Role of Adiponectin in Cardiovascular Diseases Related to Glucose and Lipid Metabolism Disorders
Abstract
1. Introduction
2. APN Structure, Receptors, and Signaling Pathways
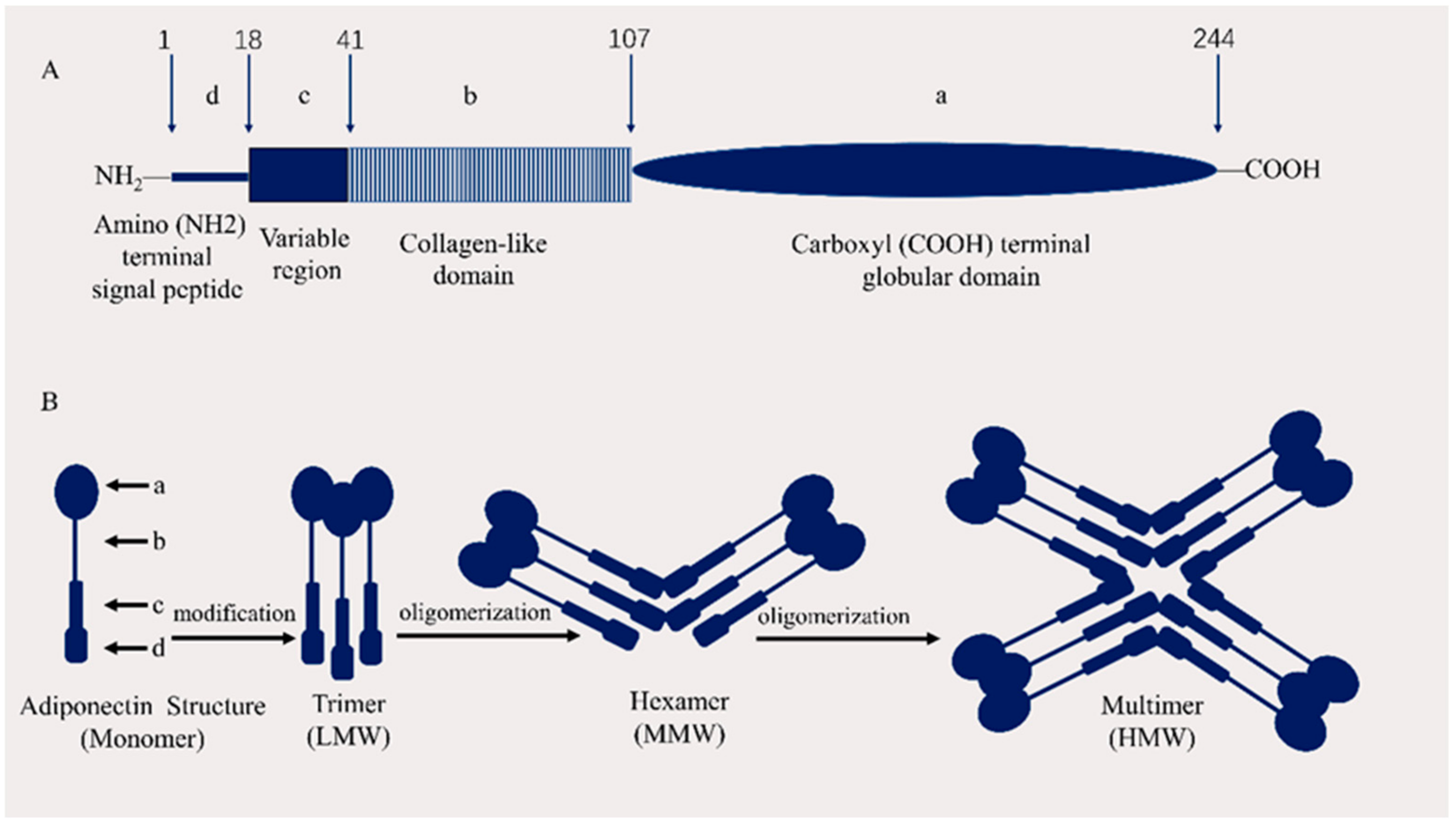
2.1. APN Structure
2.2. APN Receptors
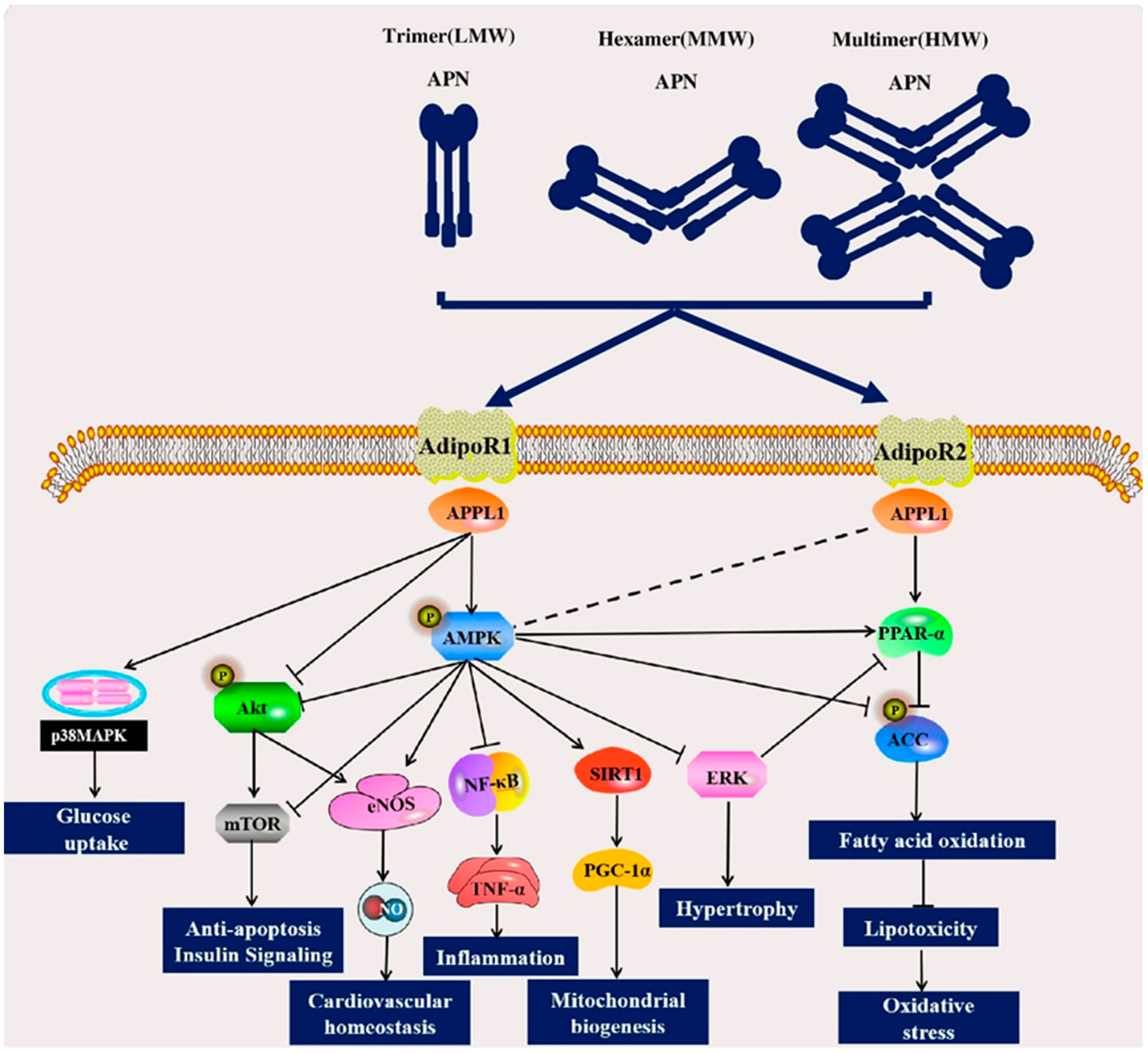
2.3. Signaling Pathways of APN
2.3.1. AMPK and PPAR Signaling Pathways
2.3.2. Akt Signaling Pathway and MAPK Signaling Pathway
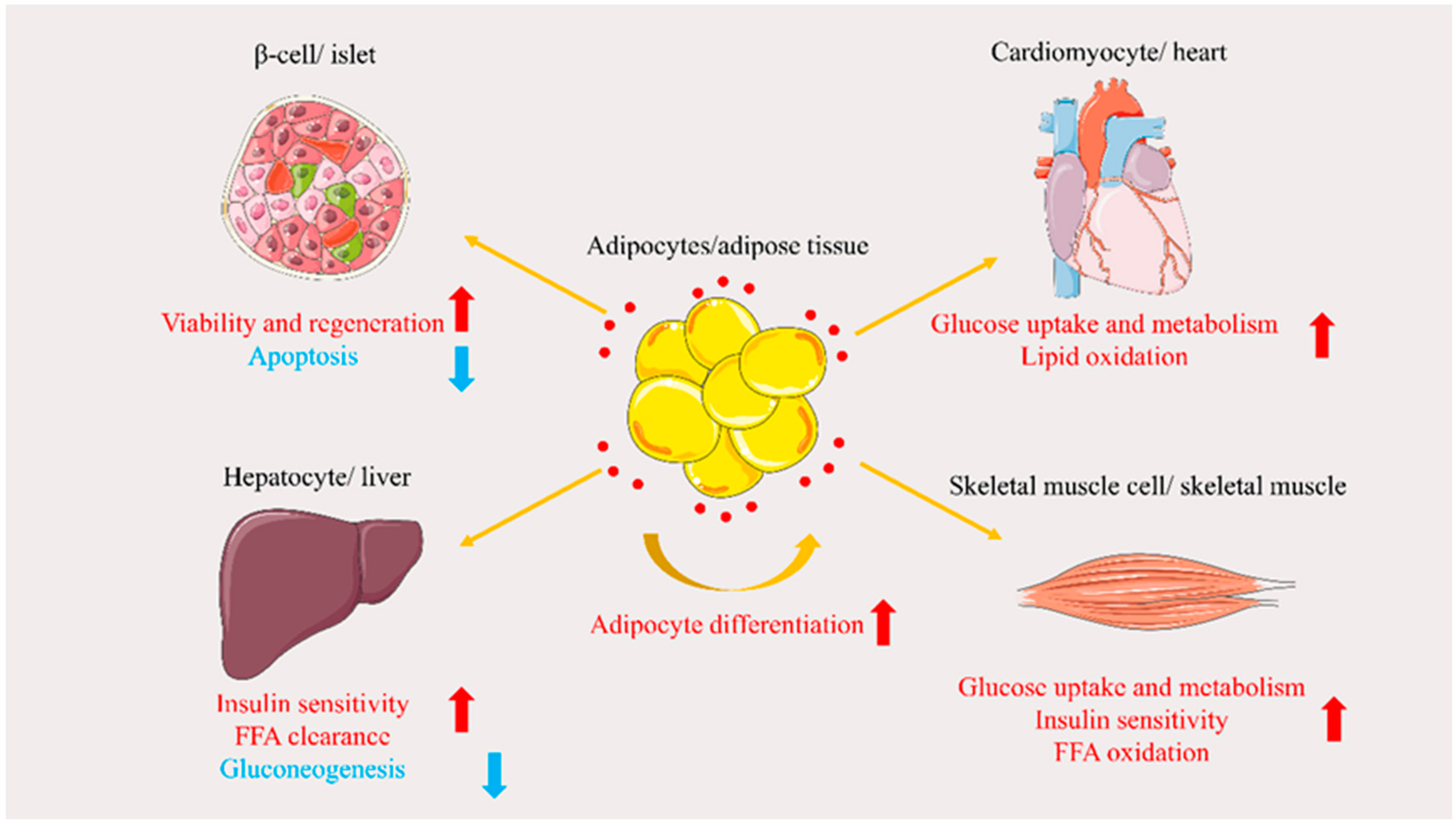
3. Role of APN in Glucose and Lipid Metabolism Disorders
3.1. Regulation of Glucose Metabolism
3.1.1. Protecting β-Cells
3.1.2. Increasing Glucose Tissue Uptake
3.1.3. Reducing Gluconeogenesis
3.2. Regulation of Lipid Metabolism
3.2.1. Promoting Adipocyte Differentiation
3.2.2. Promoting Free Fatty Acid (FFA) Oxidation and Clearance
3.2.3. Insulin Sensitization
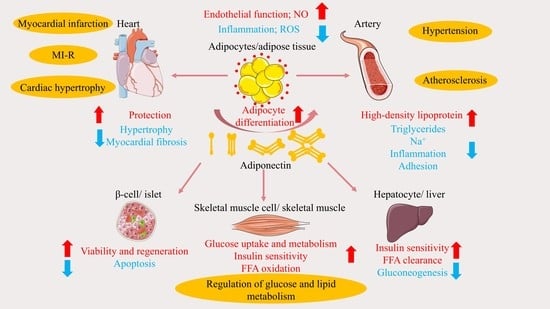
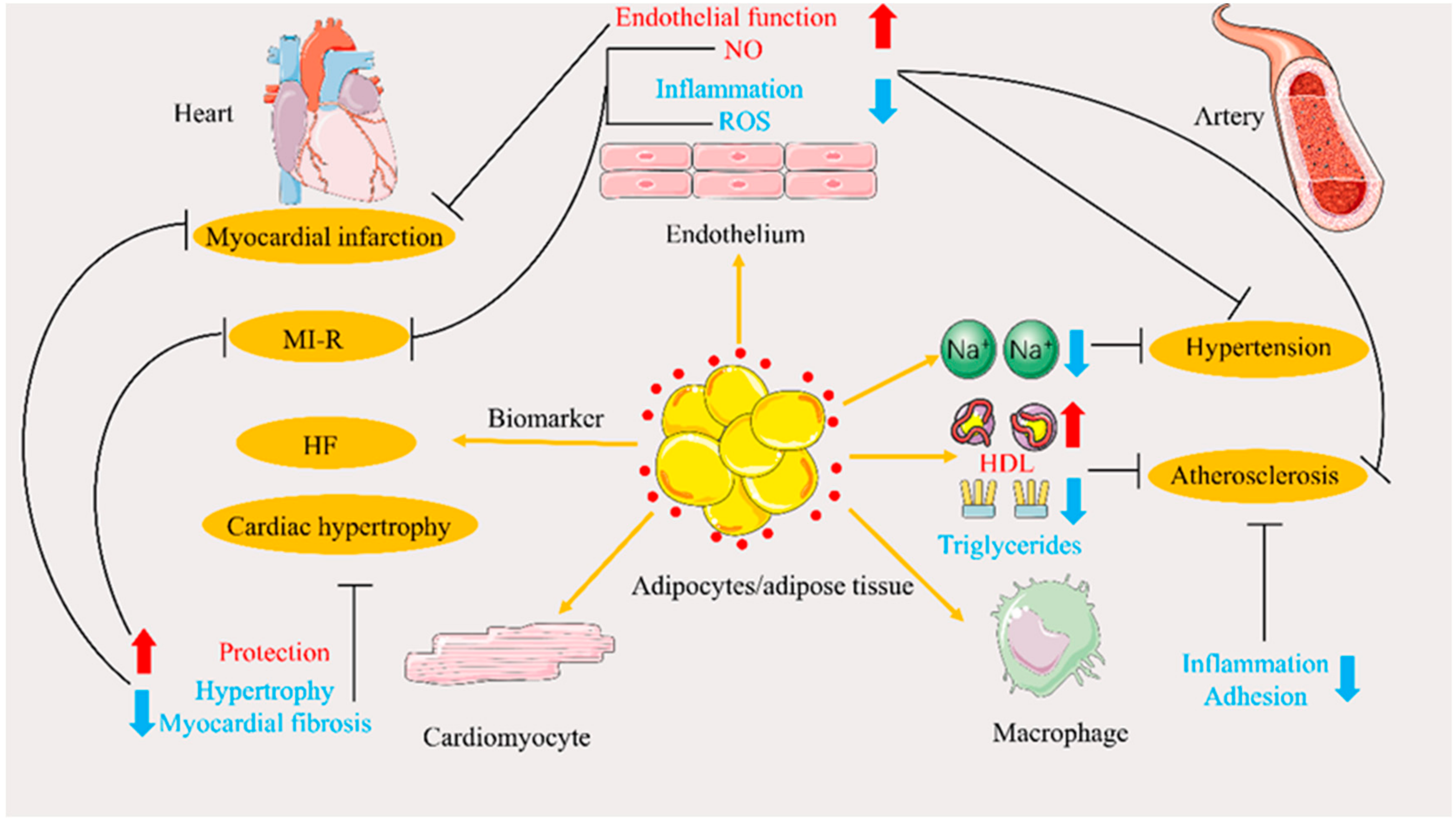
4. Role of APN in Cardiovascular Disease and Related Advances
4.1. Atherosclerosis
4.1.1. Regulation of Lipid Metabolism
4.1.2. Improvement of Endothelial Dysfunction
4.1.3. Regulation of Nitrous Oxide Production and Oxidative Stress Reduction
4.2. Hypertension
4.3. Cardiac Hypertrophy
4.3.1. Improvement of Myocardial Hypertrophy
4.3.2. Activation of AMPK and Other Related Signaling Pathways
4.4. Myocardial Ischemia/Reperfusion and Infarction
4.5. Heart Failure
5. Problems and Prospects
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Koliaki, C.; Liatis, S.; Kokkinos, A. Obesity and cardiovascular disease: Revisiting an old relationship. Metabolism 2019, 92, 98–107. [Google Scholar] [CrossRef]
- Poznyak, A.; Grechko, A.V.; Poggio, P.; Myasoedova, V.A.; Alfieri, V.; Orekhov, A.N. The Diabetes Mellitus-Atherosclerosis Connection: The Role of Lipid and Glucose Metabolism and Chronic Inflammation. Int. J. Mol. Sci. 2020, 21, 1835. [Google Scholar] [CrossRef] [PubMed]
- Freeman, A.M.; Pennings, N. Insulin Resistance. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Cheng, K.K.Y.; Lam, K.S.; Wang, B.; Xu, A. Signaling mechanisms underlying the insulin-sensitizing effects of adiponectin. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Fuster, J.J.; Walsh, K. Adipokines: A link between obesity and cardiovascular disease. J. Cardiol. 2013, 63, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Shi, Z.; Ji, X.; Zhang, W.; Luan, J.; Zahr, T.; Qiang, L. Adipokines, adiposity, and atherosclerosis. Cell. Mol. Life Sci. 2022, 79, 272. [Google Scholar] [CrossRef]
- Yan, C.-J.; Li, S.-M.; Xiao, Q.; Liu, Y.; Hou, J.; Chen, A.-F.; Xia, L.-P.; Li, X.-C. Influence of serum adiponectin level and SNP +45 polymorphism of adiponectin gene on myocardial fibrosis. J. Zhejiang Univ. Sci. B 2013, 14, 721–728. [Google Scholar] [CrossRef]
- Park, M.; Sweeney, G. Direct effects of adipokines on the heart: Focus on adiponectin. Heart Fail. Rev. 2012, 18, 631–644. [Google Scholar] [CrossRef]
- Tao, L.; Gao, E.; Jiao, X.; Yuan, Y.; Li, S.; Christopher, T.A.; Lopez, B.L.; Koch, W.; Chan, L.; Goldstein, B.J.; et al. Adiponectin Cardioprotection After Myocardial Ischemia/Reperfusion Involves the Reduction of Oxidative/Nitrative Stress. Circulation 2007, 115, 1408–1416. [Google Scholar] [CrossRef]
- Persson, J.; Lindberg, K.; Gustafsson, T.P.; Eriksson, P.; Paulsson-Berne, G.; Lundman, P. Low plasma adiponectin concentration is associated with myocardial infarction in young individuals. J. Intern. Med. 2010, 268, 194–205. [Google Scholar] [CrossRef]
- Mado, H.; Szczurek, W.; Gąsior, M.; Szyguła-Jurkiewicz, B. Adiponectin in heart failure. Futur. Cardiol. 2021, 17, 757–764. [Google Scholar] [CrossRef]
- Ghoshal, K.; Bhattacharyya, M. Adiponectin: Probe of the molecular paradigm associating diabetes and obesity. World J. Diabetes 2015, 6, 151–166. [Google Scholar] [CrossRef]
- Choi, H.M.; Doss, H.M.; Kim, K.S. Multifaceted Physiological Roles of Adiponectin in Inflammation and Diseases. Int. J. Mol. Sci. 2020, 21, 1219. [Google Scholar] [CrossRef]
- Fang, H.; Judd, R.L. Adiponectin Regulation and Function. Compr. Physiol. 2018, 8, 1031–1063. [Google Scholar]
- Chandran, M.; Phillips, S.A.; Ciaraldi, T.; Henry, R.R. Adiponectin: More than just another fat cell hormone? Diabetes Care 2003, 26, 2442–2450. [Google Scholar] [CrossRef]
- Shibata, R.; Ouchi, N.; Ohashi, K.; Murohara, T. The role of adipokines in cardiovascular disease. J. Cardiol. 2017, 70, 329–334. [Google Scholar] [CrossRef]
- Pheiffer, C.; Dias, S.; Jack, B.; Malaza, N.; Adam, S. Adiponectin as a Potential Biomarker for Pregnancy Disorders. Int. J. Mol. Sci. 2021, 22, 1326. [Google Scholar] [CrossRef]
- Wang, Z.V.; Scherer, P.E. Adiponectin, the past two decades. J. Mol. Cell. Biol. 2016, 8, 93–100. [Google Scholar] [CrossRef]
- Yamauchi, T.; Kamon, J.; Ito, Y.; Tsuchida, A.; Yokomizo, T.; Kita, S.; Sugiyama, T.; Miyagishi, M.; Hara, K.; Tsunoda, M.; et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 2003, 423, 762–769. [Google Scholar] [CrossRef]
- Hug, C.; Wang, J.; Ahmad, N.S.; Bogan, J.S.; Tsao, T.S.; Lodish, H.F. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc. Natl. Acad. Sci. USA 2004, 101, 10308–10313. [Google Scholar] [CrossRef]
- Mao, X.; Kikani, C.K.; Riojas, R.A.; Langlais, P.; Wang, L.; Ramos, F.J.; Fang, Q.; Christ-Roberts, C.Y.; Hong, J.Y.; Kim, R.Y.; et al. APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nat. Cell Biol. 2006, 8, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Roy, B.; Palaniyandi, S.S. Tissue-specific role and associated downstream signaling pathways of adiponectin. Cell Biosci. 2021, 11, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Straub, L.G.; Scherer, P.E. Metabolic Messengers: Adiponectin. Nat. Metab. 2019, 1, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Schindler, M.; Pendzialek, M.; Grybel, K.J.; Seeling, T.; Gürke, J.; Fischer, B.; Santos, A.N. Adiponectin stimulates lipid metabolism via AMPK in rabbit blastocysts. Hum. Reprod. 2017, 32, 1382–1392. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Mah, M.; Ritchie, R.H.; De Blasio, M.J. The adiponectin signalling pathway—A therapeutic target for the cardiac complications of type 2 diabetes? Pharmacol. Ther. 2022. 232, 108008. [CrossRef]
- Fujita, K.; Maeda, N.; Sonoda, M.; Ohashi, K.; Hibuse, T.; Nishizawa, H.; Nishida, M.; Hiuge, A.; Kurata, A.; Kihara, S.; et al. Adiponectin Protects Against Angiotensin II–Induced Cardiac Fibrosis Through Activation of PPAR-α. Arter. Thromb. Vasc. Biol. 2008, 28, 863–870. [Google Scholar] [CrossRef]
- Hui, X.; Lam, K.S.; Vanhoutte, P.M.; Xu, A. Adiponectin and cardiovascular health: An update. Br. J. Pharmacol. 2012, 165, 574–590. [Google Scholar] [CrossRef]
- Wang, S.; Miao, J.; Qu, M.; Yang, G.-Y.; Shen, L. Adiponectin modulates the function of endothelial progenitor cells via AMPK/eNOS signaling pathway. Biochem. Biophys. Res. Commun. 2017, 493, 64–70. [Google Scholar] [CrossRef]
- Nanayakkara, G.; Kariharan, T.; Wang, L.; Zhong, J.; Amin, R. The cardio-protective signaling and mechanisms of adiponectin. Am. J. Cardiovasc. Dis. 2012, 2, 253–266. [Google Scholar]
- Iwabu, M.; Yamauchi, T.; Okada-Iwabu, M.; Sato, K.; Nakagawa, T.; Funata, M.; Yamaguchi, M.; Namiki, S.; Nakayama, R.; Tabata, M.; et al. Adiponectin and AdipoR1 regulate PGC-1α and mitochondria by Ca2+ and AMPK/SIRT1. Nature 2010, 464, 1313–1319. [Google Scholar] [CrossRef]
- Pepin, M.; Koentges, C.; Pfeil, K.; Gollmer, J.; Kersting, S.; Wiese, S.; Hoffmann, M.M.; Odening, K.E.; Mühlen, C.V.Z.; Diehl, P.; et al. Dysregulation of the Mitochondrial Proteome Occurs in Mice Lacking Adiponectin Receptor 1. Front. Endocrinol. 2019, 10, 872. [Google Scholar] [CrossRef]
- Cui, X.; Lin, X.; Zhong, J.; Li, S.; He, J.; Ni, Y.; Zhan, J.; Liu, Y. Adiponectin attenuates the premature senescence of vascular smooth muscle cells induced by high glucose through mTOR signaling pathway. Aging Med. 2020, 3, 178–187. [Google Scholar] [CrossRef]
- Ruan, H.; Dong, L.Q. Adiponectin signaling and function in insulin target tissues. J. Mol. Cell Biol. 2016, 8, 101–109. [Google Scholar] [CrossRef]
- Yanai, H.; Yoshida, H. Beneficial Effects of Adiponectin on Glucose and Lipid Metabolism and Atherosclerotic Progression: Mechanisms and Perspectives. Int. J. Mol. Sci. 2019, 20, 1190. [Google Scholar] [CrossRef]
- Rakatzi, I.; Mueller, H.; Ritzeler, O.; Tennagels, N.; Eckel, J. Adiponectin counteracts cytokine- and fatty acid-induced apoptosis in the pancreatic beta-cell line INS-1. Diabetologia 2004, 47, 249–258. [Google Scholar] [CrossRef]
- Ye, R.; Wang, M.; Wang, Q.; Scherer, P.E. Adiponectin-Mediated Antilipotoxic Effects in Regenerating Pancreatic Islets. Endocrinology 2015, 156, 2019–2028. [Google Scholar] [CrossRef]
- Ceddia, R.B.; Somwar, R.; Maida, A.; Fang, X.; Bikopoulos, G.; Sweeney, G. Globular adiponectin increases GLUT4 translocation and glucose uptake but reduces glycogen synthesis in rat skeletal muscle cells. Diabetologia 2005, 48, 132–139. [Google Scholar] [CrossRef]
- Palanivel, R.; Ganguly, R.; Turdi, S.; Xu, A.; Sweeney, G. Adiponectin stimulates Rho-mediated actin cytoskeleton remodeling and glucose uptake via APPL1 in primary cardiomyocytes. Metabolism 2014, 63, 1363–1373. [Google Scholar] [CrossRef]
- Palanivel, R.; Fang, X.; Park, M.; Eguchi, M.; Pallan, S.; De Girolamo, S.; Liu, Y.; Wang, Y.; Xu, A.; Sweeney, G. Globular and full-length forms of adiponectin mediate specific changes in glucose and fatty acid uptake and metabolism in cardiomyocytes. Cardiovasc. Res. 2007, 75, 148–157. [Google Scholar] [CrossRef]
- Combs, T.P.; Berg, A.H.; Obici, S.; Scherer, P.E.; Rossetti, L. Endogenous glucose production is inhibited by the adipose-derived protein Acrp30. J. Clin. Invest. 2001, 108, 1875–1881. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, D.; Wang, B.; Zhang, Y.; Wang, L.; Chen, X.; Li, M.; Tang, Z.; Wang, C. APPL1-mediated activation of STAT3 contributes to inhibitory effect of adiponectin on hepatic gluconeogenesis. Mol. Cell. Endocrinol. 2016, 433, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Diep Nguyen, T.M. Adiponectin: Role in physiology and pathophysiology. Int. J. Prev. Med. 2020, 11, 136. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Luo, N.; Klein, R.L.; Garvey, W.T. Adiponectin promotes adipocyte differentiation, insulin sensitivity, and lipid accumulation. J. Lipid Res. 2005, 46, 1369–1379. [Google Scholar] [CrossRef] [PubMed]
- Avides, M.D.C.; Domingues, L.; Vicente, A.; Teixeira, J. Differentiation of human pre-adipocytes by recombinant adiponectin. Protein Expr. Purif. 2008, 59, 122–126. [Google Scholar] [CrossRef]
- Yang, W.; Yuan, W.; Peng, X.; Wang, M.; Xiao, J.; Wu, C.; Luo, L. PPAR γ/Nnat/NF-κB Axis Involved in Promoting Effects of Adiponectin on Preadipocyte Differentiation. Mediat. Inflamm. 2019, 2019, 5618023. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.J.; Lee, G.Y.; Chung, J.-J.; Ahn, Y.H.; Hong, S.H.; Kim, J.B. Adiponectin Increases Fatty Acid Oxidation in Skeletal Muscle Cells by Sequential Activation of AMP-Activated Protein Kinase, p38 Mitogen-Activated Protein Kinase, and Peroxisome Proliferator–Activated Receptor α. Diabetes 2006, 55, 2562–2570. [Google Scholar] [CrossRef]
- Shetty, S.; Ramos-Roman, M.A.; Cho, Y.-R.; Brown, J.; Plutzky, J.; Muise, E.; Horton, J.D.; Scherer, P.E.; Parks, E.J. Enhanced Fatty Acid Flux Triggered by Adiponectin Overexpression. Endocrinology 2012, 153, 113–122. [Google Scholar] [CrossRef]
- Lopez-Yus, M.; Lopez-Perez, R.; Garcia-Sobreviela, M.P.; del Moral-Bergos, R.; Lorente-Cebrian, S.; Arbones-Mainar, J.M. Adiponectin overexpression in C2C12 myocytes increases lipid oxidation and myofiber transition. J. Physiol. Biochem. 2021, 78, 517–525. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Farrokhi, F.R.; Butler, A.E.; Sahebkar, A. Insulin resistance: Review of the underlying molecular mechanisms. J. Cell. Physiol. 2018, 234, 8152–8161. [Google Scholar] [CrossRef]
- Moon, H.U.; Ha, K.H.; Han, S.J.; Kim, H.J.; Kim, D.J. The Association of Adiponectin and Visceral Fat with Insulin Resistance and beta-Cell Dysfunction. J. Korean Med. Sci. 2019, 34, e7. [Google Scholar] [CrossRef]
- Tishinsky, J.M.; Robinson, L.E.; Dyck, D.J. Insulin-sensitizing properties of adiponectin. Biochimie 2012, 94, 2131–2136. [Google Scholar] [CrossRef]
- Li, X.; Zhang, D.; Vatner, D.F.; Goedeke, L.; Hirabara, S.M.; Zhang, Y.; Perry, R.J.; Shulman, G.I. Mechanisms by which adiponectin reverses high fat diet-induced insulin resistance in mice. Proc. Natl. Acad. Sci. USA 2020, 117, 32584–32593. [Google Scholar] [CrossRef]
- Chang, E.; Choi, J.M.; Park, S.E.; Rhee, E.J.; Lee, W.Y.; Oh, K.W.; Park, S.W.; Park, C.Y. Adiponectin deletion impairs insulin signaling in insulin-sensitive but not insulin-resistant 3T3-L1 adipocytes. Life Sci. 2015, 132, 93–100. [Google Scholar] [CrossRef]
- Karwi, Q.G.; Uddin, G.M.; Ho, K.L.; Lopaschuk, G.D. Loss of Metabolic Flexibility in the Failing Heart. Front. Cardiovasc. Med. 2018, 5, 68. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Karwi, Q.G.; Tian, R.; Wende, A.R.; Abel, E.D. Cardiac Energy Metabolism in Heart Failure. Circ. Res. 2021, 128, 1487–1513. [Google Scholar] [CrossRef]
- Chanda, D.; Luiken, J.J.F.P.; Glatz, J.F.C. Signaling pathways involved in cardiac energy metabolism. FEBS Lett. 2016, 590, 2364–2374. [Google Scholar] [CrossRef]
- Nishi, H.; Higashihara, T.; Inagi, R. Lipotoxicity in Kidney, Heart, and Skeletal Muscle Dysfunction. Nutrients 2019, 11, 1664. [Google Scholar] [CrossRef]
- Ren, J.; Wu, N.N.; Wang, S.; Sowers, J.R.; Zhang, Y. Obesity cardiomyopathy: Evidence, mechanisms, and therapeutic implications. Physiol. Rev. 2021, 101, 1745–1807. [Google Scholar] [CrossRef]
- Ramírez, E.; Picatoste, B.; González-Bris, A.; Oteo, M.; Cruz, F.; Caro-Vadillo, A.; Egido, J.; Tuñón, J.; Morcillo, M.A.; Lorenzo, Ó. Sitagliptin improved glucose assimilation in detriment of fatty-acid utilization in experimental type-II diabetes: Role of GLP-1 isoforms in Glut4 receptor trafficking. Cardiovasc. Diabetol. 2018, 17, 1–13. [Google Scholar] [CrossRef]
- Ormazabal, V.; Nair, S.; Elfeky, O.; Aguayo, C.; Salomon, C.; Zuñiga, F.A. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 2018, 17, 122. [Google Scholar] [CrossRef]
- Matsuda, M.; Shimomura, I. Roles of adiponectin and oxidative stress in obesity-associated metabolic and cardiovascular diseases. Rev. Endocr. Metab. Disord. 2013, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Liu, M. Adiponectin: A versatile player of innate immunity. J. Mol. Cell Biol. 2016, 8, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Long, Y.; Yu, Y.R.; Li, M.R. Adiponectin directly improves endothelial dysfunction in obese rats through the AMPK-eNOS Pathway. Int. J. Obes. 2010, 34, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.M. Molecular pathways associated with oxidative stress in diabetes mellitus. Biomed. Pharmacother. 2018, 108, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Guo, C.; Hu, F.; Zhu, W.; Mao, S. Undernutrition-induced lipid metabolism disorder triggers oxidative stress in maternal and fetal livers using a model of pregnant sheep. FASEB J. 2020, 34, 6508–6520. [Google Scholar] [CrossRef]
- Rius-Pérez, S.; Torres-Cuevas, I.; Millán, I.; Ortega, Á.L.; Pérez, S. PGC-1alpha, Inflammation, and Oxidative Stress: An Integrative View in Metabolism. Oxidative Med. Cell. Longev. 2020, 2020, 1452696. [Google Scholar] [CrossRef]
- Xiang, M.; Lu, Y.; Xin, L.; Gao, J.; Shang, C.; Jiang, Z.; Lin, H.; Fang, X.; Qu, Y.; Wang, Y.; et al. Role of Oxidative Stress in Reperfusion following Myocardial Ischemia and Its Treatments. Oxidative Med. Cell. Longev. 2021, 2021, 1–23. [Google Scholar] [CrossRef]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef]
- Marso, S.P.; Mehta, S.K.; Frutkin, A.; House, J.A.; McCrary, J.R.; Kulkarni, K.R. Low adiponectin levels are associated with atherogenic dyslipidemia and lipid-rich plaque in nondiabetic coronary arteries. Diabetes Care 2008, 31, 989–994. [Google Scholar] [CrossRef][Green Version]
- Csongrádi, É.; Káplár, M.; Nagy Jr, B.; Koch, C.A.; Juhász, A.; Bajnok, L.; Varga, Z.; Seres, I.; Karányi, Z.; Magyar, M.T.; et al. Adipokines as atherothrombotic risk factors in obese subjects: Associations with haemostatic markers and common carotid wall thickness. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 571–580. [Google Scholar] [CrossRef]
- Hafiane, A.; Gasbarrino, K.; Daskalopoulou, S.S. The role of adiponectin in cholesterol efflux and HDL biogenesis and metabolism. Metabolism 2019, 100, 153953. [Google Scholar] [CrossRef]
- Kobayashi, T.; Imachi, H.; Fukunaga, K.; Lyu, J.; Sato, S.; Saheki, T.; Ibata, T.; Matsumoto, M.; Japar, S.B.; Murao, K. HDL promotes adiponectin gene expression via the CAMKK/CAMKIV pathway. J. Mol. Endocrinol. 2022, 68, 89–98. [Google Scholar] [CrossRef]
- Qiao, L.; Zou, C.; van der Westhuyzen, D.R.; Shao, J. Adiponectin Reduces Plasma Triglyceride by Increasing VLDL Triglyceride Catabolism. Diabetes 2008, 57, 1824–1833. [Google Scholar] [CrossRef]
- Liang, B.; Wang, X.; Guo, X.; Yang, Z.; Bai, R.; Liu, M.; Xiao, C.; Bian, Y. Adiponectin upregulates ABCA1 expression through liver X receptor alpha signaling pathway in RAW 264.7 macrophages. Int. J. Clin. Exp. Pathol. 2015, 8, 450–457. [Google Scholar]
- Christou, G.A.; Kiortsis, D.N. Adiponectin and lipoprotein metabolism. Obes. Rev. 2013, 14, 939–949. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Q.; Pu, H.; Wei, Q.; Duan, M.; Zhang, C.; Jiang, T.; Shou, X.; Zhang, J.; Yang, Y. Adiponectin improves NF-kappaB-mediated inflammation and abates atherosclerosis progression in apolipoprotein E-deficient mice. Lipids Health Dis. 2016, 15, 33. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, Y.; Liu, L.; Lin, C.; Liao, C.; Xin, L.; Zhong, S.; Cheng, Q.; Zhang, L. Adiponectin Inhibits TNF-alpha-Activated PAI-1 Expression Via the cAMP-PKA-AMPK-NF-κB Axis in Human Umbilical Vein Endothelial Cells. Cell. Physiol. Biochem. 2017, 42, 2342–2352. [Google Scholar] [CrossRef]
- Mahadev, K.; Wu, X.; Donnelly, S.; Ouedraogo, R.; Eckhart, A.D.; Goldstein, B.J. Adiponectin inhibits vascular endothelial growth factor-induced migration of human coronary artery endothelial cells. Cardiovasc. Res. 2008, 78, 376–384. [Google Scholar] [CrossRef]
- Ohashi, K.; Parker, J.L.; Ouchi, N.; Higuchi, A.; Vita, J.A.; Gokce, N.; Pedersen, A.A.; Kalthoff, C.; Tullin, S.; Sams, A.; et al. Adiponectin Promotes Macrophage Polarization toward an Anti-inflammatory Phenotype. J. Biol. Chem. 2010, 285, 6153–6160. [Google Scholar] [CrossRef]
- Tsai, J.-S.; Chen, C.-Y.; Chen, Y.-L.; Chuang, L.-M. Rosiglitazone inhibits monocyte/macrophage adhesion through de novo adiponectin production in human monocytes. J. Cell. Biochem. 2010, 110, 1410–1419. [Google Scholar] [CrossRef]
- Tsai, J.S.; Chuang, L.M.; Chen, C.S.; Liang, C.J.; Chen, Y.L.; Chen, C.Y. Troglitazone and Δ2Troglitazone enhance adiponectin expression in monocytes/macrophages through the AMP-activated protein kinase pathway. Mediat. Inflamm. 2014, 2014, 726068. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Yang, T.; Chen, H.; Fu, D.; Hu, Y.; Wang, J.; Yuan, Q.; Yu, H.; Xu, W.; Xie, X. New insights into oxidative stress and inflammation during diabetes mellitus-accelerated atherosclerosis. Redox Biol. 2018, 20, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Ouedraogo, R.; Wu, X.; Xu, S.Q.; Fuchsel, L.; Motoshima, H.; Mahadev, K.; Hough, K.; Scalia, R.; Goldstein, B.J. Adiponectin suppression of high-glucose-induced reactive oxygen species in vascular endothelial cells: Evidence for involvement of a cAMP signaling pathway. Diabetes 2006, 55, 1840–1846. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Montagnani, M.; Funahashi, T.; Shimomura, I.; Quon, M.J. Adiponectin Stimulates Production of Nitric Oxide in Vascular Endothelial Cells. J. Biol. Chem. 2003, 278, 45021–45026. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Pu, H.; Ma, C.; Jiang, T.; Wei, Q.; Duan, M.; Zhang, C.; Shou, X.; Su, L.; Zhang, J.; et al. Adiponectin Abates Atherosclerosis by Reducing Oxidative Stress. Med. Sci. Monit. 2014, 20, 1792–1800. [Google Scholar] [CrossRef]
- Cai, X.J.; Li, C.J.; Chen, L.; Rong, Y.Y.; Zhang, Y.; Zhang, M. A hypothesis: Adiponectin mediates anti-atherosclerosis via adventitia-AMPK-iNOS pathway. Med. Hypotheses 2008, 70, 1044–1047. [Google Scholar] [CrossRef]
- Al Ghorani, H.; Kulenthiran, S.; Lauder, L.; Böhm, M.; Mahfoud, F. Hypertension trials update. J. Hum. Hypertens. 2021, 35, 398–409. [Google Scholar] [CrossRef]
- Deshmukh, M.; Lee, H.W.; McFarlane, S.I.; Whaley-Connell, A. Antihypertensive medications and their effects on lipid metabolism. Curr. Diabetes Rep. 2008, 8, 214–220. [Google Scholar] [CrossRef]
- Litwin, M.; Kułaga, Z. Obesity, metabolic syndrome, and primary hypertension. Pediatr. Nephrol. 2021, 36, 825–837. [Google Scholar] [CrossRef]
- Chakraborty, S.; Mandal, J.; Yang, T.; Cheng, X.; Yeo, J.Y.; McCarthy, C.G.; Wenceslau, C.F.; Koch, L.G.; Hill, J.W.; Vijay-Kumar, M.; et al. Metabolites and Hypertension: Insights into Hypertension as a Metabolic Disorder: 2019 Harriet Dustan Award. Hypertension 2020, 75, 1386–1396. [Google Scholar] [CrossRef]
- Jung, D.-H.; Kim, J.-Y.; Kim, J.-K.; Koh, S.-B.; Park, J.-K.; Ahn, S.V. Relative contribution of obesity and serum adiponectin to the development of hypertension. Diabetes Res. Clin. Pract. 2014, 103, 51–56. [Google Scholar] [CrossRef]
- Wu, J.; Xu, G.; Cai, W.; Huang, Y.; Xie, N.; Shen, Y.; Xie, L. The association of two polymorphisms in adiponectin-encoding gene with hypertension risk and the changes of circulating adiponectin and blood pressure: A meta-analysis. Oncotarget 2017, 8, 14636–14645. [Google Scholar] [CrossRef]
- Ohashi, K.; Kihara, S.; Ouchi, N.; Kumada, M.; Fujita, K.; Hiuge, A.; Hibuse, T.; Ryo, M.; Nishizawa, H.; Maeda, N.; et al. Adiponectin Replenishment Ameliorates Obesity-Related Hypertension. Hypertension 2006, 47, 1108–1116. [Google Scholar] [CrossRef]
- Zhao, Z.Y.; Shi, Z.Y.; Zhang, Z.Z.; Li, Y.H.; Zeng, X.H.; Chen, Y.X.; Yao, N.; Zhou, M.; Su, H.; Wang, Q.H.; et al. Anti-hypertensive and endothelia protective effects of Fufang Qima capsule on primary hypertension via adiponectin/adenosine monophosphate activated protein kinase pathway. J. Tradit. Chin. Med. = Chung I Tsa Chih Ying Wen Pan 2021, 41, 919–926. [Google Scholar]
- Zhao, Y.; Gao, P.; Sun, F.; Li, Q.; Chen, J.; Yu, H.; Li, L.; Wei, X.; He, H.; Lu, Z.; et al. Sodium Intake Regulates Glucose Homeostasis through the PPARδ/Adiponectin-Mediated SGLT2 Pathway. Cell Metab. 2016, 23, 699–711. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.; Huang, H.; Zeng, A.; Han, Y.; Zeng, C.; Zheng, S.; Ren, H.; Wang, Y.; Huang, Y.; et al. GRK4-mediated adiponectin receptor-1 phosphorylative desensitization as a novel mechanism of reduced renal sodium excretion in hypertension. Clin. Sci. 2020, 134, 2453–2467. [Google Scholar] [CrossRef]
- Nakamura, M.; Sadoshima, J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat. Rev. Cardiol. 2018, 15, 387–407. [Google Scholar] [CrossRef]
- Ritterhoff, J.; Young, S.; Villet, O.; Shao, D.; Neto, F.C.; Bettcher, L.F.; Hsu, Y.-W.A.; Kolwicz, S.C., Jr.; Raftery, D.; Tian, R. Metabolic Remodeling Promotes Cardiac Hypertrophy by Directing Glucose to Aspartate Biosynthesis. Circ. Res. 2020, 126, 182–196. [Google Scholar] [CrossRef]
- Amin, R.H.; Mathews, S.T.; Alli, A.; Leff, T. Endogenously produced adiponectin protects cardiomyocytes from hypertrophy by a PPARγ-dependent autocrine mechanism. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H690–H698. [Google Scholar] [CrossRef]
- Li, H.; Yao, W.; Irwin, M.G.; Wang, T.; Wang, S.; Zhang, L.; Xia, Z. Adiponectin ameliorates hyperglycemia-induced cardiac hypertrophy and dysfunction by concomitantly activating Nrf2 and Brg1. Free. Radic. Biol. Med. 2015, 84, 311–321. [Google Scholar] [CrossRef]
- Dadson, K.; Turdi, S.; Hashemi, S.; Zhao, J.; Polidovitch, N.; Beca, S.; Backx, P.H.; McDermott, J.C.; Sweeney, G. Adiponectin is required for cardiac MEF2 activation during pressure overload induced hypertrophy. J. Mol. Cell. Cardiol. 2015, 86, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Ou-Yang, Q.; Wang, L.; Li, T.; Xie, X.; Liu, J. AdipoRon prevents l-thyroxine or isoproterenol-induced cardiac hypertrophy through regulating the AMPK-related path-way. Acta Biochim. Biophys. Sin. 2019, 51, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.; Gao, Z.; Gu, L.; Chen, M.; Yang, B.; Cao, K.; Huang, H.; Li, M. AdipoR1/APPL1 potentiates the protective effects of globular adiponectin on angiotensin II-induced cardiac hypertrophy and fibrosis in neonatal rat atrial myocytes and fibroblasts. PLoS ONE 2014, 9, e103793. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cai, X.; Guan, Y.; Wang, L.; Wang, S.; Li, Y.; Fu, Y.; Gao, X.; Su, G. Adiponectin Upregulates MiR-133a in Cardiac Hypertrophy through AMPK Activation and Reduced ERK1/2 Phosphorylation. PLoS ONE 2016, 11, e0148482. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Shibata, R.; Unno, K.; Shimano, M.; Furukawa, M.; Ohashi, T.; Cheng, X.; Nagata, K.; Ouchi, N.; Murohara, T. Evidence for the Importance of Adiponectin in the Cardioprotective Effects of Pioglitazone. Hypertension 2010, 55, 69–75. [Google Scholar] [CrossRef]
- Leffler, K.; Abdel-Rahman, A.A. Restoration of Adiponectin-Connexin43 Signaling Mitigates Myocardial Inflammation and Dysfunction in Diabetic Female Rats. J. Cardiovasc. Pharmacol. 2019, 75, 259–267. [Google Scholar] [CrossRef]
- Fujishima, Y.; Maeda, N.; Matsuda, K.; Komura, N.; Hirata, A.; Mori, T.; Sekimoto, R.; Tsushima, Y.; Nishizawa, H.; Funahashi, T.; et al. Effect of adiponectin on cardiac beta-catenin signaling pathway under angiotensin II infusion. Biochem. Biophys. Res. Commun. 2014, 444, 224–229. [Google Scholar] [CrossRef]
- Cadenas, S. ROS and redox signaling in myocardial ischemia-reperfusion injury and cardioprotection. Free. Radic. Biol. Med. 2018, 117, 76–89. [Google Scholar] [CrossRef]
- Wang, H.; Wu, W.; Duan, J.; Ma, M.; Kong, W.; Ke, Y.; Li, G.; Zheng, J. Cardioprotection of ischemic preconditioning in rats involves upregulating adiponectin. J. Mol. Endocrinol. 2017, 58, 155–165. [Google Scholar] [CrossRef]
- Zhu, K.; Guo, J.; Yu, X.; Wang, Q.; Yan, C.; Qiu, Q.; Tang, W.; Huang, X.; Mu, H.; Dou, L.; et al. Polypeptide Globular Adiponectin Ameliorates Hypoxia/Reoxygenation-Induced Cardiomyocyte Injury by Inhibiting Both Apoptosis and Necroptosis. J. Immunol. Res. 2021, 2021, 1815098. [Google Scholar] [CrossRef]
- Potenza, M.A.; Sgarra, L.; Nacci, C.; Leo, V.; De Salvia, M.A.; Montagnani, M. Activation of AMPK/SIRT1 axis is required for adiponectin-mediated preconditioning on myocardial ischemia-reperfusion (I/R) injury in rats. PLoS ONE 2019, 14, e0210654. [Google Scholar] [CrossRef]
- Zhu, Q.; Li, H.; Xie, X.; Chen, X.; Kosuru, R.; Li, S.; Lian, Q.; Cheung, C.W.; Irwin, M.G.; Ge, R.-S.; et al. Adiponectin Facilitates Postconditioning Cardioprotection through Both AMPK-Dependent Nuclear and AMPK-Independent Mitochondrial STAT3 Activation. Oxidative Med. Cell. Longev. 2020, 2020, 4253457. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, B.; Lau, W.B.; Du, Y.; Guo, R.; Yan, Z.; Gan, L.; Yan, W.; Zhao, J.; Gao, E.; et al. Restoring diabetes-induced autophagic flux arrest in ischemic/reperfused heart by ADIPOR (adiponectin receptor) activation involves both AMPK-dependent and AMPK-independent signaling. Autophagy 2017, 13, 1855–1869. [Google Scholar] [CrossRef]
- Cao, C.; Liu, H.-M.; Li, W.; Wu, Y.; Leng, Y.; Xue, R.; Chen, R.; Tang, L.-H.; Sun, Q.; Xia, Z.; et al. Role of adiponectin in diabetes myocardial ischemia-reperfusion injury and ischemic postconditioning. Acta Cir. Bras. 2020, 35, e202000107. [Google Scholar] [CrossRef] [PubMed]
- Huynh, D.N.; Bessi, V.L.; Ménard, L.; Piquereau, J.; Proulx, C.; Febbraio, M.; Lubell, W.D.; Carpentier, A.C.; Burelle, Y.; Ong, H.; et al. Adiponectin has a pivotal role in the cardioprotective effect of CP-3(iv), a selective CD36 azapeptide ligand, after transient coronary artery occlusion in mice. FASEB J. 2018, 32, 807–818. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, H.-C.; Liu, Y.; Gao, C.; Sun, L.; Tao, L. Resveratrol Cardioprotection Against Myocardial Ischemia/Reperfusion Injury Involves Upregulation of Adiponectin Levels and Multimerization in Type 2 Diabetic Mice. J. Cardiovasc. Pharmacol. 2016, 68, 304–312. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth universal definition of myocardial infarction (2018). Rev. Esp. Cardiol. 2019, 72, 72. [Google Scholar] [CrossRef]
- Shibata, R.; Izumiya, Y.; Sato, K.; Papanicolaou, K.; Kihara, S.; Colucci, W.S.; Sam, F.; Ouchi, N.; Walsh, K. Adiponectin protects against the development of systolic dysfunction following myocardial infarction. J. Mol. Cell. Cardiol. 2007, 42, 1065–1074. [Google Scholar] [CrossRef]
- Han, X.; Wu, Y.; Liu, X.; Ma, L.; Lv, T.; Sun, Q.; Xu, W.; Zhang, S.; Wang, K.; Wang, W.; et al. Adiponectin improves coronary no-reflow injury by protecting the endothelium in rats with type 2 diabetes mellitus. Biosci. Rep. 2017, 37, BSR20170282. [Google Scholar] [CrossRef]
- Zhang, C.-J.; Deng, Y.-Z.; Lei, Y.-H.; Zhao, J.-B.; Wei, W.; Li, Y.-H. The mechanism of exogenous adiponectin in the prevention of no-reflow phenomenon in type 2 diabetic patients with acute myocardial infarction during PCI treatment. Eur. Rev. Med Pharmacol. Sci. 2018, 22, 2169–2174. [Google Scholar]
- Tanai, E.; Frantz, S. Pathophysiology of Heart Failure. Compr. Physiol. 2015, 6, 187–214. [Google Scholar] [PubMed]
- Bai, W.; Huang, J.; Zhu, M.; Liu, X.; Tao, J. Association between elevated adiponectin level and adverse outcomes in patients with heart failure: A systematic review and meta-analysis. Braz. J. Med Biol. Res. 2019, 52, e8416. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Zhang, Y.; Ye, H.; Zhang, G.; Jin, H.; Chen, Z.; Yao, Y.; Tian, X.; Zhou, J.; Li, P.; et al. Adiponectin is valuable in the diagnosis of acute heart failure with renal insufficiency. Exp. Ther. Med. 2018, 16, 2725–2734. [Google Scholar] [CrossRef] [PubMed]
- Monzo, L.; Kotrc, M.; Benes, J.; Sedlacek, K.; Jurcova, I.; Franekova, J.; Jarolim, P.; Kautzner, J.; Melenovsky, V. Clinical and Humoral Determinants of Congestion in Heart Failure: Potential Role of Adiponectin. Kidney Blood Press. Res. 2019, 44, 1271–1284. [Google Scholar] [CrossRef] [PubMed]
- O'Shea, K.M.; Chess, D.J.; Khairallah, R.J.; Rastogi, S.; Hecker, P.A.; Sabbah, H.N.; Walsh, K.; Stanley, W.C. Effects of adiponectin deficiency on structural and metabolic remodeling in mice subjected to pressure overload. Am. J. Physiol. Circ. Physiol. 2010, 298, H1639–H1645. [Google Scholar] [CrossRef] [PubMed]
- Hecker, P.A.; O'Shea, K.M.; Galvao, T.F.; Brown, B.H.; Stanley, W.C. Role of Adiponectin in the Development of High Fat Diet-induced Metabolic Abnormalities in Mice. Horm. Metab. Res. 2010, 43, 100–105. [Google Scholar] [CrossRef]
- Shimano, M.; Ouchi, N.; Shibata, R.; Ohashi, K.; Pimentel, D.R.; Murohara, T.; Walsh, K. Adiponectin deficiency exacerbates cardiac dysfunction following pressure overload through disruption of an AMPK-dependent angiogenic response. J. Mol. Cell. Cardiol. 2010, 49, 210–220. [Google Scholar] [CrossRef]
- Han, X.; Wang, Y.; Fu, M.; Song, Y.; Wang, J.; Cui, X.; Fan, Y.; Cao, J.; Luo, J.; Sun, A.; et al. Effects of Adiponectin on Diastolic Function in Mice Underwent Transverse Aorta Constriction. J. Cardiovasc. Transl. Res. 2019, 13, 225–237. [Google Scholar] [CrossRef]
- Young, L.H.; Li, J.; Baron, S.J.; Russell, R.R. AMP-Activated Protein Kinase: A Key Stress Signaling Pathway in the Heart. Trends Cardiovasc. Med. 2005, 15, 110–118. [Google Scholar] [CrossRef]
- Zhao, S.; Kusminski, C.M.; Scherer, P.E. Adiponectin, Leptin and Cardiovascular Disorders. Circ. Res. 2021, 128, 136–149. [Google Scholar] [CrossRef]
- Naylor, S. Biomarkers: Current perspectives and future prospects. Expert Rev. Mol. Diagn. 2003, 3, 525–529. [Google Scholar] [CrossRef]
| Disease | Effect of APN | References |
|---|---|---|
| Atherosclerosis | Induces increased serum HDL levels, reduces serum triglyceride levels, and promotes cholesterol efflux. | [72,73,74,75,76] |
| Improves endothelial dysfunction and plays an anti-inflammatory role via the AMPK/NF-κB/TNF-α and other signaling pathways. | [77,78,79,80,81,82] | |
| Activates eNOS and inhibits iNOS to regulate NO production and maintain cardiovascular homeostasis. Reduces ROS production to reduce oxidative stress | [84,85,86,87] | |
| Hypertension | Increases NO concentration, protects vascular endothelium, and mediates sodium intake and excretion to lower the blood pressure | [94,95,96,97] |
| Cardiac hypertrophy | The effects of improving and inducing cardiomyocyte hypertrophy have both been reported. | [100,101,102] |
| Improves cardiac hypertrophy and myocardial fibrosis induced by multiple factors mainly mediated by AMPK. | [103,104,105,106,107,108] | |
| Myocardial Ischemia/reperfusionand infarction | Reduces oxidative stress and protects the heart in MI/R; works in T2DM state. | [10,110,111,112,113,114,115,116,117] |
| Protects myocardial cells and improves microcirculation to prevent the occurrence and development of myocardial infarction; it also plays a role in T2DM state. | [11,119,120,121] | |
| Heart failure | Biomarker for diagnosis and prognosis. | [123,124,125] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, W.; Yang, S.; Xiao, H.; Wang, M.; Ye, J.; Cao, L.; Sun, G. Role of Adiponectin in Cardiovascular Diseases Related to Glucose and Lipid Metabolism Disorders. Int. J. Mol. Sci. 2022, 23, 15627. https://doi.org/10.3390/ijms232415627
Han W, Yang S, Xiao H, Wang M, Ye J, Cao L, Sun G. Role of Adiponectin in Cardiovascular Diseases Related to Glucose and Lipid Metabolism Disorders. International Journal of Molecular Sciences. 2022; 23(24):15627. https://doi.org/10.3390/ijms232415627
Chicago/Turabian StyleHan, Wen, Shuxian Yang, Haiyan Xiao, Min Wang, Jingxue Ye, Li Cao, and Guibo Sun. 2022. "Role of Adiponectin in Cardiovascular Diseases Related to Glucose and Lipid Metabolism Disorders" International Journal of Molecular Sciences 23, no. 24: 15627. https://doi.org/10.3390/ijms232415627
APA StyleHan, W., Yang, S., Xiao, H., Wang, M., Ye, J., Cao, L., & Sun, G. (2022). Role of Adiponectin in Cardiovascular Diseases Related to Glucose and Lipid Metabolism Disorders. International Journal of Molecular Sciences, 23(24), 15627. https://doi.org/10.3390/ijms232415627