Healing of Ligaments and Tendons: Tissue Engineering and Models
Conflicts of Interest
Abbreviations
| ADSC | Adipose-tissue-derived stem cells |
| ACL | Anterior cruciate ligament |
| 3D | Three-dimensional |
| ECM | Extracellular matrix |
| EVs | Extracellular vesicles |
| MSCs | Mesenchymal stem cells |
| TE | Tissue engineering |
| 3R | Replace, reduce, refine |
References
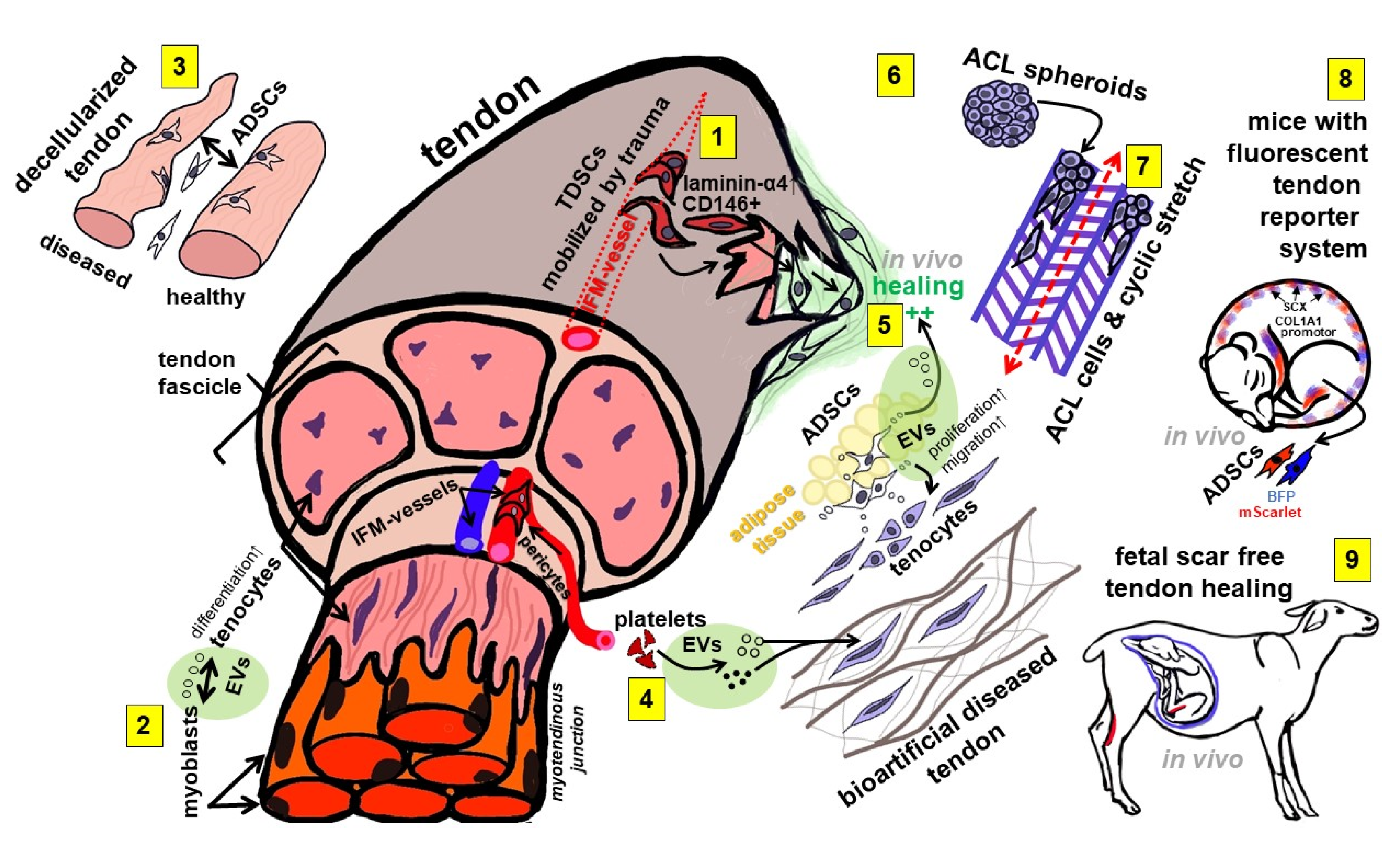
- Gögele, C.; Konrad, J.; Hahn, J.; Breier, A.; Schröpfer, M.; Meyer, M.; Merkel, R.; Hoffmann, B.; Schulze-Tanzil, G. Maintenance of Ligament Homeostasis of Spheroid-Colonized Embroidered and Functionalized Scaffolds after 3D Stretch. Int. J. Mol. Sci. 2021, 22, 8204. [Google Scholar] [CrossRef] [PubMed]
- Graça, A.L.; Domingues, R.M.A.; Calejo, I.; Gómez-Florit, M.; Gomes, M.E. Therapeutic Effects of Platelet-Derived Extracellular Vesicles in a Bioengineered Tendon Disease Model. Int. J. Mol. Sci. 2022, 23, 2948. [Google Scholar] [CrossRef] [PubMed]
- Doll, C.U.; Niebert, S.; Burk, J. Mesenchymal Stromal Cells Adapt to Chronic Tendon Disease Environment with an Initial Reduction in Matrix Remodeling. Int. J. Mol. Sci. 2021, 22, 12798. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Li, J.; Giannopoulos, A.; Kingham, P.J.; Backman, L.J. Secretome from In Vitro Mechanically Loaded Myoblasts Induces Tenocyte Migration, Transition to a Fibroblastic Phenotype and Suppression of Collagen Production. Int. J. Mol. Sci. 2021, 22, 13089. [Google Scholar] [CrossRef] [PubMed]
- Marr, N.; Meeson, R.; Kelly, E.F.; Fang, Y.; Peffers, M.J.; Pitsillides, A.A.; Dudhia, J.; Thorpe, C.T. CD146 Delineates an Interfascicular Cell Sub-Population in Tendon That Is Recruited during Injury through Its Ligand Laminin-α4. Int. J. Mol. Sci. 2021, 22, 9729. [Google Scholar] [CrossRef] [PubMed]
- Jozsa, L.; Lehto, M.; Kannus, P.; Kvist, M.; Reffy, A.; Vieno, T.; Järvinen, M.; Demel, S.; Elek, E. Fibronectin and laminin in Achilles tendon. Acta Orthop. Scand. 1989, 60, 469–471. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Chen, C.; Dong, S.; Xie, G.; Jiang, J.; Zhao, S.; Zhao, J. Differentiation Ability of Tendon-Derived Stem Cells and Histological Characteristics of Rotator Cuff Remnant on the Greater Tuberosity Degenerated with Age and Chronicity. Arthrosc. J. Arthrosc. Relat. Surg. 2021, 38, 1037–1048. [Google Scholar] [CrossRef] [PubMed]
- Ruzzini, L.; Abbruzzese, F.; Rainer, A.; Longo, U.G.; Trombetta, M.; Maffulli, N.; Denaro, V. Characterization of age-related changes of tendon stem cells from adult human tendons. Knee Surg. Sports Traumatol. Arthrosc. 2013, 22, 2856–2866. [Google Scholar] [CrossRef] [PubMed]
- Favata, M.; Beredjiklian, P.K.; Zgonis, M.H.; Beason, D.P.; Crombleholme, T.M.; Jawad, A.F.; Soslowsky, L.J. Regenerative properties of fetal sheep tendon are not adversely affected by transplantation into an adult environment. J. Orthop. Res. 2006, 24, 2124–2132. [Google Scholar] [CrossRef] [PubMed]
- Ribitsch, I.; Bileck, A.; Aldoshin, A.; Kańduła, M.; Mayer, R.; Egerbacher, M.; Gabner, S.; Auer, U.; Gültekin, S.; Huber, J.; et al. Molecular Mechanisms of Fetal Tendon Regeneration Versus Adult Fibrous Repair. Int. J. Mol. Sci. 2021, 22, 5619. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-H.; Chen, Z.-Y.; Lin, Y.-H.; Chen, S.-H.; Chou, P.-Y.; Kao, H.-K.; Lin, F.-H. Extracellular Vesicles of Adipose-Derived Stem Cells Promote the Healing of Traumatized Achilles Tendons. Int. J. Mol. Sci. 2021, 22, 12373. [Google Scholar] [CrossRef] [PubMed]
- Zahn, I.; Braun, T.; Gogele, C.; Schulze-Tanzil, G. Minispheroids as a Tool for Ligament Tissue Engineering: Do the Self-Assembly Techniques and Spheroid Dimensions Influence the Cruciate Ligamentocyte Phenotype? Int. J. Mol. Sci. 2021, 22, 11011. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zhou, X.; Skutella, T. A Tendon-Specific Double Reporter Transgenic Mouse Enables Tracking Cell Lineage and Functions Alteration In Vitro and In Vivo. Int. J. Mol. Sci. 2021, 22, 11189. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schulze-Tanzil, G.G. Healing of Ligaments and Tendons: Tissue Engineering and Models. Int. J. Mol. Sci. 2022, 23, 15503. https://doi.org/10.3390/ijms232415503
Schulze-Tanzil GG. Healing of Ligaments and Tendons: Tissue Engineering and Models. International Journal of Molecular Sciences. 2022; 23(24):15503. https://doi.org/10.3390/ijms232415503
Chicago/Turabian StyleSchulze-Tanzil, Gundula Gesine. 2022. "Healing of Ligaments and Tendons: Tissue Engineering and Models" International Journal of Molecular Sciences 23, no. 24: 15503. https://doi.org/10.3390/ijms232415503
APA StyleSchulze-Tanzil, G. G. (2022). Healing of Ligaments and Tendons: Tissue Engineering and Models. International Journal of Molecular Sciences, 23(24), 15503. https://doi.org/10.3390/ijms232415503




