Abstract
Obesity, which is considered a pandemic due to its high prevalence, is a risk factor for many types of cancers, including lymphoma, through a variety of mechanisms by promoting an inflammatory state. Specifically, over the last few decades, obesity has been suggested not only to increase the risk of lymphoma but also to be associated with poor clinical outcomes and worse responses to different treatments for those diseases. Within the extensive range of proinflammatory mediators that adipose tissue releases, leptin has been demonstrated to be a key adipokine due to its pleotropic effects in many physiological systems and diseases. In this sense, different studies have analyzed leptin levels and leptin/leptin receptor expressions as a probable bridge between obesity and lymphomas. Since both obesity and lymphomas are prevalent pathophysiological conditions worldwide and their incidences have increased over the last few years, here we review the possible role of leptin as a promising proinflammatory mediator promoting lymphomas.
1. Introduction
Lymphomas are lymphoid neoplasms that manifest as solid tumor masses. There are two variants of this disease called Hodgkin lymphoma (HL) and non-Hodgkin lymphoma (NHL). HL constitutes about 8% of all malignant lymphoid neoplasms and involves mature B lymphocytes, which correspond to the so-called Reed–Sternberg (RS) cell, which represents less than 1% of the total tumor [1,2]. By contrast, NHL represents most of the cases of malignant lymphoid neoplasms and involves not only mature B cells but also T and NK lymphocytes. Specifically, diffuse large B-cell lymphoma (DLBCL) is by far the most frequent subtype of NHL [3].
The overall incidence of HL is relatively low (two or three per 100,000 individuals), although there are different incidence peaks depending on age. The highest peak is observed in adolescents and young adults (15–35 years old), and almost 70% of them have EBV-negative HL, whereas the two lowest peaks appear in children and elderly adults, with a low prevalence (~30%) of EBV-negative cases [4]. However, the mortality rate in elderly patients is higher than in their young counterparts [5], especially in the male population [6]. By contrast, the overall incidence of NHL depends on the location, which goes from three to four per 100,000 male individuals in Vietnam or India to 17–18 per 100,000 in Israel Jews on the male population [7]. Moreover, NHL incidence increases exponentially with age. For example, 9.3 per 100,000 people under 65 years and 91.5 per 100,000 people more than 65 years had NHL from 2007 to 2011 in the USA [8].
Different risk factors have been associated with lymphoma development and progression over the years, such as immune deficiencies, viral and bacterial infections, inherited polymorphisms, acquired genetic mutations, or chemical exposures [9]. Nevertheless, another risk factor that needs to be taken into consideration is obesity, which is a proinflammatory state characterized by excessive adipose tissue that could promote not only different conditions and diseases such as cardiovascular disease, including coronary artery disease and high blood pressure, insulin resistance or type 2 diabetes mellitus [10] but also a huge variety of cancers [11,12,13], including lymphomas [14,15,16]. Interestingly, obesity-derived inflammation is potentially driven by the action of certain adipokines, such as leptin [17].
Leptin is a 16 kDa adipocyte-derived hormone that was first predicted in murine models [18,19] and described years later as the product of the obese (Ob) gene [20]. Leptin is mainly characterized having pleiotropic effects due to the existence of different leptin receptors (known as LEPR-a, LEPR-b, LEPR-c, LEPR-d, LEPR-e, and LEPR-f) [21,22]. The most important receptor is LEPR-b, which can fully transduce signals into the cell to activate a set of signaling pathways such as the Janus kinase (JAK) 2/signal transducer and activator of transcription (STAT) 3, insulin receptor substrate (IRS)/phosphatidylinositol-3 kinase (PI3K), or Src homology 2 domain-containing phosphatase 2 (SHP2)/mitogen-activated protein kinase (MAPK) [23,24]. The signaling pathways are typical of a type I cytokine receptor [25], and in fact, the receptor is present in every cell of the innate and adaptative immunological system [26], and that is why leptin is considered to be the link between the immune and metabolic system [27].
Leptin is mainly involved in the central control of energy metabolism [28] and obesity [29] but also plays a key role in other physiological systems and diseases, including autoimmune diseases [30], human dental pulp [31], bone metabolism [32], or cardiovascular diseases [33]. Of note, our group previously reviewed the striking role of leptin in other conditions that involve female reproduction [34], pregnancy [35], gestational diabetes [36], non-alcoholic fatty liver disease [37], atopic dermatitis [38], and even cancer [39,40].
Due to the increased incidence rate of both obesity and lymphomas over the past few decades, as well as the promising role of leptin in bridging obesity and many malignancies such as cancer, the aim of this article is to review the relationship between those two prevalent diseases and elucidate the role of leptin underlying this association.
2. Obesity and Lymphoma
2.1. The Relationship between Obesity and Lymphoma
The association between obesity and lymphoma has been largely discussed over the years. Overall, it seems that the risk of lymphoma increases at a higher body mass index (BMI) [41,42,43,44,45,46,47], but some studies have found non-significant positive associations or no correlations between both diseases [48,49,50]. Probably, this may be caused not only because HL and NHL are heterogenous diseases that involve many histological subtypes but also due to other variables such as location, gender, or age, as previously explained. In line with this notion, Willet et al. (2008) concluded that there was no evidence to support obesity as a determinant parameter for all types of NHL combined, whereas Ingham et al. (2011) found that obesity was associated with the risk of HL and most types of NHL, excluding FL [51].
The relationship between obesity and lymphomas was first suggested almost 50 years ago in a case–control study that evaluated 50,000 male students with different diseases, including 45 cases of HL and 89 cases of NHL (considered as “other types of lymphomas”) between 1916 and 1950, who were followed until 1974. In the study, it was found that an increased HL risk was prevalent among students who were obese, smokers, and coffee drinkers [52], whereas leanness was a predisposing factor for NHL in the same cohort [53]. Specifically, the risk of HL has significantly increased in both obese men and women [41,42,43,47], having been elevated more than two times compared with normal-BMI (18.5–25 kg/m2) patients. Interestingly, every 5 kg/m2 gained in BMI has proved to increase the risk of HL by 40% in both genders [42,47]. The British population passage cohort study, which analyzed 5.82 million patients, showed that every 5 kg/m2 increased the risk of HL by 10% [54]. Healthy adult women (considered from 19 to 44 years old) with a lower BMI also had a significantly increased risk of HL, whereas this association has been inversed in their older counterparts, suggesting that body size and strenuous physical activity may be associated with HL risk (at least in the female population), through immunologic, infectious, or genetic mechanisms [43]. By contrast, only a few studies found no significant increases in the risk of HL considering BMI as a single variant [49,50] or including other factors such as age or tobacco [55].
Regarding NHL, it has been found that the risk of the disease is elevated not only in obese (BMI ≥30 kg/m2) patients but also in the population with severe obesity (BMI≥35 kg/m2) [50] regarding both women and men [56,57], although it has also been found that obesity promotes the risk of NHL in female patients compared with their male counterparts [58]. The million women study evaluated the incidence and mortality for 17 specific types of cancer, including NHL, and found that the increase of 10 kg/m2 in BMI increased the relative risk of the disease [44]. Similarly, different studies have found that a 5 kg/m2 increase from normal BMI in men and women was also associated with the increased risk of NHL [45,46,47,59]. Of note, some factors, such as age, ethnicity, smoking status, alcohol consumption, gender [50,60,61,62], and probably menopause [44], should be considered since they have been found to increase the overall risk of the disease in obese people. Regarding responses to treatments, salvage chemotherapy and high-dose chemotherapy following autologous hematopoietic stem cell transplantation have been successfully tested as promising treatments in patients with relapsed lymphoma [63,64]. However, obesity influences responses to treatment in those patients and may impair overall survival [16].
NHL comprises a huge variety of histologic diseases that are also linked to obesity, especially DLBCL [56,65], which is aggressive cancer and the most common type of NHL since it represents about 40% of new cases. Moreover, one-third of DLBCL patients develop relapsed/refractory disease with poor prognosis, although some treatments have been successfully tested in the last few years to improve their clinical outcomes [66,67,68]. Up to now, some systematic reviews have shown limited evidence regarding the influence of obesity over DLBCL [69,70], but many studies support this association [45,46,47,59,71,72,73] even when a higher BMI has not been associated with the risk of overall NHL [74].
Similar conclusions have been extracted for FL: the second most common subtype of NHL [75]. Although it has been demonstrated that neither obesity nor height, waist/hip ratio, and physical activity has been associated with the risk of FL [76], other studies have found non-significant, positive associations between both diseases [45,57] and a higher risk of death [77]. Moreover, obesity was considered a risk factor not only for lymphoma in the head and neck (considering that the increase of 1 kg/m2 could enhance the risk of the disease by 1.3 times) [78], but also for T-cell NHL [79]. Regarding age at diagnosis, obese individuals between 40 and 49 years may have an elevated risk of DLBCL and FL [57]. Another study found that obese individuals under 45 years could have a greater probability of developing DLBCL compared to FL, especially in the male population [80].
On the other hand, obesity has also been suggested as a protective element for the development of NHL [48], and the relationship between obesity and the risk of small lymphocytic lymphoma (SLL) is de facto controversial [57,76]. In this regard, it was proposed the term “obesity paradox” to explain that obese individuals could have a more favorable prognosis compared to their healthy or underweight counterparts. The obesity paradox not only has been especially associated with cardiovascular diseases [81,82,83] but also with cancer [84,85,86]. It could be related to the inadequate use of BMI to measure obesity since protective muscle mass may also contribute to BMI [87]. Moreover, cancer produces weight loss, and it depends on the stage of the disease, so the excess of adipose tissue may represent an energy store for the survival of patients [39]. In line with those notions, other parameters, such as the waist circumference (WC) and the A body shape index (ABSI), defined by WC/ [BMI (2/3) × height (1/2)], have been analyzed in the Malmö diet and cancer study as better predictors than BMI for the risk of different hematological malignancies, including lymphomas [88]. Additionally, Alberta’s tomorrow project suggested that central adiposity, measured by WC, may be a stronger predictor of total cancer risk than BMI since men with more than 102 cm of WC had a significantly increased risk of NHL and hematological cancer [89].
2.2. Molecular Mechanisms Underlying the Association between Obesity and Lymphomas
The relationship between obesity and cancer has been extensively described in the past few decades due to the proinflammatory state that promotes tumor cell proliferation, including in lymphomas, as shown in Figure 1. It has been demonstrated that an excess of adiposity can modulate the aggressiveness of Hodgkin Reed–Sternberg lymphoma cells though different mechanisms that involve hypertrophied adipocytes, adipose stem cells, angiogenesis, and the release of pro-tumoral adipokines [90]. However, some studies have shown limited power, especially in NHL subtypes, and robust analyses to determine the etiologic mechanisms should be carried out [91].
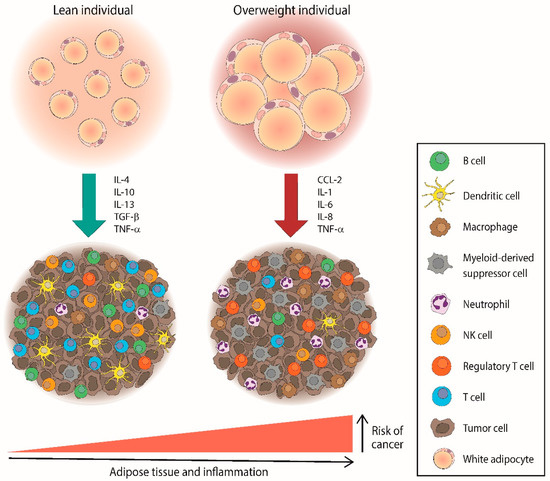
Figure 1.
Overweight individuals could increase the risk of cancer, including lymphomas, through the accumulation of pro-inflammatory mediators and immunosuppressive cells within the tumor microenvironment. CCL—C–C motif chemokine ligand; IL—interleukin; TGF—tumor growth factor; TNF—tumor necrosis factor.
In this sense, we already know that one of the most important pathways to regulate inflammatory responses associated with obesity is NF-κB, whose activity has been found to increase in mice with a high-fat diet compared with their low-fat diet counterparts [92] and could also mediate tumor cell proliferation, survival, and angiogenesis through the expression of different target genes, including TNFA, BCLXL, or BCL2, among others [93]. In many lymphoid malignancies, including HL, DLBCL, mucosa-associated lymphoid tissue (MALT) lymphoma, primary effusion lymphoma, or adult T-cell lymphoma/leukemia (ATLL), NF-κB signaling is considered a common hallmark since it is involved in lymphoma survival and growth by inducing anti-apoptotic and pro-proliferative gene programs [94,95]. In fact, many therapeutic approaches have been tested by targeting the NF-κB signaling pathway, such as rituximab in combination with ibrutinib, thalidomide, or lenalidomide in MCL [96] or the small interfering RNA (siRNA) nanotherapy in ATLL [97].
In addition, adipose tissue is one of the main sources of pro-inflammatory mediators. The major cytokine released by adipocytes is interleukin (IL)-6 [98], which could increase the risk of different cancers in obese patients, such as breast, liver, prostate, colon, and esophagus cancers [99], and lymphomas [100]. IL-6 acts as a growth factor together with IL-10 in NHL [101,102] and has been demonstrated to be involved in the resistance of PI3K pathway-targeted treatments via STAT3 or STAT5 activation [103]. Specifically, IL-6 is an important survival factor in MCL [102], and its level has been correlated with prognosis in DLBCL [104]. Moreover, pleural effusion lymphoma cell growth has been inhibited by using human IL-6 antisense oligonucleotides [105].
Circulating IL-8 is another cytokine secreted by adipocytes [106] and was found to be significantly higher in obese subjects compared with the non-obese controls [107], increasing inflammation and associated with different types of lymphomas. In this line, elevated levels of IL-8 have been found in gastrointestinal FL and MALT lymphomas [108] as well as DLBCL cells, which finally recruit neutrophils producing APRIL: a factor that promotes the development of different types of tumors and has been associated with poor survival in DLCBL due to DNA methylation and acetylation [109]. Circulating IL-8 levels have also been associated with concomitant infections and have been positively correlated with neutrophil counts in cutaneous T-cell lymphoma patients [110]. Of note, both IL-6 and IL-8 can be released by tumor-associated macrophages [111], which are widely known to promote cancer progression and metastasis and, in turn, these cytokines could participate in the recruitment and expansion of MDSCs [112], which have been extensively associated with poor clinical outcomes in both HL and NHL due to their role in immune evasion and cancer progression through different mechanisms [113]. In this context, we have recently found increased circulating levels of MDSC in DLBCL that decreased in patients with >24 months of survival [68].
Another protein, the monocyte chemoattractant protein (MCP)-1 (mostly known as C–C Motif Chemokine Ligand 2, CCL2), was also found in high concentrations in the serum of obese individuals [107] and was overexpressed in patients with triple-negative breast cancer, leading to cancer progression and metastasis [114]. Specifically, MCP-1 could be involved in the migration and localization of FL cells [115] and, together with its receptor (CCR2), has been suggested as a good factor to better identify DLBCL patients with high-risk by the international prognostic index since the high expression of these proteins has been associated with poor overall survival and progression-free survival [116]. The high expression of MCP-1 has also been found in other types of DLBCL called primary central nervous system lymphomas [117].
IL-1 also promotes inflammation in obese individuals [118] and has been demonstrated to be up-regulated in a huge variety of tumors, such as breast, head, neck, colon, pancreas, lung, melanomas, and lymphomas [119,120]. In lymphomas, IL-1α may have anti-tumoral properties [121], whereas IL-1β has been shown to be expressed in HL cells from areas of tissue with active fibrosis, and the receptor IL-1R2 may contribute to local and systemic modulation in the disease [120]. In this sense, an IL-1 blockade has been proposed with different treatments, such as chimeric antigen receptor (CAR) T cells targeting CD19 in acute lymphoblastic leukemia or DLBCL [122].
By contrast, the tumor necrosis factor (TNF)-α is also secreted by adipose tissues, and its levels correlate with the degree of adiposity [123], but its role in cancer remains controversial [124,125,126]. However, the role of anti-TNF-α therapies in increasing the risk of lymphoma has been described and seems clear in patients with autoimmune diseases, such as inflammatory bowel disease [127] or rheumatoid arthritis [128,129].
3. Leptin and Lymphoma
3.1. Leptin Signaling in Lymphoma
The metabolic abnormalities associated with an excess of adipose tissue include biochemical alterations such as high levels of plasma triglycerides [130] or peripheral insulin resistance, which lead to increased levels of insulin and glucose [131]. Importantly, other factors closely involved in obesity have been described as promoters of many diseases in the last decades, such as the adipokines leptin or adiponectin. Leptin is known to activate and promote the proliferation of monocytes and lymphocytes by activating JAK-STAT, PI3K, and MAPK [132,133]. Leptin signaling also drives the activation of many oncogenic pathways leading to the increased proliferation, epithelial-mesenchymal transition, migration, and invasion of tumor cells [134]. Specifically, leptin signaling pathways can promote lymphomas (Figure 2). Leptin binds its receptor LEPR-b to transduce activation signals into cells via JAK2, which is phosphorylated together with Tyr985, Tyr1077, and Tyr1138. STAT3 proteins bind phospho-Tyr1138 and are phosphorylated and translocated into the nucleus of dimeric units, activating the transcription of their targeting genes and leading to a huge variety of lymphomas, including DLBCL, unclassifiable diseases with features between DLBCL and Burkitt lymphoma, mantle cell (MCL), NK/T-cell (NKTCL), peripheral T-cell (PTCL), anaplastic large cell (ALCL) or intestinal T-cell lymphomas, as well as HL [135,136]. One of its targeting genes, the suppressor of cytokine signaling (SOCS)-3, has been found to be highly expressed in FL and ALCL [137,138]. Similarly, STAT5 binds phospho-Tyr1077 and is translocated into the nucleus after its phosphorylation, thus promoting not only DLBCL, PTCL, MCL, or HL (as STAT3 signaling does) but also γδ-T-cell and lymphoblastic lymphomas [139,140,141,142,143,144].
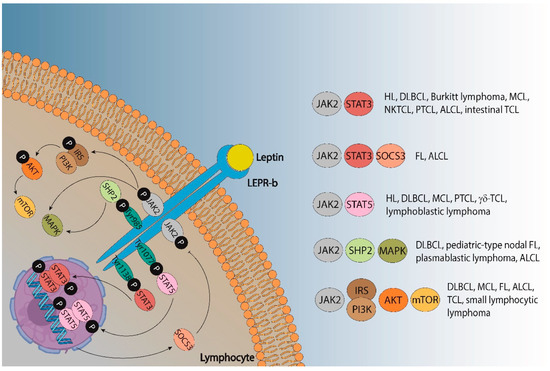
Figure 2.
Leptin signaling pathways that could promote different types of lymphoma. ALCL—anaplastic large cell lymphoma; DLBCL—diffuse large B-cell lymphoma; FL—follicular lymphoma; HL—Hodgkin lymphoma; MCL—mantle cell lymphoma; NKTCL—natural killer/T-cell lymphoma; PTCL—peripheral T cell lymphoma; TCL—T-cell lymphoma.
Moreover, SHP2 binds to phospho-Tyr985 and promotes the activation of the MAPK pathway, although leptin can also activate MAPK signaling independent of SHP2. The protein SHP2 has been associated with ALCL [145,146], whereas MAPK activity impairs outcomes in DLBCL, pediatric-type nodal FL, and plasmablastic lymphoma [147,148,149]. The phosphorylation of JAK2 also promotes the PI3K/AKT/mTOR signaling pathway via IRS activation. The IRS proteins are a family of cytoplasmic adaptor proteins with important roles in cancer [150]. Regarding lymphomas, IRS-1 has been demonstrated to activate anaplastic lymphoma kinase (ALK), which is involved in ALCL [151], and IRS-4 could mediate the mitogenic signaling of LB cells: a murine pre-T-cell lymphoma [152]. The activation of the PI3K/protein kinase B (AKT)/mammalian target of the rapamycin (mTOR) pathway also plays a key role in lymphoma, and many signaling pathway inhibitors have been developed to treat FL, DLBCL, MCL, small lymphocytic, and T-cell NHL [153,154,155,156,157].
At the cellular level, leptin signaling favors Th1 responses by enhancing IL-2, interferon (IFN)-γ synthesis, and inhibiting IL-4 production, which suggests that this adipokine may alter T-cell responses toward a proinflammatory phenotype [25,158]. The recruitment of proinflammatory cytokines by leptin could regulate the production of adhesion molecules, such as the vascular cell adhesion molecule (VCAM)-1 and intercellular cell adhesion molecule (ICAM)-1 [159,160], that have been found to be highly increased in newly diagnosed lymphoma patients and correlate with tumor dissemination, the aggressiveness of the disease, and worse response to treatments [161,162,163,164].
Leptin also induces TNF-α in many settings [165,166,167]. Although its role in cancer remains controversial (as previously explained), TNF-α has been shown to play a key role in the pathogenesis of NHL [168] and may increase the risk of disease together with leptin, especially in FL [169] and DLBCL, through polymorphisms in the TNF rs1800629G>A gene [170]. Additionally, TNF-α levels were higher in lymphoma from children compared with their solid-tumor counterparts [171], which suggests the relevant role of this protein in lymphomas. IL-10 and IFN-γ released by leptin may be implicated in lymphomagenesis since their circulating levels were increased in patients with BMI ≥ 25 kg/m2 compared to individuals with a lower BMI [172]. Although IL-10 may be associated with a higher risk of NHL, especially FL, IFN-γ was not associated with that risk [169]. The risk of lymphoma in patients with a higher BMI could be also increased by the release of IL-6 via leptin signaling [172], but it still needs to be completely elucidated since other studies did not find this association [169]. Of note, blood glucose was suggested as a prognostic biomarker for TCL [173], and the human oocyte testis gene 1, an antigen whose disruption promotes aberrant glucose homeostasis and defective hormone secretion, has been shown to decrease levels of insulin and leptin in TCL-bearing mice [174].
3.2. Leptin and LEPR Genes in Lymphoma
Leptin has been suggested to promote immune dysfunctions regarding body weight regulation and NHL: mainly DLBCL and FL. Regarding gene expression, lymphomas are mainly characterized by mutations that involve genes, such as B-cell lymphoma (bcl)-2 [175], bcl-6 [176], p15 and p16 [177], p53 [178], or myc [179], which have been widely considered as biomarkers of poor prognosis in those diseases [180,181,182,183,184,185,186]. Specifically, BCL-2 is an antiapoptotic protein that belongs to the BCL-2 family together with other proteins, including (but not limited to) CL-XL and BCL-W, with antiapoptotic properties, as well as the proapoptotic BAX, BAK, or BID proteins [187]. Leptin signaling has been demonstrated to play a key role in B-cell homeostasis through the induction of Bcl-2 [188], which could increase the risk of different pathological conditions. Leptin has demonstrated the ability to inhibit apoptosis and induce cell cycle by elevating Bcl-2 and cyclin D1 in leptin-receptor-deficient (db/db) mice [188]. Similarly, the Bcl-2 protein expression was elevated in db/db mice with diabetes [189,190,191], Which may be predisposed to develop lymphoma [192]. This adipokine also decreased the apoptosis of myocardial cells in rats via bcl-2 [193] and reduced the apoptosis of beta cells at physiological concentrations in vitro by maintaining or up-regulating bcl-2 expression, which could promote non-insulin-dependent diabetes mellitus [194,195]. Additionally, mild maternal protein deprivation during lactation in rat pups could affect thymic homeostasis by increasing the activity of leptin, which improves the levels of BCL-2 and inhibits the apoptosis of thymocytes [196]. In human trophoblasts, leptin also prevents apoptosis when elicited with high temperatures by increasing the BCL-2/BAX ratio [197]. In cancer, the silencing of leptin in HeLa cells, a cervical cancer cell line, has reduced the expression of bcl-2 and, consequently, promotes apoptosis and inhibits cell proliferation, thus suggesting the probable role of leptin in the progression of cervical cancer [198]. Those notions are especially significant since NF-kB, STAT3, PI3K, and AKT pathways are activated in lymphoma cells via leptin/LEPR signaling [199,200,201] and improving bcl-2 expression.
Several studies have analyzed the role of leptin genes in lymphomas (Table 1). Single nucleotide polymorphisms (SNPs) in leptin genes LEP 2548GA and LEP 2548AA have been shown to increase the risk of FL compared with LEP 2548GG [80]. Specifically, genetic polymorphisms in LEP 2548GA have been significantly associated with NHL under the homozygous co-dominant model and additive genetic model in the Caucasian population rather than among Asians after analyzing almost 7000 cases and 8000 controls [202]. The positive associations between LEP 2548GA and the susceptibility of NHL were also found in another study, but without statistically significant differences [203]. Moreover, SNPs in LEP 2548GA have not been suggested to increase the risk of cutaneous T cell lymphomas (TCL) but may be involved in the pharmacogenetic of different treatments for this disease since patients with AG or GG genotypes (with lower plasma leptin levels) could better respond to topical steroids (male patients) and phototherapy (female counterparts) compared with AA patients [204].

Table 1.
Leptin/LEPR gene polymorphisms analyzed in lymphomas. NHL—non-Hodgkin lymphoma; DLBCL—diffuse large B-cell lymphoma; FL—follicular lymphoma.
However, results regarding SNPs in other leptin genes, such as LEP A19G (also known as rs2167270) and its receptor LEPR Q223R, remain inconsistent. Polymorphisms in the LEP A19G gene have been correlated with BMI and an increased risk of DLCL and FL [71], but accumulating evidence from recent years has revealed that SNPs in LEP A19G are associated with a decreased risk of DLBCL [170] and FL [203]. In line with this, meta-analyses have reported that genetic polymorphisms in the LEP A19G gene were associated with a lower risk (or even decrease in the risk) of NHL among Latin American individuals [205] and Asians, Caucasians and mixed populations [207]. Additionally, polymorphisms in the LEP A19G receptor, LEPR Q223R, could not increase the susceptibility of NHL [206]. Other leptin genes, such as LEP 19AA, could decrease that risk [80], whereas the leptin receptor gene rs1327118 G>C has not been associated with susceptibility to the disease [170].
By contrast, the ghrelin GHRL SNP allele for GHRL 4427G>A has been inversely correlated with the risk of NHL, especially DLCL [208]. GHLR and leptin are hormones that play antagonistic roles in controlling energy balance [209] by increasing and decreasing the levels of neuropeptide Y (NPY), respectively [210,211]. NPY is a powerful appetite stimulator that serves as an immune mediator by releasing and inhibiting proinflammatory cytokines [208]. The role of NPY in disease risk and progression remains unclear since it has been found that NPY genes may affect the risk of NHL, especially FL [208], but no significant changes in NPY levels after treatment have been revealed in patients with different types of cancer, including NHL [168].
3.3. Serum Leptin and LEPR Expression in Lymphoma
Similar to leptin genes, the relationship between the concentration of circulating leptin or LEPR expression and lymphomas has also been studied (Table 2). It has been demonstrated that leptin levels increase the risk of NHL in individuals with BMI ≥ 25 kg/m2 [172]. In addition, the phosphorylation of STAT3 and AKT via JAK2/STAT and PI3K/AKT signaling pathways has led immunohistochemical studies to reveal high expressions of LEPR, p-STAT3, and p-AKT in many DLBCL cases [201]. In line with this notion, leptin has been demonstrated to stimulate the proliferation of DLBCL cells and inhibit apoptosis via the PI3K/AKT signaling pathway in vitro, whereas the pretreatment of DLBCL cells with LEPR-specific siRNA or the inactivation of PI3K/AKT activity depleted these responses [200]. Likewise, leptin has increased the cell viability of CTL MOLT-3 cells by promoting the recruitment and expression of Glut1, and LEPR-siRNA, which inhibited those responses [212].

Table 2.
Leptin/LEPR levels or expression analyzed in lymphomas.
By contrast, it has also been shown that leptin levels not only undergo slight changes after treatment in NHL adult patients [168] but also could be negatively correlated with the international prognostic score in HL and with the international prognostic index in NHL [214], suggesting a paradoxical role of leptin that has been previously explained not only in cancer [40] but also in other settings [218,219,220]. Also, Bertolini et al. (1999) studied patients with NHL (mainly DLBCL and FL but also other types of lymphomas such as MCL, PTCL, ALCL, large granular NK-cell lymphoma, and extranodal marginal zone lymphoma of MALT), whose leptin levels were not only similar regardless of the outcome but were also not correlated with age, gender, or even-free survival [213].
3.4. Linking Leptin, Lymphoma, and Obesity
Most of the studies have analyzed the relationship between leptin and lymphoma, obesity and lymphoma, or obesity and leptin. Therefore, only a few studies have analyzed the possible associations among leptin, lymphoma, and obesity. Recently, leptin has been positively associated with BMI and NHL risk [172]. Also, patients who survived the Burkitt type, non-Burkitt, and lymphoblastic lymphomas not only had low leptin levels but also a normal/low BMI (19.5 ± 3.4 kg/m2) [221]. On the other hand, leptin levels have been positively correlated with BMI but not associated with lymphoma risk [214]. Similarly, relationships between leptin or BMI with HL or NHL were not found in pediatric patients [216]. A BMI ranging from underweight to healthy values in children newly diagnosed with HL or NHL has been positively correlated with leptin. Thus, leptin levels were low in those patients at diagnosis [215,217] but may significantly increase after remission and predict the response to treatment or progressive disease [217]. Regarding SNP, it has been found that leptin gene polymorphisms were independent of BMI and did not alter the risk of NHL [80].
4. Conclusions and Future Perspectives
Leptin is a pleiotropic hormone released by adipocytes and not only plays critical functions in energy metabolism or appetite regulation but also takes part in multiple immune actions, including those that promote diseases such as cancer. At least in part, it seems that leptin may play a key role by increasing the risk of NHL or driving their progression, thus being associated with poor outcomes. However, leptin action in HL has been poorly studied and still needs to be completely elucidated. Also, we need to consider that adipose tissue secretes not only leptin but also other pro-inflammatory mediators that may take part in the development and progression of these diseases, such as IL-1, IL-6, IL-8, or MCP-1, as explained above.
Many factors may be implied to promote variations in leptin or LEPR expression in lymphomas, such as gene mutations since lymphomas are heterogeneous diseases with a huge variety of gene expression profiles, especially DLBCL [222,223]. In line with this notion, different gene mutations, and even the different signaling pathways activated depend on the type of lymphoma (Figure 2) and may lead to the opposite roles of the same leptin/LEPR SNPs for different lymphomas, as shown in Table 1. For example, the SNP of the LEP 2548GA gene increases the risk of FL, but cutaneous T-cell lymphoma patients with the SNP of this gene may respond better to treatments (Table 1). In this sense, further research is needed to find out the possible links between gene mutations, activated signaling pathways, and the gain-of-function or loss-of-function of those leptin/LEPR SNPs.
Other factors such as age, gender, ethnicity, or location have been proven to vary the incidence of lymphomas [224,225,226,227,228]. Similarly, although BMI includes body weight and is widely used as a marker for the development of several diseases, it might not be a good indicator of obesity because body weight also depends on muscle mass; also, BMI does not consider the fat mass of different body sites [229]. However, other parameters that have been examined to measure adiposity are increasingly used, such as WC, the waist:hip ratio, and even the waist:height ratio [230], which should be used to measure central adiposity more accurately and may better elucidate the actual relationship between obesity and lymphomas.
As previously explained, the different results obtained by linking obesity, leptin, and lymphomas highlight the paradoxical role of leptin. However, the results obtained by El Demerdash et al. (2021) further support the probable importance that leptin may have as a bridge between these pathphysiological conditions [172]. In any case, further research is needed to better elucidate this question not only in lymphomas but also in other diseases.
Interestingly, the idea that leptin plays a key role in lymphomas may be reinforced because of the use of metreleptin in other diseases. Metreleptin is a human leptin analog for the treatment of metabolic pathologies, such as acquired generalized lipodystrophy (AGL) [231]. Although AGL is associated per se with a high risk of lymphoma [232], metreleptin may accelerate that risk since AGL patients have been reported to develop NHL during metreleptin treatment, including peripheral TCL and ALK-positive ALCL [233] and may also jeopardize the recurrence of lymphoma [234], although further research is needed in this sense to completely confirm this statement.
We believe that leptin could be a potential bridge between obesity and lymphomas based on the available literature regarding leptin and LEPR genes, serum leptin, and LEPR expression in these types of cancer, as well as the different leptin signaling cascades that promote lymphoma. Overall, even though there is much evidence to support the critical role of leptin in increasing the risk of disease or being associated with a worse outcome of lymphoma, further studies, especially controlled and intervention studies, are needed to finally conclude the role of leptin as a link between obesity and lymphoma.
Author Contributions
C.J.-C., L.d.l.C.-M. and V.S.-M. Conceptualization. C.J.-C. writing the original draft. All authors participated in the investigation and revision of the draft. All authors have read and agreed to the published version of the manuscript.
Funding
The work of the group is supported by PAIDI, Junta de Andalucia (CTS-151).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available at reasonable request.
Acknowledgments
C.J.-C. is supported by a Margarita Salas Fellowship, granted by the University of Seville (Seville, Spain); L.H.-P. is supported by the Consejería de Salud y Familias, Junta de Andalucía (RH-0047-2021) (Spain).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Meng, J.; Chang, C.; Pan, H.; Zhu, F.; Xiao, Y.; Liu, T.; Nie, X.; Wu, G.; Zhang, L. Epidemiologic characteristics of malignant lymphoma in Hubei, China: A single-center 5-year retrospective study. Medicine 2018, 97, e12120. [Google Scholar] [CrossRef] [PubMed]
- Pileri, S.A.; Ascani, S.; Leoncini, L.; Sabattini, E.; Zinzani, P.L.; Piccaluga, P.P.; Pileri, A., Jr.; Giunti, M.; Falini, B.; Bolis, G.B.; et al. Hodgkin’s lymphoma: The pathologist’s viewpoint. J. Clin. Pathol. 2002, 55, 162–176. [Google Scholar] [CrossRef] [PubMed]
- Kanas, G.; Ge, W.; Quek, R.G.W.; Keeven, K.; Nersesyan, K.; Jon, E.A. Epidemiology of diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma (FL) in the United States and Western Europe: Population-level projections for 2020–2025. Leuk. Lymphoma 2022, 63, 54–63. [Google Scholar] [CrossRef]
- Connors, J.M.; Cozen, W.; Steidl, C.; Carbone, A.; Hoppe, R.T.; Flechtner, H.H.; Bartlett, N.L. Hodgkin lymphoma. Nat. Rev. Dis. Prim. 2020, 6, 61. [Google Scholar] [CrossRef] [PubMed]
- Evens, A.M.; Hong, F.; Gordon, L.I.; Fisher, R.I.; Bartlett, N.L.; Connors, J.M.; Gascoyne, R.D.; Wagner, H.; Gospodarowicz, M.; Cheson, B.D.; et al. The efficacy and tolerability of adriamycin, bleomycin, vinblastine, dacarbazine and Stanford V in older Hodgkin lymphoma patients: A comprehensive analysis from the North American intergroup trial E2496. Br. J. Haematol. 2013, 161, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Hasenclever, D.; Diehl, V. A prognostic score for advanced Hodgkin’s disease. International Prognostic Factors Project on Advanced Hodgkin’s Disease. N. Engl. J. Med. 1998, 339, 1506–1514. [Google Scholar] [CrossRef]
- Miranda-Filho, A.; Pineros, M.; Znaor, A.; Marcos-Gragera, R.; Steliarova-Foucher, E.; Bray, F. Global patterns and trends in the incidence of non-Hodgkin lymphoma. Cancer Causes Control 2019, 30, 489–499. [Google Scholar] [CrossRef]
- Chiu, B.C.; Hou, N. Epidemiology and etiology of non-hodgkin lymphoma. Cancer Treat. Res. 2015, 165, 1–25. [Google Scholar] [CrossRef]
- Dave, S.S. Host factors for risk and survival in lymphoma. Hematology 2010, 2010, 255–258. [Google Scholar] [CrossRef]
- Piche, M.E.; Tchernof, A.; Despres, J.P. Obesity Phenotypes, Diabetes, and Cardiovascular Diseases. Circ. Res. 2020, 126, 1477–1500. [Google Scholar] [CrossRef]
- Kolb, R.; Sutterwala, F.S.; Zhang, W. Obesity and cancer: Inflammation bridges the two. Curr. Opin. Pharmacol. 2016, 29, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, B.D.; Goncalves, M.D.; Cantley, L.C. Obesity and Cancer Mechanisms: Cancer Metabolism. J. Clin. Oncol. 2016, 34, 4277–4283. [Google Scholar] [CrossRef] [PubMed]
- Avgerinos, K.I.; Spyrou, N.; Mantzoros, C.S.; Dalamaga, M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism 2019, 92, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Wolk, A. Obesity and risk of non-Hodgkin’s lymphoma: A meta-analysis. Int. J. Cancer 2007, 121, 1564–1570. [Google Scholar] [CrossRef] [PubMed]
- Willett, E.V.; Morton, L.M.; Hartge, P.; Becker, N.; Bernstein, L.; Boffetta, P.; Bracci, P.; Cerhan, J.; Chiu, B.C.; Cocco, P.; et al. Non-Hodgkin lymphoma and obesity: A pooled analysis from the InterLymph Consortium. Int. J. Cancer 2008, 122, 2062–2070. [Google Scholar] [CrossRef] [PubMed]
- Scheich, S.; Enssle, J.C.; Mucke, V.T.; Acker, F.; Aspacher, L.; Wolf, S.; Wilke, A.C.; Weber, S.; Brunnberg, U.; Serve, H.; et al. Obesity is associated with an impaired survival in lymphoma patients undergoing autologous stem cell transplantation. PLoS ONE 2019, 14, e0225035. [Google Scholar] [CrossRef]
- Perez-Perez, A.; Sanchez-Jimenez, F.; Vilarino-Garcia, T.; Sanchez-Margalet, V. Role of Leptin in Inflammation and Vice Versa. Int. J. Mol. Sci. 2020, 21, 5887. [Google Scholar] [CrossRef]
- Ingalls, A.M.; Dickie, M.M.; Snell, G.D. Obese, a new mutation in the house mouse. J. Hered. 1950, 41, 317–318. [Google Scholar] [CrossRef]
- Hummel, K.P.; Dickie, M.M.; Coleman, D.L. Diabetes, a new mutation in the mouse. Science 1966, 153, 1127–1128. [Google Scholar] [CrossRef]
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef]
- Tartaglia, L.A.; Dembski, M.; Weng, X.; Deng, N.; Culpepper, J.; Devos, R.; Richards, G.J.; Campfield, L.A.; Clark, F.T.; Deeds, J.; et al. Identification and expression cloning of a leptin receptor, OB-R. Cell 1995, 83, 1263–1271. [Google Scholar] [CrossRef]
- Tartaglia, L.A. The leptin receptor. J. Biol. Chem. 1997, 272, 6093–6096. [Google Scholar] [CrossRef]
- Gorska, E.; Popko, K.; Stelmaszczyk-Emmel, A.; Ciepiela, O.; Kucharska, A.; Wasik, M. Leptin receptors. Eur. J. Med. Res. 2010, 15 (Suppl. 2), 50–54. [Google Scholar] [CrossRef] [PubMed]
- Park, H.K.; Ahima, R.S. Leptin signaling. F1000Prime Rep. 2014, 6, 73. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Margalet, V.; Martin-Romero, C.; Santos-Alvarez, J.; Goberna, R.; Najib, S.; Gonzalez-Yanes, C. Role of leptin as an immunomodulator of blood mononuclear cells: Mechanisms of action. Clin. Exp. Immunol. 2003, 133, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Riejos, P.; Najib, S.; Santos-Alvarez, J.; Martin-Romero, C.; Perez-Perez, A.; Gonzalez-Yanes, C.; Sanchez-Margalet, V. Role of leptin in the activation of immune cells. Mediat. Inflamm. 2010, 2010, 568343. [Google Scholar] [CrossRef]
- Perez-Perez, A.; Vilarino-Garcia, T.; Fernandez-Riejos, P.; Martin-Gonzalez, J.; Segura-Egea, J.J.; Sanchez-Margalet, V. Role of leptin as a link between metabolism and the immune system. Cytokine Growth Factor Rev. 2017, 35, 71–84. [Google Scholar] [CrossRef]
- Deck, C.A.; Honeycutt, J.L.; Cheung, E.; Reynolds, H.M.; Borski, R.J. Assessing the Functional Role of Leptin in Energy Homeostasis and the Stress Response in Vertebrates. Front. Endocrinol. 2017, 8, 63. [Google Scholar] [CrossRef]
- Izquierdo, A.G.; Crujeiras, A.B.; Casanueva, F.F.; Carreira, M.C. Leptin, Obesity, and Leptin Resistance: Where Are We 25 Years Later? Nutrients 2019, 11, 2704. [Google Scholar] [CrossRef]
- Cojocaru, M.; Cojocaru, I.M.; Silosi, I.; Rogoz, S. Role of leptin in autoimmune diseases. Maedica 2013, 8, 68–74. [Google Scholar]
- Martin-Gonzalez, J.; Perez-Perez, A.; Sanchez-Jimenez, F.; Diaz-Parrado, E.M.; de Miguel, M.; Sanchez-Margalet, V.; Segura-Egea, J.J. Leptin promotes dentin sialophosphoprotein expression in human dental pulp. J. Endod. 2015, 41, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, J.; Farr, O.M.; Mantzoros, C.S. The role of leptin in regulating bone metabolism. Metabolism 2015, 64, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Poetsch, M.S.; Strano, A.; Guan, K. Role of Leptin in Cardiovascular Diseases. Front. Endocrinol. 2020, 11, 354. [Google Scholar] [CrossRef] [PubMed]
- Perez-Perez, A.; Sanchez-Jimenez, F.; Maymo, J.; Duenas, J.L.; Varone, C.; Sanchez-Margalet, V. Role of leptin in female reproduction. Clin. Chem Lab. Med. 2015, 53, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Perez-Perez, A.; Toro, A.; Vilarino-Garcia, T.; Maymo, J.; Guadix, P.; Duenas, J.L.; Fernandez-Sanchez, M.; Varone, C.; Sanchez-Margalet, V. Leptin action in normal and pathological pregnancies. J. Cell Mol. Med. 2018, 22, 716–727. [Google Scholar] [CrossRef]
- Perez-Perez, A.; Vilarino-Garcia, T.; Guadix, P.; Duenas, J.L.; Sanchez-Margalet, V. Leptin and Nutrition in Gestational Diabetes. Nutrients 2020, 12, 1970. [Google Scholar] [CrossRef]
- Jimenez-Cortegana, C.; Garcia-Galey, A.; Tami, M.; Del Pino, P.; Carmona, I.; Lopez, S.; Alba, G.; Sanchez-Margalet, V. Role of Leptin in Non-Alcoholic Fatty Liver Disease. Biomedicines 2021, 9, 762. [Google Scholar] [CrossRef]
- Jimenez-Cortegana, C.; Ortiz-Garcia, G.; Serrano, A.; Moreno-Ramirez, D.; Sanchez-Margalet, V. Possible Role of Leptin in Atopic Dermatitis: A Literature Review. Biomolecules 2021, 11, 1642. [Google Scholar] [CrossRef]
- Sanchez-Jimenez, F.; Perez-Perez, A.; de la Cruz-Merino, L.; Sanchez-Margalet, V. Obesity and Breast Cancer: Role of Leptin. Front. Oncol. 2019, 9, 596. [Google Scholar] [CrossRef]
- Jimenez-Cortegana, C.; Lopez-Saavedra, A.; Sanchez-Jimenez, F.; Perez-Perez, A.; Castineiras, J.; Virizuela-Echaburu, J.A.; de la Cruz-Merino, L.; Sanchez-Margalet, V. Leptin, Both Bad and Good Actor in Cancer. Biomolecules 2021, 11, 913. [Google Scholar] [CrossRef]
- Wolk, A.; Gridley, G.; Svensson, M.; Nyren, O.; McLaughlin, J.K.; Fraumeni, J.F.; Adam, H.O. A prospective study of obesity and cancer risk (Sweden). Cancer Causes Control 2001, 12, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Willett, E.V.; Roman, E. Obesity and the risk of Hodgkin lymphoma (United Kingdom). Cancer Causes Control 2006, 17, 1103–1106. [Google Scholar] [CrossRef] [PubMed]
- Keegan, T.H.; Glaser, S.L.; Clarke, C.A.; Dorfman, R.F.; Mann, R.B.; DiGiuseppe, J.A.; Chang, E.T.; Ambinder, R.F. Body size, physical activity, and risk of Hodgkin’s lymphoma in women. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1095–1101. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Reeves, G.K.; Pirie, K.; Beral, V.; Green, J.; Spencer, E.; Bull, D.; senior statistician Million Women Study Collaboration. Cancer incidence and mortality in relation to body mass index in the Million Women Study: Cohort study. BMJ 2007, 335, 1134. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Wolk, A. Body mass index and risk of non-Hodgkin’s and Hodgkin’s lymphoma: A meta-analysis of prospective studies. Eur. J. Cancer 2011, 47, 2422–2430. [Google Scholar] [CrossRef]
- Hidayat, K.; Li, H.J.; Shi, B.M. Anthropometric factors and non-Hodgkin’s lymphoma risk: Systematic review and meta-analysis of prospective studies. Crit. Rev. Oncol. Hematol. 2018, 129, 113–123. [Google Scholar] [CrossRef]
- Psaltopoulou, T.; Sergentanis, T.N.; Ntanasis-Stathopoulos, I.; Tzanninis, I.G.; Riza, E.; Dimopoulos, M.A. Anthropometric characteristics, physical activity and risk of hematological malignancies: A systematic review and meta-analysis of cohort studies. Int. J. Cancer 2019, 145, 347–359. [Google Scholar] [CrossRef]
- Moller, H.; Mellemgaard, A.; Lindvig, K.; Olsen, J.H. Obesity and cancer risk: A Danish record-linkage study. Eur. J. Cancer 1994, 30, 344–350. [Google Scholar] [CrossRef]
- Engeland, A.; Tretli, S.; Hansen, S.; Bjorge, T. Height and body mass index and risk of lymphohematopoietic malignancies in two million Norwegian men and women. Am. J. Epidemiol. 2007, 165, 44–52. [Google Scholar] [CrossRef]
- Lim, U.; Morton, L.M.; Subar, A.F.; Baris, D.; Stolzenberg-Solomon, R.; Leitzmann, M.; Kipnis, V.; Mouw, T.; Carroll, L.; Schatzkin, A.; et al. Alcohol, smoking, and body size in relation to incident Hodgkin’s and non-Hodgkin’s lymphoma risk. Am. J. Epidemiol. 2007, 166, 697–708. [Google Scholar] [CrossRef]
- Ingham, R.R.; Reagan, J.L.; Dalia, S.; Furman, M.; Merhi, B.; Nemr, S.; Zarrabi, A.; Mitri, J.; Castillo, J.J. The Relationship Between Obesity and Lymphoma: A Meta-Analysis of Prospective Cohort Studies. Blood 2011, 118, 5198. [Google Scholar] [CrossRef]
- Paffenbarger, R.S., Jr.; Wing, A.L.; Hyde, R.T. Characteristics in youth indicative of adult-onset Hodgkin’s disease. J. Natl. Cancer Inst. 1977, 58, 1489–1491. [Google Scholar] [CrossRef]
- Paffenbarger, R.S., Jr.; Wing, A.L.; Hyde, R.T. Characteristics in youth predictive of adult-onset malignant lymphomas, melanomas, and leukemias: Brief communication. J. Natl. Cancer Inst. 1978, 60, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Strongman, H.; Brown, A.; Smeeth, L.; Bhaskaran, K. Body mass index and Hodgkin’s lymphoma: UK population-based cohort study of 5.8 million individuals. Br. J. Cancer 2019, 120, 768–770. [Google Scholar] [CrossRef] [PubMed]
- Fernberg, P.; Odenbro, A.; Bellocco, R.; Boffetta, P.; Pawitan, Y.; Adami, J. Tobacco use, body mass index and the risk of malignant lymphomas--a nationwide cohort study in Sweden. Int. J. Cancer 2006, 118, 2298–2302. [Google Scholar] [CrossRef]
- Pan, S.Y.; Mao, Y.; Ugnat, A.M.; the Canadian Cancer Registries Epidemiology Research Group. Physical activity, obesity, energy intake, and the risk of non-Hodgkin’s lymphoma: A population-based case-control study. Am. J. Epidemiol. 2005, 162, 1162–1173. [Google Scholar] [CrossRef] [PubMed]
- Chiu, B.C.; Soni, L.; Gapstur, S.M.; Fought, A.J.; Evens, A.M.; Weisenburger, D.D. Obesity and risk of non-Hodgkin lymphoma (United States). Cancer Causes Control 2007, 18, 677–685. [Google Scholar] [CrossRef]
- Rapp, K.; Schroeder, J.; Klenk, J.; Stoehr, S.; Ulmer, H.; Concin, H.; Diem, G.; Oberaigner, W.; Weiland, S.K. Obesity and incidence of cancer: A large cohort study of over 145,000 adults in Austria. Br. J. Cancer 2005, 93, 1062–1067. [Google Scholar] [CrossRef]
- Hidayat, K.; Du, X.; Shi, B.M. Body fatness at a young age and risks of eight types of cancer: Systematic review and meta-analysis of observational studies. Obes. Rev. 2018, 19, 1385–1394. [Google Scholar] [CrossRef]
- Pan, S.Y.; Johnson, K.C.; Ugnat, A.M.; Wen, S.W.; Mao, Y.; Canadian Cancer Registries Epidemiology Research Group. Association of obesity and cancer risk in Canada. Am. J. Epidemiol. 2004, 159, 259–268. [Google Scholar] [CrossRef]
- Holly, E.A.; Lele, C.; Bracci, P.M.; McGrath, M.S. Case-control study of non-Hodgkin’s lymphoma among women and heterosexual men in the San Francisco Bay Area, California. Am. J. Epidemiol. 1999, 150, 375–389. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.W.; Yoon, Y.S.; Shin, S.A. Effects of excess weight on cancer incidences depending on cancer sites and histologic findings among men: Korea National Health Insurance Corporation Study. J. Clin. Oncol. 2005, 23, 4742–4754. [Google Scholar] [CrossRef]
- Kelta, M.; Zekri, J.; Abdelghany, E.; Rehman, J.U.; Khan, Z.A.; Al-Saadi, R.; Dada, R. High-dose chemotherapy and peripheral hematopoietic stem cell transplantation in relapsed/refractory Hodgkin’s lymphoma. Tumori J. 2018, 104, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Dahi, P.B.; Lee, J.; Devlin, S.M.; Ruiz, J.; Maloy, M.; Rondon-Clavo, C.; Petrlik, E.; Tamari, R.; Shah, G.; Scordo, M.; et al. Toxicities of high-dose chemotherapy and autologous hematopoietic cell transplantation in older patients with lymphoma. Blood Adv. 2021, 5, 2608–2618. [Google Scholar] [CrossRef] [PubMed]
- Leiba, M.; Leiba, A.; Keinan-Boker, L.; Avigdor, A.; Derazne, E.; Levine, H.; Kark, J.D. Adolescent weight and height are predictors of specific non-Hodgkin lymphoma subtypes among a cohort of 2,352,988 individuals aged 16 to 19 years. Cancer 2016, 122, 1068–1077. [Google Scholar] [CrossRef] [PubMed]
- Goy, A.; Ramchandren, R.; Ghosh, N.; Munoz, J.; Morgan, D.S.; Dang, N.H.; Knapp, M.; Delioukina, M.; Kingsley, E.; Ping, J.; et al. Ibrutinib plus lenalidomide and rituximab has promising activity in relapsed/refractory non-germinal center B-cell-like DLBCL. Blood 2019, 134, 1024–1036. [Google Scholar] [CrossRef] [PubMed]
- Sehn, L.H.; Herrera, A.F.; Flowers, C.R.; Kamdar, M.K.; McMillan, A.; Hertzberg, M.; Assouline, S.; Kim, T.M.; Kim, W.S.; Ozcan, M.; et al. Polatuzumab Vedotin in Relapsed or Refractory Diffuse Large B-Cell Lymphoma. J. Clin. Oncol. 2020, 38, 155–165. [Google Scholar] [CrossRef]
- Jimenez-Cortegana, C.; Palazon-Carrion, N.; Martin Garcia-Sancho, A.; Nogales-Fernandez, E.; Carnicero-Gonzalez, F.; Rios-Herranz, E.; de la Cruz-Vicente, F.; Rodriguez-Garcia, G.; Fernandez-Alvarez, R.; Rueda Dominguez, A.; et al. Circulating myeloid-derived suppressor cells and regulatory T cells as immunological biomarkers in refractory/relapsed diffuse large B-cell lymphoma: Translational results from the R2-GDP-GOTEL trial. J. Immunother. Cancer 2021, 9, e002323. [Google Scholar] [CrossRef]
- Colditz, G.A.; Peterson, L.L. Obesity and Cancer: Evidence, Impact, and Future Directions. Clin. Chem. 2018, 64, 154–162. [Google Scholar] [CrossRef]
- Lauby-Secretan, B.; Dossus, L.; Marant-Micallef, C.; His, M. Obesity and Cancer. Bull. Cancer 2019, 106, 635–646. [Google Scholar] [CrossRef]
- Skibola, C.F.; Holly, E.A.; Forrest, M.S.; Hubbard, A.; Bracci, P.M.; Skibola, D.R.; Hegedus, C.; Smith, M.T. Body mass index, leptin and leptin receptor polymorphisms, and non-hodgkin lymphoma. Cancer Epidemiol. Biomark. Prev. 2004, 13, 779–786. [Google Scholar] [CrossRef]
- Cerhan, J.R.; Bernstein, L.; Severson, R.K.; Davis, S.; Colt, J.S.; Blair, A.; Hartge, P. Anthropometrics, physical activity, related medical conditions, and the risk of non-hodgkin lymphoma. Cancer Causes Control 2005, 16, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Kane, E.; Skibola, C.F.; Bracci, P.M.; Cerhan, J.R.; Costas, L.; Smedby, K.E.; Holly, E.A.; Maynadie, M.; Novak, A.J.; Lightfoot, T.J.; et al. Non-Hodgkin Lymphoma, Body Mass Index, and Cytokine Polymorphisms: A Pooled Analysis from the InterLymph Consortium. Cancer Epidemiol. Biomark. Prev. 2015, 24, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.T.; Hjalgrim, H.; Smedby, K.E.; Akerman, M.; Tani, E.; Johnsen, H.E.; Glimelius, B.; Adami, H.O.; Melbye, M. Body mass index and risk of malignant lymphoma in Scandinavian men and women. J. Natl. Cancer Inst. 2005, 97, 210–218. [Google Scholar] [CrossRef][Green Version]
- Bowzyk Al-Naeeb, A.; Ajithkumar, T.; Behan, S.; Hodson, D.J. Non-Hodgkin lymphoma. BMJ 2018, 362, k3204. [Google Scholar] [CrossRef]
- Cerhan, J.R.; Janney, C.A.; Vachon, C.M.; Habermann, T.M.; Kay, N.E.; Potter, J.D.; Sellers, T.A.; Folsom, A.R. Anthropometric characteristics, physical activity, and risk of non-Hodgkin’s lymphoma subtypes and B-cell chronic lymphocytic leukemia: A prospective study. Am. J. Epidemiol. 2002, 156, 527–535. [Google Scholar] [CrossRef]
- Boyle, T.; Connors, J.M.; Gascoyne, R.D.; Berry, B.R.; Sehn, L.H.; Bashash, M.; Spinelli, J.J. Physical activity, obesity and survival in diffuse large B-cell and follicular lymphoma cases. Br. J. Haematol. 2017, 178, 442–447. [Google Scholar] [CrossRef]
- Basirat, M.; Rabiei, M.; Bashardoust, N. Incidence of Head and Neck Lymphoma in Guilan Province, Iran. Asian Pac. J. Cancer Prev. 2016, 17, 1–4. [Google Scholar] [CrossRef]
- Lukanova, A.; Bjor, O.; Kaaks, R.; Lenner, P.; Lindahl, B.; Hallmans, G.; Stattin, P. Body mass index and cancer: Results from the Northern Sweden Health and Disease Cohort. Int. J. Cancer 2006, 118, 458–466. [Google Scholar] [CrossRef]
- Willett, E.V.; Skibola, C.F.; Adamson, P.; Skibola, D.R.; Morgan, G.J.; Smith, M.T.; Roman, E. Non-Hodgkin’s lymphoma, obesity and energy homeostasis polymorphisms. Br. J. Cancer 2005, 93, 811–816. [Google Scholar] [CrossRef]
- Horwich, T.B.; Fonarow, G.C.; Clark, A.L. Obesity and the Obesity Paradox in Heart Failure. Prog. Cardiovasc. Dis. 2018, 61, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Lavie, C.J.; Milani, R.V.; Ventura, H.O. Obesity and cardiovascular disease: Risk factor, paradox, and impact of weight loss. J. Am. Coll. Cardiol. 2009, 53, 1925–1932. [Google Scholar] [CrossRef] [PubMed]
- Carbone, S.; Canada, J.M.; Billingsley, H.E.; Siddiqui, M.S.; Elagizi, A.; Lavie, C.J. Obesity paradox in cardiovascular disease: Where do we stand? Vasc. Health Risk Manag. 2019, 15, 89–100. [Google Scholar] [CrossRef]
- Naik, A.; Monjazeb, A.M.; Decock, J. The Obesity Paradox in Cancer, Tumor Immunology, and Immunotherapy: Potential Therapeutic Implications in Triple Negative Breast Cancer. Front. Immunol. 2019, 10, 1940. [Google Scholar] [CrossRef]
- Lennon, H.; Sperrin, M.; Badrick, E.; Renehan, A.G. The Obesity Paradox in Cancer: A Review. Curr. Oncol. Rep. 2016, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Giovannucci, E.L. The Obesity Paradox in Cancer: Epidemiologic Insights and Perspectives. Curr. Nutr. Rep. 2019, 8, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Cespedes Feliciano, E.M.; Kroenke, C.H.; Caan, B.J. The Obesity Paradox in Cancer: How Important Is Muscle? Annu. Rev. Nutr. 2018, 38, 357–379. [Google Scholar] [CrossRef] [PubMed]
- Hagstrom, H.; Andreasson, A.; Carlsson, A.C.; Jerkeman, M.; Carlsten, M. Body composition measurements and risk of hematological malignancies: A population-based cohort study during 20 years of follow-up. PLoS ONE 2018, 13, e0202651. [Google Scholar] [CrossRef]
- Barberio, A.M.; Alareeki, A.; Viner, B.; Pader, J.; Vena, J.E.; Arora, P.; Friedenreich, C.M.; Brenner, D.R. Central body fatness is a stronger predictor of cancer risk than overall body size. Nat. Commun. 2019, 10, 1–12. [Google Scholar] [CrossRef]
- Matos, A.; Marinho-Dias, J.; Ramalheira, S.; Oliveira, M.J.; Bicho, M.; Ribeiro, R. Mechanisms underlying the association between obesity and Hodgkin lymphoma. Tumour Biol. 2016, 37, 13005–13016. [Google Scholar] [CrossRef]
- Hosgood, H.D.; Gunter, M.J.; Murphy, N.; Rohan, T.E.; Strickler, H.D. The Relation of Obesity-Related Hormonal and Cytokine Levels With Multiple Myeloma and Non-Hodgkin Lymphoma. Front. Oncol. 2018, 8, 103. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, H.; Haugen, F.; Zadelaar, S.; Kleemann, R.; Kooistra, T.; Drevon, C.A.; Blomhoff, R. Diet-induced obesity increases NF-kappaB signaling in reporter mice. Genes Nutr. 2009, 4, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Tan, S.; Zhou, Y.; Lin, J.; Wang, H.; Oyang, L.; Tian, Y.; Liu, L.; Su, M.; Wang, H.; et al. Role of the NFkappaB-signaling pathway in cancer. OncoTargets Ther. 2018, 11, 2063–2073. [Google Scholar] [CrossRef] [PubMed]
- Jost, P.J.; Ruland, J. Aberrant NF-kappaB signaling in lymphoma: Mechanisms, consequences, and therapeutic implications. Blood 2007, 109, 2700–2707. [Google Scholar] [CrossRef] [PubMed]
- Nagel, D.; Vincendeau, M.; Eitelhuber, A.C.; Krappmann, D. Mechanisms and consequences of constitutive NF-kappaB activation in B-cell lymphoid malignancies. Oncogene 2014, 33, 5655–5665. [Google Scholar] [CrossRef] [PubMed]
- Balaji, S.; Ahmed, M.; Lorence, E.; Yan, F.; Nomie, K.; Wang, M. NF-kappaB signaling and its relevance to the treatment of mantle cell lymphoma. J. Hematol. Oncol. 2018, 11, 83. [Google Scholar] [CrossRef]
- Rauch, D.A.; Harding, J.C.; Ratner, L.; Wickline, S.A.; Pan, H. Targeting NF-kappaB with Nanotherapy in a Mouse Model of Adult T-Cell Leukemia/Lymphoma. Nanomaterials 2021, 11, 1582. [Google Scholar] [CrossRef] [PubMed]
- Eder, K.; Baffy, N.; Falus, A.; Fulop, A.K. The major inflammatory mediator interleukin-6 and obesity. Inflamm. Res. 2009, 58, 727–736. [Google Scholar] [CrossRef]
- Ghosh, S.; Ashcraft, K. An IL-6 link between obesity and cancer. Front. Biosci. 2013, 5, 461–478. [Google Scholar] [CrossRef]
- Burger, R. Impact of interleukin-6 in hematological malignancies. Transfus. Med. Hemotherapy 2013, 40, 336–343. [Google Scholar] [CrossRef]
- Voorzanger, N.; Touitou, R.; Garcia, E.; Delecluse, H.J.; Rousset, F.; Joab, I.; Favrot, M.C.; Blay, J.Y. Interleukin (IL)-10 and IL-6 are produced in vivo by non-Hodgkin’s lymphoma cells and act as cooperative growth factors. Cancer Res. 1996, 56, 5499–5505. [Google Scholar] [PubMed]
- Zhang, L.; Yang, J.; Qian, J.; Li, H.; Romaguera, J.E.; Kwak, L.W.; Wang, M.; Yi, Q. Role of the microenvironment in mantle cell lymphoma: IL-6 is an important survival factor for the tumor cells. Blood 2012, 120, 3783–3792. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, W.S.; Park, C. Interleukin-6 mediates resistance to PI3K-pathway-targeted therapy in lymphoma. BMC Cancer 2019, 19, 936. [Google Scholar] [CrossRef]
- Hashwah, H.; Bertram, K.; Stirm, K.; Stelling, A.; Wu, C.T.; Kasser, S.; Manz, M.G.; Theocharides, A.P.; Tzankov, A.; Muller, A. The IL-6 signaling complex is a critical driver, negative prognostic factor, and therapeutic target in diffuse large B-cell lymphoma. EMBO Mol. Med. 2019, 11, e10576. [Google Scholar] [CrossRef] [PubMed]
- Asou, H.; Said, J.W.; Yang, R.; Munker, R.; Park, D.J.; Kamada, N.; Koeffler, H.P. Mechanisms of growth control of Kaposi’s sarcoma-associated herpes virus-associated primary effusion lymphoma cells. Blood 1998, 91, 2475–2481. [Google Scholar] [CrossRef]
- Blaszczak, A.M.; Jalilvand, A.; Hsueh, W.A. Adipocytes, Innate Immunity and Obesity: A Mini-Review. Front. Immunol. 2021, 12, 650768. [Google Scholar] [CrossRef]
- Kim, C.S.; Park, H.S.; Kawada, T.; Kim, J.H.; Lim, D.; Hubbard, N.E.; Kwon, B.S.; Erickson, K.L.; Yu, R. Circulating levels of MCP-1 and IL-8 are elevated in human obese subjects and associated with obesity-related parameters. Int. J. Obes. 2006, 30, 1347–1355. [Google Scholar] [CrossRef]
- Miyata-Takata, T.; Takata, K.; Toji, T.; Goto, N.; Kasahara, S.; Takahashi, T.; Tari, A.; Noujima-Harada, M.; Miyata, T.; Sato, Y.; et al. Elevation of serum interleukins 8, 4, and 1beta levels in patients with gastrointestinal low-grade B-cell lymphoma. Sci. Rep. 2015, 5, 18434. [Google Scholar] [CrossRef]
- Manfroi, B.; McKee, T.; Mayol, J.F.; Tabruyn, S.; Moret, S.; Villiers, C.; Righini, C.; Dyer, M.; Callanan, M.; Schneider, P.; et al. CXCL-8/IL8 Produced by Diffuse Large B-cell Lymphomas Recruits Neutrophils Expressing a Proliferation-Inducing Ligand APRIL. Cancer Res. 2017, 77, 1097–1107. [Google Scholar] [CrossRef]
- Abreu, M.; Miranda, M.; Castro, M.; Fernandes, I.; Cabral, R.; Santos, A.H.; Fonseca, S.; Rodrigues, J.; Leander, M.; Lau, C.; et al. IL-31 and IL-8 in Cutaneous T-Cell Lymphoma: Looking for Their Role in Itch. Adv. Hematol. 2021, 2021, 5582581. [Google Scholar] [CrossRef]
- Xu, H.; Lai, W.; Zhang, Y.; Liu, L.; Luo, X.; Zeng, Y.; Wu, H.; Lan, Q.; Chu, Z. Tumor-associated macrophage-derived IL-6 and IL-8 enhance invasive activity of LoVo cells induced by PRL-3 in a KCNN4 channel-dependent manner. BMC Cancer 2014, 14, 330. [Google Scholar] [CrossRef]
- Tobin, R.P.; Jordan, K.R.; Kapoor, P.; Spongberg, E.; Davis, D.; Vorwald, V.M.; Couts, K.L.; Gao, D.; Smith, D.E.; Borgers, J.S.W.; et al. IL-6 and IL-8 Are Linked With Myeloid-Derived Suppressor Cell Accumulation and Correlate With Poor Clinical Outcomes in Melanoma Patients. Front. Oncol. 2019, 9, 1223. [Google Scholar] [CrossRef] [PubMed]
- Betsch, A.; Rutgeerts, O.; Fevery, S.; Sprangers, B.; Verhoef, G.; Dierickx, D.; Beckers, M. Myeloid-derived suppressor cells in lymphoma: The good, the bad and the ugly. Blood Rev. 2018, 32, 490–498. [Google Scholar] [CrossRef]
- Dutta, P.; Sarkissyan, M.; Paico, K.; Wu, Y.; Vadgama, J.V. MCP-1 is overexpressed in triple-negative breast cancers and drives cancer invasiveness and metastasis. Breast Cancer Res. Treat. 2018, 170, 477–486. [Google Scholar] [CrossRef]
- Husson, H.; Carideo, E.G.; Cardoso, A.A.; Lugli, S.M.; Neuberg, D.; Munoz, O.; de Leval, L.; Schultze, J.; Freedman, A.S. MCP-1 modulates chemotaxis by follicular lymphoma cells. Br. J. Haematol. 2001, 115, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Shi, Z.H.; Wang, X.; Gu, K.S.; Zhai, Z.M. Prognostic significance of monocyte chemoattractant protein-1 and CC chemokine receptor 2 in diffuse large B cell lymphoma. Ann. Hematol. 2019, 98, 413–422. [Google Scholar] [CrossRef]
- Kitai, R.; Ishisaka, K.; Sato, K.; Sakuma, T.; Yamauchi, T.; Imamura, Y.; Matsumoto, H.; Kubota, T. Primary central nervous system lymphoma secretes monocyte chemoattractant protein 1. Med. Mol. Morphol. 2007, 40, 18–22. [Google Scholar] [CrossRef]
- Ghanbari, M.; Momen Maragheh, S.; Aghazadeh, A.; Mehrjuyan, S.R.; Hussen, B.M.; Abdoli Shadbad, M.; Dastmalchi, N.; Safaralizadeh, R. Interleukin-1 in obesity-related low-grade inflammation: From molecular mechanisms to therapeutic strategies. Int. Immunopharmacol. 2021, 96, 107765. [Google Scholar] [CrossRef] [PubMed]
- Gelfo, V.; Romaniello, D.; Mazzeschi, M.; Sgarzi, M.; Grilli, G.; Morselli, A.; Manzan, B.; Rihawi, K.; Lauriola, M. Roles of IL-1 in Cancer: From Tumor Progression to Resistance to Targeted Therapies. Int. J. Mol. Sci. 2020, 21, 6009. [Google Scholar] [CrossRef] [PubMed]
- Oelmann, E.; Stein, H.; Berdel, W.E.; Herbst, H. Expression of Interleukin-1 and Interleukin-1 Receptors Type 1 and Type 2 in Hodgkin Lymphoma. PLoS ONE 2015, 10, e0138747. [Google Scholar] [CrossRef][Green Version]
- Baker, K.J.; Houston, A.; Brint, E. IL-1 Family Members in Cancer; Two Sides to Every Story. Front. Immunol. 2019, 10, 1197. [Google Scholar] [CrossRef] [PubMed]
- Gottschlich, A.; Endres, S.; Kobold, S. Therapeutic Strategies for Targeting IL-1 in Cancer. Cancers 2021, 13, 477. [Google Scholar] [CrossRef]
- Tzanavari, T.; Giannogonas, P.; Karalis, K.P. TNF-alpha and obesity. Curr. Dir. Autoimmun 2010, 11, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lin, Y. Tumor necrosis factor and cancer, buddies or foes? Acta Pharmacol. Sin. 2008, 29, 1275–1288. [Google Scholar] [CrossRef]
- Balkwill, F. Tumour necrosis factor and cancer. Nat. Rev. Cancer 2009, 9, 361–371. [Google Scholar] [CrossRef]
- Montfort, A.; Colacios, C.; Levade, T.; Andrieu-Abadie, N.; Meyer, N.; Segui, B. The TNF Paradox in Cancer Progression and Immunotherapy. Front. Immunol. 2019, 10, 1818. [Google Scholar] [CrossRef] [PubMed]
- Dahmus, J.; Rosario, M.; Clarke, K. Risk of Lymphoma Associated with Anti-TNF Therapy in Patients with Inflammatory Bowel Disease: Implications for Therapy. Clin. Exp. Gastroenterol. 2020, 13, 339–350. [Google Scholar] [CrossRef]
- Calip, G.S.; Patel, P.R.; Adimadhyam, S.; Xing, S.; Wu, Z.; Sweiss, K.; Schumock, G.T.; Lee, T.A.; Chiu, B.C. Tumor necrosis factor-alpha inhibitors and risk of non-Hodgkin lymphoma in a cohort of adults with rheumatologic conditions. Int. J. Cancer 2018, 143, 1062–1071. [Google Scholar] [CrossRef]
- Song, W.K.; Cho, A.R.; Yoon, Y.H. Highly suspected primary intraocular lymphoma in a patient with rheumatoid arthritis treated with etanercept: A case report. BMC Ophthalmol. 2018, 18, 156. [Google Scholar] [CrossRef]
- van de Woestijne, A.P.; Monajemi, H.; Kalkhoven, E.; Visseren, F.L. Adipose tissue dysfunction and hypertriglyceridemia: Mechanisms and management. Obes. Rev. 2011, 12, 829–840. [Google Scholar] [CrossRef]
- Gastaldelli, A.; Gaggini, M.; DeFronzo, R.A. Role of Adipose Tissue Insulin Resistance in the Natural History of Type 2 Diabetes: Results From the San Antonio Metabolism Study. Diabetes 2017, 66, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Margalet, V.; Martin-Romero, C. Human leptin signaling in human peripheral blood mononuclear cells: Activation of the JAK-STAT pathway. Cell Immunol. 2001, 211, 30–36. [Google Scholar] [CrossRef]
- Martin-Romero, C.; Sanchez-Margalet, V. Human leptin activates PI3K and MAPK pathways in human peripheral blood mononuclear cells: Possible role of Sam68. Cell Immunol. 2001, 212, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, A.; Saeidi, J.; Azimi-Nejad, M.; Hashemy, S.I. Leptin-induced signaling pathways in cancer cell migration and invasion. Cell Oncol. 2019, 42, 243–260. [Google Scholar] [CrossRef]
- Ding, B.B.; Yu, J.J.; Yu, R.Y.; Mendez, L.M.; Shaknovich, R.; Zhang, Y.; Cattoretti, G.; Ye, B.H. Constitutively activated STAT3 promotes cell proliferation and survival in the activated B-cell subtype of diffuse large B-cell lymphomas. Blood 2008, 111, 1515–1523. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Wang, K.B.; Rui, L. STAT3 Activation and Oncogenesis in Lymphoma. Cancers 2019, 12, 19. [Google Scholar] [CrossRef] [PubMed]
- Krishnadasan, R.; Bifulco, C.; Kim, J.; Rodov, S.; Zieske, A.W.; Vanasse, G.J. Overexpression of SOCS3 is associated with decreased survival in a cohort of patients with de novo follicular lymphoma. Br. J. Haematol. 2006, 135, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Cho-Vega, J.H.; Rassidakis, G.Z.; Amin, H.M.; Tsioli, P.; Spurgers, K.; Remache, Y.K.; Vega, F.; Goy, A.H.; Gilles, F.; Medeiros, L.J. Suppressor of cytokine signaling 3 expression in anaplastic large cell lymphoma. Leukemia 2004, 18, 1872–1878. [Google Scholar] [CrossRef]
- Maurer, B.; Nivarthi, H.; Wingelhofer, B.; Pham, H.T.T.; Schlederer, M.; Suske, T.; Grausenburger, R.; Schiefer, A.I.; Prchal-Murphy, M.; Chen, D.; et al. High activation of STAT5A drives peripheral T-cell lymphoma and leukemia. Haematologica 2020, 105, 435–447. [Google Scholar] [CrossRef]
- Kucuk, C.; Jiang, B.; Hu, X.; Zhang, W.; Chan, J.K.; Xiao, W.; Lack, N.; Alkan, C.; Williams, J.C.; Avery, K.N.; et al. Activating mutations of STAT5B and STAT3 in lymphomas derived from gammadelta-T or NK cells. Nat. Commun. 2015, 6, 1–12. [Google Scholar] [CrossRef]
- Martini, M.; Hohaus, S.; Petrucci, G.; Cenci, T.; Pierconti, F.; Massini, G.; Teofili, L.; Leone, G.; Larocca, L.M. Phosphorylated STAT5 represents a new possible prognostic marker in Hodgkin lymphoma. Am. J. Clin. Pathol. 2008, 129, 472–477. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Scheeren, F.A.; Diehl, S.A.; Smit, L.A.; Beaumont, T.; Naspetti, M.; Bende, R.J.; Blom, B.; Karube, K.; Ohshima, K.; van Noesel, C.J.; et al. IL-21 is expressed in Hodgkin lymphoma and activates STAT5: Evidence that activated STAT5 is required for Hodgkin lymphomagenesis. Blood 2008, 111, 4706–4715. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.A.; Spolski, R.; Kovanen, P.E.; Suzuki, T.; Bollenbacher, J.; Pise-Masison, C.A.; Radonovich, M.F.; Lee, S.; Jenkins, N.A.; Copeland, N.G.; et al. Stat5 synergizes with T cell receptor/antigen stimulation in the development of lymphoblastic lymphoma. J. Exp. Med. 2003, 198, 79–89. [Google Scholar] [CrossRef]
- Shipp, M.A.; Ross, K.N.; Tamayo, P.; Weng, A.P.; Kutok, J.L.; Aguiar, R.C.; Gaasenbeek, M.; Angelo, M.; Reich, M.; Pinkus, G.S.; et al. Diffuse large B-cell lymphoma outcome prediction by gene-expression profiling and supervised machine learning. Nat. Med. 2002, 8, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Voena, C.; Conte, C.; Ambrogio, C.; Boeri Erba, E.; Boccalatte, F.; Mohammed, S.; Jensen, O.N.; Palestro, G.; Inghirami, G.; Chiarle, R. The tyrosine phosphatase Shp2 interacts with NPM-ALK and regulates anaplastic lymphoma cell growth and migration. Cancer Res. 2007, 67, 4278–4286. [Google Scholar] [CrossRef]
- Karaca Atabay, E.; Mecca, C.; Wang, Q.; Ambrogio, C.; Mota, I.; Prokoph, N.; Mura, G.; Martinengo, C.; Patrucco, E.; Leonardi, G.; et al. Tyrosine phosphatases regulate resistance to ALK inhibitors in ALK+ anaplastic large cell lymphoma. Blood 2022, 139, 717–731. [Google Scholar] [CrossRef]
- Vega, G.G.; Aviles-Salas, A.; Chalapud, J.R.; Martinez-Paniagua, M.; Pelayo, R.; Mayani, H.; Hernandez-Pando, R.; Martinez-Maza, O.; Huerta-Yepez, S.; Bonavida, B.; et al. P38 MAPK expression and activation predicts failure of response to CHOP in patients with Diffuse Large B-Cell Lymphoma. BMC Cancer 2015, 15, 722. [Google Scholar] [CrossRef] [PubMed]
- Louissaint, A., Jr.; Schafernak, K.T.; Geyer, J.T.; Kovach, A.E.; Ghandi, M.; Gratzinger, D.; Roth, C.G.; Paxton, C.N.; Kim, S.; Namgyal, C.; et al. Pediatric-type nodal follicular lymphoma: A biologically distinct lymphoma with frequent MAPK pathway mutations. Blood 2016, 128, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Ramis-Zaldivar, J.E.; Gonzalez-Farre, B.; Nicolae, A.; Pack, S.; Clot, G.; Nadeu, F.; Mottok, A.; Horn, H.; Song, J.Y.; Fu, K.; et al. MAPK and JAK-STAT pathways dysregulation in plasmablastic lymphoma. Haematologica 2021, 106, 2682–2693. [Google Scholar] [CrossRef]
- Shaw, L.M. The insulin receptor substrate (IRS) proteins: At the intersection of metabolism and cancer. Cell Cycle 2011, 10, 1750–1756. [Google Scholar] [CrossRef]
- Kuo, A.H.; Stoica, G.E.; Riegel, A.T.; Wellstein, A. Recruitment of insulin receptor substrate-1 and activation of NF-kappaB essential for midkine growth signaling through anaplastic lymphoma kinase. Oncogene 2007, 26, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Urso, B.; Ilondo, M.M.; Holst, P.A.; Christoffersen, C.T.; Ouwens, M.; Giorgetti, S.; Van Obberghen, E.; Naor, D.; Tornqvist, H.; De Meyts, P. IRS-4 mediated mitogenic signalling by insulin and growth hormone in LB cells, a murine T-cell lymphoma devoid of IGF-I receptors. Cell. Signal. 2003, 15, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Pongas, G.; Cheson, B.D. PI3K signaling pathway in normal B cells and indolent B-cell malignancies. Semin. Oncol. 2016, 43, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Cai, Y.; Wang, W.; Liu, Z.; Wei, P.; Bi, R.; Chen, W.; Sun, M.; Zhou, X. Frequent copy number variations of PI3K/AKT pathway and aberrant protein expressions of PI3K subunits are associated with inferior survival in diffuse large B cell lymphoma. J. Transl. Med. 2014, 12, 10. [Google Scholar] [CrossRef]
- Iyengar, S.; Clear, A.; Bodor, C.; Maharaj, L.; Lee, A.; Calaminici, M.; Matthews, J.; Iqbal, S.; Auer, R.; Gribben, J.; et al. P110alpha-mediated constitutive PI3K signaling limits the efficacy of p110delta-selective inhibition in mantle cell lymphoma, particularly with multiple relapse. Blood 2013, 121, 2274–2284. [Google Scholar] [CrossRef]
- Shah, A.; Barrientos, J.C. Oral PI3K-delta, gamma Inhibitor for the Management of People with Chronic Lymphocytic Leukemia and Small Lymphocytic Lymphoma: A Narrative Review on Duvelisib. OncoTargets Ther. 2021, 14, 2109–2119. [Google Scholar] [CrossRef]
- Huang, D.; Song, T.L.; Nairismagi, M.L.; Laurensia, Y.; Pang, W.L.; Zhe, D.C.M.; Wong, E.K.Y.; Wijaya, G.G.; Tan, J.; Tan, S.H.; et al. Evaluation of the PIK3 pathway in peripheral T-cell lymphoma and NK/T-cell lymphoma. Br. J. Haematol. 2020, 189, 731–744. [Google Scholar] [CrossRef]
- Raso, G.M.; Pacilio, M.; Esposito, E.; Coppola, A.; Di Carlo, R.; Meli, R. Leptin potentiates IFN-gamma-induced expression of nitric oxide synthase and cyclo-oxygenase-2 in murine macrophage J774A.1. Br. J. Pharmacol. 2002, 137, 799–804. [Google Scholar] [CrossRef]
- Conde, J.; Scotece, M.; Lopez, V.; Gomez, R.; Lago, F.; Pino, J.; Gomez-Reino, J.J.; Gualillo, O. Adiponectin and leptin induce VCAM-1 expression in human and murine chondrocytes. PLoS ONE 2012, 7, e52533. [Google Scholar] [CrossRef]
- Suzukawa, M.; Koketsu, R.; Baba, S.; Igarashi, S.; Nagase, H.; Yamaguchi, M.; Matsutani, N.; Kawamura, M.; Shoji, S.; Hebisawa, A.; et al. Leptin enhances ICAM-1 expression, induces migration and cytokine synthesis, and prolongs survival of human airway epithelial cells. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2015, 309, L801-811. [Google Scholar] [CrossRef]
- Terol, M.J.; Lopez-Guillermo, A.; Bosch, F.; Villamor, N.; Cid, M.C.; Rozman, C.; Campo, E.; Montserrat, E. Expression of the adhesion molecule ICAM-1 in non-Hodgkin’s lymphoma: Relationship with tumor dissemination and prognostic importance. J. Clin. Oncol. 1998, 16, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Jacob, M.C.; Agrawal, S.; Chaperot, L.; Giroux, C.; Gressin, R.; Le Marc’Hadour, F.; Favre, M.; Sotto, J.J.; Bensa, J.C.; Plumas, J. Quantification of cellular adhesion molecules on malignant B cells from non-Hodgkin’s lymphoma. Leukemia 1999, 13, 1428–1433. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, Y.; Gu, J.J.; Yang, L.; Tsai, P.C.; Guo, Y.; Xue, K.; Xia, Z.; Liu, X.; Lv, F.; Cao, J.; et al. The adhesion molecule ICAM-1 in diffuse large B-cell lymphoma post-rituximab era: Relationship with prognostic importance and rituximab resistance. Aging 2020, 13, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Syrigos, K.N.; Salgami, E.; Karayiannakis, A.J.; Katirtzoglou, N.; Sekara, E.; Roussou, P. Prognostic significance of soluble adhesion molecules in Hodgkin’s disease. Anticancer Res. 2004, 24, 1243–1247. [Google Scholar] [PubMed]
- Agrawal, S.; Gollapudi, S.; Su, H.; Gupta, S. Leptin activates human B cells to secrete TNF-alpha, IL-6, and IL-10 via JAK2/STAT3 and p38MAPK/ERK1/2 signaling pathway. J. Clin. Immunol. 2011, 31, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Choi, H.J.; Oh, C.H.; Oh, J.W.; Han, J.S. Leptin increases TNF-alpha expression and production through phospholipase D1 in Raw 264.7 cells. PLoS ONE 2014, 9, e102373. [Google Scholar] [CrossRef]
- Ziegler, J.F.; Bottcher, C.; Letizia, M.; Yerinde, C.; Wu, H.; Freise, I.; Rodriguez-Sillke, Y.; Stoyanova, A.K.; Kreis, M.E.; Asbach, P.; et al. Leptin induces TNFalpha-dependent inflammation in acquired generalized lipodystrophy and combined Crohn’s disease. Nat. Commun. 2019, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kowalczuk, A.; Wiecek, A.; Franek, E.; Kokot, F. Plasma concentration of leptin, neuropeptide Y and tumor necrosis factor alpha in patients with cancers, before and after radio- and chemotherapy. Pol. Arch. Med. Wewn. 2001, 106, 657–668. [Google Scholar]
- Conroy, S.M.; Maskarinec, G.; Morimoto, Y.; Franke, A.A.; Cooney, R.V.; Wilkens, L.R.; Goodman, M.T.; Hernadez, B.Y.; Le Marchand, L.; Henderson, B.E.; et al. Non-hodgkin lymphoma and circulating markers of inflammation and adiposity in a nested case-control study: The multiethnic cohort. Cancer Epidemiol. Biomark. Prev. 2013, 22, 337–347. [Google Scholar] [CrossRef]
- Al-Khatib, S.M.; Abdo, N.; Al-Eitan, L.N.; Al-Mistarehi, A.W.; Zahran, D.J.; Kewan, T.Z. LTA, LEP, and TNF-a Gene Polymorphisms are Associated with Susceptibility and Overall Survival of Diffuse Large B-Cell lymphoma in an Arab Population: A Case-Control Study. Asian Pac. J. Cancer Prev. 2020, 21, 2783–2791. [Google Scholar] [CrossRef]
- Barbosa-Cortes, L.; Klunder-Klunder, M.; Lopez-Alarcon, M.; Marquez, H.R.; Lopez-Aguilar, E.; Tapia-Marcial, A. Nutritional status and cytokine concentration during chemotherapy in Mexican children: A longitudinal analysis. Nutrition 2019, 57, 46–51. [Google Scholar] [CrossRef] [PubMed]
- El Demerdash, D.M.; Tawfik, N.M.; Elazab, R.; El Sissy, M.H. The Association of Pre-diagnostic Inflammatory Markers and Adipokines and the Risk of Non-Hodgkin Lymphoma Development in Egypt. Indian J. Hematol. Blood Transfus. 2021, 37, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Luo, X.; Liang, Y.; Rao, H.; Fang, X.; Jiang, W.; Lin, T.; Lin, T.; Huang, H. Fasting blood glucose is a novel prognostic indicator for extranodal natural killer/T-cell lymphoma, nasal type. Br. J. Cancer 2013, 108, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, R.; Yang, Y.; Cai, L.; Ding, S.; Xu, T.; Han, M.; Wu, X. Disruption of the Golgi protein Otg1 gene causes defective hormone secretion and aberrant glucose homeostasis in mice. Cell Biosci. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Lai, R.; Arber, D.A.; Chang, K.L.; Wilson, C.S.; Weiss, L.M. Frequency of bcl-2 expression in non-Hodgkin’s lymphoma: A study of 778 cases with comparison of marginal zone lymphoma and monocytoid B-cell hyperplasia. Mod. Pathol. 1998, 11, 864–869. [Google Scholar]
- Wagner, S.D.; Ahearne, M.; Ko Ferrigno, P. The role of BCL6 in lymphomas and routes to therapy. Br. J. Haematol. 2011, 152, 3–12. [Google Scholar] [CrossRef]
- Child, F.J.; Scarisbrick, J.J.; Calonje, E.; Orchard, G.; Russell-Jones, R.; Whittaker, S.J. Inactivation of tumor suppressor genes p15(INK4b) and p16(INK4a) in primary cutaneous B cell lymphoma. J. Investig. Dermatol. 2002, 118, 941–948. [Google Scholar] [CrossRef]
- Wang, X.J.; Medeiros, L.J.; Bueso-Ramos, C.E.; Tang, G.; Wang, S.; Oki, Y.; Desai, P.; Khoury, J.D.; Miranda, R.N.; Tang, Z.; et al. P53 expression correlates with poorer survival and augments the negative prognostic effect of MYC rearrangement, expression or concurrent MYC/BCL2 expression in diffuse large B-cell lymphoma. Mod. Pathol. 2017, 30, 194–203. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, X. The Spectrum of MYC Alterations in Diffuse Large B-Cell Lymphoma. Acta Haematol. 2020, 143, 520–528. [Google Scholar] [CrossRef]
- Sup, S.J.; Alemany, C.A.; Pohlman, B.; Elson, P.; Malhi, S.; Thakkar, S.; Steinle, R.; Hsi, E.D. Expression of bcl-2 in classical Hodgkin’s lymphoma: An independent predictor of poor outcome. J. Clin. Oncol. 2005, 23, 3773–3779. [Google Scholar] [CrossRef]
- Mahmoud, H.M.; El-Sakhawy, Y.N. Significance of Bcl-2 and Bcl-6 immunostaining in B-Non Hodgkin’s lymphoma. Hematol. Rep. 2011, 3, e26. [Google Scholar] [CrossRef]
- Xia, B.; Zhang, L.; Guo, S.Q.; Li, X.W.; Qu, F.L.; Zhao, H.F.; Zhang, L.Y.; Sun, B.C.; You, J.; Zhang, Y.Z. Coexpression of MYC and BCL-2 predicts prognosis in primary gastrointestinal diffuse large B-cell lymphoma. World J. Gastroenterol. 2015, 21, 2433–2442. [Google Scholar] [CrossRef] [PubMed]
- Patrascu, A.M.; Rotaru, I.; Olar, L.; Patrascu, S.; Ghilusi, M.C.; NeamTu, S.D.; Nacea, J.G.; Gluhovschi, A. The prognostic role of Bcl-2, Ki67, c-MYC and p53 in diffuse large B-cell lymphoma. Rom. J. Morphol. Embryol. 2017, 58, 837–843. [Google Scholar] [PubMed]
- Li, L.; Zhang, X.; Zhang, T.; Song, Z.; Hu, G.; Li, W.; Li, L.; Qiu, L.; Qian, Z.; Zhou, S.; et al. Prognostic Significance of BCL-2 and BCL-6 Expression in MYC-positive DLBCL. Clin. Lymphoma Myeloma Leuk. 2018, 18, e381–e389. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.L.; Zhang, X.Y.; Li, Y.; Song, L.L.; Wang, L. Correlations of mouse lymphoma xenografts with the expressions of MMP-9 and Bcl-2. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1176–1183. [Google Scholar] [CrossRef]
- Pileri, A.; Agostinelli, C.; Bertuzzi, C.; Grandi, V.; Maio, V.; Lastrucci, I.; Santucci, M.; Pimpinelli, N. Prognostic significance of Bcl-2 expression in primary cutaneous B-cell lymphoma: A reappraisal. Ital. J. Dermatol. Venerol. 2021, 156, 642–649. [Google Scholar] [CrossRef]
- Kale, J.; Osterlund, E.J.; Andrews, D.W. BCL-2 family proteins: Changing partners in the dance towards death. Cell Death Differ. 2018, 25, 65–80. [Google Scholar] [CrossRef]
- Lam, Q.L.; Wang, S.; Ko, O.K.; Kincade, P.W.; Lu, L. Leptin signaling maintains B-cell homeostasis via induction of Bcl-2 and Cyclin D1. Proc. Natl. Acad. Sci. USA 2010, 107, 13812–13817. [Google Scholar] [CrossRef]
- Michurina, S.V.; Ishchenko, I.Y.; Arkhipov, S.A.; Cherepanova, M.A.; Vasendin, D.V.; Zavjalov, E.L. Apoptosis in the liver of male db/db mice during the development of obesity and type 2 diabetes. Vavilov J. Genet. Breed. 2020, 24, 435–440. [Google Scholar] [CrossRef]
- Plante, E.; Menaouar, A.; Danalache, B.A.; Yip, D.; Broderick, T.L.; Chiasson, J.L.; Jankowski, M.; Gutkowska, J. Oxytocin treatment prevents the cardiomyopathy observed in obese diabetic male db/db mice. Endocrinology 2015, 156, 1416–1428. [Google Scholar] [CrossRef]
- Chen, W.; Sun, Q.; Ju, J.; Chen, W.; Zhao, X.; Zhang, Y.; Yang, Y. Effect of Astragalus Polysaccharides on Cardiac Dysfunction in db/db Mice with Respect to Oxidant Stress. Biomed. Res. Int. 2018, 2018, 8359013. [Google Scholar] [CrossRef] [PubMed]
- Sataranatarajan, K.; Ikeno, Y.; Bokov, A.; Feliers, D.; Yalamanchili, H.; Lee, H.J.; Mariappan, M.M.; Tabatabai-Mir, H.; Diaz, V.; Prasad, S.; et al. Rapamycin Increases Mortality in db/db Mice, a Mouse Model of Type 2 Diabetes. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.D.; Wu, X.H.; Zhang, L.N. Effect of leptin on expression of calpain-1 and Bcl-2 and apoptosis in myocardial tissue of neonatal rats after asphyxia. Zhongguo Dang dai er ke za zhi 2016, 18, 1044–1049. [Google Scholar]
- Shimabukuro, M.; Wang, M.Y.; Zhou, Y.T.; Newgard, C.B.; Unger, R.H. Protection against lipoapoptosis of beta cells through leptin-dependent maintenance of Bcl-2 expression. Proc. Natl. Acad. Sci. USA 1998, 95, 9558–9561. [Google Scholar] [CrossRef]
- Brown, J.E.; Dunmore, S.J. Leptin decreases apoptosis and alters BCL-2: Bax ratio in clonal rodent pancreatic beta-cells. Diabetes/Metab. Res. Rev. 2007, 23, 497–502. [Google Scholar] [CrossRef]
- da Silva, S.V.; Salama, C.; Renovato-Martins, M.; Helal-Neto, E.; Citelli, M.; Savino, W.; Barja-Fidalgo, C. Increased leptin response and inhibition of apoptosis in thymocytes of young rats offspring from protein deprived dams during lactation. PLoS ONE 2013, 8, e64220. [Google Scholar] [CrossRef] [PubMed]
- Perez-Perez, A.; Toro, A.R.; Vilarino-Garcia, T.; Guadix, P.; Maymo, J.L.; Duenas, J.L.; Varone, C.L.; Sanchez-Margalet, V. Leptin reduces apoptosis triggered by high temperature in human placental villous explants: The role of the p53 pathway. Placenta 2016, 42, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhang, J.; Cai, L.; Ding, C.; Wang, X.; Chen, H.; Wang, X.; Yan, J.; Lu, J. Leptin induces cell proliferation and reduces cell apoptosis by activating c-myc in cervical cancer. Oncol. Rep. 2013, 29, 2291–2296. [Google Scholar] [CrossRef]
- Han, S.S.; Yun, H.; Son, D.J.; Tompkins, V.S.; Peng, L.; Chung, S.T.; Kim, J.S.; Park, E.S.; Janz, S. NF-kappaB/STAT3/PI3K signaling crosstalk in iMyc E mu B lymphoma. Mol. Cancer 2010, 9, 97. [Google Scholar] [CrossRef]
- Uddin, S.; Bu, R.; Ahmed, M.; Hussain, A.R.; Ajarim, D.; Al-Dayel, F.; Bavi, P.; Al-kuraya, K.S. Leptin receptor expression and its association with PI3K/AKT signaling pathway in diffuse large B-cell lymphoma. Leuk. Lymphoma 2010, 51, 1305–1314. [Google Scholar] [CrossRef]
- Lin, S.; Li, Y.; Xing, X.M.; Ran, W.W. Expression and significance of leptin receptor, p-STAT3 and p-AKT in diffuse large B-cell lymphoma. Acta Histochem. 2014, 116, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, P.; Guo, F.; Liu, R.; Yang, Y.; Huang, C.; Shu, H.; Gong, J.; Cai, M. Genetic G2548A polymorphism of leptin gene and risk of cancer: A meta-analysis of 6860 cases and 7956 controls. J. BUON 2014, 19, 1096–1104. [Google Scholar] [PubMed]
- Lin, H.Y.; Shi, H.; Li, C.Y.; Chen, Q.C.; Huang, T.B.; Liu, P.C.; Lou, L.M. LEP and LEPR polymorphisms in non-Hodgkin lymphoma risk: A systematic review and pooled analysis. J. BUON 2015, 20, 261–268. [Google Scholar]
- Vasku, V.; Vasku, A.; Vasku, J.B. Pharmacogenetic contribution of leptin gene polymorphism in cutaneous T-cell lymphoma. Int. J. Clin. Exp. Pathol. 2009, 2, 163–168. [Google Scholar]
- Liu, P.; Shi, H.; Huang, C.; Shu, H.; Liu, R.; Yang, Y.; Gong, J.; Yang, Y.; Cai, M. Association of LEP A19G polymorphism with cancer risk: A systematic review and pooled analysis. Tumour Biol. 2014, 35, 8133–8141. [Google Scholar] [CrossRef]
- Liu, P.; Shi, H.; Liu, R.; Yang, Y.; Yang, Y.; Huang, C.; Shu, H.; Gong, J.; Cai, M. Lack of association between LEPR Q223R polymorphisms and cancer susceptibility: Evidence from a meta-analysis. J. BUON 2014, 19, 855–862. [Google Scholar]
- Yang, J.; Zhong, Z.; Tang, W.; Chen, J. Leptin rs2167270 G > A (G19A) polymorphism may decrease the risk of cancer: A case-control study and meta-analysis involving 19 989 subjects. J. Cell. Biochem. 2019, 120, 10998–11007. [Google Scholar] [CrossRef] [PubMed]
- Skibola, D.R.; Smith, M.T.; Bracci, P.M.; Hubbard, A.E.; Agana, L.; Chi, S.; Holly, E.A. Polymorphisms in ghrelin and neuropeptide Y genes are associated with non-Hodgkin lymphoma. Cancer Epidemiol. Biomark. Prev. 2005, 14, 1251–1256. [Google Scholar] [CrossRef]
- Cui, H.; Lopez, M.; Rahmouni, K. The cellular and molecular bases of leptin and ghrelin resistance in obesity. Nat. Rev. Endocrinol. 2017, 13, 338–351. [Google Scholar] [CrossRef]
- Wang, Q.; Bing, C.; Al-Barazanji, K.; Mossakowaska, D.E.; Wang, X.M.; McBay, D.L.; Neville, W.A.; Taddayon, M.; Pickavance, L.; Dryden, S.; et al. Interactions between leptin and hypothalamic neuropeptide Y neurons in the control of food intake and energy homeostasis in the rat. Diabetes 1997, 46, 335–341. [Google Scholar] [CrossRef]
- Goto, M.; Arima, H.; Watanabe, M.; Hayashi, M.; Banno, R.; Sato, I.; Nagasaki, H.; Oiso, Y. Ghrelin increases neuropeptide Y and agouti-related peptide gene expression in the arcuate nucleus in rat hypothalamic organotypic cultures. Endocrinology 2006, 147, 5102–5109. [Google Scholar] [CrossRef] [PubMed]
- Han, T.J.; Xu, H.Z.; Li, J.S.; Geng, L.Y.; Li, X.Y.; Zhou, X.X.; Wang, X. Leptin and its receptor in glucose metabolism of T-cell lymphoma. Oncol. Lett. 2018, 16, 5838–5846. [Google Scholar] [CrossRef]
- Bertolini, F.; Paolucci, M.; Peccatori, F.; Cinieri, S.; Agazzi, A.; Ferrucci, P.F.; Cocorocchio, E.; Goldhirsch, A.; Martinelli, G. Angiogenic growth factors and endostatin in non-Hodgkin’s lymphoma. Br. J. Haematol. 1999, 106, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Pamuk, G.E.; Demir, M.; Harmandar, F.; Yesil, Y.; Turgut, B.; Vural, O. Leptin and resistin levels in serum of patients with hematologic malignancies: Correlation with clinical characteristics. Exp. Oncol. 2006, 28, 241–244. [Google Scholar]
- Petridou, E.T.; Sergentanis, T.N.; Dessypris, N.; Vlachantoni, I.T.; Tseleni-Balafouta, S.; Pourtsidis, A.; Moschovi, M.; Polychronopoulou, S.; Athanasiadou-Piperopoulou, F.; Kalmanti, M.; et al. Serum adiponectin as a predictor of childhood non-Hodgkin’s lymphoma: A nationwide case-control study. J. Clin. Oncol. 2009, 27, 5049–5055. [Google Scholar] [CrossRef]
- Petridou, E.T.; Dessypris, N.; Panagopoulou, P.; Sergentanis, T.N.; Mentis, A.F.; Pourtsidis, A.; Polychronopoulou, S.; Kalmanti, M.; Athanasiadou-Piperopoulou, F.; Moschovi, M. Adipocytokines in relation to Hodgkin lymphoma in children. Pediatr. Blood Cancer 2010, 54, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Okur, F.V.; Karadeniz, C.; Buyukpamukcu, M.; Oguz, A.; Yucel, A.; Cinaz, P.; Emir, S.; Varan, A. Clinical significance of serum vascular endothelial growth factor, endostatin, and leptin levels in children with lymphoma. Pediatr. Blood Cancer 2010, 55, 1272–1277. [Google Scholar] [CrossRef]
- Head, G.A. The oestrogen-leptin paradox. J. Physiol. 2015, 593, 1523. [Google Scholar] [CrossRef]
- Ren, J. Lessons from the leptin paradox in cardiac regulation--too much versus too little. J. Physiol. 2005, 565, 347. [Google Scholar] [CrossRef]
- Zhao, S.; Kusminski, C.M.; Scherer, P.E. Adiponectin, Leptin and Cardiovascular Disorders. Circ. Res. 2021, 128, 136–149. [Google Scholar] [CrossRef]
- Yaris, N.; Sozen, E.; Erduran, E.; Okten, A.; Orem, A.; Cakirbay, H. Bone mineral metabolism and its relationship to leptin levels in survivors of childhood leukemia and lymphoma. Pediatr. Hematol. Oncol. 2005, 22, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.S.; Park, C.S.; Yoon, D.H.; Suh, C.; Huh, J. High concordance of gene expression profiling-correlated immunohistochemistry algorithms in diffuse large B-cell lymphoma, not otherwise specified. Am. J. Surg. Pathol. 2014, 38, 1046–1057. [Google Scholar] [CrossRef] [PubMed]
- Dubois, S.; Tesson, B.; Mareschal, S.; Viailly, P.J.; Bohers, E.; Ruminy, P.; Etancelin, P.; Peyrouze, P.; Copie-Bergman, C.; Fabiani, B.; et al. Refining diffuse large B-cell lymphoma subgroups using integrated analysis of molecular profiles. EBioMedicine 2019, 48, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Pahwa, P.; Karunanayake, C.P.; Spinelli, J.J.; Dosman, J.A.; McDuffie, H.H. Ethnicity and incidence of Hodgkin lymphoma in Canadian population. BMC Cancer 2009, 9, 141. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, B.; Zimmermann, M.; Oschlies, I.; Niggli, F.; Mann, G.; Parwaresch, R.; Riehm, H.; Schrappe, M.; Reiter, A.; the BFM Group. The impact of age and gender on biology, clinical features and treatment outcome of non-Hodgkin lymphoma in childhood and adolescence. Br. J. Haematol. 2005, 131, 39–49. [Google Scholar] [CrossRef]
- Wilson, L.D.; Hinds, G.A.; Yu, J.B. Age, race, sex, stage, and incidence of cutaneous lymphoma. Clin. Lymphoma Myeloma Leuk. 2012, 12, 291–296. [Google Scholar] [CrossRef]
- Li, Q.; Chang, E.T.; Bassig, B.A.; Dai, M.; Qin, Q.; Gao, Y.; Zhang, Y.; Zheng, T. Body size and risk of Hodgkin’s lymphoma by age and gender: A population-based case-control study in Connecticut and Massachusetts. Cancer Causes Control 2013, 24, 287–295. [Google Scholar] [CrossRef]
- Blansky, D.; Mantzaris, I.; Rohan, T.; Hosgood, H.D., 3rd. Influence of Rurality, Race, and Ethnicity on Non-Hodgkin Lymphoma Incidence. Clin. Lymphoma Myeloma Leuk. 2020, 20, 668–676.e665. [Google Scholar] [CrossRef]
- Nuttall, F.Q. Body Mass Index: Obesity, BMI, and Health: A Critical Review. Nutr. Today 2015, 50, 117–128. [Google Scholar] [CrossRef]
- Huxley, R.; Mendis, S.; Zheleznyakov, E.; Reddy, S.; Chan, J. Body mass index, waist circumference and waist:hip ratio as predictors of cardiovascular risk—a review of the literature. Eur. J. Clin. Nutr. 2010, 64, 16–22. [Google Scholar] [CrossRef]
- Chou, K.; Perry, C.M. Metreleptin: First global approval. Drugs 2013, 73, 989–997. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, N.X.S.; Adiyaman, S.C.; Yuksel, B.D.; Ferrari, C.T.; Eldin, A.J.; Saydam, B.O.; Altay, C.; Sharma, P.; Bhave, N.; Little, A.; et al. Unusual Presentations of Lmna-Associated Lipodystrophy with Complex Phenotypes and Generalized Fat Loss: When the Genetic Diagnosis Uncovers Novel Features. AACE Clin. Case Rep. 2020, 6, e79–e85. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.J.; Chan, J.L.; Jaffe, E.S.; Cochran, E.; DePaoli, A.M.; Gautier, J.F.; Goujard, C.; Vigouroux, C.; Gorden, P. Lymphoma in acquired generalized lipodystrophy. Leuk. Lymphoma 2016, 57, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Esfandiari, N.H.; Rubenfire, M.; Neidert, A.H.; Hench, R.; Eldin, A.J.; Meral, R.; Oral, E.A. Diagnosis of acquired generalized lipodystrophy in a single patient with T-cell lymphoma and no exposure to Metreleptin. Clin. Diabetes Endocrinol. 2019, 5, 4. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).