Increased Oxidative Stress Markers in Acute Ischemic Stroke Patients Treated with Thrombolytics
Abstract
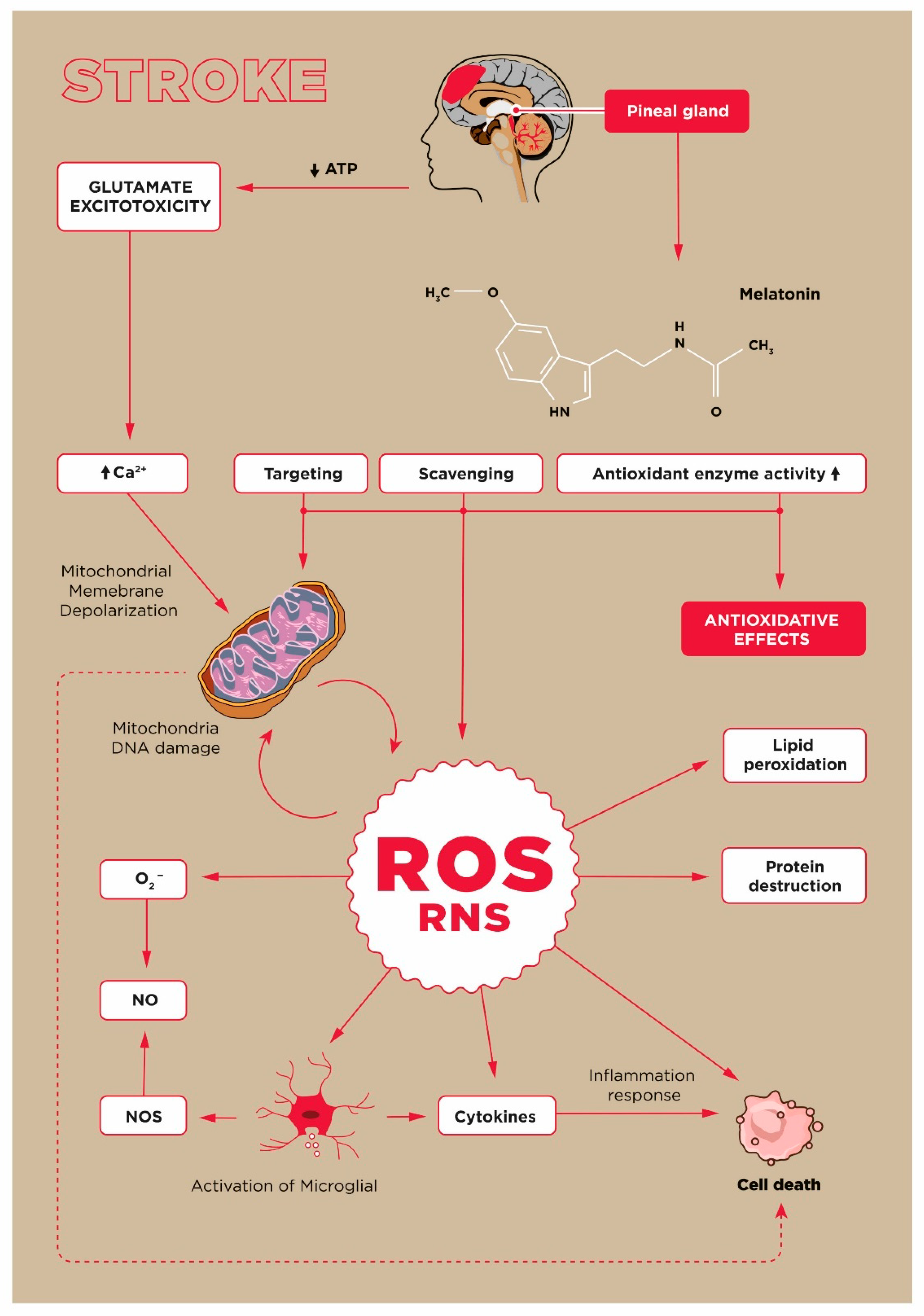
1. Introduction
2. Results
2.1. Clinical Picture of Stroke Patients Treated with Intravenous Thrombolysis
2.2. Melatonin and Carbonyl Group Levels before and after Intravenous Thrombolysis
2.3. The Levels of Biomarkers Compared to the Control Group
2.4. The Statistical Connection between the Level of Biomarkers and the Severity of Neurological Symptoms
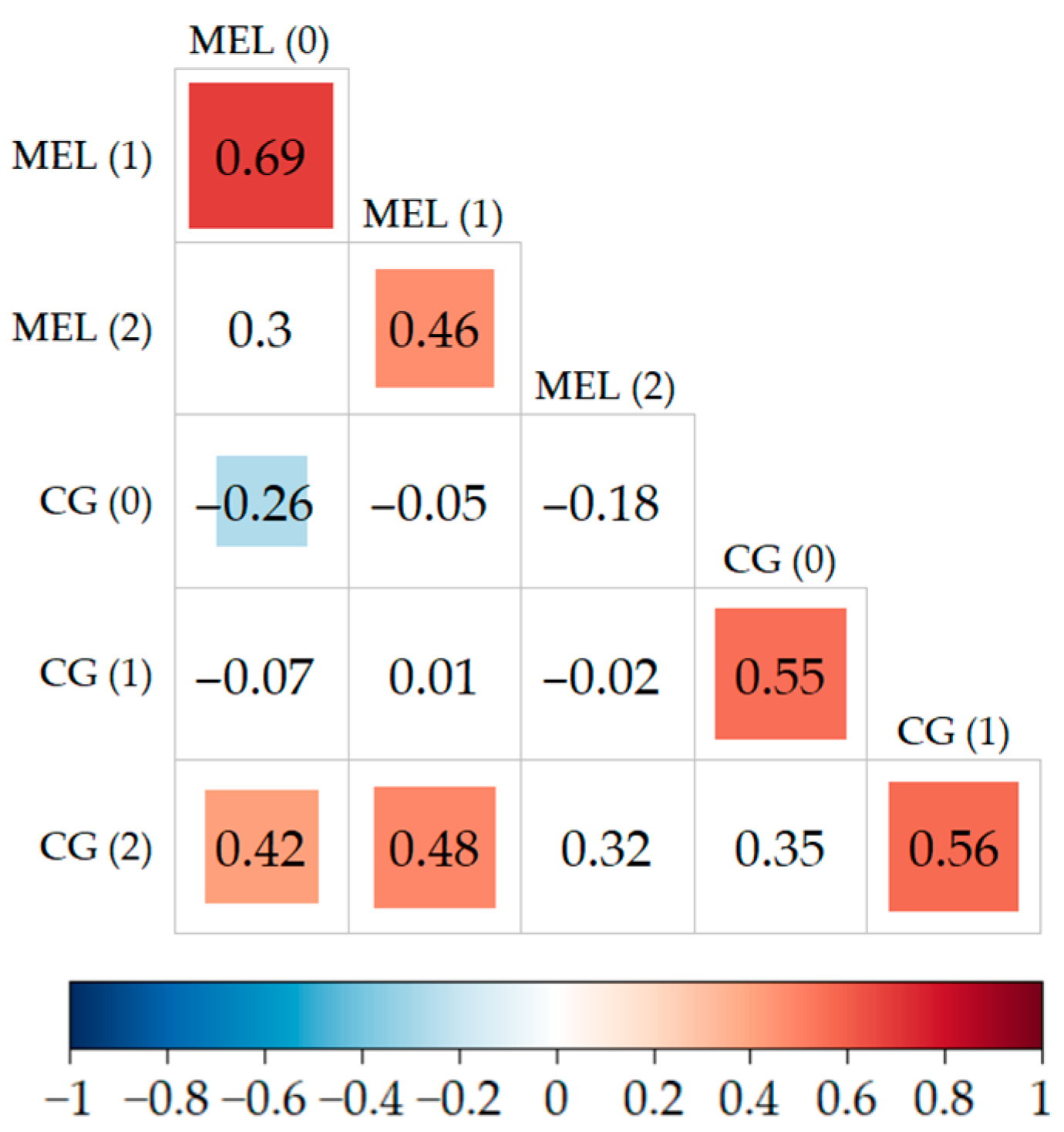
2.5. Correlations between the Levels of Biomarkers
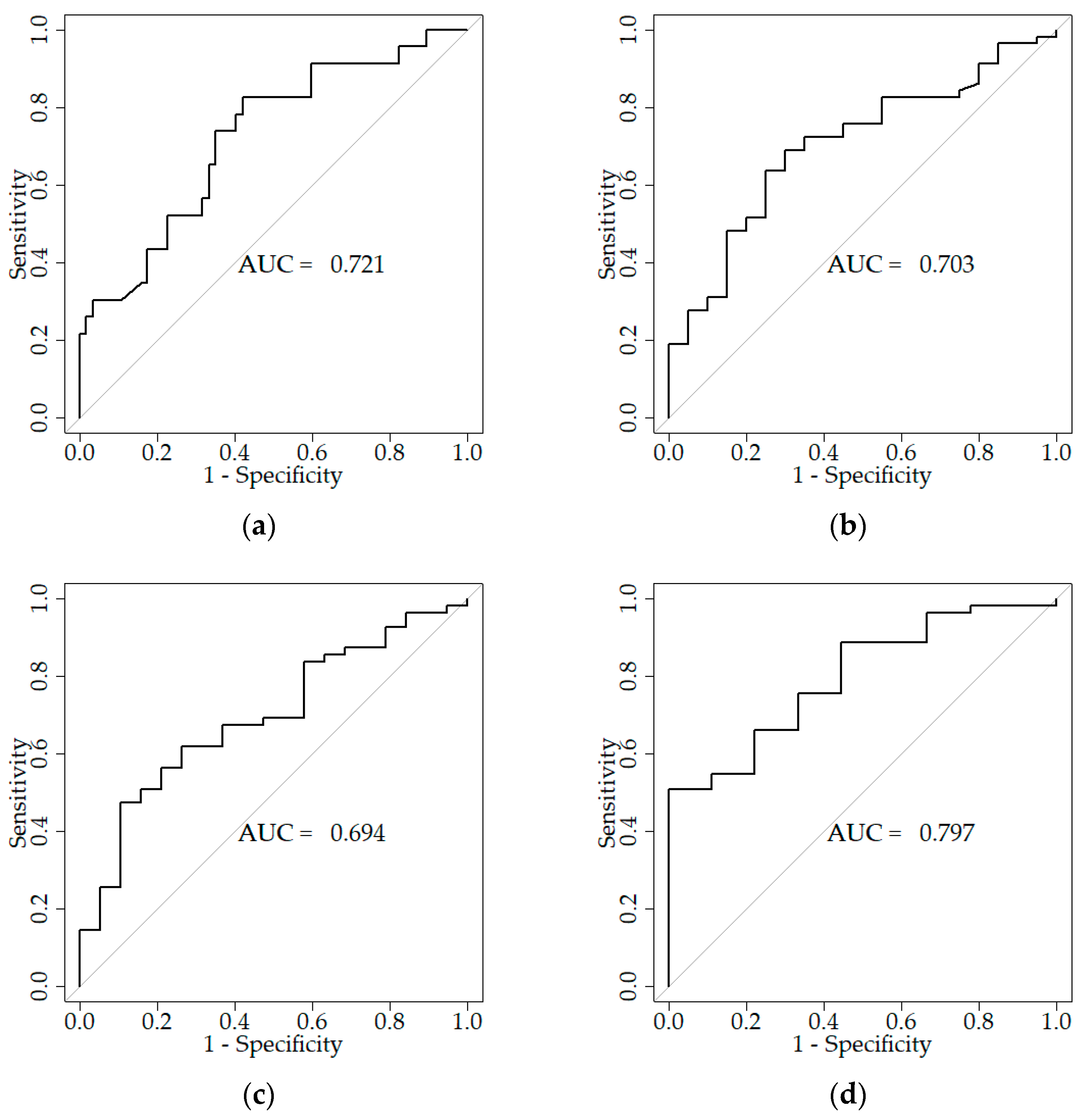
2.6. ROC Curves
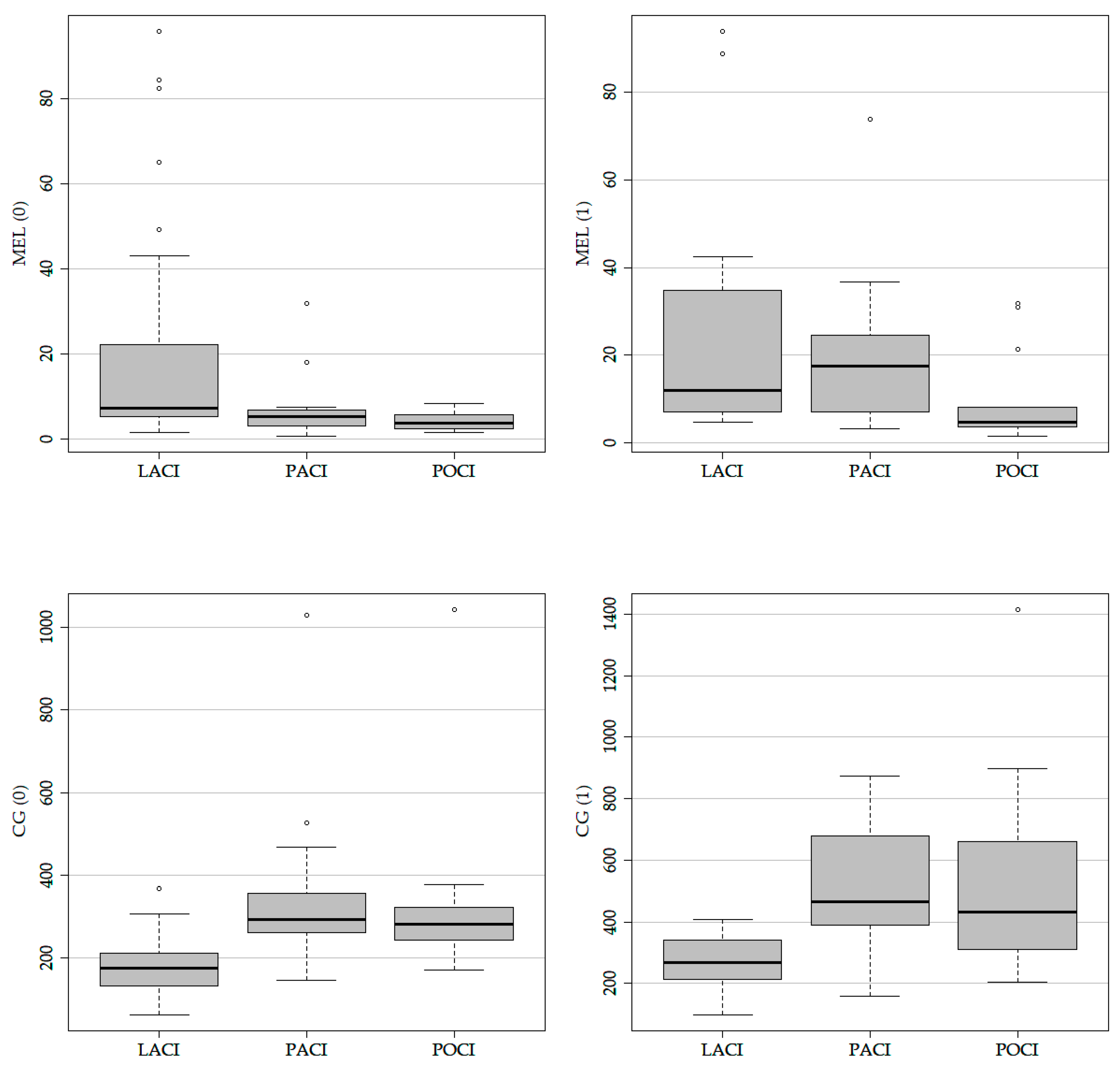
2.7. The Level of Biomarkers Depending on Subtypes of Strokes
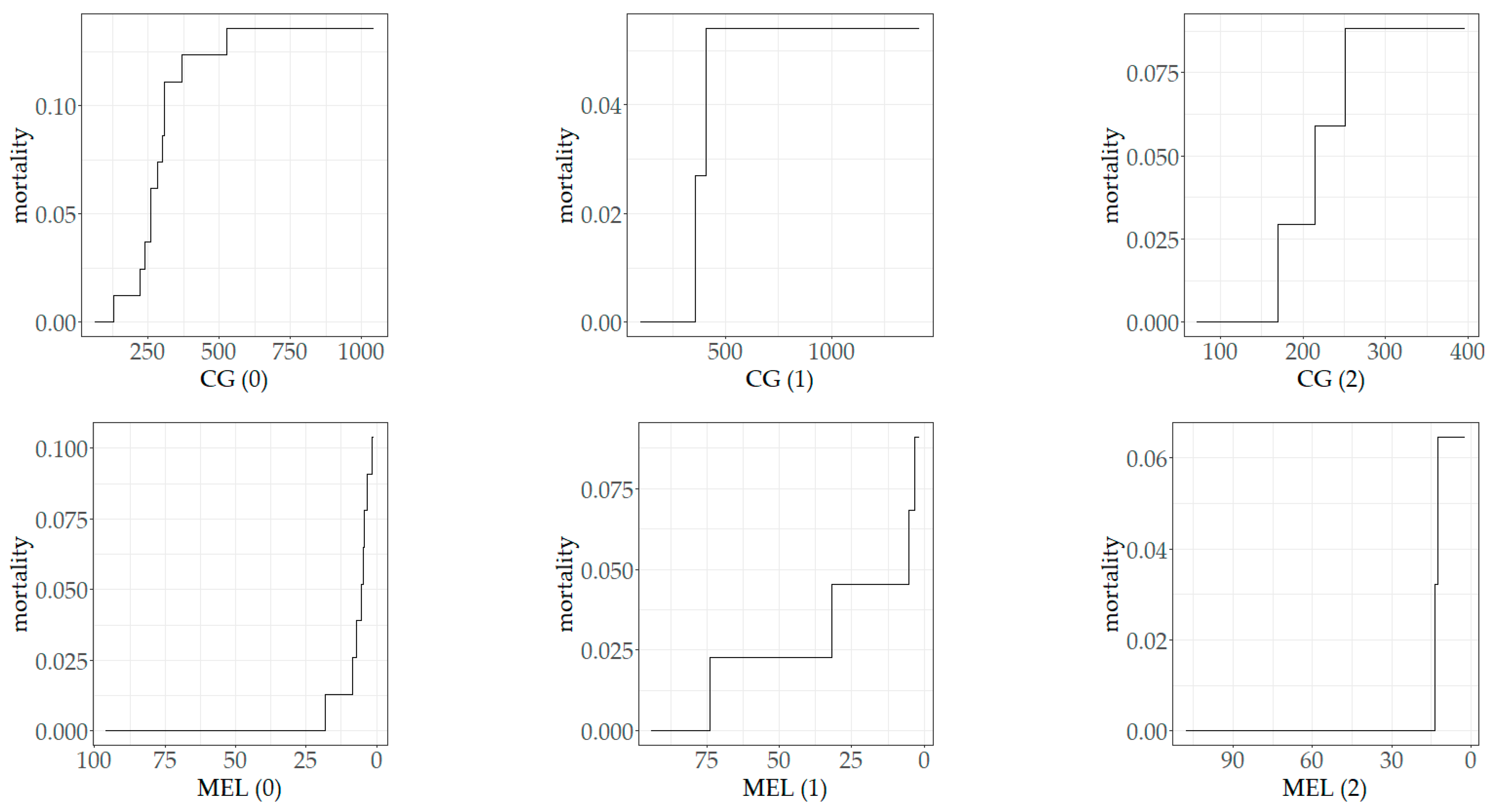
2.8. The Dependence of Mortality on Biomarker Concentrations
3. Discussion
4. Materials and Methods
4.1. Study Group
4.2. Biochemical Testing
4.3. Statistical Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pawluk, H.; Woźniak, A.; Grześk, G.; Kołodziejska, R.; Kozakiewicz, M.; Kopkowska, E.; Grzechowiak, E.; Kozera, G. The role of selected pro-inflammatory cytokines in pathogenesis of ischemic stroke. Clin. Interv. Aging. 2020, 15, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Vidale, S.; Consoli, A.; Arnaboldi, M.; Consoli, D. Postischemic inflammation in acute stroke. J. Clin. Neurol. 2017, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
- De Nigris, F.; Lerman, A.; Ignarro, L.J.; Williams-Ignarro, S.; Sica, V.; Baker, A.H.; Lilach, O.L.; Geng, Y.J.; Napoli, C. Oxida-tion-sensitive mechanisms, vascular apoptosis and atherosclerosis. Trends Mol. Med. 2003, 9, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Moon, G.J.; Shin, D.H.; Im, D.S.; Bang, O.Y.; Nam, H.S.; Lee, J.H.; Joo, I.S.; Huh, K.; Gwag, B.J. Identification of oxidized serum albumin in the cerebrospinal fluid of ischaemic stroke patients. Eur. J. Neurol. 2011, 18, 1151–1158. [Google Scholar] [CrossRef]
- Andrabi, S.S.; Parvez, S.; Tabassum, H. Melatonin and ischemic stroke: Mechanistic roles and action. Adv. Pharmacol. Sci. 2015, 2015, 384750. [Google Scholar] [CrossRef]
- Alghamdi, B.S. The neuroprotective role of melatonin in neurological disorders. J. Neurosci. Res. 2018, 96, 1136–1149. [Google Scholar] [CrossRef]
- Rodrigo, R.; Fernandez-Gajardo, R.; Gutiérrez, R.; Matamala, J.M.; Carrasco, R.; Miranda-Merchak, A.; Feuerhake, W. Oxidative stress and pathophysiology of ischemic stroke: Novel therapeutic opportunities. CNS Neurol. Disord.-Drug Targets 2013, 12, 698–714. [Google Scholar] [CrossRef]
- Ramos, E.; Patiño, P.; Reiter, R.J.; Gil-Martin, E.; Marco-Contelles, J.; Parada, E.; De los Rios, C.; Romero, A.; Egea, J. Ischemic brain injury: New insights on the protective role of melatonin. Free Radic. Biol. Med. 2017, 104, 32–53. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Rossi, R.; Giustarini, D.; Milzani, A.; Colombo, R. Protein carbonyl groups as biomarkers of oxidative stress. Clin. Chim. Acta 2003, 329, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Irazusta, V.; Moreno-Cermeño, A.; Cabiscol, E.; Tamarit, J.; Ros, J. Proteomic strategies for the analysis of carbonyl groups on proteins. Curr. Protein Pept. Sci. 2010, 11, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Moon, G.J.; Kim, S.J.; Cho, Y.H.; Ryoo, S.; Bangc, O.Y. Antioxidant effects of statins in patients with atherosclerotic cerebrovascular disease. J. Clin. Neurol. 2014, 10, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Žitňanová, I.; Šiarnik, P.; Kollár, B.; Chomová, M.; Pazderová, P.; Andrezálová, L.; Ježovičová, M.; Koňariková, K.; Laubertová, L.; Krivošíková, Z.; et al. Oxidative stress markers and their dynamic changes in patients after acute ischemic stroke oxidative. Oxid. Med. Cell. Longev. 2016, 2016, 9761697. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Liu, T.; Borjigin, J.; Wang, M.M. Ischemic stroke destabilizes circadian rhythms. J. Circadian Rhythm. 2008, 6, 9. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Manchester, L.C.; Qi, W. Biochemical reactivity of melatonin with reactive oxygen and nitrogen species. A review of the evidence. Cell Biochem. Biophys. 2001, 34, 237–256. [Google Scholar] [CrossRef]
- Ramos, E.; Farré-Alins, V.; Egea, J.; López-Muñoz, F.; Reiter, R.J.; Romero, A. Melatonin’s efficacy in stroke patients; a matter of dose? A review of the evidence. Toxicol. Appl. Pharmacol. 2020, 392, 114933. [Google Scholar] [CrossRef] [PubMed]
- Romero, A.; Ramos, E.; Patiño, P.; Oset-Gasque, M.J.; López-Muñoz, F.; Marco-Contelles, J.; Ayuso, M.I.; Alcázar, A. Melatonin and nitrones as potential therapeutic agents for stroke. Front. Aging Neurosci. 2016, 8, 27932976. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gao, S.; Lenahan, C.; Gu, Y.; Wang, X.; Fang, Y.; Xu, W.; Wu, H.; Pan, Y.; Shao, A.; et al. Melatonin as an antioxidant agent in stroke: An updated review. Aging Dis. 2022, 13, 1823–1844. [Google Scholar] [CrossRef]
- Simão, A.N.C.; Lehmann, M.F.; Alfieri, D.F.; Meloni, M.Z.; Flauzino, T.; Scavuzzi, B.M.; de Oliveira, S.R.; Lozovoy, M.A.B.; Dichi, I.; Reiche, E.M.V. Metabolic syndrome increases oxidative stress but does not influence disability and short-time outcome in acute ischemic stroke patients. Metab. Brain Dis. 2015, 30, 1409–1416. [Google Scholar] [CrossRef]
- Pouya, V.T.; Hashemy, S.I.; Shoeibi, A.; Tirkani, A.N.; Tavallaie, S.; Avval, F.Z.; Soukhtanloo, M.; Mashkani, B.A.; Alamdari, D.H. Serum pro-oxidant-antioxidant balance, advanced oxidized protein products (AOPP) and protein carbonyl in patients with stroke. Razavi Int. J. Med. 2016, 4, 21–26. [Google Scholar] [CrossRef]
- Cichoń, N.; Bijak, M.; Miller, E.; Niwald, M.; Saluk, J. Poststroke depression as a factor adversely affecting the level of oxidative damage to plasma proteins during a brain stroke. Oxid. Med. Cell. Longev. 2015, 2015, 408745. [Google Scholar] [CrossRef]
- Manolescu, B.N.; Berteanu, M.; Oprea, E.; Chiriac, N.; Dumitru, L.; Vlădoiu, S.; Popa, O.; Ianas, O. Dynamic of oxidative and nitrosative stress markers during the convalescent period of stroke patients undergoing rehabilitation. Ann. Clin. Biochem. 2011, 48, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Dringen, R. Oxidative and antioxidative potential of brain microglial cells. Antioxid. Redox Signal. 2005, 7, 1223–1233. [Google Scholar] [CrossRef] [PubMed]
- Ritzenthaler, T.; Nighoghossian, N.; Berthiller, J.; Schott, A.M.; Cho, T.H.; Derex, L.; Brun, J.; Trouillas, P.; Claustrat, B. Nocturnal urine melatonin and 6-sulphatoxymelatonin excretion at the acute stage of ischaemic stroke. J. Pineal Res. 2009, 46, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Ritzenthaler, T.; Lhommeau, I.; Douillard, S.; Cho, T.H.; Brun, J.; Patrice, T.; Nighoghossian, N.; Claustrat, B. Dynamics of oxidative stress and urinary excretion of melatonin and its metabolites during acuteischemic stroke. Neurosci. Lett. 2013, 544, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Onaolapo, O.J.; Onaolapo, A.Y.; Olowe, O.A.; Udoh, M.O.; Udoh, D.O.; Nathaniel, T.I. Melatonin and melatonergic influence on neuronal transcription factors: Implications for the development of novel therapies for neurodegenerative disorders. Curr. Neuropharmacol. 2020, 18, 563–577. [Google Scholar] [CrossRef]
- Kulesh, A.A.; Shestakov, V.V. Chronobiological parameters, cognitive emotional status and sleep quality in acute stroke. Nevrol. Psikhiatr. Im. S. S. Korsakova 2013, 113, 24–28. [Google Scholar]
- Atanassova, P.A.; Terzieva, D.D.; Dimitrov, B.D. Impaired nocturnal melatonin in acute phase of ischaemic stroke: Cross-sectional matched case-control analysis. J. Neuroendocrinol. 2009, 21, 657–663. [Google Scholar] [CrossRef]
- Lorente, L.; Martína, M.M.; Abreu-González, P.; Ramos, L.; Argueso, M.; Solé-Violán, J.; Cáceres, J.J.; Jiménez, A.; García-Marín, V. The serum melatonin levels and mortality of patients with spontaneous intracerebral hemorrhage. Brain Sci. 2020, 9, 263. [Google Scholar] [CrossRef]
- Reiter, R.J.; Sainz, R.M.; Lopez-Burillo, S.; Mayo, J.C.; Manchester, L.C.; Tan, D.X. Melatonin ameliorates neurologic damage and neurophysiologic deficits in experimental models of stroke. Ann. N. Y. Acad. Sci. 2003, 993, 35–47. [Google Scholar] [CrossRef]
- Tai, S.H.; Hung, Y.C.; Lee, E.J.; Lee, A.C.; Chen, T.Y.; Shen, C.; Chen, H.Y.; Lee, M.Y.; Huang, S.Y.; Wu, T.S. Melatonin protects against transient focal cerebral ischemia in both reproductively active and estrogen-deficient female rats: The impact of circulating estrogen on its hormetic dose-response. J. Pineal Res. 2011, 50, 292–303. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, S.; Dong, Y.; Fan, C.; Zhao, L.; Yang, X.; Li, J.; Di, S.; Yue, L.; Liang, G.; et al. Melatonin prevents cell death and mitochondrial dysfunction via a SIRT1-dependent mechanism during ischemic-stroke in mice. J. Pineal Res. 2015, 58, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Kulesh, A.A.; Shestakov, V.V. Secretion of melatonin and serum cholinesterase activity as biological markers of cognitive disorders in the acute stage of ischemic stroke. Zhurnal Nevrol. Psikhiatr. Im. S. S. Korsakova 2012, 112, 11–14. [Google Scholar]
- Arslan, C.; Altan, H.; Beşirli, K.; Aydemir, B.; Kiziler, A.R.; Denli, S. The role of oxidative stress and antioxidant defenses in Buerger disease and atherosclerotic peripheral arterial occlusive disease. Ann. Vasc. Surg. 2010, 24, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Zhan, C.P.; Zhuge, C.J.; Yan, X.J.; Dai, W.M.; Yu, G.F. Measuring serum melatonin concentrations to predict clinical outcome after aneurysmal subarachnoid hemorrhage. Clin. Chim. Acta 2021, 513, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Beloosesky, Y.; Grinblat, J.; Laudon, M.; Grosman, B.; Streifler, J.Y.; Zisapel, N. Melatonin rhythms in stroke patients. Neurosci. Lett. 2002, 319, 103–106. [Google Scholar] [CrossRef]
- Maldonado, M.D.; Murillo-Cabezas, F.; Terron, M.P.; Flores, L.J.; Tan, D.; Manchester, L.C.; Reiter, R.J. The potential of melatonin in reducing morbidity-mortality after craniocerebral trauma. J. Pineal Res. 2007, 42, 1–11. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Terron, M.P.; Flores, L.J.; Reiter, R.J. One molecule, many derivatives: A never-ending interaction of melatonin with reactive oxygen and reactive nitrogen species. J. Pineal Res. 2007, 42, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Peyrot, F.; Ducrocq, C. Potential of tryptophan derivatives in stress responses characterized by the generation of reactive oxygen and nitrogen species. J. Pineal Res. 2008, 45, 235–246. [Google Scholar] [CrossRef]
- Tan, D.X.; Reiter, R.J. Mitochondria: The birth place, battle ground and the site of melatonin metabolism in cells. Melatonin Res. 2019, 2, 44–66. [Google Scholar] [CrossRef]
- Lorente, L.; Martín, M.M.; Abreu-González, P.; Pérez-Cejas, A.; Ramos, L.; Argueso, M.; Solé-Violán, J.; Cáceres, J.J.; Jiménez, A.; García-Marín, V. Serum melatonin levels are associated with mortality in patients with malignant middle cerebral artery infarction. J. Int. Med. Res. 2018, 46, 3268–3277. [Google Scholar] [CrossRef]
| Clinical Parameters | Favourable (N = 26) (mRS 0–2 pts) | Unfavourable (N = 96) (mRS 3–6 pts) | p-Value | |
|---|---|---|---|---|
| Age | Median [Q1, Q3] | 58.8 [51.3, 69.3] | 68.3 [61.0, 77.3] | 0.002 |
| Gender (male) | n (%) | 18 (69.2%) | 54 (56.3%) | 0.268 |
| Current smoking | n (%) | 10 (38.5%) | 28 (29.2%) | 0.474 |
| SBP on admission | Median [Q1, Q3] | 145.8 [131, 155] | 159.9 [146, 171] | 0.002 |
| DBP on admission | Median [Q1, Q3] | 80.0 [75.0, 90.0] | 89.0 [79.8, 98.3] | 0.0692 |
| Hyperlipidaemia | n (%) | 2 (7.7%) | 11 (11.4%) | 1 |
| Hyperuricemia | n (%) | 1 (3.8%) | 5 (5.2%) | 1 |
| Impaired renal function | n (%) | 0 (0%) | 3 (3.1%) | 1 |
| Gout | n (%) | 1 (3.8%) | 5 (5.2%) | 1 |
| Diabetes Mellitus | n (%) | 9 (34.6%) | 41 (42.7%) | 0.507 |
| Arterial hypertension | n(%) | 18 (69.2%) | 85 (88.5%) | 0.0289 |
| Coronary heart disease | n(%) | 1 (3.8%) | 26 (27.1%) | 0.014 |
| Atrial fibrillation | n (%) | 0 (0%) | 15 (15.6%) | 0.039 |
| Statin therapy before stroke | n (%) | 6 (23.1%) | 30 (31.3%) | 0.625 |
| Statin therapy after stroke | n (%) | 26 (100%) | 88 (91.7%) | 0.584 |
| Anticoagulant therapy before the stroke | n (%) | 0 (0%) | 3 (3.1%) | 1 |
| Infection | n(%) | 0 (0%) | 20 (20.8%) | 0.007 |
| Antibiotic | n(%) | 0 (0%) | 20 (20.8%) | 0.007 |
| NIHSS on admission | Median [Q1, Q3] | 3.00 [2.00, 3.75] | 5.00 [4.00, 10.0] | <0.001 |
| NIHSS on discharge | Median [Q1, Q3] | 0 [0, 1.00] | 2.00 [1.00, 5.00] | <0.001 |
| mRS on admission | Median [Q1, Q3] | 2.00 [2.00, 2.00] | 4.00 [3.00, 5.00] | <0.001 |
| mRS on discharge | Median [Q1, Q3] | 0 [0, 0] | 1.00 [1.00, 3.00] | <0.001 |
| mRS after three months | Median [Q1, Q3] | 0 [0, 0] | 1 [0, 3.00] | <0.001 |
| mRS after a year | Median [Q1, Q3] | 0 [0, 0.250] | 1.00 [0, 2.00] | 0.003 |
| Biomarkers | Favourable (mRS 0–2 pts) (N = 26) | Unfavourable (mRS 3–6 pts) (N = 96) | p-Value | |
|---|---|---|---|---|
| CG (0) Median [Q1, Q3] [U/mL] | on admission | 180.58 [118.95, 219.67] | 255.15 [183.21, 294.12] | 0.002 |
| CG (1) Median [Q1, Q3] [U/mL] | 280.69 [259.41, 384.24] | 373.60 [223.59, 476.17] | 0.240 | |
| CG (2) Median [Q1, Q3] [U/mL] | 179.26 [98.86, 251.32] | 195.09 [137.31, 235.68] | 0.843 | |
| MEL (0) Median [Q1, Q3] [pg/mL] | 6.87 [4.95, 13.60] | 6.31 [3.98, 7.90] | 0.175 | |
| MEL (1) Median [Q1, Q3] [pg/mL] | 31.00 [8.66, 36.60] | 8.02 [4.68, 19.10] | 0.010 | |
| MEL (2) Median [Q1, Q3] [pg/mL] | 16.90 [3.39, 23.40] | 9.38 [6.56, 13.20] | 0.982 | |
| (N = 90) | (N = 29) | |||
| CG (0) Median [Q1, Q3] [U/mL] | on discharge | 202.85 [157.72, 267.31] | 274.65 [234.80, 301.28] | 0.007 |
| CG (1) Median [Q1, Q3] [U/mL] | 347.40 [250.80, 427.91] | 405.83 [219.42, 492.35] | 0.689 | |
| CG (2) Median [Q1, Q3] [U/mL] | 195.35 [119.77, 246.80] | 194.82 [137.73, 233.70] | 1 | |
| MEL (0) Median [Q1, Q3] [pg/mL] | 6.04 [4.19, 9.41] | 6.62 [5.46, 8.20] | 0.835 | |
| MEL (1) Median [Q1, Q3] [pg/mL] | 8.66 [4.98, 29.60] | 13.50 [8.29, 21.30] | 0.495 | |
| MEL (2) Median [Q1, Q3] [pg/mL] | 12.50 [6.14, 23.40] | 8.32 [5.98, 10.30] | 0.255 | |
| (N = 84) | (N = 27) | |||
| CG (0) Median [Q1, Q3] [U/mL] | after three months | 203.23 [164.19, 274.65] | 259.63 [230.33, 306.63] | 0.012 |
| CG (1) Median [Q1, Q3] [U/mL] | 347.40 [250.80, 427.91] | 438.31 [307.31, 696.33] | 0.296 | |
| CG (2) Median [Q1, Q3] [U/mL] | 169.07 [119.37, 235.57] | 214.41 [153.92, 239.03] | 0.566 | |
| MEL (0) Median [Q1, Q3] [pg/mL] | 6.38 [3.99, 9.84] | 5.93 [4.43, 8.20] | 0.940 | |
| MEL (1) Median [Q1, Q3] [pg/mL] | 8.66 [4.72, 28.10] | 8.80 [6.83, 15.90] | 0.962 | |
| MEL (2) Median [Q1, Q3] [pg/mL] | 10.80 [4.73, 17.60] | 9.15 [6.96, 9.62] | 0.607 | |
| (N = 78) | (N = 17) | |||
| CG (0) Median [Q1, Q3] [U/mL] | after a year | 197.85 [157.72, 258.25] | 291.47 [244.18, 361.20] | 0.005 |
| CG (1) Median [Q1, Q3] [U/mL] | 307.53 [222.20, 429.44] | 621.15 [421.70, 797.09] | 0.111 | |
| CG (2) Median [Q1, Q3] [U/mL] | 149.94 [113.17, 224.83] | 194.82 [140.29, 234.00] | 0.679 | |
| MEL (0) Median [Q1, Q3] [pg/mL] | 6.38 [3.99, 9.84] | 6.85 [5.58, 8.49] | 0.557 | |
| MEL (1) Median [Q1, Q3] [pg/mL] | 8.34 [4.70, 21.70] | 8.70 [7.05, 8.90] | 0.752 | |
| MEL (2) Median [Q1, Q3] [pg/mL] | 8.27 [4.50, 14.90] | 9.15 [7.79, 9.39] | 0.966 | |
| Biomarkers | Patients Group (n = 123) | Control Group (n = 35) | p-Value |
|---|---|---|---|
| CG (0) Median [Q1, Q3] [U/mL] | 222.02 [171.09, 283.47] (n = 81) | 144.18 [93.49, 191.69] (n = 22) | <0.001 |
| CG (1) Median [Q1, Q3] [U/mL] | 355.56 [224.98, 437.38] (n = 37) | 144.18 [93.49, 191.69] (n = 22) | <0.001 |
| CG (2) Median [Q1, Q3] [U/mL] | 195.08 [124.21, 242.01] (n = 34) | 144.18 [93.49, 191.69] (n = 22) | 0.032 |
| MEL (0) Median [Q1, Q3] [pg/mL] | 6.41 [4.26, 8.64] (n = 77) | 37.85 [21.46, 103.82] (n = 23) | <0.001 |
| MEL (1) Median [Q1, Q3] [pg/mL] | 9.34 [5.49, 25.42] (n = 44) | 37.85 [21.46, 103.82] (n = 23) | <0.001 |
| MEL (2) Median [Q1, Q3] [pg/mL] | 9.61 [6.04, 15.53] (n = 31) | 37.85 [21.46, 103.82] (n = 23) | <0.001 |
| CG (0) | CG (1) | CG (2) | MEL (0) | MEL (1) | MEL (2) | |
|---|---|---|---|---|---|---|
| NIHSS score on admission [pts] | R = 0.26 | R = 0.14 | R = 0.10 | R = −0.01 | R = −0.02 | R = −0.07 |
| p = 0.04 | p = 0.31 | p = 0.62 | p = 0.42 | p = 0.88 | p = 0.39 | |
| NIHSS score on discharge [pts] | R = 0.28 | R = 0.04 | R = −0.06 | R = −0.09 | R = −0.08 | R = −0.06 |
| p = 0.03 | p = 0.26 | p = 0.90 | p = 0.53 | p = 0.64 | p = 0.35 | |
| mRS on admission [pts] | R = 0.34 | R = 0.26 | R = 0.18 | R = −0.12 | R = −0.32 | R = 0.04 |
| p < 0.01 | p = 0.19 | p = 0.40 | p = 0.18 | p = 0.01 | p = 0.61 | |
| mRS on discharge [pts] | R = 0.37 | R = 0.11 | R = 0.08 | R = −0.01 | R = −0.13 | R = 0.01 |
| p < 0.01 | p = 0.53 | p = 0.74 | p = 0.51 | p = 0.67 | p = 0.32 | |
| mRS after 3 months from stroke onset [pts] | R = 0.34 | R = 0.13 | R = −0.03 | R = −0.15 | R = −0.24 | R = −0.15 |
| p = 0.04 | p = 0.48 | p = 0.81 | p = 0.22 | p = 0.94 | p = 0.29 | |
| mRS after a year from stroke on-set [pts] | R = 0.30 | R = 0.11 | R = −0.12 | R = −0.11 | R = −0.23 | R = 0.05 |
| p < 0.01 | p = 0.41 | p = 0.85 | p = 0.48 | p = 0.26 | p = 0.68 |
| Biomarker | LACI | POCI | PACI |
|---|---|---|---|
| Mel (0) Median; IQR | 7.27 [5.31, 22.29] | 5.32 [3.55, 6.84] | 3.73 [2.60, 5.61] |
| Mel (1) Median; IQR | 11.84 [7.05, 34.29] | 4.72 [3.65; 8.02] | |
| CG (0) Median; IQR | 176.89 [134.82, 211.50] | 292.84 [263.80, 344.61] | 282.67 [244.18, 323.49] |
| CG (1) Median; IQR | 268.42 [213.02, 331.31] | 464.87 [397.84, 632.60] | 432.49 [311.32, 660.82] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawluk, H.; Kołodziejska, R.; Grześk, G.; Woźniak, A.; Kozakiewicz, M.; Kosinska, A.; Pawluk, M.; Grzechowiak, E.; Wojtasik, J.; Kozera, G. Increased Oxidative Stress Markers in Acute Ischemic Stroke Patients Treated with Thrombolytics. Int. J. Mol. Sci. 2022, 23, 15625. https://doi.org/10.3390/ijms232415625
Pawluk H, Kołodziejska R, Grześk G, Woźniak A, Kozakiewicz M, Kosinska A, Pawluk M, Grzechowiak E, Wojtasik J, Kozera G. Increased Oxidative Stress Markers in Acute Ischemic Stroke Patients Treated with Thrombolytics. International Journal of Molecular Sciences. 2022; 23(24):15625. https://doi.org/10.3390/ijms232415625
Chicago/Turabian StylePawluk, Hanna, Renata Kołodziejska, Grzegorz Grześk, Alina Woźniak, Mariusz Kozakiewicz, Agnieszka Kosinska, Mateusz Pawluk, Elżbieta Grzechowiak, Jakub Wojtasik, and Grzegorz Kozera. 2022. "Increased Oxidative Stress Markers in Acute Ischemic Stroke Patients Treated with Thrombolytics" International Journal of Molecular Sciences 23, no. 24: 15625. https://doi.org/10.3390/ijms232415625
APA StylePawluk, H., Kołodziejska, R., Grześk, G., Woźniak, A., Kozakiewicz, M., Kosinska, A., Pawluk, M., Grzechowiak, E., Wojtasik, J., & Kozera, G. (2022). Increased Oxidative Stress Markers in Acute Ischemic Stroke Patients Treated with Thrombolytics. International Journal of Molecular Sciences, 23(24), 15625. https://doi.org/10.3390/ijms232415625






