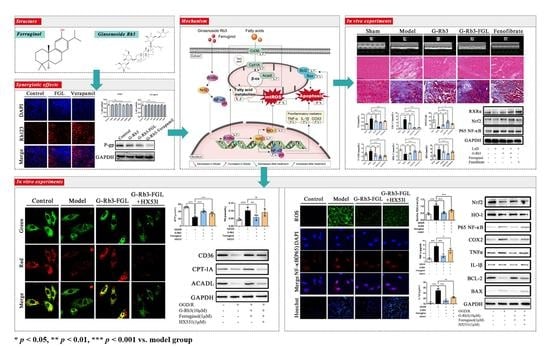
Synergistic Effects of Ginsenoside Rb3 and Ferruginol in Ischemia-Induced Myocardial Infarction
Abstract
1. Introduction
2. Results
2.1. The Combination of G-Rb3 and FGL Improved Cardiac Function and Protected Cardiac Structure in LAD-Induced MI Mice
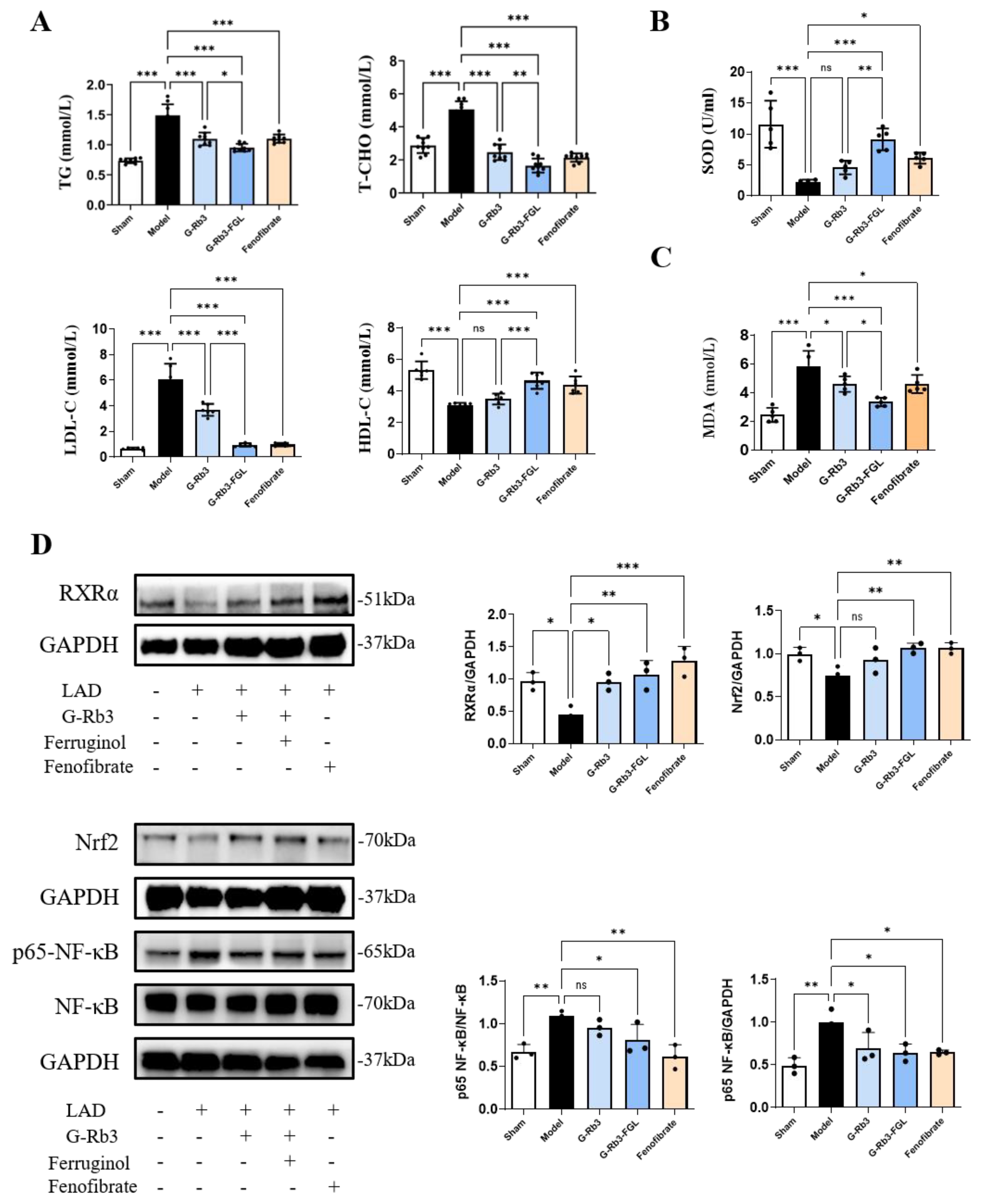
2.2. The Combination of G-Rb3 and FGL Alleviated Dyslipidemia and Oxidative Stress in LAD-Induced MI Mice
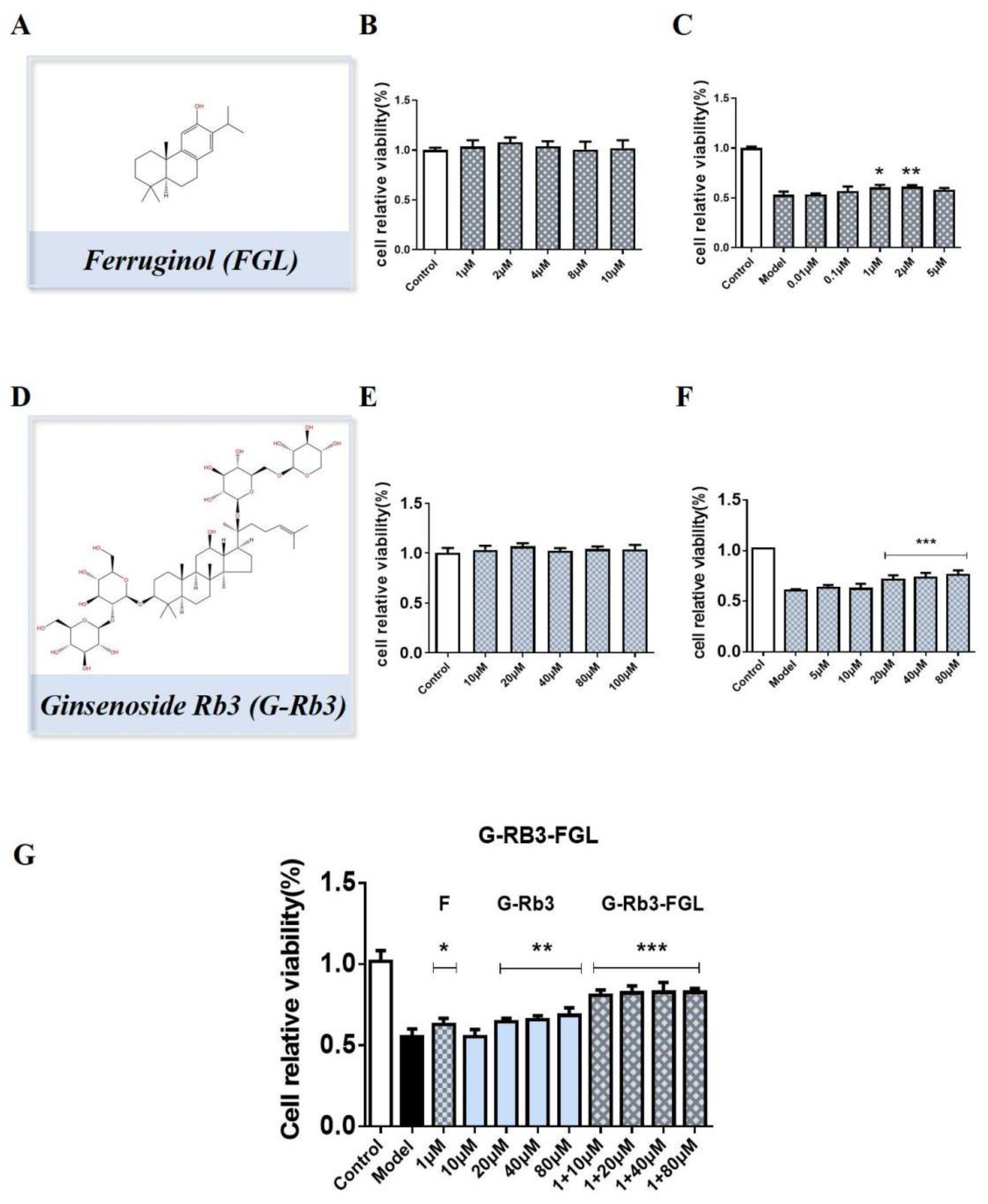
2.3. G-Rb3-FGL Protected H9C2 Cells against OGD/R-Induced Cellular Injury
2.4. Effects of G-Rb3-FGL Co-Treatment on Fatty Acid Oxidization in OGD/R-Stimulated H9C2 Cells
2.5. Effects of G-Rb3-FGL Co-Treatment on Oxidative Stress and Inflammation Response in OGD/R-Stimulated H9C2 Cells
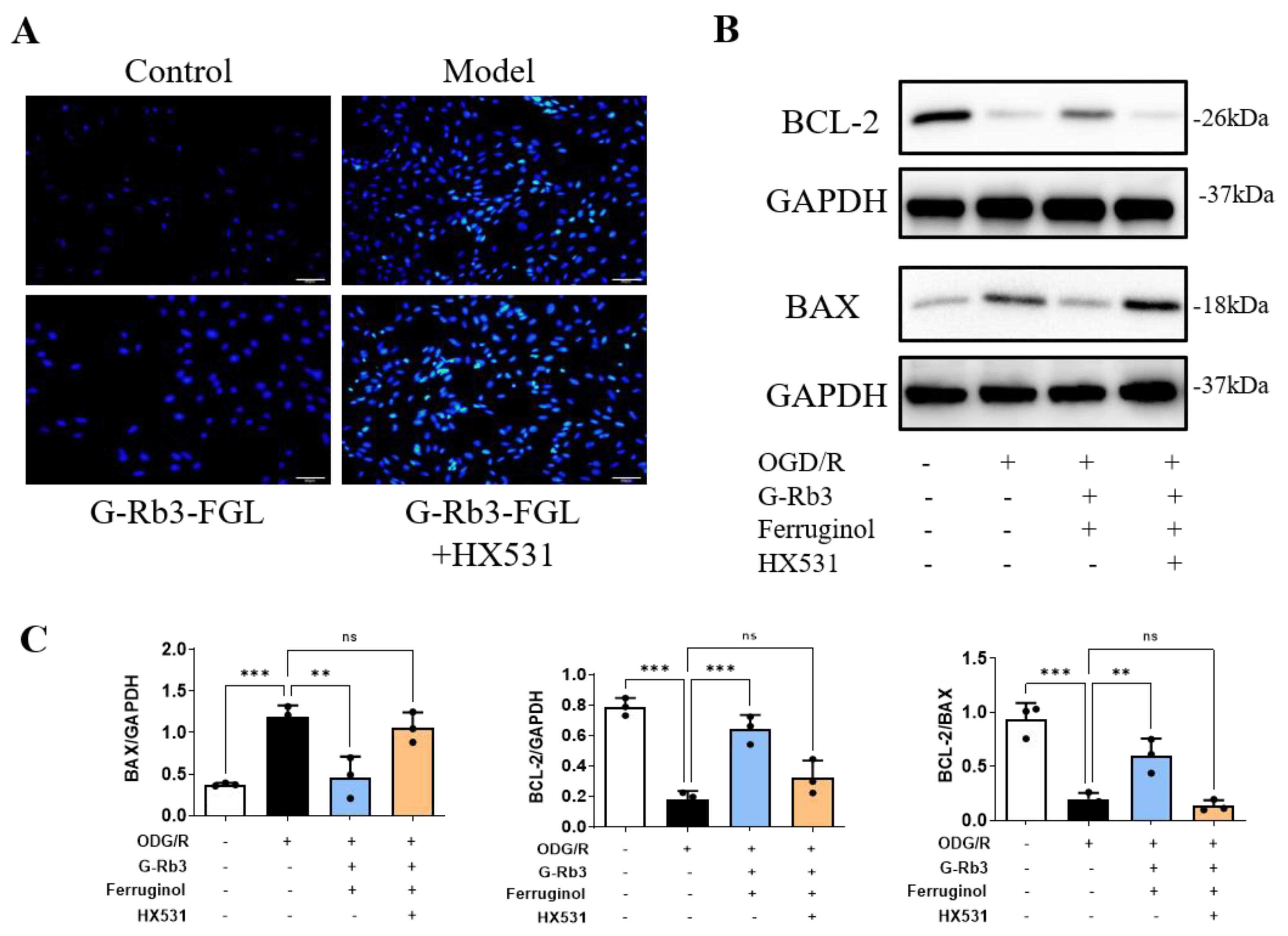
2.6. G-Rb3-FGL Inhibited Apoptosis via RXRα In Vitro
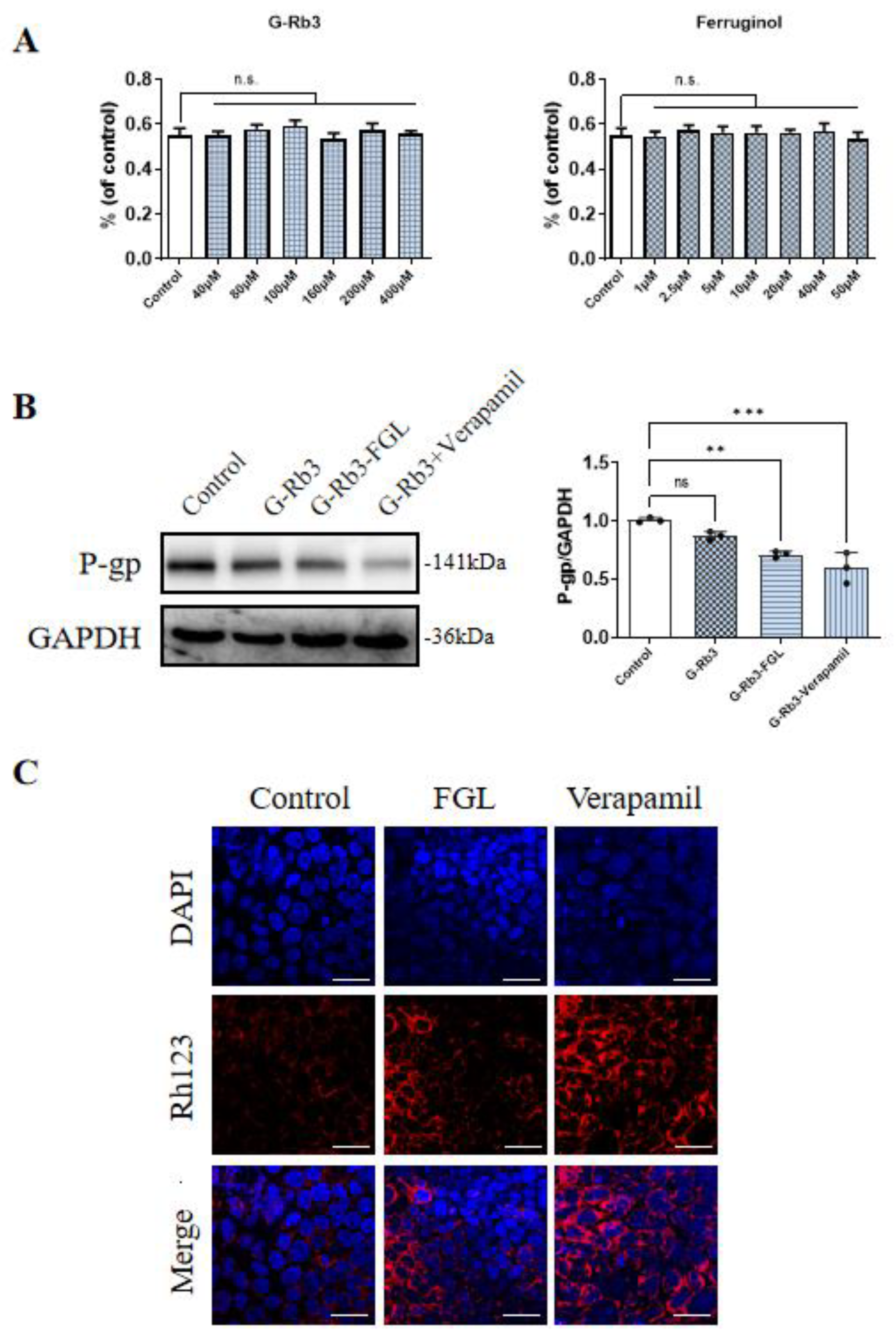
2.7. FGL Had the Potential to Improve the Oral Bioavailability of G-Rb3
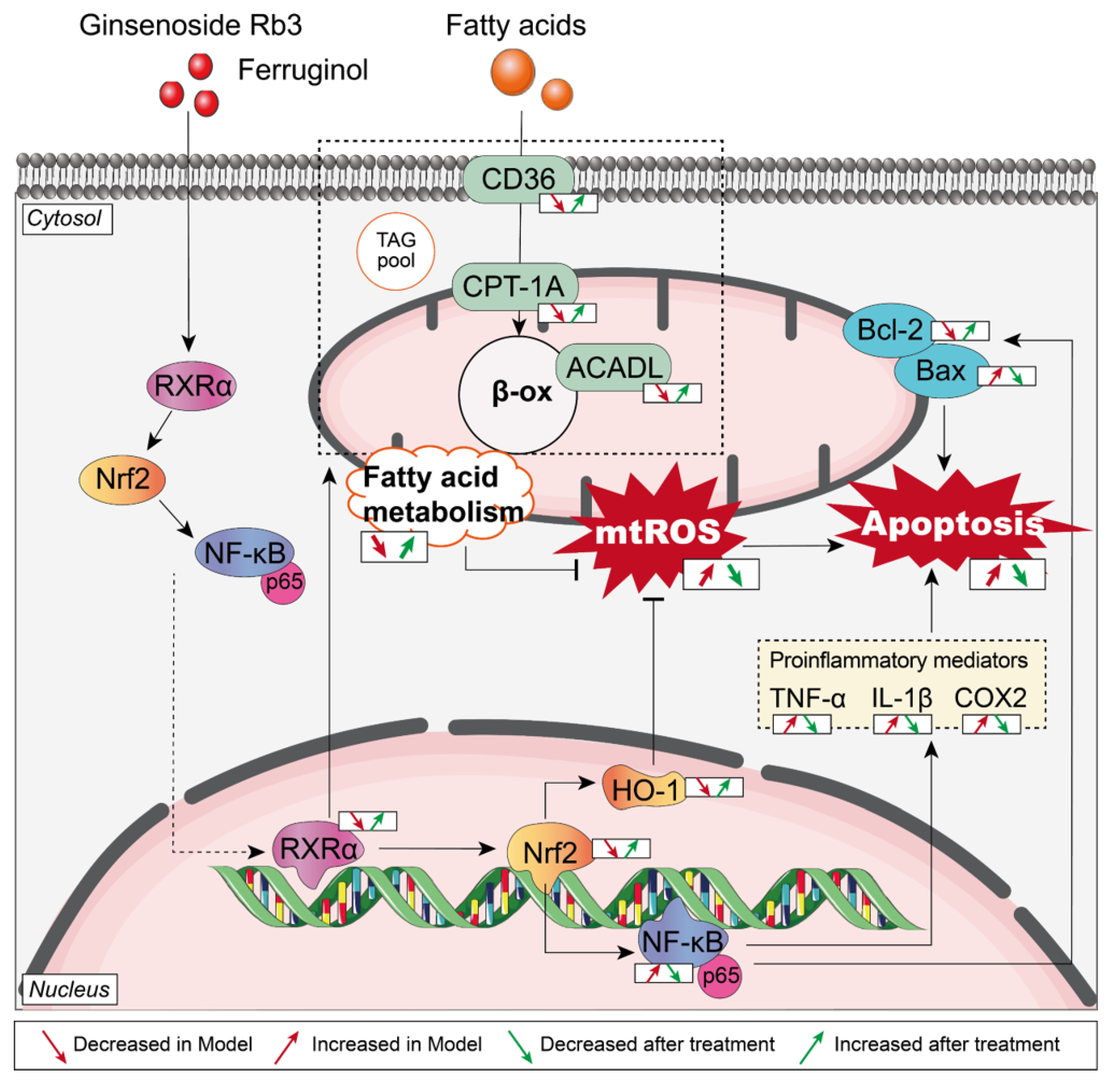
3. Discussion
4. Materials and Methods
4.1. MI Model Establishment and Animal Grouping
4.2. Cardiac Functions and Histological Assessment
4.3. Serum Detection
4.4. Cell Culture
4.5. Intracellular ROS Detection
4.6. ATP and Mitochondrial Membrane Potential Assessment
4.7. Detection of Intracellular Rh123 Content
4.8. Western Blot Analysis
| Antibodies | Source | Identifier |
| anti-RXRα antibody | Abcam, Cambrige, UK | Cat#ab125001 |
| anti-CD36 antibody | Abcam, Cambrige, UK | Cat#ab252922 |
| anti-CPT1A antibody | Abcam, Cambrige, UK | Cat#ab128568 |
| anti-ACADL antibody | Abcam, Cambrige, UK | Cat#ab196655 |
| anti-p65-NF-κB antibody | Abways, Shanghai, China | Cat#CY5034 |
| anti-TNF-α antibody | Abcam, Cambrige, UK | Cat#ab307164 |
| anti-IL-1β antibody | ProteinTech Group, Rosemont, IL, USA | Cat#10663 |
| anti-COX2 antibody | Abcam, Cambrige, UK | Cat#ab179800 |
| anti-Bax antibody | Abcam, Cambrige, UK | Cat#ab32503 |
| anti-Bcl-2 antibody | Abcam, Cambrige, UK | Cat#ab196495 |
| anti-HO-1 antibody | Abways, Shanghai, China | Cat#CY5113 |
| anti-Nrf-2 antibody | Abways, Shanghai, China | Cat#CY5136 |
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bahit, M.C.; Kochar, A.; Granger, C.B. Post-Myocardial Infarction Heart Failure. JACC Heart Fail. 2018, 6, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Hausenloy, D.J.; Bøtker, H.E.; Ferdinandy, P.; Heusch, G.; Ng, G.A.; Redington, A.; Garcia-Dorado, D. Cardiac innervation in acute myocardial ischaemia/reperfusion injury and cardioprotection. Cardiovasc. Res. 2019, 115, 1167–1177. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.B.; Hernández-Reséndiz, S.; Crespo-Avilan, G.E.; Mukhametshina, R.T.; Kwek, X.Y.; Cabrera-Fuentes, H.A.; Hausenloy, D.J. Inflammation following acute myocardial infarction: Multiple players, dynamic roles, and novel therapeutic opportunities. Pharmacol. Ther. 2018, 186, 73–87. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.; Coats, A.J.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2016, 18, 891–975. [Google Scholar] [CrossRef] [PubMed]
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, N.A.C.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; Abdollahpour, I.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.M.; Mangelsdorf, D.J. Nuclear Receptors, RXR, and the Big Bang. Cell 2014, 157, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhu, P.; Ding, Y.; Zhang, H. Retinoid X receptor alpha is a spatiotemporally predominant therapeutic target for anthracycline-induced cardiotoxicity. Sci. Adv. 2020, 6, eaay2939. [Google Scholar] [CrossRef]
- Clabby, M.L.; Robison, T.A.; Quigley, H.F.; Wilson, D.B.; Kelly, D.P. Retinoid X receptor alpha represses GATA-4-mediated transcription via a retinoid-dependent interaction with the cardiac-enriched repressor FOG-2. J. Biol. Chem. 2003, 278, 5760–5767. [Google Scholar] [CrossRef]
- Merki, E.; Zamora, M.; Raya, A.; Kawakami, Y.; Wang, J.; Zhang, X.; Burch, J.; Kubalak, S.W.; Kaliman, P.; Belmonte, J.C.I.; et al. Epicardial retinoid X receptor alpha is required for myocardial growth and coronary artery formation. Proc. Natl. Acad. Sci. USA 2005, 102, 18455–18460. [Google Scholar] [CrossRef]
- Osorio, J.C.; Stanley, W.C.; Linke, A.; Castellari, M.; Diep, Q.N.; Panchal, A.R.; Hintze, T.H.; Lopaschuk, G.D.; Recchia, F.A. Impaired myocardial fatty acid oxidation and reduced protein expression of retinoid X receptor-alpha in pacing-induced heart failure. Circulation 2002, 106, 606–612. [Google Scholar] [CrossRef]
- Shen, L.; Sun, Z.; Nie, P.; Yuan, R.; Cai, Z.; Wu, C.; Hu, L.; Jin, S.; Zhou, H.; Zhang, X.; et al. Sulindac-derived retinoid X receptor-α modulator attenuates atherosclerotic plaque progression and destabilization in ApoE−/− mice. Br. J. Pharmacol. 2019, 176, 2559–2572. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.K.; Su, Y.; Chen, L.; Chen, F.; Liu, J.; Zhou, H. Regulation of the nongenomic actions of retinoid X receptor-α by targeting the coregulator-binding sites. Acta Pharmacol. Sin. 2015, 36, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Chandra, V.; Huang, P.; Hamuro, Y.; Raghuram, S.; Wang, Y.; Burris, T.P.; Rastinejad, F. Structure of the intact PPAR-gamma-RXR- nuclear receptor complex on DNA. Nature 2008, 456, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, K.; Geng, M.; Gao, P.; Wu, X.; Hai, Y.; Li, Y.; Li, Y.; Luo, L.; Hayes, J.D.; et al. RXRα inhibits the NRF2-ARE signaling pathway through a direct interaction with the Neh7 domain of NRF2. Cancer Res. 2013, 73, 3097–3108. [Google Scholar] [CrossRef]
- Zhou, H.; Ren, J.; Toan, S.; Mui, D. Role of mitochondrial quality surveillance in myocardial infarction: From bench to bedside. Ageing Res. Rev. 2021, 66, 101250. [Google Scholar] [CrossRef]
- Shen, Y.; Liu, X.; Shi, J.; Wu, X. Involvement of Nrf2 in myocardial ischemia and reperfusion injury. Int. J. Biol. Macromol. 2019, 125, 496–502. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; Li, S.; Yu, T.; Jia, Z. Therapeutic Effects of Traditional Chinese Medicine on Cardiovascular Diseases: The Central Role of Calcium Signaling. Front. Pharmacol. 2021, 12, 682273. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Q.; Shao, M.; Ma, L.; Guo, D.; Wu, Y.; Gao, P.; Wang, X.; Li, W.; Li, C.; et al. Ginsenoside Rb3 regulates energy metabolism and apoptosis in cardiomyocytes via activating PPARα pathway. Biomed. Pharmacother. 2019, 120, 109487. [Google Scholar] [CrossRef]
- Lo, S.H.; Hsu, C.T.; Niu, H.S.; Niu, C.S.; Cheng, J.T.; Chen, Z.C. Ginsenoside Rh2 Improves Cardiac Fibrosis via PPARδ-STAT3 Signaling in Type 1-Like Diabetic Rats. Int. J. Mol. Sci. 2017, 18, 1364. [Google Scholar] [CrossRef]
- Sun, J.; Yu, X.; Huangpu, H.; Yao, F. Ginsenoside Rb3 protects cardiomyocytes against hypoxia/reoxygenation injury via activating the antioxidation signaling pathway of PERK/Nrf2/HMOX1. Biomed. Pharmacother. 2019, 109, 254–261. [Google Scholar] [CrossRef]
- Wang, Q.W.; Yu, X.F.; Xu, H.L.; Zhao, X.Z. Ginsenoside Re Improves Isoproterenol-Induced Myocardial Fibrosis and Heart Failure in Rats. Evid.-Based Complement. Altern. Med. 2019, 2019, 3714508. [Google Scholar] [CrossRef]
- Xiao, J.; Zhu, T.; Yin, Y.Z.; Sun, B. Notoginsenoside R1, a unique constituent of Panax notoginseng, blinds proinflammatory monocytes to protect against cardiac hypertrophy in ApoE−/− mice. Eur. J. Pharmacol. 2018, 833, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.L.; Fan, Y.; Liu, M.L. Ginsenoside Rg1 inhibits autophagy in H9c2 cardiomyocytes exposed to hypoxia/reoxygenation. Mol. Cell Biochem. 2012, 365, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wang, S.; Zou, X.; Jing, Y.; Yang, R.; Li, S.; Wang, F. Ginsenoside Rb1 improves cardiac function and remodeling in heart failure. Exp. Anim. 2017, 66, 217–228. [Google Scholar] [CrossRef]
- Zhong, L.; Zhou, X.L.; Liu, Y.S.; Wang, Y.M.; Ma, F.; Guo, B.L.; Yan, Z.Q.; Zhang, Q.Y. Estrogen receptor α mediates the effects of notoginsenoside R1 on endotoxin-induced inflammatory and apoptotic responses in H9c2 cardiomyocytes. Mol. Med. Rep. 2015, 12, 119–126. [Google Scholar] [CrossRef]
- Yu, S.E.; Mwesige, B.; Yi, Y.S.; Yoo, B.C. Ginsenosides: The need to move forward from bench to clinical trials. J. Ginseng. Res. 2019, 43, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.; Tang, B.; Wang, Y.; Li, C.; Zeng, Z.; Cui, L.; Zhang, X.; Shao, M.; Guo, D.; Wang, Q. Pro-angiogenic Role of Danqi Pill Through Activating Fatty Acids Oxidation Pathway Against Coronary Artery Disease. Front. Pharmacol. 2018, 9, 1414. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.J.; Li, J.H.; Zhang, Z.; Zhang, J.Y.; Liu, S.Q.; Yang, J. Suppression of MIF-induced neuronal apoptosis may underlie the therapeutic effects of effective components of Fufang Danshen in the treatment of Alzheimer’s disease. Acta Pharmacol. Sin. 2018, 39, 1421–1438. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Guo, D.; Wang, Y.; Wang, X.; Wang, Q.; Wu, Y.; Li, C.; Wang, W.; Wang, Y. Danqi Pill Protects Against Heart Failure Post-Acute Myocardial Infarction via HIF-1α/PGC-1α Mediated Glucose Metabolism Pathway. Front. Pharmacol. 2020, 11, 458. [Google Scholar] [CrossRef]
- Zhou, X.; Razmovski-Naumovski, V.; Kam, A.; Chang, D.; Li, C.G.; Chan, K.; Bensoussan, A. Synergistic study of a Danshen (Salvia Miltiorrhizae Radix et Rhizoma) and Sanqi (Notoginseng Radix et Rhizoma) combination on cell survival in EA.hy926 cells. BMC Complement Altern. Med. 2019, 19, 50. [Google Scholar] [CrossRef]
- Li, W.; Cao, J.; Wang, X.; Zhang, Y.; Sun, Q.; Jiang, Y.; Yao, J.; Li, C.; Wang, Y.; Wang, W. Ferruginol Restores SIRT1-PGC-1α-Mediated Mitochondrial Biogenesis and Fatty Acid Oxidation for the Treatment of DOX-Induced Cardiotoxicity. Front. Pharmacol. 2021, 12, 773834. [Google Scholar] [CrossRef]
- Aller, S.G.; Yu, J.; Ward, A.; Weng, Y.; Chittaboina, S.; Zhuo, R.; Harrell, P.M.; Trinh, Y.T.; Zhang, Q.; Urbatsch, I.L.; et al. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science 2009, 323, 1718–1722. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Pathophysiology of Myocardial Infarction. Compr. Physiol. 2015, 5, 1841–1875. [Google Scholar] [CrossRef]
- Shao, M.; Guo, D.; Lu, W.; Chen, X.; Ma, L.; Wu, Y.; Zhang, X.; Wang, Q.; Wang, X.; Li, W.; et al. Identification of the active compounds and drug targets of Chinese medicine in heart failure based on the PPARs-RXRα pathway. J. Ethnopharmacol. 2020, 257, 112859. [Google Scholar] [CrossRef]
- Zhao, X.R.; Gonzales, N.; Aronowski, J. Pleiotropic role of PPARγ in intracerebral hemorrhage: An intricate system involving Nrf2, RXR, and NF-κB. CNS Neurosci. Ther. 2015, 21, 357–366. [Google Scholar] [CrossRef]
- Zhang, Q.; Shao, M.; Zhang, X.; Wang, Q.; Guo, D.; Yang, X.; Li, C.; Wang, Y. The Effect of Chinese Medicine on Lipid and Glucose Metabolism in Acute Myocardial Infarction Through PPARγ Pathway. Front. Pharmacol. 2018, 9, 1209. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, H.; Li, X.; Yang, T.; Jiang, Q. Effects of PPARα/PGC-1α on the energy metabolism remodeling and apoptosis in the doxorubicin induced mice cardiomyocytes in vitro. Int. J. Clin. Exp. Pathol. 2015, 8, 12216–12224. [Google Scholar]
- Poustchi, F.; Amani, H.; Ahmadian, Z.; Niknezhad, S.V.; Mehrabi, S.; Santos, H.A.; Shahbazi, M.A. Combination Therapy of Killing Diseases by Injectable Hydrogels: From Concept to Medical Applications. Adv. Healthc. Mater. 2021, 10, e2001571. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Karwi, Q.G.; Tian, R.; Wende, A.R.; Abel, E.D. Cardiac Energy Metabolism in Heart Failure. Circ. Res. 2021, 128, 1487–1513. [Google Scholar] [CrossRef]
- Senol, S.P.; Temiz, M.; Guden, D.S.; Cecen, P.; Sari, A.N.; Sahan-Firat, S.; Falck, J.R.; Dakarapu, R.; Malik, K.U.; Tunctan, B. Contribution of PPARα/β/γ, AP-1, importin-α3, and RXRα to the protective effect of 5,14-HEDGE, a 20-HETE mimetic, against hypotension, tachycardia, and inflammation in a rat model of septic shock. Inflamm. Res. 2016, 65, 367–387. [Google Scholar] [CrossRef]
- Guleria, R.S.; Choudhary, R.; Tanaka, T.; Baker, K.M.; Pan, J. Retinoic acid receptor-mediated signaling protects cardiomyocytes from hyperglycemia induced apoptosis: Role of the renin-angiotensin system. J. Cell Physiol. 2011, 226, 1292–1307. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, L.; Wang, S.; Cheng, H.; Xu, L.; Pei, G.; Wang, Y.; Fu, C.; Jiang, Y. Signaling pathways and targeted therapy for myocardial infarction. Signal Transduct. Target. Ther. 2022, 7, 78. [Google Scholar] [CrossRef]
- De Oliveira, M.R.; de Souza, I.C.C.; Fürstenau, C.R. Carnosic Acid Induces Anti-Inflammatory Effects in Paraquat-Treated SH-SY5Y Cells Through a Mechanism Involving a Crosstalk Between the Nrf2/HO-1 Axis and NF-κB. Mol. Neurobiol. 2018, 55, 890–897. [Google Scholar] [CrossRef]
- Lee, D.Y.; Yun, S.M.; Song, M.Y.; Jung, K. Cyanidin Chloride Induces Apoptosis by Inhibiting NF-κB Signaling through Activation of Nrf2 in Colorectal Cancer Cells. Antioxidants 2020, 9, 285. [Google Scholar] [CrossRef]
- García-Varela, L.; García, D.V.; Aguiar, P.; Kakiuchi, T.; Ohba, H.; Harada, N.; Nishiyama, S.; Tago, T.; Elsinga, P.H.; Tsukada, H.; et al. Head-to-head comparison of (R)-[(11)C]verapamil and [(18)F]MC225 in non-human primates, tracers for measuring P-glycoprotein function. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4307–4317. [Google Scholar] [CrossRef]
- Elmeliegy, M.; Vourvahis, M.; Guo, C.; Wang, D.D. Effect of P-glycoprotein (P-gp) Inducers on Exposure of P-gp Substrates: Review of Clinical Drug-Drug Interaction Studies. Clin. Pharm. 2020, 59, 699–714. [Google Scholar] [CrossRef]
- Meng, H.; Du, Z.; Lu, W.; Wang, Q.; Sun, X.; Jiang, Y.; Wang, Y.; Li, C.; Tu, P. Baoyuan decoction (BYD) attenuates cardiac hypertrophy through ANKRD1-ERK/GATA4 pathway in heart failure after acute myocardial infarction. Phytomedicine 2021, 89, 153617. [Google Scholar] [CrossRef]
- Zeng, Z.; Wang, Q.; Yang, X.; Ren, Y.; Jiao, S.; Zhu, Q.; Guo, D.; Xia, K.; Wang, Y.; Li, C.; et al. Qishen granule attenuates cardiac fibrosis by regulating TGF-β /Smad3 and GSK-3β pathway. Phytomedicine 2019, 62, 152949. [Google Scholar] [CrossRef]
- Chen, X.; Ma, L.; Shao, M.; Wang, Q.; Jiang, Q.; Guo, D.; Zhang, P.; Yang, R.; Li, C.; Wang, Y.; et al. Exploring the protective effects of PNS on acute myocardial ischaemia-induced heart failure by Transcriptome analysis. J. Ethnopharmacol. 2021, 271, 113823. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Li, J.; Liu, T.; Jiang, Q.; Hong, Y.; Wang, Q.; Li, C.; Guo, D.; Wang, Y. Qishen granule (QSG) exerts cardioprotective effects by inhibiting NLRP3 inflammasome and pyroptosis in myocardial infarction rats. J. Ethnopharmacol. 2022, 285, 114841. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Liu, T.; Wang, Q.; Wang, H.; Xue, S.; Jiang, Q.; Li, J.; Li, C.; Wang, W.; Wang, Y. Synergistic Effects of Ginsenoside Rb3 and Ferruginol in Ischemia-Induced Myocardial Infarction. Int. J. Mol. Sci. 2022, 23, 15935. https://doi.org/10.3390/ijms232415935
Chen X, Liu T, Wang Q, Wang H, Xue S, Jiang Q, Li J, Li C, Wang W, Wang Y. Synergistic Effects of Ginsenoside Rb3 and Ferruginol in Ischemia-Induced Myocardial Infarction. International Journal of Molecular Sciences. 2022; 23(24):15935. https://doi.org/10.3390/ijms232415935
Chicago/Turabian StyleChen, Xu, Tiantian Liu, Qiyan Wang, Hui Wang, Siming Xue, Qianqian Jiang, Junjun Li, Chun Li, Wei Wang, and Yong Wang. 2022. "Synergistic Effects of Ginsenoside Rb3 and Ferruginol in Ischemia-Induced Myocardial Infarction" International Journal of Molecular Sciences 23, no. 24: 15935. https://doi.org/10.3390/ijms232415935
APA StyleChen, X., Liu, T., Wang, Q., Wang, H., Xue, S., Jiang, Q., Li, J., Li, C., Wang, W., & Wang, Y. (2022). Synergistic Effects of Ginsenoside Rb3 and Ferruginol in Ischemia-Induced Myocardial Infarction. International Journal of Molecular Sciences, 23(24), 15935. https://doi.org/10.3390/ijms232415935