Nanoparticles for Topical Application in the Treatment of Skin Dysfunctions—An Overview of Dermo-Cosmetic and Dermatological Products
Abstract
1. Introduction
2. The Penetration of NPs through the Skin
2.1. The Routes of Penetration
2.2. The Models of Skin Penetration
2.3. Influence of Physical–Chemical Properties of NPs on Skin Penetration Efficiency
2.3.1. Size Effect
2.3.2. Surface Charge Effect
2.3.3. Hydrophobic/Hydrophilic Effect
2.3.4. Shape Effect
2.3.5. Chemical Composition Effect
2.4. Methods to Improve the Penetration of NPs through the Skin
2.4.1. Exposure to UV Radiation
2.4.2. Local Hyperthermia
2.4.3. Iontophoresis
2.4.4. Dermaportation and Sonophoresis
2.4.5. Mechanical Permeation Enhancement
2.4.6. Chemical Permeation Enhancement
2.4.7. Thermal Ablation
3. Methods Applied to Investigate the Skin Penetration by NPs
3.1. Visualization
3.2. Quantification of NPs and Structural Changes in Skin
3.3. NPs Physicochemical Characteristics
4. Nano-Drug Therapy
4.1. The Transdermal Drug Delivery
4.1.1. Skin Cancer Imaging and Targeted Therapy
4.1.2. Immunomodulation and Topical Vaccine Delivery
4.1.3. Gene Therapy
4.2. NPs for Topical Application
4.2.1. NPs as UV Protestant (against Photoaging and Photocarcinogenesis)
4.2.2. Antimicrobials and Wound Healing
4.2.3. Treatment of Psoriasis
4.2.4. Treatment of Vitiligo
4.2.5. Baldness Treatment
5. Nano-Dermocosmetics
5.1. Nanoparticles as Anti-Aging Agents
5.1.1. Lipid NPs
5.1.2. Nanoemulsions
5.1.3. Nanoparticles of Precious Metals, i.e., Pd, Pt, and Au
5.1.4. Cerium Oxide Nanoparticles (CeO2-NPs)
5.1.5. Anti-Aging Polymeric Nanoparticles
6. NPs Protection against Pollution
7. Impact of NPs on the Natural Environment
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dowling, A.; Cliff, R.; Grobert, N.; Hutton, D.; Oliver, R.; O’Neill, O.; Pethica, J.; Pidgeon, N.; Porritt, J.; Ryan, J.; et al. Nanoscience and Nanotechnologies: Opportunities and Uncertainties; The Royal Society & The Royal Academy of Engineering: London, UK, 2004; Volume 44, pp. 7–10. Available online: http://sscottgraham.com/314/redesign4.pdf (accessed on 24 December 2021).
- Potocnik, J. Commission recommendation of 18 October 2011 on the definition of nanomaterial (2011/696/EU). Off. J. Eur. Commun. Legis. 2011, L275, 3840. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef]
- United Nations. Questions About Nanotechnology. 2012. Available online: https://www.epa.gov/chemical-research/research-nanomaterials (accessed on 21 August 2014).
- Federal Drug Administration. Considering Whether an FDA-Regulated Product Involves the Application of Nanotechnology; Federal Drug Administration: Silver Spring, MD, USA, 2011. Available online: https://www.fda.gov/RegulatoryInformation/Guidances/ucm257698.htm (accessed on 25 January 2016).
- ISO/TS 80004-1:2010; Nanotechnology—Vocabulary—Part 1: Core Terms. International Organization for Standardization: Geneva, Switzerland, 2010. Available online: https://www.iso.org/standard/51240.html (accessed on 17 July 2017).
- Menetrez, M.Y.; Foarde, K.K.; Ensor, D.S. An analytical method for the measurement of nonviable bioaerosols. J. Air Waste Manag. Assoc. 2001, 51, 1436–1442. [Google Scholar] [CrossRef]
- Baker, T.S.; Newcomb, W.W.; Olson, N.H.; Cowsert, L.M.; Olson, C.; Brown, J.C. Structures of bovine and human papillomaviruses. Analysis by cryoelectron microscopy and three-dimensional image reconstruction. Biophys. J. 1991, 60, 1445–1456. [Google Scholar] [CrossRef]
- Dubina, M.; Goldenberg, G. Viral-associated nonmelanoma skin cancers: A review. Am. J. Dermatopathol. 2009, 31, 561–573. [Google Scholar] [CrossRef]
- Buzea, C.; Pacheco, I.I.; Robbie, K. Nanomaterials and nanoparticles: Sources and toxicity. Biointerphases 2007, 2, MR17. [Google Scholar] [CrossRef]
- Singh, H.; Du, J.; Singh, P.; Yi, T.H. Role of green silver nanoparticles synthesized from Symphytum officinale leaf extract in protec tion against UVB-induced photoaging. J. Nanostruct. Chem. 2018, 8, 359–368. [Google Scholar] [CrossRef]
- Gong, T.; Xie, J.; Liao, J.; Zhang, T.; Lin, S.; Lin, Y. Nanomaterials and bone regeneration. Bone Res. 2015, 3, 15029. [Google Scholar] [CrossRef]
- Altman, K.W.; Desai, S.C.; Moline, J.; de la Hoz, R.E.; Herbert, R.; Gannon, P.J.; Doty, R.L. Odor identification ability and self-reported upper respiratory symptoms in workers at the post-9/11 World Trade Center site. Int. Arch. Occup. Environ. Health. 2011, 84, 131–137. [Google Scholar] [CrossRef]
- Cone, J.E.; Farfel, M. World Trade Center Health Registry--a model for a nanomaterials exposure registry. J. Occup. Environ. Med. 2011, 53, S48–S51. [Google Scholar] [CrossRef]
- Heiligtag, F.J.; Niederberger, M. The fascinating world of nanoparticle research. Mater. Today 2013, 16, 262–271. [Google Scholar] [CrossRef]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef]
- Misra, S.K.; Mohn, D.; Brunner, T.J.; Stark, W.J.; Philip, S.E.; Roy, I.; Salih, V.; Knowles, J.C.; Boccaccini, A.R. Comparison of nanoscale and microscale bioactive glass on the properties of P(3HB)/Bioglass composites. Biomaterials 2008, 29, 1750–1761. [Google Scholar] [CrossRef]
- Yadwade, R.; Gharpure, S.; Ankamwar, B. Nanotechnology in cosmetics pros and cons. Nano Express 2021, 2, 022003. [Google Scholar] [CrossRef]
- DeLouise, L.A. Applications of nanotechnology in dermatology. J. Investig. Dermatol. 2012, 132, 964–975. [Google Scholar] [CrossRef]
- Zhang, L.; Gu, F.X.; Chan, J.M.; Wang, A.Z.; Langer, R.S.; Farokhzad, O.C. Nanoparticles in medicine: Therapeutic applications and developments. Clin. Pharmacol. Ther. 2008, 83, 761–769. [Google Scholar] [CrossRef]
- Parveen, S.; Misra, R.; Sahoo, S.K. Nanoparticles: A boon to drug delivery, therapeutics, diagnostics and imaging. Nanomedicine 2012, 8, 147–166. [Google Scholar] [CrossRef]
- Huang, H.C.; Barua, S.; Sharma, G.; Dey, S.K.; Rege, K. Inorganic nanoparticles for cancer imaging and therapy. J. Control. Release 2011, 155, 344–357. [Google Scholar] [CrossRef]
- Flores-Rojas, G.G.; López-Saucedo, F.; Vera-Graziano, R.; Mendizabal, E.; Bucio, E. Magnetic Nanoparticles for Medical Applications: Updated Review. Macromol 2022, 2, 374–390. [Google Scholar] [CrossRef]
- Huang, X.; Peng, X.; Wang, Y.; Wang, Y.; Shin, D.M.; El-Sayed, M.A.; Nie, S. A reexamination of active and passive tumor targeting by using rod-shaped gold nanocrystals and covalently conjugated peptide ligands. ACS Nano 2010, 4, 5887–5896. [Google Scholar] [CrossRef]
- Al-Jamal, W.T.; Al-Jamal, K.T.; Tian, B.; Cakebread, A.; Halket, J.M.; Kostarelos, K. Tumor targeting of functionalized quantum dot-liposome hybrids by intravenous administration. Mol. Pharm. 2009, 6, 520–530. [Google Scholar] [CrossRef]
- Debbage, P. Targeted drugs and nanomedicine: Present and future. Curr. Pharm. Des. 2009, 15, 153–172. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Potential of Nanoparticles as Permeation Enhancers and Targeted Delivery Options for Skin: Advantages and Disadvantages. Drug Des. Devel. Ther. 2020, 14, 3271–3289. [Google Scholar] [CrossRef]
- Long, T.C.; Saleh, N.; Tilton, R.D.; Lowry, G.V.; Veronesi, B. Titanium dioxide (P25) produces reactive oxygen species in immortalized brain microglia (BV2): Implications for nanoparticle neurotoxicity. Environ. Sci. Technol. 2006, 40, 4346–4352. [Google Scholar] [CrossRef]
- Jin, C.Y.; Zhu, B.S.; Wang, X.F.; Lu, Q.H. Cytotoxicity of titanium dioxide nanoparticles in mouse fibroblast cells. Chem. Res. Toxicol. 2008, 21, 1871–1877. [Google Scholar] [CrossRef]
- AshaRani, P.V.; Low Kah Mun, G.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 2009, 3, 279–290. [Google Scholar] [CrossRef]
- Xu, A.; Chai, Y.; Nohmi, T.; Hei, T.K. Genotoxic responses to titanium dioxide nanoparticles and fullerene in gpt delta transgenic MEF cells. Part. Fibre Toxicol. 2009, 6, 3. [Google Scholar] [CrossRef]
- Pan, Y.; Neuss, S.; Leifert, A.; Fischler, M.; Wen, F.; Simon, U.; Schmid, G.; Brandau, W.; Jahnen-Dechent, W. Size-dependent cytotoxicity of gold nanoparticles. Small 2007, 3, 1941–1949. [Google Scholar] [CrossRef]
- Cui, H.F.; Vashist, S.K.; Al-Rubeaan, K.; Luong, J.H.; Sheu, F.S. Interfacing carbon nanotubes with living mammalian cells and cytotoxicity issues. Chem. Res. Toxicol. 2010, 23, 1131–1147. [Google Scholar] [CrossRef]
- Pedata, P.; Boccellino, M.; La Porta, R.; Napolitano, M.; Minutolo, P.; Sgro, L.A.; Zei, F.; Sannolo, N.; Quagliuolo, L. Interaction between combustion-generated organic nanoparticles and biological systems: In vitro study of cell toxicity and apoptosis in human keratinocytes. Nanotoxicology 2012, 6, 338–352. [Google Scholar] [CrossRef]
- Gupta, V.; Mohapatra, S.; Mishra, H.; Farooq, U.; Kumar, K.; Ansari, M.J.; Aldawsari, M.F.; Alalaiwe, A.S.; Mirza, M.A.; Iqbal, Z. Nanotechnology in Cosmetics and Cosmeceuticals-A Review of Latest Advancements. Gels 2022, 8, 173. [Google Scholar] [CrossRef]
- Thomas, T.; Thomas, K.; Sadrieh, N.; Savage, N.; Adair, P.; Bronaugh, R. Research strategies for safety evaluation of nanomaterials, part VII: Evaluating consumer exposure to nanoscale materials. Toxicol. Sci. 2006, 91, 14–19. [Google Scholar] [CrossRef]
- Scientific Committee on Emerging and Newly Identified Health Risks SCENIHR, Risk Assessment of Products of Nanotechnologies. 2009. Available online: http://ec.europa.eu/health/ph_risk/committees/04_scenihr/docs/scenihr_o_023.pdf (accessed on 17 July 2017).
- Lee, C.C.; Lin, Y.H.; Hou, W.C.; Li, M.H.; Chang, J.W. Exposure to ZnO/TiO2 Nanoparticles Affects Health Outcomes in Cosmetics Salesclerks. Int. J. Environ. Res. Public Health 2020, 17, 6088. [Google Scholar] [CrossRef]
- Shakeel, M.; Jabeen, F.; Shabbir, S.; Asghar, M.S.; Khan, M.S.; Chaudhry, A.S. Toxicity of Nano-Titanium Dioxide (TiO2-NP) Through Various Routes of Exposure: A Review. Biol. Trace Elem. Res. 2016, 172, 1–36. [Google Scholar] [CrossRef]
- Jansen, R.; Osterwalder, U.; Wang, S.Q.; Burnett, M.; Lim, H.W. Photoprotection: Part II. Sunscreen: Development, efficacy, and controversies. J. Am. Acad. Dermatol. 2013, 69, 867.e1–867.e14. [Google Scholar] [CrossRef]
- Mohd Nordin, U.U.; Ahmad, N.; Salim, N.; Mohd Yusof, N.S. Lipid-based nanoparticles for psoriasis treatment: A review on conventional treatments, recent works, and future prospects. RSC Adv. 2021, 11, 29080–29101. [Google Scholar] [CrossRef]
- Wang, M.; Marepally, S.K.; Vemula, P.K.; Xu, C. Inorganic Nanoparticles for Transdermal Drug Delivery and Topical Application. In Nanoscience in Dermatology; Hamblin, M.R., Avci, P., Prow, T.W., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 57–72. [Google Scholar] [CrossRef]
- Dianzani, C.; Zara, G.P.; Maina, G.; Pettazzoni, P.; Pizzimenti, S.; Rossi, F.; Gigliotti, C.L.; Ciamporcero, E.S.; Daga, M.; Barrera, G. Drug delivery nanoparticles in skin cancers. Biomed. Res. Int. 2014, 2014, 895986. [Google Scholar] [CrossRef]
- Robichaud, C.O.; Uyar, A.E.; Darby, M.R.; Zucker, L.G.; Wiesner, M.R. Estimates of upper bounds and trends in nano-TiO2 production as a basis for exposure assessment. Environ. Sci. Technol. 2009, 43, 4227–4233. [Google Scholar] [CrossRef]
- Vierkötter, A.; Schikowski, T.; Ranft, U.; Sugiri, D.; Matsui, M.; Krämer, U.; Krutmann, J. Airborne particle exposure and extrinsic skin aging. J. Investig. Dermatol. 2010, 130, 2719–2726. [Google Scholar] [CrossRef]
- Patzelt, A.; Richter, H.; Knorr, F.; Schäfer, U.; Lehr, C.M.; Dähne, L.; Sterry, W.; Lademann, J. Selective follicular targeting by modification of the particle sizes. J. Control. Release 2011, 150, 45–48. [Google Scholar] [CrossRef]
- Todo, H.; Kimura, E.; Yasuno, H.; Tokudome, Y.; Hashimoto, F.; Ikarashi, Y.; Sugibayashi, K. Permeation pathway of macromolecules and nanospheres through skin. Biol. Pharm. Bull. 2010, 33, 1394–1399. [Google Scholar] [CrossRef]
- Lademann, J.; Richter, H.; Schanzer, S.; Knorr, F.; Meinke, M.; Sterry, W.; Patzelt, A. Penetration and storage of particles in human skin: Perspectives and safety aspects. Eur. J. Pharm. Biopharm. 2011, 77, 465–468. [Google Scholar] [CrossRef]
- Lademann, J.; Richter, H.; Teichmann, A.; Otberg, N.; Blume-Peytavi, U.; Luengo, J.; Weiss, B.; Schaefer, U.F.; Lehr, C.M.; Wepf, R.; et al. Nanoparticles--an efficient carrier for drug delivery into the hair follicles. Eur. J. Pharm. Biopharm. 2007, 66, 159–164. [Google Scholar] [CrossRef]
- Lademann, J.; Otberg, N.; Richter, H.; Weigmann, H.J.; Lindemann, U.; Schaefer, H.; Sterry, W. Investigation of follicular penetration of topically applied substances. Skin Pharmacol. Appl. Skin Physiol. 2001, 14, S17–S22. [Google Scholar] [CrossRef]
- Prow, T.W.; Grice, J.E.; Lin, L.L.; Faye, R.; Butler, M.; Becker, W.; Wurm, E.M.; Yoong, C.; Robertson, T.A.; Soyer, H.P.; et al. Nanoparticles and microparticles for skin drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 470–491. [Google Scholar] [CrossRef]
- Schneider, M.; Stracke, F.; Hansen, S.; Schaefer, U.F. Nanoparticles and their interactions with the dermal barrier. Dermato-endocrinology 2009, 1, 197–206. [Google Scholar] [CrossRef]
- Schulz, J.; Hohenberg, H.; Pflücker, F.; Gärtner, E.; Will, T.; Pfeiffer, S.; Wepf, R.; Wendel, V.; Gers-Barlag, H.; Wittern, K.P. Distribution of sunscreens on skin. Adv. Drug Deliv. Rev. 2002, 54, S157–S163. [Google Scholar] [CrossRef]
- Filipe, P.; Silva, J.N.; Silva, R.; Cirne de Castro, J.L.; Marques Gomes, M.; Alves, L.C.; Santus, R.; Pinheiro, T. Stratum corneum is an effective barrier to TiO2 and ZnO nanoparticle percutaneous absorption. Skin Pharmacol. Physiol. 2009, 22, 266–275. [Google Scholar] [CrossRef]
- Sadrieh, N.; Wokovich, A.M.; Gopee, N.V.; Zheng, J.; Haines, D.; Parmiter, D.; Siitonen, P.H.; Cozart, C.R.; Patri, A.K.; McNeil, S.E.; et al. Lack of significant dermal penetration of titanium dioxide from sunscreen formulations containing nano- and submicron-size TiO2 particles. Toxicol. Sci. 2010, 115, 156–166. [Google Scholar] [CrossRef]
- Lopez, R.F.; Seto, J.E.; Blankschtein, D.; Langer, R. Enhancing the transdermal delivery of rigid nanoparticles using the simultaneous application of ultrasound and sodium lauryl sulfate. Biomaterials 2011, 32, 933–941. [Google Scholar] [CrossRef]
- Monteiro-Riviere, N.A.; Wiench, K.; Landsiedel, R.; Schulte, S.; Inman, A.O.; Riviere, J.E. Safety evaluation of sunscreen formulations containing titanium dioxide and zinc oxide nanoparticles in UVB sunburned skin: An in vitro and in vivo study. Toxicol. Sci. 2011, 123, 264–280. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.W.; Monteiro-Riviere, N.A. Assessment of quantum dot penetration into intact, tape-stripped, abraded and flexed rat skin. Skin Pharmacol. Physiol. 2008, 21, 166–180. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.W.; Yu, W.W.; Colvin, V.L.; Monteiro-Riviere, N.A. Biological interactions of quantum dot nanoparticles in skin and in human epidermal keratinocytes. Toxicol. Appl. Pharmacol. 2008, 228, 200–211. [Google Scholar] [CrossRef]
- Gopee, N.V.; Roberts, D.W.; Webb, P.; Cozart, C.R.; Siitonen, P.H.; Latendresse, J.R.; Warbitton, A.R.; Yu, W.W.; Colvin, V.L.; Walker, N.J.; et al. Quantitative determination of skin penetration of PEG-coated CdSe quantum dots in dermabraded but not intact SKH-1 hairless mouse skin. Toxicol. Sci. 2009, 111, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Prow, T.W.; Monteiro-Riviere, N.A.; Inman, A.O.; Grice, J.E.; Chen, X.; Zhao, X.; Sanchez, W.H.; Gierden, A.; Kendall, M.A.; Zvyagin, A.V.; et al. Quantum dot penetration into viable human skin. Nanotoxicology 2012, 6, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, L.J.; Oberdörster, G.; Pentland, A.P.; Delouise, L.A. In vivo skin penetration of quantum dot nanoparticles in the murine model: The effect of UVR. Nano Lett. 2008, 8, 2779–2787. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, L.J.; Glazowski, C.E.; Zavislan, J.M.; Delouise, L.A. Near-IR fluorescence and reflectance confocal microscopy for imaging of quantum dots in mammalian skin. Biomed. Opt. Express 2011, 2, 1610–1625. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, S.; Mortensen, L.J.; DeLouise, L.A. Quantification of human skin barrier function and susceptibility to quantum dot skin penetration. Nanotoxicology 2011, 5, 675–686. [Google Scholar] [CrossRef]
- Huang, Y.; Yu, F.; Park, Y.S.; Wang, J.; Shin, M.C.; Chung, H.S.; Yang, V.C. Co-administration of protein drugs with gold nanoparticles to enable percutaneous delivery. Biomaterials 2010, 31, 9086–9091. [Google Scholar] [CrossRef]
- Sonavane, G.; Tomoda, K.; Sano, A.; Ohshima, H.; Terada, H.; Makino, K. In vitro permeation of gold nanoparticles through rat skin and rat intestine: Effect of particle size. Colloids Surf. B Biointerfaces 2008, 65, 1–10. [Google Scholar] [CrossRef]
- Bronaugh, R.L.; Stewart, R.F.; Congdon, E.R. Methods for in vitro percutaneous absorption studies. II. Animal models for human skin. Toxicol. Appl. Pharmacol. 1982, 62, 481–488. [Google Scholar] [CrossRef]
- Otberg, N.; Richter, H.; Schaefer, H.; Blume-Peytavi, U.; Sterry, W.; Lademann, J. Variations of hair follicle size and distribution in different body sites. J. Investig. Dermatol. 2004, 122, 14–19. [Google Scholar] [CrossRef]
- Labouta, H.I.; Schneider, M. Interaction of inorganic nanoparticles with the skin barrier: Current status and critical review. Nanomedicine 2013, 9, 39–54. [Google Scholar] [CrossRef]
- Izquierdo, M.C.; Lillo, C.R.; Bucci, P.; Gómez, G.E.; Martínez, L.; Alonso, S.D.V.; Calienni, M.N.; Montanari, J. Comparative skin penetration profiles of formulations including ultradeformable liposomes as potential nanocosmeceutical carriers. J. Cosmet. Dermatol. 2020, 19, 3127–3137. [Google Scholar] [CrossRef]
- Caussin, J.; Gooris, G.S.; Janssens, M.; Bouwstra, J.A. Lipid organization in human and porcine stratum corneum differs widely, while lipid mixtures with porcine ceramides model human stratum corneum lipid organization very closely. Biochim. Biophys. Acta 2008, 1778, 1472–1482. [Google Scholar] [CrossRef]
- Jacobi, U.; Kaiser, M.; Toll, R.; Mangelsdorf, S.; Audring, H.; Otberg, N.; Sterry, W.; Lademann, J. Porcine ear skin: An in vitro model for human skin. Skin Res. Technol. 2007, 13, 19–24. [Google Scholar] [CrossRef]
- Senzui, M.; Tamura, T.; Miura, K.; Ikarashi, Y.; Watanabe, Y.; Fujii, M. Study on penetration of titanium dioxide (TiO(2)) nanoparticles into intact and damaged skin in vitro. J. Toxicol. Sci. 2010, 35, 107–113. [Google Scholar] [CrossRef]
- Labouta, H.I.; el-Khordagui, L.K.; Kraus, T.; Schneider, M. Mechanism and determinants of nanoparticle penetration through human skin. Nanoscale 2011, 3, 4989–4999. [Google Scholar] [CrossRef]
- Larese Filon, F.; Mauro, M.; Adami, G.; Bovenzi, M.; Crosera, M. Nanoparticles skin absorption: New aspects for a safety profile evaluation. Regul. Toxicol. Pharmacol. 2015, 72, 310–322. [Google Scholar] [CrossRef]
- Labouta, H.I.; Liu, D.C.; Lin, L.L.; Butler, M.K.; Grice, J.E.; Raphael, A.P.; Kraus, T.; El-Khordagui, L.K.; Soyer, H.P.; Roberts, M.S.; et al. Gold nanoparticle penetration and reduced metabolism in human skin by toluene. Pharm. Res. 2011, 28, 2931–2944. [Google Scholar] [CrossRef]
- Barbero, A.M.; Frasch, H.F. Effect of Frozen Human Epidermis Storage Duration and Cryoprotectant on Barrier Function Using Two Model Compounds. Skin Pharmacol. Physiol. 2016, 29, 31–40. [Google Scholar] [CrossRef]
- Abd, E.; Yousef, S.A.; Pastore, M.N.; Telaprolu, K.; Mohammed, Y.H.; Namjoshi, S.; Grice, J.E.; Roberts, M.S. Skin models for the testing of transdermal drugs. Clin. Pharmacol. 2016, 8, 163–176. [Google Scholar] [CrossRef]
- Lauterbach, A.; Müller-Goymann, C.C. Applications and limitations of lipid nanoparticles in dermal and transdermal drug delivery via the follicular route. Eur. J. Pharm. Biopharm. 2015, 97, 152–163. [Google Scholar] [CrossRef]
- Krugluger, W.; Rohrbacher, W.; Laciak, K.; Moser, K.; Moser, C.; Hugeneck, J. Reorganization of hair follicles in human skin organ culture induced by cultured human follicle-derived cells. Exp. Dermatol. 2005, 14, 580–585. [Google Scholar] [CrossRef]
- Hewitt, N.J.; Grégoire, S.; Cubberley, R.; Duplan, H.; Eilstein, J.; Ellison, C.; Lester, C.; Fabian, E.; Fernandez, J.; Géniès, C.; et al. Measurement of the penetration of 56 cosmetic relevant chemicals into and through human skin using a standardized protocol. J. Appl. Toxicol. 2020, 40, 403–415. [Google Scholar] [CrossRef]
- Veryser, L.; Boonen, J.; Taevernier, L.; Guillaume, J.; Risseeuw, M.; Shah, S.N.; Roche, N.; Van Calenbergh, S.; De Spiegeleer, B. The influence of the acyl chain on the transdermal penetration-enhancing effect of synthetic phytoceramides. Skin Pharmacol. Physiol. 2015, 28, 124–136. [Google Scholar] [CrossRef]
- Esposto Biondo, N.; Fretes Argenta, D.; Schneider Rauber, G.; Caon, T. How to define the experimental conditions of skin permeation assays for drugs presenting biopharmaceutical limitations? The experience with testosterone. Int. J. Pharm. 2021, 607, 120987. [Google Scholar] [CrossRef]
- Van de Sandt, J.J.; van Burgsteden, J.A.; Cage, S.; Carmichael, P.L.; Dick, I.; Kenyon, S.; Korinth, G.; Larese, F.; Limasset, J.C.; Maas, W.J.; et al. In vitro predictions of skin absorption of caffeine, testosterone, and benzoic acid: A multi-centre comparison study. Regul. Toxicol. Pharmacol. 2004, 39, 271–281. [Google Scholar] [CrossRef]
- Organisation for Economic Co-operation and Development. OECD Guidelines for the Testing of Chemicals, Section 4. Test No. 428: Skin Absorption: In Vitro Method. Available online: http://www.oecd-ilibrary.org/environment/test-no-428-skin-absorptionin-vitro-method_9789264071087-en (accessed on 23 November 2004).
- SCCS. Basic Criteria for the In Vitro Assessment of Dermal Absorption of Cosmetic Ingredients; SCCS: Brussels, Belgium, 2010; pp. 1–14. Available online: https://op.europa.eu/en/publication-detail/-/publication/91793089-8206-4975-a6c9-078770655851 (accessed on 22 June 2010).
- Schreiber, S.; Mahmoud, A.; Vuia, A.; Rübbelke, M.K.; Schmidt, E.; Schaller, M.; Kandárová, H.; Haberland, A.; Schäfer, U.F.; Bock, U.; et al. Reconstructed epidermis versus human and animal skin in skin absorption studies. Toxicol. Vitr. 2005, 19, 813–822. [Google Scholar] [CrossRef]
- Simard, M.; Julien, P.; Fradette, J.; Pouliot, R. Modulation of the Lipid Profile of Reconstructed Skin Substitutes after Essential Fatty Acid Supplementation Affects Testosterone Permeability. Cells 2019, 8, 1142. [Google Scholar] [CrossRef]
- Bouwstra, J.A.; Helder, R.W.J.; El Ghalbzouri, A. Human skin equivalents: Impaired barrier function in relation to the lipid and protein properties of the stratum corneum. Adv. Drug Deliv. Rev. 2021, 175, 113802. [Google Scholar] [CrossRef] [PubMed]
- Colvin, V.L. The potential environmental impact of engineered nanomaterials. Nat. Biotechnol. 2003, 21, 1166–1170. [Google Scholar] [CrossRef] [PubMed]
- Gwinn, M.R.; Vallyathan, V. Nanoparticles: Health effects--pros and cons. Environ. Health Perspect. 2006, 114, 1818–1825. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, J.S.; Maynard, A.D.; Howard, P.C.; James, J.T.; Lam, C.W.; Warheit, D.B.; Santamaria, A.B. Research strategies for safety evaluation of nanomaterials, part IV: Risk assessment of nanoparticles. Toxicol. Sci. 2006, 89, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Nohynek, G.J.; Lademann, J.; Ribaud, C.; Roberts, M.S. Grey goo on the skin? Nanotechnology, cosmetic and sunscreen safety. Crit. Rev. Toxicol. 2007, 37, 251–277. [Google Scholar] [CrossRef]
- Stern, S.T.; McNeil, S.E. Nanotechnology safety concerns revisited. Toxicol. Sci. 2008, 101, 4–21. [Google Scholar] [CrossRef]
- Adiseshaiah, P.P.; Hall, J.B.; McNeil, S.E. Nanomaterial standards for efficacy and toxicity assessment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010, 2, 99–112. [Google Scholar] [CrossRef]
- Baroli, B. Penetration of nanoparticles and nanomaterials in the skin: Fiction or reality? J. Pharm Sci. 2010, 99, 21–50. [Google Scholar] [CrossRef]
- Smijs, T.G.; Bouwstra, J.A. Focus on skin as a possible port of entry for solid nanoparticles and the toxicological impact. J. Biomed. Nanotechnol. 2010, 6, 469–484. [Google Scholar] [CrossRef]
- Burnett, M.E.; Wang, S.Q. Current sunscreen controversies: A critical review. Photodermatol. Photoimmunol. Photomed. 2011, 27, 58–67. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Azadi, A.; Daneshamouz, S.; Heidari, R.; Azarpira, N.; Mohammadi-Samani, S. Cyproterone acetate-loaded nanostructured lipid carriers: Effect of particle size on skin penetration and follicular targeting. Pharm. Dev. Technol. 2019, 24, 812–823. [Google Scholar] [CrossRef] [PubMed]
- Patzelt, A.; Mak, W.C.; Jung, S.; Knorr, F.; Meinke, M.C.; Richter, H.; Rühl, E.; Cheung, K.Y.; Tran, N.B.N.N.; Lademann, J. Do nanoparticles have a future in dermal drug delivery? J. Control. Release. 2017, 246, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Monteiro-Riviere, N.A. Structure and function of skin. In Toxicology of the Skin—Target Organ Series; Monteiro-Riviere, N.A., Ed.; Informa Healthcare: New York, NY, USA, 2010; pp. 1–18. [Google Scholar]
- Beck, R.; Guterres, S.; Pohlmann, A. Nanocosmetics and Nanomedicines: New Approaches for Skin Care; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Baroli, B.; Ennas, M.G.; Loffredo, F.; Isola, M.; Pinna, R.; López-Quintela, M.A. Penetration of metallic nanoparticles in human full-thickness skin. J. Investig. Dermatol. 2007, 127, 1701–1712. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R. Overcoming the stratum corneum barrier: A nano approach. Drug Deliv. Transl. Res. 2013, 3, 205–208. [Google Scholar] [CrossRef]
- Gamer, A.O.; Leibold, E.; van Ravenzwaay, B. The in vitro absorption of microfine zinc oxide and titanium dioxide through porcine skin. Toxicol. Vitr. 2006, 20, 301–307. [Google Scholar] [CrossRef]
- Rancan, F.; Vogt, A. Getting under the skin: What is the potential of the transfollicular route in drug delivery? Ther. Deliv. 2014, 5, 875–877. [Google Scholar] [CrossRef]
- Mardhiah Adib, Z.; Ghanbarzadeh, S.; Kouhsoltani, M.; Yari Khosroshahi, A.; Hamishehkar, H. The Effect of Particle Size on the Deposition of Solid Lipid Nanoparticles in Different Skin Layers: A Histological Study. Adv. Pharm. Bull. 2016, 6, 31–36. [Google Scholar] [CrossRef]
- Jenning, V.; Gysler, A.; Schäfer-Korting, M.; Gohla, S.H. Vitamin A loaded solid lipid nanoparticles for topical use: Occlusive properties and drug targeting to the upper skin. Eur. J. Pharm. Biopharm. 2000, 49, 211–218. [Google Scholar] [CrossRef]
- Yang, Y.; Sunoqrot, S.; Stowell, C.; Ji, J.; Lee, C.W.; Kim, J.W.; Khan, S.A.; Hong, S. Effect of size, surface charge, and hydrophobicity of poly(amidoamine) dendrimers on their skin penetration. Biomacromolecules 2012, 13, 2154–2162. [Google Scholar] [CrossRef]
- Takeuchi, I.; Suzuki, T.; Makino, K. Skin permeability and transdermal delivery route of 50-nm indomethacin-loaded PLGA nanoparticles. Colloids Surf. B Biointerfaces 2017, 159, 312–317. [Google Scholar] [CrossRef]
- Takeuchi, I.; Kagawa, A.; Makino, K. Skin permeability and transdermal delivery route of 30-nm cyclosporin A-loaded nanoparticles using PLGA-PEG-PLGA triblock copolymer. Colloids Surf. A Physicochem. Eng. Asp. 2020, 600, 124866. [Google Scholar] [CrossRef]
- Shanmugam, S.; Song, C.K.; Nagayya-Sriraman, S.; Baskaran, R.; Yong, C.S.; Choi, H.G.; Kim, D.D.; Woo, J.S.; Yoo, B.K. Physicochemical characterization and skin permeation of liposome formulations containing clindamycin phosphate. Arch. Pharm. Res. 2009, 32, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.; Smyth, N.R.; Muskens, O.L.; Nitti, S.; Heuer-Jungemann, A.; Ardern-Jones, M.R.; Kanaras, A.G. Interactions of skin with gold nanoparticles of different surface charge, shape, and functionality. Small 2015, 11, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Talarska, P.; Boruczkowski, M.; Żurawski, J. Current Knowledge of Silver and Gold Nanoparticles in Laboratory Research-Application, Toxicity, Cellular Uptake. Nanomaterials 2021, 11, 2454. [Google Scholar] [CrossRef]
- Ryman-Rasmussen, J.P.; Riviere, J.E.; Monteiro-Riviere, N.A. Penetration of intact skin by quantum dots with diverse physicochemical properties. Toxicol. Sci. 2006, 91, 159–165. [Google Scholar] [CrossRef]
- Baspinar, Y.; Borchert, H.H. Penetration and release studies of positively and negatively charged nanoemulsions--is there a benefit of the positive charge? Int. J. Pharm. 2012, 430, 247–252. [Google Scholar] [CrossRef]
- Luesakul, U.; Puthong, S.; Sansanaphongpricha, K.; Muangsin, N. Quaternized chitosan-coated nanoemulsions: A novel platform for improving the stability, anti-inflammatory, anti-cancer and transdermal properties of Plai extract. Carbohydr. Polym. 2020, 230, 115625. [Google Scholar] [CrossRef]
- Shaker, D.S.; Ishak, R.A.H.; Ghoneim, A.; Elhuoni, M.A. Nanoemulsion: A review on mechanisms for the transdermal delivery of hydrophobic and hydrophilic drugs. Sci. Pharm. 2019, 87, 17. [Google Scholar] [CrossRef]
- Wu, X.; Landfester, K.; Musyanovych, A.; Guy, R.H. Disposition of charged nanoparticles after their topical application to the skin. Skin Pharmacol. Physiol. 2010, 23, 117–123. [Google Scholar] [CrossRef]
- Gillet, A.; Compère, P.; Lecomte, F.; Hubert, P.; Ducat, E.; Evrard, B.; Piel, G. Liposome surface charge influence on skin penetration behaviour. Int. J. Pharm. 2011, 411, 223–231. [Google Scholar] [CrossRef]
- Lee, O.; Jeong, S.H.; Shin, W.U.; Lee, G.; Oh, C.; Son, S.W. Influence of surface charge of gold nanorods on skin penetration. Skin Res. Technol. 2013, 19, e390–e396. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Han, G.; Toley, B.J.; Kim, C.K.; Rotello, V.M.; Forbes, N.S. Tuning payload delivery in tumour cylindroids using gold nanoparticles. Nat. Nanotechnol. 2010, 5, 465–472. [Google Scholar] [CrossRef]
- Sinico, C.; Manconi, M.; Peppi, M.; Lai, F.; Valenti, D.; Fadda, A.M. Liposomes as carriers for dermal delivery of tretinoin: In vitro evaluation of drug permeation and vesicle-skin interaction. J. Control. Release 2005, 103, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Farkas, J.; Peter, H.; Christian, P.; Gallego Urrea, J.A.; Hassellöv, M.; Tuoriniemi, J.; Gustafsson, S.; Olsson, E.; Hylland, K.; Thomas, K.V. Characterization of the effluent from a nanosilver producing washing machine. Environ. Int. 2011, 37, 1057–1062. [Google Scholar] [CrossRef] [PubMed]
- Iannuccelli, V.; Bertelli, D.; Romagnoli, M.; Scalia, S.; Maretti, E.; Sacchetti, F.; Leo, E. In vivo penetration of bare and lipid-coated silica nanoparticles across the human stratum corneum. Colloids Surf. B Biointerfaces 2014, 122, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Teng, X.; Chen, D.; Tang, F.; He, J. The effect of the shape of mesoporous silica nanoparticles on cellular uptake and cell function. Biomaterials 2010, 31, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Liao, J.; Shao, X.; Li, Q.; Lin, Y. The Effect of shape on Cellular Uptake of Gold Nanoparticles in the forms of Stars, Rods, and Triangles. Sci. Rep. 2017, 7, 3827. [Google Scholar] [CrossRef]
- Wang, W.; Gaus, K.; Tilley, R.D.; Gooding, J.J. The impact of nanoparticle shape on cellular internalisation and transport: What do the different analysis methods tell us? Mater. Horiz. 2019, 6, 1538–1547. [Google Scholar] [CrossRef]
- Tak, Y.K.; Pal, S.; Naoghare, P.K.; Rangasamy, S.; Song, J.M. Shape-dependent skin penetration of silver nanoparticles: Does it really matter? Sci. Rep. 2015, 5, 16908. [Google Scholar] [CrossRef]
- Costa, R.; Santos, L. Delivery systems for cosmetics—From manufacturing to the skin of natural antioxidants. Powder Technol. 2017, 322, 402–416. [Google Scholar] [CrossRef]
- Chantaburanan, T.; Teeranachaideekul, V.; Chantasart, D.; Jintapattanakit, A.; Junyaprasert, V.B. Effect of binary solid lipid matrix of wax and triglyceride on lipid crystallinity, drug-lipid interaction and drug release of ibuprofen-loaded solid lipid nanoparticles (SLN) for dermal delivery. J. Colloid Interface Sci. 2017, 504, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Lombardi Borgia, S.; Regehly, M.; Sivaramakrishnan, R.; Mehnert, W.; Korting, H.C.; Danker, K.; Röder, B.; Kramer, K.D.; Schäfer-Korting, M. Lipid nanoparticles for skin penetration enhancement-correlation to drug localization within the particle matrix as determined by fluorescence and parelectric spectroscopy. J. Control. Release 2005, 110, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Arora, R.; Katiyar, S.S.; Kushwah, V.; Jain, S. Solid lipid nanoparticles and nanostructured lipid carrier-based nanotherapeutics in treatment of psoriasis: A comparative study. Expert Opin. Drug Deliv. 2017, 14, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Pokharkar, V.B.; Mendiratta, C.; Kyadarkunte, A.Y.; Bhosale, S.H.; Barhate, G.A. Skin delivery aspects of benzoyl peroxide-loaded solid lipid nanoparticles for acne treatment. Ther. Deliv. 2014, 5, 635–652. [Google Scholar] [CrossRef]
- Garcês, A.; Amaral, M.H.; Sousa Lobo, J.M.; Silva, A.C. Formulations based on solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for cutaneous use: A review. Eur. J. Pharm. Sci. 2018, 112, 159–167. [Google Scholar] [CrossRef]
- Chen, J.; Wei, N.; Lopez-Garcia, M.; Ambrose, D.; Lee, J.; Annelin, C.; Peterson, T. Development and evaluation of resveratrol, Vitamin E, and epigallocatechin gallate loaded lipid nanoparticles for skin care applications. Eur. J. Pharm. Biopharm. 2017, 117, 286–291. [Google Scholar] [CrossRef]
- Kelidari, H.R.; Saeedi, M.; Akbari, J.; Morteza-Semnani, K.; Gill, P.; Valizadeh, H.; Nokhodchi, A. Formulation optimization and in vitro skin penetration of spironolactone loaded solid lipid nanoparticles. Colloids Surf. B Biointerfaces 2015, 128, 473–479. [Google Scholar] [CrossRef]
- Schwarz, J.C.; Baisaeng, N.; Hoppel, M.; Löw, M.; Keck, C.M.; Valenta, C. Ultra-small NLC for improved dermal delivery of coenyzme Q10. Int. J. Pharm. 2013, 447, 213–217. [Google Scholar] [CrossRef]
- Pivetta, T.P.; Simões, S.; Araújo, M.M.; Carvalho, T.; Arruda, C.; Marcato, P.D. Development of nanoparticles from natural lipids for topical delivery of thymol: Investigation of its anti-inflammatory properties. Colloids Surf. B Biointerfaces 2018, 164, 281–290. [Google Scholar] [CrossRef]
- Shah, S.M.; Ashtikar, M.; Jain, A.S.; Makhija, D.T.; Nikam, Y.; Gude, R.P.; Steiniger, F.; Jagtap, A.A.; Nagarsenker, M.S.; Fahr, A. LeciPlex, invasomes, and liposomes: A skin penetration study. Int. J. Pharm. 2015, 490, 391–403. [Google Scholar] [CrossRef]
- El Maghraby, G.M.; Williams, A.C.; Barry, B.W. Can drug-bearing liposomes penetrate intact skin? J. Pharm. Pharmacol. 2006, 58, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Som, I.; Bhatia, K.; Yasir, M. Status of surfactants as penetration enhancers in transdermal drug delivery. J. Pharm. Bioallied Sci. 2012, 4, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Ternullo, S.; de Weerd, L.; Holsæter, A.M.; Flaten, G.E.; Škalko-Basnet, N. Going skin deep: A direct comparison of penetration potential of lipid-based nanovesicles on the isolated perfused human skin flap model. Eur. J. Pharm. Biopharm. 2017, 121, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Cristiano, M.C.; Froiio, F.; Spaccapelo, R.; Mancuso, A.; Nisticò, S.P.; Udongo, B.P.; Fresta, M.; Paolino, D. Sulforaphane-Loaded Ultradeformable Vesicles as A Potential Natural Nanomedicine for the Treatment of Skin Cancer Diseases. Pharmaceutics 2019, 12, 6. [Google Scholar] [CrossRef] [PubMed]
- Di Francesco, M.; Primavera, R.; Fiorito, S.; Cristiano, M.C.; Taddeo, V.A.; Epifano, F.; Di Marzio, L.; Genovese, S.; Celia, C. Acronychiabaueri Analogue Derivative-Loaded Ultradeformable Vesicles: Physicochemical Characterization and Potential Applications. Planta Med. 2017, 83, 482–491. [Google Scholar] [CrossRef]
- Paolino, D.; Celia, C.; Trapasso, E.; Cilurzo, F.; Fresta, M. Paclitaxel-loaded ethosomes®: Potential treatment of squamous cell carcinoma, a malignant transformation of actinic keratoses. Eur. J. Pharm. Biopharm. 2012, 81, 102–112. [Google Scholar] [CrossRef]
- Celia, C.; Cilurzo, F.; Trapasso, E.; Cosco, D.; Fresta, M.; Paolino, D. Ethosomes® and transfersomes® containing linoleic acid: Physicochemical and technological features of topical drug delivery carriers for the potential treatment of melasma disorders. Biomed. Microdev. 2012, 14, 119–130. [Google Scholar] [CrossRef]
- Amoabediny, G.; Haghiralsadat, F.; Naderinezhad, S.; Helder, M.N.; Akhoundi Kharanaghi, E.; Mohammadnejad Arough, J.; Zandieh-Doulabi, B. Overview of preparation methods of polymeric and lipid-based (niosome, solid lipid, liposome) nanoparticles: A comprehensive review. Int. J. Polym. Mater. 2018, 67, 383–400. [Google Scholar] [CrossRef]
- Muzzalupo, R.; Pérez, L.; Pinazo, A.; Tavano, L. Pharmaceutical versatility of cationic niosomes derived from amino acid-based surfactants: Skin penetration behavior and controlled drug release. Int. J. Pharm. 2017, 529, 245–252. [Google Scholar] [CrossRef]
- Tavano, L.; Picci, N.; Ioele, G.; Muzzalupo, R. Tetracycline-niosomes versus tetracycline hydrochlo-ride-niosomes: How to modulate encapsulation and percutaneous permeation properties. J. Drug. 2017, 1, 1–6. [Google Scholar] [CrossRef]
- Eid, R.K.; Essa, E.A.; El Maghraby, G.M. Essential oils in niosomes for enhanced transdermal delivery of felodipine. Pharm. Dev. Technol. 2019, 24, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Bragagni, M.; Scozzafava, A.; Mastrolorenzo, A.; Supuran, C.T.; Mura, P. Development and ex vivo evaluation of 5-aminolevulinic acid-loaded niosomal formulations for topical photodynamic therapy. Int. J. Pharm. 2015, 494, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Junyaprasert, V.B.; Singhsa, P.; Suksiriworapong, J.; Chantasart, D. Physicochemical properties and skin permeation of Span 60/Tween 60 niosomes of ellagic acid. Int. J. Pharm. 2012, 423, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.J.; Maibach, H.I. Liposomes and niosomes as topical drug delivery systems. Skin Pharmacol. Physiol. 2005, 18, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.; Sharma, O.P.; Mehta, T. Nanocrystal: A novel approach to overcome skin barriers for improved topical drug delivery. Expert Opin. Drug Deliv. 2018, 15, 351–368. [Google Scholar] [CrossRef] [PubMed]
- Döge, N.; Hönzke, S.; Schumacher, F.; Balzus, B.; Colombo, M.; Hadam, S.; Rancan, F.; Blume-Peytavi, U.; Schäfer-Korting, M.; Schindler, A.; et al. Ethyl cellulose nanocarriers and nanocrystals differentially deliver dexamethasone into intact, tape-stripped or sodium lauryl sulfate-exposed ex vivo human skin—assessment by intradermal microdialysis and extraction from the different skin layers. J. Control. Release 2016, 242, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Mitri, K.; Shegokar, R.; Gohla, S.; Anselmi, C.; Müller, R.H. Lutein nanocrystals as antioxidant formulation for oral and dermal delivery. Int. J. Pharm. 2011, 420, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Pyo, S.M.; Hespeler, D.; Keck, C.M.; Müller, R.H. Dermal miconazole nitrate nanocrystals—formulation development, increased antifungal efficacy & skin penetration. Int. J. Pharm. 2017, 531, 350–359. [Google Scholar] [CrossRef]
- Guterres, S.S.; Alves, M.P.; Pohlmann, A.R. Polymeric nanoparticles, nanospheres and nanocapsules, for cutaneous applications. Drug Target Insights 2007, 2, 147–157. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Hydrogels as drug delivery systems; pros and cons. Trends Pharm. Sci. 2019, 5, 7–24. [Google Scholar] [CrossRef]
- Chellappan, D.K.; Yee, N.J.; Kaur Ambar Jeet Singh, B.J.; Panneerselvam, J.; Madheswaran, T.; Chellian, J.; Satija, S.; Mehta, M.; Gulati, M.; Gupta, G.; et al. Formulation and characterization of glibenclamide and quercetin-loaded chitosan nanogels targeting skin permeation. Ther. Deliv. 2019, 10, 281–293. [Google Scholar] [CrossRef]
- Dave, K.; Krishna Venuganti, V.V. Dendritic polymers for dermal drug delivery. Ther. Deliv. 2017, 8, 1077–1096. [Google Scholar] [CrossRef] [PubMed]
- Sheihet, L.; Chandra, P.; Batheja, P.; Devore, D.; Kohn, J.; Michniak, B. Tyrosine-derived nanospheres for enhanced topical skin penetration. Int. J. Pharm. 2008, 350, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Batheja, P.; Sheihet, L.; Kohn, J.; Singer, A.J.; Michniak-Kohn, B. Topical drug delivery by a polymeric nanosphere gel: Formulation optimization and in vitro and in vivo skin distribution studies. J. Control. Release 2011, 149, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Rai, B. Effect of Size and Surface Charge of Gold Nanoparticles on their Skin Permeability: A Molecular Dynamics Study. Sci. Rep. 2017, 7, 45292. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Sugihara, F.; Matsushita, H.; Yoshioka, Y.; Mizukami, S.; Kikuchi, K. Mesoporous silica nanoparticles for 19F magnetic resonance imaging, fluorescence imaging, and drug delivery. Chem. Sci. 2015, 6, 1986–1990. [Google Scholar] [CrossRef] [PubMed]
- Rancan, F.; Gao, Q.; Graf, C.; Troppens, S.; Hadam, S.; Hackbarth, S.; Kembuan, C.; Blume-Peytavi, U.; Rühl, E.; Lademann, J.; et al. Skin penetration and cellular uptake of amorphous silica nanoparticles with variable size, surface functionalization, and colloidal stability. ACS Nano 2012, 6, 6829–6842. [Google Scholar] [CrossRef]
- Park, Y.H.; Bae, H.C.; Jang, Y.; Jeong, S.H.; Lee, H.N.; Ryu, W.I.; Yoo, M.G.; Kim, Y.R.; Kim, M.K.; Lee, J.K.; et al. Effect of the size and surface charge of silica nanoparticles on cutaneous toxicity. Mol. Cell. Toxicol. 2013, 9, 67–74. [Google Scholar] [CrossRef]
- Hirai, T.; Yoshikawa, T.; Nabeshi, H.; Yoshida, T.; Tochigi, S.; Ichihashi, K.; Uji, M.; Akase, T.; Nagano, K.; Abe, Y.; et al. Amorphous silica nanoparticles size-dependently aggravate atopic dermatitis-like skin lesions following an intradermal injection. Part Fibre Toxicol. 2012, 9, 3. [Google Scholar] [CrossRef]
- Ghosh, P.; Han, G.; De, M.; Kim, C.K.; Rotello, V.M. Gold nanoparticles in delivery applications. Adv. Drug Deliv. Rev. 2008, 60, 1307–1315. [Google Scholar] [CrossRef]
- Pissuwan, D.; Nose, K.; Kurihara, R.; Kaneko, K.; Tahara, Y.; Kamiya, N.; Goto, M.; Katayama, Y.; Niidome, T. A solid-in-oil dispersion of gold nanorods can enhance transdermal protein delivery and skin vaccination. Small 2011, 7, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.W.; Monteiro-Riviere, N.A. Mechanisms of quantum dot nanoparticle cellular uptake. Toxicol. Sci. 2009, 110, 138–155. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, L.; Zheng, H.; Faulknor, R.; De Benedetto, A.; Beck, L.; DeLouise, L.A. Increased in vivo skin penetration of quantum dots with UVR and in vitro quantum dot cytotoxicity. Colloid. Quantum Dots Biomed. Appl. IV 2009, 7189, 234–245. [Google Scholar]
- Joshi, N.; Duhan, V.; Lingwal, N.; Bhaskar, S.; Upadhyay, P. Adjuvant properties of thermal component of hyperthermia enhanced transdermal immunization: Effect on dendritic cells. PLoS ONE 2012, 7, e32067. [Google Scholar] [CrossRef]
- Upadhyay, P. Enhanced transdermal-immunization with diptheria-toxoid using local hyperthermia. Vaccine 2006, 24, 5593–5598. [Google Scholar] [CrossRef]
- Alkilani, A.Z.; Nasereddin, J.; Hamed, R.; Nimrawi, S.; Hussein, G.; Abo-Zour, H.; Donnelly, R.F. Beneath the Skin: A Review of Current Trends and Future Prospects of Transdermal Drug Delivery Systems. Pharmaceutics 2022, 14, 1152. [Google Scholar] [CrossRef]
- Rodríguez-Cruz, I.M.; Merino, V.; Merino, M.; Díez, O.; Nácher, A.; Quintanar-Guerrero, D. Polymeric nanospheres as strategy to increase the amount of triclosan retained in the skin: Passive diffusion vs. iontophoresis. J. Microencapsul. 2013, 30, 72–80. [Google Scholar] [CrossRef]
- Balzus, B.; Sahle, F.F.; Hönzke, S.; Gerecke, C.; Schumacher, F.; Hedtrich, S.; Kleuser, B.; Bodmeier, R. Formulation and ex vivo evaluation of polymeric nanoparticles for controlled delivery of corticosteroids to the skin and the corneal epithelium. Eur. J. Pharm. Biopharm. 2017, 115, 122–130. [Google Scholar] [CrossRef]
- Chen, H.Y.; Zhao, Q.; Su, K.L.; Lin, Y.-C. Development of transdermal delivery chip system: Deliver gold nanoparticles into human stratum corneume. In Proceedings of the 3rd IEEE International Conference on Nano/Micro Engineered and Molecular Systems, NEMS 2008, Sanya, China, 6–9 January 2008; pp. 996–999. [Google Scholar] [CrossRef]
- Krishnan, G.; Edwards, J.; Chen, Y.; Benson, H.A. Enhanced skin permeation of naltrexone by pulsed electromagnetic fields in human skin in vitro. J. Pharm. Sci. 2010, 99, 2724–2731. [Google Scholar] [CrossRef]
- Seto, J.E.; Polat, B.E.; Lopez, R.F.; Blankschtein, D.; Langer, R. Effects of ultrasound and sodium lauryl sulfate on the transdermal delivery of hydrophilic permeants: Comparative in vitro studies with full-thickness and split-thickness pig and human skin. J. Control. Release 2010, 145, 26–32. [Google Scholar] [CrossRef]
- Gulson, B.; McCall, M.; Korsch, M.; Gomez, L.; Casey, P.; Oytam, Y.; Taylor, A.; McCulloch, M.; Trotter, J.; Kinsley, L.; et al. Small amounts of zinc from zinc oxide particles in sunscreens applied outdoors are absorbed through human skin. Toxicol. Sci. 2010, 118, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Lademann, J.; Patzelt, A.; Richter, H.; Antoniou, C.; Sterry, W.; Knorr, F. Determination of the cuticula thickness of human and porcine hairs and their potential influence on the penetration of nanoparticles into the hair follicles. J. Biomed. Opt. 2009, 14, 021014. [Google Scholar] [CrossRef] [PubMed]
- Gratieri, T.; Schaefer, U.F.; Jing, L.; Gao, M.; Kostka, K.H.; Lopez, R.F.; Schneider, M. Penetration of quantum dot particles through human skin. J. Biomed. Nanotechnol. 2010, 6, 586–595. [Google Scholar] [CrossRef]
- Lindemann, U.; Wilken, K.; Weigmann, H.J.; Schaefer, H.; Sterry, W.; Lademann, J. Quantification of the horny layer using tape stripping and microscopic techniques. J. Biomed. Opt. 2003, 8, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Polat, B.E.; Hart, D.; Langer, R.; Blankschtein, D. Ultrasound-mediated transdermal drug delivery: Mechanisms, scope, and emerging trends. J. Control. Release 2011, 152, 330–348. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Jarrahian, C.; Zehrung, D.; Mitragotri, S.; Prausnitz, M.R. Delivery systems for intradermal vaccination. Curr. Top. Microbiol. Immunol. 2012, 351, 77–112. [Google Scholar] [CrossRef] [PubMed]
- Gill, H.S.; Andrews, S.N.; Sakthivel, S.K.; Fedanov, A.; Williams, I.R.; Garber, D.A.; Priddy, F.H.; Yellin, S.; Feinberg, M.B.; Staprans, S.I.; et al. Selective removal of stratum corneum by microdermabrasion to increase skin permeability. Eur. J. Pharm. Sci. 2009, 38, 95–103. [Google Scholar] [CrossRef]
- Tsai, J.C.; Shen, L.C.; Sheu, H.M.; Lu, C.C. Tape stripping and sodium dodecyl sulfate treatment increase the molecular weight cutoff of polyethylene glycol penetration across murine skin. Arch. Dermatol. Res. 2003, 295, 169–174. [Google Scholar] [CrossRef]
- Jeong, S.H.; Kim, J.H.; Yi, S.M.; Lee, J.P.; Kim, J.H.; Sohn, K.H.; Park, K.L.; Kim, M.K.; Son, S.W. Assessment of penetration of quantum dots through in vitro and in vivo human skin using the human skin equivalent model and the tape stripping method. Biochem. Biophys. Res. Commun. 2010, 394, 612–615. [Google Scholar] [CrossRef]
- Kuo, T.R.; Wu, C.L.; Hsu, C.T.; Lo, W.; Chiang, S.J.; Lin, S.J.; Dong, C.Y.; Chen, C.C. Chemical enhancer induced changes in the mechanisms of transdermal delivery of zinc oxide nanoparticles. Biomaterials 2009, 30, 3002–3008. [Google Scholar] [CrossRef]
- Labouta, H.I.; El-Khordagui, L.K.; Schneider, M. Could chemical enhancement of gold nanoparticle penetration be extrapolated from established approaches for drug permeation? Skin Pharmacol. Physiol. 2012, 25, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Melancon, M.P.; Zhou, M.; Li, C. Cancer theranostics with near-infrared light-activatable multimodal nanoparticles. Acc. Chem. Res. 2011, 44, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, S.; Guo, L.; Li, Y.; Yan, B.; Lu, W. Hollow copper sulfide nanoparticle-mediated transdermal drug delivery. Small 2012, 8, 3143–3150. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.K.; Lee, K.S.; El-Sayed, I.H.; El-Sayed, M.A. Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: Applications in biological imaging and biomedicine. J. Phys. Chem. B. 2006, 110, 7238–7248. [Google Scholar] [CrossRef]
- Thorling, C.; Holmes, A.; Studier, H.; Liu, D.; Liang, X.; Roberts, M.S. Multiphoton and Fluorescence Lifetime Imaging Microscopy in Studying Nanoparticle Pharmacokinetics in Skin and Liver. In Micro and Nanotechnologies for Biotechnology; Stanciu, S., Ed.; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef]
- Pérez-Medina, C.; Teunissen, A.J.P.; Kluza, E.; Mulder, W.J.M.; van der Meel, R. Nuclear imaging approaches facilitating nanomedicine translation. Adv. Drug Deliv. Rev. 2020, 154, 123–141. [Google Scholar] [CrossRef]
- Norlén, L.; Al-Amoudi, A.; Dubochet, J. A cryotransmission electron microscopy study of skin barrier formation. J. Investig. Dermatol. 2003, 120, 555–560. [Google Scholar] [CrossRef]
- Uchechi, O.; Ogbonna, J.D.N.; Attama, A.A. Nanoparticles for Dermal and Transdermal Drug Delivery. In Application of Nanotechnology in Drug Delivery; Demir Sezer, A., Ed.; IntechOpen: London, UK, 2014; Available online: https://www.intechopen.com/chapters/47025 (accessed on 25 July 2014).
- Cheng, Y.C.; Li, T.S.; Su, H.L.; Lee, P.C.; Wang, H.D. Transdermal Delivery Systems of Natural Products Applied to Skin Therapy and Care. Molecules 2020, 25, 5051. [Google Scholar] [CrossRef]
- Mittal, A.; Raber, A.S.; Schaefer, U.F.; Weissmann, S.; Ebensen, T.; Schulze, K.; Guzmán, C.A.; Lehr, C.M.; Hansen, S. Non-invasive delivery of nanoparticles to hair follicles: A perspective for transcutaneous immunization. Vaccine 2013, 31, 3442–3451. [Google Scholar] [CrossRef]
- Lev, D.C.; Onn, A.; Melinkova, V.O.; Miller, C.; Stone, V.; Ruiz, M.; McGary, E.C.; Ananthaswamy, H.N.; Price, J.E.; Bar-Eli, M. Exposure of melanoma cells to dacarbazine results in enhanced tumor growth and metastasis in vivo. J. Clin. Oncol. 2004, 22, 2092–2100. [Google Scholar] [CrossRef]
- Stracke, F.; Weiss, B.; Lehr, C.M.; König, K.; Schaefer, U.F.; Schneider, M. Multiphoton microscopy for the investigation of dermal penetration of nanoparticle-borne drugs. J. Investig. Dermatol. 2006, 126, 2224–2233. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, N.; Ogawa, M.; Sato, N.; Choyke, P.L.; Kobayashi, H. In vivo real-time, multicolor, quantum dot lymphatic imaging. J. Investig. Dermatol. 2009, 129, 2818–2822. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.B.; Aplin, A.E. Paying "particle" attention to novel melanoma treatment strategies. J. Investig. Dermatol. 2010, 130, 2699–2701. [Google Scholar] [CrossRef] [PubMed]
- Schmieder, A.H.; Winter, P.M.; Caruthers, S.D.; Harris, T.D.; Williams, T.A.; Allen, J.S.; Lacy, E.K.; Zhang, H.; Scott, M.J.; Hu, G.; et al. Molecular MR imaging of melanoma angiogenesis with alphanubeta3-targeted paramagnetic nanoparticles. Magn. Reson. Med. 2005, 53, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Boles, K.S.; Schmieder, A.H.; Koch, A.W.; Carano, R.A.; Wu, Y.; Caruthers, S.D.; Tong, R.K.; Stawicki, S.; Hu, G.; Scott, M.J.; et al. MR angiogenesis imaging with Robo4- vs. alphaVbeta3-targeted nanoparticles in a B16/F10 mouse melanoma model. FASEB J. 2010, 24, 4262–4270. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Huang, P.; Lin, J.; He, R.; Liu, B.; Zhang, X.; Yang, S.; Xi, P.; Zhang, X.; Ren, Q.; et al. Arginine-glycine-aspartic acid-conjugated dendrimer-modified quantum dots for targeting and imaging melanoma. J. Nanosci. Nanotechnol. 2010, 10, 4859–4867. [Google Scholar] [CrossRef]
- Benezra, M.; Penate-Medina, O.; Zanzonico, P.B.; Schaer, D.; Ow, H.; Burns, A.; DeStanchina, E.; Longo, V.; Herz, E.; Iyer, S.; et al. Multimodal silica nanoparticles are effective cancer-targeted probes in a model of human melanoma. J. Clin. Investig. 2011, 121, 2768–2780. [Google Scholar] [CrossRef]
- Saraceno, R.; Chiricozzi, A.; Gabellini, M.; Chimenti, S. Emerging applications of nanomedicine in dermatology. Skin Res. Technol. 2013, 19, e13–e19. [Google Scholar] [CrossRef]
- Lovrić, J.; Bazzi, H.S.; Cuie, Y.; Fortin, G.R.; Winnik, F.M.; Maysinger, D. Differences in subcellular distribution and toxicity of green and red emitting CdTe quantum dots. J. Mol. Med. 2005, 83, 377–385. [Google Scholar] [CrossRef]
- Low, S.P.; Voelcker, N.H.; Canham, L.T.; Williams, K.A. The biocompatibility of porous silicon in tissues of the eye. Biomaterials 2009, 30, 2873–2880. [Google Scholar] [CrossRef]
- Rosenholm, J.M.; Sahlgren, C.; Lindén, M. Multifunctional mesoporous silica nanoparticles for combined therapeutic, diagnostic and targeted action in cancer treatment. Curr. Drug Targets 2011, 12, 1166–1186. [Google Scholar] [CrossRef]
- Choi, M.J.; Maibach, H.I. Elastic vesicles as topical/transdermal drug delivery systems. Int. J. Cosmet. Sci. 2005, 27, 211–221. [Google Scholar] [CrossRef]
- Benson, H.A. Elastic liposomes for topical and transdermal drug delivery. Curr. Drug Deliv. 2009, 6, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Rancan, F.; Papakostas, D.; Hadam, S.; Hackbarth, S.; Delair, T.; Primard, C.; Verrier, B.; Sterry, W.; Blume-Peytavi, U.; Vogt, A. Investigation of polylactic acid (PLA) nanoparticles as drug delivery systems for local dermatotherapy. Pharm. Res. 2009, 26, 2027–2036. [Google Scholar] [CrossRef] [PubMed]
- Camerin, M.; Magaraggia, M.; Soncin, M.; Jori, G.; Moreno, M.; Chambrier, I.; Cook, M.J.; Russell, D.A. The in vivo efficacy of phthalocyanine-nanoparticle conjugates for the photodynamic therapy of amelanotic melanoma. Eur. J. Cancer 2010, 46, 1910–1918. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Ng, S.S.; Huo, L.F.; Chow, B.K.; Shen, Z.; Yang, M.; Sze, J.; Ko, O.; Li, M.; Yue, A.; et al. Effective melanoma immunotherapy with interleukin-2 delivered by a novel polymeric nanoparticle. Mol. Cancer Ther. 2011, 10, 1082–1092. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhu, X.; Zhang, X.; Liu, B.; Huang, L. Nanoparticles modified with tumor-targeting scFv deliver siRNA and miRNA for cancer therapy. Mol. Ther. 2010, 18, 1650–1656. [Google Scholar] [CrossRef]
- Chen, Y.; Bathula, S.R.; Yang, Q.; Huang, L. Targeted nanoparticles deliver siRNA to melanoma. J. Investig. Dermatol. 2010, 130, 2790–2798. [Google Scholar] [CrossRef]
- Davis, M.E.; Zuckerman, J.E.; Choi, C.H.; Seligson, D.; Tolcher, A.; Alabi, C.A.; Yen, Y.; Heidel, J.D.; Ribas, A. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature 2010, 464, 1067–1070. [Google Scholar] [CrossRef]
- Kaul, G.; Amiji, M. Long-circulating poly(ethylene glycol)-modified gelatin nanoparticles for intracellular delivery. Pharm. Res. 2002, 19, 1061–1067. [Google Scholar] [CrossRef]
- Cho, K.; Wang, X.; Nie, S.; Chen, Z.G.; Shin, D.M. Therapeutic nanoparticles for drug delivery in cancer. Clin. Cancer Res. 2008, 14, 1310–1316. [Google Scholar] [CrossRef]
- De Jong, W.H.; Borm, P.J. Drug delivery and nanoparticles:applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Nasir, A. Dermatologic toxicity of nanoengineered materials. Arch. Dermatol. 2008, 144, 253–254. [Google Scholar] [CrossRef] [PubMed]
- Dhar, S.; Kolishetti, N.; Lippard, S.J.; Farokhzad, O.C. Targeted delivery of a cisplatin prodrug for safer and more effective prostate cancer therapy in vivo. Proc. Natl. Acad. Sci. USA 2011, 108, 1850–1855. [Google Scholar] [CrossRef]
- Nasir, A. Nanotechnology and dermatology: Part I--potential of nanotechnology. Clin. Dermatol. 2010, 28, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Cho, E.C.; Chen, J.; Song, K.H.; Au, L.; Favazza, C.; Zhang, Q.; Cobley, C.M.; Gao, F.; Xia, Y.; et al. In vivo molecular photoacoustic tomography of melanomas targeted by bioconjugated gold nanocages. ACS Nano 2010, 4, 4559–4564. [Google Scholar] [CrossRef]
- Lu, W.; Xiong, C.; Zhang, G.; Huang, Q.; Zhang, R.; Zhang, J.Z.; Li, C. Targeted photothermal ablation of murine melanomas with melanocyte-stimulating hormone analog-conjugated hollow gold nanospheres. Clin. Cancer Res. 2009, 15, 876–886. [Google Scholar] [CrossRef]
- Zhou, M.; Nakatani, E.; Gronenberg, L.S.; Tokimoto, T.; Wirth, M.J.; Hruby, V.J.; Roberts, A.; Lynch, R.M.; Ghosh, I. Peptide-labeled quantum dots for imaging GPCRs in whole cells and as single molecules. Bioconjug. Chem. 2007, 18, 323–332. [Google Scholar] [CrossRef]
- Zheng, H.; Chen, G.; DeLouise, L.A.; Lou, Z. Detection of the cancer marker CD146 expression in melanoma cells with semiconductor quantum dot label. J. Biomed. Nanotechnol. 2010, 6, 303–311. [Google Scholar] [CrossRef]
- Zhu, X.; Bidlingmaier, S.; Hashizume, R.; James, C.D.; Berger, M.S.; Liu, B. Identification of internalizing human single-chain antibodies targeting brain tumor sphere cells. Mol. Cancer Ther. 2010, 9, 2131–2141. [Google Scholar] [CrossRef][Green Version]
- Siegrist, W.; Stutz, S.; Eberle, A.N. Homologous and heterologous regulation of alpha-melanocyte-stimulating hormone receptors in human and mouse melanoma cell lines. Cancer Res. 1994, 54, 2604–2610. [Google Scholar]
- Wong, W.; Minchin, R.F. Binding and internalization of the melanocyte stimulating hormone receptor ligand [Nle4, D-Phe7] alpha-MSH in B16 melanoma cells. Int. J. Biochem. Cell Biol. 1996, 28, 1223–1232. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.L.; Tao, X.; Lakkis, F.; Kiyokawa, T.; Murphy, J.R. Diphtheria toxin-related alpha-melanocyte-stimulating hormone fusion toxin. Internal in-frame deletion from Thr387 to His485 results in the formation of a highly potent fusion toxin which is resistant to proteolytic degradation. J. Biol. Chem. 1991, 266, 12289–12293. [Google Scholar] [CrossRef] [PubMed]
- Cone, R.D. Anatomy and regulation of the central melanocortin system. Nat. Neurosci. 2005, 8, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Neumann Andersen, G.; Nagaeva, O.; Mandrika, I.; Petrovska, R.; Muceniece, R.; Mincheva-Nilsson, L.; Wikberg, J.E. MC(1) receptors are constitutively expressed on leucocyte subpopulations with antigen presenting and cytotoxic functions. Clin. Exp. Immunol. 2001, 126, 441–446. [Google Scholar] [CrossRef]
- Carlson, J.A.; Linette, G.P.; Aplin, A.; Ng, B.; Slominski, A. Melanocyte receptors: Clinical implications and therapeutic relevance. Dermatol. Clin. 2007, 25, 541–557. [Google Scholar] [CrossRef]
- Hoch, M.; Eberle, A.N.; Wagner, U.; Bussmann, C.; Peters, T.; Peterli, R. Expression and localization of melanocortin-1 receptor in human adipose tissues of severely obese patients. Obesity 2007, 15, 40–49. [Google Scholar] [CrossRef]
- Li, D.; Taylor, A.W. Diminishment of alpha-MSH anti-inflammatory activity in MC1r siRNA-transfected RAW264.7 macrophages. J. Leukoc. Biol. 2008, 84, 191–198. [Google Scholar] [CrossRef]
- Iwasaki, A.; Medzhitov, R. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 2004, 5, 987–995. [Google Scholar] [CrossRef]
- Schröder, J.M.; Reich, K.; Kabashima, K.; Liu, F.T.; Romani, N.; Metz, M.; Kerstan, A.; Lee, P.H.; Loser, K.; Schön, M.P.; et al. Who is really in control of skin immunity under physiological circumstances—lymphocytes, dendritic cells or keratinocytes? Exp. Dermatol. 2006, 15, 913–929. [Google Scholar] [CrossRef]
- Gallo, R.L.; Nakatsuji, T. Microbial symbiosis with the innate immune defense system of the skin. J. Investig. Dermatol. 2011, 131, 1974–1980. [Google Scholar] [CrossRef]
- Beck, L.A.; Leung, D.Y. Allergen sensitization through the skin induces systemic allergic responses. J. Allergy Clin. Immunol. 2000, 106, S258–S263. [Google Scholar] [CrossRef] [PubMed]
- Warbrick, E.V.; Dearman, R.J.; Kimber, I. Induced changes in total serum IgE concentration in the Brown Norway rat: Potential for identification of chemical respiratory allergens. J. Appl. Toxicol. 2002, 22, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Arts, J.H.; Bloksma, N.; Leusink-Muis, T.; Kuper, C.F. Respiratory allergy and pulmonary irritation to trimellitic anhydride in Brown Norway rats. Toxicol. Appl. Pharmacol. 2003, 187, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A. Mucosal dendritic cells. Annu. Rev. Immunol. 2007, 25, 381–418. [Google Scholar] [CrossRef] [PubMed]
- Romani, N.; Clausen, B.E.; Stoitzner, P. Langerhans cells and more: Langerin-expressing dendritic cell subsets in the skin. Immunol. Rev. 2010, 234, 120–141. [Google Scholar] [CrossRef]
- Vogt, A.; Combadiere, B.; Hadam, S.; Stieler, K.M.; Lademann, J.; Schaefer, H.; Autran, B.; Sterry, W.; Blume-Peytavi, U. 40 nm, but not 750 or 1500 nm, nanoparticles enter epidermal CD1a+ cells after transcutaneous application on human skin. J. Investig. Dermatol. 2006, 126, 1316–1322. [Google Scholar] [CrossRef]
- Mahe, B.; Vogt, A.; Liard, C.; Duffy, D.; Abadie, V.; Bonduelle, O.; Boissonnas, A.; Sterry, W.; Verrier, B.; Blume-Peytavi, U.; et al. Nanoparticle-based targeting of vaccine compounds to skin antigen-presenting cells by hair follicles and their transport in mice. J. Investig. Dermatol. 2009, 129, 1156–1164. [Google Scholar] [CrossRef]
- Reilly, D.M.; Green, M.R. Eicosanoid and cytokine levels in acute skin irritation in response to tape stripping and capsaicin. Acta Derm. Venereol. 1999, 79, 187–190. [Google Scholar] [CrossRef]
- Streilein, J.W.; Lonsberry, L.W.; Bergstresser, P.R. Depletion of epidermal langerhans cells and Ia immunogenicity from tape-stripped mouse skin. J. Exp. Med. 1982, 155, 863–871. [Google Scholar] [CrossRef]
- Holzmann, S.; Tripp, C.H.; Schmuth, M.; Janke, K.; Koch, F.; Saeland, S.; Stoitzner, P.; Romani, N. A model system using tape stripping for characterization of Langerhans cell-precursors in vivo. J. Investig. Dermatol. 2004, 122, 1165–1174. [Google Scholar] [CrossRef]
- Nygaard, U.C.; Hansen, J.S.; Samuelsen, M.; Alberg, T.; Marioara, C.D.; Løvik, M. Single-walled and multi-walled carbon nanotubes promote allergic immune responses in mice. Toxicol. Sci. 2009, 109, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Grecco, A.C.; Paula, R.F.; Mizutani, E.; Sartorelli, J.C.; Milani, A.M.; Longhini, A.L.; Oliveira, E.C.; Pradella, F.; Silva, V.D.; Moraes, A.S.; et al. Up-regulation of T lymphocyte and antibody production by inflammatory cytokines released by macrophage exposure to multi-walled carbon nanotubes. Nanotechnology 2011, 22, 265103. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, R.; Takano, H.; Inoue, K.; Koike, E.; Kamachi, T.; Sadakane, K.; Ichinose, T. Titanium dioxide nanoparticles aggravate atopic dermatitis-like skin lesions in NC/Nga mice. Exp. Biol. Med. 2009, 234, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Kambara, T.; Aihara, M.; Matsukura, S.; Sato, I.; Kubota, Y.; Hirasawa, T.; Ikezawa, Z. Effects of photocatalytic agent on DS-Nh mice, developing atopic dermatitis-like eruption with an increase of Staphylococcus aureus. Int. Arch. Allergy Immunol. 2006, 141, 151–157. [Google Scholar] [CrossRef]
- Holleran, W.M.; Uchida, Y.; Halkier-Sorensen, L.; Haratake, A.; Hara, M.; Epstein, J.H.; Elias, P.M. Structural and biochemical basis for the UVB-induced alterations in epidermal barrier function. Photodermatol. Photoimmunol. Photomed. 1997, 13, 117–128. [Google Scholar] [CrossRef]
- Lynch, I.; Cedervall, T.; Lundqvist, M.; Cabaleiro-Lago, C.; Linse, S.; Dawson, K.A. The nanoparticle-protein complex as a biological entity; a complex fluids and surface science challenge for the 21st century. Adv. Colloid Interface Sci. 2007, 134, 167–174. [Google Scholar] [CrossRef]
- McNeela, E.A.; Lavelle, E.C. Recent advances in microparticle and nanoparticle delivery vehicles for mucosal vaccination. Curr. Top. Microbiol. Immunol. 2012, 354, 75–99. [Google Scholar] [CrossRef]
- Chen, X.; Prow, T.W.; Crichton, M.L.; Jenkins, D.W.; Roberts, M.S.; Frazer, I.H.; Fernando, G.J.; Kendall, M.A. Dry-coated microprojection array patches for targeted delivery of immunotherapeutics to the skin. J. Control. Release 2009, 139, 212–220. [Google Scholar] [CrossRef]
- Geusens, B.; Sanders, N.; Prow, T.; Van Gele, M.; Lambert, J. Cutaneous short-interfering RNA therapy. Expert Opin. Drug Deliv. 2009, 6, 1333–1349. [Google Scholar] [CrossRef]
- Jang, J.; Lim, D.H.; Choi, I.H. The impact of nanomaterials in immune system. Immune Netw. 2010, 10, 85–91. [Google Scholar] [CrossRef]
- Zolnik, B.S.; González-Fernández, A.; Sadrieh, N.; Dobrovolskaia, M.A. Nanoparticles and the immune system. Endocrinology 2010, 151, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Özbaş-Turan, S.; Akbuğa, J. Plasmid DNA-loaded chitosan/TPP nanoparticles for topical gene delivery. Drug Deliv. 2011, 18, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Nasir, A. Nanotechnology in vaccine development: A step forward. J. Investig. Dermatol. 2009, 129, 1055–1059. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fernando, G.J.; Chen, X.; Prow, T.W.; Crichton, M.L.; Fairmaid, E.J.; Roberts, M.S.; Frazer, I.H.; Brown, L.E.; Kendall, M.A. Potent immunity to low doses of influenza vaccine by probabilistic guided micro-targeted skin delivery in a mouse model. PLoS ONE 2010, 5, e10266. [Google Scholar] [CrossRef]
- Liard, C.; Munier, S.; Arias, M.; Joulin-Giet, A.; Bonduelle, O.; Duffy, D.; Shattock, R.J.; Verrier, B.; Combadière, B. Targeting of HIV-p24 particle-based vaccine into differential skin layers induces distinct arms of the immune responses. Vaccine 2011, 29, 6379–6391. [Google Scholar] [CrossRef]
- Jung, S.; Patzelt, A.; Otberg, N.; Thiede, G.; Sterry, W.; Lademann, J. Strategy of topical vaccination with nanoparticles. J. Biomed. Opt. 2009, 14, 021001. [Google Scholar] [CrossRef][Green Version]
- Peek, L.J.; Middaugh, C.R.; Berkland, C. Nanotechnology in vaccine delivery. Adv. Drug Deliv. Rev. 2008, 60, 915–928. [Google Scholar] [CrossRef]
- De Vries, J.J.; Bungener, L.; Ter Veer, W.; van Alphen, L.; van der Ley, P.; Wilschut, J.; Huckriede, A. Incorporation of LpxL1, a detoxified lipopolysaccharide adjuvant, in influenza H5N1 virosomes increases vaccine immunogenicity. Vaccine 2009, 27, 947–955. [Google Scholar] [CrossRef]
- Ludwig, C.; Wagner, R. Virus-like particles-universal molecular toolboxes. Curr. Opin. Biotechnol. 2007, 18, 537–545. [Google Scholar] [CrossRef]
- Bolzinger, M.A.; Briançon, S.; Chevalier, Y. Nanoparticles through the skin: Managing conflicting results of inorganic and organic particles in cosmetics and pharmaceutics. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2011, 3, 463–478. [Google Scholar] [CrossRef]
- Chourasia, R.; Jain, S.K. Drug targeting through pilosebaceous route. Curr. Drug Targets 2009, 10, 950–967. [Google Scholar] [CrossRef] [PubMed]
- Soria, A.; Vogt, A.; Nasir, A.; Combadičre, B. Nanoparticle-Based Epidermal and Dermal Vaccination. In Nanotechnology in Dermatology; Nasir, A., Friedman, A., Wang, S., Eds.; Springer: New York, NY, USA, 2013. [Google Scholar]
- Riemondy, K.; Hoefert, J.E.; Yi, R. Not miR-ly micromanagers: The functions and regulatory networks of microRNAs in mammalian skin. Wiley Interdiscip. Rev. RNA 2014, 5, 849–865. [Google Scholar] [CrossRef] [PubMed]
- Ross, K. Towards topical microRNA-directed therapy for epidermal disorders. J. Control. Release 2018, 269, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Leachman, S.A.; Hickerson, R.P.; Schwartz, M.E.; Bullough, E.E.; Hutcherson, S.L.; Boucher, K.M.; Hansen, C.D.; Eliason, M.J.; Srivatsa, G.S.; Kornbrust, D.J.; et al. First-in-human mutation-targeted siRNA phase Ib trial of an inherited skin disorder. Mol. Ther. 2010, 18, 442–446. [Google Scholar] [CrossRef]
- Domashenko, A.; Gupta, S.; Cotsarelis, G. Efficient delivery of transgenes to human hair follicle progenitor cells using topical lipoplex. Nat. Biotechnol. 2000, 18, 420–423. [Google Scholar] [CrossRef]
- Hachiya, A.; Sriwiriyanont, P.; Patel, A.; Saito, N.; Ohuchi, A.; Kitahara, T.; Takema, Y.; Tsuboi, R.; Boissy, R.E.; Visscher, M.O.; et al. Gene transfer in human skin with different pseudotyped HIV-based vectors. Gene Ther. 2007, 14, 648–656. [Google Scholar] [CrossRef][Green Version]
- Branski, L.K.; Masters, O.E.; Herndon, D.N.; Mittermayr, R.; Redl, H.; Traber, D.L.; Cox, R.A.; Kita, K.; Jeschke, M.G. Pre-clinical evaluation of liposomal gene transfer to improve dermal and epidermal regeneration. Gene Ther. 2010, 17, 770–778. [Google Scholar] [CrossRef][Green Version]
- Ding, Y.; Jiang, Z.; Saha, K.; Kim, C.S.; Kim, S.T.; Landis, R.F.; Rotello, V.M. Gold nanoparticles for nucleic acid delivery. Mol. Ther. 2014, 22, 1075–1083. [Google Scholar] [CrossRef]
- Singh, Y.; Tomar, S.; Khan, S.; Meher, J.G.; Pawar, V.K.; Raval, K.; Sharma, K.; Singh, P.K.; Chaurasia, M.; Surendar Reddy, B.; et al. Bridging small interfering RNA with giant therapeutic outcomes using nanometric liposomes. J. Control. Release 2015, 220, 368–387. [Google Scholar] [CrossRef]
- Vemula, P.K.; Anderson, R.R.; Karp, J.M. Nanoparticles reduce nickel allergy by capturing metal ions. Nat. Nanotechnol. 2011, 6, 291–295. [Google Scholar] [CrossRef]
- Papakostas, D.; Rancan, F.; Sterry, W.; Blume-Peytavi, U.; Vogt, A. Nanoparticles in dermatology. Arch. Dermatol. Res. 2011, 303, 533. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B.; Fernandes, A.R.; Martins-Gomes, C.; Coutinho, T.E.; Durazzo, A.; Lucarini, M.; Souto, S.B.; Silva, A.M.; Santini, A. Nanomaterials for Skin Delivery of Cosmeceuticals and Pharmaceuticals. Appl. Sci. 2020, 10, 1594. [Google Scholar] [CrossRef]
- Fisher, G.J.; Kang, S.; Varani, J.; Bata-Csorgo, Z.; Wan, Y.; Datta, S.; Voorhees, J.J. Mechanisms of photoaging and chronological skin aging. Arch. Dermatol. 2002, 138, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- González, S.; Fernández-Lorente, M.; Gilaberte-Calzada, Y. The latest on skin photoprotection. Clin. Dermatol. 2008, 26, 614–626. [Google Scholar] [CrossRef] [PubMed]
- Edlich, R.F.; Winters, K.L.; Lim, H.W.; Cox, M.J.; Becker, D.G.; Horowitz, J.H.; Nichter, L.S.; Britt, L.D.; Long, W.B. Photoprotection by sunscreens with topical antioxidants and systemic antioxidants to reduce sun exposure. J. Long Term Eff. Med. Implant. 2004, 14, 317–340. [Google Scholar] [CrossRef]
- Havenga, D.; Akoba, R.; Menzi, L.; Azizi, S.; Sackey, J.; Swanepoel, N.; Gibaud, A.; Maaza, M. From Himba indigenous knowledge to engineered Fe2O3 UV-blocking green nanocosmetics. Sci. Rep. 2022, 12, 2259. [Google Scholar] [CrossRef]
- Smijs, T.G.; Pavel, S. Titanium dioxide and zinc oxide nanoparticles in sunscreens: Focus on their safety and effectiveness. Nanotechnol. Sci. Appl. 2011, 4, 95–112. [Google Scholar] [CrossRef]
- Bucci, P.; Prieto, M.J.; Milla, L.; Calienni, M.N.; Martinez, L.; Rivarola, V.; Alonso, S.; Montanari, J. Skin penetration and UV-damage prevention by nanoberries. J. Cosmet. Dermatol. 2018, 17, 889–899. [Google Scholar] [CrossRef]
- Shetty, P.K.; Venuvanka, V.; Jagani, H.V.; Chethan, G.H.; Ligade, V.S.; Musmade, P.B.; Nayak, U.Y.; Reddy, M.S.; Kalthur, G.; Udupa, N.; et al. Development and evaluation of sunscreen creams containing morin-encapsulated nanoparticles for enhanced UV radiation protection and antioxidant activity. Int. J. Nanomed. 2015, 10, 6477–6491. [Google Scholar] [CrossRef]
- Wang, S.Q.; Balagula, Y.; Osterwalder, U. Photoprotection: A review of the current and future technologies. Dermatol. Ther. 2010, 23, 31–47. [Google Scholar] [CrossRef]
- Detoni, C.B.; Paese, K.; Beck, R.C.R.; Pohlmann, A.R.; Guterres, S.S. Nanosized and Nanoencapsulated sunscreens. In NanoCosmetics and Nanomedicines; Beck, R.C.R., Guterres, S.S., Pohlmann, A.R., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 333–362. [Google Scholar]
- Marcato, P.D.; Caverzan, J.; Rossi-Bergmann, B.; Pinto, E.F.; Machado, D.; Silva, R.A.; Justo, G.Z.; Ferreira, C.V.; Durán, N. Nanostructured polymer and lipid carriers for sunscreen. Biological effects and skin permeation. J. Nanosci. Nanotechnol. 2011, 11, 1880–1886. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Ediriwickrema, A.; Yang, F.; Lewis, J.; Girardi, M.; Saltzman, W.M. A sunblock based on bioadhesive nanoparticles. Nat. Mater. 2015, 14, 1278–1285. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Yoo, E.; Lee, S.H.; Won, K. Preparation and Application of Light-Colored Lignin Nanoparticles for Broad-Spectrum Sunscreens. Polymers 2020, 12, 699. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.L.; Lim, H.W. A review of inorganic UV filters zinc oxide and titanium dioxide. Photodermatol. Photoimmunol. Photomed. 2019, 35, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Hanigan, D.; Truong, L.; Schoepf, J.; Nosaka, T.; Mulchandani, A.; Tanguay, R.L.; Westerhoff, P. Trade-offs in ecosystem impacts from nanomaterial versus organic chemical ultraviolet filters in sunscreens. Water Res. 2018, 139, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Bennett, S.W.; Zhou, D.; Mielke, R.; Keller, A.A. Photoinduced disaggregation of TiO₂ nanoparticles enables transdermal penetration. PLoS ONE 2012, 7, e48719. [Google Scholar] [CrossRef] [PubMed]
- Abendrot, M.; Kalinowska-Lis, U. Zinc-containing compounds for personal care applications. Int. J. Cosmet. Sci. 2018, 40, 319–327. [Google Scholar] [CrossRef] [PubMed]
- James, S.A.; Feltis, B.N.; de Jonge, M.D.; Sridhar, M.; Kimpton, J.A.; Altissimo, M.; Mayo, S.; Zheng, C.; Hastings, A.; Howard, D.L.; et al. Quantification of ZnO nanoparticle uptake, distribution, and dissolution within individual human macrophages. ACS Nano 2013, 7, 10621–10635. [Google Scholar] [CrossRef] [PubMed]
- Ilves, M.; Palomäki, J.; Vippola, M.; Lehto, M.; Savolainen, K.; Savinko, T.; Alenius, H. Topically applied ZnO nanoparticles suppress allergen induced skin inflammation but induce vigorous IgE production in the atopic dermatitis mouse model. Part. Fibre Toxicol. 2014, 11, 38. [Google Scholar] [CrossRef]
- Jo, H.-J.; Joo, S.-M.; Kim, J.Y.; Yu, K.-H.; Kim, S.W. Development of a hybrid chitosan- and niacinamide-coupled ZnO nanoparticle composite for sun protection application. J. Nanomater. 2019, 2019, 5957606. [Google Scholar] [CrossRef]
- Aditya, A.; Chattopadhyay, S.; Gupta, N.; Alam, S.; Veedu, A.P.; Pal, M.; Singh, A.; Santhiya, D.; Ansari, K.M.; Ganguli, M. ZnO Nanoparticles Modified with an Amphipathic Peptide Show Improved Photoprotection in Skin. ACS Appl. Mater. Interfaces 2019, 11, 56–72. [Google Scholar] [CrossRef] [PubMed]
- Ditlopo, N.; Sintwa, N.; Khamlich, S.; Manikandan, E.; Gnanasekaran, K.; Henini, M.; Gibaud, A.; Krief, A.; Maaza, M. From Khoi-San indigenous knowledge to bioengineered CeO2 nanocrystals to exceptional UV-blocking green nanocosmetics. Sci. Rep. 2022, 12, 3468. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.Z.; Nafisi, S.; Maibach, H.I. Fullerene nanoparticle in dermatological and cosmetic applications. Nanomedicine 2017, 13, 1071–1087. [Google Scholar] [CrossRef] [PubMed]
- Krusic, P.J.; Wasserman, E.; Keizer, P.N.; Morton, J.R.; Preston, K.F. Radical reactions of c60. Science 1991, 254, 1183–1185. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Takada, H.; Gan, X.; Miwa, N. The water-soluble fullerene derivative "Radical Sponge" exerts cytoprotective action against UVA irradiation but not visible-light-catalyzed cytotoxicity in human skin keratinocytes. Bioorg. Med. Chem. Lett. 2006, 16, 1590–1595. [Google Scholar] [CrossRef] [PubMed]
- Tzirakis, M.D.; Orfanopoulos, M. Radical reactions of fullerenes: From synthetic organic chemistry to materials science and biology. Chem. Rev. 2013, 113, 5262–5321. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, Y.; Miyanishi, A.; Mizuno, H.; Kato, S.; Aoshima, H.; Kokubo, K.; Miwa, N. Super-highly hydroxylated fullerene derivative protects human keratinocytes from UV-induced cell injuries together with the decreases in intracellular ROS generation and DNA damages. J. Photochem. Photobiol. B. 2011, 102, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Hyodo, S.; Fujikawa, Y.; Fujimoto, T.; Maeda, K. Photoprotective effects of inclusion complexes of fullerenes with polyvinylpyrrolidone. Photodermatol. Photoimmunol. Photomed. 2013, 29, 196–203. [Google Scholar] [CrossRef] [PubMed]
- O’Meara, S.; Cullum, N.; Majid, M.; Sheldon, T. Systematic reviews of wound care management: (3) antimicrobial agents for chronic wounds; (4) diabetic foot ulceration. Health Technol. Assess. 2000, 4, 1–237. [Google Scholar] [CrossRef]
- Breuer, K.; Haussler, S.; Kapp, A.; Werfel, T. Staphylococcus aureus: Colonizing features and influence of an antibacterial treatment in adults with atopic dermatitis. Br. J. Dermatol. 2002, 147, 55–61. [Google Scholar] [CrossRef]
- Nowack, B.; Krug, H.F.; Height, M. 120 years of nanosilver history: Implications for policy makers. Environ. Sci. Technol. 2011, 45, 1177–1183. [Google Scholar] [CrossRef] [PubMed]
- Hamad, A.; Khashan, K.S.; Hadi, A. Silver Nanoparticles and Silver Ions as Potential Antibacterial Agents. J. Inorg. Organomet. Polym. 2020, 30, 4811–4828. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Levard, C.; Hotze, E.M.; Lowry, G.V.; Brown, G.E., Jr. Environmental transformations of silver nanoparticles: Impact on stability and toxicity. Environ. Sci. Technol. 2012, 46, 6900–6914. [Google Scholar] [CrossRef] [PubMed]
- Benn, T.M.; Westerhoff, P. Nanoparticle silver released into water from commercially available sock fabrics. Environ. Sci. Technol. 2008, 42, 4133–4139. [Google Scholar] [CrossRef]
- Lubick, N. Silver socks have cloudy lining. Environ. Sci. Technol. 2008, 42, 3910. [Google Scholar] [CrossRef]
- Costa, C.; Conte, A.; Buonocore, G.G.; Del Nobile, M.A. Antimicrobial silver-montmorillonite nanoparticles to prolong the shelf life of fresh fruit salad. Int. J. Food Microbiol. 2011, 148, 164–167. [Google Scholar] [CrossRef]
- Li, Y.; Leung, P.; Yao, L.; Song, Q.W.; Newton, E. Antimicrobial effect of surgical masks coated with nanoparticles. J. Hosp. Infect. 2006, 62, 58–63. [Google Scholar] [CrossRef]
- Elliott, C. The effects of silver dressings on chronic and burns wound healing. Br. J. Nurs. 2010, 19, S32–S36. [Google Scholar] [CrossRef]
- Tian, J.; Wong, K.K.; Ho, C.M.; Lok, C.N.; Yu, W.Y.; Che, C.M.; Chiu, J.F.; Tam, P.K. Topical delivery of silver nanoparticles promotes wound healing. ChemMedChem. 2007, 2, 129–136. [Google Scholar] [CrossRef]
- Jung, W.K.; Koo, H.C.; Kim, K.W.; Shin, S.; Kim, S.H.; Park, Y.H. Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli. Appl. Environ. Microbiol. 2008, 74, 2171–2178. [Google Scholar] [CrossRef] [PubMed]
- Chamakura, K.; Perez-Ballestero, R.; Luo, Z.; Bashir, S.; Liu, J. Comparison of bactericidal activities of silver nanoparticles with common chemical disinfectants. Colloids Surf. B Biointerfaces 2011, 84, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Hachicho, N.; Hoffmann, P.; Ahlert, K.; Heipieper, H.J. Effect of silver nanoparticles and silver ions on growth and adaptive response mechanisms of Pseudomonas putida mt-2. FEMS Microbiol. Lett. 2014, 355, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Fung, M.C.; Bowen, D.L. Silver products for medical indications: Risk-benefit assessment. J. Toxicol. Clin. Toxicol. 1996, 34, 119–126. [Google Scholar] [CrossRef]
- Rahman, M.F.; Wang, J.; Patterson, T.A.; Saini, U.T.; Robinson, B.L.; Newport, G.D.; Murdock, R.C.; Schlager, J.J.; Hussain, S.M.; Ali, S.F. Expression of genes related to oxidative stress in the mouse brain after exposure to silver-25 nanoparticles. Toxicol. Lett. 2009, 187, 15–21. [Google Scholar] [CrossRef]
- Jun, E.A.; Lim, K.M.; Kim, K.; Bae, O.N.; Noh, J.Y.; Chung, K.H.; Chung, J.H. Silver nanoparticles enhance thrombus formation through increased platelet aggregation and procoagulant activity. Nanotoxicology 2011, 5, 157–167. [Google Scholar] [CrossRef]
- Teow, Y.; Asharani, P.V.; Hande, M.P.; Valiyaveettil, S. Health impact and safety of engineered nanomaterials. Chem. Commun. 2011, 47, 7025–7038. [Google Scholar] [CrossRef]
- Samberg, M.E.; Oldenburg, S.J.; Monteiro-Riviere, N.A. Evaluation of silver nanoparticle toxicity in skin in vivo and keratinocytes in vitro. Environ. Health Perspect. 2010, 118, 407–413. [Google Scholar] [CrossRef]
- Korani, M.; Rezayat, S.M.; Gilani, K.; Arbabi Bidgoli, S.; Adeli, S. Acute and subchronic dermal toxicity of nanosilver in guinea pig. Int. J. Nanomed. 2011, 6, 855–862. [Google Scholar] [CrossRef]
- Morales-Avila, E.; Ferro-Flores, G.; Ocampo-García, B.E.; López-Téllez, G.; López-Ortega, J.; Rogel-Ayala, D.G.; Sánchez-Padilla, D. Antibacterial Efficacy of Gold and Silver Nanoparticles Functionalized with the Ubiquicidin (29–41) Antimicrobial Peptide. J. Nanomater. 2017, 2017, 5831959. [Google Scholar] [CrossRef]
- Tsuang, Y.H.; Sun, J.S.; Huang, Y.C.; Lu, C.H.; Chang, W.H.; Wang, C.C. Studies of photokilling of bacteria using titanium dioxide nanoparticles. Artif. Organs 2008, 32, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Nhung, D.T.; Freydiere, A.M.; Constant, H.; Falson, F.; Pirot, F. Sustained antibacterial effect of a hand rub gel incorporating chlorhexdine-loaded nanocapsules (Nanochlorex). Int. J. Pharm. 2007, 334, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Martinez, L.R.; Han, G.; Chacko, M.; Mihu, M.R.; Jacobson, M.; Gialanella, P.; Friedman, A.J.; Nosanchuk, J.D.; Friedman, J.M. Antimicrobial and healing efficacy of sustained release nitric oxide nanoparticles against Staphylococcus aureus skin infection. J. Investig. Dermatol. 2009, 129, 2463–2469. [Google Scholar] [CrossRef] [PubMed]
- Gnanamoorthy, G.; Kumar Yadav, V.; Ali, D.; Parthiban, E.; Kumar, G.; Narayanan, V. New orchestrated of X-CuTiAP (en, trien, ETA and DMA) nanospheres with enhanced photocatalytic and antimicrobial activities. J. Ind. Eng. Chem. 2022, 110, 503–519. [Google Scholar] [CrossRef]
- Gnanamoorthy, G.; Ramar, K.; Ali, D.; Kumar Yadav, V.; Jafar, A.A.; Kumar, G. Synthesis and effective performance of Photocatalytic and Antimicrobial activities of Bauhinia tomentosa Linn plants using of gold nanoparticles. Opt. Mater. 2022, 123, 111945. [Google Scholar] [CrossRef]
- Ege, D.; Zheng, K.; Boccaccini, A.R. Borate Bioactive Glasses (BBG): Bone Regeneration, Wound Healing Applications, and Future Directions. ACS Appl. Bio. Mater. 2022, 5, 3608–3622. [Google Scholar] [CrossRef]
- Okonkwo, U.A.; DiPietro, L.A. Diabetes and Wound Angiogenesis. Int. J. Mol. Sci. 2017, 18, 1419. [Google Scholar] [CrossRef]
- Schuhladen, K.; Stich, L.; Schmidt, J.; Steinkasserer, A.; Boccaccini, A.R.; Zinser, E. Cu, Zn Doped Borate Bioactive Glasses: Antibacterial Efficacy and Dose-Dependent in Vitro Modulation of Murine Dendritic Cells. Biomater. Sci. 2020, 8, 2143–2155. [Google Scholar] [CrossRef]
- Hu, H.; Tang, Y.; Pang, L.; Lin, C.; Huang, W.; Wang, D.; Jia, W. Angiogenesis and Full-Thickness Wound Healing Efficiency of a Copper-Doped Borate Bioactive Glass/Poly(lactic- co-glycolic acid) Dressing Loaded with Vitamin E in Vivo and in Vitro. ACS Appl. Mater. Interfaces 2018, 10, 22939–22950. [Google Scholar] [CrossRef]
- Mehrabi, T.; Mesgar, A.S.; Mohammadi, Z. Bioactive Glasses: A Promising Therapeutic Ion Release Strategy for Enhancing Wound Healing. ACS Biomater. Sci. Eng. 2020, 6, 5399–5430. [Google Scholar] [CrossRef]
- Bi, L.; Rahaman, M.N.; Day, D.E.; Brown, Z.; Samujh, C.; Liu, X.; Mohammadkhah, A.; Dusevich, V.; Eick, J.D.; Bonewald, L.F. Effect of bioactive borate glass microstructure on bone regeneration, angiogenesis, and hydroxyapatite conversion in a rat calvarial defect model. Acta Biomater. 2013, 9, 8015–8026. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Michalek, M.; Liverani, L.; Švančárek, P.; Boccaccini, A.R.; Galusek, D. Preparation and Characterization of Sintered Bioactive Borate Glass Tape. Mater. Lett. 2021, 282, 128843–128853. [Google Scholar] [CrossRef]
- Zhao, S.; Li, L.; Wang, H.; Zhang, Y.; Cheng, X.; Zhou, N.; Rahaman, M.N.; Liu, Z.; Huang, W.; Zhang, C. Wound dressings composed of copper-doped borate bioactive glass microfibers stimulate angiogenesis and heal full-thickness skin defects in a rodent model. Biomaterials 2015, 53, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Rau, J.V.; De Bonis, A.; Curcio, M.; Schuhladen, K.; Barbaro, K.; De Bellis, G.; Teghil, R.; Boccaccini, A.R. Borate and Silicate Bioactive Glass Coatings Prepared by Nanosecond Pulsed Laser Deposition. Coatings 2020, 10, 1105. [Google Scholar] [CrossRef]
- Naseri, S.; Griffanti, G.; Lepry, W.C.; Maisuria, V.B.; Tufenkji, N.; Nazhat, S.N. Silver-Doped Sol-Gel Borate Glasses: Dose-Dependent Effect on Pseudomonas Aeruginosa Biofilms and Keratinocyte Function. J. Am. Ceram. Soc. 2022, 105, 1711–1722. [Google Scholar] [CrossRef]
- Ippagunta, S.K.; Gangwar, R.; Finkelstein, D.; Vogel, P.; Pelletier, S.; Gingras, S.; Redecke, V.; Häcker, H. Keratinocytes contribute intrinsically to psoriasis upon loss of Tnip1 function. Proc. Natl. Acad. Sci. USA 2016, 113, E6162–E6171. [Google Scholar] [CrossRef]
- Gabros, S.; Nessel, T.A.; Zito, P.M. Topical Corticosteroids; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Al-Dhubaibi, M.S. Association between Vitamin D deficiency and psoriasis: An exploratory study. Int. J. Health Sci. 2018, 12, 33–39. [Google Scholar]
- Kadian, V.; Kumar, S.; Saini, K.; Kakkar, V.; Rao, R. Dithranol: An Insight into its Novel Delivery Cargos for Psoriasis Management. Curr. Drug Res. Rev. 2020, 12, 82–96. [Google Scholar] [CrossRef]
- Menter, A.; Strober, B.E.; Kaplan, D.H.; Kivelevitch, D.; Prater, E.F.; Stoff, B.; Armstrong, A.W.; Connor, C.; Cordoro, K.M.; Davis, D.M.R.; et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J. Am. Acad. Dermatol. 2019, 80, 1029–1072. [Google Scholar] [CrossRef]
- Sun, M.C.; Xu, X.L.; Lou, X.F.; Du, Y.Z. Recent Progress and Future Directions: The Nano-Drug Delivery System for the Treatment of Vitiligo. Int. J. Nanomed. 2020, 15, 3267–3279. [Google Scholar] [CrossRef]
- Daniel, B.S.; Wittal, R. Vitiligo treatment update. Australas. J. Dermatol. 2015, 56, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Sathe, P.; Saka, R.; Kommineni, N.; Raza, K.; Khan, W. Dithranol-loaded nanostructured lipid carrier-based gel ameliorate psoriasis in imiquimod-induced mice psoriatic plaque model. Drug Dev. Ind. Pharm. 2019, 45, 826–838. [Google Scholar] [CrossRef] [PubMed]
- Walunj, M.; Doppalapudi, S.; Bulbake, U.; Khan, W. Preparation, characterization, and in vivo evaluation of cyclosporine cationic liposomes for the treatment of psoriasis. J. Liposome Res. 2020, 30, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Gupta, M.; Mangal, S.; Agrawal, U.; Vyas, S.P. Capsaicin-loaded vesicular systems designed for enhancing localized delivery for psoriasis therapy. Artif. Cells Nanomed. Biotechnol. 2016, 44, 825–834. [Google Scholar] [CrossRef]
- Srisuk, P.; Thongnopnua, P.; Raktanonchai, U.; Kanokpanont, S. Physico-chemical characteristics of methotrexate-entrapped oleic acid-containing deformable liposomes for in vitro transepidermal delivery targeting psoriasis treatment. Int. J. Pharm. 2012, 427, 426–434. [Google Scholar] [CrossRef]
- Moghddam, S.R.; Ahad, A.; Aqil, M.; Imam, S.S.; Sultana, Y. Formulation and optimization of niosomes for topical diacerein delivery using 3-factor, 3-level Box-Behnken design for the management of psoriasis. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 69, 789–797. [Google Scholar] [CrossRef]
- Abu Hashim, I.I.; Abo El-Magd, N.F.; El-Sheakh, A.R.; Hamed, M.F.; Abd El-Gawad, A.E.H. Pivotal role of Acitretin nanovesicular gel for effective treatment of psoriasis: Ex vivo-in vivo evaluation study. Int. J. Nanomed. 2018, 13, 1059–1079. [Google Scholar] [CrossRef]
- Meng, S.; Sun, L.; Wang, L.; Lin, Z.; Liu, Z.; Xi, L.; Wang, Z.; Zheng, Y. Loading of water-insoluble celastrol into niosome hydrogels for improved topical permeation and anti-psoriasis activity. Colloids Surf. B Biointerfaces 2019, 182, 110352. [Google Scholar] [CrossRef]
- Chandra, A.; Aggarwal, G.; Manchanda, S.; Narula, A. Development of Topical Gel of Methotrexate Incorporated Ethosomes and Salicylic Acid for the Treatment of Psoriasis. Pharm. Nanotechnol. 2019, 7, 362–374. [Google Scholar] [CrossRef]
- Fathalla, D.; Youssef, E.M.K.; Soliman, G.M. Liposomal and Ethosomal Gels for the Topical Delivery of Anthralin: Preparation, Comparative Evaluation and Clinical Assessment in Psoriatic Patients. Pharmaceutics 2020, 12, 446. [Google Scholar] [CrossRef]
- Doppalapudi, S.; Jain, A.; Chopra, D.K.; Khan, W. Psoralen loaded liposomal nanocarriers for improved skin penetration and efficacy of topical PUVA in psoriasis. Eur. J. Pharm. Sci. 2017, 96, 515–529. [Google Scholar] [CrossRef] [PubMed]
- Rashid, S.A.; Bashir, S.; Naseem, F.; Farid, A.; Rather, I.A.; Hakeem, K.R. Olive Oil Based Methotrexate Loaded Topical Nanoemulsion Gel for the Treatment of Imiquimod Induced Psoriasis-like Skin Inflammation in an Animal Model. Biology 2021, 10, 1121. [Google Scholar] [CrossRef] [PubMed]
- Algahtani, M.S.; Ahmad, M.Z.; Nourein, I.H.; Ahmad, J. Co-Delivery of Imiquimod and Curcumin by Nanoemugel for Improved Topical Delivery and Reduced Psoriasis-Like Skin Lesions. Biomolecules 2020, 10, 968. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S.; Katiyar, S.S.; Kushwah, V.; Jain, S. Active natural oil-based nanoemulsion containing tacrolimus for synergistic antipsoriatic efficacy. Nanomedicine 2018, 13, 1985–1998. [Google Scholar] [CrossRef]
- Somagoni, J.; Boakye, C.H.; Godugu, C.; Patel, A.R.; Mendonca Faria, H.A.; Zucolotto, V.; Singh, M. Nanomiemgel--a novel drug delivery system for topical application--in vitro and in vivo evaluation. PLoS ONE 2014, 9, e115952. [Google Scholar] [CrossRef]
- Desmet, E.; Bracke, S.; Forier, K.; Taevernier, L.; Stuart, M.C.; De Spiegeleer, B.; Raemdonck, K.; Van Gele, M.; Lambert, J. An elastic liposomal formulation for RNAi-based topical treatment of skin disorders: Proof-of-concept in the treatment of psoriasis. Int. J. Pharm. 2016, 500, 268–274. [Google Scholar] [CrossRef]
- Parkash, V.; Maan, S.; Chaudhary, V.; Jogpal, V.; Mittal, G.; Jain, V. Implementation of Design of Experiments in Development and Optimization of Transfersomal Carrier System of Tacrolimus for the Dermal Management of Psoriasis in Albino Wistar Rat. J. Bioequiv. Availab. 2018, 10, 98–106. [Google Scholar] [CrossRef]
- Wang, W.; Shu, G.F.; Lu, K.J.; Xu, X.L.; Sun, M.C.; Qi, J.; Huang, Q.L.; Tan, W.Q.; Du, Y.Z. Flexible liposomal gel dual-loaded with all-trans retinoic acid and betamethasone for enhanced therapeutic efficiency of psoriasis. J. Nanobiotechnol. 2020, 18, 80. [Google Scholar] [CrossRef]
- Anwer, M.K.; Mohammad, M.; Ezzeldin, E.; Fatima, F.; Alalaiwe, A.; Iqbal, M. Preparation of sustained release apremilast-loaded PLGA nanoparticles: In vitro characterization and in vivo pharmacokinetic study in rats. Int. J. Nanomed. 2019, 14, 1587–1595. [Google Scholar] [CrossRef]
- Tomoda, K.; Terashima, H.; Suzuki, K.; Inagi, T.; Terada, H.; Makino, K. Enhanced transdermal delivery of indomethacin-loaded PLGA nanoparticles by iontophoresis. Colloids Surf. B Biointerfaces 2011, 88, 706–710. [Google Scholar] [CrossRef]
- Kilfoyle, B.E.; Sheihet, L.; Zhang, Z.; Laohoo, M.; Kohn, J.; Michniak-Kohn, B.B. Development of paclitaxel-TyroSpheres for topical skin treatment. J. Control. Release 2012, 163, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Lapteva, M.; Mondon, K.; Möller, M.; Gurny, R.; Kalia, Y.N. Polymeric micelle nanocarriers for the cutaneous delivery of tacrolimus: A targeted approach for the treatment of psoriasis. Mol. Pharm. 2014, 11, 2989–3001. [Google Scholar] [CrossRef] [PubMed]
- Beber, T.C.; de Andrade, D.F.; Chaves Pdos, S.; Pohlmann, A.R.; Guterres, S.S.; Beck, R.C. Cationic Polymeric Nanocapsules as a Strategy to Target Dexamethasone to Viable Epidermis: Skin Penetration and Permeation Studies. J. Nanosci. Nanotechnol. 2016, 16, 1331–1338. [Google Scholar] [CrossRef]
- Jain, A.; Pooladanda, V.; Bulbake, U.; Doppalapudi, S.; Rafeeqi, T.A.; Godugu, C.; Khan, W. Liposphere mediated topical delivery of thymoquinone in the treatment of psoriasis. Nanomedicine 2017, 13, 2251–2262. [Google Scholar] [CrossRef]
- Raghuwanshi, N.; Yadav, T.C.; Srivastava, A.K.; Raj, U.; Varadwaj, P.; Pruthi, V. Structure-based drug designing and identification of Woodfordia fruticosa inhibitors targeted against heat shock protein (HSP70-1) as suppressor for Imiquimod-induced psoriasis like skin inflammation in mice model. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 95, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Fratoddi, I.; Benassi, L.; Botti, E.; Vaschieri, C.; Venditti, I.; Bessar, H.; Samir, M.A.; Azzoni, P.; Magnoni, C.; Costanzo, A.; et al. Effects of topical methotrexate loaded gold nanoparticle in cutaneous inflammatory mouse model. Nanomedicine 2019, 17, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Doppalapudi, S.; Mahira, S.; Khan, W. Development and in vitro assessment of psoralen and resveratrol co-loaded ultradeformable liposomes for the treatment of vitiligo. J. Photochem. Photobiol. B 2017, 174, 44–57. [Google Scholar] [CrossRef]
- Mir-Palomo, S.; Nácher, A.; Ofelia Vila Busó, M.A.; Caddeo, C.; Manca, M.L.; Manconi, M.; Díez-Sales, O. Baicalin and berberine ultradeformable vesicles as potential adjuvant in vitiligo therapy. Colloids Surf. B Biointerfaces 2019, 175, 654–662. [Google Scholar] [CrossRef]
- Manosroi, J.; Khositsuntiwong, N.; Götz, F.; Werner, R.G.; Manosroi, W.; Manosroi, A. Potent melanin production enhancement of human tyrosinase gene by Tat and an entrapment in elastic cationic niosomes: Potential application in vitiligo gene therapy. Chem. Biol. Drug Des. 2012, 80, 953–960. [Google Scholar] [CrossRef]
- Patel, H.K.; Barot, B.S.; Parejiya, P.B.; Shelat, P.K.; Shukla, A. Topical delivery of clobetasol propionate loaded microemulsion based gel for effective treatment of vitiligo--part II: Rheological characterization and in vivo assessment through dermatopharmacokinetic and pilot clinical studies. Colloids Surf. B Biointerfaces 2014, 119, 145–153. [Google Scholar] [CrossRef]
- Garg, B.J.; Garg, N.K.; Beg, S.; Singh, B.; Katare, O.P. Nanosized ethosomes-based hydrogel formulations of methoxsalen for enhanced topical delivery against vitiligo: Formulation optimization, in vitro evaluation and preclinical assessment. J. Drug Target. 2016, 24, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Matos, B.N.; Reis, T.A.; Gratieri, T.; Gelfuso, G.M. Chitosan nanoparticles for targeting and sustaining minoxidil sulphate delivery to hair follicles. Int. J. Biol. Macromol. 2015, 75, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, P.M.; Alencar-Silva, T.; Pires, F.Q.; Cunha-Filho, M.; Gratieri, T.; Carvalho, J.L.; Gelfuso, G.M. Nanostructured lipid carriers loaded with an association of minoxidil and latanoprost for targeted topical therapy of alopecia. Eur. J. Pharm. Biopharm. 2022, 172, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.C.S.; Oliveira, P.M.; Cunha-Filho, M.; Gratieri, T.; Gelfuso, G.M. Latanoprost Loaded in Polymeric Nanocapsules for Effective Topical Treatment of Alopecia. AAPS PharmSciTech 2020, 21, 305. [Google Scholar] [CrossRef]
- Wang, W.; Chen, L.; Huang, X.; Shao, A. Preparation and Characterization of Minoxidil Loaded Nanostructured Lipid Carriers. AAPS PharmSciTech 2017, 18, 509–516. [Google Scholar] [CrossRef]
- Nagai, N.; Iwai, Y.; Sakamoto, A.; Otake, H.; Oaku, Y.; Abe, A.; Nagahama, T. Drug Delivery System Based on Minoxidil Nanoparticles Promotes Hair Growth in C57BL/6 Mice. Int. J. Nanomed. 2019, 14, 7921–7931. [Google Scholar] [CrossRef]
- Tsujimoto, H.; Hara, K.; Tsukada, Y.; Huang, C.C.; Kawashima, Y.; Arakaki, M.; Okayasu, H.; Mimura, H.; Miwa, N. Evaluation of the permeability of hair growing ingredient encapsulated PLGA nanospheres to hair follicles and their hair growing effects. Bioorg. Med. Chem. Lett. 2007, 17, 4771–4777. [Google Scholar] [CrossRef]
- Nakamura, M.; Jo, J.; Tabata, Y.; Ishikawa, O. Controlled delivery of T-box21 small interfering RNA ameliorates autoimmune alopecia (Alopecia Areata) in a C3H/HeJ mouse model. Am. J. Pathol. 2008, 172, 650–658. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, B.; Bakshi, G.; Katare, O.P. Development of liposomal systems of finasteride for topical applications: Design, characterization, and in vitro evaluation. Pharm. Dev. Technol. 2007, 12, 591–601. [Google Scholar] [CrossRef]
- Pornpattananangkul, D.; Fu, V.; Thamphiwatana, S.; Zhang, L.; Chen, M.; Vecchio, J.; Gao, W.; Huang, C.M.; Zhang, L. In vivo treatment of Propionibacterium acnes infection with liposomal lauric acids. Adv. Healthc. Mater. 2013, 2, 1322–1328. [Google Scholar] [CrossRef]
- Castro, G.A.; Coelho, A.L.; Oliveira, C.A.; Mahecha, G.A.; Oréfice, R.L.; Ferreira, L.A. Formation of ion pairing as an alternative to improve encapsulation and stability and to reduce skin irritation of retinoic acid loaded in solid lipid nanoparticles. Int. J. Pharm. 2009, 381, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Bikowski, J.; Del Rosso, J.Q. Benzoyl peroxide microsphere cream as monotherapy and combination treatment of acne. J. Drugs Dermatol. 2008, 7, 590–595. [Google Scholar] [PubMed]
- Huber, L.A.; Pereira, T.A.; Ramos, D.N.; Rezende, L.C.; Emery, F.S.; Sobral, L.M.; Leopoldino, A.M.; Lopez, R.F. Topical Skin Cancer Therapy Using Doxorubicin-Loaded Cationic Lipid Nanoparticles and lontophoresis. J. Biomed. Nanotechnol. 2015, 11, 1975–1988. [Google Scholar] [CrossRef] [PubMed]
- Jose, A.; Labala, S.; Ninave, K.M.; Gade, S.K.; Venuganti, V.V.K. Effective Skin Cancer Treatment by Topical Co-delivery of Curcumin and STAT3 siRNA Using Cationic Liposomes. AAPS PharmSciTech 2018, 19, 166–175. [Google Scholar] [CrossRef]
- Caddeo, C.; Nacher, A.; Vassallo, A.; Armentano, M.F.; Pons, R.; Fernàndez-Busquets, X.; Carbone, C.; Valenti, D.; Fadda, A.M.; Manconi, M. Effect of quercetin and resveratrol co-incorporated in liposomes against inflammatory/oxidative response associated with skin cancer. Int. J. Pharm. 2016, 513, 153–163. [Google Scholar] [CrossRef]
- Petrilli, R.; Eloy, J.O.; Saggioro, F.P.; Chesca, D.L.; de Souza, M.C.; Dias, M.V.S.; daSilva, L.L.P.; Lee, R.J.; Lopez, R.F.V. Skin cancer treatment effectiveness is improved by iontophoresis of EGFR-targeted liposomes containing 5-FU compared with subcutaneous injection. J. Control. Release 2018, 283, 151–162. [Google Scholar] [CrossRef]
- Nirmala, J.G.; Akila, S.; Nadar, M.S.A.M.; Narendhirakannan, R.T.; Chatterjee, S. Biosynthesized Vitis vinifera seed gold nanoparticles induce apoptotic cell death in A431 skin cancer cells. RSC Adv. 2016, 6, 82205–82218. [Google Scholar] [CrossRef]
- Rao, Y.F.; Chen, W.; Liang, X.G.; Huang, Y.Z.; Miao, J.; Liu, L.; Lou, Y.; Zhang, X.G.; Wang, B.; Tang, R.K.; et al. Epirubicin-loaded superparamagnetic iron-oxide nanoparticles for transdermal delivery: Cancer therapy by circumventing the skin barrier. Small 2015, 11, 239–247. [Google Scholar] [CrossRef]
- Gamal-Eldeen, A.M.; El-Daly, S.M.; Borai, I.H.; Wafay, H.A.; Abdel-Ghaffar, A.R. Photodynamic therapeutic effect of indocyanine green entrapped in polymeric nanoparticles and their anti-EGFR-conjugate in skin cancer in CD1 mice. Photodiagnosis Photodyn. Ther. 2013, 10, 446–459. [Google Scholar] [CrossRef]
- Bhatnagar, P.; Pant, A.B.; Shukla, Y.; Chaudhari, B.; Kumar, P.; Gupta, K.C. Bromelain nanoparticles protect against 7,12-dimethylbenz[a]anthracene induced skin carcinogenesis in mouse model. Eur. J. Pharm. Biopharm. 2015, 91, 35–46. [Google Scholar] [CrossRef]
- Dubey, S.K.; Dey, A.; Singhvi, G.; Pandey, M.M.; Singh, V.; Kesharwani, P. Emerging trends of nanotechnology in advanced cosmetics. Colloids Surf. B Biointerfaces 2022, 214, 112440. [Google Scholar] [CrossRef] [PubMed]
- Mavridi-Printezi, A.; Guernelli, M.; Menichetti, A.; Montalti, M. Bio-Applications of Multifunctional Melanin Nanoparticles: From Nanomedicine to Nanocosmetics. Nanomaterials 2020, 10, 2276. [Google Scholar] [CrossRef] [PubMed]
- Mohd-Setapar, S.H.; John, C.P.; Mohd-Nasir, H.; Azim, M.M.; Ahmad, A.; Alshammari, M.B. Application of Nanotechnology Incorporated with Natural Ingredients in Natural Cosmetics. Cosmetics 2022, 9, 110. [Google Scholar] [CrossRef]
- Angelopoulou, P.; Giaouris, E.; Gardikis, K. Applications and Prospects of Nanotechnology in Food and Cosmetics Preservation. Nanomaterials 2022, 12, 1196. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.; Coelho, C.; Teixeira, J.A.; Ferreira-Santos, P.; Botelho, C.M. Nanocarriers as Active Ingredients Enhancers in the Cosmetic Industry—The European and North America Regulation Challenges. Molecules 2022, 27, 1669. [Google Scholar] [CrossRef]
- Ahamad, N.; Bhardwaj, P.; Bhatia, E.; Banerjee, R. Clinical toxicity of nanomedicines. In Nano Medicine and Nano Safety; Das, M.K., Pathak, Y.V., Eds.; Springer: Singapore, 2020; pp. 533–560. [Google Scholar]
- Gurunathan, S.; Qasim, M.; Park, C.; Yoo, H.; Choi, D.Y.; Song, H.; Park, C.; Kim, J.H.; Hong, K. Cytotoxicity and Transcriptomic Analysis of Silver Nanoparticles in Mouse Embryonic Fibroblast Cells. Int. J. Mol. Sci. 2018, 19, 3618. [Google Scholar] [CrossRef]
- Abbasi, B.H.; Shah, M.; Hashmi, S.S.; Nazir, M.; Naz, S.; Ahmad, W.; Khan, I.U.; Hano, C. Green Bio-Assisted Synthesis, Characterization and Biological Evaluation of Biocompatible ZnO NPs Synthesized from Different Tissues of Milk Thistle (Silybum marianum). Nanomaterials 2019, 9, 1171. [Google Scholar] [CrossRef]
- Jiménez, Z.; Kim, Y.J.; Mathiyalagan, R.; Seo, K.H.; Mohanan, P.; Ahn, J.C.; Kim, Y.J.; Yang, D.C. Assessment of radical scavenging, whitening and moisture retention activities of Panax ginseng berry mediated gold nanoparticles as safe and efficient novel cosmetic material. Artif. Cells Nanomed. Biotechnol. 2018, 46, 333–340. [Google Scholar] [CrossRef]
- Radwan, R.A.; El-Sherif, Y.A.; Salama, M.M. A Novel Biochemical Study of Anti-Ageing Potential of Eucalyptus Camaldulensis Bark Waste Standardized Extract and Silver Nanoparticles. Colloids Surf. B Biointerfaces 2020, 191, 111004. [Google Scholar] [CrossRef]
- Shibuya, S.; Ozawa, Y.; Watanabe, K.; Izuo, N.; Toda, T.; Yokote, K.; Shimizu, T. Palladium and platinum nanoparticles attenuate aging-like skin atrophy via antioxidant activity in mice. PLoS ONE 2014, 9, e109288. [Google Scholar] [CrossRef]
- Jiménez-Pérez, Z.E.; Singh, P.; Kim, Y.J.; Mathiyalagan, R.; Kim, D.H.; Lee, M.H.; Yang, D.C. Applications of Panax ginseng leaves-mediated gold nanoparticles in cosmetics relation to antioxidant, moisture retention, and whitening effect on B16BL6 cells. J. Ginseng Res. 2018, 42, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hou, X.; Yang, C.; Pang, Y.; Li, X.; Jiang, G.; Liu, Y. Photoprotection of Cerium Oxide Nanoparticles against UVA radiation-induced Senescence of Human Skin Fibroblasts due to their Antioxidant Properties. Sci. Rep. 2019, 9, 2595. [Google Scholar] [CrossRef] [PubMed]
- Elnaggar, Y.S.; El-Refaie, W.M.; El-Massik, M.A.; Abdallah, O.Y. Lecithin-based nanostructured gels for skin delivery: An update on state of art and recent applications. J. Control. Release 2014, 180, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Xia, H.; Li, L.; Lee, R.J.; Xie, J.; Liu, Z.; Qiu, Z.; Teng, L. Liposomal Vitamin D3 as an Anti-aging Agent for the Skin. Pharmaceutics 2019, 11, 311. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.C.; Tsai, T.H. Preparation and characterization of liposomal coenzyme Q10 for in vivo topical application. Int. J. Pharm. 2010, 395, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.K.; Nanda, S.; Sharma, G.; Katare, O.P. Systematically optimized coenzyme q10-loaded novel proniosomal formulation for treatment of photo-induced aging in mice: Characterization, biocompatibility studies, biochemical estimations and anti-aging evaluation. J. Drug Target. 2016, 24, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Yücel, Ç.; Şeker Karatoprak, G.; Değim, İ.T. Anti-aging formulation of rosmarinic acid-loaded ethosomes and liposomes. J. Microencapsul. 2019, 36, 180–191. [Google Scholar] [CrossRef]
- Sherif, S.; Bendas, E.R.; Badawy, S. The clinical efficacy of cosmeceutical application of liquid crystalline nanostructured dispersions of alpha lipoic acid as anti-wrinkle. Eur. J. Pharm. Biopharm. 2014, 86, 251–259. [Google Scholar] [CrossRef]
- Manosroi, A.; Chutoprapat, R.; Abe, M.; Manosroi, W.; Manosroi, J. Anti-aging efficacy of topical formulations containing niosomes entrapped with rice bran bioactive compounds. Pharm. Biol. 2012, 50, 208–224. [Google Scholar] [CrossRef]
- Simões, S.I.; Marques, C.M.; Cruz, M.E.; Cevc, G.; Martins, M.B. The effect of cholate on solubilisation and permeability of simple and protein-loaded phosphatidylcholine/sodium cholate mixed aggregates designed to mediate transdermal delivery of macromolecules. Eur. J. Pharm. Biopharm. 2004, 58, 509–519. [Google Scholar] [CrossRef]
- Gupta, A. Nanoemulsions. In Nanoparticles for Biomedical Applications, 1st ed.; Chung, E.-J., Leon, L., Rinaldi, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 371–378. [Google Scholar]
- Ali, A.; Iqbal, S.; Ilyas, A.; Khan, H.; Asad, M.H.H.B.; Fatima, N.; Akhtar, N. Anti-pollution cosmetic-based one-step formation of w/o/w multiple emulsion containing D-biotin for skin protection: Fabrication and in vitro and in vivo evaluation. Drug Deliv. Transl. Res. 2019, 9, 1117–1132. [Google Scholar] [CrossRef] [PubMed]
- Baccarin, T.; Mitjans, M.; Ramos, D.; Lemos-Senna, E.; Vinardell, M.P. Photoprotection by Punica granatum seed oil nanoemulsion entrapping polyphenol-rich ethyl acetate fraction against UVB-induced DNA damage in human keratinocyte (HaCaT) cell line. J. Photochem. Photobiol. B. 2015, 153, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Klang, V.; Matsko, N.; Zimmermann, A.M.; Vojnikovic, E.; Valenta, C. Enhancement of stability and skin permeation by sucrose stearate and cyclodextrins in progesterone nanoemulsions. Int. J. Pharm. 2010, 393, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Mou, D.; Chen, H.; Du, D.; Mao, C.; Wan, J.; Xu, H.; Yang, X. Hydrogel-thickened nanoemulsion system for topical delivery of lipophilic drugs. Int. J. Pharm. 2008, 353, 270–276. [Google Scholar] [CrossRef]
- Hoppel, M.; Reznicek, G.; Kählig, H.; Kotisch, H.; Resch, G.P.; Valenta, C. Topical delivery of acetyl hexapeptide-8 from different emulsions: Influence of emulsion composition and internal structure. Eur. J. Pharm. Sci. 2015, 68, 27–35. [Google Scholar] [CrossRef]
- Fernandes, M.M.; Rivera, D.; Francesko, A.; Slizyte, R.; Mozuraityte, R.; Rommi, K.; Lantto, R.; Tzanov, T. Bio/sonochemical conversion of fish backbones into bioactive nanospheres. Process Biochem. 2015, 50, 1843–1851. [Google Scholar] [CrossRef]
- Abbas, H.; Kamel, R.; El-Sayed, N. Dermal anti-oxidant, anti-inflammatory and anti-aging effects of Compritol ATO-based Resveratrol colloidal carriers prepared using mixed surfactants. Int. J. Pharm. 2018, 541, 37–47. [Google Scholar] [CrossRef]
- Roucoux, A.; Schulz, J.; Patin, H. Reduced transition metal colloids: A novel family of reusable catalysts? Chem. Rev. 2002, 102, 3757–3778. [Google Scholar] [CrossRef]
- Yoshihisa, Y.; Honda, A.; Zhao, Q.L.; Makino, T.; Abe, R.; Matsui, K.; Shimizu, H.; Miyamoto, Y.; Kondo, T.; Shimizu, T. Protective effects of platinum nanoparticles against UV-light-induced epidermal inflammation. Exp. Dermatol. 2010, 19, 1000–1006. [Google Scholar] [CrossRef]
- Yoshihisa, Y.; Zhao, Q.L.; Hassan, M.A.; Wei, Z.L.; Furuichi, M.; Miyamoto, Y.; Kondo, T.; Shimizu, T. SOD/catalase mimetic platinum nanoparticles inhibit heat-induced apoptosis in human lymphoma U937 and HH cells. Free Radic. Res. 2011, 45, 326–335. [Google Scholar] [CrossRef]
- Elhusseiny, A.F.; Hassan, H.H. Antimicrobial and antitumor activity of platinum and palladium complexes of novel spherical aramides nanoparticles containing flexibilizing linkages: Structure-property relationship. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 103, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Ishizuka, S. (Ed.) Generation of PAPLAL; Juseikai: Tokyo, Japan, 1956. (In Japanese) [Google Scholar]
- Tajima, K.; Watabe, R.; Kanaori, K. Antioxidant activity of PAPLAL a colloidal mixture of Pt and Pd metal to superoxide anion radical as studied by quantitative spin trapping ESR measurements. Clin. Phamacol. Ther. 2005, 15, 635–642. (In Japanese) [Google Scholar]
- Cao, M.; Li, B.; Guo, M.; Liu, Y.; Zhang, L.; Wang, Y.; Hu, B.; Li, J.; Sutherland, D.S.; Wang, L.; et al. In vivo percutaneous permeation of gold nanomaterials in consumer cosmetics: Implication in dermal safety assessment of consumer nanoproducts. Nanotoxicology 2021, 15, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Flieger, J.; Flieger, W.; Baj, J.; Maciejewski, R. Antioxidants: Classification, Natural Sources, Activity/Capacity Measurements, and Usefulness for the Synthesis of Nanoparticles. Materials 2021, 14, 4135. [Google Scholar] [CrossRef]
- Flieger, J.; Franus, W.; Panek, R.; Szymańska-Chargot, M.; Flieger, W.; Flieger, M.; Kołodziej, P. Green Synthesis of Silver Nanoparticles Using Natural Extracts with Proven Antioxidant Activity. Molecules 2021, 26, 4986. [Google Scholar] [CrossRef]
- Celardo, I.; Pedersen, J.Z.; Traversa, E.; Ghibelli, L. Pharmacological potential of cerium oxide nanoparticles. Nanoscale 2011, 3, 1411–1420. [Google Scholar] [CrossRef]
- Kirilov, P.; Rum, S.; Gilbert, E.; Roussel, L.; Salmon, D.; Abdayem, R.; Serre, C.; Villa, C.; Haftek, M.; Falson, F.; et al. Aqueous dispersions of organogel nanoparticles–potential systems for cosmetic and dermo-cosmetic applications. Int. J. Cosmet. Sci. 2014, 36, 336–346. [Google Scholar] [CrossRef]
- Yang, L.; Zheng, Z.; Qian, C.; Wu, J.; Liu, Y.; Guo, S.; Li, G.; Liu, M.; Wang, X.; Kaplan, D.L. Curcumin-functionalized silk biomaterials for anti-aging utility. J. Colloid Interface Sci. 2017, 496, 66–77. [Google Scholar] [CrossRef]
- Kaul, S.; Gulati, N.; Verma, D.; Mukherjee, S.; Nagaich, U. Role of Nanotechnology in Cosmeceuticals: A Review of Recent Advances. J. Pharm. 2018, 2018, 3420204. [Google Scholar] [CrossRef]
- Lohani, A.; Verma, A.; Joshi, H.; Yadav, N.; Karki, N. Nanotechnology-based cosmeceuticals. ISRN Dermatol. 2014, 2014, 843687. [Google Scholar] [CrossRef]
- Ferraris, C.; Rimicci, C.; Garelli, S.; Ugazio, E.; Battaglia, L. Nanosystems in Cosmetic Products: A Brief Overview of Functional, Market, Regulatory and Safety Concerns. Pharmaceutics 2021, 13, 1408. [Google Scholar] [CrossRef] [PubMed]
- English, J.S.; Dawe, R.S.; Ferguson, J. Environmental effects and skin disease. Br. Med. Bull. 2003, 68, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Jessop, P.G.; Ahmadpour, F.; Buczynski, M.A.; Burns, T.J.; Green, N.B., II; Korwin, R.; Long, D.; Massad, S.K.; Manley, J.B.; Omidbakhsh, N.; et al. Opportunities for greener alternatives in chemical formulations. Green Chem. 2015, 17, 2664–2678. [Google Scholar] [CrossRef]
- Suter, F.; Schmid, D.; Wandrey, F.; Zülli, F. Heptapeptide-loaded solid lipid nanoparticles for cosmetic anti-aging applications. Eur. J. Pharm. Biopharm. 2016, 108, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.M.; O’Donovan, M.; Sun, L.; Choi, J.Y.; Ren, M.; Cao, K. Anti-Aging Potentials of Methylene Blue for Human Skin Longevity. Sci. Rep. 2017, 7, 2475. [Google Scholar] [CrossRef] [PubMed]
- Rembiesa, J.; Ruzgas, T.; Engblom, J.; Holefors, A. The Impact of Pollution on Skin and Proper Efficacy Testing for Anti-Pollution Claims. Cosmetics 2018, 5, 4. [Google Scholar] [CrossRef]
- Fernandez-Mejia, C.; Lazo-de-la-Vega-Monroy, M.L. Biological effects of pharmacological concentrations of biotin. J. Evid. Based Complement Altern. Med. 2011, 16, 40–48. [Google Scholar] [CrossRef]
- Lee, C.W.; Lin, Z.C.; Hsu, L.F.; Fang, J.Y.; Chiang, Y.C.; Tsai, M.H.; Lee, M.H.; Li, S.Y.; Hu, S.C.; Lee, I.T.; et al. Eupafolin ameliorates COX-2 expression and PGE2 production in particulate pollutants-exposed human keratinocytes through ROS/MAPKs pathways. J. Ethnopharmacol. 2016, 189, 300–309. [Google Scholar] [CrossRef]
- Lin, Z.C.; Lee, C.W.; Tsai, M.H.; Ko, H.H.; Fang, J.Y.; Chiang, Y.C.; Liang, C.J.; Hsu, L.F.; Hu, S.C.; Yen, F.L. Eupafolin nanoparticles protect HaCaT keratinocytes from particulate matter-induced inflammation and oxidative stress. Int. J. Nanomed. 2016, 11, 3907–3926. [Google Scholar] [CrossRef]
- Lee, C.W.; Su, Y.H.; Chiang, Y.C.; Lee, I.T.; Li, S.Y.; Lee, H.C.; Hsu, L.F.; Yan, Y.L.; Li, H.Y.; Chen, M.C.; et al. Glycofullerenes Inhibit Particulate Matter Induced Inflammation and Loss of Barrier Proteins in HaCaT Human Keratinocytes. Biomolecules 2020, 10, 514. [Google Scholar] [CrossRef]
- Ramsden, C.S.; Henry, T.B.; Handy, R.D. Sub-lethal effects of titanium dioxide nanoparticles on the physiology and reproduction of zebrafish. Aquat. Toxicol. 2013, 126, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Iswarya, V.; Bhuvaneshwari, M.; Alex, S.A.; Iyer, S.; Chaudhuri, G.; Chandrasekaran, P.T.; Bhalerao, G.M.; Chakravarty, S.; Raichur, A.M.; Chandrasekaran, N.; et al. Combined toxicity of two crystalline phases (anatase and rutile) of Titania nanoparticles towards freshwater microalgae: Chlorella sp. Aquat. Toxicol. 2015, 161, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, N.I.; Allen, G.J.; Robart, T.E.; Dieni, C.A.; MacCormack, T.J. Zinc oxide nanoparticles trigger cardiorespiratory stress and reduce aerobic scope in the white sucker, Catostomus commersonii. NanoImpact 2016, 2, 29–37. [Google Scholar] [CrossRef]
- Galhano, V.; Zeumer, R.; Monteiro, M.S.; Knopf, B.; Meisterjahn, B.; Soares, A.M.V.M.; Loureiro, S.; Schlechtriem, C.; Lopes, I. Effects of wastewater-spiked nanoparticles of silver and titanium dioxide on survival, growth, reproduction and biochemical markers of Daphnia magna. Sci. Total. Environ. 2022, 839, 156079. [Google Scholar] [CrossRef] [PubMed]
- Zeumer, R.; Galhano, V.; Monteiro, M.S.; Kuehr, S.; Knopf, B.; Meisterjahn, B.; Soares, A.M.V.M.; Loureiro, S.; Lopes, I.; Schlechtriem, C. Chronic effects of wastewater-borne silver and titanium dioxide nanoparticles on the rainbow trout (Oncorhynchus mykiss). Sci. Total. Environ. 2020, 723, 137974. [Google Scholar] [CrossRef] [PubMed]
- Gojznikar, J.; Zdravković, B.; Vidak, M.; Leskošek, B.; Ferk, P. TiO2 Nanoparticles and Their Effects on Eukaryotic Cells: A Double-Edged Sword. Int. J. Mol. Sci. 2022, 23, 12353. [Google Scholar] [CrossRef]
- Wang, K.; Ruan, J.; Song, H.; Zhang, J.; Wo, Y.; Guo, S.; Cui, D. Biocompatibility of Graphene Oxide. Nanoscale Res. Lett. 2011, 6, 8. [Google Scholar] [CrossRef]
- Kumar Yadav, V.; Gnanamoorthy, G.; Ali, D.; Parimita Bera, S.; Roy, A.; Kumar, G.; Choudhary, N.; Kalasariya, H.; Basnet, A. Cytotoxicity, Removal of Congo Red Dye in Aqueous Solution Using Synthesized Amorphous Iron Oxide Nanoparticles from Incense Sticks Ash Waste. J. Nanomater. 2022, 2022, 5949595. [Google Scholar] [CrossRef]
- Paramo, L.A.; Feregrino-Pérez, A.A.; Guevara, R.; Mendoza, S.; Esquivel, K. Nanoparticles in Agroindustry: Applications, Toxicity, Challenges, and Trends. Nanomaterials 2020, 10, 1654. [Google Scholar] [CrossRef]
- Vineeth Kumar, C.M.; Karthick, V.; Kumar, V.G.; Inbakandan, D.; Rene, E.R.; Suganya, K.S.U.; Embrandiri, A.; Dhas, T.S.; Ravi, M.; Sowmiya, P. The impact of engineered nanomaterials on the environment: Release mechanism, toxicity, transformation, and remediation. Environ. Res. 2022, 212, 113202. [Google Scholar] [CrossRef]
- Rajput, V.; Minkina, T.; Sushkova, S.; Mandzhieva, S.; Rensing, C. Toxicity of Nanoparticles in Plants; Academic Press: London, UK, 2022. [Google Scholar]
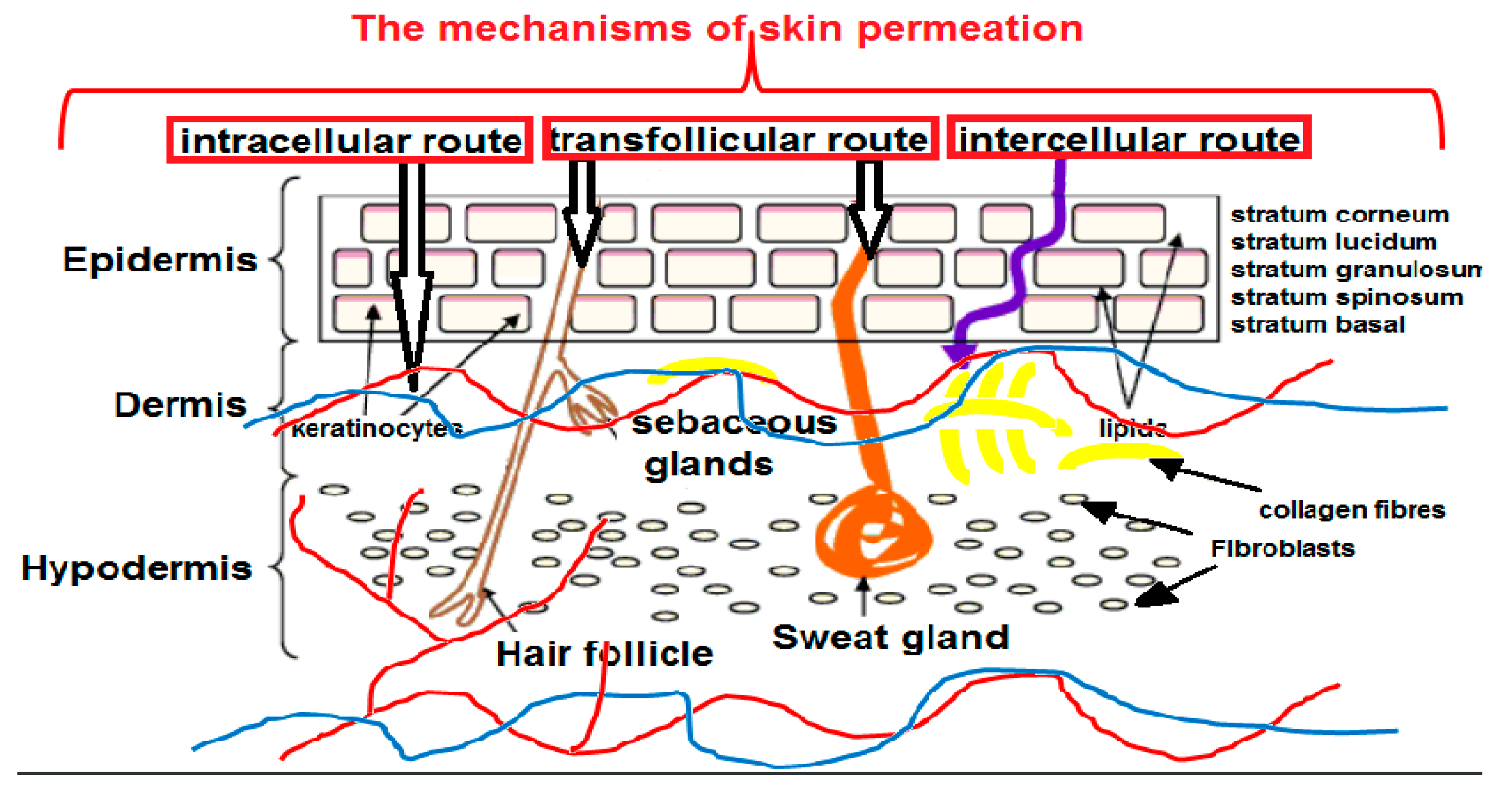
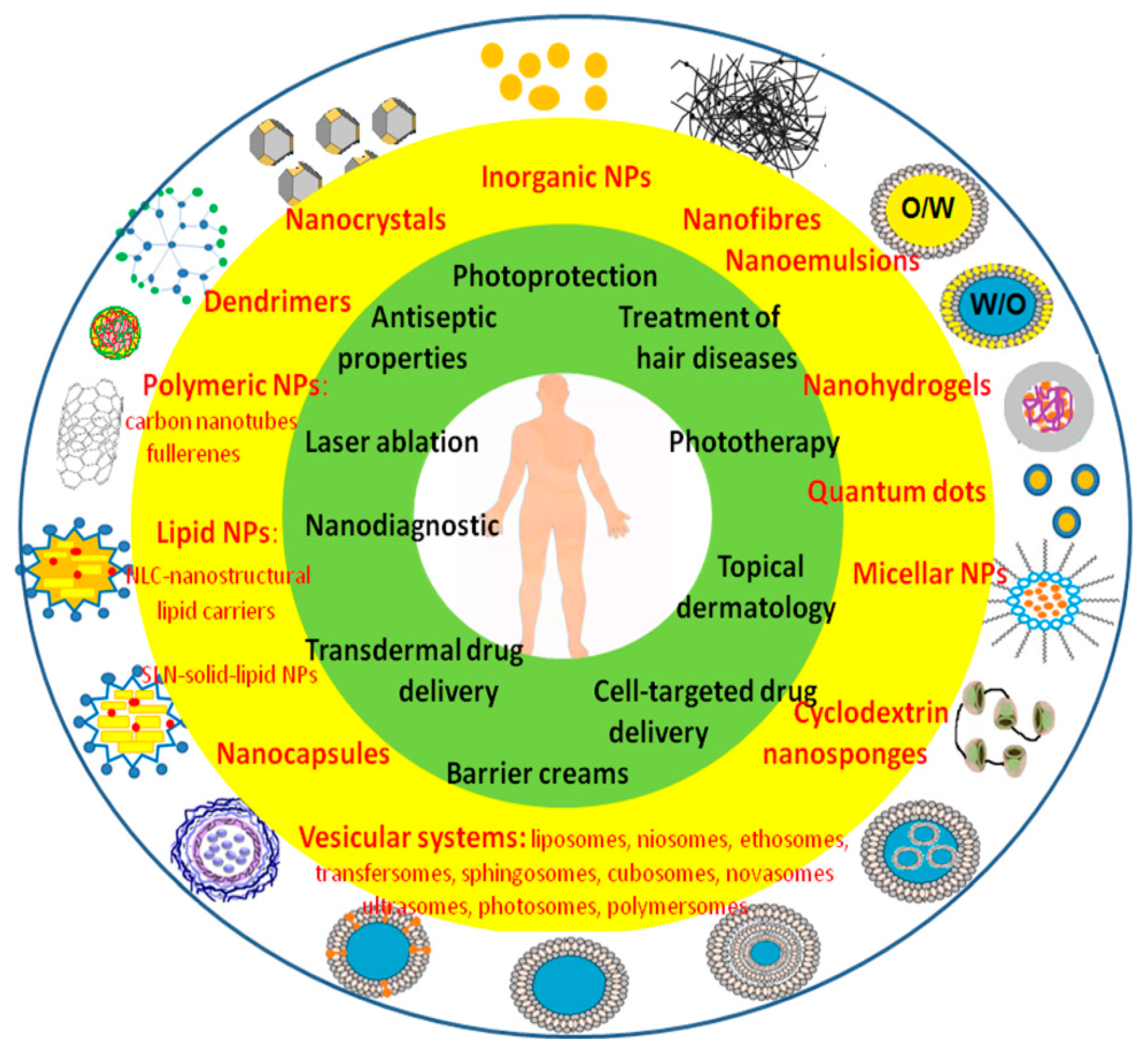
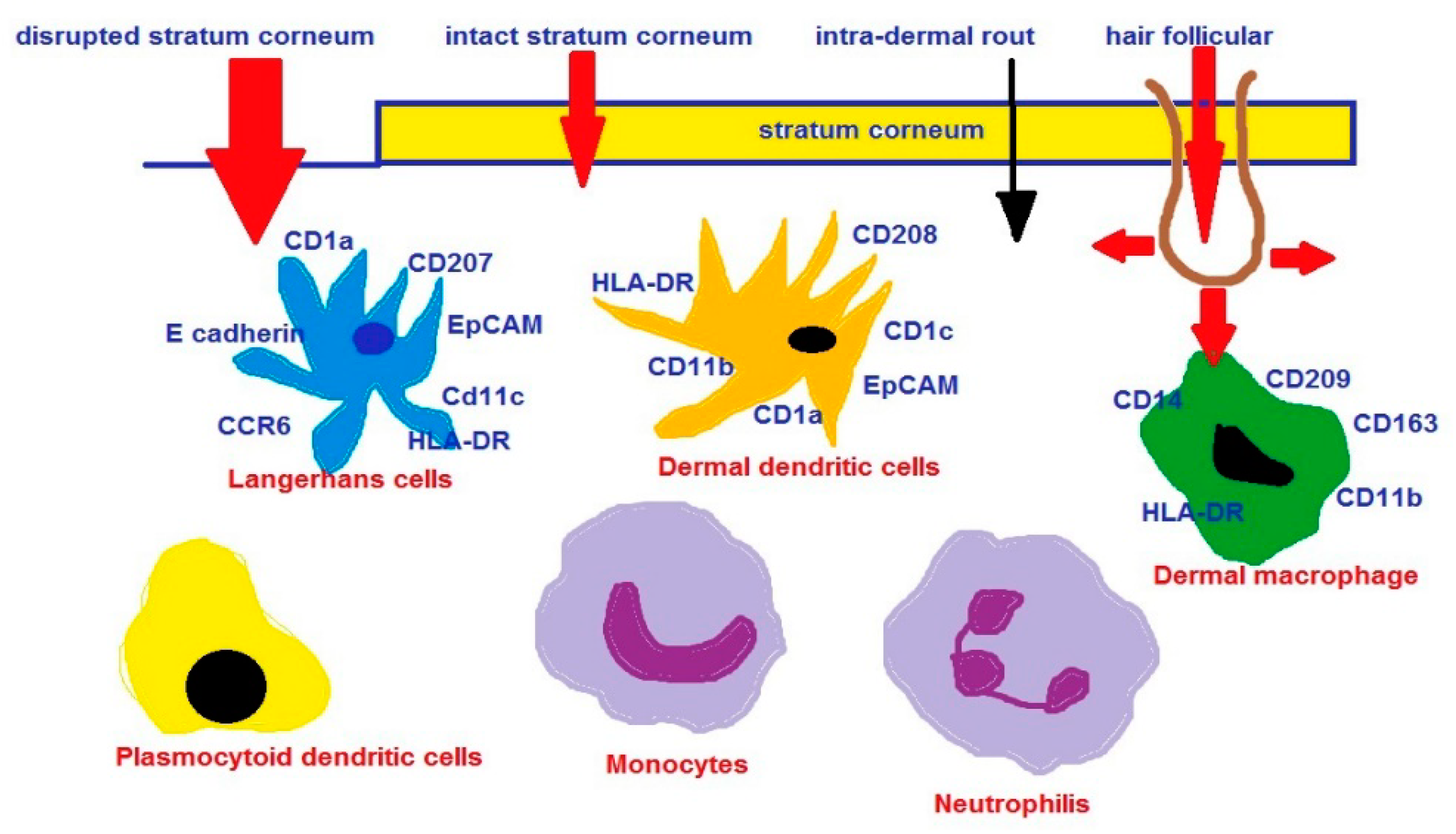
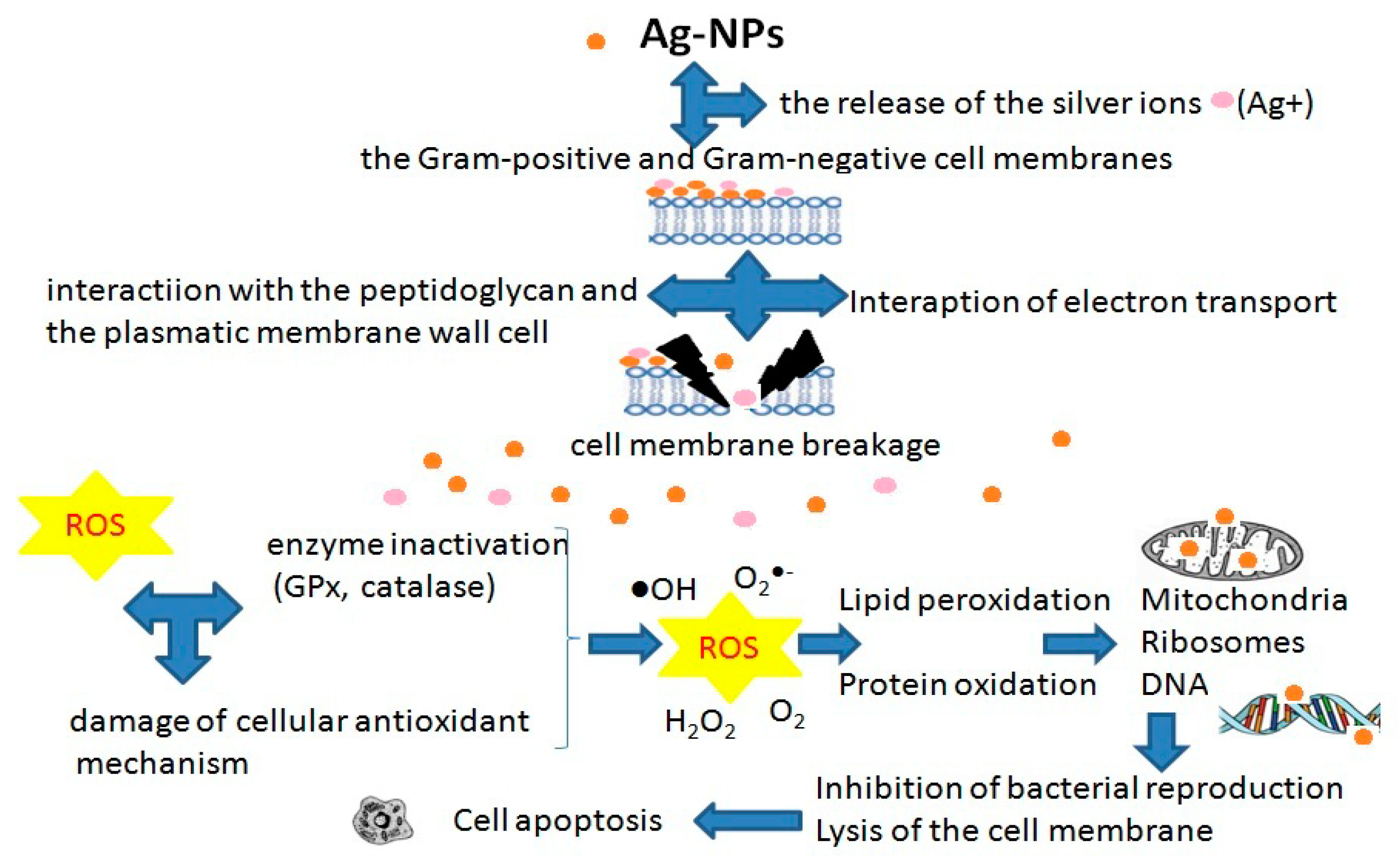
| Nanocarriers | NPs Parameters | Drug Loaded | Skin Permeation Studies | Ref |
|---|---|---|---|---|
| therapy of psoriasis | ||||
| NLCs (Precirol VR ATO 5, LabrafacTM PG, TweenVR 20) | 203.3 ± 1.20 nm; PDI 0.29 ± 0.04; EE% 100% | dithranol | IMQ induced a murine model of human psoriasis | [358] |
| liposomes (DOTAP + CHl) | 111 ± 1.62 nm; PDI 0.27 ± 0.08; EE% 93 ± 2.12%; ZP 41.12 ± 3.56 mV | cyclosporine | IMQ induced a murine model of human psoriasis | [359] |
| 1-liposomes (PC + CH), 2-niosomes (Span 80 + CH), 3-emulsomes (PC + CH + tristearin) | 1-(368.5 ± 43 nm, PDI 0.136 ± 0.024, EE% 70.98 ± 2.36%); 2-(342.7 ± 35 nm, PDI 0.167 ± 0.045, EE% 54.30 ± 2.16%); 3-(172.8 ± 10 nm, PDI 0.116 ± 0.019; EE% 83.79 ± 3.58%) | capsaicin | hairless albino rat skin from the abdominal and dorsal area after removing underlying fat and subcutaneous tissues | [360] |
| liposomes (PC + OA), (PC + CH) | 80–140 nm; PDI 0.14−0.37; ZP 6.64–2.56 mV; EE mol% 2.41–10.20% | methotrexate | the abdominal skin of newly born pig, the hairs, and lipid layer were removed | [361] |
| niosomes (Span 60 + CH) | 477.8 nm; EE% 83.02% | diacerein | in vitro-the albino rats; the hairs, and subcutaneous tissues were removed | [362] |
| niosomes (Span 60 + CH) | 369.73 ± 45.45 nm; EE% 90.32 ± 3.80%; ZP −36.33 ± 1.80 mV | acitretin | ex vivo- HaCaT cells (a keratinocyte cell line); in vivo-mouse tail model | [363] |
| niosome hydrogels (Span 20, Span 60, CH) | 147.4 nm ± 5.6 nm; PDI 0.258 ± 0.02; ZP 48.9 mV ± 1.1 mV; Y% 90.42% ± 3.38%. | celastrol | female C57/BL6 mice, the dorsal hair was removed (a razor + depilatory cream) | [364] |
| ethosomes (soya lecithin + ethanol) | 376.04 ± 3.47 nm; EE% 91.77 ± 0.02% | methotrexate + salicylic acid | ex-vivo-pig ear skin; in vivo-the shaved dorsal surface of Albino mice | [365] |
| ethosomes, liposomes | 116 to 199 nm (liposomes); 146 to 381 nm (ethosomes); EE% ≥ 97.2% (liposomes), ≥77% (ethosomes) | anthralin (1,8-dihydroxy-9-anthrone) | in vitro-a dialysis membrane (MWCO: 6–8 kDa); ex vivo-Wistar male albino rats with abdominal skin freed from hair; the connective tissue, fat, and subcutaneous tissues | [366] |
| cationic liposomes (DC-CH, CH), anionic liposomes (egg lecithin, CH, tetramyristoyl cardiolipin) | 100 nm; ZP + 25.8 mV, EE% 75.12% (cationic); ZP −28.5 mV, EE% 60.08% (anionic) | psoralen + UVA (PUVA) | IMQ-induced psoriatic plaque model | [367] |
| nanoemulsion | 202.6 ± 11.59 nm; PDI 0.233 ± 0.01; EE% 76.57 ± 2.48% | methotrexate | in vivo, in vitro: male rabbits, male Sprague Dawley rats; the abdomen skin cleaned of lipids and connective tissues | [368] |
| nanoemugel | 76.93 nm; PDI 0.121; ZP −20.5 mV | Curcumin + imiquimod | ex vivo, in vivo-BALB/c mice; the abdominal, and the dorsal skin hair shaved, the subcutaneous tissue, and fat at the dermis removed | [369] |
| nanoemusion | 93.37 ± 2.58 nm; PDI 0.330 ± 0.025. | tacrolimus and kalonji oil | in vitro-a dialysis bag membrane with a molecular weight cut-off 12,000 Da; in vitro-A-431 cell lines; ex vivo-the dorsal portion of pig ear skin shaved; subcutaneous fat removed; in vivo-IMQ induced psoriasis model on BALB/c mice shaved on their back | [370] |
| the nanomiemgel composed of nanomicelle-NMI (with Vit.E TPGS) + nanoemulsion – NEM (olive oil + miglyol + Polysorbate80 + Transcutol) | 229 ± 16 nm (NEM); 185 ± 10 nm (NMI); PDI 0.18 ± 0.06 (NEM); PDI 0.12 ± 0.08 (NMI); EE% 96.74 ± 4.24% (ACE-NEM); EE% 95.72 ± 3.58% (CAP-NEM); NMI, 94.88 ± 3.76% (ACE-NMI); 92.69 ± 3.08% (CAP-NMI) | aceclofenac (ACE) capsaicin (CAP) | ex vivo-human skin with a thickness of 0.5–0.1 mm; in vivo-C57BL/6 mice with shaved backs | [371] |
| liposomes (DDC642) | 100 nm | RNA interference (RNAi) molecule | in vitro-psoriasis-induced keratinocytes, and melanocytes cultured | [372] |
| transfersomes | 94.49 ± 6 nm–154.65 ± 8.46 nm; EE% 59.17 ± 5.03% | tacrolimus | ex vivo, in vivo-Albino Wistar Rat, the hairs trimmed | [373] |
| flexible liposomes (lecithin, Tween-80) | 76.1 ± 0.5 nm; PDI 0.251 ± 0.009; EE% 99% | all-trans retinoic acid, betamethasone | in vitro-immortalized human keratinocytes (HaCaT), full-thickness skin from the ventral part of rats with removed extraneous subcutaneous fat; in vivo-BALB/c mice model induced by IQM | [374] |
| polymeric NPs (PLGA) | 307.3 ± 8.5 nm; PDI 0.317; ZP −43.4 ± 2.6 mV; EE% 61.1 ± 1.9%; DL 1.9 ± 0.1% | apremilast | male Wistar albino rats | [375] |
| PLGA NPs | 100 nm | indomethacin | rat skin/iontophoresis | [376] |
| polymeric micelles (poly(ethylene glycol)-b-oligo(desaminotyrosyl-tyrosine octyl ester suberate)-b-poly(ethylene glycol) | 70 nm; PDI ≤ 0.22 | paclitaxel | HaCaT (a cell line of human keratinocytes); human cadaver skin samples | [377] |
| polymeric micelles (methoxy-poly(ethylene glycol)-dihexyl substituted polylactide (MPEG-dihexPLA) diblock copolymer) | 10–50 nm | tacrolimus | human skin | [378] |
| polymeric nanocapsules (Eudragit RS 100) | 139 ±3.6 nm; ZP +11.38 ± 1.7 mV; EE% 81 ± 2% | dexamethasone | in vitro | [379] |
| Nanospheres; lipoglobules | 70 nm | thymoquinone | in vitro-cell lines and IMQ-induced psoriatic plaque model | [380] |
| biogenically obtained Au-NPs (ethanolic extract of Woodfordia fruticosa flowers) | 10–20 nm | myricetin quercetin ellagic acid | in vivo-Swiss albino mice, IMQ-induced psoriasis-like skin inflammation model; shaved dorsum surface of the mice skin | [381] |
| Au-NPs functionalized by 3-mercapto-1-propansulfonate (AuNPs-3MPS) | 5 nm | methotrexate | in vivo-C57BL/6 mice (Charles River) with shaved backs; in vitro-acute toxicity studied on human skin equivalents (HSEs) after 21-day culture period (adult human keratinocytes seeded on a dermal substitute consisting of a collagen type I matrix and fibroblasts) | [382] |
| therapy of vitiligo | ||||
| liposomes (DC-CH, CH + sodium deoxy cholate) | 120–130 nm; ZP +46.2 mV; EE% 74.09% (psoralen), 76.91% (resveratrol) | psoralen, resveratrol | B16F10 cell line | [383] |
| deformable liposomes | 64.8 ± 1.3 nm; PDI 0.14 ± 0.03; ZP −27.0 ± 1.1; EE% 82.7 ± 0.4 (baicalin); 61.1 ± 1.4 nm; PDI 0.24 ± 0.01; ZP −38.1 ± 1.4; EE% 87.1 ± 1.2 (berberine) | baicalin, berberine | ex vivo-the epidermis (thickness ~200 μm) from newborn pig; in vitro-human immortalized keratinocytes (HaCaT) | [384] |
| elastic cationic niosomes (Tween61/CH/dodecyldimethyl ammonium bromide) | 163.5 ± 1.8 nm; ZP (+) 37.0 ± 0.6 (PE); 101.5 ± 0.5 nm; ZP (+) 36.1 ± 7.1 (TPE) | human tyrosinase plasmid (PE) Tat/human tyrosinase plasmid (TPE) | the melanoma (B16F10) cell | [385] |
| microemulsion | 18.26 nm | clobetasol propionate | in vivo-human | [386] |
| ethosomes | 80 ± 1.2 nm; ZP −0.531 ± 0.10 mV; EE% 99.14 ± 0.32% | methoxsalen | ex vivo, in vivo-shaved skin of Wistar rats | [387] |
| therapy of alopecia | ||||
| chitosan nanoparticles | 235.5 ± 99.9 nm; PDI of 0.31 ± 0.01; ZP +38.6 ± 6.0 mV | minoxidil sulphate | in vitro-porcine ear skin | [388] |
| NLCs | 393.5 ± 36.0 nm; PDI < 0.4; ZP +22.5 ± 0.2 mV; EE% 86.9% (minoxidil), 99.9% (latanoprost) | minoxidil + latanoprost | in vitro-porcine ear skin | [389] |
| polymeric nanocapsules (poly-ε-caprolactone) /manual massage | 197.8 ± 1.2 nm; PDI 0.15 ± 0.01; ZP −30.1 ± 1.8 mV; EE% 93.9 ± 0.4% | latanoprost | in vitro-the full-thickness skin of the porcine ears | [390] |
| NLCs (stearic acid + oleic acid) | 281.4 ± 7.4 nm; PDI 0.207 ± 0.009; ZP −32.90 ± 1.23 mV; EE% 92.48 ± 0.31%; DL 13.85 ± 0.47% | minoxidil | in vitro-the abdominal full-thickness skins of male Sprague Dawley rats | [391] |
| polymeric NPs | 90–300 nm | minoxidil | C57BL/6 mice | [392] |
| PLGA nanospheres | 182–205 nm | hinokitiol, glycyrrhetinic acid, 6-benzyl-amino- purine | in-vitro, in-vivo -C3H mice, extracted human scalp skin | [393] |
| cationized gelatin microspheres | - | Tbx21 siRNA | in vivo-C3H/HeJ mice, a mouse model of alopecia areata; ex vivo-skin samples obtained from the peripheral region of the alopecic skin of patients | [394] |
| liposomes (saturated phospholipid + CH) | 6.1 ± 1.8−16.6 ± 3.4 µm; PDE 88.6% | finasteride | in vitro-the full-thickness abdominal, and the dorsal mice skin after hair, and subcutaneous fat removal | [395] |
| therapy of acne | ||||
| Liposomes (egg PC + CH) | 120 nm; ZP −43 mV | lauric acids (anti-Propionibacterium acnes (P. acnes) | in vitro, in vivo-a mouse ear model | [396] |
| SLNs | 180 ± 2 nm; ZP 47 ± 4; EE% 100 ± 1% | retinoic acid | in vivo-a mutant strain of hairless mouse (rhino mice) | [397] |
| niosomes | - | benzoyl peroxide, clindamycin | in vivo-110 patients | [398] |
| therapy of skin cancer | ||||
| cationic lipid NPs (CTAB + stearic acid + monoolein) | 196.90 ± 39.73 nm; PDI 0.26 ± 0.02; ZP +63.85 ± 12.37 mV; EE% 47.39 ± 2.52% | doxorubicin | in vitro-the pig ears skin with removed hair; in vivo-female nude BALB/c/iontophoresis | [399] |
| cationic liposomes/iontophoresis | 192.6 ± 9.0 nm; PDI 0.326 ± 0.004; ZP 56.4 ± 8.0 mV; EE% (curcumin) 86.8 ± 6.0% | curcumin + STAT3 siRNA | in vitro-mouse melanoma cells (B16F10), ex vivo-porcine ear skin; in vivo- C57BL/6 mice | [400] |
| liposomes (lipoid S75 + oleic acid) | 79.0 ± 4.1 nm; PID 0.12; ZP 40.06.7 mV; EE% 71.2 ± 10.9% (QUE); 72.1 ±6.6% (RSV) | quercetin (QUE)+ resveratrol (RSV) | in vitro-human dermal fibroblasts culture; in vivo-female CD-1 mice with the back skin shaved | [401] |
| Immunoliposome /iontophoresis | 137 ± 25 nm; PDI 0.26 ± 0.04; ZP −6 mV | 5-fluorouracil+ cetuximab | in vitro-A431 (EGFR positive) and B16F10 (EGFR negative) cell lines; in vitro- dermatomed skin of the outer portion of porcine ears; in vivo-immunosuppressed Swiss nude mice | [402] |
| Au-NPs | 55.1 ± 5.1 nm; ZP −15 ± 1 mV | phytochemicals present in Vitis vinifera seeds | in vitro-A431 cancer cell line (skin carcinoma, human); HaCaT (normal, human immortalized keratinocyte cell line) | [403] |
| Fe2O3-NPs | 10 ± 2.5 nm; ZP 7.9 ± 0.4 mV | epirubicin | in vitro-melanoma WM266cells; ex vivo-human cadaver skin without subcutaneous fat | [404] |
| polymeric NPs with EGFR antibody/photodynamic therapy | - | indocyanine green | in vitro-shaved the dorsal skin area of the Female CD1 mice | [405] |
| poly (lactic-co-glycolic acid) NPs | 130.4 ± 10.5 nm; PDI 0.095; EE% 52 ± 3.34% | bromelain | in vivo-Male Swiss albino mice | [406] |
| The Active Ingredients | Nanoformulations |
|---|---|
| vit.E, panthenol | nanocapsules |
| coenzyme Q10, vitamins (A, C and E), natural extracts (Symphytum officinale, Camellia sinensis, Gingko biloba, Chondrus crispus, Rosa damascena, Aloe barbadensis), natural oils (Helianthus, Prunus Armeniaca, Symphytum officinale), hyaluronic acid, SOD | liposomes |
| plant extracts (Canadian Willow, Camellia sinensis), olive oil, vit.E, hyaluronic acid, soy firming agent, UVA/UVB filters | nanospheres |
| gold powder (24 k), silk, plant extracts (Coffee arabica, Aloe barbadensis, Cucurbita pepo), vitamins (A, C and E), hyaluronic acid, plant extracts, plant stem cell extracts | Au-NPs |
| coenzyme Q10, natural oils (hemp, macadamia nut), plant extracts (Leontopodium nivale) | NLCs, SNLs |
| ZnO-NPs, Fe2O3-NPs, TiO2-NPs, plant extracts, vitamins | nanocomplexes |
| vitamins (E, B3, provitamin B5), UVA/UVB filters, bepanthol | nanoemulsions |
| plant extracts (Gingko biloba), oils (almond, lavender), natural compounds (caffeine, amino acids, polyphenols) | nanosomes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raszewska-Famielec, M.; Flieger, J. Nanoparticles for Topical Application in the Treatment of Skin Dysfunctions—An Overview of Dermo-Cosmetic and Dermatological Products. Int. J. Mol. Sci. 2022, 23, 15980. https://doi.org/10.3390/ijms232415980
Raszewska-Famielec M, Flieger J. Nanoparticles for Topical Application in the Treatment of Skin Dysfunctions—An Overview of Dermo-Cosmetic and Dermatological Products. International Journal of Molecular Sciences. 2022; 23(24):15980. https://doi.org/10.3390/ijms232415980
Chicago/Turabian StyleRaszewska-Famielec, Magdalena, and Jolanta Flieger. 2022. "Nanoparticles for Topical Application in the Treatment of Skin Dysfunctions—An Overview of Dermo-Cosmetic and Dermatological Products" International Journal of Molecular Sciences 23, no. 24: 15980. https://doi.org/10.3390/ijms232415980
APA StyleRaszewska-Famielec, M., & Flieger, J. (2022). Nanoparticles for Topical Application in the Treatment of Skin Dysfunctions—An Overview of Dermo-Cosmetic and Dermatological Products. International Journal of Molecular Sciences, 23(24), 15980. https://doi.org/10.3390/ijms232415980





