Detection and pH-Thermal Characterization of Proteinases Exclusive of Honeybee Worker-Fate Larvae (Apis mellifera L.)
Abstract
1. Introduction
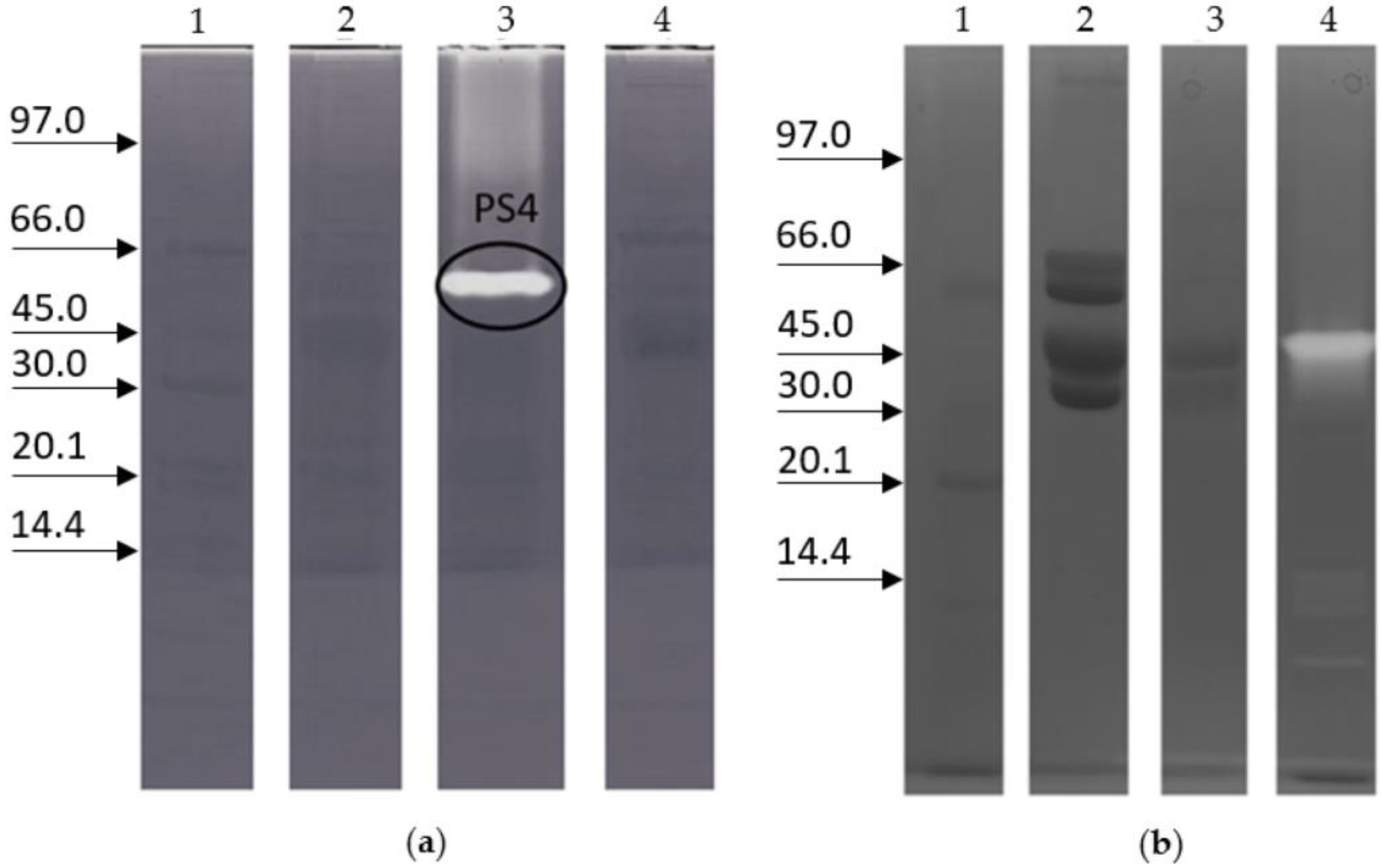
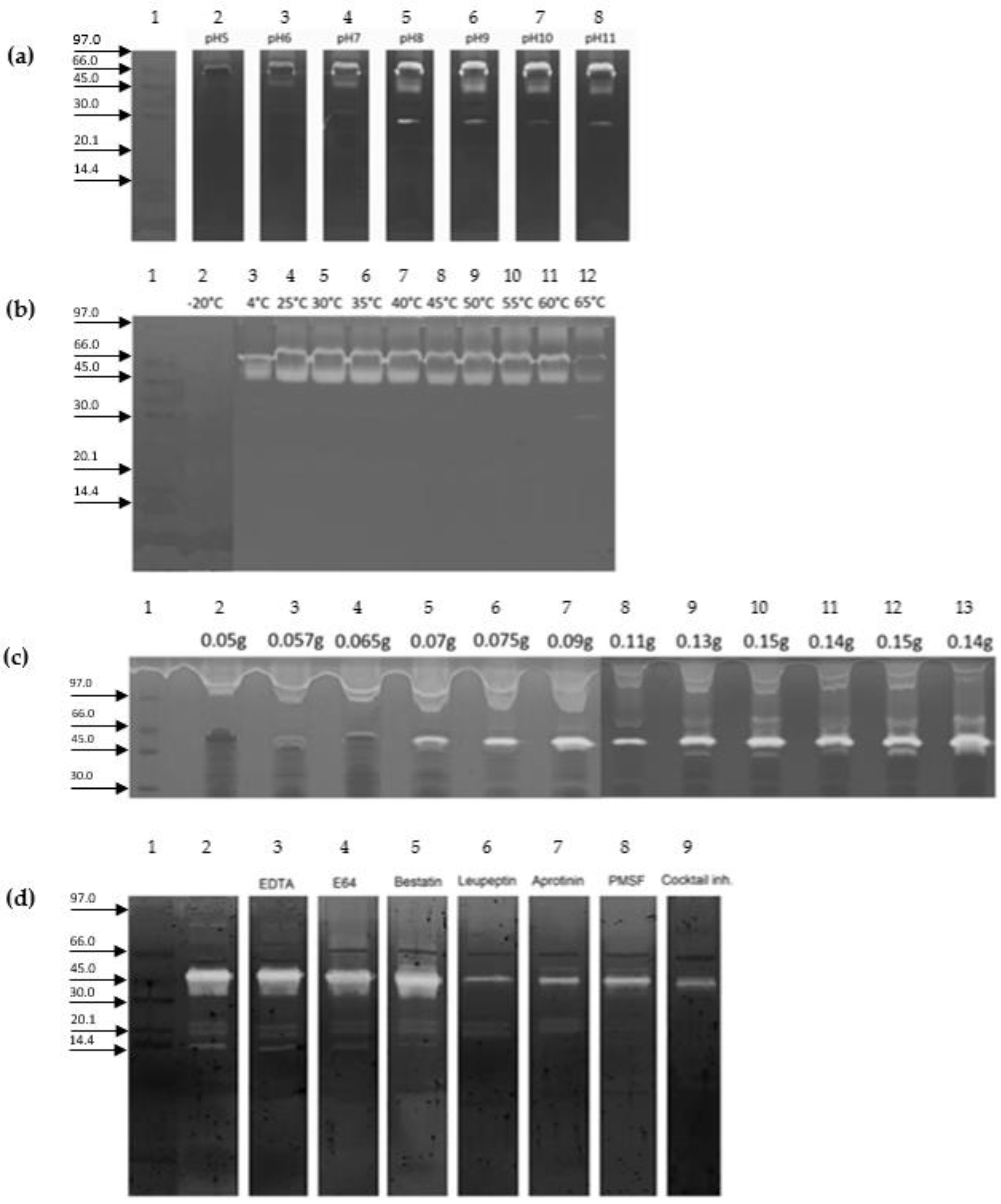
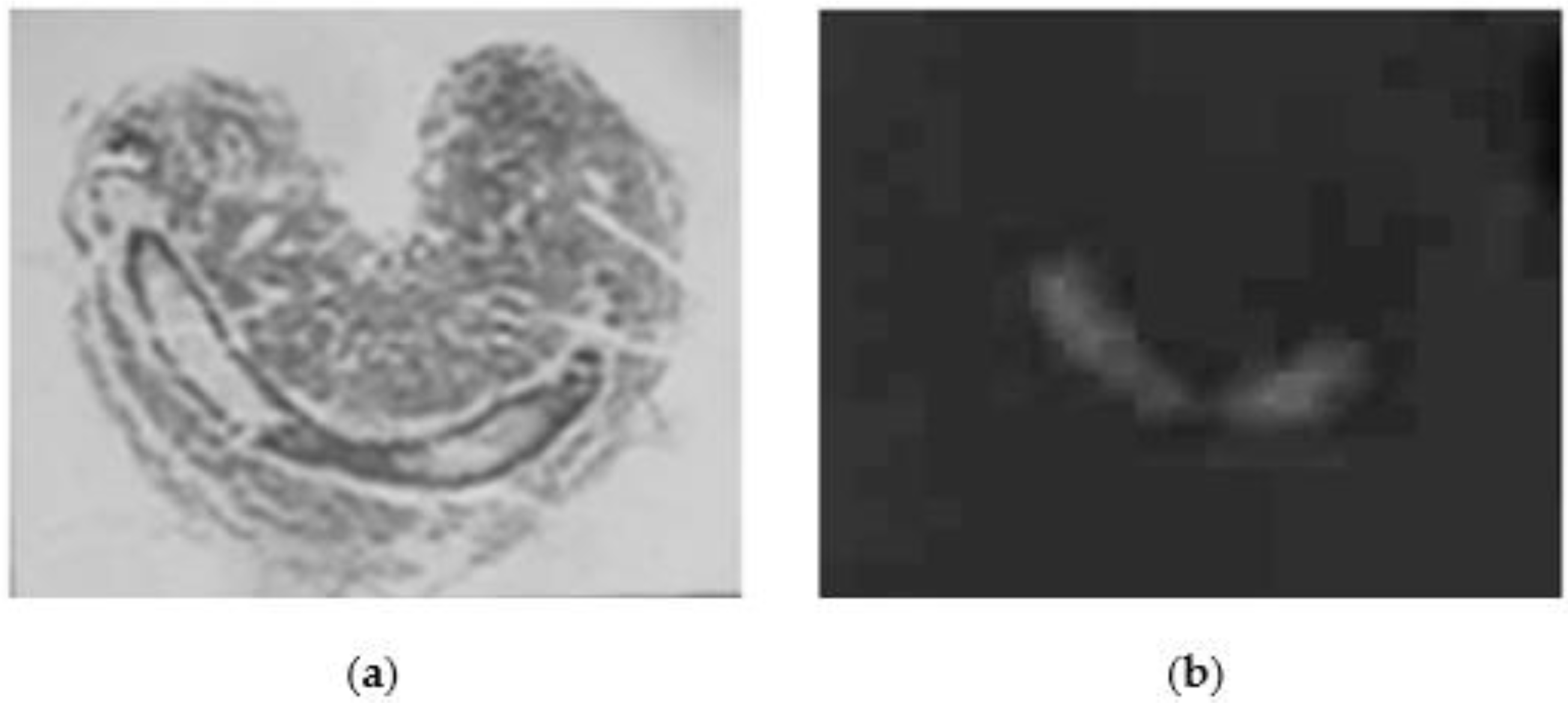
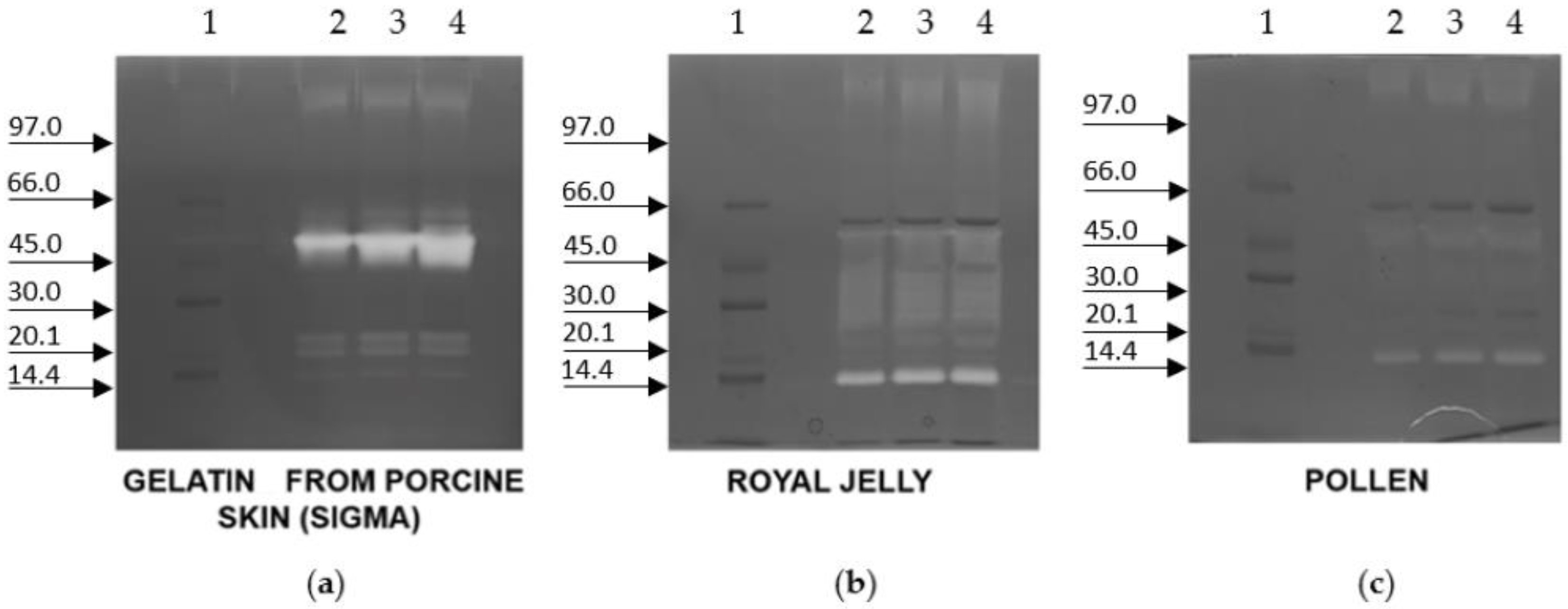
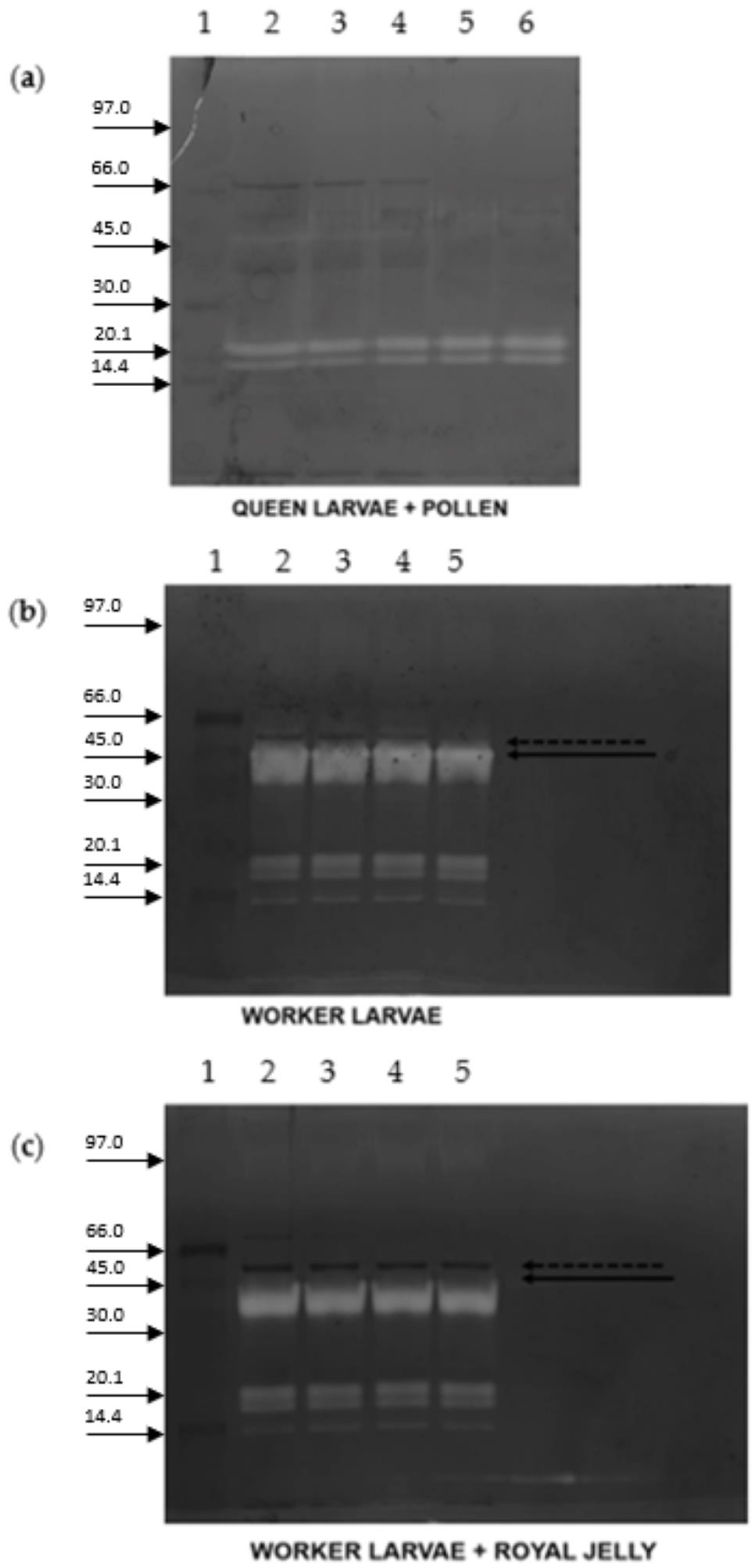
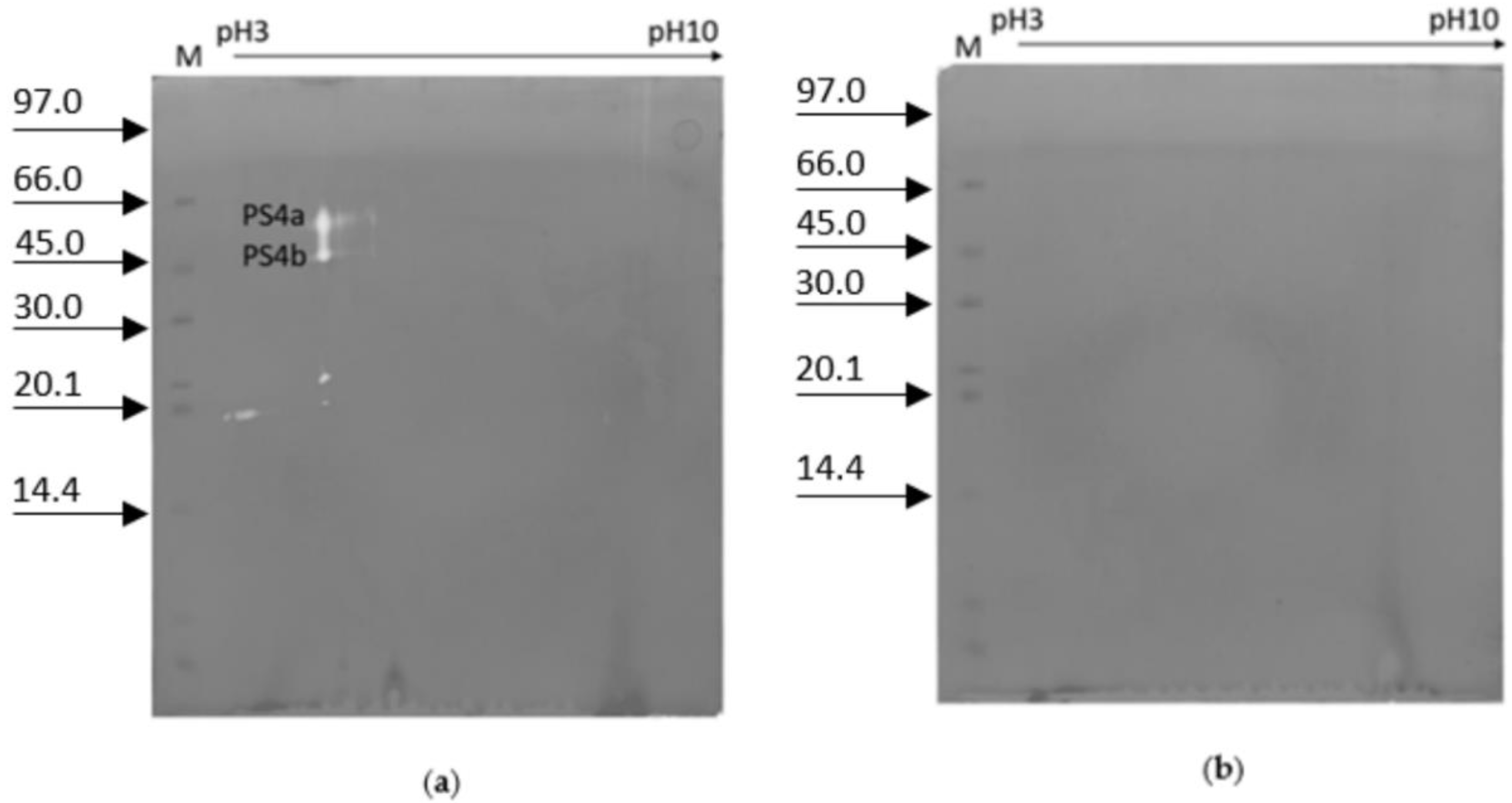
2. Results
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Chemicals
5.2. Biological Samples
5.3. Sample Preparation
5.4. Zymography
5.5. 2-DE (Two-Dimensional Gel Electrophoresis) Zymography
5.6. Histochemistry
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Evans, J.D.; Wheeler, D.E. Gene expression and the evolution of insect polyphenisms. BioEssays 2001, 23, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.D.; Wheeler, D.E. Differential gene expression between developing queens and workers in the honey bee, Apis mellifera. Proc. Natl. Acad. Sci. USA 1999, 96, 5575–5580. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.D.; Wheeler, D.E. Expression profiles during honeybee caste determination. Genome Biol. 2001, 2, 1–6. [Google Scholar]
- Nijhout, H.F. Control mechanisms of polyphenic development in insects: In polyphenic development, environmental factors alter some aspects of development in an orderly and predictable way. Bioscience 1999, 49, 181–192. [Google Scholar] [CrossRef]
- Engels, W.; Imperatriz-Fonseca, V.L. Caste Development, Reproductive Strategies, and Control of Fertility in Honey Bees and Stingless Bees. In Social Insects; Engels, E., Ed.; Springer: Berlin/Heidelberg, Germany, 1990; pp. 167–230. [Google Scholar]
- Beetsma, J. The Process of Queen-Worker Differentiation in the Honeybee. Bee World 1979, 60, 24–39. [Google Scholar] [CrossRef]
- Hartfelder, K.; Engels, W. Social insect polymorphism: Hormonal regulation of plasticity in development and reproduction in the honeybee. Curr. Top. Dev. Biol. 1998, 40, 45–77. [Google Scholar]
- Wang, Y.; Ma, L.; Zhang, W.; Cui, X.; Wang, H.; Xu, B. Comparison of the nutrient composition of royal jelly and worker jelly of honey bees (Apis mellifera). Apidologie 2016, 47, 48–56. [Google Scholar] [CrossRef]
- Chen, C.S.; Chen, S.Y. Changes in Protein-Components and Storage Stability of Royal Jelly under Various Conditions. Food Chem. 1995, 54, 195–200. [Google Scholar] [CrossRef]
- Howe, S.R.; Dimick, P.S.; Benton, A.W. Composition of Freshly Harvested and Commercial Royal Jelly. J. Apic. Res. 1985, 24, 52–61. [Google Scholar] [CrossRef]
- Koya-Miyata, S.; Okamoto, I.; Ushio, S.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Identification of a collagen production-promoting factor from an extract of royal jelly and its possible mechanism. Biosci. Biotechnol. Biochem. 2004, 68, 767–773. [Google Scholar] [CrossRef]
- Nagai, T.; Inoue, R. Preparation and the functional properties of water extract and alkaline extract of royal jelly. Food Chem. 2004, 84, 181–186. [Google Scholar] [CrossRef]
- Šimúth, J.; Bíliková, K.; Kováčová, E.; Kuzmová, Z.; Schroder, W. Immunochemical approach to detection of adulteration in honey: Physiologically active royal jelly protein stimulating TNF-alpha release is a regular component of honey. J. Agric. Food Chem. 2004, 52, 2154–2158. [Google Scholar] [CrossRef] [PubMed]
- Albert, S.; Bhattacharya, D.; Klaudiny, J.; Schmitzová, J.; Simúth, J. The family of major royal jelly proteins and its evolution. J. Mol. Evol. 1999, 49, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Šimúth, J. Some properties of the main protein of honeybee (Apis mellifera) royal jelly. Apidologie 2001, 32, 69–80. [Google Scholar] [CrossRef]
- Vezeteu, T.V.; Bobiş, O.; Moritz, R.F.; Buttstedt, A. Food to some, poison to others—Honeybee royal jelly and its growth inhibiting effect on European Foulbrood bacteria. Microbiologyopen 2017, 6, e00397. [Google Scholar] [CrossRef]
- Kim, B.Y.; Lee, K.S.; Jung, B.; Choi, Y.S.; Kim, H.K.; Yoon, H.J.; Gui, Z.Z.; Lee, J.; Jin, B.R. Honeybee (Apis cerana) major royal jelly protein 4 exhibits antimicrobial activity. J. Asia Pac. Entomol. 2019, 22, 175–182. [Google Scholar] [CrossRef]
- Rawlings, N.D.; Barrett, A.J. Evolutionary families of peptidases. Biochem. J. 1993, 290, 205–218. [Google Scholar] [CrossRef]
- Krem, M.M.; Cera, E.D. Evolution of enzyme cascades from embryonic development to blood coagulation. Trends Biochem. Sci. 2002, 27, 67–74. [Google Scholar] [CrossRef]
- Dahlmann, B.; Jany, K.D.; Pfleiderer, G. The midgut endopeptidases of the honey bee (Apis mellifica): Comparison of the enzymes in different ontogenetic stages. Insect Biochem. 1978, 8, 203–211. [Google Scholar] [CrossRef]
- Chen, X.; Hu, Y.; Zheng, H.; Cao, L.; Niu, D.; Yu, D.; Sun, Y.; Hu, S.; Hu, F. Transcriptome comparison between honey bee queen- and worker-destined larvae. Insect Biochem. Mol. Biol. 2012, 42, 665–673. [Google Scholar] [CrossRef]
- Matsuoka, T.; Takasaki, A.; Mishima, T.; Kawashima, T.; Kanamaru, Y.; Nakamura, T.; Yabe, T. Expression and characterization of honeybee, Apis mellifera, larva chymotrypsin-like protease. Apidologie 2015, 46, 167–176. [Google Scholar] [CrossRef][Green Version]
- Matsuoka, T.; Kawashima, T.; Nakamura, T.; Yabe, T. Characterization and comparison of recombinant honeybee chymotrypsin-like protease (HCLPase) expressed in Escherichia coli and insect cells. Biosci. Biotechnol. Biochem. 2017, 81, 1401–1404. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Felicioli, A.; Turchi, B.; Fratini, F.; Giusti, M.; Nuvoloni, R.; Dani, F.R.; Sagona, S. Proteinase pattern of honeybee prepupae from healthy and American Foulbrood infected bees investigated by zymography. Electrophoresis 2018, 39, 2160–2167. [Google Scholar] [CrossRef] [PubMed]
- Grzywnowicz, K.; Ciołek, A.; Tabor, A.; Jaszek, M. Profiles of the body-surface proteolytic system of honey bee queens, workers and drones: Ontogenetic and seasonal changes in proteases and their natural inhibitors. Apidologie 2009, 40, 4–19. [Google Scholar] [CrossRef]
- Giebel, W.; Zwilling, R.; Pfleiderer, G. The evolution of endopeptidases—XII. The proteolytic enzymes of the honeybee (Apis mellifica L.): Purification and characterization of endopeptidases in the midgut of adult workers and comparative studies on the endopeptidase-pattern of different castes and on different ontogenetic stages. Comp. Biochem. Physiol. B Biochem. 1971, 38, 197–210. [Google Scholar]
- Polanowski, A.; Wilusz, T.; Blum, M.S.; Escoubas, P.; Schmidt, J.O.; Travis, J. Serine proteinase inhibitor profiles in the hemolymph of a wide range of insect species. Comp. Biochem. Physiol. B Biochem. 1992, 102, 757–760. [Google Scholar] [CrossRef]
- Bania, J.; Stachowiak, D.; Polanowski, A. Primary structure and properties of the cathepsin G/chymotrypsin inhibitor from the larval hemolymph of Apis mellifera. Eur. J. Biochem. 1999, 262, 680–687. [Google Scholar] [CrossRef]
- Felicioli, A.; Donadio, E.; Balestreri, E.; Montagnoli, G.; Felicioli, R.; Podestà, A. Expression profile of water-soluble proteinases during ontogenesis of Megachile rotundata: An electrophoretic investigation. Apidologie 2004, 35, 595–604. [Google Scholar] [CrossRef][Green Version]
- Rembold, H.; Kremer, J.-P.; Ulrich, G.M. Characterization of postembryonic developmental stages of the female castes of the honey bee, Apis mellifera L. Apidologie 1980, 11, 29–38. [Google Scholar] [CrossRef]
- Matsuoka, T.; Kawashima, T.; Nakamura, T.; Kanamaru, Y.; Yabe, T. Isolation and characterization of proteases that hydrolyze royal jelly proteins from queen bee larvae of the honeybee, Apis mellifera. Apidologie 2012, 43, 685–697. [Google Scholar] [CrossRef]
- Rossano, R.; Larocca, M.; Polito, T.; Perna, A.M.; Padula, M.C.; Martelli, G.; Riccio, P. What are the proteolytic enzymes of honey and what they do tell us? A fingerprint analysis by 2-D zymography of unifloral honeys. PLoS ONE 2012, 7, e49164. [Google Scholar] [CrossRef] [PubMed]
- Erban, T.; Shcherbachenko, E.; Talacko, P.; Harant, K. The Unique Protein Composition of Honey Revealed by Comprehensive Proteomic Analysis: Allergens, Venom-like Proteins, Antibacterial Properties, Royal Jelly Proteins, Serine Proteases, and Their Inhibitors. J. Nat. Prod. 2019, 82, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Funakoshi, T.; Shimada, H.; Kojima, S. Proteolytic activity of royal jelly. Med. Biol. 1993, 127, 5. [Google Scholar]
- Li, J.; Feng, M.; Begna, D.; Fang, Y.; Zheng, A. Proteome comparison of hypopharyngeal gland development between Italian and royal jelly producing worker honeybees (Apis mellifera L.). J. Proteome Res. 2010, 9, 6578–6594. [Google Scholar]
- Li, J.K.; Feng, M.; Zhang, L.; Zhang, Z.H.; Pan, Y.H. Proteomics analysis of major royal jelly protein changes under different storage conditions. J. Proteome Res. 2008, 7, 3339–3353. [Google Scholar] [CrossRef]
- Scarselli, R.; Donadio, E.; Giuffrida, M.G.; Fortunato, D.; Conti, A.; Balestreri, E.; Felicioli, R.; Pinzauti, M.; Sabatini, A.G.; Felicioli, A. Towards royal jelly proteome. Proteomics 2005, 5, 769–776. [Google Scholar] [CrossRef]
- Vinhas, R.; Cortes, L.; Cardoso, I.; Mendes, V.M.; Manadas, B.; Todo-Bom, A.; Pires, E.; Verissimo, P. Pollen proteases compromise the airway epithelial barrier through degradation of transmembrane adhesion proteins and lung bioactive peptides. Allergy 2011, 66, 1088–1098. [Google Scholar] [CrossRef]
- Grogan, D.E.; Hunt, J.H. Pollen proteases: Their potential role in insect digestion. Insect Biochem. 1979, 9, 309–313. [Google Scholar] [CrossRef]
- McKenna, O.E.; Posselt, G.; Briza, P.; Lackner, P.; Schmitt, A.O.; Gadermaier, G.; Wessler, S.; Ferreira, F. Multi-Approach Analysis for the Identification of Proteases within Birch Pollen. Int. J. Mol. Sci. 2017, 18, 1433. [Google Scholar] [CrossRef]
- Höllbacher, B.; Schmitt, A.O.; Hofer, H.; Ferreira, F.; Lackner, P. Identification of Proteases and Protease Inhibitors in Allergenic and Non-Allergenic Pollen. Int. J. Mol. Sci. 2017, 18, 1199. [Google Scholar] [CrossRef]
- Barcia, C.; Coelho, A.S.; Barberis, S.; Veríssimo, P. Acaciain peptidase: The first South American pollen peptidase potentially involved in respiratory allergy. Biotechnol. Appl. Biochem. 2020, 67, 224–233. [Google Scholar] [CrossRef]
- Crailsheim, K.; Schneider, L.H.W.; Hrassnigg, N.; Bühlmann, G.; Brosch, U.; Gmeinbauer, R.; Schöffmann, B. Pollen consumption and utilization in worker honeybees (Apis mellifera carnica): Dependence on individual age and function. J. Insect Physiol. 1992, 38, 409–419. [Google Scholar] [CrossRef]
- Di Pasquale, G.; Salignon, M.; Le Conte, Y.; Belzunces, L.P.; Decourtye, A.; Kretzschmar, A.; Suchail, S.; Brunet, J.L.; Alaux, C. Influence of pollen nutrition on honey bee health: Do pollen quality and diversity matter? PLoS ONE 2013, 8, e72016. [Google Scholar] [CrossRef] [PubMed]
- Mattila, H.R.; Otis, G.W. The effects of pollen availability during larval development on the behaviour and physiology of spring-reared honey bee workers. Apidologie 2006, 37, 533–546. [Google Scholar] [CrossRef]
- Crailsheim, K.; Stolberg, E. Influence of diet, age and colony condition upon intestinal proteolytic activity and size of the hypopharyngeal glands in the honeybee (Apis mellifera L.). J. Insect Physiol. 1989, 35, 595–602. [Google Scholar] [CrossRef]
- Dietz, A.; Haydak, M.H. Caste determination in honey bees. I. The significance of moisture in larval food. J. Exp. Zool. 1971, 177, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Heussen, C.; Dowdle, E.B. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal. Biochem. 1980, 102, 196–202. [Google Scholar] [CrossRef]
- Jousson, O.; Di Bello, D.; Donadio, E.; Felicioli, A.; Pretti, C. Differential expression of cysteine proteases in developmental stages of the parasitic ciliate Ichthyophthirius multifiliis. FEMS Microbiol. Lett. 2007, 269, 77–84. [Google Scholar] [CrossRef]
- Barrett, A.J. Classification of peptidases. Meth. Enzymol. 1994, 244, 1–15. [Google Scholar]
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image processing with ImageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sagona, S.; D’Onofrio, C.; Miragliotta, V.; Felicioli, A. Detection and pH-Thermal Characterization of Proteinases Exclusive of Honeybee Worker-Fate Larvae (Apis mellifera L.). Int. J. Mol. Sci. 2022, 23, 15546. https://doi.org/10.3390/ijms232415546
Sagona S, D’Onofrio C, Miragliotta V, Felicioli A. Detection and pH-Thermal Characterization of Proteinases Exclusive of Honeybee Worker-Fate Larvae (Apis mellifera L.). International Journal of Molecular Sciences. 2022; 23(24):15546. https://doi.org/10.3390/ijms232415546
Chicago/Turabian StyleSagona, Simona, Chiara D’Onofrio, Vincenzo Miragliotta, and Antonio Felicioli. 2022. "Detection and pH-Thermal Characterization of Proteinases Exclusive of Honeybee Worker-Fate Larvae (Apis mellifera L.)" International Journal of Molecular Sciences 23, no. 24: 15546. https://doi.org/10.3390/ijms232415546
APA StyleSagona, S., D’Onofrio, C., Miragliotta, V., & Felicioli, A. (2022). Detection and pH-Thermal Characterization of Proteinases Exclusive of Honeybee Worker-Fate Larvae (Apis mellifera L.). International Journal of Molecular Sciences, 23(24), 15546. https://doi.org/10.3390/ijms232415546





