Antipsychotic- and Anxiolytic-like Properties of a Multimodal Compound JJGW08 in Rodents
Abstract
1. Introduction
2. Results
2.1. JJGW08 Showed Antagonistic Properties at 5-HT1A, 5-HT2A, 5-HT7, and D2 Receptors
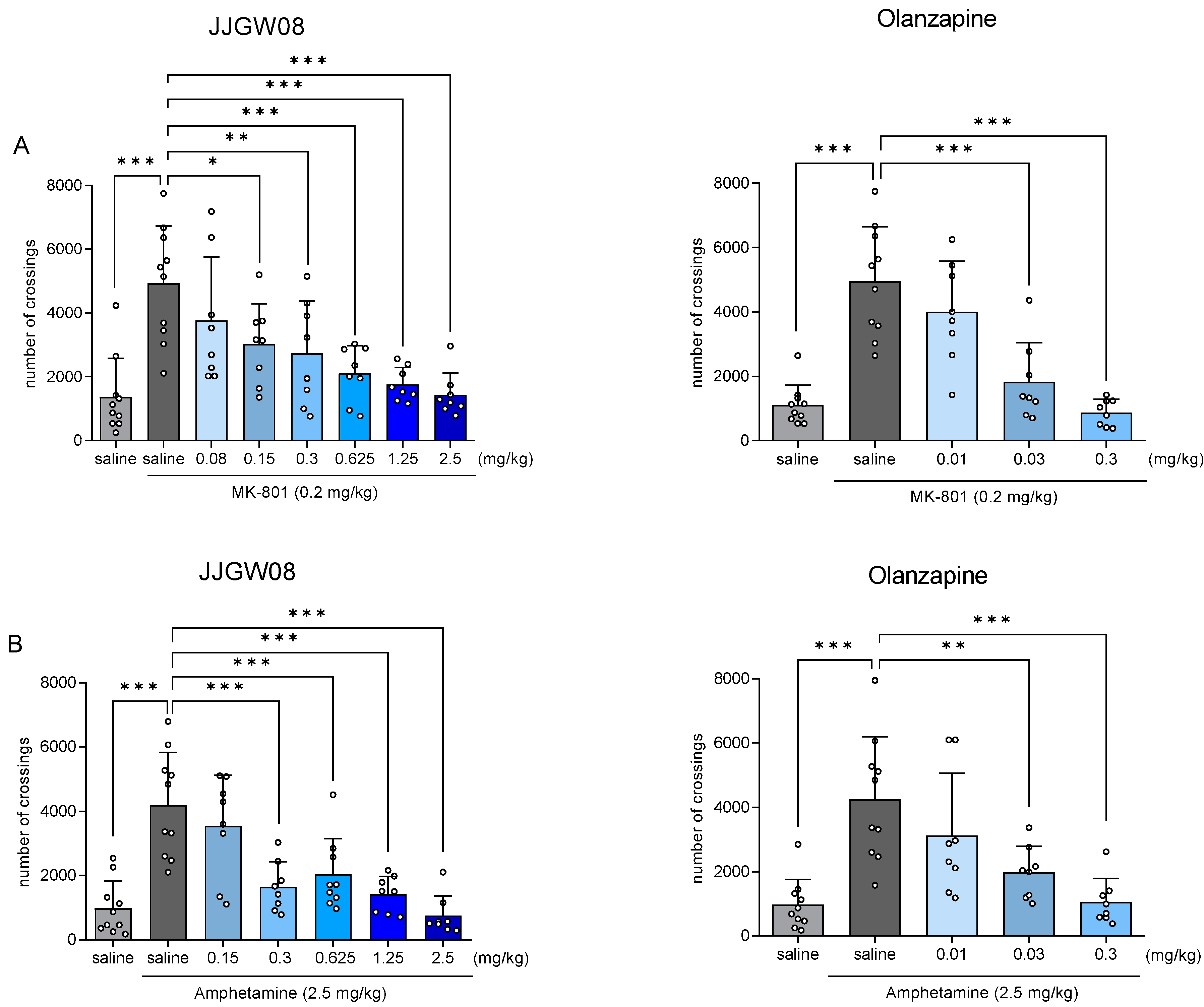
2.2. JJGW08 Reversed MK-801- and Amphetamine-Induced Hyperlocomotion in Mice
2.3. JJGW08 Did Not Induce Catalepsy in the Bar Test in Mice at Antipsychotic-like Doses
2.4. JJGW08 Did Not Influence the Motor Coordination in Mice
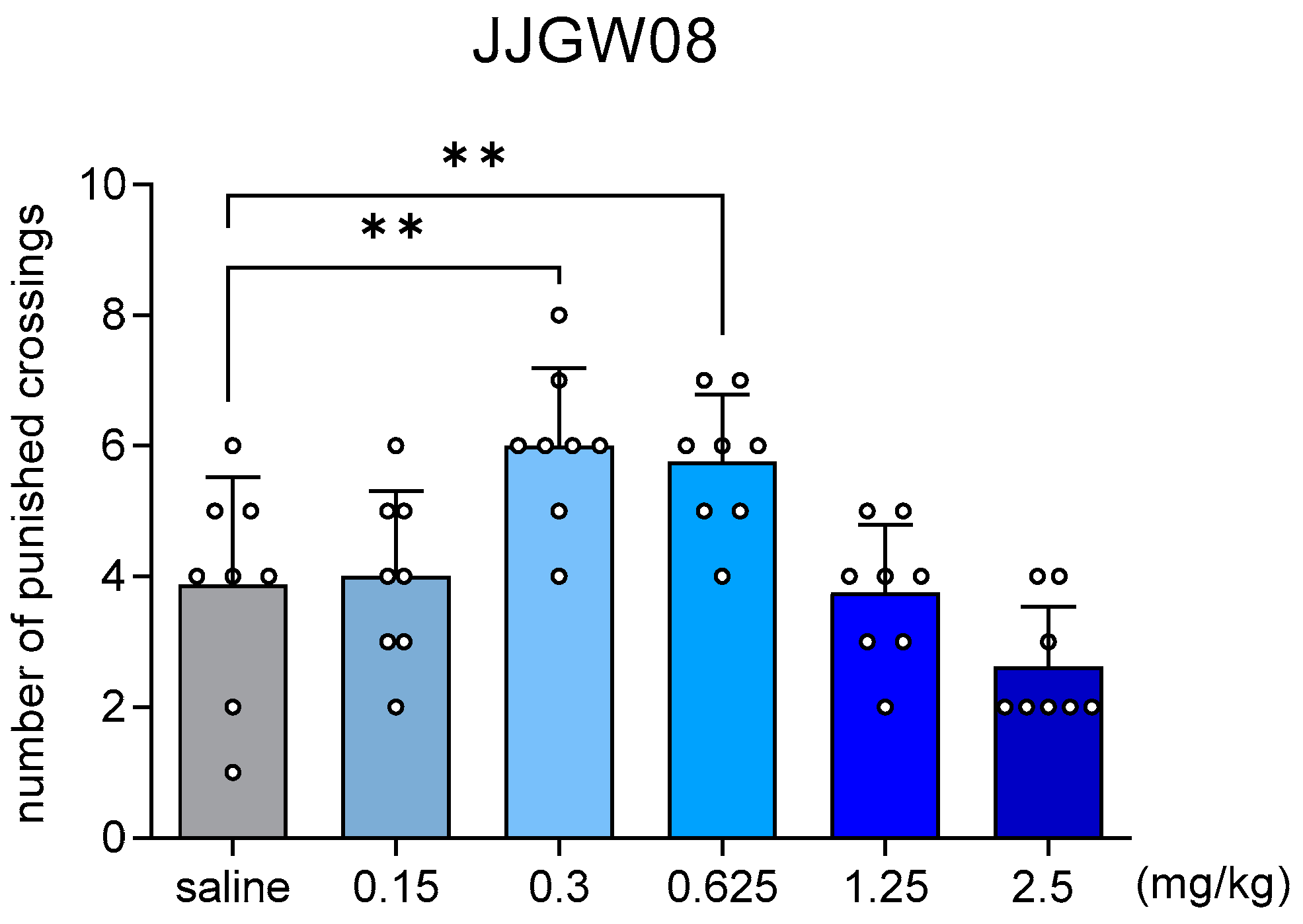
2.5. JJGW08 Increased the Number of Punished Crossings in Four-Plate Test in Mice
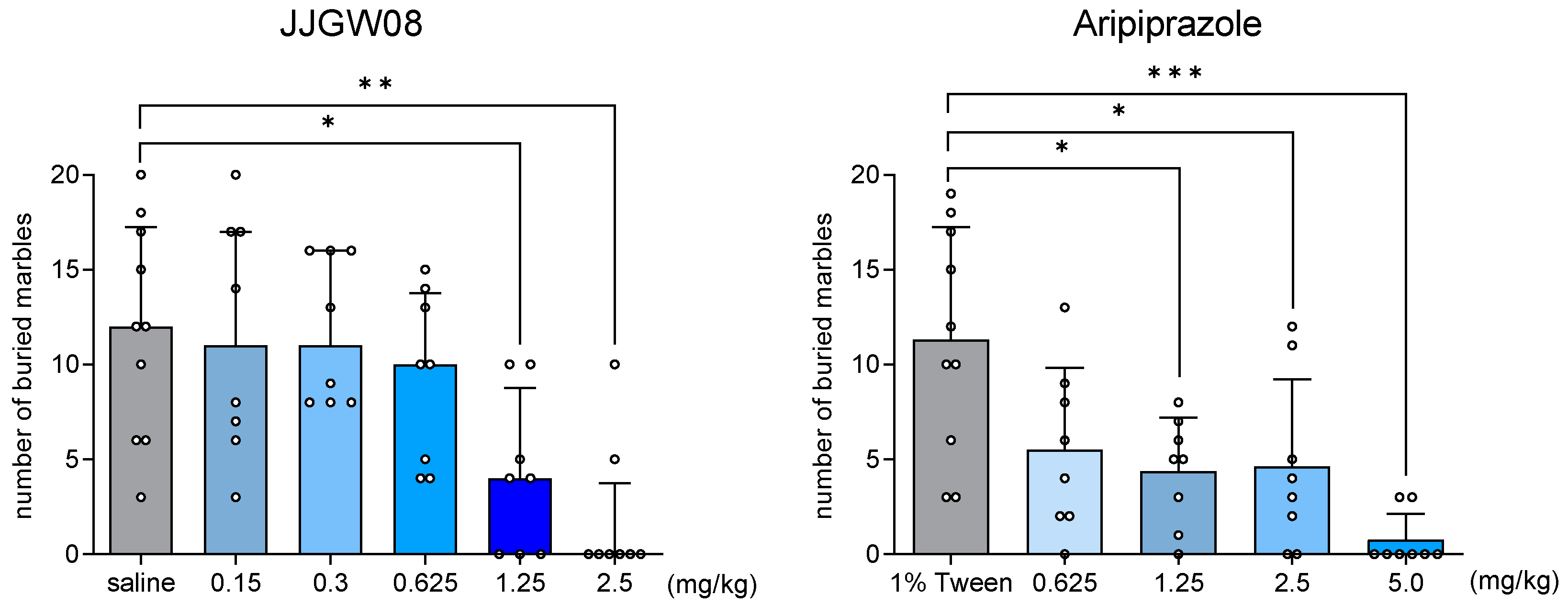
2.6. JJGW08 Decreased the Number of Buried Marbles by Mice
2.7. JJGW08 Did Not Influence Locomotor Activity in Mice
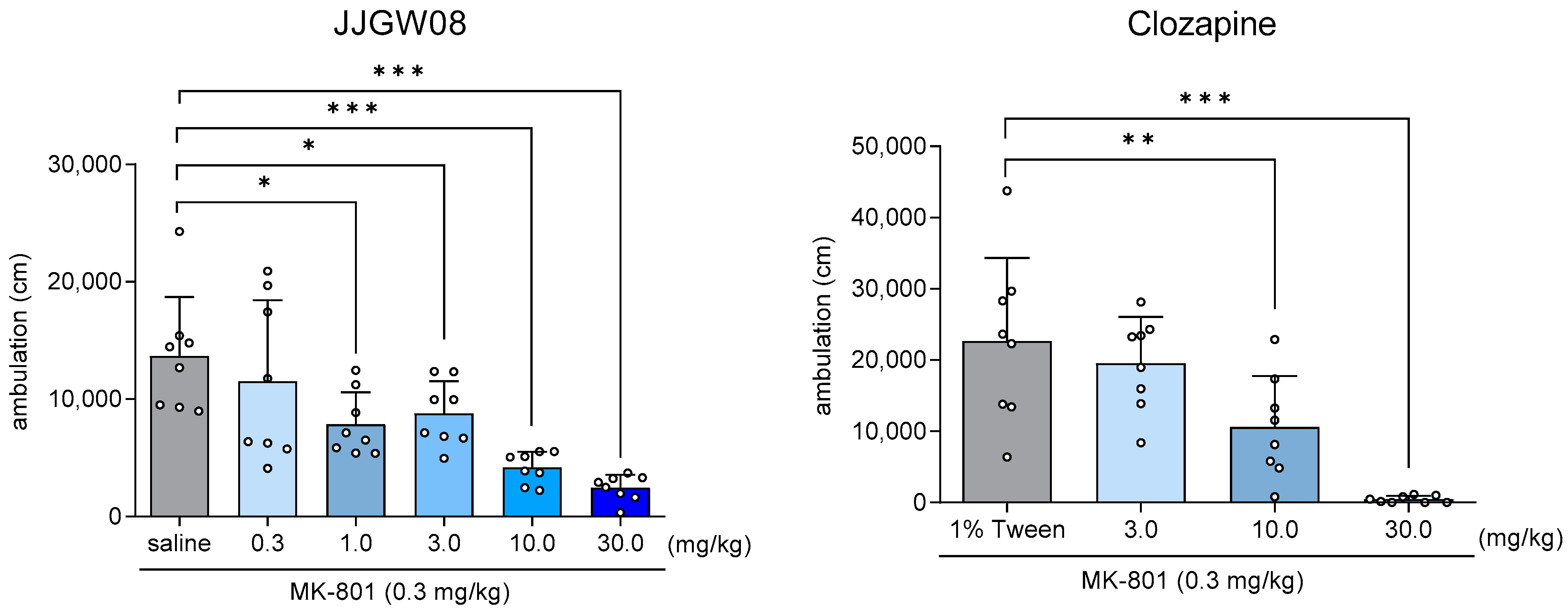
2.8. JJGW08 Reduced MK-801-Induced Hyperlocomotion in the Open Field in Rats
2.9. JJGW08 Did Not Reverse Deficits in Sensorimotor Gating Induced by MK-801 in Rats
3. Discussion
4. Materials and Methods
4.1. Drugs
4.2. Animals
4.3. Functional Assay for 5-HT1A, 5-HT2A and D2 Receptor
4.4. Functional Assays for 5-HT7 Receptors
4.5. MK-801-and Amphetamine-Induced Hyperlocomotion Test in Mice
4.6. Catalepsy Bar Test
- -
- 0 points if the animals held the constrained position < 15 s;
- -
- 1 point when the animal stayed on the bar for 15–29.9 s;
- -
- 2 points for when the animal stayed on the bar for 30–59.9 s;
- -
- 3 points for staying on the bar for more than 60 s.
4.7. Rotarod Test
4.8. Four-Plate Test
4.9. Marble Burying Test
4.10. Spontaneous Locomotor Activity in Mice
4.11. Open Field Test after MK-801 Administration in Rats
4.12. Prepulse Inhibition Test in Rats
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Schizophrenia. Available online: https://www.who.int/news-room/fact-sheets/detail/schizophrenia (accessed on 28 October 2022).
- Kahn, R.S.; Sommer, I.E.; Murray, R.M.; Meyer-Lindenberg, A.; Weinberger, D.R.; Cannon, T.D.; O’Donovan, M.; Correll, C.U.; Kane, J.M.; van Os, J.; et al. Schizophrenia. Nat. Rev. Dis. Prim. 2015, 1, 15067. [Google Scholar] [CrossRef]
- Bowie, C.R.; Harvey, P.D. Cognitive deficits and functional outcome in schizophrenia. Neuropsychiatr. Dis. Treat. 2006, 2, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, S.; E Duncan, G.; E Marx, C.; A Lieberman, J. Treatments for schizophrenia: A critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol. Psychiatry 2004, 10, 79–104. [Google Scholar] [CrossRef] [PubMed]
- Lavretsky, H. History of Schizophrenia as a Psychiatric Disorder; Guilford Press: New York, NY, USA, 2008. [Google Scholar]
- Stahl, S.M. Essential Psychopharmacology Neuroscientific Basis and Practical Applications, 2nd ed.; Cambridge University Press: Cambridge, UK, 2000; 152.—References—Scientific Research Publishing; Available online: https://www.scirp.org/(S(351jmbntvnsjt1aadkposzje))/reference/ReferencesPapers.aspx?ReferenceID=2599428 (accessed on 28 October 2022).
- Lau, C.-I.; Wang, H.-C.; Hsu, J.-L.; Liu, M.-E. Does the dopamine hypothesis explain schizophrenia? Rev. Neurosci. 2013, 24, 389–400. [Google Scholar] [CrossRef]
- Meltzer, H.; Massey, B. The role of serotonin receptors in the action of atypical antipsychotic drugs. Curr. Opin. Pharmacol. 2011, 11, 59–67. [Google Scholar] [CrossRef]
- Stępnicki, P.; Kondej, M.; Kaczor, A.A. Current Concepts and Treatments of Schizophrenia. Molecules 2018, 23, 2087. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wang, Y.; Yang, F.; Shi, W.; Wang, Z.; He, L.; He, Y.; Shen, J. Synthesis and Biological Evaluation of Five-Atom-Linker-Based Arylpiperazine Derivatives with an Atypical Antipsychotic Profile. ChemMedChem 2019, 14, 2042–2051. [Google Scholar] [CrossRef] [PubMed]
- Taverne, T.; Diouf, O.; Depreux, P.; Poupaert, J.H.; Lesieur, D.; Guardiola-Lemaître, B.; Renard, P.; Rettori, M.-C.; Caignard, D.-H.; Pfeiffer, B. Novel Benzothiazolin-2-one and Benzoxazin-3-one Arylpiperazine Derivatives with Mixed 5HT1A/D2 Affinity as Potential Atypical Antipsychotics. J. Med. Chem. 1998, 41, 2010–2018. [Google Scholar] [CrossRef]
- Zajdel, P.; Partyka, A.; Marciniec, K.; Bojarski, A.J.; Pawlowski, M.; Wesolowska, A. Quinoline- and isoquinoline-sulfonamide analogs of aripiprazole: Novel antipsychotic agents? Futur. Med. Chem. 2014, 6, 57–75. [Google Scholar] [CrossRef]
- González-Gómez, J.; Santana, L.; Uriarte, E.; Brea, J.; Villazón, M.; Loza, M.; De Luca, M.; Rivas, M.; Montenegro, G.; Fontenla, J. New arylpiperazine derivatives with high affinity for α1A, D2 and 5-HT2A receptors. Bioorg. Med. Chem. Lett. 2003, 13, 175–178. [Google Scholar] [CrossRef]
- Reitz, A.B.; Bennett, D.J.; Blum, P.S.; Codd, E.E.; Maryanoff, C.A.; Ortegon, M.E.; Renzi, M.J.; Scott, M.K.; Shank, R.P.; Vaught, J.L. A New Arylpiperazine Antipsychotic with High D2/D3/5-HT1A/α1A-Adrenergic Affinity and a Low Potential for Extrapyramidal Effects. J. Med. Chem. 1994, 37, 1060–1062. [Google Scholar] [CrossRef] [PubMed]
- Popovic, M.; Stanojevic, Z.; Tosic, J.; Isakovic, A.; Paunovic, V.; Petricevic, S.; Martinovic, T.; Ciric, D.; Kravic-Stevovic, T.; Soskic, V.; et al. Neuroprotective arylpiperazine dopaminergic/serotonergic ligands suppress experimental autoimmune encephalomyelitis in rats. J. Neurochem. 2015, 135, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Pytka, K.; Partyka, A.; Jastrzębska-Więsek, M.; Siwek, A.; Głuch-Lutwin, M.; Mordyl, B.; Kazek, G.; Rapacz, A.; Olczyk, A.; Gałuszka, A.; et al. Antidepressant- and Anxiolytic-Like Effects of New Dual 5-HT1A and 5-HT7 Antagonists in Animal Models. PLoS ONE 2015, 10, e0142499. [Google Scholar] [CrossRef]
- Sałaciak, K.; Malikowska-Racia, N.; Lustyk, K.; Siwek, A.; Głuch-Lutwin, M.; Kazek, G.; Popiół, J.; Sapa, J.; Marona, H.; Żelaszczyk, D.; et al. Synthesis and Evaluation of the Antidepressant-like Properties of HBK-10, a Novel 2-Methoxyphenylpiperazine Derivative Targeting the 5-HT1A and D2 Receptors. Pharmaceuticals 2021, 14, 744. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, P.; Jaśkowska, J.; Bojarski, A.J.; Duszyńska, B.; Bucki, A.; Kołaczkowski, M. Evaluation of 1-arylpiperazine derivative of hydroxybenzamides as 5-HT1A and 5-HT7 serotonin receptor ligands: An experimental and molecular modeling approach. J. Heterocycl. Chem. 2011, 48, 192–198. [Google Scholar] [CrossRef]
- Jaśkowska, J.; Drabczyk, A.K.; Śliwa, P.; Jodłowski, P.; Pindelska, E.; Kułaga, D.; Zaręba, P.; Majka, Z.; Siwek, A.; Wolak, M.; et al. Ultrasound assisted one-pot synthesis and preliminary in vitro studies of salicylamide arylpiperazines as dual 5-HT1A/5-HT7 ligands. J. Mol. Struct. 2023, 1275, 134585. [Google Scholar] [CrossRef]
- Cheng, H.C. The power issue: Determination of KB or Ki from IC50: A closer look at the Cheng–Prusoff equation, the Schild plot and related power equations. J. Pharmacol. Toxicol. Methods 2001, 46, 61–71. [Google Scholar] [CrossRef]
- Magnusson, O.; Ögren, S.O.; Hall, H.; Köhler, C.; Sjöstrand, S.-E. The selective dopamine D2 receptor antagonist raclopride discriminates between dopamine-mediated motor functions. Psychopharmacology 1986, 90, 287–294. [Google Scholar] [CrossRef]
- Litchfield, J.T.; Wilcoxon, F. A simplified method of evaluating dose-effect experiments. J. Pharmacol. Exp. Ther. 1949, 96, 99–113. [Google Scholar]
- Casey, A.B.; Cui, M.; Booth, R.G.; Canal, C.E. “Selective” serotonin 5-HT2A receptor antagonists. Biochem. Pharmacol. 2022, 200, 115028. [Google Scholar] [CrossRef]
- Seeman, P. Dopamine D2 Receptors as Treatment Targets in Schizophrenia. Clin. Schizophr. Relat. Psychoses 2010, 4, 56–73. [Google Scholar] [CrossRef] [PubMed]
- Kotańska, M.; Mika, K.; Sałaciak, K.; Wheeler, L.; Sapa, J.; Kieć-Kononowicz, K.; Pytka, K. Pitolisant protects mice chronically treated with corticosterone from some behavioral but not metabolic changes in corticosterone-induced depression model. Pharmacol. Biochem. Behav. 2020, 196, 172974. [Google Scholar] [CrossRef] [PubMed]
- Ellison, G. The N-methyl-d-aspartate antagonists phencyclidine, ketamine and dizocilpine as both behavioral and anatomical models of the dementias. Brain Res. Rev. 1995, 20, 250–267. [Google Scholar] [CrossRef]
- Yan, Q.-S.; Reith, M.E.; Jobe, P.C.; Dailey, J.W. Dizocilpine (MK-801) increases not only dopamine but also serotonin and norepinephrine transmissions in the nucleus accumbens as measured by microdialysis in freely moving rats. Brain Res. 1997, 765, 149–158. [Google Scholar] [CrossRef]
- Criswell, H.E.; Johnson, K.B.; Mueller, R.A.; Breese, G.R. Evidence for involvement of brain dopamine and other mechanisms in the behavioral action of the N-methyl-D-aspartic acid antagonist MK-801 in control and 6-hydroxydopamine-lesioned rats. J. Pharmacol. Exp. Ther. 1993, 265, 1001–1010. Available online: https://pubmed.ncbi.nlm.nih.gov/8098756/ (accessed on 28 October 2022). [PubMed]
- Svensson, T. Dysfunctional brain dopamine systems induced by psychotomimetic NMDA-receptor antagonists and the effects of antipsychotic drugs. Brain Res. Rev. 2000, 31, 320–329. [Google Scholar] [CrossRef]
- Gururajan, A.; Taylor, D.A.; Malone, D.T. Effect of testing conditions on the propsychotic action of MK-801 on prepulse inhibition, social behaviour and locomotor activity. Physiol. Behav. 2010, 99, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Pijnenburg, A.J.J.; Honig, W.M.M.; Van Rossum, J.M. Inhibition of d-amphetamine-induced locomotor activity by injection of haloperidol into the nucleus accumbens of the rat. Psychopharmacology 1975, 41, 87–95. [Google Scholar] [CrossRef]
- Strömberg, C. Interactions of antidepressants and ethanol on spontaneous locomotor activity and rotarod performance in NMRI and C57BL/6 mice. J. Psychopharmacol. 1988, 2, 61–66. [Google Scholar] [CrossRef]
- Szkutnik-Fiedler, D.; Kus, K.; Balcerkiewicz, M.; Grzeskowiak, E.; Nowakowska, E.; Burda, K.; Ratajczak, P.; Sadowski, C. Concomitant use of tramadol and venlafaxine—Evaluation of antidepressant-like activity and other behavioral effects in rats. Pharmacol. Rep. 2012, 64, 1350–1358. [Google Scholar] [CrossRef]
- Galeotti, N.; Ghelardini, C.; Bartolini, A. Involvement of potassium channels in amitriptyline and clomipramine analgesia. Neuropharmacology 2000, 40, 75–84. [Google Scholar] [CrossRef]
- Vogel, H.G. Drug Discovery and Evaluation: Pharmacological Assays|SpringerLink. Available online: https://link.springer.com/referencework/10.1007/978-3-540-70995-4 (accessed on 28 October 2022).
- Tibbo, P.; Swainson, J.; Chue, P.; LeMelledo, J.-M. Prevalence and relationship to delusions and hallucinations of anxiety disorders in schizophrenia. Depress. Anxiety 2003, 17, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Bulbena, A.; Sperry, L.; Anguiano, B.; Pailhez, G.; Gago, J. Joint Hypermobility in Schizophrenia: A Potential Marker for Co-Morbid Anxiety. Open Psychiatry J. 2007, 1, 31–33. [Google Scholar] [CrossRef]
- Norman, R.M.G.; Malla, A.K.; Cortese, L.; Diaz, F. Aspects of dysphoria and symptoms of schizophrenia. Psychol. Med. 1998, 28, 1433–1441. [Google Scholar] [CrossRef]
- Siris, S.G. Depression in Schizophrenia: Perspective in the Era of “Atypical” Antipsychotic Agents. Am. J. Psychiatry 2000, 157, 1379–1389. [Google Scholar] [CrossRef] [PubMed]
- Katzman, M.A. Aripiprazole: A clinical review of its use for the treatment of anxiety disorders and anxiety as a comorbidity in mental illness. J. Affect. Disord. 2011, 128 (Suppl. 1), S11–S20. [Google Scholar] [CrossRef] [PubMed]
- Geyer, M.A.; Krebs-Thomson, K.; Braff, D.L.; Swerdlow, N.R. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: A decade in review. Psychopharmacology 2001, 156, 117–154. [Google Scholar] [CrossRef]
- Chen, C.-H.; Lee, P.-W.; Liao, H.-M.; Chang, P.-K. Neuroligin 2 R215H Mutant Mice Manifest Anxiety, Increased Prepulse Inhibition, and Impaired Spatial Learning and Memory. Front. Psychiatry 2017, 8, 257. [Google Scholar] [CrossRef]
- Bubeníková, V.; Votava, M.; Horácek, J.; Pálenícek, T.; Dockery, C. The effect of zotepine, risperidone, clozapine and olanzapine on MK-801-disrupted sensorimotor gating. Pharmacol. Biochem. Behav. 2005, 80, 591–596. [Google Scholar] [CrossRef]
- Fu, K.; Miyamoto, Y.; Sumi, K.; Saika, E.; Muramatsu, S.-I.; Uno, K.; Nitta, A. Overexpression of transmembrane protein 168 in the mouse nucleus accumbens induces anxiety and sensorimotor gating deficit. PLoS ONE 2017, 12, e0189006. [Google Scholar] [CrossRef]
- Kołaczkowski, M.; Marcinkowska, M.; Bucki, A.; Pawłowski, M.; Mitka, K.; Jaśkowska, J.; Kowalski, P.; Kazek, G.; Siwek, A.; Wasik, A.; et al. Novel Arylsulfonamide Derivatives with 5-HT6/5-HT7 Receptor Antagonism Targeting Behavioral and Psychological Symptoms of Dementia. J. Med. Chem. 2014, 57, 4543–4557. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, M.L.; Martin, P.; Nilsson, M.; Sorensen, S.M.; Carlsson, A.; Waters, S.; Waters, N. The 5-HT2A receptor antagonist M100907 is more effective in counteracting NMDA antagonist- than dopamine agonist-induced hyperactivity in mice. J. Neural Transm. 1999, 106, 123–129. [Google Scholar] [CrossRef]
- Nilsson, M.; Carlsson, A.; Carlsson, M.L. Glycine and D-serine decrease MK-801-induced hyperactivity in mice. J. Neural Transm. 1997, 104, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Ueki, S.; Fujiwara, M.; Inoue, K.; Kataoka, Y.; Ibii, N.; Wada, Y. Behavior pharmacology of maprotiline, a new antidepressant. Nihon Yakurigaku Zasshi 1975, 71, 789–815. [Google Scholar] [CrossRef] [PubMed]
- Czopek, A.; Kołaczkowski, M.; Bucki, A.; Byrtus, H.; Pawłowski, M.; Kazek, G.; Bojarski, A.J.; Piaskowska, A.; Kalinowska-Tłuścik, J.; Partyka, A.; et al. Novel spirohydantoin derivative as a potent multireceptor-active antipsychotic and antidepressant agent. Bioorg. Med. Chem. 2015, 23, 3436–3447. [Google Scholar] [CrossRef] [PubMed]
- Pytka, K.; Rapacz, A.; Zygmunt, M.; Olczyk, A.; Waszkielewicz, A.; Sapa, J.; Filipek, B. Antidepressant-like activity of a new piperazine derivative of xanthone in the forced swim test in mice: The involvement of serotonergic system. Pharmacol. Rep. 2015, 67, 160–165. [Google Scholar] [CrossRef]
- Aron, C.; Simon, P.; Larousse, C.; Boissier, J. Evaluation of a rapid technique for detecting minor tranquilizers. Neuropharmacology 1971, 10, 459–469. [Google Scholar] [CrossRef]
- Bourin, M.; Masse, F.; Dailly, E.; Hascoët, M. Anxiolytic-like effect of milnacipran in the four-plate test in mice: Mechanism of action. Pharmacol. Biochem. Behav. 2005, 81, 645–656. [Google Scholar] [CrossRef]
- Broekkamp, C.L.; Rijk, H.W.; Joly-Gelouin, D.; Lloyd, K.L. Major tranquillizers can be distinguished from minor tranquillizers on the basis of effects on marble burying and swim-induced grooming in mice. Eur. J. Pharmacol. 1986, 126, 223–229. [Google Scholar] [CrossRef]
- Waszkielewicz, A.M.; Pytka, K.; Rapacz, A.; Wełna, E.; Jarzyna, M.; Satała, G.; Bojarski, A.; Sapa, J.; Żmudzki, P.; Filipek, B.; et al. Synthesis and Evaluation of Antidepressant-like Activity of Some 4-Substituted 1-(2-methoxyphenyl)Piperazine Derivatives. Chem. Biol. Drug Des. 2014, 85, 326–335. [Google Scholar] [CrossRef]
- Acewicz, A.; Mierzejewski, P.; Jastrzebska, A.; Korkosz, I.; Karas, K.; Sienkiewicz-Jarosz, H.; Samochowiec, J.; Kolaczkowski, M.; Bienkowski, P. Anxiety- and depressive-like traits in Warsaw alcohol high-preferring (WHP) and Warsaw alcohol low-preferring (WLP) rats. Pharmacol. Biochem. Behav. 2014, 122, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Mierzejewski, P.; Kolaczkowski, M.; Marcinkowska, M.; Wesołowska, A.; Samochowiec, J.; Pawlowski, M.; Bienkowski, P. Antipsychotic-like effects of zolpidem in Wistar rats. Eur. J. Pharmacol. 2016, 773, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Sienkiewicz-Jarosz, H.; Członkowska, A.I.; Siemiątkowski, M.; Maciejak, P.; Szyndler, J.; Planik, A. The effects of physostigmine and cholinergic receptor ligands on novelty-induced neophobia. J. Neural Transm. 2000, 107, 1403–1412. [Google Scholar] [CrossRef] [PubMed]
- Sienkiewicz-Jarosz, H.; Maciejak, P.; Krząścik, P.; I Członkowska, A.; Szyndler, J.; Bidziński, A.; Kostowski, W.; Płaźnik, A. The effects of central administration of physostigmine in two models of anxiety. Pharmacol. Biochem. Behav. 2003, 75, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Andiné, P.; Widermark, N.; Axelsson, R.; Nyberg, G.; Olofsson, U.; Mårtensson, E.; Sandberg, M. Characterization of MK-801-induced behavior as a putative rat model of psychosis. J. Pharmacol. Exp. Ther. 1999, 290, 1393–1408. [Google Scholar] [PubMed]
- Acewicz, A.; Mierzejewski, P.; Jastrzebska, A.; Kolaczkowski, M.; Wesolowska, A.; Korkosz, I.; Samochowiec, J.; Bienkowski, P. Acoustic Startle Responses and Prepulse Inhibition of Acoustic Startle Responses in Warsaw Alcohol High-Preferring (WHP) and Warsaw Alcohol Low-Preferring (WLP) Rats. Alcohol Alcohol. 2012, 47, 386–389. [Google Scholar] [CrossRef][Green Version]

| Receptor | Treatment | Agonist Mode | Antagonist Mode | ||||
|---|---|---|---|---|---|---|---|
| Emax% | pEC50 ± Range | Emax% | pIC50 ± Range | Kb [nM] | R2Kb | ||
| 5-HT1A | Serotonin | 100 | 7.6 ± 1.1 | 0 | n.c. | n.c. | n.c. |
| NAN-190 | 6 | n.c. | 0 | 9.0 ± 0.1 | 0 | 0.99 | |
| JJGW08 | 9 | n.c. | 1 | 8.3 ± 0.1 | 0 | 0.98 | |
| 5-HT2A | α-methylserotonin | 100 | 8.5 ± 0.3 | 2 | n.c. | n.c. | n.c. |
| Serotonin | 112 | 8.4 ± 0.0 | 1 | n.c. | n.c. | n.c. | |
| Mianserin | 3 | n.c. | 3 | 8.1 ± 0.1 | 2 | 0.91 | |
| JJGW08 | 21 | 5.9 ± 0.1 | 5 | 5.7 ± 0.4 | 290 | 0.93 | |
| 5-HT7 | Serotonin | 100 | 8.1 ± 0.1 | 0 | n.c. | n.c. | n.c. |
| SB-269970 | 0 | n.c. | 9 | 9.3 ± 0.2 | 0.2 | 0.94 | |
| JJGW08 | 1 | n.c. | 4 | 6.4 ± 0.1 | 190 | 0.97 | |
| D2 | Quiniprole | 100 | 8.7 ± 0.1 | 0 | n.c. | n.c. | n.c. |
| Apomorphine | 100 | 7.5 ± 0.1 | 0 | n.c. | n.c. | n.c. | |
| Chlorpromazine | 2 | n.c. | 0 | 9.8 ± 0.4 | 0 | 0.94 | |
| JJGW08 | 13 | n.c. | 0 | 7.9 ± 0.0 | 2 | 0.97 | |
| Treatment | Dose (mg/kg) | Mean Score | ||
|---|---|---|---|---|
| 30 min | 60 min | 120 min | ||
| 5 | 0.0 | 0.2 | 0.2 | |
| JJGW08 | 10 | 0.7 | 0.9 | 1.4 |
| 20 | 2.1 | 2.1 | 1.5 | |
| 5 | 0.8 | 0.8 | 0.7 | |
| Olanzapine | 10 | 0.7 | 1.6 | 1.3 |
| 20 | 0.7 | 2.0 | 1.8 |
| Treatment | Dose (mg/kg) | Animals That Fell from Rotating Rod | Time before Animals Fell (s) | TD50 (mg/kg) |
|---|---|---|---|---|
| JJGW08 | 10 | 2/8 | 58 ± 4 | 18 (11–29) |
| 20 | 4/8 | 41 ± 24 | ||
| 30 | 6/8 | 20 ± 25 |
| Treatment | Dose (mg/kg) | Number of Crossings ± SD | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 60 min | 30 min | 1 min | ||||||||
| Saline | - | 1140 | ± | 600 | 866 | ± | 653 | 45 | ± | 21 |
| 0.15 | 1346 | ± | 886 | 547 | ± | 343 | 38 | ± | 15 | |
| 0.3 | 1588 | ± | 772 | 1027 | ± | 733 | 46 | ± | 21 | |
| JJGW08 | 0.625 | 1671 | ± | 923 | 634 | ± | 716 | 27 | ± | 14 |
| 1.25 | 1769 | ± | 835 | 1181 | ± | 732 | 22 | ± | 14 | |
| 2.5 | 1450 | ± | 1252 | 437 | ± | 588 | 19 | ± | 24 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żmudzka, E.; Lustyk, K.; Głuch-Lutwin, M.; Mordyl, B.; Zakrzewska-Sito, A.; Mierzejewski, P.; Jaśkowska, J.; Kołaczkowski, M.; Sapa, J.; Pytka, K. Antipsychotic- and Anxiolytic-like Properties of a Multimodal Compound JJGW08 in Rodents. Int. J. Mol. Sci. 2022, 23, 15929. https://doi.org/10.3390/ijms232415929
Żmudzka E, Lustyk K, Głuch-Lutwin M, Mordyl B, Zakrzewska-Sito A, Mierzejewski P, Jaśkowska J, Kołaczkowski M, Sapa J, Pytka K. Antipsychotic- and Anxiolytic-like Properties of a Multimodal Compound JJGW08 in Rodents. International Journal of Molecular Sciences. 2022; 23(24):15929. https://doi.org/10.3390/ijms232415929
Chicago/Turabian StyleŻmudzka, Elżbieta, Klaudia Lustyk, Monika Głuch-Lutwin, Barbara Mordyl, Alicja Zakrzewska-Sito, Paweł Mierzejewski, Jolanta Jaśkowska, Marcin Kołaczkowski, Jacek Sapa, and Karolina Pytka. 2022. "Antipsychotic- and Anxiolytic-like Properties of a Multimodal Compound JJGW08 in Rodents" International Journal of Molecular Sciences 23, no. 24: 15929. https://doi.org/10.3390/ijms232415929
APA StyleŻmudzka, E., Lustyk, K., Głuch-Lutwin, M., Mordyl, B., Zakrzewska-Sito, A., Mierzejewski, P., Jaśkowska, J., Kołaczkowski, M., Sapa, J., & Pytka, K. (2022). Antipsychotic- and Anxiolytic-like Properties of a Multimodal Compound JJGW08 in Rodents. International Journal of Molecular Sciences, 23(24), 15929. https://doi.org/10.3390/ijms232415929






