Degradation of the Selected Antibiotic in an Aqueous Solution by the Fenton Process: Kinetics, Products and Ecotoxicity
Abstract
1. Introduction
2. Results and Discussion
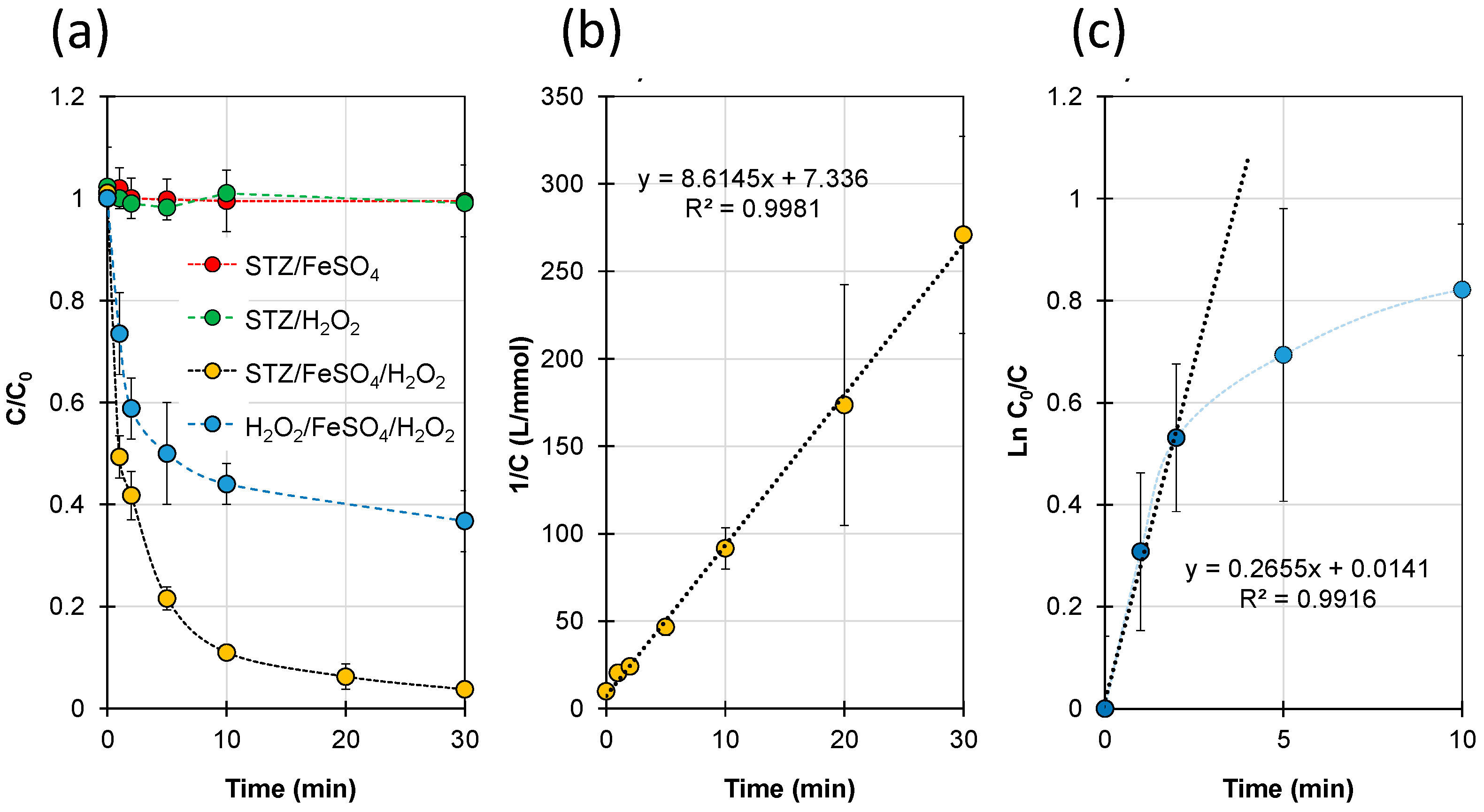
2.1. Kinetics of STZ Degradation and H2O2 Decomposition
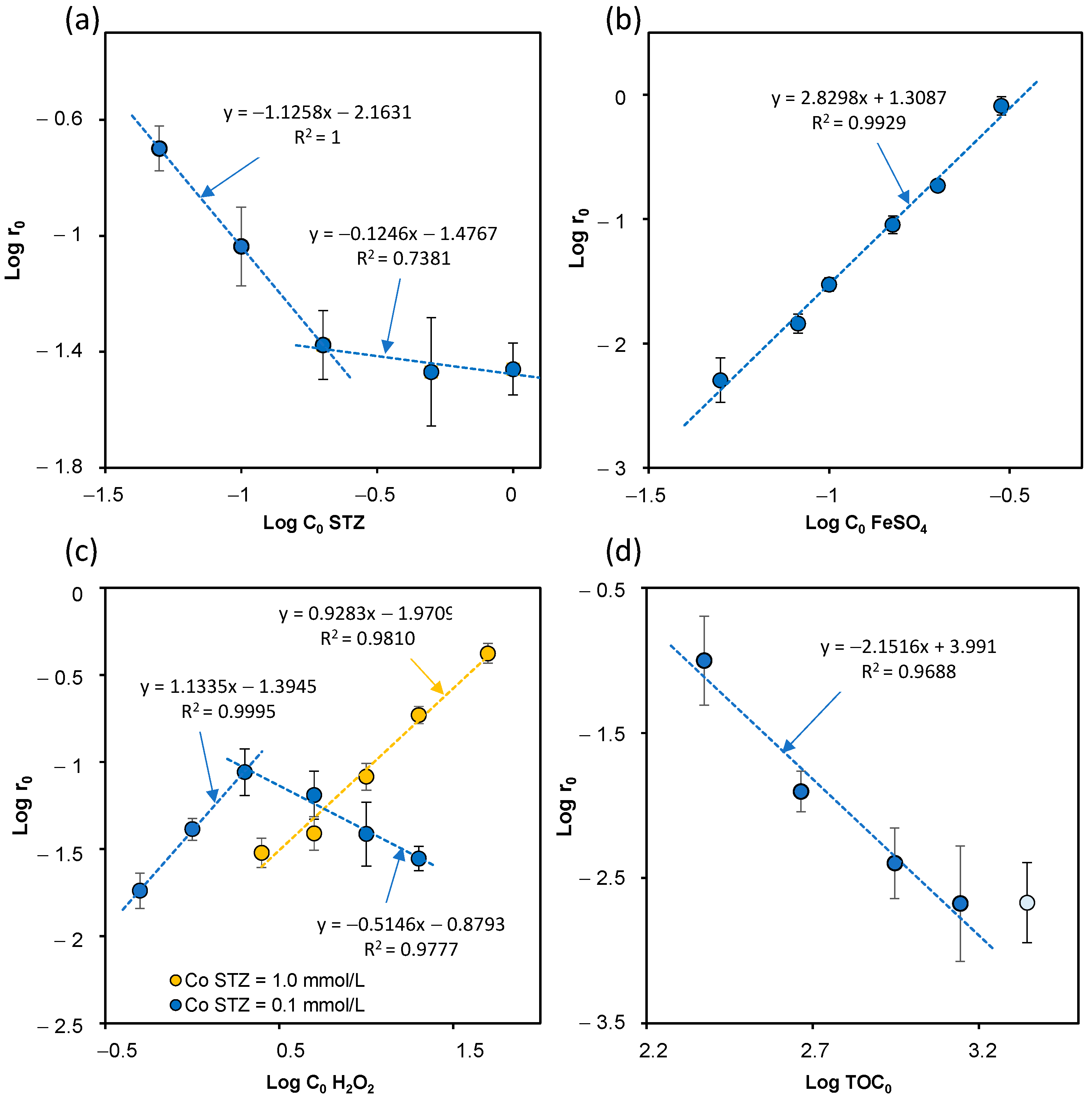
2.2. Effect of the Initial STZ Concentration
2.3. Effect of the Initial FeSO4 Concentration
2.4. Effect of the Initial H2O2 Concentration
2.5. Effect of the Initial TOC Value
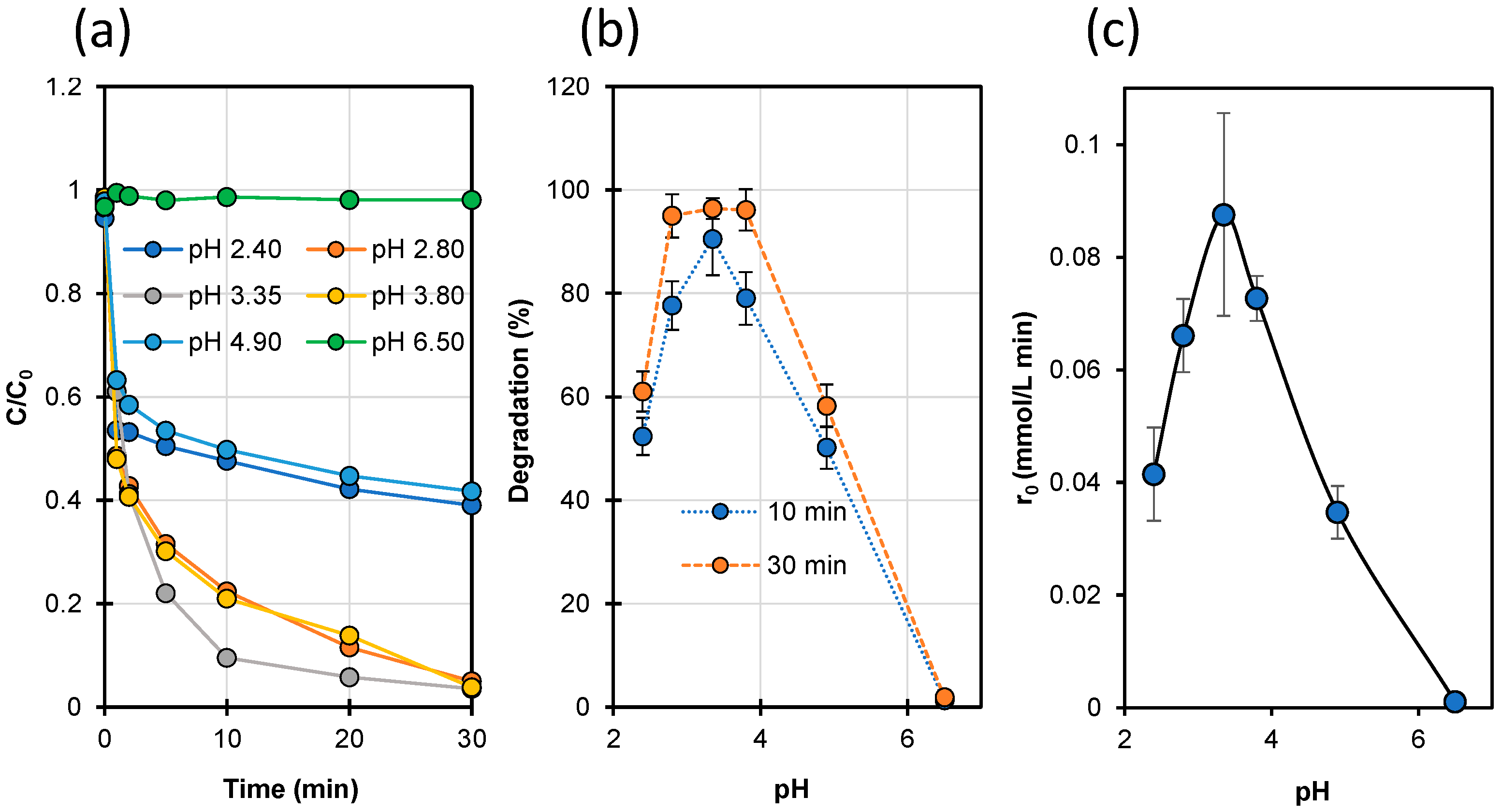
2.6. Effect of pH
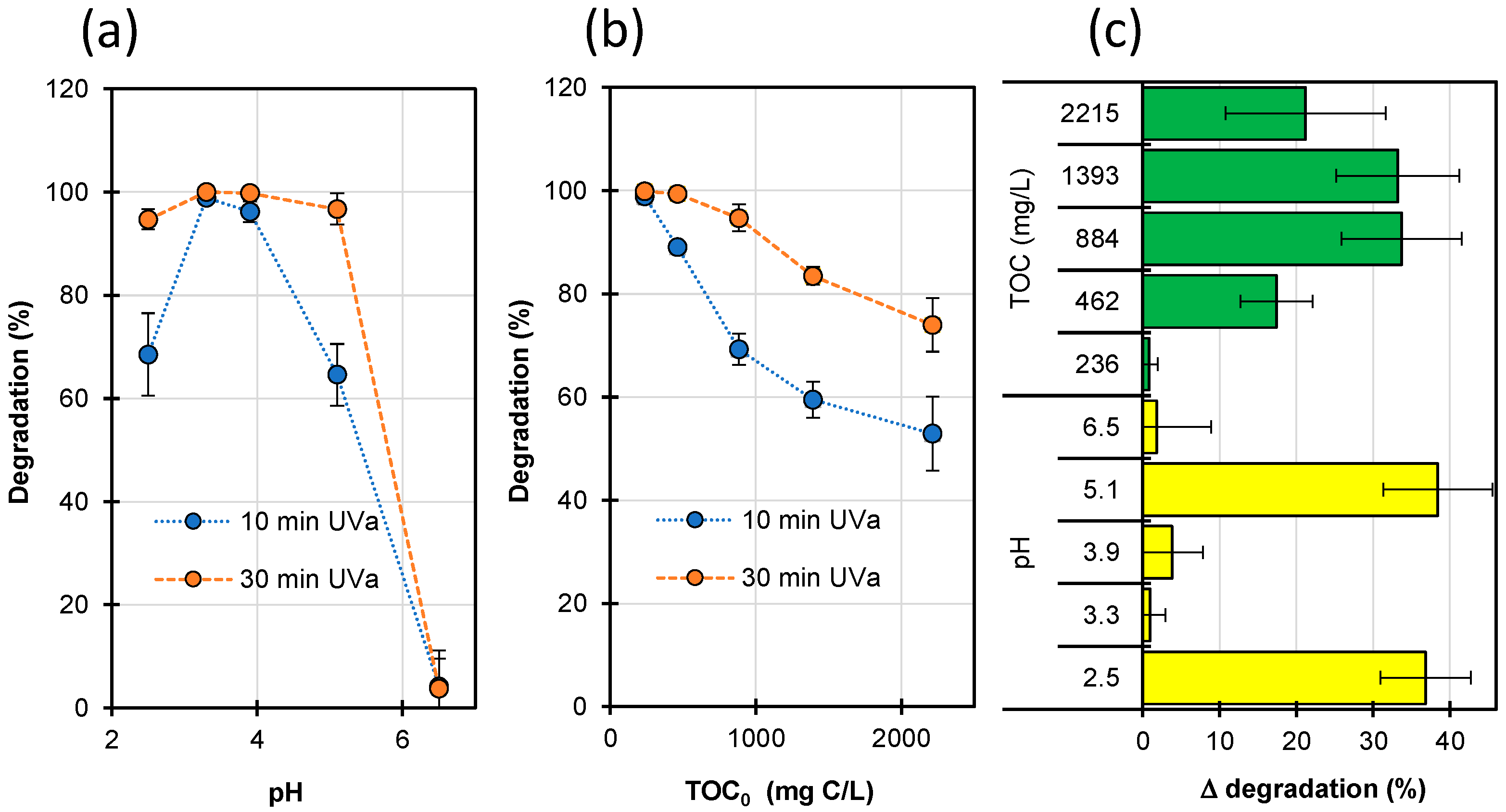
2.7. Photo-Fenton Process
2.8. Antimicrobial Activity and Ecotoxicity Evaluation
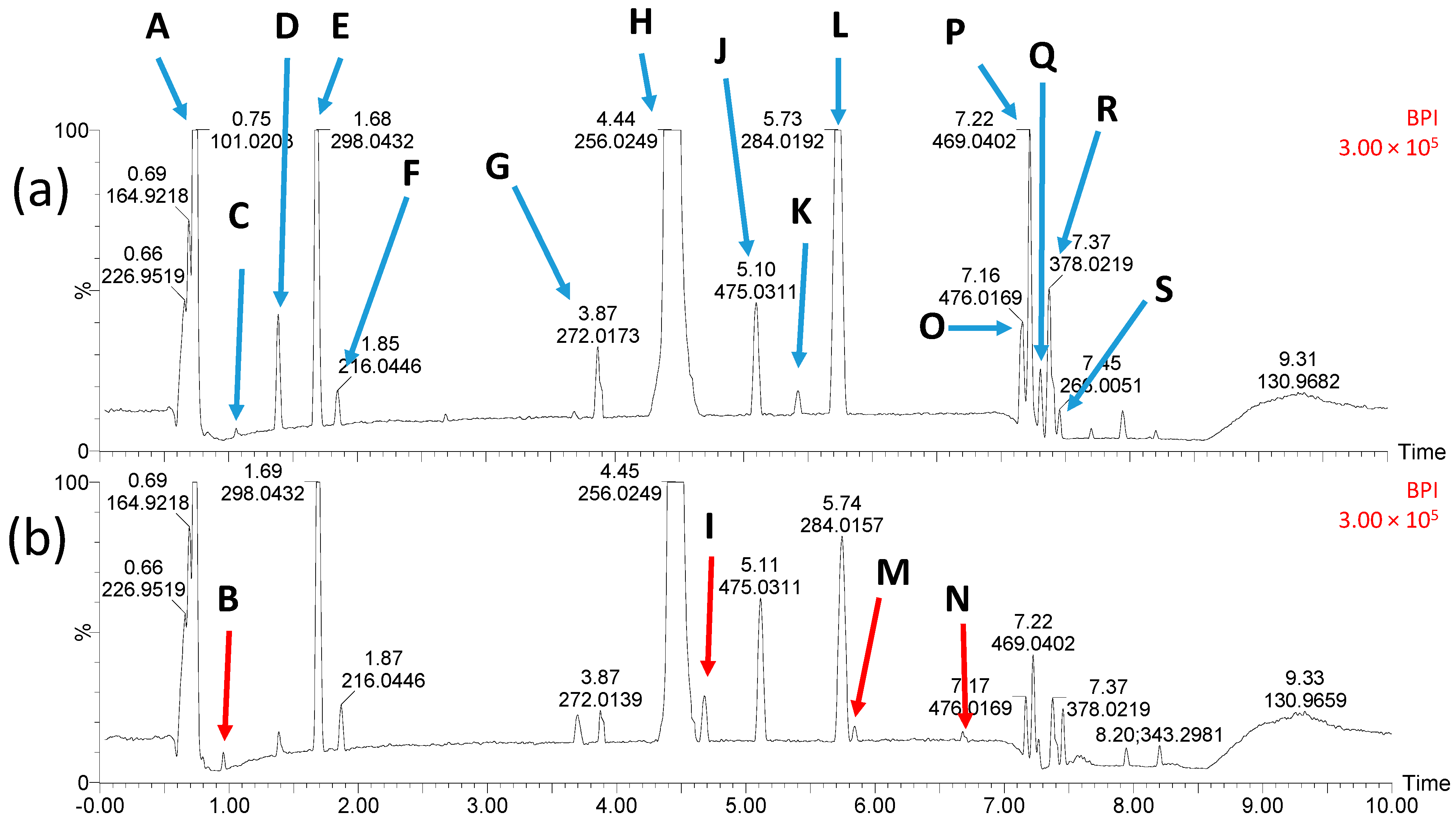
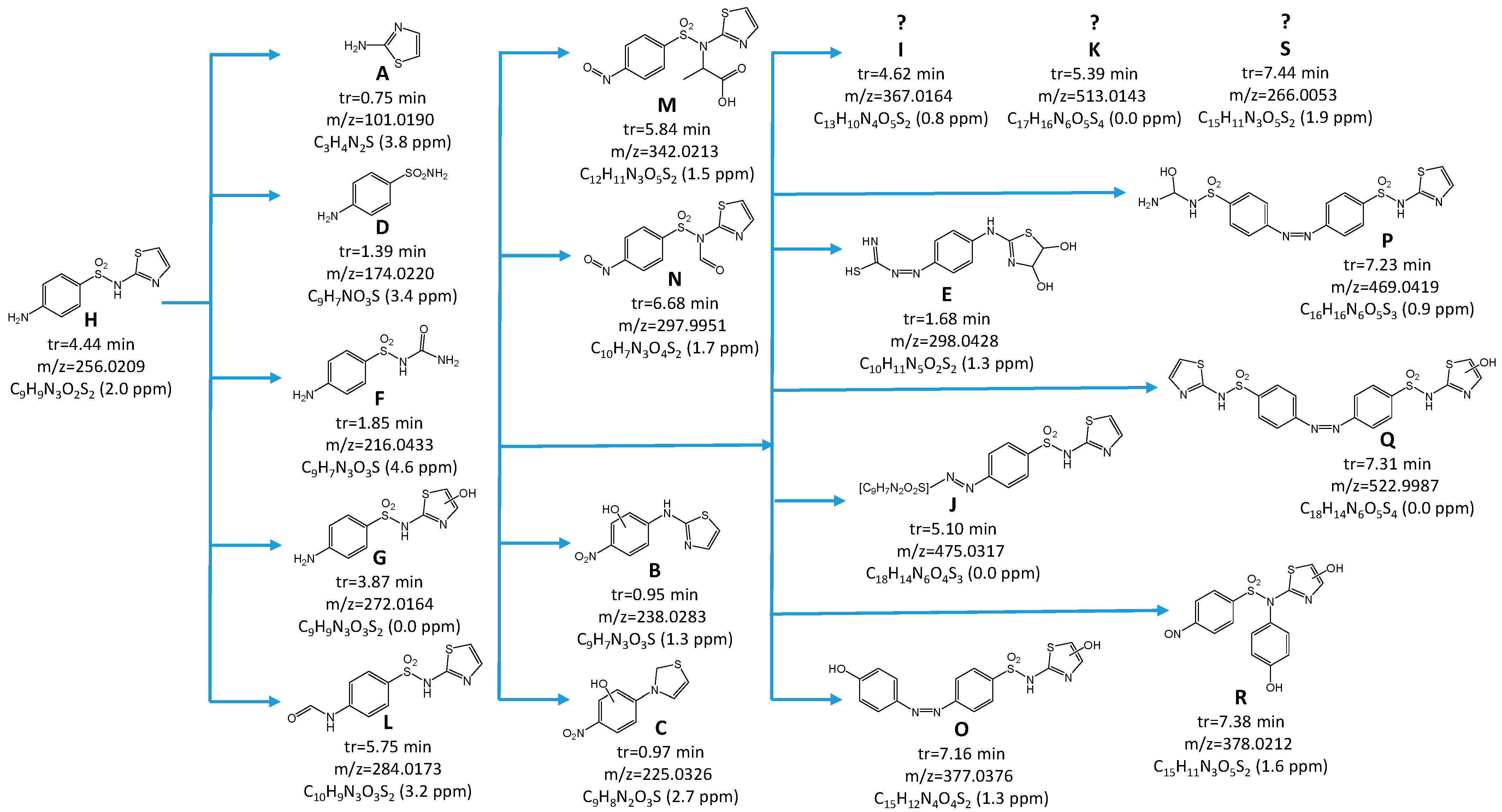
2.9. Identification of STZ Degradation Products
3. Materials and Methods
3.1. Reactants
3.2. Fenton Process
3.3. Photo-Fenton Process
3.4. Sampling and Preparation of Samples for Analysis
3.5. UPLC Analysis
3.6. Total Organic Carbon Analysis
3.7. Determination of Residual H2O2
3.8. Assessment of Chronic Toxicity
3.9. Assessment of Acute Toxicity (Microtox® assay)
3.10. Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hruska, K.; Franek, M. Sulfonamides in the Environment: A Review and a Case Report. Vet. Med. 2012, 57, 1–35. [Google Scholar] [CrossRef]
- Baran, W.; Adamek, E.; Ziemiańska, J.; Sobczak, A. Effects of the Presence of Sulfonamides in the Environment and Their Influence on Human Health. J. Hazard. Mater. 2011, 196, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Attauabi, M.; Høg, B.B.; Müller-Pebody, B.; Duarte, A.S.R.; Korsgaard, H.B.; Boel, J.; Dalby, T.; Hammerum, A.M.; Hansen, F.; Hasman, H.; et al. DANMAP 2020 Use of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Bacteria from Food Animals, Food and Humans in Denmark; Statens Serum Institut og Technical University of Denmark: Copenhagen, Denmark; Kongens Lyngby, Denmark, 2021; p. 174. Available online: https://www.danmap.org/reports (accessed on 14 November 2022).
- Białk-Bielińska, A.; Stolte, S.; Matzke, M.; Fabiańska, A.; Maszkowska, J.; Kołodziejska, M.; Liberek, B.; Stepnowski, P.; Kumirska, J. Hydrolysis of Sulphonamides in Aqueous Solutions. J. Hazard. Mater. 2012, 221–222, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Na, G.; Wang, C.; Gao, H.; Li, R.; Jin, S.; Zhang, W.; Zong, H. The Occurrence of Sulfonamide and Quinolone Resistance Genes at the Fildes Peninsula in Antarctica. Mar. Pollut. Bull. 2019, 149, 110503. [Google Scholar] [CrossRef] [PubMed]
- Thiele-Bruhn, S.; Beck, I.-C. Effects of Sulfonamide and Tetracycline Antibiotics on Soil Microbial Activity and Microbial Biomass. Chemosphere 2005, 59, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Kemper, N. Veterinary Antibiotics in the Aquatic and Terrestrial Environment. Ecol. Indic. 2008, 8, 1–13. [Google Scholar] [CrossRef]
- Wu, L.; Xiao, X.; Chen, F.; Zhang, H.; Huang, L.; Rong, L.; Zou, X. New Parameters for the Quantitative Assessment of the Proliferation of Antibiotic Resistance Genes Dynamic in the Environment and Its Application: A Case of Sulfonamides and Sulfonamide Resistance Genes. Sci. Total. Environ. 2020, 726, 138516. [Google Scholar] [CrossRef]
- Vilela, P.B.; Martins, A.S.; Starling, M.C.V.M.; de Souza, F.A.R.; Pires, G.F.F.; Aguilar, A.P.; Pinto, M.E.A.; Mendes, T.A.O.; de Amorim, C.C. Solar Photon-Fenton Process Eliminates Free Plasmid DNA Harboring Antimicrobial Resistance Genes from Wastewater. J. Environ. Manag. 2021, 285, 112204. [Google Scholar] [CrossRef]
- Nieto-Juarez, J.I.; Kohn, T. Virus Removal and Inactivation by Iron (Hydr)Oxide-Mediated Fenton-like Processes under Sunlight and in the Dark. Photochem. Photobiol. Sci. 2013, 12, 1596–1605. [Google Scholar] [CrossRef]
- Pei, M.; Zhang, B.; He, Y.; Su, J.; Gin, K.; Lev, O.; Shen, G.; Hu, S. State of the Art of Tertiary Treatment Technologies for Controlling Antibiotic Resistance in Wastewater Treatment Plants. Environ. Int. 2019, 131, 105026. [Google Scholar] [CrossRef]
- Homem, V.; Santos, L. Degradation and Removal Methods of Antibiotics from Aqueous Matrices-A Review. J. Environ. Manag. 2011, 92, 2304–2347. [Google Scholar] [CrossRef] [PubMed]
- Karkman, A.; Do, T.T.; Walsh, F.; Virta, M.P.J. Antibiotic-Resistance Genes in Waste Water. Trends. Microbiol. 2018, 26, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Paździor, K.; Bilińska, L.; Ledakowicz, S. A Review of the Existing and Emerging Technologies in the Combination of AOPs and Biological Processes in Industrial Textile Wastewater Treatment. Chem. Eng. J. 2019, 376, 120597. [Google Scholar] [CrossRef]
- Mirzaei, A.; Chen, Z.; Haghighat, F.; Yerushalmi, L. Removal of Pharmaceuticals from Water by Homo/Heterogonous Fenton-Type Processes-A Review. Chemosphere 2017, 174, 665–688. [Google Scholar] [CrossRef]
- Mousset, E.; Loh, W.H.; Lim, W.S.; Jarry, L.; Wang, Z.; Lefebvre, O. Cost Comparison of Advanced Oxidation Processes for Wastewater Treatment Using Accumulated Oxygen-Equivalent Criteria. Water. Res. 2021, 200, 117234. [Google Scholar] [CrossRef] [PubMed]
- Cassano, D.; Zapata, A.; Brunetti, G.; del Moro, G.; di Iaconi, C.; Oller, I.; Malato, S.; Mascolo, G. Comparison of Several Combined/Integrated Biological-AOPs Setups for the Treatment of Municipal Landfill Leachate: Minimization of Operating Costs and Effluent Toxicity. Chem. Eng. J. 2011, 172, 250–257. [Google Scholar] [CrossRef]
- Cañizares, P.; Paz, R.; Sáez, C.; Rodrigo, M.A. Costs of the Electrochemical Oxidation of Wastewaters: A Comparison with Ozonation and Fenton Oxidation Processes. J. Environ. Manag. 2009, 90, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Gar Alalm, M.; Tawfik, A.; Ookawara, S. Comparison of Solar TiO2 Photocatalysis and Solar Photo-Fenton for Treatment of Pesticides Industry Wastewater: Operational Conditions, Kinetics, and Costs. J. Water Process. Eng. 2015, 8, 55–63. [Google Scholar] [CrossRef]
- Tejera, J.; Miranda, R.; Hermosilla, D.; Urra, I.; Negro, C.; Blanco, Á. Treatment of a Mature Landfill Leachate: Comparison between Homogeneous and Heterogeneous Photo-Fenton with Different Pretreatments. Water 2019, 11, 1849. [Google Scholar] [CrossRef]
- Noman, D.-S.; Yasar, A.; Yousaf, S. Solar Assisted Photo Fenton for Cost Effective Degradation of Textile Effluents in Comparison to AOPs. Glob. Nest. J. 2012, 14, 477–486. [Google Scholar]
- Kwan, W.P.; Voelker, B.M. Rates of Hydroxyl Radical Generation and Organic Compound Oxidation in Mineral-Catalyzed Fenton-like Systems. Environ. Sci. Technol. 2003, 37, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Perini, J.A.L.; Tonetti, A.L.; Vidal, C.; Montagner, C.C.; Nogueira, R.F.P. Simultaneous Degradation of Ciprofloxacin, Amoxicillin, Sulfathiazole and Sulfamethazine, and Disinfection of Hospital Effluent after Biological Treatment via Photo-Fenton Process under Ultraviolet Germicidal Irradiation. Appl. Catal. B Environ. 2018, 224, 761–771. [Google Scholar] [CrossRef]
- Yang, J.-F.; Zhou, S.-B.; Xiao, A.-G.; Li, W.-J.; Ying, G.-G. Chemical Oxidation of Sulfadiazine by the Fenton Process: Kinetics, Pathways, Toxicity Evaluation. J. Environ. Sci. Health Part B 2014, 49, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Jain, B.; Singh, A.K.; Kim, H.; Lichtfouse, E.; Sharma, V.K. Treatment of Organic Pollutants by Homogeneous and Heterogeneous Fenton Reaction Processes. Environ. Chem. Lett. 2018, 16, 947–967. [Google Scholar] [CrossRef]
- Khankhasaeva, S.T.; Dambueva, D.V.; Dashinamzhilova, E.T.; Gil, A.; Vicente, M.A.; Timofeeva, M.N. Fenton Degradation of Sulfanilamide in the Presence of Al, Fe-Pillared Clay: Catalytic Behavior and Identification of the Intermediates. J. Hazard. Mater. 2015, 293, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Liu, X. Progress in the Mechanism and Kinetics of Fenton Reaction. MOJ Ecol. Environ. Sci. 2018, 3, 60. [Google Scholar] [CrossRef]
- Michael, I.; Frontistis, Z.; Fatta-Kassinos, D. Removal of Pharmaceuticals from Environmentally Relevant Matrices by Advanced Oxidation Processes (AOPs). Compr. Anal. Chem. 2013, 62, 345–407. [Google Scholar]
- Su, R.; Dai, X.; Wang, H.; Wang, Z.; Li, Z.; Chen, Y.; Luo, Y.; Ouyang, D. Metronidazole Degradation by UV and UV/H2O2 Advanced Oxidation Processes: Kinetics, Mechanisms, and Effects of Natural Water Matrices. Int. J. Environ. Res. Public Health 2022, 19, 12354. [Google Scholar] [CrossRef]
- Rozas, O.; Contreras, D.; Mondaca, M.A.; Pérez-Moya, M.; Mansilla, H.D. Experimental Design of Fenton and Photo-Fenton Reactions for the Treatment of Ampicillin Solutions. J. Hazard. Mater. 2010, 177, 1025–1030. [Google Scholar] [CrossRef] [PubMed]
- Batista, A.P.S.; Nogueira, R.F.P. Parameters Affecting Sulfonamide Photo-Fenton Degradation-Iron Complexation and Substituent Group. J. Photochem. Photobiol. A Chem. 2012, 232, 8–13. [Google Scholar] [CrossRef]
- Sági, G.; Csay, T.; Szabó, L.; Pátzay, G.; Csonka, E.; Takács, E.; Wojnárovits, L. Analytical Approaches to the OH Radical Induced Degradation of Sulfonamide Antibiotics in Dilute Aqueous Solutions. J. Pharm. Biomed. Anal. 2015, 106, 52–60. [Google Scholar] [CrossRef]
- Salgado, P.; Melin, V.; Contreras, D.; Moreno, Y.; Mansilla, H.D. Fenton Reaction Driven By Iron Ligands. J. Chil. Chem. Soc. 2013, 58, 2096–2101. [Google Scholar] [CrossRef]
- Lian, L.; Yao, B.; Hou, S.; Fang, J.; Yan, S.; Song, W. Kinetic Study of Hydroxyl and Sulfate Radical-Mediated Oxidation of Pharmaceuticals in Wastewater Effluents. Environ. Sci. Technol. 2017, 51, 2954–2962. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Zhang, X.; Yang, X.; Shang, C.; Song, W.; Fang, J.; Pan, Y. The Multiple Role of Bromide Ion in PPCPs Degradation under UV/Chlorine Treatment. Environ. Sci. Technol. 2018, 52, 1806–1816. [Google Scholar] [CrossRef] [PubMed]
- Kiwi, J.; Lopez, A.; Nadtochenko, V. Mechanism and Kinetics of the OH-Radical Intervention during Fenton Oxidation in the Presence of a Significant Amount of Radical Scavenger (Cl−). Environ. Sci. Technol. 2000, 34, 2162–2168. [Google Scholar] [CrossRef]
- Babuponnusami, A.; Muthukumar, K. A Review on Fenton and Improvements to the Fenton Process for Wastewater Treatment. J. Environ. Chem. Eng. 2014, 2, 557–572. [Google Scholar] [CrossRef]
- Wang, N.; Zheng, T.; Zhang, G.; Wang, P. A Review on Fenton-like Processes for Organic Wastewater Treatment. J. Environ. Chem. Eng. 2016, 4, 762–787. [Google Scholar] [CrossRef]
- Elmolla, E.; Chaudhuri, M. Optimization of Fenton Process for Treatment of Amoxicillin, Ampicillin and Cloxacillin Antibiotics in Aqueous Solution. J. Hazard. Mater. 2009, 170, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Burbano, A.A.; Dionysiou, D.D.; Suidan, M.T.; Richardson, T.L. Oxidation Kinetics and Effect of PH on the Degradation of MTBE with Fenton Reagent. Water Res. 2005, 39, 107–118. [Google Scholar] [CrossRef]
- Zazo, J.A.; Casas, J.A.; Mohedano, A.F.; Gilarranz, M.A.; Rodríguez, J.J. Chemical Pathway and Kinetics of Phenol Oxidation by Fenton’s Reagent. Environ. Sci. Technol. 2005, 39, 9295–9302. [Google Scholar] [CrossRef]
- Duesterberg, C.K.; Mylon, S.E.; Waite, T.D. pH Effects on Iron-Catalyzed Oxidation Using Fenton’s Reagent. Environ. Sci. Technol. 2008, 42, 8522–8527. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Yao, H.; Li, X.; Deng, S.; Zhao, S.; Zhang, W. Enhanced Degradation of Sulfamethoxazole (SMX) in Toilet Wastewater by Photo-Fenton Reactive Membrane Filtration. Nanomaterials 2020, 10, 180. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, A.; Esteban, B.; Garrido-Cardenas, J.A.; Kowalska, K.; Rizzo, L.; Aguera, A.; Pérez, J.A.S. Effect of Solar Photo-Fenton Process in Raceway Pond Reactors at Neutral pH on Antibiotic Resistance Determinants in Secondary Treated Urban Wastewater. J. Hazard. Mater. 2019, 378, 120737. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Brigante, M.; Wu, F.; Mousty, C.; Hanna, K.; Mailhot, G. Assessment of the Fe(III)–EDDS Complex in Fenton-Like Processes: From the Radical Formation to the Degradation of Bisphenol A. Environ. Sci. Technol. 2013, 47, 1952–1959. [Google Scholar] [CrossRef] [PubMed]
- Urbano, V.R.; Maniero, M.G.; Guimarães, J.R.; del Valle, L.J.; Pérez-Moya, M. Sulfaquinoxaline Oxidation and Toxicity Reduction by Photo-Fenton Process. Int. J. Environ. Res. Public Health 2021, 18, 1005. [Google Scholar] [CrossRef] [PubMed]
- Trovó, A.G.; Pupo Nogueira, R.F.; Agüera, A.; Fernandez-Alba, A.R.; Malato, S. Degradation of the Antibiotic Amoxicillin by Photo-Fenton Process-Chemical and Toxicological Assessment. Water Res. 2011, 45, 1394–1402. [Google Scholar] [CrossRef]
- Michael, I.; Hapeshi, E.; Aceña, J.; Perez, S.; Petrović, M.; Zapata, A.; Barceló, D.; Malato, S.; Fatta-Kassinos, D. Light-Induced Catalytic Transformation of Ofloxacin by Solar Fenton in Various Water Matrices at a Pilot Plant: Mineralization and Characterization of Major Intermediate Products. Sci. Total. Environ. 2013, 461–462, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Moya, M.; Graells, M.; Castells, G.; Amigó, J.; Ortega, E.; Buhigas, G.; Pérez, L.M.; Mansilla, H.D. Characterization of the Degradation Performance of the Sulfamethazine Antibiotic by Photo-Fenton Process. Water. Res. 2010, 44, 2533–2540. [Google Scholar] [CrossRef]
- Batista, A.P.S.; Pires, F.C.C.; Teixeira, A.C.S.C. Photochemical Degradation of Sulfadiazine, Sulfamerazine and Sulfamethazine: Relevance of Concentration and Heterocyclic Aromatic Groups to Degradation Kinetics. J. Photochem. Photobiol. A Chem. 2014, 286, 40–46. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J. Comparative Study on Sulfamethoxazole Degradation by Fenton and Fe(II)-Activated Persulfate Process. RSC Adv. 2017, 7, 48670–48677. [Google Scholar] [CrossRef]
- Wang, S. A Comparative Study of Fenton and Fenton-like Reaction Kinetics in Decolourisation of Wastewater. Dye. Pigment. 2008, 76, 714–720. [Google Scholar] [CrossRef]
- Anotai, J.; Lu, M.C.; Chewpreecha, P. Kinetics of Aniline Degradation by Fenton and Electro-Fenton Processes. Water Res. 2006, 40, 1841–1847. [Google Scholar] [CrossRef] [PubMed]
- Abdili, T.; Rahmani, A.; Rahmani, H.; Alighadri, M.; Rahmani, K. Heterogeneous Oxidation of Sulfacetamide in Aquatic Environment Using Ultrasonic and Nano-Fenton: Kinetics Intermediates and Bioassay Test. Desalin. Water Treat. 2019, 166, 158–167. [Google Scholar] [CrossRef]
- Baran, W.; Adamek, E.; Sobczak, A.; Sochacka, J. The Comparison of Photocatalytic Activity of Fe-Salts, TiO2 and TiO2/FeCl3 during the Sulfanilamide Degradation Process. Catal. Commun. 2009, 10, 811–814. [Google Scholar] [CrossRef]
- Acosta-Rangel, A.; Sánchez-Polo, M.; Polo, A.M.S.; Rivera-Utrilla, J.; Berber-Mendoza, M.S. Sulfonamides Degradation Assisted by UV, UV/H2O2 and UV/K2S2O8: Efficiency, Mechanism and Byproducts Cytotoxicity. J. Environ. Manag. 2018, 225, 224–231. [Google Scholar] [CrossRef]
- Bach, A.; Shemer, H.; Semiat, R. Kinetics of Phenol Mineralization by Fenton-like Oxidation. Desalination 2010, 264, 188–192. [Google Scholar] [CrossRef]
- Pervaiz, M.; Riaz, A.; Munir, A.; Saeed, Z.; Hussain, S.; Rashid, A.; Younas, U.; Adnan, A. Synthesis and Characterization of Sulfonamide Metal Complexes as Antimicrobial Agents. J. Mol. Struct. 2020, 1202, 127284. [Google Scholar] [CrossRef]
- Engelmann, M.D.; Bobier, R.T.; Hiatt, T.; Cheng, I.F. Variability of the Fenton Reaction Characteristics of the EDTA, DTPA, and Citrate Complexes of Iron. BioMetals 2003, 16, 519–527. [Google Scholar] [CrossRef]
- Velásquez, M.; Santander, I.P.; Contreras, D.R.; Yáñez, J.; Zaror, C.; Salazar, R.A.; Pérez-Moya, M.; Mansilla, H.D. Oxidative Degradation of Sulfathiazole by Fenton and Photo-Fenton Reactions. J. Environ. Sci. Health A 2014, 49, 661–670. [Google Scholar] [CrossRef]
- Rokhina, E.V.; Virkutyte, J. Environmental Application of Catalytic Processes: Heterogeneous Liquid Phase Oxidation of Phenol With Hydrogen Peroxide. Crit. Rev. Environ. Sci. Technol. 2010, 41, 125–167. [Google Scholar] [CrossRef]
- Trovó, A.G.; de Paiva, V.A.B.; Machado, A.E.H.; de Oliveira, C.A.; Santos, R.O. Degradation of the Antibiotic Chloramphenicol by Photo-Fenton Process at Lab-Scale and Solar Pilot Plant: Kinetic, Toxicity and Inactivation Assessment. Sol. Energy 2013, 97, 596–604. [Google Scholar] [CrossRef]
- González, O.; Sans, C.; Esplugas, S. Sulfamethoxazole Abatement by Photo-Fenton: Toxicity, Inhibition and Biodegradability Assessment of Intermediates. J. Hazard. Mater. 2007, 146, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Ben, W.; Qiang, Z.; Pan, X.; Chen, M. Removal of Veterinary Antibiotics from Sequencing Batch Reactor (SBR) Pretreated Swine Wastewater by Fenton’s Reagent. Water Res. 2009, 43, 4392–4402. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yao, H.; Sun, P.; Pei, J.; Li, D.; Huang, C.H. Oxidation of Tetracycline Antibiotics Induced by Fe(III) Ions without Light Irradiation. Chemosphere 2015, 119, 1255–1261. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Zhang, P.; Halsall, C.; Li, Y.; Chen, C.E.; Li, J.; Sun, H.; Yao, Z. The Importance of Reactive Oxygen Species on the Aqueous Phototransformation of Sulfonamide Antibiotics: Kinetics, Pathways, and Comparisons with Direct Photolysis. Water Res. 2019, 149, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Guo, W.; Wang, H.; Du, J.; Zhou, X.; Wu, Q.; Zheng, H.; Chang, J.; Ren, N. Selective Degradation of Sulfonamide Antibiotics by Peroxymonosulfate Alone: Direct Oxidation and Nonradical Mechanisms. Chem. Eng. J. 2018, 334, 2539–2546. [Google Scholar] [CrossRef]
- Zhang, K.; Luo, Z.; Zhang, T.; Gao, N.; Ma, Y. Degradation Effect of Sulfa Antibiotics by Potassium Ferrate Combined with Ultrasound (Fe(VI)-US). Biomed. Res. Int. 2015, 2015, 169215. [Google Scholar] [CrossRef] [PubMed]
- García-Galán, M.J.; Díaz-Cruz, M.S.; Barceló, D. Identification and Determination of Metabolites and Degradation Products of Sulfonamide Antibiotics. TrAC Trends. Anal. Chem. 2008, 27, 1008–1022. [Google Scholar] [CrossRef]
- Duca, G. Homogeneous Redox Catalysis with Transition Metal Compounds in Oxide and Peroxide Systems. In Homogeneous Catalysis with Metal Complexes; Springer Series in Chemical Physics; Springer: Berlin/Heidelberg, Germany, 2012; Volume 102, pp. 11–121. [Google Scholar]
- Wadhia, K.; Dando, T.; Clive Thompson, K. Intra-Laboratory Evaluation of Microbial Assay for Risk Assessment (MARA) for Potential Application in the Implementation of the Water Framework Directive (WFD). J. Environ. Monit. 2007, 9, 953–958. [Google Scholar] [CrossRef]
- Adamek, E.; Baran, W.; Sobczak, A. Photocatalytic Degradation of Veterinary Antibiotics: Biodegradability and Antimicrobial Activity of Intermediates. Process. Saf. Environ. 2016, 103, 1–9. [Google Scholar] [CrossRef]
- Weltens, R.; Deprez, K.; Michiels, L. Validation of Microtox as a First Screening Tool for Waste Classification. Waste Manag. 2014, 34, 2427–2433. [Google Scholar] [CrossRef] [PubMed]
- Fai, P.B.; Grant, A. An Assessment of the Potential of the Microbial Assay for Risk Assessment (MARA) for Ecotoxicological Testing. Ecotoxicology 2010, 19, 1626–1633. [Google Scholar] [CrossRef] [PubMed]
- Nałȩcz-Jawecki, G.; Wadhia, K.; Adomas, B.; Piotrowicz-Cieślak, A.I.; Sawicki, J. Application of Microbial Assay for Risk Assessment Biotest in Evaluation of Toxicity of Human and Veterinary Antibiotics. Environ. Toxicol. 2010, 25, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Baran, W.; Adamek, E.; Sobczak, A. Sobczak A Assessment of Sensitivity of Environmental Microorganisms to Antibiotics Using the Platelet Assays. Proc. ECOpole 2015, 9, 533–540. [Google Scholar]
- Wadhia, K. ISTA13-International Interlaboratory Comparative Evaluation of Microbial Assay for Risk Assessment (MARA). Environ. Toxicol. 2008, 23, 626–633. [Google Scholar] [CrossRef] [PubMed]
 ), on relative TOC content (
), on relative TOC content ( ) and H2O2 concentration (
) and H2O2 concentration ( ) (a) and relative inhibition determined for microorganisms from the Brynica River (
) (a) and relative inhibition determined for microorganisms from the Brynica River ( ), for microorganisms from effluent (
), for microorganisms from effluent ( ), V fischeri (
), V fischeri ( ), on mean relative inhibition determined for B. diminuta, D. acidovorans and P. aurantiaca (
), on mean relative inhibition determined for B. diminuta, D. acidovorans and P. aurantiaca ( ) (b), in samples after 30 min of Fenton process in the presence of FeSO4. [STZ]0 = 1.0 mmol/L, pH = 3.35 ± 0.05.
) (b), in samples after 30 min of Fenton process in the presence of FeSO4. [STZ]0 = 1.0 mmol/L, pH = 3.35 ± 0.05.
 ), on relative TOC content (
), on relative TOC content ( ) and H2O2 concentration (
) and H2O2 concentration ( ) (a) and relative inhibition determined for microorganisms from the Brynica River (
) (a) and relative inhibition determined for microorganisms from the Brynica River ( ), for microorganisms from effluent (
), for microorganisms from effluent ( ), V fischeri (
), V fischeri ( ), on mean relative inhibition determined for B. diminuta, D. acidovorans and P. aurantiaca (
), on mean relative inhibition determined for B. diminuta, D. acidovorans and P. aurantiaca ( ) (b), in samples after 30 min of Fenton process in the presence of FeSO4. [STZ]0 = 1.0 mmol/L, pH = 3.35 ± 0.05.
) (b), in samples after 30 min of Fenton process in the presence of FeSO4. [STZ]0 = 1.0 mmol/L, pH = 3.35 ± 0.05.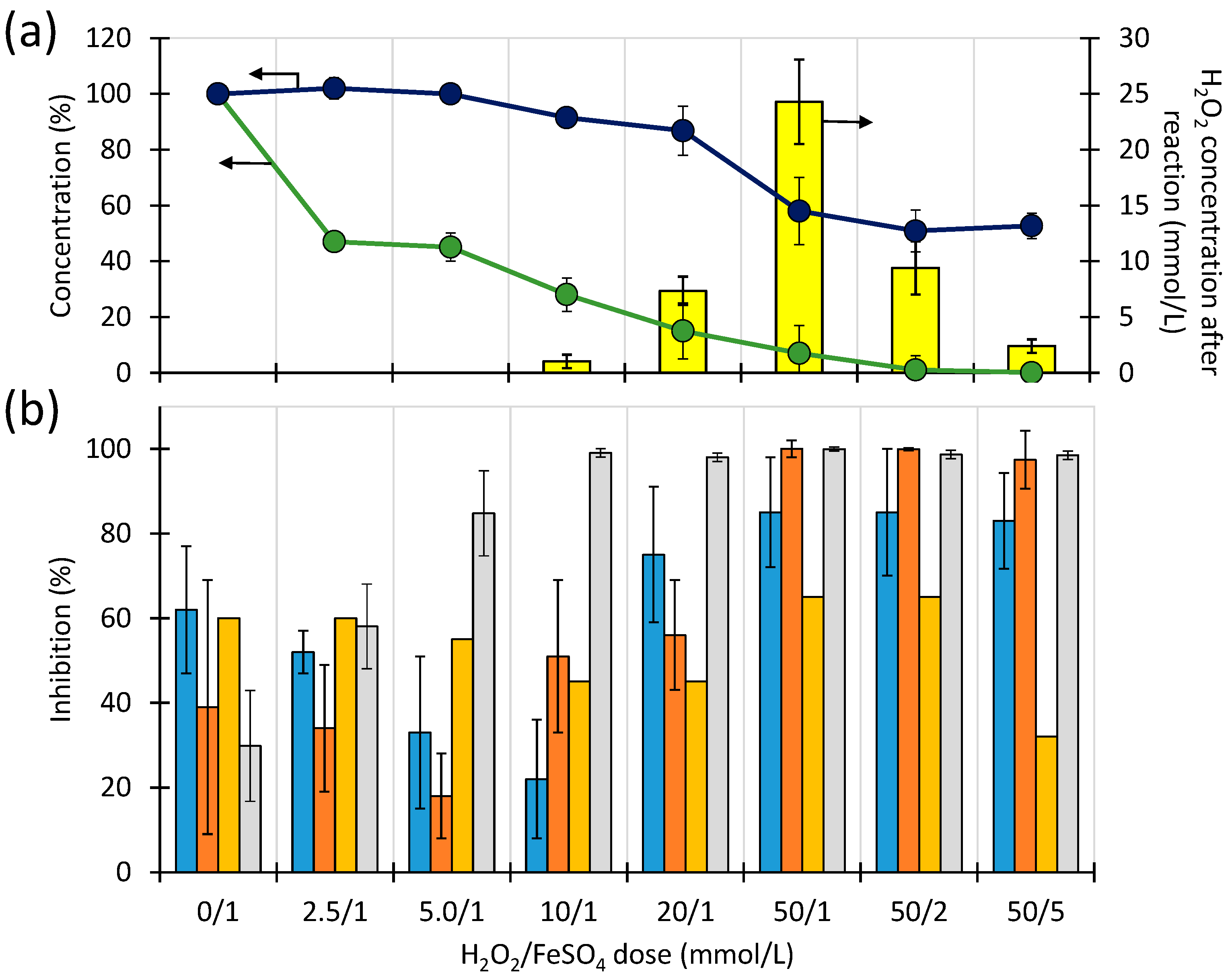
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamek, E.; Masternak, E.; Sapińska, D.; Baran, W. Degradation of the Selected Antibiotic in an Aqueous Solution by the Fenton Process: Kinetics, Products and Ecotoxicity. Int. J. Mol. Sci. 2022, 23, 15676. https://doi.org/10.3390/ijms232415676
Adamek E, Masternak E, Sapińska D, Baran W. Degradation of the Selected Antibiotic in an Aqueous Solution by the Fenton Process: Kinetics, Products and Ecotoxicity. International Journal of Molecular Sciences. 2022; 23(24):15676. https://doi.org/10.3390/ijms232415676
Chicago/Turabian StyleAdamek, Ewa, Ewa Masternak, Dominika Sapińska, and Wojciech Baran. 2022. "Degradation of the Selected Antibiotic in an Aqueous Solution by the Fenton Process: Kinetics, Products and Ecotoxicity" International Journal of Molecular Sciences 23, no. 24: 15676. https://doi.org/10.3390/ijms232415676
APA StyleAdamek, E., Masternak, E., Sapińska, D., & Baran, W. (2022). Degradation of the Selected Antibiotic in an Aqueous Solution by the Fenton Process: Kinetics, Products and Ecotoxicity. International Journal of Molecular Sciences, 23(24), 15676. https://doi.org/10.3390/ijms232415676







