular Ultrasound. Ultrasound Med. Biol. 2020, 46, 2801–2809. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Budoff, M.J.; Reilly, M.P.; Yang, W.; Rosas, S.E.; Rahman, M.; Zhang, X.; Roy, J.A.; Lustigova, E.; Nessel, L.; et al. Coronary Artery Calcification and Risk of Cardiovascular Disease and Death Among Patients With Chronic Kidney Disease. JAMA Cardiol. 2017, 2, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Budoff, M.J.; Nasir, K.; Katz, R.; Takasu, J.; Carr, J.J.; Wong, N.D.; Allison, M.; Lima, J.A.C.; Detrano, R.; Blumenthal, R.S.; et al. Thoracic aortic calcification and coronary heart disease events: The multi-ethnic study of atherosclerosis (MESA). Atherosclerosis 2011, 215, 196–202. [Google Scholar] [CrossRef]
- Buijs, R.V.C.; Leemans, E.L.; Greuter, M.; Tielliu, I.F.J.; Zeebregts, C.J.; Willems, T.P. Quantification of abdominal aortic calcification: Inherent measurement errors in current computed tomography imaging. PLoS ONE 2018, 13, e0193419. [Google Scholar] [CrossRef]
- O’Connor, S.D.; Graffy, P.M.; Zea, R.; Pickhardt, P.J. Does nonenhanced CT-based quantification of abdominal aortic calcification outperform the framingham risk score in predicting cardiovascular events in asymptomatic adults? Radiology 2019, 290, 108–115. [Google Scholar] [CrossRef]
- Agatston, A.S.; Janowitz, W.R.; Hildner, F.J.; Zusmer, N.R.; Viamonte, M.; Detrano, R. Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. 1990, 15, 827–832. [Google Scholar] [CrossRef]
- Hensen, L.C.R.; El Mahdiui, M.; van Rosendael, A.R.; Smit, J.M.; Jukema, J.W.; Bax, J.J.; Delgado, V. Prevalence and Prognostic Implications of Mitral and Aortic Valve Calcium in Patients With Chronic Kidney Disease. Am. J. Cardiol. 2018, 122, 1732–1737. [Google Scholar] [CrossRef]
- Callister, T.Q.; Cooil, B.; Raya, S.P.; Lippolis, N.J.; Russo, D.J.; Raggi, P. Coronary artery disease: Improved reproducibility of calcium scoring with an electron-beam CT volumetric method. Radiology 1998, 208, 807–814. [Google Scholar] [CrossRef]
- Hong, C.; Bae, K.T.; Pilgram, T.K. Coronary Artery Calcium: Accuracy and Reproducibility of Measurements with Multi–Detector Row CT—Assessment of Effects of Different Thresholds and Quantification Methods1. Radiology 2003, 227, 795–801. [Google Scholar] [CrossRef]
- Ketteler, M.; Block, G.A.; Evenepoel, P.; Fukagawa, M.; Herzog, C.A.; Mccann, L.; Moe, S.M.; Shroff, R.; Tonelli, M.A.; Toussaint, N.D.; et al. Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder: Synopsis of the Kidney Disease: Improving Global Outcomes 2017 Clinical Practice Guideline Update. Ann. Intern. Med. 2018, 168, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.P.; Viegas, C.S.B.; Mendes, F.; Macedo, A.; Guilherme, P.; Tavares, N.; Dias, C.; Rato, F.; Santos, N.; Faísca, M.; et al. Gla-Rich Protein (GRP) as an Early and Novel Marker of Vascular Calcification and Kidney Dysfunction in Diabetic Patients with CKD: A Pilot Cross-Sectional Study. J. Clin. Med. 2020, 9, 635. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Ogawa, T.; Ishida, H.; Ando, Y.; Nitta, K. Aortic arch calcification evaluated on chest X-ray is a strong independent predictor of cardiovascular events in chronic hemodialysis patients. Heart Vessel. 2012, 27, 135–142. [Google Scholar] [CrossRef]
- Adragão, T.; Pires, A.; Birne, R.; Dias Curto, J.; Lucas, C.; Gonçalves, M.; Negrão, A.P. A plain X-ray vascular calcification score is associated with arterial stiffness and mortality in dialysis patients. Nephrol. Dial. Transpl. 2009, 24, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Yan, H.; Yang, X.; Yu, Z.; Ni, Z.; Fang, W. Abdominal aortic calcification score as a predictor of clinical outcome in peritoneal dialysis patients: A prospective cohort study. BMC Nephrol. 2020, 21, 151. [Google Scholar] [CrossRef] [PubMed]
- Bellasi, A.; Di Lullo, L.; Russo, D.; Ciarcia, R.; Magnocavallo, M.; Lavalle, C.; Ratti, C.; Fusaro, M.; Cozzolino, M.; Di Iorio, B.R. Predictive Value of Measures of Vascular Calcification Burden and Progression for Risk of Death in Incident to Dialysis Patients. J. Clin. Med. 2021, 10, 376. [Google Scholar] [CrossRef]
- Liabeuf, S.; Desjardins, L.; Diouf, M.; Temmar, M.; Renard, C.; Choukroun, G.; Massy, Z.A. The Addition of Vascular Calcification Scores to Traditional Risk Factors Improves Cardiovascular Risk Assessment in Patients with Chronic Kidney Disease. PLoS ONE 2015, 10, e0131707. [Google Scholar] [CrossRef]
- Adragao, T.; Ferreira, A.; Frazao, J.M.; Papoila, A.L.; Pinto, I.; Monier-Faugere, M.-C.; Malluche, H.H. Higher mineralized bone volume is associated with a lower plain X-Ray vascular calcification score in hemodialysis patients. PLoS ONE 2017, 12, e0179868. [Google Scholar] [CrossRef]
- Marinelli, A.; Pistolesi, V.; Pasquale, L.; Di Lullo, L.; Ferrazzano, M.; Baudena, G.; Della Grotta, F.; Napoli, A. Di Diagnosis of Arterial Media Calcification in Chronic Kidney Disease. Cardiorenal. Med. 2013, 3, 89–95. [Google Scholar] [CrossRef]
- Wang, Y.; Osborne, M.T.; Tung, B.; Li, M.; Li, Y. Imaging Cardiovascular Calcification. J. Am. Heart Assoc. 2018, 7, e008564. [Google Scholar] [CrossRef] [PubMed]
- Agricola, E.; Stella, S. Ultrasound-based aortic valve calcium scoring method: Are we ready to use it? Int. J. Cardiol. 2018, 252, 72–73. [Google Scholar] [CrossRef] [PubMed]
- Moe, S.M.; O’Neill, K.D.; Duan, D.; Ahmed, S.; Chen, N.X.; Leapman, S.B.; Fineberg, N.; Kopecky, K. Medial artery calcification in ESRD patients is associated with deposition of bone matrix proteins. Kidney Int. 2002, 61, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Janda, K.; Krzanowski, M.; Gajda, M.; Dumnicka, P.; Fedak, D.; Lis, G.J.; Jaśkowski, P.; Pietrzycka, A.; Litwin, J.A.; Sułowicz, W. Cardiovascular risk in chronic kidney disease patients: Intima-media thickness predicts the incidence and severity of histologically assessed medial calcification in radial arteries. BMC Nephrol. 2015, 16, 78. [Google Scholar] [CrossRef]
- Hirschberg, K.; Manuel, R.; Mereles, D.; Uhlmann, L.; André, F.; Riffel, J.; Ochs, M.; Katus, H.A. Echocardiographic calcification score in patients with low/ intermediate cardiovascular risk. Clin. Res. Cardiol. 1234, 108, 194–202. [Google Scholar] [CrossRef]
- Flore, R.; Ponziani, F.R.; Tinelli, G.; Arena, V.; Fonnesu, C.; Nesci, A.; Santoro, L.; Tondi, P.; Santoliquido, A. New Modalities of Ultrasound-Based Intima-Media Thickness, Arterial Stiffness and Non-Coronary Vascular Calcifications Detection to Assess Cardiovascular Risk. Available online: https://www.europeanreview.org/article/8807 (accessed on 20 May 2022).
- Raggi, P.; Bellasi, A. Clinical Assessment of Vascular Calcification. Adv. Chronic Kidney Dis. 2007, 14, 37–43. [Google Scholar] [CrossRef]
- Becker, C.R.; Knez, A.; Ohnesorge, B.; Schoepf, U.J.; Flohr, T.; Bruening, R.; Haberl, R.; Reiser, M.F. Visualization and quantification of coronary calcifications with electron beam and spiral computed tomography. Eur. Radiol. 2000, 10, 629–635. [Google Scholar] [CrossRef]
- Pawade, T.; Clavel, M.A.; Tribouilloy, C.; Dreyfus, J.; Mathieu, T.; Tastet, L.; Renard, C.; Gun, M.; Jenkins, W.S.A.; MacRon, L.; et al. Computed Tomography Aortic Valve Calcium Scoring in Patients with Aortic Stenosis. Circ. Cardiovasc. Imaging 2018, 11, e007146. [Google Scholar] [CrossRef]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef]
- Hong, C.; Bae, K.T.; Pilgram, T.K.; Suh, J.; Bradley, D. Coronary Artery Calcium Measurement with Multi–Detector Row CT: In Vitro Assessment of Effect of Radiation Dose1. Radiology 2002, 225, 901–906. [Google Scholar] [CrossRef]
- Yoon, H.C.; Greaser, L.E.; Mather, R.; Sinha, S.; McNitt-Gray, M.F.; Goldin, J.G. Coronary Artery Calcium: Alternate Methods for Accurate and Reproducible Quantitation. Acad. Radiol. 1997, 4, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Koos, R.; Mahnken, A.H.; Sinha, A.M.; Wildberger, J.E.; Hoffmann, R.; Kühl, H.P. Aortic Valve Calcification as a Marker for Aortic Stenosis Severity: Assessment on 16-MDCT. Am. J. Roentgenol. 2012, 183, 1813–1818. [Google Scholar] [CrossRef] [PubMed]
- Peng, A.W.; Mirbolouk, M.; Orimoloye, O.A.; Osei, A.D.; Dardari, Z.; Dzaye, O.; Budoff, M.J.; Shaw, L.; Miedema, M.D.; Rumberger, J.; et al. Long-Term All-Cause and Cause-Specific Mortality in Asymptomatic Patients With CAC ≥1000: Results From the CAC Consortium. JACC Cardiovasc. Imaging 2020, 13, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Budoff, M.J.; Shaw, L.J.; Liu, S.T.; Weinstein, S.R.; Mosler, T.P.; Tseng, P.H.; Flores, F.R.; Callister, T.Q.; Raggi, P.; Berman, D.S. Long-Term Prognosis Associated With Coronary Calcification: Observations From a Registry of 25,253 Patients. J. Am. Coll. Cardiol. 2007, 49, 1860–1870. [Google Scholar] [CrossRef] [PubMed]
- Stompór, T. Coronary artery calcification in chronic kidney disease: An update. World J. Cardiol. 2014, 6, 115. [Google Scholar] [CrossRef]
- Ho, C.Y.; Shanahan, C.M. Medial Arterial Calcification: An Overlooked Player in Peripheral Arterial Disease. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1475–1482. [Google Scholar] [CrossRef]
- Irkle, A.; Vesey, A.T.; Lewis, D.Y.; Skepper, J.N.; Bird, J.L.E.; Dweck, M.R.; Joshi, F.R.; Gallagher, F.A.; Warburton, E.A.; Bennett, M.R.; et al. Identifying active vascular microcalcification by 18F-sodium fluoride positron emission tomography. Nat. Commun. 2015, 6, 1–11. [Google Scholar] [CrossRef]
- Kaiser, Y.; Verberne, H.J. Arterial 18 F-NaF-uptake as a marker for vascular calcification activity. Can a little salt cut the sweet: A golden bullet to evaluate vascular mineralization? J. Nucl. Cardiol. 2021, 29, 1867–1869. [Google Scholar] [CrossRef]
- Nadar, S.K.; Shaikh, M.M. Biomarkers in Routine Heart Failure Clinical Care. Card. Fail. Rev. 2019, 5, 50. [Google Scholar] [CrossRef]
- Luca Salvagno, G.; Pavan, C. Prognostic biomarkers in acute coronary syndrome. Ann. Transl. Med. 2016, 4, 9. [Google Scholar] [CrossRef]
- Scialla, J.J.; Linda Kao, W.H.; Crainiceanu, C.; Sozio, S.M.; Oberai, P.C.; Shafi, T.; Coresh, J.; Powe, N.R.; Plantinga, L.C.; Jaar, B.G.; et al. Biomarkers of vascular calcification and mortality in patients with ESRD. Clin. J. Am. Soc. Nephrol. 2014, 9, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Araújo, N.; Marreiros, C.; Simes, D. The interplay between mineral metabolism, vascular calcification and inflammation in Chronic Kidney Disease (CKD): Challenging old concepts with new facts. Aging (Albany. NY) 2019, 11, 4274–4299. [Google Scholar] [CrossRef] [PubMed]
- Clemente, A.; Traghella, I.; Mazzone, A.; Sbrana, S.; Vassalle, C. Vascular and valvular calcification biomarkers. Adv. Clin. Chem. 2020, 95, 73–103. [Google Scholar] [PubMed]
- Small, A.; Kiss, D.; Giri, J.; Anwaruddin, S.; Siddiqi, H.; Guerraty, M.; Chirinos, J.A.; Ferrari, G.; Rader, D.J. Biomarkers of calcific aortic valve disease. Arter. Thromb. Vasc. Biol. 2017, 37, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Neyra, J.A.; Hu, M.C.; Moe, O.W. Klotho in Clinical Nephrology: Diagnostic and Therapeutic Implications. Clin. J. Am. Soc. Nephrol. 2021, 16, 162. [Google Scholar] [CrossRef]
- Vervloet, M. Renal and extrarenal effects of fibroblast growth factor 23. Nat. Rev. Nephrol. 2018, 15, 109–120. [Google Scholar] [CrossRef]
- Gutiérrez, O.M.; Mannstadt, M.; Isakova, T.; Rauh-Hain, J.A.; Tamez, H.; Shah, A.; Smith, K.; Lee, H.; Thadhani, R.; Jüppner, H.; et al. Fibroblast Growth Factor 23 and Mortality among Patients Undergoing Hemodialysis. N. Engl. J. Med. 2008, 359, 584. [Google Scholar] [CrossRef]
- Richter, B.; Faul, C. FGF23 Actions on Target Tissues-With and Without Klotho. Front. Endocrinol. (Lausanne) 2018, 9, 189. [Google Scholar] [CrossRef]
- Han, X.; Li, L.; Yang, J.; King, G.; Xiao, Z.; Quarles, L.D. Counter-regulatory paracrine actions of FGF-23 and 1,25(OH)2D in macrophages. FEBS Lett. 2016, 590, 53–67. [Google Scholar] [CrossRef]
- Hamano, T.; Matsui, I.; Mikami, S.; Tomida, K.; Fujii, N.; Imai, E.; Rakugi, H.; Isaka, Y. Fetuin-mineral complex reflects extraosseous calcification stress in CKD. J. Am. Soc. Nephrol. 2010, 21, 1998–2007. [Google Scholar] [CrossRef]
- Lv, Q.; Zhou, J.; Liu, J.; Kang, D.; Zhang, H. Serum osteocalcin is inversely associated with lower extremity atherosclerotic disease in Chinese patients with type 2 diabetes mellitus. Endocr. J. 2021, 68, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Schurgers, L.J.; Barreto, D.V.; Barreto, F.C.; Liabeuf, S.; Renard, C.; Magdeleyns, E.J.; Vermeer, C.; Choukroun, G.; Massy, Z.A. The Circulating Inactive Form of Matrix Gla Protein Is a Surrogate Marker for Vascular Calcification in Chronic Kidney Disease: A Preliminary Report. Clin. J. Am. Soc. Nephrol. 2010, 5, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Bi, X.; Liu, Y.; Huang, Y.; Xiong, J.; Xu, X.; Xiao, T.; Yu, Y.; Jiang, W.; Huang, Y.; et al. High Phosphate-Induced Calcification of Vascular Smooth Muscle Cells is Associated with the TLR4/NF-κb Signaling Pathway. Kidney Blood Press. Res. 2017, 42, 1205–1215. [Google Scholar] [CrossRef] [PubMed]
- Shroff, G.R.; Sanchez, O.A.; Miedema, M.D.; Kramer, H.; Ix, J.H.; Duprez, D.A.; Jacobs, D.R. Coronary artery calcium progresses rapidly and discriminates incident cardiovascular events in chronic kidney disease regardless of diabetes: The Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis 2020, 310, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Ganidagli, B.; Nacar, H.; Yildiz, Y.S.; Dagli, H.; Erken, E.; Altunoren, O.; Gungor, O. The relationship between serum osteopontin and FGF 23 levels with valvular calcification in hemodialysis patients. Clin. Nephrol. 2019, 91, 9–16. [Google Scholar] [CrossRef]
- Ozkok, A.; Caliskan, Y.; Sakaci, T.; Erten, G.; Karahan, G.; Ozel, A.; Unsal, A.; Yildiz, A. Osteoprotegerin/RANKL axis and progression of coronary artery calcification in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2012, 7, 965–973. [Google Scholar] [CrossRef]
- Westenfeld, R.; Krueger, T.; Schlieper, G.; Cranenburg, E.C.M.; Magdeleyns, E.J.; Heidenreich, S.; Holzmann, S.; Vermeer, C.; Jahnen-Dechent, W.; Ketteler, M.; et al. Effect of Vitamin K2 Supplementation on Functional Vitamin K Deficiency in Hemodialysis Patients: A Randomized Trial. Am. J. Kidney Dis. 2012, 59, 186–195. [Google Scholar] [CrossRef]
- Price, P.A.; Urist, M.R.; Otawara, Y. Matrix Gla protein, a new gamma-carboxyglutamic acid-containing protein which is associated with the organic matrix of bone. Biochem. Biophys. Res. Commun. 1983, 117, 765–771. [Google Scholar] [CrossRef]
- Viegas, C.S.B.; Rafael, M.S.; Enriquez, J.L.; Teixeira, A.; Vitorino, R.; Luís, I.M.; Costa, R.M.; Santos, S.; Cavaco, S.; Neves, J.; et al. Gla-Rich Protein acts as a Calcification Inhibitor in the Human Cardiovascular System. Arter. Thromb. Vasc. Biol. 2015, 35, 399–408. [Google Scholar] [CrossRef]
- Viegas, C.S.B.; Simes, D.C. Inflammation and Calcification in the Vascular Tree; Insights Into Atherosclerosis. In Immunity and Inflammation in Health and Disease; Emerging Roles of Nutraceuticals and Functional Foods in Immune Support; Academic Press: Cambridge, MA, USA, 2018; pp. 189–201. [Google Scholar]
- Vahed, S.Z.; Mostafavi, S.; Khatibi, S.M.H.; Shoja, M.M.; Ardalan, M. Vascular Calcification: An Important Understanding in Nephrology. Vasc. Health Risk Manag. 2020, 16, 167–180. [Google Scholar] [CrossRef]
- Smith, E.R.; Hanssen, E.; McMahon, L.P.; Holt, S.G. Fetuin-A-Containing Calciprotein Particles Reduce Mineral Stress in the Macrophage. PLoS ONE 2013, 8, e60904. [Google Scholar] [CrossRef] [PubMed]
- Adeney, K.L.; Siscovick, D.S.; Ix, J.H.; Seliger, S.L.; Shlipak, M.G.; Jenny, N.S.; Kestenbaum, B.R. Association of Serum Phosphate with Vascular and Valvular Calcification in Moderate CKD. J. Am. Soc. Nephrol. 2009, 20, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Gόrriz, J.L.; Molina, P.; Cerverόn, M.J.; Vila, R.; Bover, J.; Nieto, J.; Barril, G.; Martínez-Castelao, A.; Fernández, E.; Escudero, V.; et al. Vascular calcification in patients with nondialysis CKD over 3 years. Clin. J. Am. Soc. Nephrol. 2015, 10, 654–666. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Canton, C.; Bosch, E.; Ramirez, A.; Gonzalez, Y.; Auyanet, I.; Guerra, R.; Perez, M.A.; Fernandez, E.; Toledo, A.; Lago, M.; et al. Vascular calcification and 25-hydroxyvitamin D levels in non-dialysis patients with chronic kidney disease stages 4 and 5. Nephr Dial Transpl. 2011, 26, 2250–2256. [Google Scholar] [CrossRef]
- Fitzpatrick, J.; Kim, E.D.; Sozio, S.M.; Jaar, B.G.; Estrella, M.M.; Monroy-Trujillo, J.M.; Parekh, R.S. Calcification Biomarkers, Subclinical Vascular Disease, and Mortality Among Multiethnic Dialysis Patients. Kidney Int. Rep. 2020, 5, 1729–1737. [Google Scholar] [CrossRef]
- Coen, G.; de Paolis, P.; Ballanti, P.; Pierantozzi, A.; Pisanò, S.; Sardella, D.; Mantella, D.; Pellegrino, L.; Silvestrini, G.; Iappelli, M.; et al. Peripheral artery calcifications evaluated by histology correlate to those detected by CT: Relationships with fetuin-A and FGF-23. J. Nephrol. 2011, 24, 313–321. [Google Scholar] [CrossRef]
- Zheng, S.; Zheng, Y.; Jin, L.; Zhou, Z.; Li, Z. Relationship between Serum Soluble Klotho Protein and Coronary Artery Calcification and Prognosis in Patients on Maintenance Hemodialysis. Iran. J. Public Health 2018, 47, 510. [Google Scholar]
- Keryakos, H.K.H.; Okaily, N.I.; Boulis, M.A.Y.; Salama, A.M.S. Osteocalcin and vascular calcification in hemodialysis patients: An observational cohort study. Int. Urol. Nephrol. 2021, 53, 1015–1023. [Google Scholar] [CrossRef]
- Oikonomaki, T.; Papasotiriou, M.; Ntrinias, T.; Kalogeropoulou, C.; Zabakis, P.; Kalavrizioti, D.; Papadakis, I.; Goumenos, D.S.; Papachristou, E. The effect of vitamin K2 supplementation on vascular calcification in haemodialysis patients: A 1-year follow-up randomized trial. Int. Urol. Nephrol. 2019, 51, 2037–2044. [Google Scholar] [CrossRef]
- Kurnatowska, I.; Grzelak, P.; Masajtis-Zagajewska, A.; Kaczmarska, M.; Stefañczyk, L.; Vermeer, C.; Maresz, K.; Nowicki, M. Effect of Vitamin K2 on progression of atherosclerosis and vascular calcification in nondialyzed patients with chronic kidney disease stages 3-5. Pol. Arch. Med. Wewn. 2015, 125, 631–640. [Google Scholar] [CrossRef]
- de Vriese, A.S.; Caluwé, R.; Pyfferoen, L.; de Bacquer, D.; de Boeck, K.; Delanote, J.; de Surgeloose, D.; van Hoenacker, P.; van Vlem, B.; Verbeke, F. Multicenter randomized controlled trial of Vitamin K antagonist replacement by rivaroxaban with or without vitamin K2 in hemodialysis patients with atrial fibrillation: The Valkyrie study. J. Am. Soc. Nephrol. 2020, 31, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Jaminon, A.M.G.; Dai, L.; Rashid Qureshi, A.; Evenepoel, P.; Ripsweden, J.; Söderberg, M.; Witasp, A.; Olauson, H.; Schurgers, L.J.; Stenvinkel, P. Matrix Gla protein is an independent predictor of both intimal and medial vascular calcification in chronic kidney disease. Nat.Res. Sci. Rep. 2020, 10, 6586. [Google Scholar] [CrossRef] [PubMed]
- Delanaye, P.; Krzesinski, J.M.; Warling, X.; Moonen, M.; Smelten, N.; Médart, L.; Pottel, H.; Cavalier, E. Dephosphorylated-uncarboxylated Matrix Gla protein concentration is predictive of vitamin K status and is correlated with vascular calcification in a cohort of hemodialysis patients. BMC Nephrol. 2014, 15, 145. [Google Scholar] [CrossRef] [PubMed]
- Zhelyazkova-Savova, M.D.; Yoto, T.Y.; Nikolova, M.N.; Nazifova-Tasinova, N.F.; Vankova, D.G.; Atanasov, A.A.; Galunska, B.T. Statins, vascular calcification, and vitamin K-dependent proteins: Is there a relation? Kaohsiung J. Med. Sci. 2021, 37, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Demirci, R.; Sevinc, C. The Relationship Between Carotid Intima Media Thickness, Inflammation and GLA Rich Protein Levels in Chronic Kidney Disease. Int. J. Gen. Med. 2021, 14, 5119–5126. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.P.; Viegas, C.S.B.; Guilherme, P.; Tavares, N.; Dias, C.; Rato, F.; Santos, N.; Faísca, M.; de Almeida, E.; Neves, P.L.; et al. Gla-Rich Protein, Magnesium and Phosphate Associate with Mitral and Aortic Valves Calcification in Diabetic Patients with Moderate CKD. Diagnostics 2022, 12, 496. [Google Scholar] [CrossRef]
- Maréchal, C.; Schlieper, G.; Nguyen, P.; Krüger, T.; Coche, E.; Robert, A.; Floege, J.; Goffin, E.; Jadoul, M.; Devuyst, O. Serum Fetuin-A Levels Are Associated with Vascular Calcifications and Predict Cardiovascular Events in Renal Transplant Recipients. Clin. J. Am. Soc. Nephrol. 2011, 6, 974. [Google Scholar] [CrossRef]
- Mann, A.; Makkar, V.; Mann, S.; Dhamija, P.; Soundarajan, P. Fetuin-A and vascular calcification in Indian end-stage renal disease population. Indian J. Nephrol. 2016, 26, 33–38. [Google Scholar]
- Simes, D.C.; Viegas, C.S.B.; Araújo, N.; Marreiros, C. Vitamin K as a powerful micronutrient in aging and age-related diseases: Pros and cons from clinical studies. Int. J. Mol. Sci. 2019, 20, 4150. [Google Scholar] [CrossRef]
- Cozzolino, M.; Mangano, M.; Galassi, A.; Ciceri, P.; Messa, P.; Nigwekar, S. Vitamin K in Chronic Kidney Disease. Nutrients 2019, 11, 168. [Google Scholar] [CrossRef]
- Caluwe, R.; Vandecasteele, S.; Van Vlem, B.; Vermeer, C.; De Vriese, A.S. Vitamin K2 supplementation in haemodialysis patients: A randomized dose-finding study. Nephrol. Dial. Transplant. 2014, 29, 1385–1390. [Google Scholar] [CrossRef] [PubMed]
- Boxma, P.Y.; van den Berg, E.; Geleijnse, J.M.; Laverman, G.D.; Schurgers, L.J.; Vermeer, C.; Kema, I.P.; Muskiet, F.A.; Navis, G.; Bakker, S.J.L.; et al. Vitamin k intake and plasma desphospho-uncarboxylated matrix Gla-protein levels in kidney transplant recipients. PLoS ONE 2012, 7, e47991. [Google Scholar] [CrossRef] [PubMed]
- Thamratnopkoon, S.; Susantitaphong, P.; Tumkosit, M.; Katavetin, P.; Tiranathanagul, K.; Praditpornsilpa, K.; Eiam-Ong, S. Correlations of Plasma Desphosphorylated Uncarboxylated Matrix Gla Protein with Vascular Calcification and Vascular Stiffness in Chronic Kidney Disease. Nephron 2017, 135, 167–172. [Google Scholar] [CrossRef]
- Roumeliotis, S.; Roumeliotis, A.; Stamou, A.; Leivaditis, K.; Kantartzi, K.; Panagoutsos, S.; Liakopoulos, V. The Association of dp-ucMGP with Cardiovascular Morbidity and Decreased Renal Function in Diabetic Chronic Kidney Disease. Int. J. Mol. Sci. 2020, 21, 6035. [Google Scholar] [CrossRef] [PubMed]
- Puzantian, H.; Akers, S.R.; Oldland, G.; Javaid, K.; Miller, R.; Ge, Y.; Ansari, B.; Lee, J.; Suri, A.; Hasmath, Z.; et al. Circulating Dephospho-Uncarboxylated Matrix Gla-Protein Is Associated With Kidney Dysfunction and Arterial Stiffness. Am. J. Hypertens. 2018, 31, 988–994. [Google Scholar] [CrossRef]
- Kurnatowska, I.; Grzelak, P.; Masajtis-Zagajewska, A.; Kaczmarska, M.; Stefańczyk, L.; Vermeer, C.; Maresz, K.; Nowicki, M. Plasma Desphospho-Uncarboxylated Matrix Gla Protein as a Marker of Kidney Damage and Cardiovascular Risk in Advanced Stage of Chronic Kidney Disease. Kidney Blood Press. Res. 2016, 41, 231–239. [Google Scholar] [CrossRef]
- Vlasschaert, C.; Goss, C.J.; Pilkey, N.G.; McKeown, S.; Holden, R.M. Vitamin K Supplementation for the Prevention of Cardiovascular Disease: Where Is the Evidence? A Systematic Review of Controlled Trials. Nutrients 2020, 12, 2909. [Google Scholar] [CrossRef]
- Roumeliotis, S.; Roumeliotis, A.; Panagoutsos, S.; Giannakopoulou, E.; Papanas, N.; Manolopoulos, V.G.; Passadakis, P.; Tavridou, A. Matrix Gla protein T-138C polymorphism is associated with carotid intima media thickness and predicts mortality in patients with diabetic nephropathy. J. Diabetes Complicat. 2017, 31, 1527–1532. [Google Scholar] [CrossRef]
- Vermeer, C.; Vik, H. Effect of Menaquinone-7 (vitamin K2) on vascular elasticity in healthy subjects: Results from a one-year study. Vasc. Dis. Ther. 2020, 5, 1–4. [Google Scholar] [CrossRef]
- Viegas, C.; Simes, D. A dual role for GRP in cardiovascular disease. Aging 2019, 11, 1323–1324. [Google Scholar] [CrossRef]
- Viegas, C.S.B.; Santos, L.; Macedo, A.L.; Matos, A.A.; Silva, A.P.; Neves, P.L.; Staes, A.; Gevaert, K.; Morais, R.; Vermeer, C.; et al. Chronic kidney disease circulating calciprotein particles and extracellular vesicles promote vascular calcification: A role for GRP (Gla-Rich Protein). Arterioscler. Thromb. Vasc. Biol. 2018, 38, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Ter Braake, A.D.; Shanahan, C.M.; De Baaij, J.H.F. Magnesium Counteracts Vascular Calcification: Passive Interference or Active Modulation? Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1431–1445. [Google Scholar] [CrossRef] [PubMed]
- Heiss, A.; Pipich, V.; Jahnen-dechent, W.; Schwahn, D. Fetuin-A Is a Mineral Carrier Protein: Small Angle Neutron Scattering Provides New Insight on Fetuin-A Controlled Calcification Inhibition. Biophys. J. 2010, 99, 3986–3995. [Google Scholar] [CrossRef] [PubMed]
- Jahnen-Dechent, W.; Büscher, A.; Köppert, S.; Heiss, A.; Kuro-o, M.; Smith, E.R. Mud in the blood: The role of protein-mineral complexes and extracellular vesicles in biomineralisation and calcification. J. Struct. Biol. 2020, 212. [Google Scholar] [CrossRef] [PubMed]
- Caglar, K.; Yilmaz, M.I.; Saglam, M.; Cakir, E.; Kilic, S.; Sonmez, A.; Eyileten, T.; Yenicesu, M.; Oguz, Y.; Tasar, M.; et al. Serum Fetuin-A Concentration and Endothelial Dysfunction in Chronic Kidney Disease. Nephron Clin. Pract. 2008, 108, c233–c240. [Google Scholar] [CrossRef]
- Ketteler, M.; Bongartz, P.; Westenfeld, R.; Wildberger, J.E.; Mahnken, A.H.; Böhm, R.; Metzger, T.; Wanner, C.; Jahnen-Dechent, W.; Floege, J. Association of low fetuin-A (AHSG) concentrations in serum with cardiovascular mortality in patients on dialysis: A cross-sectional study. Lancet 2003, 361, 827–833. [Google Scholar] [CrossRef]
- Kranz, C.; Jiang, H.; Zhou, Z.; Ji, Y.; Ju, H.; Chen, H.; Sun, M. Circulating Fetuin-A and Risk of All-Cause Mortality in Patients With Chronic Kidney Disease: A Systematic Review and Meta-Analysis. Front. Physiol. 2019, 1, 966. [Google Scholar]
- Kutikhin, A.G.; Krenning, G.; Feenstra, L.; Kostyunin, A.E.; Yuzhalin, A.E.; Hillebrands, J.-L. Arteriosclerosis, Thrombosis, and Vascular Biology Calciprotein Particles Balancing Mineral Homeostasis and Vascular Pathology. Arter. Thromb. Vasc. Biol. 2021, 41, 1607–1624. [Google Scholar] [CrossRef]
- Miura, Y.; Iwazu, Y.; Shiizaki, K.; Akimoto, T.; Kotani, K.; Kurabayashi, M.; Kurosu, H.; Kuro-O, M. Identification and quantification of plasma calciprotein particles with distinct physical properties in patients with chronic kidney disease OPEN. Sci. Rep. 2018, 8, 1256. [Google Scholar] [CrossRef]
- Aghagolzadeh, P.; Bachtler, M.; Bijarnia, R.; Jackson, C.; Smith, E.R.; Odermatt, A.; Radpour, R.; Pasch, A. Calcification of vascular smooth muscle cells is induced by secondary calciprotein particles and enhanced by tumor necrosis factor-α. Atherosclerosis 2016, 251, 404–414. [Google Scholar] [CrossRef]
- Köppert, S.; Büscher, A.; Babler, A.; Ghallab, A.; Buhl, E.M.; Latz, E.; Hengstler, J.G.; Smith, E.R.; Jahnen-Dechent, W. Cellular clearance and biological activity of calciprotein particles depend on their maturation state and crystallinity. Front. Immunol. 2018, 9, 1991. [Google Scholar] [CrossRef]
- Pasch, A.; Farese, S.; Gräber, S.; Wald, J.; Richtering, W.; Floege, J.; Jahnen-Dechent, W. Nanoparticle-Based Test Measures Overall Propensity for Calcification in Serum. J. Am. Soc. Nephrol. 2012, 23, 1744–1752. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.R.; Ford, M.L.; Tomlinson, L.A.; Rajkumar, C.; Mcmahon, L.P.; Holt, S.G. Phosphorylated fetuin-A-containing calciprotein particles are associated with aortic stiffness and a procalcific milieu in patients with pre-dialysis CKD. Nephrol. Dial. Transplant. 2012, 27, 1957–1966. [Google Scholar] [CrossRef] [PubMed]
- Thiem, U.; Soellradl, I.; Robl, B.; Watorek, E.; Blum, S.; Dumfarth, A.; Marculescu, R.; Pasch, A.; Haller, M.C.; Cejka, D. The effect of phosphate binder therapy with sucroferric oxyhydroxide on calcification propensity in chronic haemodialysis patients: A randomized, controlled, crossover trial. Clin. Kidney J. 2020, 14, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.R.; Ford, M.L.; Tomlinson, L.A.; Bodenham, E.; McMahon, L.P.; Farese, S.; Rajkumar, C.; Holt, S.G.; Pasch, A. Serum calcification propensity predicts all-cause mortality in predialysis CKD. J. Am. Soc. Nephrol. 2014, 25, 339–348. [Google Scholar] [CrossRef]
- Dahle, D.O.; Åsberg, A.; Hartmann, A.; Holdaas, H.; Bachtler, M.; Jenssen, T.G.; Dionisi, M.; Pasch, A. Serum Calcification Propensity Is a Strong and Independent Determinant of Cardiac and All-Cause Mortality in Kidney Transplant Recipients. Am. J. Transplant. 2016, 16, 204–212. [Google Scholar] [CrossRef]
- Keyzer, C.A.; de Borst, M.H.; van den Berg, E.; Jahnen-Dechent, W.; Arampatzis, S.; Farese, S.; Bergmann, I.P.; Floege, J.; Navis, G.; Bakker, S.J.; et al. Calcification Propensity and Survival among Renal Transplant Recipients. J. Am. Soc. Nephrol. 2016, 27, 239–248. [Google Scholar] [CrossRef]
- Pruijm, M.; Lu, Y.; Megdiche, F.; Piskunowicz, M.; Milani, B.; Stuber, M.; Bachtler, M.; Vogt, B.; Burnier, M.; Pasch, A. Serum calcification propensity is associated with renal tissue oxygenation and resistive index in patients with arterial hypertension or chronic kidney disease. J. Hypertens. 2017, 35, 2044–2052. [Google Scholar] [CrossRef]
- Berchtold, L.; Ponte, B.; Moll, S.; Hadaya, K.; Seyde, O.; Bachtler, M.; Vallée, J.P.; Martin, P.Y.; Pasch, A.; De Seigneux, S. Phosphocalcic Markers and Calcification Propensity for Assessment of Interstitial Fibrosis and Vascular Lesions in Kidney Allograft Recipients. PLoS ONE 2016, 11, e0167929. [Google Scholar] [CrossRef]
- Pasch, A. Novel assessments of systemic calcification propensity. Curr. Opin. Nephrol. Hypertens. 2016, 25, 278–284. [Google Scholar] [CrossRef]
| Clinical Assessment | Anatomical Sites | Population | VC Scores | Refs. |
|---|---|---|---|---|
| X-Ray Techniques | ||||
| Plain X-ray | Hands, pelvis, femur | Hemodialysis patients | Adragão Score | [28,29] |
| Lateral Dual-energy X-ray Absorptiometry | Abdominal aorta artery | Dialysis patients | Kauppila Score | [30,31] |
| Ultrasonography Techniques | ||||
| Duplex Ultrasound | Common femoral artery, proximal and distal superficial femoral artery and popliteal artery | Peripheral arterial disease patients | DULLAC Score | [32] |
| Echocardiography | Aortic valve Mitral annular and aortic root | Coronary disease patients | THC Score | [33] |
| Transthoracic Echocardiography | Coronary arteries | Coronary disease patients | Echo Score | [34] |
| B-mode Doopler Ultrasound | Carotid arteries, abdominal aorta and lower limbs vessels | Low-intermediate cardiovascular risk patients | CALC Score | [35] |
| Intravascular Ultrasound | Coronary arteries | Coronary disease patients | IVUS Calcium Score | [36] |
| Molecular Imaging Techniques | ||||
| Electron Beam or Multislice Computed Tomography | Coronary arteries | CKD patients | Agatston Score | [37] |
| Coronary arteries | Asymptomatic patients | Agatston Score | [38,39,40] | |
| Coronary arteries | Coronary artery disease patients | Agatston Score | [41] | |
| Thoracic aorta | Peritoneal dialysis patients | Agatston Score | [38] | |
| Abdominal aorta | Asymptomatic patients | Agatston Score | [40] | |
| Mitral and aortic valve | CKD | Agatston Score | [42] | |
| Coronary arteries | Coronary calcification patients | Volume Score | [43] | |
| Coronary arteries | Asymptomatic patients | Mass Score | [44] |
| Promoters | Role in Vascular Calcification | Inhibitors | Role in Vascular Calcification |
|---|---|---|---|
| FGF-23 [80] | Hormone that functions as a central endocrine factor that regulates phosphate balance by modulating phosphate reabsorption in the kidney, parathyroid hormone (PTH) secretion and vitamin D metabolism. | Fetuin-A [84] | Binds directly to calcium and phosphate forming mineral complexes (Calciprotein Particles). It represses growth of crystal and mineral deposition. |
| OC [85] | Vitamin K-dependent protein (VKDP) secreted by osteoblasts functioning as a negative regulator of bone formation, regulator of mineral maturation rate and mechanical stabilizer of bone matrix; regulator of glucose metabolism. | MGP [86] | VKDP functioning as an inhibitor of soft tissue calcification involved in the VSMCs phenotypic differentiation, binding to calcium and calcium-phosphate circulating forms, thereby preventing its growth and matrix deposition. |
| Phosphate [87] | Promotes VSMC differentiation and apoptosis; elevates FGF-23; decreases Klotho expression; clusters with calcium in mineral complexes and promotes matrix mineralization. | GRP [46] | VKDP functioning as an inhibitor of soft tissue calcification involved in the VSMCs phenotypic differentiation and mineralization competence of extracellular vesicles; inhibitor of mineral crystal maturation and growth in blood; anti-inflammatory agent. |
| Calcium [88] | Clusters with phosphate in mineral complexes and promotes matrix mineralization; influences parathyroid hormone and Vitamin D production stimulus affecting FGF-23 levels. | OPN [89] | Prevents calcium crystal growth and accelerates osteoclast function. |
| OPG [90] | Binds to the RANKL, thereby interfering with RANK-RANKL axis. Inhibition of RANKL-RANK inhibits the differentiation of osteoclast maturation, and thus, bone resorption. | ||
| α-Klotho [79] | Klotho decreases kidney phosphate reabsorption by acting as a coreceptor for FGF23. Reduced levels are associated with cell senescence, cell apoptosis, oxidation induced cell damage and lower autophagy activity. | ||
| Vitamin K [91] | Is the co-factor of γ-glutamyl carboxylase enzyme, responsible for the y-carboxylation of VKDPs. |
| Clinical Markers | Population | VC Detection Method | Evidence of VC Marker and Score Association | Refs. |
|---|---|---|---|---|
| Phosphate | CKD | CAC | Yes | [97] |
| Nondialysis CKD | Kauppila and Adragão | Yes | [98] | |
| Nondialysis CKD | Kauppila and Adragão | No | [99] | |
| GF23 | CKD | Adragão | Yes | [46] |
| Hemodialysis | CAC-Agatston | Yes | [100] | |
| Hemodialysis | Echocardiography | No | [89] | |
| Hemodialysis | CAC-Agatston | Yes | [101] | |
| α-Klotho | Hemodialysis | CAC | Yes | [102] |
| OPN | Hemodialysis | Echocardiography | Yes | [89] |
| OC | Hemodialysis | Transthoracic Echocardiography | No | [103] |
| OPG | Hemodialysis | CAC-Agatston | Yes | [90] |
| Hemodialysis | CAC-Agatston | Yes | [100] | |
| Vitamin K status/ dp-ucMGP | Hemodialysis | CAC-Agatston | No | [104] |
| CKD | CAC-Agatston | No | [105] | |
| Hemodialysis | CAC-Agatston | No | [106] | |
| CKD | CAC | Yes | [86,107] | |
| Hemodialysis | Kauppila | Yes | [108] | |
| Hemodialysis | CAC-Agatston | No | [100] | |
| GRP | CKD | Adragão | Yes | [46] |
| CVD | CAC | No | [109] | |
| CKD | Ultrasound | Yes | [110] | |
| CKD | Echocardiography | Yes | [111] | |
| Fetuin-A | Renal Transplant | CAC | Yes | [112] |
| ESRD | CAC | No | [113] | |
| CKD | CAC | Yes | [84] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marreiros, C.; Viegas, C.; Simes, D. Targeting a Silent Disease: Vascular Calcification in Chronic Kidney Disease. Int. J. Mol. Sci. 2022, 23, 16114. https://doi.org/10.3390/ijms232416114
Marreiros C, Viegas C, Simes D. Targeting a Silent Disease: Vascular Calcification in Chronic Kidney Disease. International Journal of Molecular Sciences. 2022; 23(24):16114. https://doi.org/10.3390/ijms232416114
Chicago/Turabian StyleMarreiros, Catarina, Carla Viegas, and Dina Simes. 2022. "Targeting a Silent Disease: Vascular Calcification in Chronic Kidney Disease" International Journal of Molecular Sciences 23, no. 24: 16114. https://doi.org/10.3390/ijms232416114
APA StyleMarreiros, C., Viegas, C., & Simes, D. (2022). Targeting a Silent Disease: Vascular Calcification in Chronic Kidney Disease. International Journal of Molecular Sciences, 23(24), 16114. https://doi.org/10.3390/ijms232416114







