Iron Restriction Alleviates Atherosclerosis in ApoE KO Mice: An iTRAQ Proteomic Analysis
Abstract
1. Introduction
2. Results
2.1. Mouse Body Weight
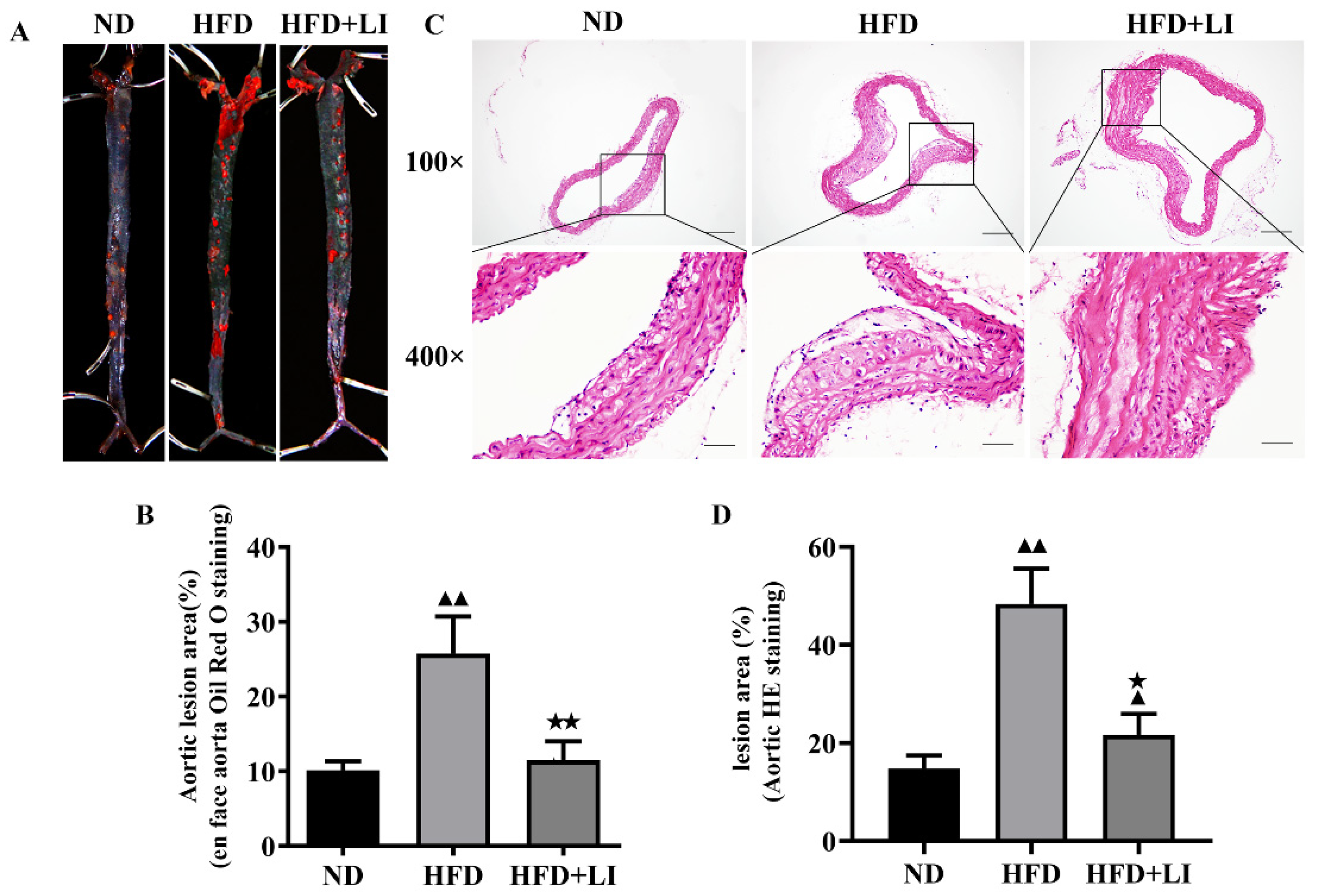
2.2. Low-Iron Diet Improves Atherosclerosis in ApoE KO Mice
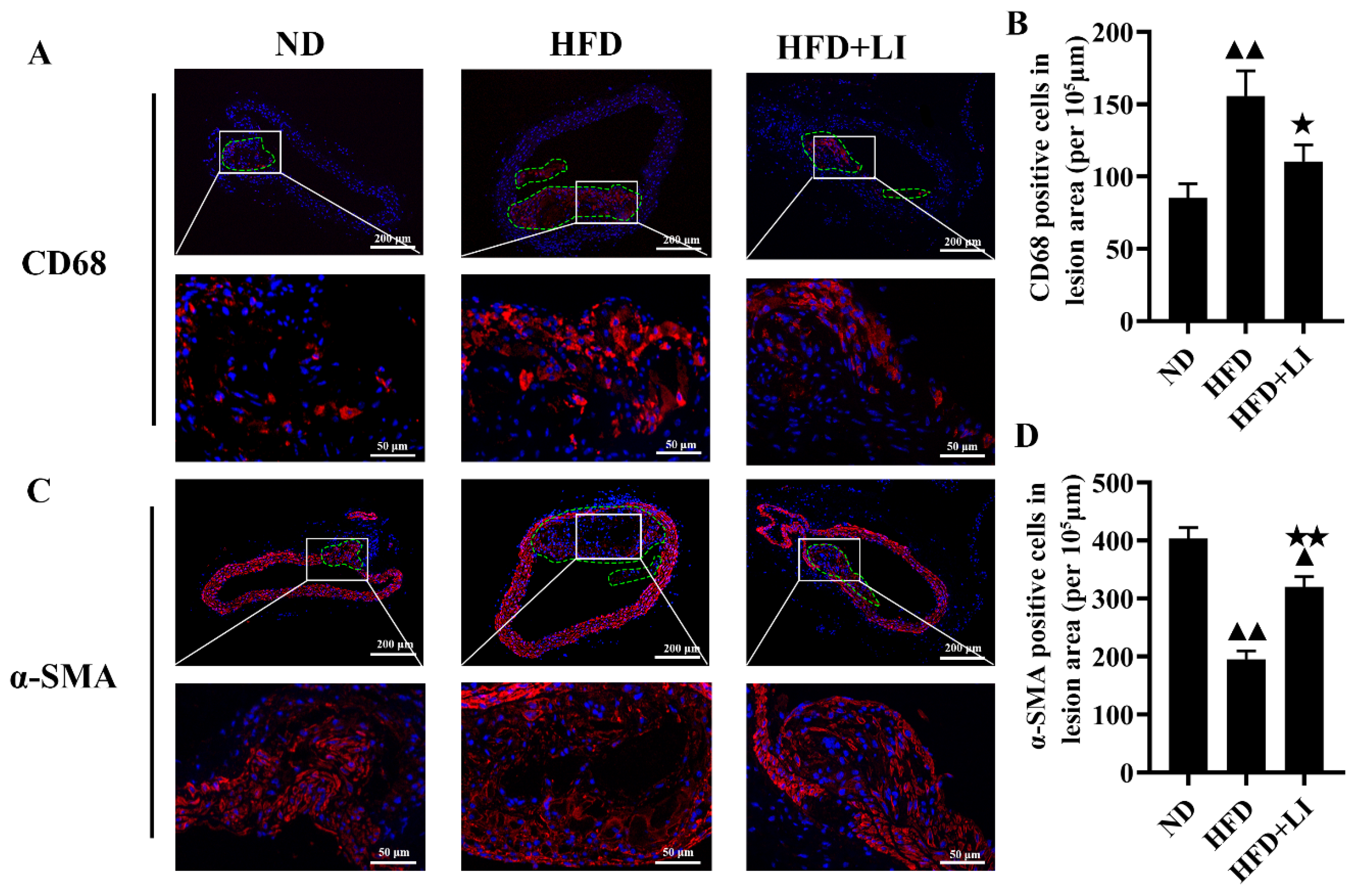
2.3. Dietary Low Iron Reduces the Macrophage Accumulation versus the HFD Diet in ApoE KO Mice
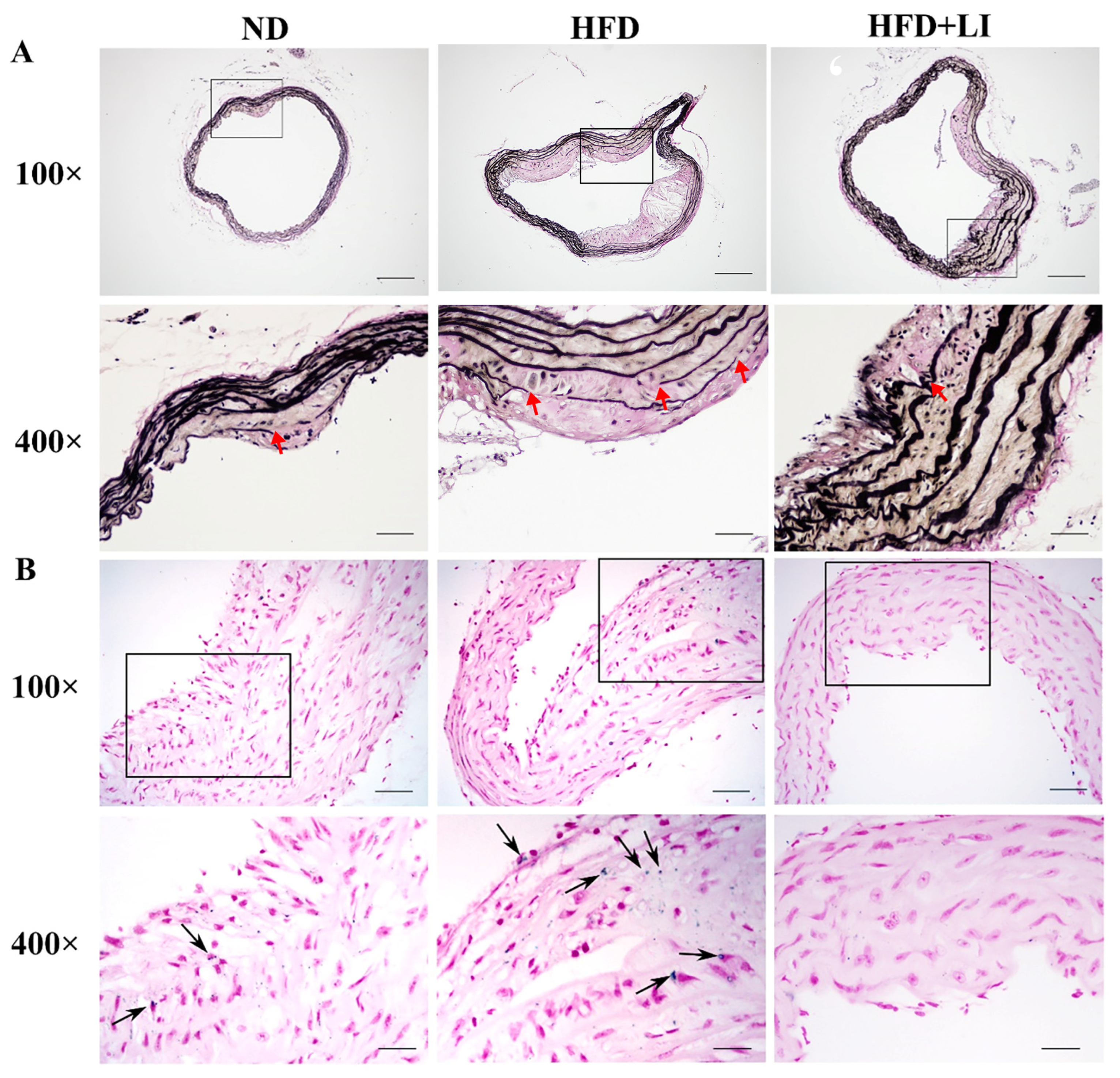
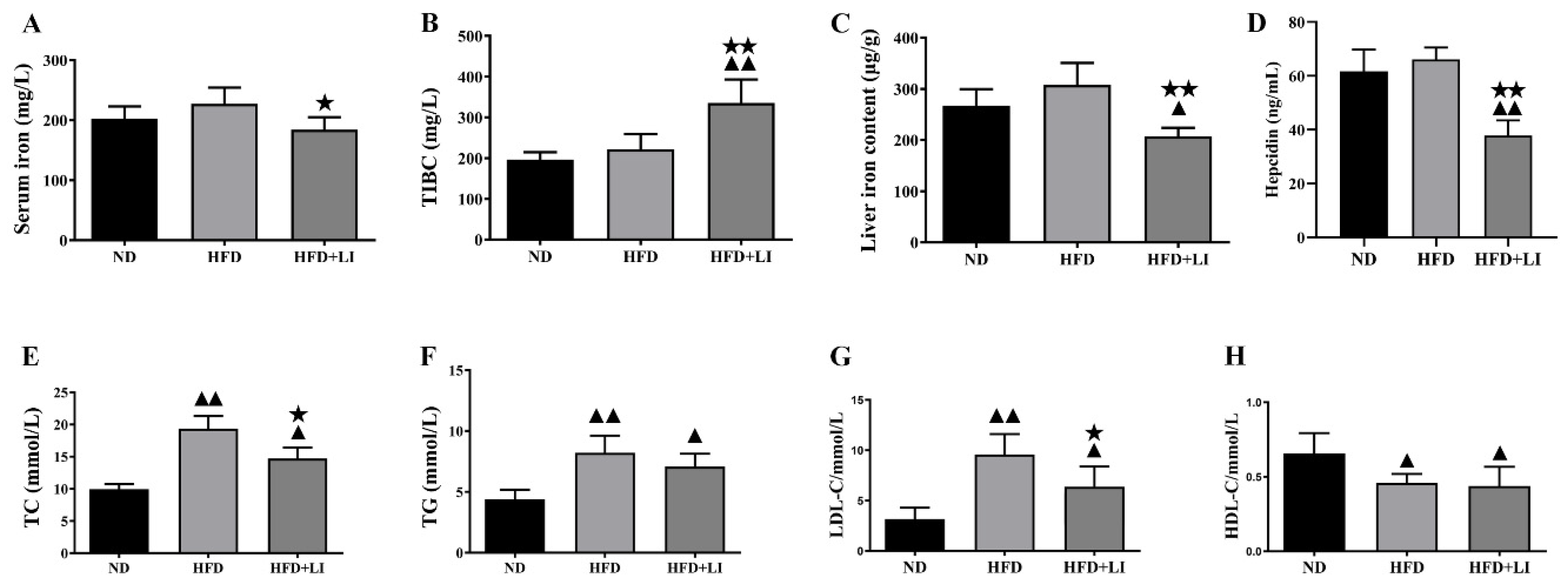
2.4. Iron Parameters and Lipid Profiles
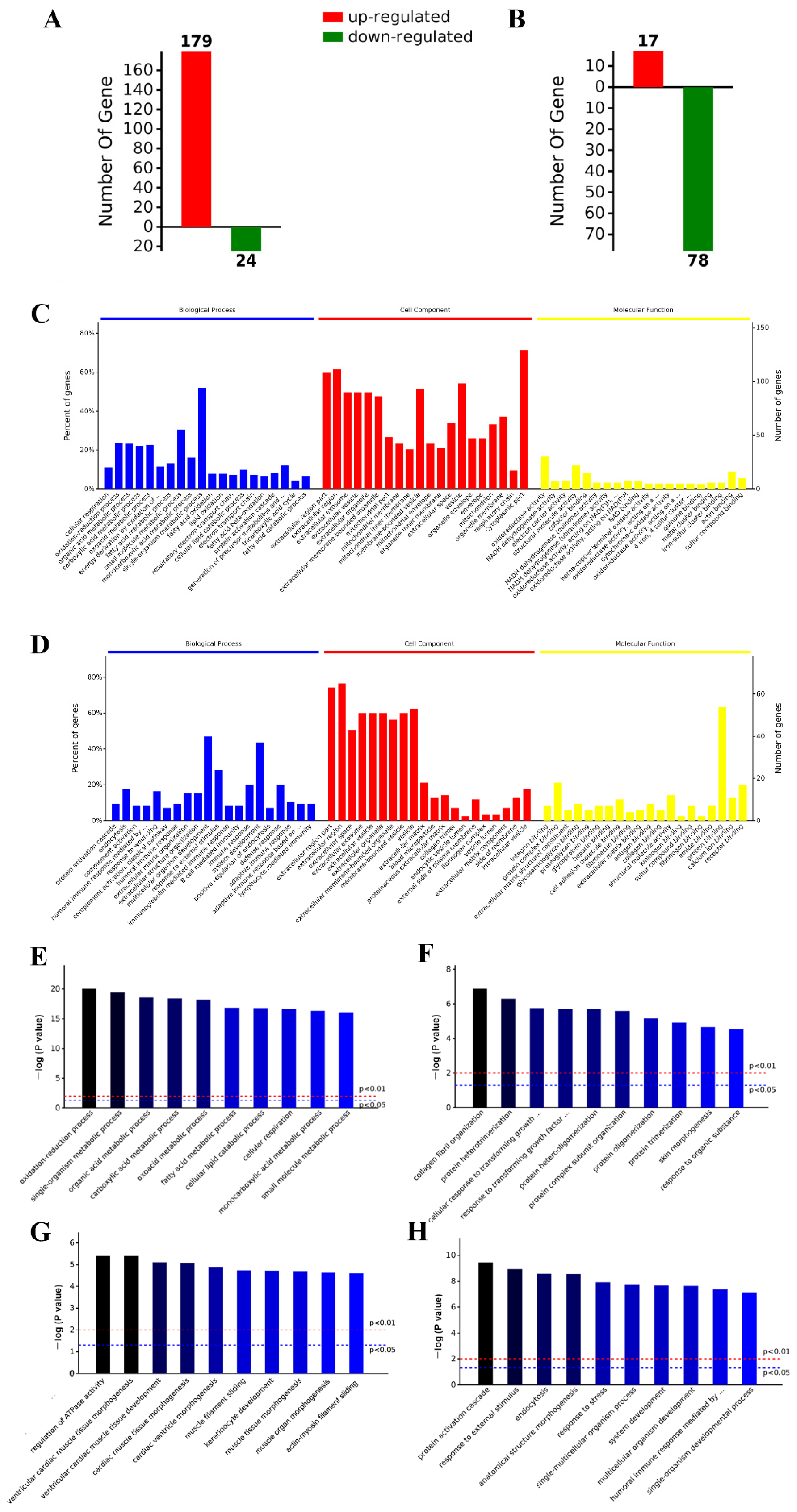
2.5. iTRAQ Analysis Identified Differentially Expressed Aortic Proteins after ND, HFD, and HFD-LI
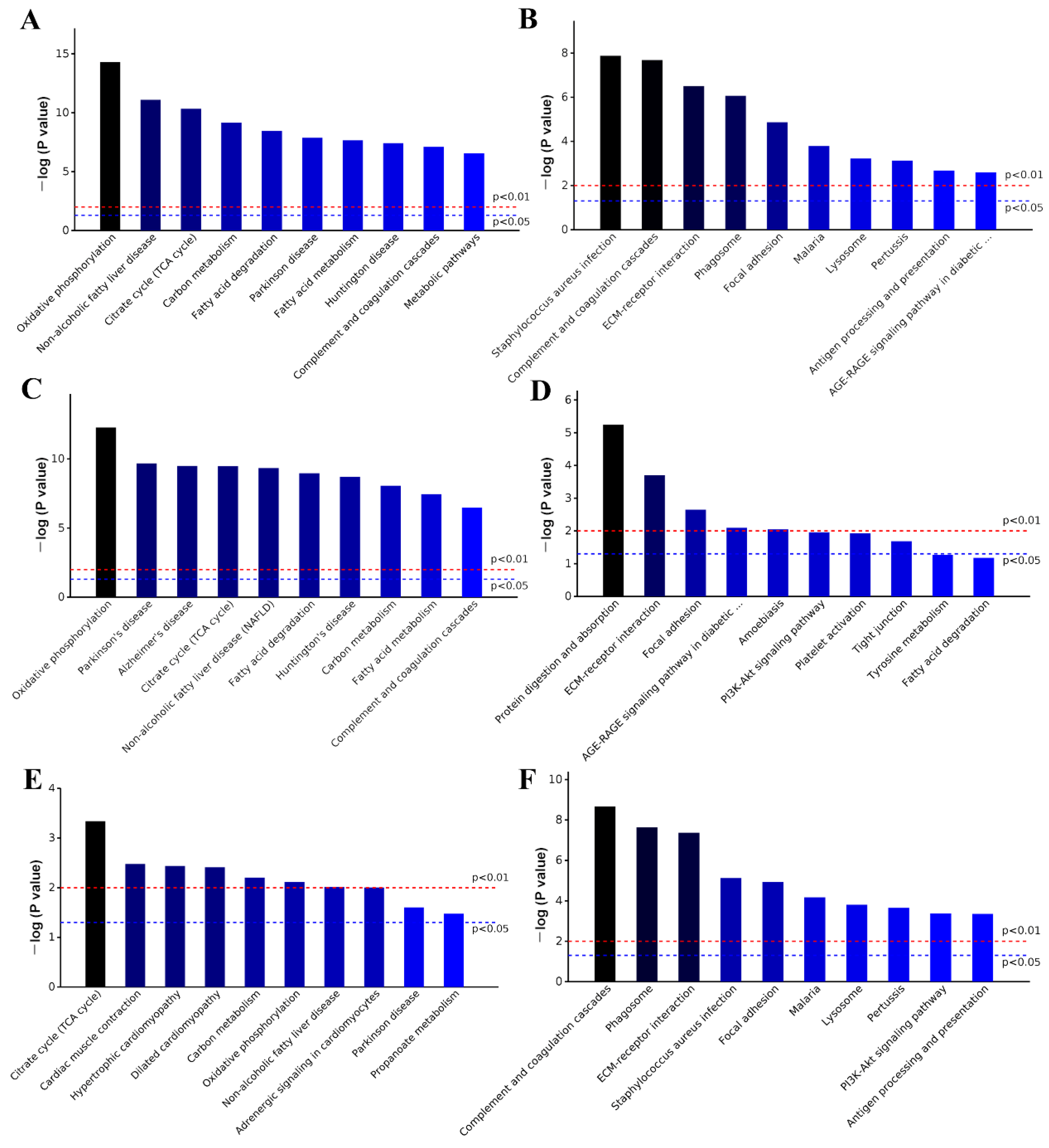
2.6. GO and KEGG Analysis
2.7. Validation of the Differentially Expressed Proteins Identified by iTRAQ Analysis
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Atherosclerosis Lesion Evaluation
4.3. Hematoxylin-Eosin and Immunofluorescence Staining
4.4. Lipid Measurement
4.5. Quantification of Serum Iron, Liver Iron Content, Total Iron-Binding Capacity (TIBC), and Iron Deposition in Atherosclerotic Plaques
4.6. Verhoeff’s Van Gieson Staining
4.7. Protein Digestion and iTRAQ Labeling
4.8. LC-MS/MS Analysis
4.9. Protein Identification and Quantification
4.10. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway Analysis
4.11. qRT PCR
4.12. Western Blot
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dai, H.; Much, A.A.; Maor, E.; Asher, E.; Younis, A.; Xu, Y.; Lu, Y.; Liu, X.; Shu, J.; Bragazzi, N.L. Global, regional, and national burden of ischaemic heart disease and its attributable risk factors, 1990–2017: Results from the global burden of disease study 2017. Eur. Heart J. Qual. Care Clin. Outcomes 2022, 8, 50–60. [Google Scholar] [CrossRef]
- Libby, P. The changing landscape of atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef]
- Arora, S.; Stouffer, G.A.; Kucharska-Newton, A.M.; Qamar, A.; Vaduganathan, M.; Pandey, A.; Porterfield, D.; Blankstein, R.; Rosamond, W.D.; Bhatt, D.L.; et al. Twenty year trends and sex differences in young adults hospitalized with acute myocardial infarction. Circulation 2019, 139, 1047–1056. [Google Scholar] [CrossRef]
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart disease and stroke statistics-2020 update: A report from the american heart association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: Update from the gbd 2019 study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Bergheanu, S.C.; Bodde, M.C.; Jukema, J.W. Pathophysiology and treatment of atherosclerosis: Current view and future perspective on lipoprotein modification treatment. Neth. Heart J. 2017, 25, 231–242. [Google Scholar] [CrossRef]
- Sullivan, J.L. Iron and the sex difference in heart disease risk. Lancet 1981, 1, 1293–1294. [Google Scholar] [CrossRef]
- Sullivan, J.L. The iron paradigm of ischemic heart disease. Am. Heart J. 1989, 117, 1177–1188. [Google Scholar] [CrossRef]
- Vinchi, F.; Porto, G.; Simmelbauer, A.; Altamura, S.; Passos, S.T.; Garbowski, M.; Silva, A.M.N.; Spaich, S.; Seide, S.E.; Sparla, R.; et al. Atherosclerosis is aggravated by iron overload and ameliorated by dietary and pharmacological iron restriction. Eur. Heart J. 2020, 41, 2681–2695. [Google Scholar] [CrossRef]
- Lee, T.S.; Shiao, M.S.; Pan, C.C.; Chau, L.Y. Iron-deficient diet reduces atherosclerotic lesions in apoe-deficient mice. Circulation 1999, 99, 1222–1229. [Google Scholar] [CrossRef]
- Zhang, W.J.; Wei, H.; Frei, B. The iron chelator, desferrioxamine, reduces inflammation and atherosclerotic lesion development in experimental mice. Exp. Biol. Med. 2010, 235, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Cai, X.; Ma, R.; Fu, W.; Zhang, C.; Du, X. Iron-load exacerbates the severity of atherosclerosis via inducing inflammation and enhancing the glycolysis in macrophages. J. Cell. Physiol. 2019, 234, 18792–18800. [Google Scholar] [CrossRef] [PubMed]
- Araujo, J.A.; Romano, E.L.; Brito, B.E.; Parthé, V.; Romano, M.; Bracho, M.; Montaño, R.F.; Cardier, J. Iron overload augments the development of atherosclerotic lesions in rabbits. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 1172–1180. [Google Scholar] [CrossRef]
- Minqin, R.; Rajendran, R.; Pan, N.; Tan, B.K.; Ong, W.Y.; Watt, F.; Halliwell, B. The iron chelator desferrioxamine inhibits atherosclerotic lesion development and decreases lesion iron concentrations in the cholesterol-fed rabbit. Free Radic. Biol. Med. 2005, 38, 1206–1211. [Google Scholar] [CrossRef]
- Kirk, E.A.; Heinecke, J.W.; LeBoeuf, R.C. Iron overload diminishes atherosclerosis in apoe-deficient mice. J. Clin. Investig. 2001, 107, 1545–1553. [Google Scholar] [CrossRef]
- Kautz, L.; Gabayan, V.; Wang, X.; Wu, J.; Onwuzurike, J.; Jung, G.; Qiao, B.; Lusis, A.J.; Ganz, T.; Nemeth, E. Testing the iron hypothesis in a mouse model of atherosclerosis. Cell Rep. 2013, 5, 1436–1442. [Google Scholar] [CrossRef]
- Bagheri, B.; Shokrzadeh, M.; Mokhberi, V.; Azizi, S.; Khalilian, A.; Akbari, N.; Habibi, V.; Yousefnejad, K.; Tabiban, S.; Nabati, M. Association between serum iron and the severity of coronary artery disease. Int. Cardivascular Res. J. 2013, 7, 95–98. [Google Scholar]
- Kiechl, S.; Willeit, J.; Egger, G.; Poewe, W.; Oberhollenzer, F. Body iron stores and the risk of carotid atherosclerosis: Prospective results from the bruneck study. Circulation 1997, 96, 3300–3307. [Google Scholar] [CrossRef]
- Salonen, J.T.; Nyyssönen, K.; Korpela, H.; Tuomilehto, J.; Seppänen, R.; Salonen, R. High stored iron levels are associated with excess risk of myocardial infarction in eastern finnish men. Circulation 1992, 86, 803–811. [Google Scholar] [CrossRef]
- Sung, K.C.; Kang, S.M.; Cho, E.J.; Park, J.B.; Wild, S.H.; Byrne, C.D. Ferritin is independently associated with the presence of coronary artery calcium in 12,033 men. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2525–2530. [Google Scholar] [CrossRef]
- Duffy, S.J.; Biegelsen, E.S.; Holbrook, M.; Russell, J.D.; Gokce, N.; Keaney, J.F., Jr.; Vita, J.A. Iron chelation improves endothelial function in patients with coronary artery disease. Circulation 2001, 103, 2799–2804. [Google Scholar] [CrossRef] [PubMed]
- Houschyar, K.S.; Lüdtke, R.; Dobos, G.J.; Kalus, U.; Broecker-Preuss, M.; Rampp, T.; Brinkhaus, B.; Michalsen, A. Effects of phlebotomy-induced reduction of body iron stores on metabolic syndrome: Results from a randomized clinical trial. BMC Med. 2012, 10, 54. [Google Scholar] [CrossRef] [PubMed]
- Meyers, D.G.; Jensen, K.C.; Menitove, J.E. A historical cohort study of the effect of lowering body iron through blood donation on incident cardiac events. Transfusion 2002, 42, 1135–1139. [Google Scholar] [CrossRef] [PubMed]
- Ekblom, K.; Marklund, S.L.; Jansson, J.H.; Hallmans, G.; Weinehall, L.; Hultdin, J. Iron stores and hfe genotypes are not related to increased risk of first-time myocardial infarction: A prospective nested case-referent study. Int. J. Cardiol. 2011, 150, 169–172. [Google Scholar] [CrossRef]
- Kim, K.S.; Son, H.G.; Hong, N.S.; Lee, D.H. Associations of serum ferritin and transferrin % saturation with all-cause, cancer, and cardiovascular disease mortality: Third national health and nutrition examination survey follow-up study. J. Prev. Med. Public Health 2012, 45, 196–203. [Google Scholar] [CrossRef]
- Marques, V.B.; Leal, M.A.S.; Mageski, J.G.A.; Fidelis, H.G.; Nogueira, B.V.; Vasquez, E.C.; Meyrelles, S.D.S.; Simões, M.R.; Dos Santos, L. Chronic iron overload intensifies atherosclerosis in apolipoprotein e deficient mice: Role of oxidative stress and endothelial dysfunction. Life Sci. 2019, 233, 116702. [Google Scholar] [CrossRef]
- Dabbagh, A.J.; Shwaery, G.T.; Keaney, J.F., Jr.; Frei, B. Effect of iron overload and iron deficiency on atherosclerosis in the hypercholesterolemic rabbit. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 2638–2645. [Google Scholar] [CrossRef]
- Xiao, L.; Luo, G.; Guo, X.; Jiang, C.; Zeng, H.; Zhou, F.; Li, Y.; Yu, J.; Yao, P. Macrophage iron retention aggravates atherosclerosis: Evidence for the role of autocrine formation of hepcidin in plaque macrophages. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158531. [Google Scholar] [CrossRef]
- Agarwal, A.K.; Yee, J. Hepcidin. Adv. Chronic Kidney Dis. 2019, 26, 298–305. [Google Scholar] [CrossRef]
- Britton, L.J.; Subramaniam, V.N.; Crawford, D.H. Iron and non-alcoholic fatty liver disease. World J. Gastroenterol. 2016, 22, 8112–8122. [Google Scholar] [CrossRef]
- Cocato, M.L.; Lobo, A.R.; Azevedo-Martins, A.K.; Filho, J.M.; de Sá, L.R.M.; Colli, C. Effects of a moderate iron overload and its interaction with yacon flour, and/or phytate, in the diet on liver antioxidant enzymes and hepatocyte apoptosis in rats. Food Chem. 2019, 285, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.T.; Qian, Z.M.; He, X.; Gong, Q.; Wu, K.C.; Jiang, L.R.; Lu, L.N.; Zhu, Z.J.; Zhang, H.Y.; Yung, W.H.; et al. Reducing iron in the brain: A novel pharmacologic mechanism of huperzine a in the treatment of alzheimer’s disease. Neurobiol. Aging 2014, 35, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Luo, G.; Li, H.; Yao, P.; Tang, Y. Dietary iron overload mitigates atherosclerosis in high-fat diet-fed apolipoprotein e knockout mice: Role of dysregulated hepatic fatty acid metabolism. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 159004. [Google Scholar] [CrossRef] [PubMed]
- Badria, F.A.; Ibrahim, A.S.; Badria, A.F.; Elmarakby, A.A. Curcumin attenuates iron accumulation and oxidative stress in the liver and spleen of chronic iron-overloaded rats. PLoS ONE 2015, 10, e0134156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, D.; Yi, B.; Liao, Z.; Tang, L.; Yin, D.; He, M. Taurine supplementation reduces oxidative stress and protects the liver in an iron-overload murine model. Mol. Med. Rep. 2014, 10, 2255–2262. [Google Scholar] [CrossRef] [PubMed]
- Madamanchi, N.R.; Runge, M.S. Mitochondrial dysfunction in atherosclerosis. Circ. Res. 2007, 100, 460–473. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; She, Z.G.; Li, H.; Zhang, X.J. Non-alcoholic fatty liver disease: A metabolic burden promoting atherosclerosis. Clin. Sci. 2020, 134, 1775–1799. [Google Scholar] [CrossRef]
- Mu, W.; Chen, M.; Gong, Z.; Zheng, F.; Xing, Q. Expression of vascular cell adhesion molecule-1 in the aortic tissues of atherosclerotic patients and the associated clinical implications. Exp. Ther. Med. 2015, 10, 423–428. [Google Scholar] [CrossRef]
- Robinet, P.; Milewicz, D.M.; Cassis, L.A.; Leeper, N.J.; Lu, H.S.; Smith, J.D. Consideration of sex differences in design and reporting of experimental arterial pathology studies-statement from atvb council. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 292–303. [Google Scholar] [CrossRef]
- Caligiuri, G.; Nicoletti, A.; Zhou, X.; Törnberg, I.; Hansson, G.K. Effects of sex and age on atherosclerosis and autoimmunity in apoe-deficient mice. Atherosclerosis 1999, 145, 301–308. [Google Scholar] [CrossRef]
- Surra, J.C.; Guillén, N.; Arbonés-Mainar, J.M.; Barranquero, C.; Navarro, M.A.; Arnal, C.; Orman, I.; Segovia, J.C.; Osada, J. Sex as a profound modifier of atherosclerotic lesion development in apolipoprotein e-deficient mice with different genetic backgrounds. J. Atheroscler. Thromb. 2010, 17, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Liu, L.; Guo, X.; Zhang, S.; Wang, J.; Zhou, F.; Liu, L.; Tang, Y.; Yao, P. Quercetin attenuates high fat diet-induced atherosclerosis in apolipoprotein e knockout mice: A critical role of nadph oxidase. Food Chem. Toxicol. 2017, 105, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Fang, L.; Zhu, J.; Xiang, Y.; Xu, H.; Sun, X.; Chen, H.; Yang, B. Total flavone of abelmoschus manihot ameliorates tnbs-induced colonic fibrosis by regulating th17/treg balance and reducing extracellular matrix. Front. Pharmacol. 2021, 12, 769793. [Google Scholar] [CrossRef] [PubMed]
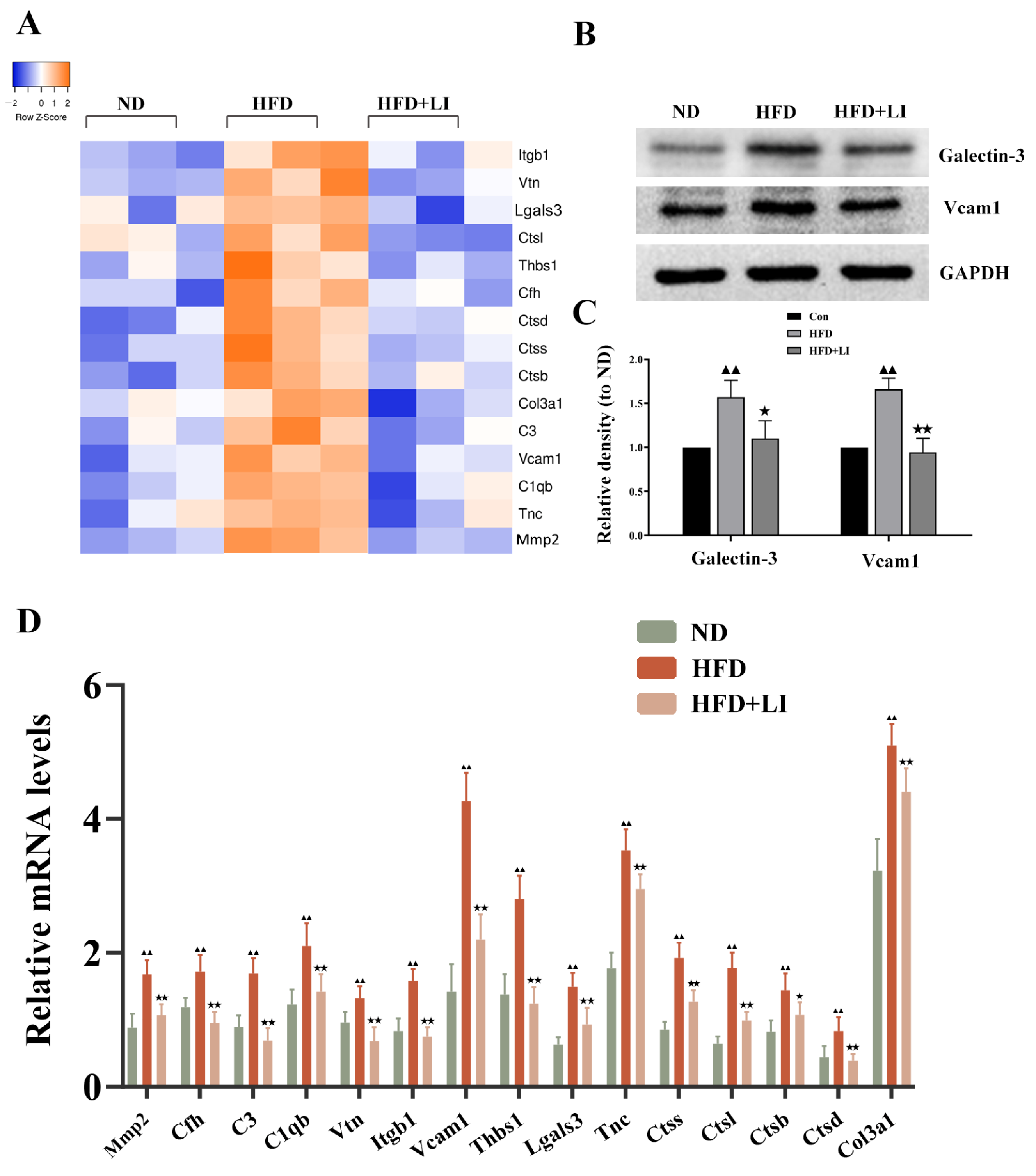
| Protein Name | Gene Name | NCBI Accession | Roles in Atherosclerosis | HFD vs. ND | HFD + LI vs. HFD | ||||
|---|---|---|---|---|---|---|---|---|---|
| Ave Fold Change | p Value | Ave Fold Change | p Value | ||||||
| Collagen alpha-1(IV)chain | Col4a1 | P02463 | vascular remodeling/Focal adhesion | 1.27 | ↑ | 0.0122 | 0.83 | ↓ | 0.0039 |
| Cathepsin S | Ctss | F6WR04 | vascular remodeling | 1.22 | ↑ | 0.0108 | 0.82 | ↓ | 0.0464 |
| Collagen alpha-1(III)chain | Col3a1 | P08121 | Focal adhesion | 1.22 | ↑ | 0.0160 | 0.82 | ↓ | 0.0099 |
| Vitronectin | Vtn | P29788 | inflammation/vascular remodeling/Focal adhesion | 1.23 | ↑ | 0.0061 | 0.81 | ↓ | 0.0048 |
| Complement C3 | C3 | P01027 | inflammation/vascular remodeling | 1.23 | ↑ | 0.0002 | 0.81 | ↓ | 0.0002 |
| Cathepsin L1 | Ctsl | P06797 | vascular remodeling | 1.43 | ↑ | 0.0023 | 0.80 | ↓ | 0.0437 |
| Integrin beta-1 | Itgb1 | P09055 | inflammation/vascular remodeling | 1.31 | ↑ | 0.0141 | 0.80 | ↓ | 0.0294 |
| Cathepsin B | Ctsb | P10605 | vascular remodeling | 1.39 | ↑ | 0.0012 | 0.80 | ↓ | 0.0007 |
| Myosin regulatory light chain 2,atrial isoform | Myl7 | Q9QVP4 | Focal adhesion | 1.25 | ↑ | 0.0200 | 0.80 | ↓ | 0.0001 |
| Cathepsin D | Ctsd | P18242 | vascular remodeling | 1.47 | ↑ | <0.0001 | 0.78 | ↓ | 0.0001 |
| Complement factor H | Cfh | E9Q8I0 | inflammation | 1.28 | ↑ | 0.0008 | 0.76 | ↓ | 0.0003 |
| Coagulation factor XIII B chain | F13b | Q07968 | inflammation | 1.58 | ↑ | 0.0005 | 0.75 | ↓ | 0.0070 |
| Complement C1q subcomponent subunit B | C1qb | P14106 | inflammation | 1.35 | ↑ | 0.0009 | 0.71 | ↓ | 0.0018 |
| Beta-2-microglobulin | B2m | P01887 | vascular remodeling | 1.32 | ↑ | 0.0267 | 0.69 | ↓ | 0.0117 |
| Chondroadherin | Chad | O55226 | vascular remodeling/Focal adhesion | 1.70 | ↑ | 0.0003 | 0.69 | ↓ | 0.0007 |
| Thrombospondin 1 | Thbs1 | Q80YQ1 | inflammation/vascular remodeling/Focal adhesion | 1.39 | ↑ | <0.0001 | 0.69 | ↓ | <0.0001 |
| Vascular cell adhesion protein 1 | Vcam1 | Q544V4 | inflammation/Focal adhesion | 1.54 | ↑ | 0.0089 | 0.68 | ↓ | 0.0150 |
| Tenascin | Tnc | Q80YX1 | vascular remodeling/Focal adhesion | 1.34 | ↑ | 0.0001 | 0.68 | ↓ | <0.0001 |
| Mannose-binding protein A | Mbl1 | P39039 | inflammation/vascular remodeling | 1.21 | ↑ | 0.0044 | 0.67 | ↓ | 0.0036 |
| Complement C1q subcomponent subunit A | C1qa | P98086 | inflammation | 1.45 | ↑ | 0.0149 | 0.66 | ↓ | 0.0081 |
| Tubulin beta-3 chain | Tubb3 | Q9ERD7 | vascular remodeling | 1.47 | ↑ | 0.0027 | 0.66 | ↓ | 0.0007 |
| Galectin-3 | Lgals3 | P16110 | vascular remodeling/Focal adhesion | 1.23 | ↑ | <0.0001 | 0.66 | ↓ | <0.0001 |
| V-type proton ATPase 16 kDa proteolipid subunit | Atp6v0c | P63082 | vascular remodeling | 1.65 | ↑ | 0.0241 | 0.65 | ↓ | 0.0320 |
| Cartilage oligomeric matrix protein | Comp | Q9R0G6 | inflammation/vascular remodeling/Focal adhesion | 1.69 | ↑ | 0.0013 | 0.58 | ↓ | 0.0018 |
| 72 kDa type IV collagenase | Mmp2 | P33434 | inflammation/vascular remodeling | 1.42 | ↑ | <0.0001 | 0.58 | ↓ | 0.0018 |
| Gene | Forward Primer 5′-3′ | Reverse Primer 5′-3′ |
|---|---|---|
| Mmp2 | GTCGCCCCTAAAACAGACAA | GGTCTCGATGGTGTTCTGGT |
| Cfh | CGTGAATGTGGTGCAGATGGG | AGAATTTCCACACATCGTGGCT |
| C3 | AGCAGGTCATCAAGTCAGGC | GATGTAGCTGGTGTTGGGCT |
| C1qb | ATAAAGGGGGAGAAAGGGCT | CGTTGCGTGGCTCATAGTT |
| Vtn | TGCAAGCCCCAAGTAACGCG | TGCCGTCCGTCCGAGGATTT |
| Itgb1 | TGCTTAGTCTTACTGACAGAGG | CAATCAGCGATCCACAAACC |
| Vcam1 | TTGGGAGCCTCAACGGTACT | GCAATCGTTTTGTATTCAGGGGA |
| Thbs1 | GGGGAGATAACGGTGTGTTTG | CGGGGATCAGGTTGGCATT |
| Lgals3 | GATCACAATCATGGGCACAG | ATTGAAGCGGGGGTTAAAGT |
| Tnc | TCATTGTGGGTCCAGATACC | GGAGTCCAATTGTGGTGAAG |
| Ctss | ATAAGATGGCTGTTTTGGATG | TTCTTTTCCCAGATGAGACGC |
| Ctsl | GTCAACATATTGGTCAAGCCGC | CCACTCCAATCCCAAGTAAGGC |
| Ctsb | TGCCGAGAGTGGAACACAC | AAAAGCAGCTGGACCCTACA |
| Ctsd | AGAGCCAGGAGAACTTTGAGCC | GAACTCATTGCGGACCACTTTG |
| Col3a1 | GAGCTCGGTGTAAACTTTCCCTA | CTTAAAGAATCGCTTGGCCTCA |
| β-actin | TTCGTTGCCGGTCCACACCC | GCTTTGCACATGCCGGAGCC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, G.; Xiang, L.; Xiao, L. Iron Restriction Alleviates Atherosclerosis in ApoE KO Mice: An iTRAQ Proteomic Analysis. Int. J. Mol. Sci. 2022, 23, 15915. https://doi.org/10.3390/ijms232415915
Luo G, Xiang L, Xiao L. Iron Restriction Alleviates Atherosclerosis in ApoE KO Mice: An iTRAQ Proteomic Analysis. International Journal of Molecular Sciences. 2022; 23(24):15915. https://doi.org/10.3390/ijms232415915
Chicago/Turabian StyleLuo, Gang, Lu Xiang, and Lin Xiao. 2022. "Iron Restriction Alleviates Atherosclerosis in ApoE KO Mice: An iTRAQ Proteomic Analysis" International Journal of Molecular Sciences 23, no. 24: 15915. https://doi.org/10.3390/ijms232415915
APA StyleLuo, G., Xiang, L., & Xiao, L. (2022). Iron Restriction Alleviates Atherosclerosis in ApoE KO Mice: An iTRAQ Proteomic Analysis. International Journal of Molecular Sciences, 23(24), 15915. https://doi.org/10.3390/ijms232415915






